Abstract
Cissus quadrangularis L., a member of the Vitaceae family, is an important medicinal plant with widespread application in Indian traditional medicines. C. quadrangularis L. whole chloroplast genome of 160,404 bp was assembled using a genome skimming approach from the whole genome library. The assembled chloroplast genome contained a large single-copy region (88,987 bp), a small single-copy region (18,621 bp), and pairs of inverted repeat regions (26,398 bp). It also comprised 133 genes, including 37 tRNAs, eight rRNAs, and 88 protein-coding genes. Aside from that, we annotated three genes atpH, petB, and psbL, as well as one duplicated copy of the ycf1 gene in C. quadrangularis L. that had previously been missing from the annotation of compared Cissus chloroplast genomes. Five divergent hotspot regions such as petA_psbJ (0.1237), rps16_trnQ-UUG (0.0913), psbC_trnS-UGA (0.0847), rps15_ycf1 (0.0788), and rps2_rpoC2 (0.0788) were identified in the investigation that could aid in future species discrimination. Surprisingly, we found the overlapping genes ycf1 and ndhF on the IRb/SSC junction, rarely seen in angiosperms. The results of the phylogenetic study showed that the genomes of the Cissus species under study formed a single distinct clade. The detailed annotations given in this study could be useful in the future for genome annotations of Cissus species. The current findings of the study have the potential to serve as a useful resource for future research in the field of population genetics and the evolutionary relationships in the Cissus genus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01312-w.
Keywords: Cissus quadrangularis L., Inverted repeats, Phylogeny, Simple sequence repeats, Tandem repeats
Introduction
Cissus quadrangularis L. is a perennial evergreen fleshy climber that belongs to the family Vitaceae. In common parlance, it is referred to as veldt grape, adamant creeper, and hadjod etc. The genus Cissus, formerly placed in the family Ampelidaceae, is thought to contain around 350 species that can be found in various habitats around the world (Onyeweaku et al. 2020). The family Vitaceae is comprised of 14 different genera, with Cissus being the most abundant genus (Onyeweaku et al. 2020). Members of this genus are found in South-East Asia, the Arabian Peninsula, tropical and subtropical Africa, and the Indian subcontinent. The plant grows in plain coastal regions, woodlands, and wastelands up to 500 m in elevation. It generates adventitious roots at nodes, which makes the process of vegetative propagation quite simple.
Since antiquity, people have used C. quadrangularis L. for therapeutic purposes in a number of traditional Ayurvedic medications. Its name, asthisamharaka, translates to "that which avoids the deterioration of bones" since it is supposed to help cure shattered bones and is used as a tonic and painkiller in Siddha medicine (Sivarajan and Balachandran 1994). Some compounds in Cissus act as glucocorticoid receptor antagonists and promote bone development and fracture healing due to their anabolic and/or androgenic properties (Tiwari et al. 2018). In addition, different parts of the plant have been utilized to treat conditions such as piles, blindness, tumours, muscular discomfort, asthma, chronic ulcers, epileptic fits, appetite loss, constipation, and sexually transmitted diseases (Sundaran et al. 2020). The stem of C. quadrangularis L. contains various important antioxidants, including vitamin C, carotenoids, calcium, and steroids (Dhanasekaran 2020). Previously, researchers isolated bioactive compounds from C. quadrangularis L. and its associated endophytes, including quercetin, daidzein quadrangularin-A, myristic acid, 2-phenylethanol, 2-naphthol, methyl palmitate, betulinaldehyde, and -amyrin acetate (Bafna et al. 2021; Purohit et al. 2022). Interestingly, there are no harmful consequences associated with its consumption (Sawangjit et al. 2017; Sundaran et al. 2020).
The amount of genomic data available online has grown dramatically in recent years as a result of the low cost and advancement of second and third-generation high-throughput DNA sequencing technologies. These large whole genome-sequencing projects are regularly used to infer phylogenetic relations, evolutionary trends, genetic diversity studies, etc. (Gichuki et al. 2019; Muraguri et al. 2020; Shelke et al. 2020). Due to the chloroplast genome’s modest size (107–218 kb), it is considerably easier to sequence and assemble than the nuclear genome, resulting in the submission of 3823 chloroplast genomes to the Comprehensive Database of Chloroplast Genomes (http://www.gndu.ac.in/CpGDB) (Daniell et al. 2016). Chloroplast genomes differ from mitochondrial and nuclear genomes in a number of significant ways, including low nucleotide substitution rates, uniparental heredity, little homologous recombination, and a significantly preserved genomic makeup (Daniell et al. 2016). In contrast to nuclear genes, homologous genetic elements in chloroplast genomes evolved on average at a rate that was about one-third that of nuclear and three times that of mitochondrial genes, making it the perfect for molecular taxonomic investigations in resolving phylogenetic connections (Palmer et al. 1988). Molecular markers such as simple sequence repeats (SSRs) and single-nucleotide polymorphisms (SNPs), as well as intergenic spacers and introns, have a higher mutation rate and are thus useful for population genetics studies (Muraguri et al. 2020; Shelke et al. 2020; Li et al. 2021). Since the majority of medicinal plants are rare species with only a limited amount of information available to confirm their identity, chloroplast DNA is widely used in the field of herbal medicine for species identification and evolutionary research (Li et al. 2021). Based on chloroplast genome sequencing, it is possible to design species- or genus-specific barcodes that are extremely reliable, durable, and affordable (Li et al. 2021; Shelke and Rangan 2022). Additionally, using the complete chloroplast genome as a super barcode in situations when universal or single barcodes are unable to offer sufficient information for species discrimination opens up new possibilities for resolving the intricate connections between taxa (Krawczyk et al. 2018; Zhang et al. 2021).
The current study involved the assembly of the C. quadrangularis L. chloroplast genome and its comparison to the chloroplast genomes of five other Cissus sp. Furthermore, we performed pairwise comparisons of the protein-coding sequences from these species to identify candidate genes that had been subjected to either positive or negative selection. Through our data analysis, we found a plethora of species-specific repeat sequences and barcodes that could be used as markers. The evolutionary connections among the members of the Vitoideae subfamily were also examined using the chloroplast genome data. In general, the findings of our study provide in-depth genome annotations of C. quadrangularis L. and contribute to resources that can be used in future evolutionary research for Cissus and other related species in the Vitoideae subfamily.
Materials and methods
C. quardrangularis L. chloroplast genome assembly and annotation
In this investigation, we retrieved SRA read of C. quadrangularis L. (SRR8573652) from the SRA database submitted by Gichuki et al. (2019). The reads were generated on Novogene's Illumina HiSeq X Ten platform as paired-end 2X150 bp reads with 400–450 bp insert size (Gichuki et al. 2019). The downloaded raw Illumina reads were quality inspected using the FastQC (0.11.8) tool before being de novo assembled using the NOVOplasty assembler (version 3.7) with a rbcL sequence as a seed (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Using the NCBI BLASTN software, the resulting chloroplast genome assembly sequence was checked against the non-redundant (nr) database. After that, the GeSeq tool was used to annotate the whole chloroplast genome (Tillich et al. 2017). Eventually, the OGDRAWv1.2 tool was used to create the circular genome map for C. quadrangularis L. (Dierckxsens et al. 2017). The whole chloroplast genome sequence of C. quadrangularis L. was submitted to GenBank along with its gene annotation (OP414588).
Structural comparison of Cissus chloroplast genomes
We have included six genomes of Cissus species in the current investigation for structural comparison of chloroplast genomes: C. quadrangularis L. (OP414588), C. antarctica Vent. (NC_061724.1), C. discolor Blume (NC_061723.1), C. microcarpa Vahl (NC_061722.1), C. trifoliata (L.) L. (NC_061720.1), and C. tuberosa Moc. & Sessé ex DC. (NC_061719.1). Synteny studies of Cissus chloroplast genomes were carried out through the MAUVE tool (https://darlinglab.org/mauve/mauve.html). While the borders between inverted repeats (IR) and short single copy (SSC) regions in these species were also compared and studied to assess the evolution of genes found in IR boundary areas.
Tandem repeats and simple sequence repeats mining
The six Cissus chloroplast genomes were examined for the presence of forward, reverse, palindromic, and complementary repeats using the REPuter software. The parameters used to identify repeats were more than 30 bp and a hamming distance of three (https://bibiserv.cebitec.uni-bielefeld.de/reputer/). MISA software v1.0.6 was used to identify SSRs in the six compared genomes using the following parameters: 10 for mono-, 5 for di-, 4 for tri-, and 3 for each of the following nucleotides: penta-, hexa-, septa-, octa-, nona-, and deca- (https://pgrc.ipk-gatersleben.de/misa/misa.html).
Identification of divergent hotspot regions
C. quadrangularis L. was compared using the mVISTA program with five Cissus genomes such as by keeping the C. quadrangularis L. annotation as a reference (http://genome.lbl.gov/vista/mvista/submit.shtml). The DnaSP v5.10 tool was utilized to determine the level of nucleotide diversity of genic and intergenic regions separately present in the compared chloroplast genomes (http://www.ub.edu/dnasp/).
Characterization of substitution rates and synonymous codon usage analysis
In six comparable species of the genus Cissus, the evolutionary forces operating on common protein-coding genes were examined. The rate of synonymous to nonsynonymous nucleotide substitution (Ka/Ks) was calculated with the help of KaKs_calculator 3.0 (Zhang 2022). Common protein-coding genes from the six Cissus species were selected for codon usage bias analysis. Average guanine-cytosine (GC) content and codon usage bias were determined for all protein-coding genes by nucleotide composition and relative synonymous codon usage (RSCU) analysis using MEGA X programme (https://www.megasoftware.net/).
Phylogenetic analyses
The newly assembled C. quadrangularis L. chloroplast genomes, as well as other Vitoideae genomes, were used in the study to construct a phylogenetic tree. We chose one chloroplast genome of Leea guineensis G. Don as an out-group species belonging to the Leeoideae subfamily of Vitaceae. The compared sequences were first aligned using MAUVE to find the locally collinear blocks (LCBs) in the HomBlocks pipeline (Bi et al. 2018). Then the model of substitution was figured out with the assistance of the MEGA-X tool (version 10.2.4), and it was done so in accordance with what the Akaike information criterion (AIC) indicated. Finally, the phylogenetic tree was built using the maximum-likelihood method (ML) in the MEGA X programme with 500 bootstraps (https://www.megasoftware.net/). The resulting phylogenetic tree was then depicted visually via the iTOL server (https://itol.embl.de/).
Results
C. quadrangularis L. chloroplast genome assembly and annotation features
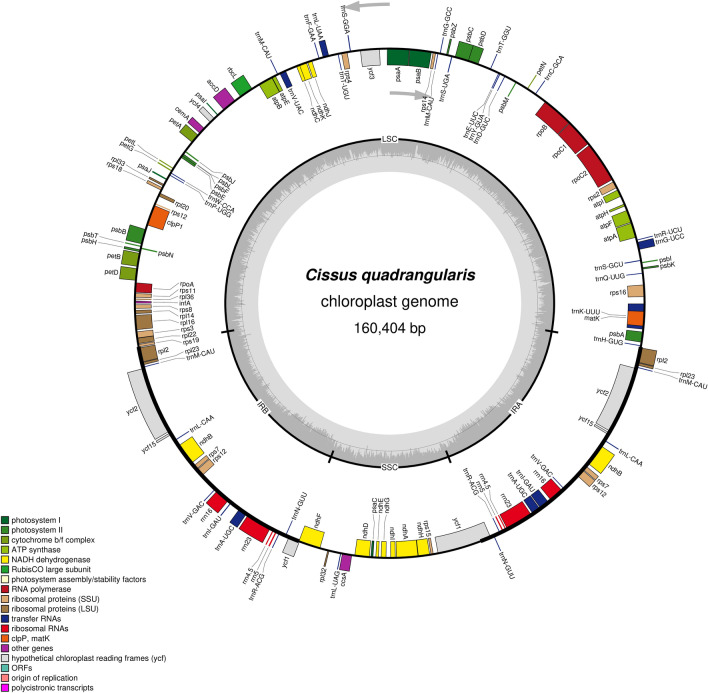
Using the NOVOplasty assembler with 18,978,233 raw paired-end reads, the whole chloroplast genome of C. quadrangularis L. was successfully assembled. The size of the C. quadrangularis L. chloroplast genome was 160,404 bp in length (Fig. 1). And its average mean coverage depth was around 2793 X. The newly assembled chloroplast genome was highly comparable in sequence to the Cissus chloroplast genomes included in the Genbank database. The whole chloroplast genome sequence of C. quadrangularis L. was submitted to GenBank with the accession number OP414588. The chloroplast genome of C. quadrangularis L. is arranged in the usual quadripartite pattern, with two IR regions (26,398 bp) that are separated by LSC (88,987 bp) and SSC (18,621 bp) regions. The average percentage of GC in the whole chloroplast genome was 37.2%, which was 42.8% in IR, 35.07% in LSC, and 31.3% in SSC.
Fig. 1.
Circular map of C. quadrangularis whole chloroplast genome along with gene annotations. Genes shown outside the outer circle are transcribed anti-clockwise, and those inside the circle are transcribed clockwise. Genes belonging to different functional groups are colour coded. The grey colour inside the circle indicates the GC content and the lighter grey depicts the AT content
In the C. quadrangularis L. chloroplast genome, there are a total of 133 genes; 88 of these genes code for proteins, while the remaining 37 and 8 code for tRNA and rRNA, respectively (Table 1). The genome was revealed to have duplications in four rRNA, seven tRNA and nine protein-coding genes. In total, 18 genes were observed in IRb and IRa regions, respectively. The genes that were found duplicated in IR regions were seven protein-coding (ndhB, rpl2, rpl23, rps7, rps12, ycf2, and ycf15), four rRNA (rRNA 4.5, rRNA 5, rRNA 16, and rRNA 23), and seven tRNA (trnA-ACG, trnA-UGC, trnM-CAU, trnI-GAU, trnL-CAA, trnN-GUU, and trnV-GAC) genes. Two genes (clpP1 and ycf3) have double introns, and the rest twenty genes (atpF, rpoC1, petB, petD, rpl2, rpl2_copy, rpl16, ndhA, ndhB, ndhB_copy, rps12, rps12_copy, rps16, rps16_copy, trnA-UGC, trnA-UGC_copy, trnI-GAU, trnI-GAU_copy, trnK-UUU and trnV-UAC) include single intron. The LSC and IRa area contains the trans-spliced rps12 gene. The matK gene is located in the biggest intron of the gene trnK-UUU. No gene loss was observed in the C. quadrangularis L. genome.
Table 1.
Annotated genes and their functions in C. quandrangularis chloroplast genome
| Gene category | Group of genes | Name of genes | ||||
|---|---|---|---|---|---|---|
| Self-replication | rRNA genes | rrn16 D | rrn23 D | rrn4.5 D | rrn5 D | |
| tRNA genes | trnA-UGC D* | trnC-GCA | trnD-GUC | trnE-UUC | trnF-GAA | |
| trnG-GCC | trnG-UCC | trnH-GUG | ||||
| trnI-GAU D* | trnK-UUU * | trnL-CAA D | trnL-UAA | trnL-UAG | ||
| trnM-CAU # | trnN-GUU D | trnP-UGG | trnQ-UUG | trnR-ACG D | ||
| trnR-UCU | trnS-GCU | trnS-GGA | trnS-UGA | trnT-GGU | ||
| trnT-UGU | trnV-GAC D | trnV-UAC * | trnW-CCA | trnY-GUA | ||
| Large subunit of ribosome (LSU) | rpl2 D* | rpl14 | rpl16 * | rpl20 | rpl22 | |
| rpl23 D | rpl32 | rpl33 | rpl36 | |||
| Small subunit of ribosome (SSU) | rps2 | rps3 | rps4 | rps7 D | rps8 | |
| rps11 | rps12 D* | rps14 | rps15 | rps16 * | ||
| rps18 | rps19 D | |||||
| RNA polymerase | rpoA | rpoB | rpoC1 * | rpoC2 | ||
| Translational initiation factor | infA | |||||
| Photosynthetic genes | Photosystem I | psaA | psaB | psaC | psaI | psaJ |
| Photosystem II | psbA | psbB | psbC | psbD | psbE | |
| psbF | psbH | psbI | psbJ | psbK | ||
| psbL | psbM | psbN/pbf1 | psbT | psbZ | ||
| Cytochrome b/f complex | petA | petB * | petD * | petG | petL | |
| petN | ||||||
| ATP synthase | atpA | atpB | atpE | atpF * | atpH | |
| atpI | ||||||
| ATP-dependent protease (p subunit) | clpP1 ** | |||||
| RubisCO large subunit | rbcL | |||||
| NADH dehydrogenase | ndhA * | ndhB D* | ndhC | ndhD | ndhE | |
| ndhF | ndhG | ndhH | ndhI | ndhJ | ||
| ndhK | ||||||
| Other genes | Maturase | matK | ||||
| Protein in envelop membrane | cemA | |||||
| Acetyl-CoA-carboxylase subunit | accD | |||||
| Cytochrome synthesis gene ( C-type) | ccsA | |||||
| Genes with unknown function | Hypothetical reading frames for chloroplast | ycf1D | ycf2 D | ycf3 ** | ycf4 | Ycf15 D |
⁎Genes having one intron
⁎⁎Gene having two introns
DGene having duplicated copies
#Gene having quadruplicated copies
Structural comparison of Cissus chloroplast genomes
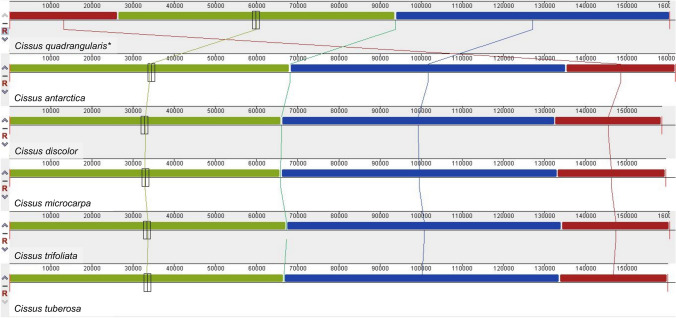
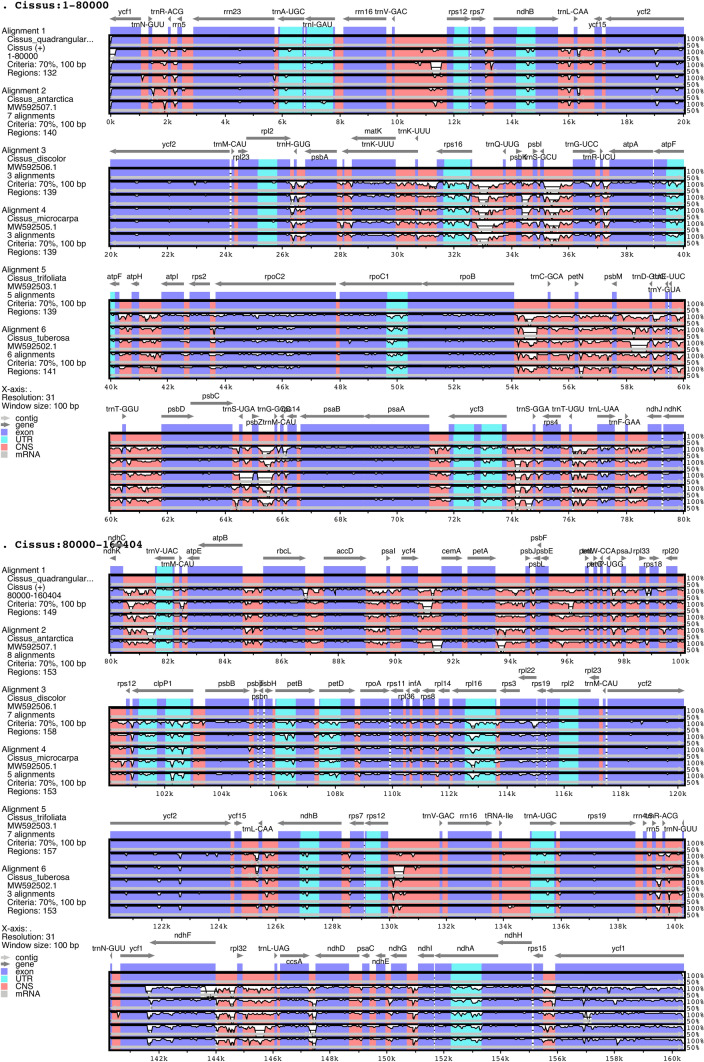
The Mauve alignment of Cissus chloroplast genomes found no major inversions; with the exception of one inversion that was around 250 bp long in C. trifoliata (L.) L. and C. tuberosa Moc. & Sessé ex DC. (Fig. 2). Remaining compared genomes showed no inversion. The observed inversion that encompassed the intergenic region can be found in the LSC region of C. trifoliata (L.) L. and C. tuberosa Moc. & Sessé ex DC. (Online Resource 1).
Fig. 2.
MAUVE alignment of C. quadrangularis and five chloroplast genomes of Cissus species. The gene arrangement in chloroplast genomes is represented by different coloured local collinear blocks. ( * denotes target species)
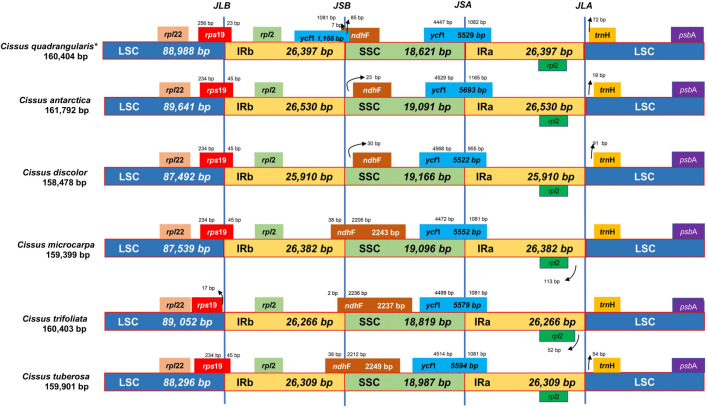
In all six studied genomes, the IR/SSC boundary included two genes, ycf1 and ndhF (Fig. 3). The contraction and expansion of the ycf1 gene at the LSC/IRa boundary in six Cissus species showed distinct patterns occupying the varying number of bp in SSC and IRa regions, with a larger part in SSC region in all cases. The ycf1 gene was found in the Cissus species at positions 4447 to 4568 bp in the SSC region and 965 to 1082 bp in the IRa region. The ndhF gene was discovered at the junction of IRb/SSC in C. quadrangularis L., C. tuberosa Moc. & Sessé ex DC., C. trifoliata (L.) L., and C. microcarpa Vahl. It extended 2–38 bp in the IRb area and the bulk remained in the SSC region. On the other hand, the ndhF of C. discolor Blume and C. antarctica Vent. was seen in the SSC region.
Fig. 3.
Comparisons of LSC, SSC, and IR region borders among six chloroplast genomes of Cissus species. Colour boxes present the genes. ( * denotes target species)
In all species, the rps19 gene extended 23–45 bp in the IRb area across the LSC/IRb junction except C. trifoliata (L.) L., which was present entirely at 17 bp inside the LSC region from the border. The IRa and IRb regions included the full rpl2 gene. The trnH gene was entirely localized in the LSC area just outside the IRa/LSC border in all the species that were investigated. The findings demonstrated that these six species of Cissus shared conserved chloroplast genomes, with only minor changes at the junctions.
Tandem repeats and simple sequence repeats mining
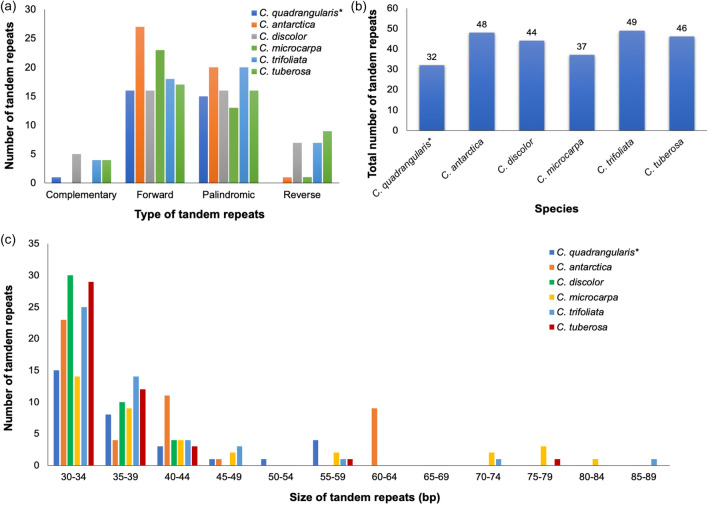
There were 32 tandem repeats in the chloroplast genome of C. quadrangularis L., 16 of which were forward repeats, 15 were palindromic, single complement, and there were no reverse repeats (Fig. 4a). In general, the total number of tandem repeats ranged from 32 in C. quadrangularis L. to 49 in C. trifoliata (L.) L. (Fig. 4b). The majority of tandem repeats were found to be of the forward type across all six analysed genomes, with maximum forward repeats in C. antarctica Vent. (27) and the minimum in C. discolor Blume and C. quadrangularis L. (16). On the other hand, the highest numbers of palindromic repeats were recorded in C. antarctica Vent. and C. trifoliata (L.) L. (20 repeats) and the lowest in C. microcarpa Vahl (13 repeats). C. tuberosa Moc. & Sessé ex DC. had the most reverse repeats (9) and no reverse repeat was observed in C. quadrangularis L. The complimentary repetitions were absent in both C. antarctica Vent. and C. microcarpa Vahl, while C. discolor Blume has a maximum of five repeats. Tandem repeats of length 30–34 bp emerged as the most frequent repetitions, followed by 35–39 bp and 40–44 bp fragments (Fig. 4c). Tandem repetitions within 45–49 bp and 55–59 bp ranges were uncommon in all species except C. quadrangularis L., and only C. discolor Blume had nine repetitions within 60–64 bp range. Additionally, a few repetitions can be observed in the range of 70–74 bp, 75–79 bp, 80–84 bp, and 85–89 bp, respectively.
Fig. 4.
Comparison of tandem repeats of six chloroplast genomes of Cissus species, a Number of tandem repeats by repeat types; b Number of tandem repeats by sequence length. ( * denotes target species)
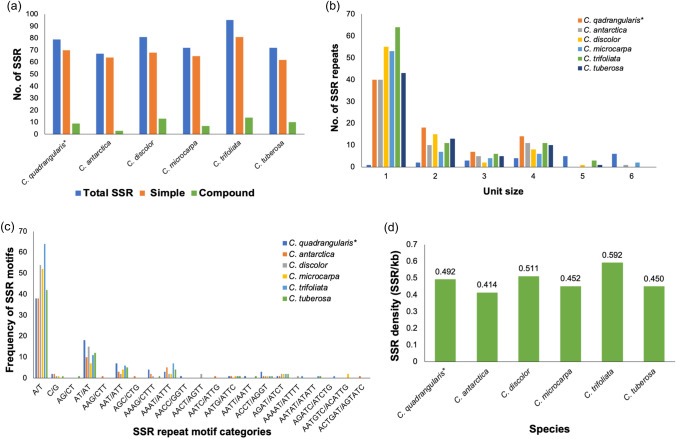
In the chloroplast genome of C. quadrangularis L., we found 70 simple and 9 compound SSRs (Fig. 5a). Out of 70 simple SSRs, 40 (50.6%) were mononucleotides, 18 (22.8%) were dinucleotides, 7 (8.9%) were tri-nucleotides, and 14 (14.1%) were tetra-nucleotides (Fig. 5b). Comparative analysis of SSRs found that C. antarctica Vent., C. discolor Blume, C. microcarpa Vahl, C. trifoliata (L.) L., and C. tuberosa Moc. & Sessé ex DC. contained 67, 81, 72, 95, and 72 SSRs, respectively. C. microcarpa Vahl was discovered to have the highest percentage of mononucleotide SSRs (73.6%), followed by C. discolor Blume (67.9%) and C. trifoliata (L.) L. (67.37%). The least mononucleotide percentage was recorded in C. quadrangularis L. (50.6%), followed by C. tuberosa Moc. & Sessé ex DC. (59.72%). In all cases, A/T motifs were abundant, whereas C/G repeat motifs were rare. C. trifoliata (L.) L. had the most A/T rich repeats (64), followed by C. discolor Blume (54), and C. antarctica Vent. along with C. quadrangularis L. had the fewest (38). Similarly, the AT/AT repeats were higher in C. quadrangularis L. (18) than C. discolor Blume (15) and least in C. microcarpa Vahl (7). AAT/ATT repeats were the most common trinucleotide repeats, whereas others were rarely present (Fig. 5c). C. trifoliata (L.) L. had a higher SSR density (0.6 SSR/kb) than the other species. However, C. antarctica Vent. had the lowest SSR density (0.41 SSR/kb) (Fig. 5d).
Fig. 5.
Comparison of SSR repeats between C. quadrangularis and five chloroplast genomes of Cissus species. a Number of SSR repeats in regular and compound formation; b The total number of SSR repeat types; c The number of SSR motifs, and d SSR density. ( * denotes target species)
Comparative genome analysis and DNA diversity
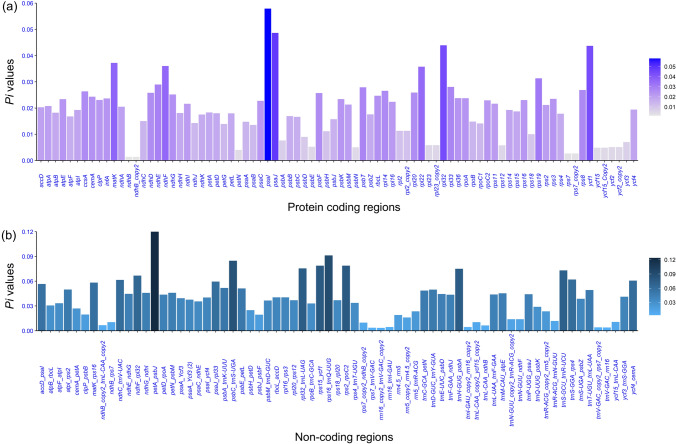
Comparing the nucleotide diversity in coding and non-coding regions identified hotspot regions in the Cissus chloroplast genomes. Protein-coding genes' nucleotide diversity (Pi) ranged from 0.0013 (ndhB) to 0.0579 (psaI) (Fig. 6a). The average diversity in protein-coding regions was calculated to be 0.0191. The five most diverged coding regions in Cissus genomes were psaI (0.0579), psaJ (0.0486), rpl32 (0.0439), ycf1 (0.0437), and matK (0.03721). The least diverse coding regions were ndhB (0.0013), rps7 (0.0027), petN (0.0040), ycf15 (0.0048), and psbN (0.0050).
Fig. 6.
Nucleotide diversity (Pi) values of Cissus species. The nucleotide diversity of, a protein-coding regions; b non-coding regions
Non-coding region nucleotide diversity (Pi) was found to be higher in Cissus genomes than in coding regions, ranging from 0.0034 (rrn16_copy2_trnV_GAC_copy2) to 0.1237 (petA_psbJ) (Fig. 6b). It was discovered that non-coding regions had an average diversity of 0.0391. The five regions that had the maximum nucleotide diversity were as follows: petA_psbJ (0.1237), rps16_trnQ-UUG (0.0913), psbC_trnS-UGA (0.0847), rps15_ycf1 (0.0788), and rps2_rpoC2 (0.0788). Non-coding regions that had the lowest Pi value were as follows: rrn16_copy2_trnV-GAC_copy2 (0.0034), rps7_trnV-GAC (0.0036), trnV-GAC_rrn16 (0.0039), trnV-GAC_copy2_rps7_copy2 (0.0040), and rrn16_trnI-GAU (0.0044). The investigation of the chloroplast genome of Cissus performed with mVISTA produced remarkably similar results with DNA diversity, with a significant drop in the percentage of similarity at non-coding regions and high conservation in coding regions (Fig. 7).
Fig. 7.
Chloroplast genome alignment between C. quadrangularis and five chloroplast genomes using the mVISTA tool with C. quadrangularis as a reference. The grey arrows represent the direction of gene transcription. The y-axis depicts the percentage identity ranging between 50 and 100%. ( * denotes target species)
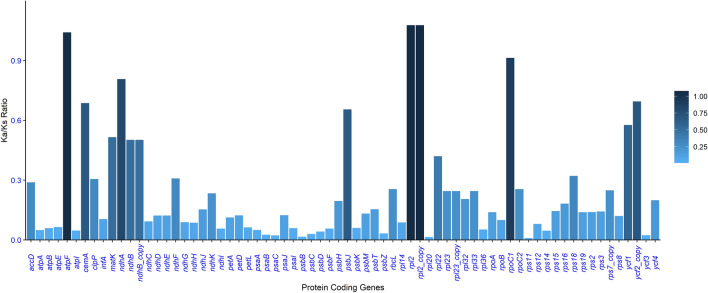
Ka/Ks ratios in Cissus genomes
The Ka/Ks ratio was determined by examining 82 common genes from compared Cissus genomes. Out of 82 genes, 12 had a 0 or no Ka/Ks ratio, 70 genes had a Ka/Ks ratio, wherein three genes had more than one ratio (atpF, rpl2, and rpl2_copy) and sixty-seven had less than one ratio (Fig. 8). The Ka/Ks ratio varied between the six Cissus species, ranging from 0.009 (rps11) to 1.077. (rpl2). Purifying selection is therefore acting on the vast majority of genes with a Ka/Ks ratio lower than one. When the Ka/Ks ratio is greater than 1, the selection is working positively on the gene under study; when it is equal to 1, the selection is neutral. There were a few genes with Ka/Ks ratios that were either zero or non-existent; these values were obtained when Ks values were exceedingly low or when there was no substitution in matched sequences.
Fig. 8.
Comparison of Ka/Ks ratio generated in protein-coding genes aligned from Cissus species genomes
Synonymous codon usage
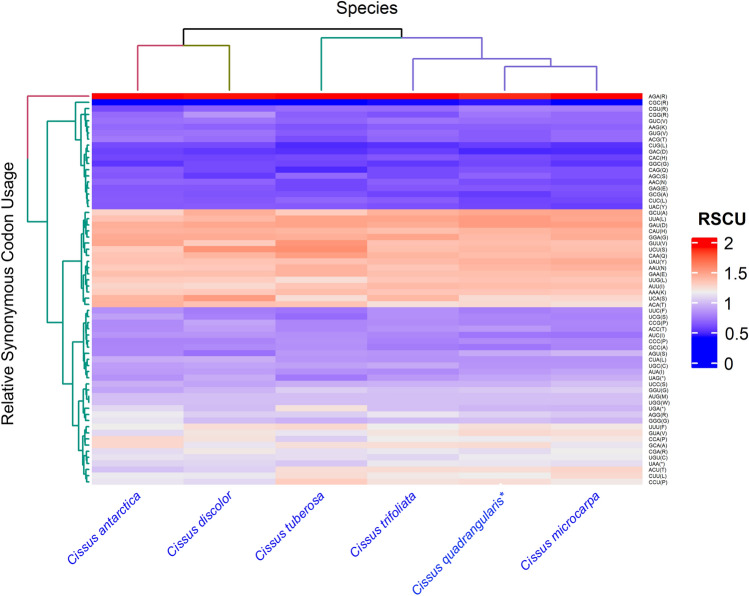
The six Cissus chloroplast protein-coding genes were discovered to preferentially use A/T bases or A/U-ending codons over GC bases at all three codon positions; as a result, the overall average GC content of codons was less than 40% (Online Resource 2). In the compared genomes, the GC content in the first position (GC1) ranged from 36.2% to 39.5% in C. antarctica Vent and C. microcarpa Vahl, respectively. While the GC content in the second position (GC2) was lowest in C. discolor Blume (36.4%) and highest in C. tuberosa Moc. & Sessé ex DC. (39.7%). The highest concentration of GC for the third position (GC3) was found in C. antarctica Vent (38.8%), whereas the lowest concentration was found in C. tuberosa Moc. & Sessé ex DC. (36.4%). Mutations are reported to have an essential role in codon usage bias (Li et al. 2017). There were 64 codons including three stop codons, present across the six Cissus species encoding 20 amino acids. C. quadrangularis L. and C. antarctica Vent each had 25,256 codons, C. microcarpa Vahl and C. tuberosa Moc. & Sessé ex DC. each had 25,174 codons, C. discolor Blume had 25,010 codons, and C. trifoliata (L.) L. had 25,420 codons. In the studied genomes, A and U bases were prevalent at the third position of codons (RCSU > 1). Analyses revealed that leucine was the most prevalent amino acid (9.01%—10.85%), followed by tryptophan (1.89%—2.11%) and serine (1.73%—2.34%). Tryptophan and methionine had only one codon each, so they had no preference for codon usage (Fig. 9). In contrast, the remaining amino acids contained multiple synonymous codons. For leucine and arginine, there were six synonymous codons, with biasness towards UUA and AGA respectively. While valine, serine, proline, threonine, alanine, and glycine had four synonymous codons each (Online Resource 3). Out of the six Cissus species, C. discolor Blume used 28 codons, C. antarctica Vent and C. tuberosa Moc. & Sessé ex DC. used 30 codons frequently (RSCU > 1), and the other three species used 29 codons more often than expected at equilibrium (RSCU > 1). The start codons AUG and UGG, which code for the amino acids methionine and tryptophan, were used equally often by all species of Cissus (RSCU = 1).
Fig. 9.
Heat map of relative synonymous codon usage (RSCU) values among six Cissus species blue to red colour indicates low to high RSCU values of codons. (* denotes target species)
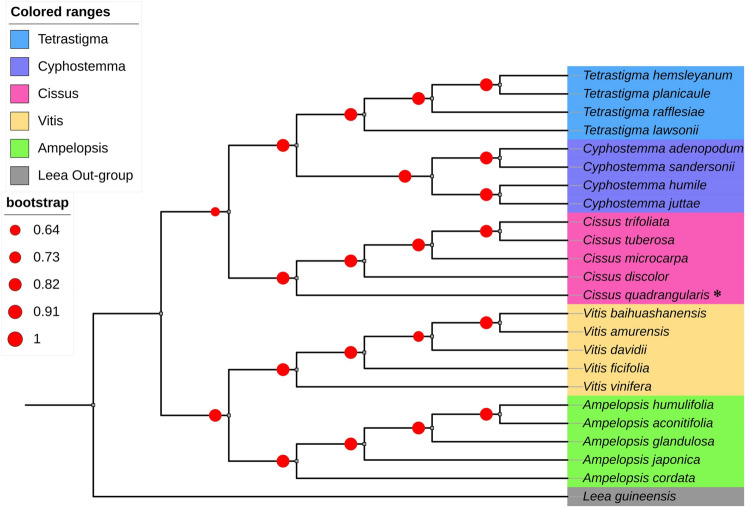
Phylogenetic analysis
The ML phylogenetic tree was created using the chloroplast genomes of 24 different species as a super barcode. Unaligned sequences were removed from the final alignment. The GTR + G + I model was determined to be the best-fit model for further phylogenetic tree construction based on the results of an alignment of 1,37,163 bp of nucleotides from the 24 whole genome sequences. The ML analysis tree was resolved, and the majority of nodes received maximum support (Fig. 10). The Leeoideae, a subfamily of the Vitaceae family served as an out-group. The phylogenetic tree recovered the clustering of Cissus species in one group clade that included C. quadrangularis L., C. discolor Blume, C. tuberosa Moc. & Sessé ex DC. and C. trifoliata (L.) L., sharing a common node with the Tetrastigma and Cyphostemma genera. Within the Cissus group, the species C. quadrangularis L. was found to have a close association with C. discolor Blume. On the other hand, the Vitis and Amplelopsis genus formed their own separate group but shared a node.
Fig. 10.
Phylogenetic tree was drawn based on the whole chloroplast genomes from 24 Vitaceae species constructed through the maximum likelihood method. (* denotes target species)
Discussion
The chloroplast genome of C. quadrangularis L. is circular and exhibits a quadripartite genome structure consistent with the angiosperm chloroplast genomes (Shelke and Rangan 2022). The chloroplast genome of C. quadrangularis L. reported in this study was 160,404 bp in length, which is within predicated range of angiosperm chloroplast genomes (107–218 kb) (Daniell et al. 2016). Among the Cissus genomes, the highest genome size of 161,792 bp was recorded in C. antarctica Vent. and lowest 158,478 bp in C. discolor Blume.
The chloroplast genome of C. quadrangularis L. encoded 37 tRNA genes and 8 rRNA genes, similar to the majority of angiosperms, including Cissus species (Daniell et al. 2016; Shelke and Rangan 2022). The fact that previous Cissus genomes were missing four genes was intriguing. If they were lost, were their genes still in the nuclear or mitochondrial genomes? While looking for an answer, we were able to identify four extra protein-coding genes, including atpH, petB, and psbL, as well as one duplicated copy of the ycf1 gene, which had previously gone unnoticed in the annotation of compared Cissus chloroplast genomes. The genes in question are, in fact, present in the Cissus chloroplast genomes; however, it's possible that previous reports failed to include them in their annotation database. Through BLASTX analysis, we confirmed the presence of these genes in the compared genomes in the current study. We conclude that the Cissus genomes have not lost any protein-coding genes. When introns are located in certain regions, they have a significant impact on the expression of genes. The chloroplast genomes of typical angiosperms contain around 23 introns in tRNA and protein-coding genes (Wang et al. 2022). From the 133 genes found in the C. quadrangularis L. genome, 22 were found to have introns which included 20 genes with a single intron and two genes (clpP1 and ycf3) with two introns. Intriguingly, we noticed two copies of the ycf15 gene in Cissus genomes, a gene that is typically absent from many angiosperm genomes (Jin et al. 2020; Wang et al. 2021; Shelke and Rangan 2022). However, the presence of the ycf15 gene was observed in many members of the Vitaceae family, with its size ranging from 234 to 488 bp. It is also present in full copies in the plastome genomes of Nicotiana, Epifagus, Cuscuta etc. (Schmitz-Linneweber et al. 2001).
Many chloroplast genome inversions have been fully characterized, revealing that this process is critical in genome rearrangement (Kim et al. 2005; Jin et al. 2020; Jiang et al. 2022). We were able to observe an inversion of approximately 250 bp in length in both C. trifoliata (L.) L. and C. tuberosa Moc. & Sessé ex DC. A similar, albeit smaller, inversion in an intergenic region was documented in Mesua ferrea L. in the past (Shelke and Rangan 2022). On the other hand, larger inversions of up to 50 kb and even more have been reported previously in some angiosperm species (Jin et al. 2020; Tian et al. 2021; Wu et al. 2022). The inversion appears to be conserved because it is located in the same place between the petA gene and the psbJ gene in both C. trifoliata (L.) L. and C. tuberosa Moc. & Sessé ex DC. genomes. Such inversion plays a crucial role in plant evolution as a mechanism for the establishment and preservation of interspecific differentiation and is likely to be related to the formation of plant groupings (Kim et al. 2005). Overall the Cissus chloroplast genomes had identical gene orders, and most of the chloroplast region’s sequence identities were also similar across species. These findings imply that the chloroplast genomes of Cissus are substantially conserved.
The IR junctions in chloroplast genomes are typically the site of structural changes. The expansion and contraction of IR junctions in higher plants usually contribute to these changes. It was discovered that the diversity of the genome's size and structure was influenced by the length of the IR regions (Jin et al. 2020). On the IR junctions, there was no evidence of gene loss in any of the six compared species of Cissus, with the exception of the ycf1 gene, which was present on the IRb/LSC border of C. quadrangularis L. but absent in other species. There is a high degree of homogeneity between LSC/IRs and SSC/IRs borders in Cissus and angiosperm chloroplast genomes, with most of these boundaries located in rps19 or ycf1 (Downie and Jansen 2015; Alzahrani et al. 2020; Shelke and Rangan 2022). In C. quadrangularis L., it was found that the ndhF gene and the ycf1 gene both crossed the IRb/SSC junction by 7 and 85 bp, respectively. In this event, the two genes overlapped each other and shared a 92 bp coding region, as documented in some species of Andrographis, Barleria and Justicia genera (Alzahrani et al. 2020). We also observed such overlapping sequences for atpE_atpB, ndhK_ndhC and psbD_psbC genes in Cissus chloroplast and other angiosperm genomes (Rossini et al. 2021). In contrast to rps19 and ndhF genes, which were occasionally found crossing the IR regions in some compared genomes, rpl2 and trnH were found to be located entirely within their respective regions. The ycf1 gene, which lies at the IRa/SSC intersection, entered the IRa area in the compared genomes with varying lengths ranging from 965 to 1082 bp, which is a regular phenomenon in angiosperms (Alzahrani et al. 2020; Li et al. 2022; Xu et al. 2022). These instances of ycf1 crossing the IRb/SSC junction were found to occur frequently in the genomes of numerous angiosperm species (Jin et al. 2020; Shelke and Rangan 2022). Here, there was no noticeable difference in IR length between Cissus genomes. It was found that the length of the ycf1 gene ranged from 5522 bp (C. discolor Blume) to 5693 bp (C. antarctica Vent.) across the analyzed genomes. The ycf1 gene was found in most of the Vitaceae species and varied from 3617 to 5811 bp. Despite its presence in many angiosperm species, it has been confirmed to be absent in some members of the Poaceae family (Goremykin et al. 2005; Guisinger et al. 2011).
The chloroplast genome contains a variety of repeated sequences, including SSRs, homo-polymeric repeats, and long repeats. These sequences serve as a source of variations for genome evolution and rearrangement (Das et al. 2018; Shelke and Rangan 2020; Shelke et al. 2020). Therefore, repeat elements may be used to make molecular markers for analyzing population genetic structure and evolutionary relationships between distinct species (Shelke and Das 2015; Shelke and Rangan 2019; Shelke et al. 2020; Wu et al. 2021). Long tandem repeats are frequently found in the chloroplast genome in association with intermolecular recombination to produce sequence diversity (Guo et al. 2021). The number of tandem repetitions in all of the Cissus genomes ranged from 32 in C. quadrangularis L. to 49 in C. trifoliata (L.) L. Despite having the largest genome size, C. antarctica Vent. appears to have the fewest number of SSRs of all the genomes studied, which is consistent with previous research in M. ferrea L. (Khan et al. 2019; Shelke and Rangan 2022). Therefore, the sequence variability caused by the tandem repeats could aid in the design of species-specific markers.
SSRs are also tandem repeats that are found in the genome in significantly greater abundances than long tandem repeats (Khan et al. 2019; Wang et al. 2021; Shelke and Rangan 2022). SSRs ranged from 67 in C. antarctica Vent. to 95 in C. trifoliata (L.) L. in the genomes of the Cissus species. It is interesting to note that the genome of C. trifoliata (L.) L. was found to contain the highest number of tandem and SSR repeats among those that were compared. Similarly, although the genomes of C. trifoliata (L.) L. and C. quadrangularis L. are roughly the same size, but there was a substantial difference in the number of repeats. This fact is supported further by the observation that C. trifoliata (L.) L. has the highest SSR density (0.6 SSR/kb). Generally, it is difficult to compare the results of repeat populations obtained from different publications because different authors use a wide range of mining parameters for their repeat analysis. Such comparisons do not provide sufficient information regarding comparative studies in such circumstances. The emergence of a significant number of SSRs in the genome is most likely the result of adaptations that were developed over the course of evolution in response to specific factors of the surrounding environment (Shelke et al. 2020). These new SSR mutations can lead to variations that are species-specific and can assist in the designing of barcodes as a marker for species delimitation.
A significant level of sequence similarity was observed among the Cissus chloroplast genomes. The non-coding areas were more diverged than the coding sections, and the IR regions were more preserved than the LSC and SSC regions, which is consistent with other angiosperms (Guan et al. 2022; Shelke and Rangan 2022). Then, comparable outcomes were also seen in DNA diversity analysis, where it was shown that coding regions were more conserved than non-coding areas (Rossini et al. 2021; Guan et al. 2022). The Cissus chloroplast genome's most varied coding and non-coding regions have been identified in the current study. However, some parts of the chloroplast genome change at a significantly faster rate and hence meet the criteria for being used as a DNA barcode (Senapati et al. 2021; Guan et al. 2022). In light of the fact that many universal DNA barcodes are often insufficient to resolve species-level distinctions in many angiosperms due to low levels of nucleotide diversity. Therefore such genus-specific DNA barcodes have the potential to be utilised in the future for discriminating Cissus species (Li et al. 2021).
Nucleotide substitution rate changes have been linked to selective pressures on protein-coding regions of genomes over evolutionary time (Shidhi et al. 2021). The Cissus genus was found to be under purifying selection on the vast majority of its protein-coding genes, a trend that has been outlined frequently in many plant species, indicating that the overall conserved nature of plastid genes in angiosperms (Shidhi et al. 2021; Shelke and Rangan 2022). As a result, plastid function is under less selective pressure leading to purifying selection on genes producing proteins for DNA maintenance and expression that may eventually be lowered.
Codon usage is extremely important in expressing genetic information correctly. The codons used by all six Cissus species were the nearly same, with 61 amino acid codons, three termination codons (UAA, UAG, and UGA). In addition to preferred codon usage, the number and type of codons that code for the 20 amino acids varied among Cissus species and similar observations were also reported in Physalis species (Feng et al. 2020). Most codons that were preferred for encoding amino acids had either an A or a T(U) as their third nucleotide. Out of all the biased codons (RSCU > 1) in Cissus species 45% had A and 48% had U at third position. Such observations have previously been made in a number of angiosperms, including the Poaceae, Asteraceae, Euphorbiaceae, and Oryza species families (Zhang et al. 2012; Nie et al. 2014; Ma et al. 2020; Chakraborty et al. 2020). Codon usage frequencies differ in the chloroplast genomes of Cissus species, which may be related to hydrophilicity, base substitution, natural selection, tRNA abundance, gene length, mRNA secondary structure, and random genetic drift (Feng et al. 2020; Li et al. 2022). The genetic makeup of a population, as well as selection in favor of enhanced translation, are said to be determinants of the trend in codon usage (Lee et al. 2019). There was also evidence from early studies that monocots favored GC-rich codons and dicots favored AT-rich ones (Wang and Roossinck 2006).
Chloroplast genomes have been found to be a very helpful tool for understanding the evolutionary connections of many angiosperm plants (Jin et al. 2020; Li et al. 2021; Shelke and Rangan 2022). In an attempt to provide light on the evolutionary position of the Cissus species, an ML tree was built utilizing the whole chloroplast genome sequences of 5 Cissus and 18 additional species from the Vitoideae subfamily. The phylogenetic grouping of Cissus genomes demonstrated that the chloroplast genome is sufficient to establish the relationships between Cissus species and the remainder of the Vitoideae subfamily with significant bootstrap support. Within the Cissus clade, C. quadrangularis L. was found closely related to C. discolor Blume. The phylogenetic analysis utilizing different DNA barcodes, as well as the study based on the entire chloroplast genomes, are in agreement with our findings (Zhang et al. 2016a; Lu et al. 2018; Wen et al. 2018). In the current study, the Cissus genus was discovered to be the sister to the Cayratieae tribe, which comprises the Tetrastigma species. This relationship was also brought up in the earlier exploration that was conducted to understand the phylogeny of the Vitoideae subfamily (Zhang et al. 2016b; Wen et al. 2018). However, we have removed the C. antarctica Vent. species from the current phylogenetic tree since it was difficult to resolve within the Cissus clade. A similar type of resolution difficulties involving C. antarctica Vent. were also documented in earlier studies, and for that reason, the Cissus is considered a polyphyletic clade as well (Rossetto et al. 2002, 2007; Liu et al. 2013). In the future, we believe that resolving this evolutionary uncertainty will be made easier by increasing the number of Cissus species, as well as with the inclusion of organellar and nuclear markers.
Conclusion
In summary, we assembled a whole chloroplast genome of C. quadrangularis L. spanning 160,404 bp from low-coverage whole genome data using a genome skimming approach. Our investigation has identified the previously missing annotations of three genes atpH, petB, and psbL, as well as an additional copy of the ycf1 gene in C. quadrangularis L., which was previously reported to be absent from Cissus genomes. C. quadrangularis L. has the second largest genome size after C. antarctica Vent. among the genomes studied. Comparative genomics identifies the genetic differences between the chloroplast genomes of the different Cissus species in terms of the number of repeat sequences and the level of DNA diversity found in the non-coding sequences. The present knowledge of the evolutionary lineage of C. quadrangularis L. in the Vitoideae subfamily is supported by the phylogenetic study focused on 24 complete chloroplast genomes. The comprehensive annotation of the genome discussed in this study could be useful as a reference for the annotation of Cissus chloroplast genomes in future research.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AS expresses gratitude to the Ministry of Education, Government of India, for supporting the student fellowship. BKC is grateful to Sherubtse College, Royal University of Bhutan, for providing the fellowship. Authors would like to thank the IIT Guwahati, Assam, India, for providing an institutional computational facility. No funding was received to assist with the preparation of this manuscript.
Author contributions
AS, and RGS assembled and annotated the chloroplast genomes. AS, RGS, SM and BKC analyzed and interpreted the data. LR and RGS conceived and designed the study. LR conceptualized, supervised and revised the manuscript. All authors have read and approved the final manuscript.
Declarations
Conflict of interests
Authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rahul G. Shelke, Email: rahul.sg98@gmail.com
Latha Rangan, Email: latha_rangan@yahoo.com, Email: lrangan@iitg.ac.in.
References
- Alzahrani DA, Yaradua SS, Yaradua SS, et al. Complete chloroplast genome sequence of Barleria prionitis, comparative chloroplast genomics and phylogenetic relationships among Acanthoideae. BMC Genom. 2020;21:1–20. doi: 10.1186/s12864-020-06798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna PS, Patil PH, Maru SK, Mutha RE. Cissus quadrangularis L.: A comprehensive multidisciplinary review. J Ethnopharmacol. 2021;279:114355. doi: 10.1016/j.jep.2021.114355. [DOI] [PubMed] [Google Scholar]
- Bi G, Mao Y, Xing Q, Cao M. HomBlocks: A multiple-alignment construction pipeline for organelle phylogenomics based on locally collinear block searching. Genomics. 2018;110:18–22. doi: 10.1016/j.ygeno.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Yengkhom S, Uddin A. Analysis ofcodon usage bias of chloroplast genes in Oryza species. Planta. 2020;252:67. doi: 10.1007/s00425-020-03470-7. [DOI] [PubMed] [Google Scholar]
- Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:1–29. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Shelke RG, Rangan L, Mitra S. Estimation of nuclear genome size and characterization of Ty1-copia like LTR retrotransposon in Mesua ferrea L. J Plant Biochem Biotechnol. 2018;27:478–487. doi: 10.1007/s13562-018-0457-7. [DOI] [Google Scholar]
- Dhanasekaran S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L.) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi J Biol Sci. 2020;27:1302–1309. doi: 10.1016/j.sjbs.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckxsens N, Mardulyn P, Smits G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkw955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie SR, Jansen RK. A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. Syst Bot. 2015;40:336–351. doi: 10.1600/036364415X686620. [DOI] [Google Scholar]
- Feng S, Zheng K, Jiao K, et al. Complete chloroplast genomes of four Physalis species (Solanaceae): lights into genome structure, comparative analysis, and phylogenetic relationships. BMC Plant Biol. 2020;20:242. doi: 10.1186/s12870-020-02429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichuki DK, Ma L, Zhu Z, et al. Genome size, chromosome number determination, and analysis of the repetitive elements in Cissus quadrangularis. PeerJ. 2019;7:e8201. doi: 10.7717/peerj.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goremykin VV, Holland B, Hirsch-Ernst KI, Hellwig FH. Analysis of Acorus calamus chloroplast genome and its phylogenetic implications. Mol Biol Evol. 2005;22:1813–1822. doi: 10.1093/molbev/msi173. [DOI] [PubMed] [Google Scholar]
- Guan Y, Liu W, Duan B, et al. The first complete chloroplast genome of Vicatia thibetica de Boiss.: genome features, comparative analysis, and phylogenetic relationships. Physiol Mol Biol Plants. 2022;28:439–454. doi: 10.1007/s12298-022-01154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisinger MM, Kuehl JV, Boore JL, Jansen RK. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol Biol Evol. 2011;28:583–600. doi: 10.1093/molbev/msq229. [DOI] [PubMed] [Google Scholar]
- Jiang H, Tian J, Yang J, et al. Comparative and phylogenetic analyses of six Kenya Polystachya (Orchidaceae) species based on the complete chloroplast genome sequences. BMC Plant Biol. 2022;22:177. doi: 10.1186/s12870-022-03529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D-M, Jin J, Yi T. Plastome structural conservation and evolution in the clusioid clade of Malpighiales. Sci Rep. 2020;10:9091. doi: 10.1038/s41598-020-66024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Asaf S, Khan AL, et al. Complete chloroplast genomes of medicinally important Teucrium species and comparative analyses with related species from Lamiaceae. PeerJ. 2019;7:7260. doi: 10.7717/peerj.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-J, Choi K-S, Jansen RK. Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae) Mol Biol Evol. 2005;22:1783–1792. doi: 10.1093/molbev/msi174. [DOI] [PubMed] [Google Scholar]
- Krawczyk K, Nobis M, Myszczyński K, et al. Plastid super-barcodes as a tool for species discrimination in feather grasses (Poaceae: Stipa) Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-20399-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kim K, Lee BY, et al. Complete chloroplast genomes of all six Hosta species occurring in Korea: molecular structures, comparative, and phylogenetic analyses. BMC Genomics. 2019;20:833. doi: 10.1186/s12864-019-6215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lin F, Huang P, et al. Complete chloroplast genome sequence of Decaisnea insignis: Genome organization, genomic resources and comparative analysis. Sci Rep. 2017;7:10073. doi: 10.1038/s41598-017-10409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xiao W, Tong T, et al. The specific DNA barcodes based on chloroplast genes for species identification of Orchidaceae plants. Sci Rep. 2021;11:1424. doi: 10.1038/s41598-021-81087-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang LN, Wang TX, et al. The complete chloroplast genome sequences of three lilies: Genome structure, comparative genomic and phylogenetic analyses. J Plant Res. 2022;135:723–737. doi: 10.1007/s10265-022-01417-5. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Ickert-Bond SM, Chen LQ, Wen J. Molecular phylogeny of Cissus L. of Vitaceae (the grape family) and evolution of its pantropical intercontinental disjunctions. Mol Phylogenet Evol. 2013;66:43–53. doi: 10.1016/j.ympev.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Lu L, Cox CJ, Mathews S, et al. Optimal data partitioning, multispecies coalescent and Bayesian concordance analyses resolve early divergences of the grape family (Vitaceae) Cladistics. 2018;34:57–77. doi: 10.1111/cla.12191. [DOI] [PubMed] [Google Scholar]
- Ma W, Lv C, Jiang D, Kang C, Zhao D (2020) The complete chloroplast genome sequence of Euphorbia lathyris L. Mito DNA B Resour5(3): 3678–3680 10.1080/23802359.2020.1832601 [DOI] [PMC free article] [PubMed]
- Muraguri S, Xu W, Chapman M, et al. Intraspecific variation within Castor bean (Ricinus communis L.) based on chloroplast genomes. Ind Crops Prod. 2020;155:112779. doi: 10.1016/j.indcrop.2020.112779. [DOI] [Google Scholar]
- Nie X, Deng P, Feng K, et al. Comparative analysis of codon usage patterns in chloroplast genomes of the Asteraceae family. Plant Mol Biol Rep. 2014;32:828–840. doi: 10.1007/s11105-013-0691-z. [DOI] [Google Scholar]
- Onyeweaku GC, Nyananyo BL, Ozimede CO. Taxonomic studies on the genus Cissus L. (Vitaceae) present in Obio/Akpor local government area of Rivers state Nigeria. J Appl Sci Environ Manag. 2020;24:139. doi: 10.4314/jasem.v24i1.20. [DOI] [Google Scholar]
- Palmer JD, Jansen RK, Michaels HJ, et al. Chloroplast DNA variation and plant phylogeny. Annals Missouri Bot. Gard. 1988;75:1180–1206. doi: 10.2307/2399279. [DOI] [Google Scholar]
- Purohit S, Bohra MK, Jain R. Identification of bioactive pentacyclic triterpenoids and fatty acid derivatives from Cissus quadrangularis and C. rotundifolia through untargeted metabolite profiling. Appl Biochem Biotechnol. 2022 doi: 10.1007/s12010-022-03940-6. [DOI] [PubMed] [Google Scholar]
- Rossetto M, Jackes BR, Scott KD, Henry RJ. Is the genus Cissus (Vitaceae) monophyletic? evidence from plastid and nuclear ribosomal DNA. Syst Bot. 2002;27:522–533. doi: 10.1043/0363-6445-27.3.522. [DOI] [Google Scholar]
- Rossetto M, Crayn DM, Jackes BR, Porter C. An updated estimate of intergeneric phylogenetic relationships in the Australian Vitaceae. symposium on Vitis at the XVII international botanical congress-2005, Vienna. Austria Can J Bot. 2007;85:722–730. doi: 10.1139/B07-022. [DOI] [Google Scholar]
- Rossini BC, de Moraes MLT, Marino CL. Complete chloroplast genome of Myracrodruon urundeuva and its phylogenetics relationships in Anacardiaceae family. Physiol Mol Biol Plants. 2021;27:801–814. doi: 10.1007/s12298-021-00989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawangjit R, Puttarak P, Saokaew S, Chaiyakunapruk N. Efficacy and safety of Cissus quadrangularis L. in clinical use: A systematic review and meta-analysis of randomized controlled trials. Phyther Res. 2017;31:555–567. doi: 10.1002/ptr.5783. [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Maier RM, Alcaraz JP, et al. The plastid chromosome of spinach (Spinacia oleracea): complete nucleotide sequence and gene organization. Plant Mol Biol. 2001;45:307–315. doi: 10.1023/A:1006478403810. [DOI] [PubMed] [Google Scholar]
- Senapati A, Basak S, Rangan L. A review on application of DNA barcoding technology for rapid molecular diagnostics of adulterants in herbal medicine. Drug Saf. 2021 doi: 10.1007/s40264-021-01133-4. [DOI] [PubMed] [Google Scholar]
- Shelke RG, Das AB. Analysis of genetic diversity in 21 genotypes of Indian banana using RAPDs and IRAPs markers. Proc Natl Acad Sci India Sect B Biol Sci. 2015;85:1027–1038. doi: 10.1007/s40011-015-0505-1. [DOI] [Google Scholar]
- Shelke RG, Rangan L. Isolation and characterisation of Ty1-copia retrotransposons from Pongamia pinnata. Trees. 2019;33:1559–1570. doi: 10.1007/s00468-019-01878-7. [DOI] [Google Scholar]
- Shelke RG, Rangan L. The role of transposable elements in Pongamia unigenes and protein diversity. Mol Biotechnol. 2020;62:31–42. doi: 10.1007/s12033-019-00223-0. [DOI] [PubMed] [Google Scholar]
- Shelke RG, Rangan L. The whole chloroplast genome of Mesua ferrea: Insight into the dynamic pattern of evolution and its comparison with species from recently diverged families. Gene. 2022;846:146866. doi: 10.1016/j.gene.2022.146866. [DOI] [PubMed] [Google Scholar]
- Shelke RG, Basak S, Rangan L. Development of EST-SSR markers for Pongamia pinnata by transcriptome database mining: Cross-species amplification and genetic diversity. Physiol Mol Biol Plants. 2020;26:2225–2241. doi: 10.1007/s12298-020-00889-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidhi PR, Nadiya F, Biju VC, et al. Complete chloroplast genome of the medicinal plant Evolvulus alsinoides: Comparative analysis, identification of mutational hotspots and evolutionary dynamics with species of Solanales. Physiol Mol Biol Plants. 2021;27:1867–1884. doi: 10.1007/s12298-021-01051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarajan VV, Balachandran I. Ayurvedic drugs and their plant sources. Oxford and IBH publishing; 1994. [Google Scholar]
- Sundaran J, Begum R, Vasanthi M, et al. A short review on pharmacological activity of Cissus quadrangularis. Bioinformation. 2020;16(8):579–585. doi: 10.6026/97320630016579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Lu P, Zhang Z, et al. Chloroplast genome sequence of Chongming lima bean (Phaseolus lunatus L.) and comparative analyses with other legume chloroplast genomes. BMC Genom. 2021;22:194. doi: 10.1186/s12864-021-07467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillich M, Lehwark P, Pellizzer T, et al. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45:W6–W11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M, Gupta PS, Sharma N. Ethnopharmacological, phytochemical and pharmacological review of plant Cissus quadrangularis L. Res J Pharmacogn Phytochem. 2018;10:81. doi: 10.5958/0975-4385.2018.00014.6. [DOI] [Google Scholar]
- Wang L, Roossinck MJ. Comparative analysis of expressed sequences reveals a conserved pattern of optimal codon usage in plants. Plant Mol Biol. 2006;61:699–710. doi: 10.1007/s11103-006-0041-8. [DOI] [PubMed] [Google Scholar]
- Wang L, Liang J, Sa W, Wang L. Sequencing and comparative analysis of the chloroplast genome of Ribes odoratum provide insights for marker development and phylogenetics in Ribes. Physiol Mol Biol Plants. 2021;27:81–92. doi: 10.1007/s12298-021-00932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang D, Gao N, et al. Identification of the complete chloroplast genome of Malus zhaojiaoensis Jiang and its comparison and evolutionary analysis with other Malus species. Genes. 2022 doi: 10.3390/genes13040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Lu LM, Nie ZL, et al. A new phylogenetic tribal classification of the grape family (Vitaceae) J Syst Evol. 2018;56:262–272. doi: 10.1111/jse.12427. [DOI] [Google Scholar]
- Wu F, Zhang S, Gao Q, et al. Genetic diversity and population structure analysis in a large collection of Vicia amoena in China with newly developed SSR markers. BMC Plant Biol. 2021;21:544. doi: 10.1186/s12870-021-03330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-Y, Wong K-H, Kong BL, et al. Comparative analysis of chloroplast genomes of Dalbergia species for identification and phylogenetic analysis. Plants. 2022;11:1109. doi: 10.3390/plants11091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Sun M, Mei Y, et al. The complete chloroplast genome sequence of the medicinal plant Abrus pulchellus subsp. cantoniensis: genome structure, comparative and phylogenetic relationship analysis. J Plant Res. 2022;135:443–452. doi: 10.1007/s10265-022-01385-w. [DOI] [PubMed] [Google Scholar]
- Zhang Z. KaKs_calculator 30: Calculating selective pressure on coding and non-coding sequences. Genom Proteom Bioinform. 2022 doi: 10.1016/j.gpb.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Fang Y, Wang X, et al. The complete chloroplast and mitochondrial genome sequences of Boea hygrometrica: Insights into the evolution of plant organellar genomes. PLoS ONE. 2012;7(1):e30531. doi: 10.1371/journal.pone.0030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wen J, Zimmer EA. Another look at the phylogenetic position of the grape order Vitales: chloroplast phylogenomics with an expanded sampling of key lineages. Mol Phylogenet Evol. 2016;101:216–223. doi: 10.1016/j.ympev.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wen J, Zimmer EA. Correction: congruent deep relationships in the grape family (Vitaceae) based on sequences of chloroplast genomes and mitochondrial genes via genome skimming. PLoS ONE. 2016;11:1–12. doi: 10.1371/journal.pone.0152059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sun Y, Liu J, et al. DNA barcoding of Oryza: conventional, specific, and super barcodes. Plant Mol Biol. 2021;105:215–228. doi: 10.1007/s11103-020-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.