Abstract
To evaluate the feasibility administering single-dose intraoperative intraperitoneal carboplatin (IP) in advanced epithelial ovarian cancer (EOC) after optimal primary or interval debulking surgery. A phase II non-randomized prospective study conducted at a regional cancer institute from January 2015 to December 2019. The advanced high-grade epithelial ovarian cancer FIGO stage IIIB–IVA was included. A total of 86 consented patients with optimal primary and interval cytoreductive surgeries received single-dose intraoperative IP carboplatin. The immediate (< 6 h), early (6–48 h), and late (48 h–21 days) perioperative complications were recorded and analyzed. The severity of adverse events was graded on the basis of National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). A total of 86 patients received single-dose intra-operative IP carboplatin during the study period. The 12 (14%) patients underwent primary debulking surgery and 74(86%) interval debulking surgery (IDS). The 13 (15.1%) patients underwent laparoscopic/robotic IDS. All the patients tolerated the intraperitoneal carboplatin well with no or minimal adverse events. Three cases (3.5%) needed resuturing for the burst abdomen, three cases (3.5%) had paralytic ileus for 3–4 days, one case (1.2%) underwent re-explorative laparotomy for hemorrhage, and one case (1.2%) mortality due to due late sepsis. The 84 (97.7%) of 86 cases received scheduled IV chemotherapy on time. Single-dose intraoperative IP carboplatin is a feasible procedure with no or minimal manageable morbidity. The procedure is user friendly combining the prognostic benefits of IP chemotherapy with assurance of earliest timely administration of chemotherapy in advanced EOC. Our study is a hypothesis generating for the future clinical trials comparing single-dose NIPEC versus HIPEC in advanced EOC.
Keywords: Epithelial ovarian cancer, Intraperitoneal carboplatin, Normothermic intraoperative IP chemotherapy
Introduction
According to latest report of National Cancer Registry Programme 2019, ovarian cancer constitutes the third most common cancer among women of India [1]. The standard chemotherapy for epithelial ovarian cancer is a combination of a platinum analogue with paclitaxel [2]. The peritoneal cavity is the principal site of disease in ovarian cancer [3]. The rationale for intraperitoneal chemotherapy in ovarian cancer is that the peritoneum, the predominant site of tumor, receives sustained exposure to high concentrations of antitumor agents while normal tissues, such as the bone marrow, are relatively spared [4].
The landmark Gynecological Oncology Group (GOG) 172 randomized trial compared intravenous chemotherapy with intraperitoneal chemotherapy arms in advanced epithelial ovarian cancer (EOC) [4]. This trial revealed the median duration of progression free survival in the intravenous (IV) and intraperitoneal (IP) therapy as 18.3 and 23.8 months, the overall survival as 49.7 and 65.6 months, respectively [4]. The toxicity profile was higher with intraperitoneal route due to cisplatin usage. The prognostic advantage of intraperitoneal route was evident in this study. Suidan et al. from Memorial Sloan-Kettering Cancer Centre (MSKCC) evaluated the prognostic significance of the number of IV/IP cycles administered, and they did not detect a significant survival difference between patients who received 1–2, 3–4, or 5–6 IV/IP chemotherapy cycles, and concluded that women may still derive a survival benefit even if they receive < 6 IV/IP cycles. [5]. The subset analysis of GOG 172 and the MSKCC studies revealed that the patients receiving 1–2 courses of IP chemotherapy also had significant overall survival advantages compared with IV arms. However, IP arm patient’s experienced substantial toxicity as the cisplatin was used in these studies. The IP chemotherapy is not very popularly practiced by gynecological oncologists due to the logistical difficulties involved with IP catheter placement, maintenances, toxicity, and higher cost [4].
In GOG 252 study, the IV therapy was compared with IP carboplatin and cisplatin in three different arms [6]. The median progression free survival was 24.9 months in IV carboplatin, 27.4 months in IP carboplatin, and 26.2 months in IP cisplatin arm. Median OS as 75.5 months, 78.9 months, and 72.9 months, respectively. This study showed benefits of IP carboplatin over other two arms in respect of better toxicity profile and marginally better PFS and OS.
The studies reveals that prolonged initiation time (more than 25 days) of adjuvant chemotherapy is associated with a decreased overall survival rate of ovarian cancer, especially in patients with advanced stage ovarian cancer [7–9].
This guided us to choose single-dose intraoperative IP carboplatin over cisplatin in our study. Our study is designed with a hypothesis that the single-dose intraoperative IP carboplatin will prognostically benefit the advanced epithelial ovarian cancer (EOC) patients with advantages of intraperitoneal dose and assurance of on time chemotherapy administration in advanced EOC.
We report the results of a phase II prospective trial conducted at our cancer institute where optimally debulked patients (< 1 cm residual disease) of EOC were subjected to single-dose of IP carboplatin intraoperatively. The perioperative events were analyzed in immediate, early, and late categories to assess the feasibility of single-dose IP chemotherapy intraoperatively. It helps in administering the chemotherapy timely as per 3 weekly regimen with primary debulking surgery (PDS) and interval debulking surgery (IDS).
Methods
This is a phase II non-randomized prospective study of 86 patients with advanced EOC (FIGO stage IIIB–IVA) conducted at the Department of Gynaecological Oncology at a regional cancer institute from January 2015 to December 2019. The departmental board approval was taken. A total of 356 cases underwent cytoreductive surgeries for the advanced stage high-grade epithelial ovarian cancers during the study period. The complete cytoreduction (residual disease nil) was achieved in 171 (48%) cases, optimal cytoreduction (residual disease < 1 cm) in 124 (34.8%) cases, and suboptimal cytoreduction (residual disease > 1 cm) in 61 (17.1%) cases. The 86 (24.2%) optimal cytoreduction cases consented for the single-dose intra-operative intraperitoneal carboplatin. The patients not willing for the enrolment in the studies due to the logistics related insurance approvals and the sub optimally debulked cases were excluded from the study. The departmental policy for selecting patients to primary debulking surgery is based on patients’ good performance status ECOG 0–1, optimal resectibility on CT scan study, clinical examination, and serum albumin > 3 g/dl, and the patients with poor performance status ECOG > 2, serum albumin < 3 g/dl, malignant pleural effusion, liver parenchymal metastasis, and optimally non-resectable disease on CT scan, and age > 80 years will receive 2–3 courses of neoadjuvant chemotherapy (NACT) followed by interval cytoreduction (IDS). The patients having complete and near complete response to NACT were subjected for the laparoscopic/robotic IDS. The surgical procedure performed to attain optimal cytoreduction included total hysterectomy with bilateral salpingo-oophorectomy, total omentectomy, and tumor debulking. Tumor debulking included removal of peritoneal deposits, diaphragmatic stripping/resection, bowel resection, splenectomy, and peritonectomy wherever it was required for optimal debulking (Fig. 1). The lymph node debulking was done on evidence of enlarged node (> 1 cm) on imaging/and or surgical exploration. The patients with suboptimal cytoreduction (residual disease > 1 cm) were excluded from the study. The IP carboplatin dose (AUC-6) with 1000 ml normal saline was administered intra-abdominally after the optimal PDS/IDS. All these patients received pre-chemotherapy medication intravenous hydrocortisone 100 mg, pheniramine maleate (avil) 25 mg, and ranitidine 75 mg. The carboplatin was thoroughly circulated in the whole abdomen and left intraperitoneally for absorption (Fig. 2). The abdomen was closed with a sealed drain, and the drain was open released after 16–24 h. The prophylactic subcutaneous injection granulocyte stimulating factor was administered for 3 days on post-operative 2–4th days. No intraperitoneal catheter was placed as subsequent adjuvant chemotherapy was given by intravenous (IV) route. The patients were scheduled to receive next cycle IV chemotherapy after 21 days from the IP carboplatin. The complete blood count with renal and liver function tests were done before giving next cycle of IV chemotherapy, and all these patients were required to have healed surgical wounds, normal haemogram, and renal and liver function tests. The patients undergoing PDS received five courses of IV adjuvant chemotherapy, and patients planed for IDS received two courses neoadjuvant with 3 courses adjuvant chemotherapy. All the patients received total six courses of chemotherapy (one intraoperative single-dose IP carboplatin with five courses of IV chemotherapy). The adjuvant chemotherapy would be modified or postponed in case of delayed wound healing, surgical site infections, neuropathy, nephrotoxicity, neutropenia, or any hematological toxicity. The immediate (< 6 h), early (6–48 h), and late (48 h–21 days) perioperative complications were recorded and analyzed. The severity of adverse events was graded on the basis of NCI Common Terminology Criteria for Adverse Events (version 3.0) [10].
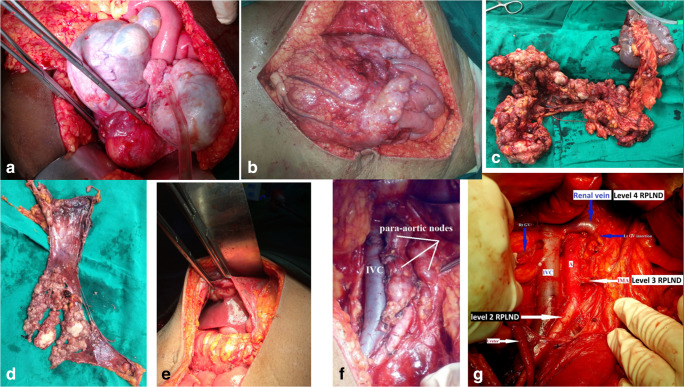
Fig. 1.
a Bilateral ovarian tumor, b omental cake, c omental cake and splenectomy enbloc excision, d diaphragm peritoneal deposit excision, e perihepatic deposit excision, f significantly enlarged para aortic nodes, and g para aortic anatomy after the nodal removal
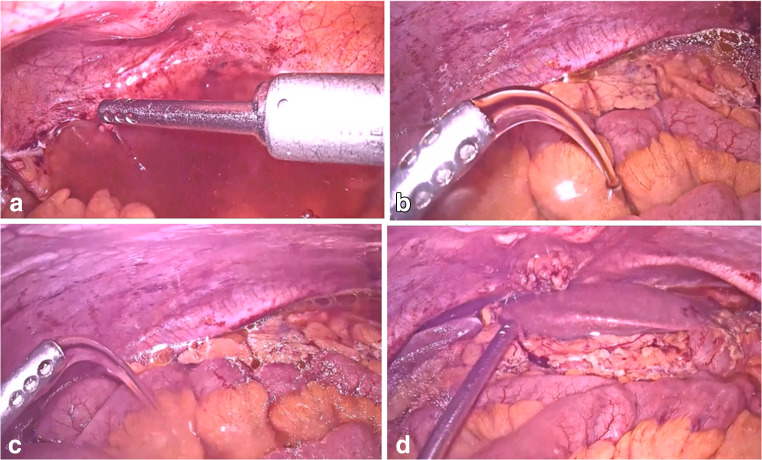
Fig. 2.
Intraperitoneal carboplatin instillation after cytoreduction surgery: a pelvic cavity, b right paracolic gutter, c Upper abdomen, and d hepatic and diaphragmatic region
Results
A total of 86 patients received single-dose intra-operative IP carboplatin during the study period. The median age of patients was 53 years (range38–76). Table 1 reveals the summarized patients characteristics; the FIGO stage distribution was 14%—IIIB, 65.1%—IIIC, and 20.9%—IVA. The 84 cases (97.7%) were high-grade serous ovarian cancers, and two cases (2.3%) were high-grade endometroid ovarian cancers. The mean Ca 125 levels at the time of diagnosis was 532 μ/ml (range 386–3031 μ/ml). All the eligible patients underwent cytoreductive surgery by both open laparotomy and laparoscopy/robotic methods. The 12 (14%) patients underwent PDS and 74 (86%) IDS. The 13 (15.1%) of 86 IDS patients were performed by laparoscopic/robotic method. All the patients tolerated the intraperitoneal carboplatin well with minimal adverse events (Table 2). In the immediate set (< 6 h) of adverse events, the 12 patients had abdominal discomfort and 14 patients had nausea with grade 1 or 2 severity, and there was no notable hypersensitivity reactions, cardiorespiratory adverse events, and soakage of dressing/and or wound dehiscence. In the early set (6–48 h postop) of adverse events, one patient (1.2%) hemodynamically stable developed grade 3 intraperitoneal hemorrhage detected on opening the pelvic drain and this patient underwent re-exploration; intraoperatively, the bleeder from splenic hilum was treated with splenectomy with 2 pints blood transfusion and had an uneventful postop period. The six patients experienced abdominal discomfort and two cases nausea with grade 1–2 severity, and three patients (3.5%) had paralytic ileus (Table 2). There were no other grade 3 or 4 adverse events noted. In the late set (> 48 h–21 days postop) three patients (3.5%) had burst abdomen which was attributed to persistent ascites in post-operative period and needed resuturing, and seven patients experienced grade 2 fatigue. One patient had a prolonged stay in ICU for 2 weeks due to poor nutritional status and extensive surgery. She had undergone recto-sigmoid resection anastomosis, peritonectomy, and end colostomy in addition to usual debulking procedure and developed low serum albumin levels post-operatively which was corrected by total parenteral nutrition and per oral high protein diet. The patient recovered and was sent home on 18th post-operative day in good health, and the adjuvant chemotherapy was delayed for 9 days. Four cases had fever one to two spikes, but there was no detectable septic focus. None had neutropenia, vomiting, neuropathy, nephrotoxicity, and cardiorespiratory or thromboembolic (Table 2). There were no severe grade 3/4 neutropenia, neuropathy, and nephrotoxicity in our study. The 84 (97.7%) of 86 cases received scheduled IV chemotherapy on time after 21 days of IP carboplatin.
Table 1.
The patient’s characteristics
| Mean age (years) | 53 |
| Age range (years) | 38–76 |
| Cytoreductive surgeries | |
| *PDS | 12(14%) |
| *IDS | 61 (70.9%) |
| Laparoscopic IDS | 13 (15.1%) |
| *FIGO stage | |
| IIIB | 12 (14%) |
| IIIC | 56 (65.1%) |
| IVA | 18 (20.9%) |
| Histopathology type | |
| High-grade serous ovarian cancer | 84 (97.7%) |
| Low-grade serous ovarian cancers | 00 |
| High-grade endometroid ovarian cancer | 02 (2.3%) |
| Others | 00 |
| Mean Ca 125 levels prior to cytoreduction | 532 u/mL |
| Ca 125 range | 386–3031 u/ml |
| Median study follow-up from the IP chemotherapy to first adjuvant IV chemotherapy | 21 days (range 1–42 days) |
PDS primary debulking surgery, IDS interval debulking surgery, FIGO International Federation of Obstetrics and Gynaecology, IP intraperitoneal chemotherapy, IV intravenous
Table 2.
Perioperative events following IP carboplatin instillation
| S. no | Adverse events | Immediate < 6 h postop | Early 6 h–48 h postop | Late 48 h–21 days postop |
|---|---|---|---|---|
| 1. | Abdominal discomfort | 12 | 06 | - |
| 2. | Hypersentivity reaction | - | - | - |
| 3. | Nausea | 14 | 02 | - |
| 4. | Hemorrhage | - | 01* | - |
| 5. | Pain | - | - | - |
| 6. | Paralytic ileus | - | 03 | - |
| 7. | Burst abdomen/ wound dehiscence | - | - | 03 |
| 8. | Sepsis | - | - | 01# |
| 9. | Cardio pulmonary event | - | - | - |
| 10. | Thromboembolic event | - | - | - |
| 11. | Nephrotoxicity | - | - | - |
| 12. | Fatigue | - | - | 07 |
| 13. | Neuropathy | - | - | - |
| 14. | Neutropenia (ANC < 1500 cells) | - | - | - |
| 15. | Fever | - | 04 | - |
*Case with grade-3 splenic hilum hemorrhage, underwent re-exploration after 24 h
# Case of sepsis, reported after 28 days, died
There was one (1.2%) late mortality; this particular patient was discharged on 7th post-operative day in a satisfactory health but developed fever and generalized sepsis after 4 weeks (28 days postop) and expired. The mortality may be due to late infection and generalized sepsis.
Discussion
Ovarian cancer commonly spreads within the peritoneal cavity; there is a reduced likelihood of substantial hematogenous or lymphatic dissemination. Successful tumor cytoreduction with modern surgical approaches allows chemotherapy to be administered in the setting of complete cytoreduction and optimally debulked (residual disease < 1 cm) cases within the peritoneal cavity. Among all randomized phase 3 trials conducted by the GOG among patients with advanced epithelial ovarian cancer, GOG 172 yielded the longest median survival: 65.6 months, in the group of patients who received intraperitoneal therapy [4], and following this study there was a NCI alert in 2006 to all the gynecological oncologist to practice IP chemotherapy for the management of advanced EOC [11]. The subset of patients who could not complete all the planned 6 courses IP chemotherapy in GOG 172 study also had improved overall survival, and the MSKCC study published patients with least number of IP chemotherapy 1–2 courses with IV chemotherapy also had significantly improved overall survival compared with pure IV chemotherapy arm [5]. The GOG-252 study revealed that IP carboplatin is equally efficacious and had marginally better DFS and OS benefits compared with IP cisplatin arm [6]. In our study all the cases received single-dose IP carboplatin with manageable grade 1–2 adverse events in few cases.
The 13 patients (17.6%) with complete response to NACT underwent laparoscopic/robotic IDS in our study. The endoscopic IDS is feasible, and the IP carboplatin was administered with a suction irrigation cannula to all the quadrants in the abdomen (Fig. 2). The global literature is growing on the safety and feasibility of endoscopic IDS in select complete response advanced EOC [12–14]. The IP chemotherapy was circulated in the abdomen thoroughly, and the abdominal drain was sealed for 16–24 h. The drain was released after 16–24 h as the IP carboplatin completely gets absorbed by < 16 h of administration [15].
The phase III Danish trial on hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced EOC in interval debulking surgery revealed significant improvement in DFI and OS [16]. Similarly, prospective and retrospective HIPEC studies have revealed improved DFI and OS in PDS settings [17–19]. The single-dose HIPEC with 5 courses of IV adjuvant chemotherapy is known to improve the survival in advanced EOC, and the NCCN guidelines include the option of HIPEC in IDS settings [20].
In our study, we administered a single dose of IP carboplatin intraoperatively in optimally debulked advanced EOC in both the PDS and IDS settings. The present study design assured timely delivery of chemotherapy combining the benefits IP chemotherapy which is known to improve the survival in advanced EOC [7–9]. The 97.7% (84/86) cases received scheduled IV chemotherapy on time after 21 days of intraoperative IP carboplatin. The delayed adjuvant chemotherapy > 25 days is known to decrease the survival in advanced EOC [7–9]. We highlight the importance of administering on time chemotherapy. And in our study design all patients by default received first dose effective IP carboplatin at the time of cytoreduction itself and subsequent IV chemotherapy. The procedure is simple, with low learning curve and a feasible option in low resource setting hospital where hyperthermic intraperitoneal chemotherapy (HIPEC) is not available or cannot be afforded by the patient. This study was designed to evaluate the feasibility of single-dose intraoperative IP carboplatin and the perioperative outcomes. All the patients tolerated the procedure well with negligible or no grade 3–4 complications. The morbidity and mortality rates are well within the acceptable limit. The long-term outcome disease-free survival and overall survival benefits will be studied in second phase at later date.
The HIPEC in advanced epithelial ovarian cancer is not popularly practiced globally due the morbidity, mortality, cost, and logistics associated with it. The single-dose intraoperative IP carboplatin used in our study is physician friendly, simpler, well tolerated by the patients. The present study procedure is normothermic intraoperative intraperitoneal chemotherapy (NIPEC) without a heated chemotherapy but has the prognostic advantages of intraperitoneal chemotherapy which is closely comparable to HIPEC. Therefore, our study is a hypothesis generating for the future trials to compare the NIPEC versus HIPEC in advanced EOC.
Conclusion
Single-dose intraoperative IP carboplatin is a feasible procedure with no or minimal manageable morbidity. The procedure is user friendly combining the prognostic benefits of IP chemotherapy with assurance of earliest timely administration of chemotherapy in advanced epithelial ovarian cancers. Our study is a hypothesis generating for the future clinical trials comparing single-dose normothermic intraoperative intra-peritoneal chemotherapy (NIPEC) versus hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in advanced EOC.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramnath Takiar (2019) Status of ovarian cancer in India (2012–14). EC Gynaecology 8.5. https://www.ecronicon.com/ecgy/pdf/ECGY-08-00336.pdf
- 2.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R, Gynecologic Oncology Group Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Suidan, et al. Prognostic significance of the number of postoperative intraperitoneal chemotherapy cycles for patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2015;25(4):599–606. doi: 10.1097/IGC.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker JL, Brady MF, Wenzel L, Fleming GF, Huang HQ, DiSilvestro PA, Fujiwara K, Alberts DS, Zheng W, Tewari KS, Cohn DE, Powell MA, van le L, Davidson SA, Gray HJ, Rose PG, Aghajanian C, Myers T, Alvarez Secord A, Rubin SC, Mannel RS. Randomized trial of intravenous versus Intraperitoneal chemotherapy plus Bevacizumab in advanced ovarian carcinoma: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2019;37(16):1380–1390. doi: 10.1200/JCO.18.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhang T, Wu Q, Jiao Y, Gong T, Ma X, Li D. Relationship between initiation time of adjuvant chemotherapy and survival in ovarian cancer patients: a dose-response meta-analysis of cohort studies. Sci Rep. 2017;7(1):9461. doi: 10.1038/s41598-017-10197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann Oncol. 2016;27:114–121. doi: 10.1093/annonc/mdv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y-Y, Lee J-W, Lu L, Xu W, Kollara A, Brown T, Heo E-J, May T. Impact of interval from primary cytoreductive surgery to initiation of adjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Gynecol Obstet. 2018;143:325–332. doi: 10.1002/ijgo.12653. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Therapy Evaluation Program: Common terminology criteria for adverse events v3.0 (CTCAE) (2006). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- 11.Clinical Advisory: NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. https://www.nlm.nih.gov/databases/alerts/ovarian_ip_chemo.html
- 12.Fagotti A, Gueli Alletti S, Corrado G, Cola E, Vizza E, Vieira M, Andrade CE, Tsunoda A, Favero G, Zapardiel I, Pasciuto T, Scambia G. The INTERNATIONAL MISSION study: minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2019;29(1):5–9. doi: 10.1136/ijgc-2018-000012. [DOI] [PubMed] [Google Scholar]
- 13.Corrado G, Mancini E, Cutillo G, Baiocco E, Vici P, Sergi D, Patrizi L, Saltari M, Baffa A, Vizza E. Laparoscopic debulking surgery in the management of advanced ovarian cancer after neoadjuvant chemotherapy. Int J Gynecol Cancer. 2015;25(7):1253–1257. doi: 10.1097/IGC.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 14.Carbajal-Mamani SL, Schweer D, Markham MJ, Esnakula AK, Grajo JR, Castagno JC, Cardenas-Goicoechea J. Robotic-assisted interval cytoreductive surgery in ovarian cancer: a feasibility study. Obstet Gynecol Sci. 2020;63(2):150–157. doi: 10.5468/ogs.2020.63.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elferink F, van der Vijgh WJ, et al. Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol. 1988;21(1):57–60. doi: 10.1007/BF00262740. [DOI] [PubMed] [Google Scholar]
- 16.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 17.Somashekhar SP, Prasanna G, Jaka R, Rauthan A, Murthy HS, Karanth S. Hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: a single institution Indian experience. Natl Med J India. 2016;29:262–266. [PubMed] [Google Scholar]
- 18.Manzanedo I, Pereira F, et al. Hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) with primary or secondary cytoreductive surgery in the treatment of advanced epithelial ovarian cancer. Minerva Ginecol. 2017;69(2):119–127. doi: 10.23736/S0026-4784.16.03959-9. [DOI] [PubMed] [Google Scholar]
- 19.Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(12):1578–1589. doi: 10.1016/j.ejso.2015.08.172. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong DK, Alvarez RD, et al. NCCN guidelines insights: Ovarian cancer, Version 1.2019. https://jnccn.org/view/journals/jnccn/17/8/article-p896.xml [DOI] [PubMed]