Abstract
To report a case series of patients with pseudomyxoma peritonei (PMP) from urachal mucinous neoplasm (UMN) treated with CRS and HIPEC at a high-volume referral centre, along with an updated literature review. Retrospective review of cases treated between 2000 and 2021. A literature review using MEDLINE and Google Scholar databases was performed. Clinical presentation of PMP from UMN is heterogeneous, and common symptoms are abdominal distension, weight loss, fatigue and haematuria. At least one tumour marker among CEA, CA 19.9, and CA 125 was elevated in the six cases reported, and 5/6 had a preoperative working diagnosis of urachal mucinous neoplasm suspected on detailed cross-sectional imaging. Complete cytoreduction was achieved in five cases, while one patient underwent maximal tumour debulking. Histological findings mirrored the findings of PMP from appendiceal mucinous neoplasms (AMN). Overall survival ranged between 43 and 141 months after complete cytoreduction. On literature review, 76 cases have been reported to date. Complete cytoreduction is associated with good prognosis for patients with PMP from UMN. A definitive classification system is still not available.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13193-022-01694-5.
Keywords: Pseudomyxoma peritonei, Urachal adenocarcinoma
Introduction
The urachus is the fibrous remnant of the allantois, a tubular structure that connects the bladder with the umbilicus in foetal life. The intramural portion of the urachus runs within the bladder wall, while the extramural part lies in the subperitoneal space. Transitional-type urothelium lines the urachal remnant and neoplasms may develop from focal glandular metaplasia. Similarly to many abdominal neoplasms, such as appendiceal neoplasms and colorectal cancer, a proportion of urachal tumours may present with overproduction of mucin. Perforation of such mucinous neoplasms followed by the spread of mucin and tumour deposit throughout the peritoneum leads to the development of a syndrome known as pseudomyxoma peritonei (PMP), in which the peritoneal cavity is filled with mucinous ascites; the clinical course is indolent but progressive, and the accumulation of disease is associated with severe abdominal distension, intestinal obstruction, and malnutrition.
Urachal mucinous neoplasms (UMN) are a rare cause of pseudomyxoma peritonei. The clinical presentation is heterogeneous and includes signs and symptoms associated with the primary tumour, such as haematuria, mucosuria, pelvic pain, or to peritoneal disease, such as abdominal distension and ascites. [1, 2] Cross-sectional imaging may be helpful, but can be difficult to interpret accurately. Histopathological features of urachal adenocarcinoma are similar to colorectal mucinous adenocarcinoma making histopathological diagnosis challenging. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have shown promising survival outcomes in selected patients with UMN [3]. In the current study, we present a case series of patients with UMN treated with CRS and HIPEC at the Peritoneal Malignancy Institute, Basingstoke.
Materials and Methods
A retrospective review of patients with PMP arising from urachal neoplasms treated at Basingstoke Peritoneal Malignancy Institute between 2000 and 2021 was performed. The prospective database, as well as hospital notes, histopathology slides, and relevant imaging, was reviewed to collect significant information. A literature review of studies regarding PMP from UMN published in English within the MEDLINE and Google Scholar databases up to May 2022 was also carried out. The following keywords were used: ‘pseudomyxoma peritonei OR peritoneal carcinomatosis’ AND ‘urachal’ OR ‘urachus’ OR ‘urachal adenocarcinoma’ OR ‘urachus adenocarcinoma’ OR ‘urachus neoplasm’ OR ‘urachal neoplasm’ SPSS Statistics for Windows, version 26 (IBM Corp., NY, USA) were used for statistical analyses.
Results
Six patients presenting with PMP from UMN were treated with CRS and HIPEC between 2000 and 2021 (Table 1). Following cytoreduction, all patients received HIPEC with mitomycin C (MMC) following the PMI Basingstoke protocol (10 mg/m2) at 42 °C for 60 min.
Table 1.
Clinical, pathological, surgical, and survival features of patients included in the case series (M, male; F, female; PCI, peritoneal carcinomatosis index; CCS, completeness of cytoreduction score; DFS, disease-free survival; OS, overall survival; MCP, mucinous carcinoma peritonei)
| Case number (sex, age) | Cytoreductive surgery | Tumour markers histology | Recurrence (DFS, months) |
|---|---|---|---|
| Mortality (OS, months) | |||
| 1 (M, 33) |
First operation (2000): parietal peritonectomy, greater and lesser omentectomy, splenectomy, right hemicolectomy, cholecystectomy, pelvic peritonectomy, resection of urachal mass, partial cystectomy, HIPEC (PCI: 14/CCS 1) Second operation (2000): reversal of ileostomy, cytoreduction of peritoneal nodules, HIPEC; 4-day EPIC with 5-FU Third operation (2002): partial cystectomy, small bowel resection, partial gastrectomy, excision of skin and subcutaneous tissue, cytoreduction with excision of multiple peritoneal mucoid nodules, HIPEC Fourth operation (2011): relaparotomy, en-bloc resection of sigmoid colon, small bowel, bladder dome, and pelvic mass |
CEA 179 µg/L CA 19.9 2.5 kU/L CA 125 40 kU/L Low grade adenocarcinoma of the urachus giving rise to PMP (2000) Low-grade PMP (2002) High-grade mucin secreting adenocarcinoma with PMP (2011) |
Yes (17) |
| Dead (141) | |||
| 2 (F,34) |
Bilateral parietal peritonectomy, pelvic peritonectomy, anterior resection of the rectum, histerectomy, bilateral salpingo-oophorectomy, defunctioning ileostomy (PCI: 15/complete cytoreduction) |
CEA 175 µg/L CA 19.9 176 kU/L CA 125 16 kU/L High-grade PMP arising from urachal mucinous adenocarcinoma |
Yes (19) |
| Dead (108) | |||
| 3 (M, 48) |
Major tumour debulking: bilateral parietal peritonectomy, greater and lesser omentectomy, splenectomy, total colectomy with end ileostomy, partial cystectomy, HIPEC (PCI: 32/CCS 2) |
CEA 1.7 µg/L CA 19.9 2901 kU/L CA 125 54 kU/L Poorly differentiated high grade signet ring adenocarcinoma of the urachus with PMP CK-20, CDX-2 + CK-7 - Positive lymph nodes: 8/22 |
Yes – MTD |
| Dead (7) | |||
| 4 (M, 65) |
Bilateral parietal, diaphragmatic and pelvic peritonectomy, resection of periumbilical nodule, greater and lesser omentectomy, liver capsulectomy, splenectomy, cholecystectomy, right hemicolectomy, anterior resection of the rectum, HIPEC (PCI: 12/complete cytoreduction) |
CEA 3.7 µg/L CA 19.9 3 kU/L CA 125 351 kU/L Adenocarcinoma of the urachus with PMP (high-grade MCP) CK-20, CDX-2 + , CK-7 - Positive lymph nodes: 5/38 |
Yes (7) |
| Alive (57) | |||
| 5 (F, 69) |
Bilateral parietal, bilateral diaphragmatic and pelvic peritonectomy, greater and lesser omentectomy, liver capsulectomy splenectomy, urachal mass and partial cystectomy, histerectomy and bilateral salpingo-oophorectomy, cholecystectomy, right hemicolectomy, anterior resection of the rectum, HIPEC (PCI: 32/complete cytoreduction) |
CEA 13 µg/L CA 19.9 253 kU/L CA 125 kU/L 88 kU/L Low-grade MCP CK-20, CDX-2 + , CK-7 - Positive lymph nodes: 0/4 |
Yes (37) |
| Alive (43) | |||
| 6 (M, 66) |
Bilateral parietal, diaphragmatic and pelvic peritonectomy, greater and lesser omentectomy, liver capsulectomy, splenectomy, cholecystectomy, HIPEC (PCI: 25/complete cytoreduction) |
CEA 5.5 µg/L CA 19.9 698 kU/L CA 125 143 kU/L Low-grade MCP CK-20, CDX-2 + , CK-7 - |
Yes (12) |
| Alive (54) |
All patients received HIPEC with mitomycin C according the PMI Basingstoke protocol (10 mg/m2 at 42 °C for 60 min)
Case 1
A 32-year-old male was referred with a history of abdominal distension, weight loss, fatigue, and raised CEA. A CT showed PMP and a bladder lesion compatible with a urachal cyst. He underwent complete cytoreduction and HIPEC in 2000 (see Table 1); he received further intraperitoneal chemotherapy and EPIC with 5-FU after some small-volume peritoneal disease was found at reversal of ileostomy 5 months later. In 2002, he developed peritoneal recurrence, and he underwent a repeat complete cytoreduction. He developed a second peritoneal recurrence in 2008, and consensus for conservative management was reached at multi-disciplinary discussion. In July 2011, he presented with a pelvic mass causing bowel obstruction and had a maximal tumour debulking. Histopathology in 2000 showed PMP from low-grade adenocarcinoma of the urachus. During follow-up, the tumour de-differentiated and in 2011 histology revealed high-grade adenocarcinoma with PMP. He died 2 months after maximal tumour debulking.
Case 2
A 34 year-old female was referred with mucosuria, haematuria, abdominal distension, and a palpable pelvic mass. At previous laparotomy, she was found to have a frozen pelvis and a mass arising from the bladder with some mucinous peritoneal deposits; an appendectomy was performed; and histology was normal. She was suspected to have PMP arising from a urachal primary and underwent complete cytoreduction, including total cystectomy, and HIPEC at our centre. Histology showed high-grade PMP (HG-PMP) arising from a urachal mucinous adenocarcinoma. One and a half year after cytoreduction, she developed lung metastases, which were treated with a right lower lobectomy; she thereafter received adjuvant chemotherapy, but unfortunately developed further lung metastases. She died 9 years after primary cytoreduction.
Case 3
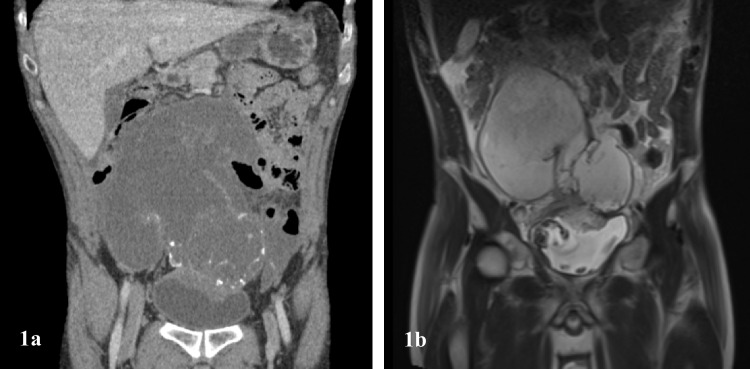
A 48-year-old male patient was referred with a history of macroscopic haematuria and a suprapubic mass. His CA 19.9 levels were elevated. Abdominal CT scan and MRI showed a urachal cyst and peritoneal deposits (Fig. 1). Intraoperatively, he was found to have unresectable disease and underwent maximal tumour debulking. His postoperative course was complicated by a fistula into the bladder, which was managed with a urethral catheter. Histology showed poorly differentiated high-grade signet ring adenocarcinoma of the urachus. He died 6 months after surgery.
Fig. 1.
a Low-grade mucinous tumour of urachus. The epithelium closely resembles that seen in low-grade appendiceal neoplasms. Haematoxylin and eosin × 20. b Low-grade mucinous carcinoma peritonei of urachal origin (same patient as a). Strips of columnar epithelium are associated with abundant extracellular mucin. Haematoxylin and eosin × 2. c Mucinous adenocarcinoma of the urachus. Malignant cells, including signet ring cells, are surrounded by extracellular mucin. Haematoxylin and eosin × 40
Case 4
A 65-year-old male was referred with a positive faecal immunochemical stool test and abdominal distension; colorectal cancer was suspected, and a colonoscopy showed a mass in the sigmoid colon, with inconclusive biopsies. His CA 125 was raised, and cross-sectional imaging of the abdomen showed widespread peritoneal disease with diffuse peritoneal deposits. He received complete cytoreduction, including the excision of a periumbilical nodule. At histologic examination, the sigmoid mass was found to be a peritoneal nodule with erosion into the bowel wall; the primary was deemed to be a urachal adenocarcinoma arising from the extramural portion of the urachus near the umbilicus, associated with HG-MCP. Seven months after primary surgery, he developed liver metastases, which were treated with parenchyma-sparing resection. Fifty-seven months after cytoreduction, he is currently alive and receiving systemic chemotherapy with FOLFOX.
Case 5
A 69-year-old female with a previous history of appendectomy presented with abdominal distension, weight loss, and fatigue. A CT scan showed a cystic lesion on the dome of the bladder and four-quadrant mucin deposits. A suspected diagnosis of PMP from UMN was made and she had complete cytoreduction. Histology showed low-grade mucinous carcinoma peritonei (LG-MCP) arising from the urachus. A small-volume recurrence in the right lower quadrant was identified 3 years after the primary operation at routine radiographic follow-up and she is currently symptom-free.
Case 6
A 66-year-old male with a past medical history of appendectomy and prostatectomy for prostate cancer presented with increasing abdominal girth and bilateral inguinal hernias. An abdominal CT scan showed a suspected urachal neoplasm with diffuse peritoneal mucinous deposits; his CA 19.9 was raised. He underwent complete cytoreduction and excision of the urachal neoplasm, which resided in the extramural portion of the urachus. Histology showed LG-MCP. At 1-year imaging, he was found to have low-volume peritoneal recurrence in the pelvis and over the right mesocolon. He is currently alive, four and a half years after cytoreduction.
Literature review
A literature review retrieved 76 published cases in the English literature: 32 patients from previous case reports and case series [1, 2, 4–23], 8 included in large cohort studies [24–27], and 36 from a large review of a multi-institutional database. [3] The results are summarised in Table 2.
Table 2.
Literature review: the first coloumn is a summary of clinical, pathological, surgical, and survival features of patients from case reports and series, including the current case series
| Previous case reports/case series (including this case series) (N = 38) | Large multicentre review Mercier et al. [3] (N = 36) | |
|---|---|---|
|
Sex Male Female |
27 (71.1%) 11 (28.9%) |
24 (66.7%) 12 (33.3%) |
|
Signs and symptoms Urinary Constitutional PMP-related Other (inguinal hernia, back pain, etc.) |
9 (23.7%) 4 (10.5%) 14 (36.8%) 13 (34.2%) |
NA |
|
Positive tumour markers CEA CA 19.9 CA125 |
(N = 28) 12 (42.8%) 12 (42.8%) 3 (10.7%) |
NA |
|
Surgical procedure Tumour resection/en-bloc resection Cytoreduction + peritonectomy + HIPEC Tumour debulking |
12 (31.6%) 22 (57.9%) 4 (10.5%) |
0 (0%) 31 (86.1%) 5 (13.9%) |
|
Cystectomy No Partial Total |
14 (36.8%) 22 (57.9%) 2 (5.3) |
NA |
|
HIPEC received Yes No |
24 (63.2%) 14 (36.8%) |
36 (100%) 0 (0%) |
| PCI—median (IQR) |
(N = 18) 15 (7–25) |
8.5 (1–33) |
|
Complete cytoreduction (CCS 0–1) Yes No |
(N = 23) 18 (78.3%) 5 (21.7%) |
31 (86.1%) 5 (13.9) |
|
Histology Mucinous adenocarcinoma Low-grade mucinous neoplasm/adenocarcinoma Mucinous adenocarcinoma with PMP Low-grade mucinous adenocarcinoma with PMP Mucinous tumour of low malignant potential Mucinous cyst of low malignant potential Cystadenoma Mucinous cystadenocarcinoma Peritoneal adenomucinosis PMP Well-differentiated adenocarcinoma Signet-ring adenocarcinoma LG-MCP HG-MCP HG-MCP with SRC |
5 (13.2%) 3 (7.9%) 3 (7.9%) 1 (2.6%) 1 (2.6%) 1 (2.6%) 1 (2.6%) 2 (5.2%) 1 (2.6%) 2 (5.2%) 2 (5.2%) 1 (2.7%) 7 (18.4%) 3 (7.9%) 5 (13.2%) |
NA |
|
Recurrence Yes No |
(N = 28) 20 (71.4%) 8 (28.6%) |
NA |
|
Survival 5-y DFS 5-y OS |
(N = 28) 40.9% 73% |
55.6% 46.2% |
| Follow-up time – median (IQR), months | 36 (18–55) | 48 (35–69) |
The second coloumn summarises results by Mercier et al. Patients included in large reviews of patients with PMP treated with CRS and HIPEC (i.e. patients included in the studies by Smeenk et al., Elias et al., Carr et al., and Delhorme et al.) are not included in this table, as characteristics could not be deducted from published data (CCS, completeness of cytoreduction score; DFS, disease-free survival; MCG-HG, mucinous carcinoma peritonei–high grade; MCG-LG, mucinous carcinoma peritonei–low grade; OS, overall survival; PMP, pseudomyxoma peritonei; SRC, signet ring cells)
Discussion
PMP arising from UMN is a very rare condition: in large case series reporting PMP from extra-appendiceal origin, the urachus was the primary site in 0.4–4.8% of cases. [26, 27] The pathophysiology of PMP from UMN is similar to PMP arising from appendiceal mucinous neoplasms (AMN)1: after rupture of the primary urachal mass, the development of peritoneal disease follows the classic pattern according to the redistribution phenomenon. From a histologic standpoint, the distinction between urachal neoplasms and glandular neoplasms of the bladder is difficult, and a lesion should be classified as urachal primary if it is located over the dome or anterior wall of the bladder, with its epicentre away from the bladder mucosa [28]. The histologic characteristics of PMP arising from UMN are non-specific, and many appearances are shared with PMP from AMN (see Fig. 2) [29]. In published case reports and in the current study, patients have heterogeneous histopathology (see Table 2), ranging from well-differentiated non-invasive neoplasms to aggressive adenocarcinoma with signet ring morphology. Sugarbaker et al. [2] suggested the use of the term ‘mucinous urachal neoplasm’ to describe this entity, and later Vora et al. [28] proposed to classify neoplasms without infiltrative invasion as ‘low-grade UMN’ and ‘high-grade UMN’—in the presence of cytologic atypia, mirroring the classification of AMN. In a recent case series, Liu et al. [1] adopted a classification similar to PMP from AMN [30]. Another similarity to AMN reported in the literature and confirmed in the current series is elevated tumour markers, namely, serum CEA, CA 19.9, and CA 125. Agrawal et al. [16] reported raised tumour markers in 40% of patients. All six patients in this series presented with elevation of one of the three markers, namely, CEA, CA 19.9, and CA 125. The immunohistochemistry (IHC) profile of our patients (CK-20 + , CDX-2 + , CK-7 -) is consistent with previous literature; IHC of normal urachus has been previously reported as CK-7 + and CDX-2 and CK-20-. [1]
Fig. 2.
Abdominal CT (a) and MRI (b) imaging of a large complex cystic mass arising from the intramural portion of the urachus with peritoneal mucinous deposits, compatible with PMP arising from UMN. The communication between the primary tumour over the dome of the bladder and the intraperitoneal mass is evident (case 3)
Preoperative diagnosis of PMP from UMN may be challenging. However, three main features are associated with this entity: urinary signs and symptoms, a midline cystic mass in the lower abdomen at CT scan or MRI, and elevated tumour markers [1–3]. All but one of the patients in the current series were found to have a lower abdominal cystic mass. This finding, especially in the absence of appendiceal or colonic pathology, should raise the suspicion of a urachal primary.
CRS and HIPEC have shown to be associated with good long-term survival [1, 3], and en-bloc total urachal resection, which encompasses excision of the bladder dome, along with the urachal remnant and umbilicus, is advocated [2]. As long as surgical margins are clear and functional capacity of the bladder is preserved, simple resection of the involved bladder (i.e. partial cystectomy) seems to be an acceptable surgical option, as opposed to total cystectomy [2], and this is supported by published cases. The clinical course and prognosis of PMP from UMN are driven by the peritoneal disease and its treatment with CRS and HIPEC [1, 2, 16]. The choice of the HIPEC regimen is highly heterogeneous: Mercier et al. [3] report the use of mitomycin C as a single agent or in combination in over 59% of cases in their multi-centre case series. Although it seems appropriate to use the same regimen used for PMP from AMN, definitive data on the best HIPEC regimen is currently lacking.
Prognosis of PMP from UMN is associated to pathological and surgery-related factors. Histologic grade and invasive features are negative prognostic features. [16] In the largest case series available, [3] the median overall survival (OS) was 58.8 months, and a 5-year DFS of 55.6% was achieved with CRS and HIPEC. Prognosis was significantly better when complete cytoreduction was achieved, while PCI did not influence survival. This finding is confirmed in the present series: all patients who had a complete cytoreduction survived more than 3 years. In the current study, LG-PMP was associated with long survivals, underscoring that the biology of peritoneal disease represents a significant driver. Interestingly, one of our long-term survivors died 11 years after his first CRS and HIPEC in 2000, and his death was likely related to de-differentiation, and this mimics the behaviour in some patients with PMP arising from AMN. [31]
Conclusion
PMP from UMN shares several pathophysiological, histopathological, and prognostic features with PMP from AMN. Preoperative suspicion of urachal primary of PMP should be raised in the presence of urinary symptoms, elevated markers, and a median pelvic cystic mass, when an appendiceal or colonic origin is excluded. CRS and HIPEC are recommended as optimal standard of care and are related to good prognosis if complete cytoreduction can be achieved. Standardised classification of this rare entity is still lacking, but the use of a standardised terminology derived from the classification of PMP from AMN may be justified.
Supplementary Information
Below is the link to the electronic supplementary material.
Data Availability
Data supporting this study may be available on request. Please contact the corresponding author.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y, Ishibashi H, Hirano M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei arising from urachus. Ann Surg Oncol. 2015;22(8):2799–2805. doi: 10.1245/s10434-014-4336-8. [DOI] [PubMed] [Google Scholar]
- 2.Sugarbaker PH, Verghese M, Yan TD, Brun E. Management of mucinous urachal neoplasm presenting as pseudomyxoma peritonei. Tumori. 2008;94:732–736. doi: 10.1177/030089160809400515. [DOI] [PubMed] [Google Scholar]
- 3.Mercier F, Passot G, Villeneuve L, et al. Peritoneal carcinomatosis of urachus origin treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): an international registry of 36 Patients. Ann Surg Oncol. 2018;25(4):1094–1100. doi: 10.1245/s10434-017-6299-z. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner J, Greene L, McDonald J. Mucinous adenocarcinoma in a urachal cyst producing pseudomyxoma peritonaei. J Urol. 1954;72(2):217–221. doi: 10.1016/S0022-5347(17)67568-2. [DOI] [PubMed] [Google Scholar]
- 5.Mendelhof J, McSwain N. Pseudomyxoma peritonei due to mucinous adenocarcinoma of the urachus. South Med J. 1971;64(4):497–498. doi: 10.1097/00007611-197104000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Loggie BW, Fleming RA, Hosseinian AA. Peritoneal carcinomatosis with urachal signet-ring cell adenocarcinoma. Urology. 1997;50(3):446–448. doi: 10.1016/S0090-4295(97)00247-1. [DOI] [PubMed] [Google Scholar]
- 7.Sasano H, Shizawa S, Nagura H, Yamaki T. Mucinous adenocarcinoma arising in a giant urachal cyst associated with pseudomyxoma peritonei and stromal osseous metaplasia. Pathol Int. 1997;47(7):502–505. doi: 10.1111/j.1440-1827.1997.tb04531.x. [DOI] [PubMed] [Google Scholar]
- 8.Stenhouse G, McRae D, Pollock AM. Urachal adenocarcinoma in situ with pseudomyxoma peritonei: a case report. J Clin Pathol. 2003;56(2):152–153. doi: 10.1136/jcp.56.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi M, Matsuzaki K, Yoshida S, Nishitani H, Uehara H. Imaging findings of urachal mucinous cystadenocarcinoma associated with pseudomyxoma peritonei. Acta Radiol. 2004;45(3):348–350. doi: 10.1080/02841850410004959. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara T, Misawa K, Sano H, Okawa Y, Takada A. Pseudomyxoma peritonei due to mucinous cystadenocarcinoma in situ of the urachus presenting as an inguinal hernia. Int J Clin Oncol. 2006;11(5):416–419. doi: 10.1007/s10147-006-0594-1. [DOI] [PubMed] [Google Scholar]
- 11.Khalid K, Ahmed M, Malik M. Adenocarcinoma of urachal cyst associated with pseudomyxoma peritonei masquerading as abdominal tuberculosis: a case report and review of literature. Indian J Urol. 2008;24(2):258–260. doi: 10.4103/0970-1591.40626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama K, Ito N. Mucinous cystadenocarcinoma of the urachus associated with pseudomyxoma peritonei with emphasis on MR findings. Magn Reson Med Sci. 2009;8(2):85–89. doi: 10.2463/mrms.8.85. [DOI] [PubMed] [Google Scholar]
- 13.Lamb BW, Vaidyanathan R, Laniado M, Karim O, Motiwala H, Lamb B. Case report mucinous adenocarcinoma of the urachal remnant with pseudomyxoma peritonei. Urol J. 2010;7(2):138–147. [PubMed] [Google Scholar]
- 14.Nozaki T, Yasuda K, Watanabe A, Fuse H. Laparoscopic management of urachal mucinous borderline tumor associated with pseudomyxoma peritonei. Surg Laparosc Endosc Percutan Tech. 2011;21:152–155. doi: 10.1097/SLE.0b013e3182222bd4. [DOI] [PubMed] [Google Scholar]
- 15.Kebapçı M, Can C, Şaylısoy S, Dündar E. Radiologic findings of urachal mucinous cystadenocarcinoma causing pseudomyxoma peritonei. Jpn J Radiol. 2012;30(4):345–348. doi: 10.1007/s11604-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Bobinski P, Grzebienak Z, et al. Pseudomyxoma peritonei originating from urachus - case report and review of the literature. Curr Oncol. 2014;21:155–165. doi: 10.3747/co.21.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelekhova KV, Zhuravlev AS, Krylova DD, Konstantinov AS, Shtan LV, Kheinshtein VA. Pseudomyxoma peritonei as a first manifestation of KRAS -mutated urachal mucinous cystadenocarcinoma of the bladder: a case report. Int J Surg Pathol. 2017;25(6):563–566. doi: 10.1177/1066896917707041. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos P, Kampantais S, Payne S, Liyanage S. Pseudomyxoma peritonei arising from urachal mucinous adenocarcinoma. Urology. 2018;111:e3–e4. doi: 10.1016/j.urology.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Yasui M, Jikuya R, Tatenuma T, et al. Urachal carcinoma with peritoneal dissemination treated with chemotherapy and surgical resection leading to prolonged survival with no recurrence. Case Reports Urol. 2018;2018:1–5. doi: 10.1155/2018/9836154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez A, Ferron G, Mery E, Gladieff L, Delord JP, Querleu D. Peritoneal pseudomyxoma arising from the urachus. Surg Oncol. 2012;21(1):1–5. doi: 10.1016/j.suronc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Zhou N, Xu H et al (2017) Urachal mucinous adenocarcinoma with pseudomyxoma peritonei. Medicine 96(35):e7548. 10.1097/MD.0000000000007548 [DOI] [PMC free article] [PubMed]
- 22.Yanagisawa S, Fujinaga Y, Kadoya M (2003) Urachal mucinous cystadenocarcinoma with a cystic ovarian metastasis. AJR Am J Roentgenol 180(4):1183–1184 [DOI] [PubMed]
- 23.de Bree E, Witkamp A, van de Vijver M, Zoetmulde F. Unusual origins of pseudomyxoma peritonei. J Surg Oncol. 2000;75:270–274. doi: 10.1002/1096-9098(200012)75:4<270::AID-JSO9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 24.Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FAN. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245(1):104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elias D, Honoré C, Ciuchendéa R, et al. Peritoneal pseudomyxoma: Results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2008;95(9):1164–1171. doi: 10.1002/bjs.6235. [DOI] [PubMed] [Google Scholar]
- 26.Carr NJ, Finch J, Ilesley IC, et al. Pathology and prognosis in pseudomyxoma peritonei: a review of 274 cases. J Clin Pathol. 2012;65(10):919–923. doi: 10.1136/jclinpath-2012-200843. [DOI] [PubMed] [Google Scholar]
- 27.Delhorme J, Severac F, Averous G, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritoneai of appendicular and extra-appendicular origin. BJS. 2018;105:668–676. doi: 10.1002/bjs.10716. [DOI] [PubMed] [Google Scholar]
- 28.Vora C, Tzivanakis A, Dayal S, Carr NJ. Pseudomyxoma peritonei arising from a low-grade mucinous neoplasm of the urachus. AJSP: Rev Reports. 2019;24(3):117–120. doi: 10.1097/PCR.0000000000000312. [DOI] [Google Scholar]
- 29.Carr NJ, McLean A. A mucinous tumour of the urachus: adenoma or low grade mucinous cystic tumour of uncertain malignant potential? Adv Clin Path. 2001;5(3):93–97. [PubMed] [Google Scholar]
- 30.Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia the results of the peritoneal surface oncology group international (PSOGI) modified delphi process. Am J Surg Pathol. 2016;40:14–26. doi: 10.1097/PAS.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 31.Memon AA, Godbole C, Cecil T, et al. Overall survival is more closely associated with peritoneal than primary appendiceal pathological grade in pseudomyxoma peritonei with discordant pathology. Ann Surg Oncol. 2022;29(4):2607–2613. doi: 10.1245/s10434-021-10994-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this study may be available on request. Please contact the corresponding author.