Summary
Background
The risk of death from spontaneous intracerebral haemorrhage is increased for people taking antiplatelet drugs. We aimed to assess the feasibility of randomising patients on antiplatelet drug therapy with spontaneous intracerebral haemorrhage to desmopressin or placebo to reduce the antiplatelet drug effect.
Methods
DASH was a phase 2, randomised, placebo-controlled, multicentre feasibility trial. Patients were recruited from ten acute stroke centres in the UK and were eligible if they had an intracerebral haemorrhage with stroke symptom onset within 24 h of randomisation, were aged 18 years or older, and were taking an antiplatelet drug. Participants were randomly assigned (1:1) to a single dose of intravenous desmopressin 20 μg or matching placebo. Treatment allocation was concealed from all staff and patients involved in the trial. The primary outcome was feasibility, which was measured as the number of eligible patients randomised and the proportion of eligible patients approached, and analysis was by intention to treat. The trial was prospectively registered with ISRCTN (reference ISRCTN67038373), and it is closed to recruitment.
Findings
Between April 1, 2019, and March 31, 2022, 1380 potential participants were screened for eligibility. 176 (13%) participants were potentially eligible, of whom 57 (32%) were approached, and 54 (31%) consented and were subsequently recruited and randomly assigned to receive desmopressin (n=27) or placebo (n=27). The main reason for eligible patients not being recruited was the patient arriving out of hours (74 [61%] of 122 participants). The recruitment rate increased after the enrolment period was extended from 12 h to 24 h, but it was then impaired due to the COVID-19 pandemic. Of the 54 participants included in the analysis (mean age 76·4 years [SD 11·3]), most were male (36 [67%]) and White (50 [93%]). 53 (98%) of 54 participants received all of their allocated treatment (one participant assigned desmopressin only received part of the infusion). No participants were lost to follow-up or withdrew from the trial. Death or dependency on others for daily activities at day 90 (modified Rankin Scale score >4) occurred in six (22%) of 27 participants in the desmopressin group and ten (37%) of 27 participants in the placebo group. Serious adverse events occurred in 12 (44%) participants in the desmopressin group and 13 (48%) participants in the placebo group. The most common adverse events were expansion of the haemorrhagic stroke (four [15%] of 27 participants in the desmopressin group and six [22%] of 27 participants in the placebo group) and pneumonia (one [4%] of 27 participants in the desmopressin group and six [22%] of 27 participants in the placebo group).
Interpretation
Our results show it is feasible to randomise patients with spontaneous intracerebral haemorrhage who are taking antiplatelet drugs to desmopressin or placebo. Our findings support the need for a definitive trial to determine if desmopressin improves outcomes in patients with intracerebral haemorrhage on antiplatelet drug therapy.
Funding
National Institute for Health Research.
Introduction
In 2019, approximately 3 million deaths were due to spontaneous intracerebral haemorrhage worldwide.1 Two-thirds of survivors are left dependent on others.2 Approximately a quarter of patients are taking antiplatelet drugs at the time of intracerebral haemorrhage in high-income countries, and this proportion is increasing.3 Prestroke antiplatelet drug use is associated with a 27% relative increase in 1-month case fatality, compared with patients who do not use antithrombotic drugs.4 There is no proven effective drug treatment for intracerebral haemorrhage, although blood pressure lowering might improve outcomes.5 This topic area has been highly prioritised by patient and public involvement groups.6
Desmopressin is a licensed prohaemostatic drug that is commonly used in the treatment of inherited bleeding disorders. In other settings, such as for patients on antiplatelet drugs undergoing cardiac surgery, use of desmopressin can reduce the risk of bleeding for patients taking antiplatelet drugs.7, 8 The outcome after intracerebral haemorrhage is closely related to haematoma expansion, which is a predictor of poor prognosis and is associated with death and disability.9 Use of antiplatelet drugs or anticoagulants, time from onset of symptoms to baseline imaging, and intracerebral haematoma volume on baseline imaging are independent predictors of haematoma expansion.10
Research in context.
Evidence before this study
We searched MEDLINE Ovid (from database inception) and Embase Ovid (from database inception) on Feb 10, 2023, combining search terms for desmopressin, intracerebral haemorrhage, and randomised controlled trials. No language restrictions were applied. Desmopressin reduces bleeding in some settings (eg, patients taking antiplatelet drugs undergoing cardiac surgery) and has a well established role in inherited coagulopathies. No randomised trials have been completed that compared desmopressin with placebo for reversing antiplatelet drug effects in spontaneous intracerebral haemorrhage.
Added value of this study
DASH was designed to demonstrate the feasibility of performing a large randomised trial to assess efficacy of desmopressin for spontaneous intracerebral haemorrhage in patients exposed to antiplatelet drugs. To our knowledge, this is the first randomised trial comparing desmopressin with placebo in patients with intracerebral haemorrhage who are taking antiplatelet drugs. No safety concerns or clinically relevant hyponatraemia occurred.
Implications of all the available evidence
Given sufficient recruiting centres and pragmatic trial methodology, it should be feasible to conduct a definitive trial to determine if desmopressin improves outcome in patients on antiplatelet drug therapy with intracerebral haemorrhage. Important considerations for a definitive trial are allowing 24 h after stroke symptoms onset for recruitment, facilitating recruitment out of hours, and using simplified temperature monitoring for desmopressin.
Recommendations of international guidelines for the management of intracerebral haemorrhage in people taking antiplatelets are conflicting.11, 12, 13, 14 We hypothesised that desmopressin might reduce bleeding and improve outcomes in people with intracerebral haemorrhage. To our knowledge, no randomised trials comparing desmopressin with placebo have been conducted in people with intracerebral haemorrhage. Before embarking on a definitive randomised controlled trial, to determine the effectiveness of desmopressin, we conducted a feasibility trial to test the methods for a full trial, to understand whether participants could be recruited to such a trial and, if so, the rate of recruitment. The primary objective was to assess the feasibility of randomising, administering the intervention, and completing follow-up for patients treated with desmopressin or placebo, to inform a definitive trial.
Methods
Study design and participants
DASH was a phase 2, randomised, placebo-controlled, multicentre feasibility trial. Participants were enrolled by investigators from emergency departments or acute stroke units at ten hospitals in the UK. The trial was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki, and ethics approval was obtained from the East Midlands–Nottingham 2 research ethics committee (18/EM/0184). The DASH trial protocol has been published elsewhere.15 Four substantial amendments were planned by the trial management committee, reviewed and supported by the trial steering committee, and approved by the funder and research ethics committee: SA/01/19 (addition of day 90 postal follow-up form); SA/02/19 (change of site principal investigator); SA/03/19 (increase in recruitment time window from 12 h to 24 h after stroke, update to Investigational Medicinal Products storage guidelines, and removal of day 90 questions on postal follow-up questionnaire regarding low sodium and fluid overload); and SA/04/21 (temporary change of chief investigator; closure of one recruiting centre). Ten sites were open to recruitment before the pandemic and before all research was suspended. The study was paused due to the COVID-19 pandemic from March 26, 2020, to June 29, 2020, after which centres reopened according to capacity at sites. One site did not reopen. Even though the other nine sites reopened on June 2, 2020, they had reduced capacity and recruitment rates never reached pre-pandemic levels. To help mitigate the impact of COVID-19 on recruitment, the trial end date was extended from: Dec 31, 2020, to Dec 31, 2021 (costed extension), and Dec 31, 2021, to June 30, 2022 (no-cost extension).
Patients were included in the trial if they met all inclusion criteria and no exclusion criteria. Inclusion criteria were that the patient was an adult (aged ≥18 years), with intracerebral haemorrhage confirmed on brain CT, and prescribed and thought to be taking a daily oral antiplatelet drug in the preceding 7 days (ie, cyclooxygenase inhibitors, phosphodiesterase inhibitors, or P2Y12 inhibitors). In the initial version of the protocol, patients were eligible if they could undergo randomisation within 12 h of symptom onset. After a substantial amendment (SA/03/19 on Nov 22, 2019) due to slow enrolment, participants could be recruited if they could undergo randomisation within 24 h from onset of symptoms. Exclusion criteria were known secondary causes of intracerebral haemorrhage, patients at risk of fluid retention, systolic blood pressure of less than 90 mm Hg, known drug-eluting stent in previous 3 months, allergy to desmopressin, pregnant or breastfeeding, life expectancy of less than 4 h or planned for palliative care only, Glasgow Coma Scale score of less than 5, and modified Rankin Scale score of more than 4.
Patients with the capacity to give informed written consent were approached directly; otherwise, a relative or close friend was approached for consent. If a relative or friend could not be identified, a professional representative (ie, doctor) not associated with the trial could be approached for proxy consent. Patients were approached again for consent when they regained capacity. This procedure is in accordance with emergency consent approvals.
Randomisation and masking
Participants were randomised centrally to receive either desmopressin or placebo (1:1), with a 5% random flip, using a computer-generated randomisation sequence in real time. Treatment allocation was concealed from all staff and participants in the trial. Randomisation involved minimisation on key prognostic risk factors: age (≥70 years); sex (male); time since onset (≥3 h); systolic blood pressure (≥170 mm Hg); and presence (or no information on presence or absence) of intraventricular haemorrhage. Randomisation allocated a number corresponding to a treatment pack and the participant received treatment from the allocated numbered pack. It was considered not possible to mask personnel preparing the injection for administration to the treatment allocation because the ampoules of active and placebo injections were different and carried the manufacturer's identifying information. Clinicians, patients, and outcome assessors (ie, clinical, radiological, and haematological assessors) were blinded to treatment allocation.
Procedures
Participants allocated desmopressin received a 20 μg dose intravenously. Desmopressin (Ferring Pharmaceuticals, West Drayton, UK) was supplied in 1 mL glass ampoules at a concentration of 4 μg/mL; five ampoules were added to 50 mL sodium chloride 0·9%, and the infusion was done for 20 min. Participants allocated placebo received sodium chloride 0·9% intravenously. The placebo was prepared by adding three 2 mL ampoules of sodium chloride 0·9% to 50 mL sodium chloride 0·9%, and the infusion was done for 20 min. In clinical trials of desmopressin for intracerebral haemorrhage, a dose of up to 0·4 μg/kg was used.11 The desmopressin dose was capped at 20 μg. Capping the dose has similar efficacy to using a weight-adjusted dose for patients with inherited bleeding disorders,16 and we considered there would be a lower risk of errors in the emergency setting using a standard dose. All patients received usual standard care, including venous thromboembolism prophylaxis with intermittent pneumatic compression devices, blood pressure lowering therapy, and neurosurgery if appropriate.
According to the summary of product characteristics,17 desmopressin should be stored at 2–8°C. We developed a pharmacy manual such that desmopressin or placebo was quarantined in the event the storage temperature was less than 2°C or higher than 8°C for 28 days cumulatively (as per stability data) or any temperature higher than 25°C. We summarised temperature excursions, including total number of temperature excursions, number of desmopressin or placebo packs that were exposed, quarantined, or destroyed, and the number of participants receiving desmopressin or placebo who had been exposed to temperature excursion. Although not listed as a specific outcome in the protocol, the incidence of temperature excursions contributes to the overall assessment of feasibility and is presented.
Sites were required to complete screening logs for consecutive patients with intracerebral haemorrhage. Participants’ age, sex, medical history, antiplatelet drug use, intracerebral haemorrhage location, intraventricular haemorrhage, and modified Rankin scale score were obtained at baseline by local investigators. Participants were reviewed again on day 2, at hospital discharge, and on day 90 to gather information on interventions, adverse events, discharge date, and discharge destination (eg, patient's own home or to nursing home care).
At day 90, central assessors trained in administering the appropriate questionnaires, and who were masked to treatment allocation, followed up each participant by telephone. Questionnaires administered were the Barthel index (to measure disability; 0–100, with 0 indicating severe disability and 100 indicting no disability), the modified Rankin scale (to measure functional independence; 0–6, with 0 indicating no disability through to 6 indicating death), the EuroQol-5D health state utility and visual analogue scale (to measure quality of life), the telephone interview for cognitive status-modified (to assess cognition), and the Zung depression scale (to assess mood). Serious adverse events were also reported by telephone up to day 90 (and could be reported at any time).
Brain imaging (CT head scan) was performed before randomisation and 24 h after administration of the allocated treatment to measure haematoma volume. Haematoma volume was measured centrally, by a trained assessor who was masked to treatment allocation, using semi-automated segmentation. Haematoma expansion was defined as an absolute increase in haematoma volume of greater than 6 mL or relative growth of greater than 33%.18
To assess baseline platelet function, platelets were stimulated with arachidonic acid or adenosine diphosphate. Platelet cell surface P-selectin expression was then measured using a standardised assay (Platelet Solutions, Nottingham, UK) to assess retrospectively whether patients were taking antiplatelet drugs, as previously described (appendix p 5).19 Von Willebrand factor antigen (VWF Ag Kit, Siemens, Marburg, Germany), Von Willebrand factor activity (Innovance VWF Ac kit, Siemens, Marburg, Germany), and chromogenic factor VIII were measured centrally, at baseline and 1 h after allocated treatment, by assessors who were masked to treatment allocation. Serum sodium was assessed locally 24 h after administration of the desmopressin or placebo, and these results were made available to the clinical team. Hyponatraemia was defined as a sodium concentration less than 135 mmol/L. Moderate to severe hyponatraemia was defined as a sodium concentration less than 125 mmol/L.
Outcomes
The primary outcome measures of this feasibility trial were the proportion of eligible patients who were enrolled and received their allocated treatment, the proportion of eligible patients who underwent randomisation (and reasons for non-randomisation), the proportion of screened patients who were eligible, the rate of participant recruitment per month per site, time to randomisation after hospital admission, adherence to the allocated treatment, the proportion of participants followed up to 90 days (and reasons for loss to follow up), and the proportion of randomised participants with full outcome data available (and reasons for non-availability of data).
Secondary outcomes were change in haematoma volume at 24 h (including changes meeting the definition of haematoma expansion), hyponatraemia at 24 h, length of hospital stay, discharge destination, death or dependency at day 90 (modified Rankin scale score >4), mortality within 28 days and up to day 90, serious adverse events up to day 90 (including thromboembolic events), disability at day 90 (Barthel index), quality of life at day 90 (EuroQol), cognition at day 90 (telephone interview for cognitive status-modified), and mood at day 90 (Zung depression scale). Other secondary outcome measures were baseline platelet dysfunction (measured by P-selectin assay), and change in factor VIII, Von Willebrand factor antigen, and Von Willebrand factor activity at 1 h after administration of desmopressin or placebo. The manufacturer of the P-selectin assay closed their business before the end of the trial, so analysis of this outcome in all participants was not completed. A protocol-defined health economic assessment will be reported separately.
All adverse events on day 1 (including during infusion) and for the 24-h period after dose administration were collected. All adverse events were assessed for seriousness, expectedness, and causality by adjudicators masked to treatment allocation. Serious adverse events were categorised in accordance with the Medical Dictionary for Regulatory Authorities. Serious adverse events occurring within the first 7 days were assessed for seriousness, expectedness, and causality. Fatal serious adverse events and safety outcome events (eg, fluid overload and hyponatraemia) were reported until day 90.
Statistical analysis
No formal sample size was calculated because this study was a feasibility trial to inform potential recruitment rates for a definitive trial. However, we considered that if more than 50 participants were randomised from ten centres in a 12-month period, it would be probable that, assuming similar recruitment rates in additional centres, a larger study recruiting approximately 1200 participants in around 50 centres recruiting for 60 months would be feasible.
Data were analysed by a qualified statistician, using a validated software package (SAS version 9.4). A statistical analysis plan (SAP) was agreed on before database lock and release of randomisation codes. An independent data monitoring committee reviewed the data during the trial on July 14, 2019 (n=3), and March 13, 2020 (n=24). A further Chairman's review was conducted on Oct 4, 2021 (n=44).
All available data were used, including overall numbers of patients presenting to the participating hospital stroke services and screening data. Sites completed and returned monthly screening logs on consecutive patients with intracerebral haemorrhage and included ineligibility or reasons for non-inclusion. When summaries by treatment group were provided, these were based on an intention-to-treat population, with the exception of safety data. A safety population was defined to summarise the safety data in this study, and these participants were summarised according to the treatment they received irrespective of randomisation. Variables were summarised by treatment group, using summary statistics. Counts were summarised using n (%), and continuous variables were summarised using mean (SD) or median (IQR) depending on their distribution.
Because the proposed primary efficacy outcome in a definitive trial would be death or dependency at day 90, measured using the modified Rankin scale, we compared between treatment groups the full modified Rankin scale at day 90, using unadjusted ordinal logistic regression, and death and dependency at day 90 (modified Rankin scale score >4), using unadjusted binary logistic regression. Results of these analyses are reported as odds ratios (ORs) with corresponding 95% CIs. No formal statistical comparisons were made for any of the other outcome measures. The occurrence of missing data was also reported, with no imputation of missing data.
The DASH trial was prospectively registered with ISRCTN, ISRCTN67038373.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
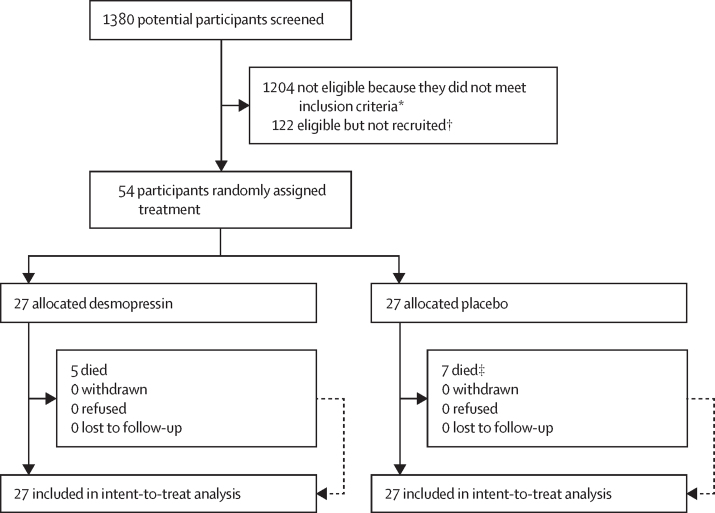
Between April 1, 2019, and March 31, 2022, 1380 potential participants were screened for eligibility at ten hospitals in the UK (appendix p 1). After the pandemic, 13 participants were potentially eligible but not approached for recruitment due to sites being on hold, no investigational product being available, or not having capacity, compared with no eligible patients missed for these capacity reasons before the pandemic. 1204 (87%) of 1380 participants were considered ineligible. The most common reasons for ineligibility were no history of antiplatelet use (843 [70%] participants) or presentation to hospital more than 24 h after symptom onset (152 [13%] participants; appendix p 2). 176 (13%) of 1380 participants were potentially eligible, of whom 57 (32%) were approached for consent. 54 (31%) consented and were subsequently recruited and underwent randomisation (figure 1). The most common reasons for the 122 eligible patients not being recruited were the patients arriving out of hours (74 [61%] participants), no doctor being available (17 [14%] participants), and recruitment being on hold (ten [8%] participants; appendix p 4). Patients who were missed were potentially eligible but were not recruited.
Figure 1.
Trial profile
*Further details provided in appendix (p 2). †Further details in appendix (pp 2, 4). ‡6 died in the placebo group before day 28 and 1 died between day 28 and day 90.
Patient consent was obtained from 23 (43%) of 54 participants before enrolment in the trial. Proxy consent was used for the other 31 (57%) participants and was provided by personal nominees for 27 (50%) and by professional legal nominees for four (7%).
Participant characteristics are summarised in table 1. Of the 54 participants included in the analysis (mean age 76·4 years [SD 11·3]), 36 (67%) were male and 50 (93%) were White). The median time from stroke onset to randomisation across all sites was 10·2 h (IQR 4·5–16·3; desmopressin group, 10·2 h [IQR 5·0–16·1]; placebo group, 9·5 h [IQR 4·3–17·6]). The rate of recruitment increased after the enrolment time was extended from 12 h to 24 h. 14 patients were enrolled in 8 months at nine sites (1·8 patients per month) before the amendment, and 11 patients were enrolled in 4 months (2·8 patients per month) after the amendment and before the COVID-19 pandemic. Post-pandemic, 29 patients were enrolled in 22 months (1·3 patient per month). Mean recruitment rate per centre per month was the same pre-pandemic, post-pandemic, and overall: 0·2 (95% CI 0·1–0·3) in each case (appendix p 3).
Table 1.
Baseline characteristics of participants
| All participants (n=54) | Desmopressin group (n=27) | Placebo group (n=27) | ||
|---|---|---|---|---|
| Age, years | 76·4 (11·3) | 76·4 (10·7) | 72·7 (11·7) | |
| Sex | ||||
| Male | 36 (67%) | 16 (59%) | 20 (74%) | |
| Female | 18 (33%) | 11 (41%) | 7 (26%) | |
| Ethnicity | ||||
| White | 50 (93%) | 25 (93%) | 25 (93%) | |
| Asian | 2 (4%) | 1 (4%) | 1 (4%) | |
| Black | 1 (2%) | 1 (4%) | 0 | |
| Other | 1 (2%) | 0 | 1 (4%) | |
| Weight, kg | 75·7 (18·6); n=51 | 74·8 (19·2); n=25 | 76·5 (18·3); n=26 | |
| <50 kg | 2 (4%); n=51 | 1 (4%); n=25 | 1 (4%); n=26 | |
| Time from stroke onset to randomisation, h | 10·2 (4·5–16·3) | 10·2 (5·0–16·1) | 9·5 (4·3–17·6) | |
| ≥3 h | 44 (81%) | 23 (85%) | 21 (78%) | |
| ≥12 h (after the protocol change to 24 h recruitment) | 21 (53%); n=40 | 10 (5); n=20 | 11 (55%); n=20 | |
| Time from stroke onset to treatment, h | 10·7 (5·3–17·3) | 10·8 (5·4–16·4) | 10·4 (4·6–18·9) | |
| Time from randomisation to treatment, h | 0·7 (0·3–1·0) | 0·6 (0·3–0·9) | 0·8 (0·3–1·0) | |
| Prestroke modified Rankin scale score | 1·0 (0·0–1·0) | 1·0 (0·0–2·0) | 0·0 (0·0–1·0) | |
| Glasgow Coma Scale score | 15·0 (13·0–15·0) | 15·0 (13·0–15·0) | 15·0 (13·0–15·0) | |
| Systolic blood pressure, mm Hg | 151·7 (22·0) | 151·5 (19·8) | 151·9 (24·4) | |
| Diastolic blood pressure, mm Hg | 81·1 (14·6) | 75·0 (11·4) | 87·2 (15·0) | |
| History of ischaemic stroke or transient ischaemic attack | 20 (37%) | 10 (37%) | 10 (37%) | |
| History of ischaemic heart disease | 22 (41%) | 15 (56%) | 7 (26%) | |
| History of hypertension | 39 (72%) | 20 (74%) | 19 (7) | |
| History of diabetes | 17 (31%) | 9 (33%) | 8 (3) | |
| History of atrial fibrillation | 2 (4%) | 0 | 2 (7%) | |
| History of haemorrhagic stroke | 2 (4%) | 0 | 2 (7%) | |
| History of peripheral arterial disease | 0 | 0 | 0 | |
| History of hyperlipidaemia | 28 (53%); n=53 | 14 (54%); n=26 | 14 (52%); n=27 | |
| History of statin use | 39 (72%) | 21 (78%) | 18 (67%) | |
| Antiplatelet therapy on admission | ||||
| Aspirin alone | 25 (46%) | 13 (48%) | 12 (44%) | |
| Clopidogrel alone | 23 (43%) | 14 (52%) | 9 (33%) | |
| Aspirin and dipyridamole | 1 (2%) | 0 | 1 (4%) | |
| Aspirin and clopidogrel | 5 (9%) | 0 | 5 (19%) | |
| Stroke severity | ||||
| National Institutes of Health Stroke Scale score | 7·0 (5·0–15·0); n=53 | 7·5 (4·0–16·0); n=26 | 7·0 (6·0–15·0); n=27 | |
| Baseline CT scan | ||||
| Time from stroke onset to CT scan, h | 2·3 (1·5–5·3); n=53 | 2·8 (1·7–6·5); n=26 | 1·9 (1·2–4·5); n=27 | |
| Time from scan to randomisation, h | 3·4 (1·4–10·6); n=53 | 3·3 (1·5–9·1); n=26 | 3·4 (1·1–12·3); n=27 | |
| Time from scan to treatment, h | 4·3 (2·2–11·9)] | 4·0 (2·0–10·0); n=26 | 4·9 (2·6–14·6); n=27 | |
| Location of stroke | ||||
| Lobar | 22 (41%) | 11 (41%) | 11 (41%) | |
| Deep | 29 (54%) | 16 (59%) | 13 (48%) | |
| Infratentorial | 5 (%) | 1 (4%) | 4 (15%) | |
| Intraventricular haemorrhage | 20 (39%); n=51 | 8 (32%); n=25 | 12 (46%); n=26 | |
| Haematoma volume, mL | 8·9 (3·3–28·5); n=51 | 13·3 (4·4–32·0); n=25 | 8·1 (3·3–17·0); n=26 | |
| >60 mL | 6 (12%); n=51 | 3 (12%); n=25 | 3 (12%); n=26 | |
Data are mean (SD), median (IQR), or n (%).
26 of 27 participants in the desmopressin group received all the trial treatment; one patient received a part infusion because of a fault with a leaking bag of sodium chloride. All participants in the placebo group received the full allocated treatment. All participants remained in their allocated group for the duration of the trial, with no participants lost to follow-up or withdrawing from the trial (figure 1). All 54 participants had day 90 outcome data (the anticipated primary outcome in a definitive trial).
100 (71%) of 140 treatment packs of desmopressin or placebo were exposed to at least one temperature excursion. 69 (49%) treatment packs were quarantined at least once and 59 (42%) treatment packs were destroyed. 25 (46%) of 54 participants received treatment from a pack that had been exposed to a temperature of less than 2°C or more than 8°C at some point during its storage.
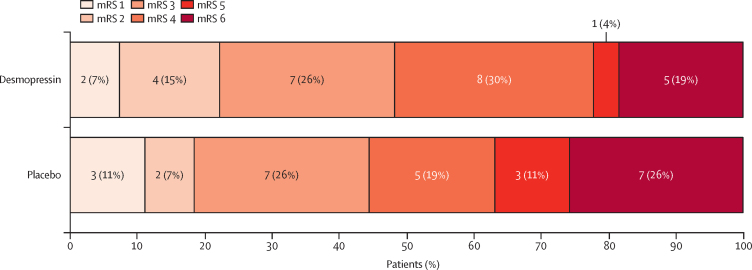
Secondary outcome data are summarised in table 2. One (4%) patient in the placebo group required neurosurgery. Median change in haematoma volume at 24 h was 0 mL (IQR –1·4 to 2·0) in the desmopressin group and 0·5 mL (IQR –0·7 to 2·1) in the placebo group. Haematoma expansion occurred in five (21%) of 24 participants in the desmopressin group and five (23%) of 22 participants in the placebo group. Median length of hospital stay was 9 days (IQR 3–15) in the desmopressin group and 6 days (2–22) in the placebo group. Five patients in each treatment group died before 28 days. At day 90, in the desmopressin group, 18 (67%) patients had been discharged home, four (15%) were in hospital or residential care, and five (19%) had died. In the placebo group, 16 (59%) patients were at home, four (15%) were in hospital or residential care, and seven (26%) had died. Death or dependency at day 90 (modified Rankin Scale score >4) occurred in six (22%) of 27 participants in the desmopressin group and ten (37%) of 27 participants in the placebo group. Shift in modified Rankin Scale score is summarised in figure 2. No difference was seen between the treatment groups for either the full-scale modified Rankin Scale score (OR 0·74 [95% CI 0·29–1·93]; table 2), or death and dependency (OR 0·49 [95% CI 0·15–1·61]; table 2).
Table 2.
Secondary outcome measures
| All participants (n=54) | Desmopressin group (n=27) | Placebo group (n=27) | ||
|---|---|---|---|---|
| Outcomes measured at 24 h | ||||
| Sodium concentration, mmol/L | 136·9 (4·1); n=47 | 135·6 (3·6); n=23 | 138·1 (4·2); n=24 | |
| Any hyponatraemia, <135 mmol/L | 11 (23%); n=47 | 8 (35%); n=23 | 3 (13%); n=24 | |
| Moderate to severe hyponatraemia, <125 mmol/L | 0; n=47 | 0; n=23 | 0; n=24 | |
| Outcomes measured at discharge | ||||
| Length of hospital stay, days | 8·0 (3·0–15·0) | 9·0 (3·0–15·0) | 6·0 (2·0–22·0) | |
| Discharge destination | ||||
| Home, independent | 17 (4%); n=42 | 11 (5%); n=22 | 6 (3%); n=20 | |
| Home, needing care | 17 (4%); n=42 | 7 (32%); n=22 | 10 (5%); n=20 | |
| Hospital, rehabilitation | 5 (12%); n=42 | 3 (14%); n=22 | 2 (1%); n=20 | |
| Hospital, readmission | 2 (5%); n=42 | 0; n=22 | 2 (1%); n=20 | |
| Hospital, not discharged | 1 (2%); n=42 | 1 (5%); n=22 | 0; n=20 | |
| Outcomes measured at day 90 | ||||
| Modified Rankin Scale score* | 4·0 (3·0–5·0) | 4·0 (3·0–4·0) | 4·0 (3·0–6·0) | |
| 0 | 0 | 0 | 0 | |
| 1 | 5 (9%) | 2 (7%) | 3 (11%) | |
| 2 | 6 (11%) | 4 (15%) | 2 (7%) | |
| 3 | 14 (26%) | 7 (26%) | 7 (26%) | |
| 4 | 13 (24%) | 8 (3) | 5 (19%) | |
| 5 | 4 (7%) | 1 (4%) | 3 (11%) | |
| 6 | 12 (22%) | 5 (19%) | 7 (26%) | |
| Death or dependency (modified Rankin Scale score >4)† | 16 (3) | 6 (22%) | 10 (37%) | |
| Barthel index | 69·6 (33·0); n=41 | 71·6 (33·1); n=22 | 67·4 (33·6); n=19 | |
| EuroQol-5D health state utility | 0·36 (0·37); n=53 | 0·37 (0·40); n=27 | 0·34 (0·35); n=26 | |
| EuroQol visual analogue scale | 63·5 (17·9); n=37 | 62·0 (19·5); n=20 | 65·2 (16·3); n=17 | |
| Telephone interview for cognitive status-modified | 22·5 (5·4); n=26 | 22·3 (6·4); n=15 | 22·9 (4·0); n=11 | |
| Zung depression scale | 49·1 (13·8); n=28 | 52·2 (15·5); n=16 | 45·0 (10·4); n=12 | |
| Mortality | 12 (22%) | 5 (19%) | 7 (26%) | |
| At 28 days | 10 (19%) | 5 (19%) | 5 (19%) | |
| Serious adverse events | 25 (46%) | 12 (44%) | 13 (48%) | |
| Thrombotic events | 2 (4%) | 1 (4%) | 1 (4%) | |
| Change in haematoma volume, mL | 0·1 (−1·3 to 2·1); n=46 | 0·0 (−1·4 to 2·0); n=24 | 0·5 (−0·7 to 2·1) n=22 | |
| Haematoma expansion‡ | 10 (22%); n=46 | 5 (21%); n=24 | 5 (23%); n=22 | |
Data are mean (SD), median (IQR), or n (%).
Odds ratio 0·74 (95% CI 0·29–1·93).
Odds ratio 0·49 (95% CI 0·15–1·61).
Haematoma expansion was defined as an absolute increase in haematoma volume of greater than 6 mL or relative growth of greater than 33%.
Figure 2.
Shift analysis of the modified Rankin Scale score in participants at day 90
mRS= modified Rankin Scale.
Serious adverse events had occurred by day 90 in 12 (44%) of 27 participants in the desmopressin group and 13 (48%) of 27 participants in the placebo group. After 24 h, mild hyponatraemia (sodium concentration 125–134 mmol/L) was present in eight (35%) of 23 participants in the desmopressin group and three (13%) of 24 participants in the placebo group. No patient in either group had a sodium concentration of less than 125 mmol/L. Thrombotic events were reported for one (4%) patient in the desmopressin group (non-ST-elevation myocardial infarction) and one (4%) patient in the placebo group (ischaemic stroke; table 3).
Table 3.
Serious adverse events
|
All participants (n=54) |
Desmopressin group (n=27) |
Placebo group (n=27) |
|||||
|---|---|---|---|---|---|---|---|
| Events (n) | Patients (n [%]) | Events | Patients (n [%]) | Events (n) | Patients (n [%]) | ||
| Total serious adverse events | 38 | 25 (46%) | 16 | 12 (44%) | 22 | 13 (48%) | |
| Cardiovascular events | 5 | 5 (9%) | 3 | 3 (11%) | 2 | 2 (7%) | |
| Cardiac failure or pulmonary oedema | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Hypervolemia (fluid overload) | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Hypotension | 2 | 2 (4%) | 2 | 2 (7%) | 0 | 0 | |
| Myocardial infarction (Non-ST-elevation myocardial infarction) | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Nervous system events | 19 | 17 (31%) | 8 | 7 (26%) | 11 | 10 (37%) | |
| Complication of initial stroke | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Expansion of haemorrhagic stroke | 10 | 10 (19%) | 4 | 4 (15%) | 6 | 6 (22%) | |
| Ischaemic stroke | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Neurological deterioration | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Seizure or convulsions | 5 | 4 (7%) | 2 | 2 (7%) | 3 | 2 (7%) | |
| Weakness | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Respiratory events | 11 | 9 (17%) | 4 | 3 (11%) | 7 | 6 (22%) | |
| Chest infection | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| COVID-19 or SARS-CoV-2 infection | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Pneumonia | 8 | 7 (13%) | 1 | 1 (4%) | 7 | 6 (22%) | |
| Lower respiratory tract infection | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Gastrointestinal events | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Diarrhoea | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Genitourinary events | 0 | 0 | 0 | 0 | 0 | 0 | |
| Haematological or immunological events | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metabolic or endocrine events | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Hyponatraemia (low sodium) | 1 | 1 (2%) | 0 | 0 | 1 | 1 (4%) | |
| Musculoskeletal or cutaneous events | 0 | 0 | 0 | 0 | 0 | 0 | |
| Miscellaneous | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
| Possible sepsis or possible hyperosmolar hyperglycaemic state | 1 | 1 (2%) | 1 | 1 (4%) | 0 | 0 | |
Serious adverse events were categorised as per the Medical Dictionary for Regulatory Authorities.
At day 90, the mean Barthel index score was 71·6 (SD 33·1) in the desmopressin group (n=22), and 67·4 (33·6) in the placebo group (n=19). Mean EuroQol-5D was 0·37 (SD 0·40) in the desmopressin group (n=27) and 0·34 (SD 0·35) in the placebo group (n=26). Mean score on the telephone interview for cognitive status-modified score was 22·3 (SD 6·4) in the desmopressin group and 22·9 (4·0) in the placebo group. The mean Zung depression scale score was 52·2 (SD 15·5) in the desmopressin group and 45·0 (10·4) in the placebo group.
The first 28 patients recruited into the DASH trial were eligible for P-selectin testing, and the tests were done in 14 (50%). Table 4 presents results of haemostatic marker testing at baseline and 1 h after treatment. Changes from baseline to 1 h in these markers were greater in the desmopressin group than with placebo.
Table 4.
Changes in haemostatic markers before and after desmopressin or placebo
| All participants (n=24) | Desmopressin group (n=10) | Placebo group (n=14) | |
|---|---|---|---|
| Von Willebrand factor antigen, IU/mL | |||
| Before treatment | 2·2 (1·0); 1·9 (1·5 to 2·7) | 2·7 (1·1); 2·7 (2·1 to 3·4) | 1·8 (0·7); 1·7 (1·3 to 1·9) |
| 1 h after treatment | 2·2 (1·1); 1·9 (1·5 to 2·9) | 2·8 (1·2); 2·9 (1·8 to 3·4) | 1·8 (0·7); 1·8 (1·2 to 2·0) |
| Change | 0·1 (0·4); 0·1 (0·0 to 0·3) | 0·1 (0·7); 0·2 (0·0 to 0·7) | 0·0 (0·2); 0·0 (0·0 to 0·1) |
| Von Willebrand factor activity, IU/mL | |||
| Before treatment | 2·3 (1·2); 1·9 (1·6 to 3·0) | 3·0 (1·4); 3·0 (1·9 to 3·6) | 1·7 (0·6); 1·8 (1·2 to 1·9) |
| 1 h after treatment | 2·4 (1·2); 2·1 (1·7 to 3·0) | 3·2 (1·4); 3·0 (2·1 to 4·1) | 1·8 (0·6); 1·8 (1·6 to 2·1) |
| Change | 0·1 (0·7); 0·1 (−0·1 to 0·4) | 0·2 (1·1); 0·3 (−0·7 to 1·1) | 0·1 (0·3); 0·0 (−0·1 to 0·2) |
| Chromogenic factor VIII, IU/mL | |||
| Before treatment | 2·4 (1·1); 1·9 (1·7 to 2·8) | 3·0 (1·3); 3·1 (1·7 to 4·0) | 1·9 (0·4); 1·8 (1·7 to 2·2) |
| 1 h after treatment | 2·6 (1·1); 2·2 (1·8 to 3·0) | 3·3 (1·3); 3·3 (2·5 to 4·2) | 2·0 (0·4); 2·0 (1·8 to 2·2) |
| Change | 0·2 (0·9); 0·1 (0·0 to 0·6) | 0·3 (1·4); 0·5 (−0·4 to 1·2) | 0·1 (0·3); 0·1 (0·0 to 0·3) |
Data are mean (SD) or median (IQR).
Discussion
The findings of the DASH trial showed the feasibility of running a large randomised trial of desmopressin in patients on antiplatelet drug therapy with intracerebral haemorrhage. 176 (13%) of 1380 patients with intracerebral haemorrhage were eligible for the DASH trial. Of these, 54 (31%) participants were recruited. The measures in place to follow up participants worked well, and no patients were lost to follow-up. Desmopressin and placebo infusions were administered in full for all but one participant. No safety concerns, such as thrombosis, were reported. Hyponatraemia is a known side-effect of desmopressin, and our results showed the incidence of mild hyponatraemia was higher for those treated with desmopressin, with no instances of moderate or severe hyponatraemia, suggesting its safety in this clinical setting. To our knowledge, the DASH trial is the first randomised trial to compare desmopressin with placebo in patients on antiplatelet drug therapy with intracerebral haemorrhage. A 2021 systematic review20 identified three retrospective controlled studies (including 263 patients in total) assessing desmopressin in intracerebral haemorrhage. No randomised controlled trials have been done since this systematic review, although further case series have been published.21
The proposed primary efficacy outcome in a definitive trial would be death or dependency at day 90, measured using the modified Rankin scale. For a definitive study, we estimate approximately 1500 eligible patients would need to be recruited, a recruitment rate of up to 500 participants per year in the UK. Notably, DASH was conducted before, during, and after the global COVID-19 pandemic, during which time staff were redeployed to work in alternative areas. Therefore, the participant recruitment rate for a definitive trial could be increased from that observed in DASH. Furthermore, a future clinical trial to assess efficacy would be most likely to be successful if it were embedded into clinical practice, which would increase recruitment because it would be possible for clinical teams to randomly assign patients out of hours. In a definitive trial, a more pragmatic approach to temperature monitoring should be used, requiring that desmopressin be stored in a fridge, in line with current clinical practice.
A pragmatic decision was made in the DASH trial to administer desmopressin or placebo as soon as possible after diagnosis, but to allow enrolment up to 24 h after symptom onset for patients who presented late or for whom enrolment was not possible in the first few hours. This decision is in contrast to ongoing trials of prohaemostatic drugs, such as the FASTEST trial of recombinant factor VIIa (NCT03496883) and the TICH-3 trial of tranexamic acid (ISRCTN97695350), in which much tighter periods (2 h and 4·5 h, respectively) have been selected. There is growing awareness that the greatest risk of haematoma expansion occurs early after intracerebral haemorrhage, with previous clinical trials22, 23 suggesting administration of prohaemostatic therapy occurred too late after stroke onset to improve outcome. Although the risk of haematoma expansion is greatest in the first few hours after intracerebral haemorrhage onset, it can occur for the first 24 h, particularly in patients on antiplatelet therapy. The largest systematic review, including 30 000 patients with intracerebral haemorrhage on antiplatelet therapy from 23 studies, reported that such patients had larger baseline haematoma volume and were more likely to have haematoma expansion over 24 h compared with patients not on antiplatelet dugs (haematoma expansion OR 2·58 (95% CI 1·18–5·67), but not beyond this timepoint.24 In TICH-2,25 165 (35%) of 611 patients with intracerebral haemorrhage on antiplatelet treatments underwent haematoma expansion after enrolment (mean 4·1 h) within the first 24 h.
Several mechanisms have been proposed for how desmopressin might potentially reduce bleeding for patients on antiplatelet drugs. For instance, desmopressin stimulates release of Von Willebrand factor and factor VIII from endothelial Weibel-Palade bodies. Von Willebrand factor causes platelet adhesion to collagen through platelet glycoprotein 1b-V-IX receptors, and might also bind platelets together directly through their glycoprotein IIb/IIIa receptors.19 Increased concentrations of Von Willebrand factor, therefore, have the potential to compensate for the platelet function defect associated with antiplatelet drugs. Another suggested mechanism is that desmopressin might increase the formation of procoagulant platelets.26 Participants treated with desmopressin had a greater increase in Von Willebrand factor and factor VIII than did those treated with placebo, although no formal statistical comparison was made.
Guidelines currently provide conflicting guidance on use of desmopressin for patients on antiplatelet drugs with intracerebral haemorrhage. The Neurocritical Care Society and Society of Critical Care Medicine recommend a single dose of 0·4 μg/kg desmopressin for patients with intracranial haemorrhage who were exposed to antiplatelet agents;11 for patients undergoing a neurosurgical procedure, these guidelines recommend that desmopressin can be used in addition to platelet transfusion. The American Heart Association and American Stroke Association advise that further studies are necessary to determine if there is a subgroup of patients taking antiplatelet drugs with intracerebral haemorrhage who might benefit from desmopressin.12 The Intercollegiate Stroke Working Party of the Royal College of Physicians guidelines13 and British Society for Haematology guidelines27 make no recommendations.
The DASH trial has several strengths. First, it provides randomised data for the use of desmopressin for acute intracerebral haemorrhage. Second, the methodology of the trial was rigorous, and the feasibility of the approach under investigation was demonstrated. Finally, there is substantial worldwide mortality and a large morbidity burden from intracerebral haemorrhage, and a lack of consensus in guidelines, so the study is of great importance for increasing knowledge.
The DASH trial has some limitations. First, only research-active stroke units participated in the trial, so it is possible recruitment rates would be different in a definitive study. Recruitment was put on hold midway through the trial because of the COVID-19 pandemic and recruitment rates never reached pre-pandemic levels. The prolonged recruitment period associated with the funding extensions was an important modification that had meaningful implications for the ability of the trial to surpass the recruitment target and address its objectives. Furthermore, information provided in screening logs was restricted to data provided by each study site; these data were collected locally and it was not possible for the central research team to verify it. Second, the placebo was non-identical to desmopressin, although the risks of unmasking were minimised by fully concealing treatment allocation from clinicians, patients, and outcome assessors. Third, one of the most common reasons for patient exclusion was presenting out of hours or more than 24 h after symptom onset and, therefore, our data might not be representative of all patients. Streamlining trial processes for a future trial might reduce the time from confirmation of intracerebral haemorrhage on imaging to administration of desmopressin or placebo. Finally, the six patients who were taking dual antiplatelet therapy were all randomised to placebo. In a large definitive trial, we would consider minimisation on additional prognostic factors, such as antiplatelet therapy and baseline stroke severity.
In summary, given sufficient recruiting centres and pragmatic trial methodology, it should be feasible to conduct a definitive trial to determine if desmopressin improves outcomes in patients on antiplatelet drug therapy with intracerebral haemorrhage. Although this trial could be delivered as a standalone study, it might be more efficient as part of a platform trial or to embed in an existing intracerebral haemorrhage trial. Given the high mortality and morbidity after intracerebral haemorrhage in patients exposed to antiplatelet drugs, and the increased frequency of patients taking antiplatelet drugs, even a small reduction in morbidity or mortality could reduce the risk of death and leave tens of thousands of patients free from disability.
Data sharing
The trial chief investigators (MJRD and NS) will consider requests to share anonymised individual participant data via email at dash@nottingham.ac.uk. Submitted requests will require a protocol detailing hypothesis, aims, analyses, and intended tables and figures. Where possible, we will perform the analyses; alternatively, deidentified data and a data dictionary will be supplied for the necessary variables for remote analysis. Any sharing will be subject to a signed data access agreement. Individual participant data will eventually be shared with the Virtual International Stroke Trials Archive (VISTA) collaboration.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (grant reference number PB-PG-0816-20011). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. We thank Sharon Ellender (trial co-ordinator), Lee J Haywood (web application and database programmer), Patricia Robinson (trial administrator), Iris Mhlanga (statistician), Cameron Skinner (trial co-ordinator), Emily Stanyard (trial co-ordinator), Tray Morgan (trial administrator), Peter Burt (haemostasis laboratory analysis), Colin Baigent (trial steering committee chair), Christine Knott (patient and public involvement representative), Matthew Walters (independent member of trial steering committee), Angela Shone (sponsor representative), Laura Green (independent member of trial steering committee), Phil Johnson (patient and public involvement representative), John Bamford (chair of data monitoring committee), Graham Venables (data monitoring committee), Martin Bland (data monitoring committee), Wei Tan (independent statistician), Cydney Bruce (independent statistician), Sheila Hodgson (trial pharmacist), Bernie Cook (trial pharmacist), and Sally Hodgkinson (trial pharmacist). We also thank the participating principal investigators: German Guzman Gutierrez (NHS Grampian, Aberdeen, UK); David Werring (University College London Hospitals NHS Foundation Trust, London, UK); Neshika Samarasekera (NHS Lothian, Edinburgh, UK); Jane Sword and Martin James (Royal Devon and Exeter NHS Foundation Trust, Exeter, UK); Amit Mistry (University Hospitals of Leicester NHS Trust, Leicester, UK); Rahulan Dharmarajah (University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, UK); Timothy England (University Hospitals of Derby and Burton NHS Foundation Trust, Derby, UK); Alexander Dyker (Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK); and Adrian Blight (St George's University Hospitals NHS Foundation Trust, London, UK).
Contributors
MJRD, SJS, RA-SS, and NS had the ideas for the study. MJRD wrote the first draft of the manuscript with NS. DH and JC provided trial management. LJW and TH did the statistical analysis. PMBr, RAD, TJC, and PMBa provided expert input in trial design. MJRD oversaw the laboratory analysis. KK and RD centrally assessed the imaging. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. MJRD, NS, and LJW directly accessed and verified the underlying data reported in the manuscript.
Contributor Information
Michael J R Desborough, Email: michael.desborough@ouh.nhs.uk.
DASH trial investigators:
Adrian Blight, Rahulan Dharmarajah, Alexander Dyker, Timothy England, German Guzman Gutierrez, Martin James, Amit Mistry, Neshika Samarasekera, Jane Sword, and David Werring
Supplementary Material
References
- 1.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Royal College of Physicians Sentinel Stroke National Audit Programme (SSNAP) 2016. https://www.strokeaudit.org/Documents/National/AcuteOrg/2016/2016-AOANationalReport.aspx
- 3.Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the Global Burden of Disease Study 2017. Neuroepidemiology. 2020;54:171–179. doi: 10.1159/000506396. [DOI] [PubMed] [Google Scholar]
- 4.Thompson BB, Béjot Y, Caso V, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology. 2010;75:1333–1342. doi: 10.1212/WNL.0b013e3181f735e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 6.Stroke Association Shaping stroke research to rebuild lives: The Stroke Priority Setting Partnership results for investment. 2021. https://www.jla.nihr.ac.uk/priority-setting-partnerships/stroke/downloads/Stroke-PSP-results-Full-Report.pdf
- 7.Desborough MJ, Oakland K, Brierley C, et al. Desmopressin use for minimising perioperative blood transfusion. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD001884.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desborough MJ, Oakland KA, Landoni G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost. 2017;15:263–272. doi: 10.1111/jth.13576. [DOI] [PubMed] [Google Scholar]
- 9.Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke. 2010;41:402–409. doi: 10.1161/STROKEAHA.109.552919. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17:885–894. doi: 10.1016/S1474-4422(18)30253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frontera JA, Lewin JJ, 3rd, Rabinstein AA, et al. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24:6–46. doi: 10.1007/s12028-015-0222-x. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022;53:e282–e361. doi: 10.1161/STR.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 13.Intercollegiate Stroke Working Party . Royal College of Physicians; London, UK: 2023. National clinical guideline for stroke. [Google Scholar]
- 14.Al-Shahi Salman R, Law ZK, Bath PM, Steiner T, Sprigg N. Haemostatic therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD005951.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desborough MJR, Al-Shahi Salman R, Stanworth SJ, et al. Desmopressin for reversal of Antiplatelet drugs in Stroke due to Haemorrhage (DASH): protocol for a phase II double-blind randomised controlled feasibility trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siew DA, Mangel J, Laudenbach L, Schembri S, Minuk L. Desmopressin responsiveness at a capped dose of 15 μg in type 1 von Willebrand disease and mild hemophilia A. Blood Coagul Fibrinolysis. 2014;25:820–823. doi: 10.1097/MBC.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Ferring Pharmaceuticals Octim injection summary of product characteristics. 2012. https://www.medicines.org.uk/emc/product/111/smpc#gref
- 18.Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–2115. doi: 10.1016/S0140-6736(18)31033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider SW, Nuschele S, Wixforth A, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loggini A, El Ammar F, Darzi AJ, et al. Effect of desmopressin on hematoma expansion in antiplatelet-associated intracerebral hemorrhage: a systematic review and meta-analysis. J Clin Neurosci. 2021;86:116–121. doi: 10.1016/j.jocn.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Summers A, Singh J, Lai M, et al. A multicenter retrospective study evaluating the impact of desmopressin on hematoma expansion in patients with antiplatelet-associated intracranial hemorrhage. Thromb Res. 2023;222:96–101. doi: 10.1016/j.thromres.2022.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Sprigg N, Flaherty K, Appleton JP, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–2115. doi: 10.1016/S0140-6736(18)31033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral haemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 24.Goeldlin MB, Siepen BM, Mueller M, et al. Intracerebral haemorrhage volume, haematoma expansion and 3-month outcomes in patients on antiplatelets. A systematic review and meta-analysis. Eur Stroke J. 2021;6:333–342. doi: 10.1177/23969873211061975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law ZK, Desborough M, Roberts I, et al. Outcomes in antiplatelet-associated intracerebral hemorrhage in the TICH-2 randomized controlled trial. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colucci G, Stutz M, Rochat S, et al. The effect of desmopressin on platelet function: a selective enhancement of procoagulant COAT platelets in patients with primary platelet function defects. Blood. 2014;123:1905–1916. doi: 10.1182/blood-2013-04-497123. [DOI] [PubMed] [Google Scholar]
- 27.Makris M, Van Veen JJ, Tait CR, Mumford AD, Laffan M. Guideline on the management of bleeding in patients on antithrombotic agents. Br J Haematol. 2013;160:35–46. doi: 10.1111/bjh.12107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The trial chief investigators (MJRD and NS) will consider requests to share anonymised individual participant data via email at dash@nottingham.ac.uk. Submitted requests will require a protocol detailing hypothesis, aims, analyses, and intended tables and figures. Where possible, we will perform the analyses; alternatively, deidentified data and a data dictionary will be supplied for the necessary variables for remote analysis. Any sharing will be subject to a signed data access agreement. Individual participant data will eventually be shared with the Virtual International Stroke Trials Archive (VISTA) collaboration.