Abstract
Agriculture sector is facing a lot of constraints such as climate change, increasing population and the use of chemicals, and fertilizers which have significant influence on sustainability. The excessive usage of chemical fertilizers and pesticides has created a significant risk to humans, animals, plants, and the environment. To reduce the dependency on chemical fertilizers and pesticides a biological-based alternative is required. Seaweeds are essential marine resources that contain bioactive compounds and they have several uses in agriculture. The use of seaweed extracts in agriculture can mitigate stress, enhance nutrient efficiency, and boost plant growth. The use of seaweed extracts and their components activate several signaling pathways and defense-related genes/enzymes. In this review, an attempt has been made to explain how seaweed extracts and their bioactive components induce tolerance and promote growth under stress conditions.
Keywords: Seaweeds, Biostimulant, Plant defense, Elicitors, Gene regulation
Introduction
The agricultural industry is confronted with several challenges including increasing the production to feed world’s rising population and increasing resource use efficiency, all while limiting the environmental impact on ecosystems and human health. The agro-ecosystem is consequently disturbed by extreme climatic events and such changes might have impact on both the quality and quantity of crops. The increasing level of pollutants, as well as the negative impact of climate change on soil microbiome, are additional constraints to agriculture. Increase in atmospheric carbon dioxide (CO2), temperature, and humidity, in particularly, are predicted to exacerbate the frequency of major plant diseases, insect infestation, and the migration of major pathogens and insect pests into new geographic areas. To increase agricultural production and fulfil the food requirement of increasing population inorganic fertilizer and pesticides are used liberally and it has serious implications on both agro-ecosystem and human health. In developing countries, farmers rely on the use of inorganic pesticides, insecticides, chemical fertilizers, and herbicides and their use has become an unavoidable risk in agriculture. Moreover, agriculture World is the backbone of our economy, and extended and excessive use of these inorganic fertilizers results in massive economic losses. Though their use has led to increased crop production their improper employment has caused agro contamination which is a troublesome issue. The use of chemical fertilizer could impact the agricultural environment by changing its parameter such as eutrophication, reducing the crop yield, salinization of soil, and increasing in pH of the soil (Prakash et al. 2018). To enhance agricultural production several technological advancements have been proposed which also include the identification of new plant biostimulants and efficient methods for their application. The use of plant bio-stimulants is promising and environmental friendly (Colla and Rouphael 2015) and the alternative to chemical fertilizer would be the use of organic fertilizer such as seaweed liquid fertilizer, humic acid, vermicompost, and essential microorganisms and these are biodegradable, non-toxic, and non-polluting. Kauffman et al.(2007) reported that bio-stimulants are not fertilizers, but when applied in low quantities improve plant growth (Kauffman et al. 2007). The biostimulant sector is expected to develop at an annual pace of 11.24% and reach USD 4.9 billion by 2025 (Caradonia et al. 2018). There is a growing interest in the farming sector for novel biostimulant compounds and much research is being conducted in this emerging sector of the business. Seaweeds serve as a valuable source of several elicitors that may be used for inducing physiological development and multitude of defensive responses. This review discusses the utilisation of various seaweed extracts (SEs) as biostimulants to mitigate biotic and abiotic stress. The mechanism of action, composition and function of several seaweed are discussed elaborately.
Seaweeds
Seaweeds or marine algae are marine aquatic plants that inhabit the coasts of oceans and seas. These are macroscopic algae that are found attached to the solid bottom of rocks, shells, and other plant material. It constitutes about 6000 species with a great diversity of forms and size and only 5% of it is being used. Seaweeds are a rich source of bioactive compounds and produce secondary metabolites with a varying range of biological functions such as antibacterial (Singh and Chaudhary 2010), antifungal (De Corato et al. 2017), antiviral, anti-inflammatory, nematocidal, and anticoagulant (Caijiao et al. 2021). They are divided into three categories based on their pigmentation, such as red algae (Rhodophyta), green algae (Chlorophyta), and brown algae (Phaeophyta) (Kim 2011). They are rich in minerals (Mg, Ca, P, K, and I), proteins, vitamins, undigestable carbohydrates, and fibers. The composition of minerals varies from species to species and the source of dietary fibers varies chemically and physiochemically from those of the plants found in the land (Jiménez-Escrig and Cambrodón 1999). Seaweed active compounds are used in wide range of sector such as food, wastewater treatments, agriculture, pharmaceutical, and cosmetics (Pereira and Correia 2015). Beyond these applications, the global demand for seaweed usage has increased. Seaweeds are beneficial alternative feeds for domesticated animals, because they contain valuable source of nutrients, particularly chelated microminerals, the availability of which is greater than that of inorganic minerals; complex carbohydrates with prebiotic properties; and pigments and polyunsaturated fatty acids that are beneficial to consumer health.
Seaweed is extremely important in agriculture and human lives and the huge seaweed reserve along the world's coastal areas should be used more effectively and strategically to reduce the waste of these vital resources (Parmar et al. 2017). The seaweeds have been utilized as organic fertilizer for centuries and in coastal regions seaweeds have been used either directly or in the composted form to improve crop productivity (Craigie 2010). Seaweeds are employed as biofertilizers to compensate for soil nutrient deficiencies. Seaweed extract (SEs) includes regulators, plant growth hormones, carbohydrates, auxins, gibberellins, and vitamins which can help to improve crop productivity and to maintain soil fertility. The SEs target several pathways to improve tolerance under stress and the certain plundering caused by bacteria, fungi, insects, and parasites can be reduced in plants. SEs biochemical composition is complex and as a result, understanding seaweed mode of action is exceedingly complex. Because of the various interactions between a large number of bioactive compounds within the same extract, a multidisciplinary approach is required.
The extraction of seaweed is still the most important phase in the production of agronomically efficient products. The seaweed extract is obtained through various processes but the widely used method is alkali extraction with or without heating (Khan et al. 2009). The seaweed-based biostimulant is produced in either liquid or soluble powder from a different range of seaweeds (Michalak et al. 2016). The use of seaweed extract’s intrinsic chelating characteristics, which prevent trace metal ions from precipitating, the extract is commonly fortified with plant fertilizer and micronutrients. The raw materials used and the extraction procedure have a significant impact on the various mechanism of action of seaweed-derived biostimulants (Shukla et al. 2019).
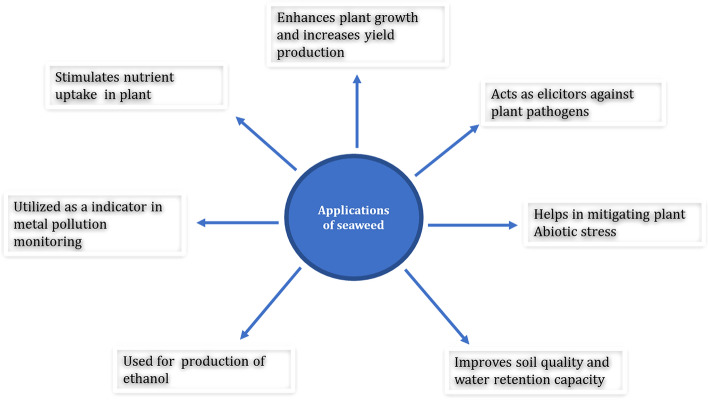
There are many seaweeds extracts available in the market and a considerable amount of seaweed is used as nutrient supplements and as biostimulants or biofertilizers. The most commonly manufactured seaweed species include Ascophyllum nodosum, Macrocystis pyrifera, Ecklonia msaxima, Sargassum species, Laminaria species, and Fucus serratus (Sharma et al. 2013). Numerous studies have found that seaweed extract applications have a wide range of favourable impact on crops (Fig. 1) which include early seed germination and establishment, improved crop performance and yield, increased resilience to biotic and abiotic stress and increased postharvest shelf-life of perishable products. For example A. nodosum one of the most studied seaweed when used as foliar spray improved the flowering phenomenon in tomatoes (Dookie et al. 2021).
Fig. 1.
The schematic illustration of different application of seaweed extracts in plants
The biostimulant prepared from different seaweeds elicits plant defense through a distinct mode of action. The effect of specified applications of seaweed extract (rates and timings are crucial for specific responses to be evoked) is also linked to the activation of many molecular and biochemical changes in the treated plants, demonstrating the possible impact of SEs on genetic pathways.
Effect of seaweed extract on plant growth and development
The seaweed extract has been demonstrated to positively affect seed germination and plant development at all phases including harvest and post-harvest (Table 1). Seaweed products have been demonstrated to improve germination rate and a considerable increase in seedling vigor by enhancing root length and density. Recently it was reported that Padina gymnospora, Gracilaria edulis, and Ulva fasciata aqueous extracts are used as seaweed biofertilizers for the seed germination of Capsicum annuum (Shamya Arokia rajan et al. 2020). Rengasamy et al.(2015) reported that the use of eckol and phloroglucinol isolated from the Ecklonia maxima increased the germination rate in maize seeds (Rengasamy et al. 2015). This has been linked to the activity of the enzyme α-amylase, which facilitates the conversion of starch to simple sugars in the roots of maize seedlings treated with eckol and phloroglucinol-treated. The sugar produced was transported to the embryo in order to provide the necessary energy for metabolism. The use of Kappaphycus alvarezii seaweed extract on maize during the grain-filling stage boosted yield attributes such as cob length, the number of grains per cob, and length of grain fill (Trivedi et al. 2018). Different concentration of K. alvarezii was applied together with 100% of the required amount of fertilizer on maize (Singh et al. 2015) and potato tuber (Pramanick et al. 2017) and increased growth, yield, and quality was observed in both studies. Treatment with A. nodosum has been shown to promote growth and nutrient uptake from the soil in Brassica napus (winter rapeseed) and strawberry (Jannin et al. 2012). Application of A.nodosum to wheat increased the nutrient uptake and the grain nutrient accumulation was linearly associated with grain production showing enhanced soil nutrient absorption and remobilization to the reproductive organs (Stamatiadis et al. 2018).
Table 1.
The effects of different seaweed extracts on plant growth and development
| Algal species (extract/commercial name) | Plant | Bioactive molecules | Mode of application | Response of plant’s trait | Gene/proteins/enzymes | References |
|---|---|---|---|---|---|---|
| A. nodosum | Moth bean | Alginic acid, fucosterol, mannitol and laminarin | Root application and Foliar application | Increased leaf number, leaf area, photosynthetic pigment, number of pods and nodules | Nod genes | Verma et al. (2021) |
| Soybean | Foliar spray | Increased photosynthetic activity and improved antioxidant efficiency |
Catalase, Superoxide dismutase, ascorbate peroxide and peroxidases |
do Rosário Rosa et al. (2021) | ||
| Maize | Foliar spray | Stimulated root growth and morphology | Chlorophyll and carotenoid content | Ertani et al. (2018) | ||
| Sugarcane | Foliar spray | Increased plant height, leaf area index and SPAD | – | Gomathi et al. (2017) | ||
| Sunflower | Foliar spray | Seed germination and seedling development were enhanced | – | Santos et al. (2019) | ||
| Wheat | Foliar application | Increased yield and grain nutrient accumulation | – | Stamatiadis et al. (2018) | ||
| Onion | Foliar application | Increased photosynthesis, nutrient accumulation and root development | – | Abbas et al. (2020) | ||
| winter rapeseed | Foliar spray | Increase in root, shoot and nutrient uptake |
Early light inducible protein (ELIP) ferredoxin-2, chloroplast precursor and serine decarboxylase |
Jannin et al. (2012) | ||
| Capsicum annuum L | Soil drench/Foliar spray | Increase in plant biomass, root, shoot and chlorophyll content | – | Ozbay and Demirkiran (2019) | ||
| Soybean |
Granule/ Foliar spray |
Increased shoot and root mass and increase in chlorophyll content | Cytokinin and Abscisic acid | Tandon and Dubey (2015) | ||
| Potato | Foliar spray | Increase in tuber weight and yield | – | Dziugieł and Wadas (2020) | ||
| Sweet pepper | Seed priming | Increased chlorophyll content, reducing sugar and defense related enzymes | Chitinase, β-1,3 glucanase, peroxidase, polyphenol oxidase and phenylalanine ammonia-lyase | Rajendran et al. (2021) | ||
| Cabbage | Foliar spray | Increased accumulation of phytochemicals | – | Lola-Luz et al. (2013) | ||
| Apple | Foliar spray | Increase in number of fruits, weight and length of fruits | – | de Sousa et al. (2019) | ||
| Orange | Foliar spray | Increase in yield | – | Fornes et al. (2002) | ||
|
Kelpak® (Ecklonia maxima) |
Onion | Eckol and phloroglucinol | Foliar spray | Increased chlorophyll content and highest mineral concentration in leaf and bulb | – | Gupta et al. (2021) |
| Smooth pigweed | Foliar spray | Increased growth parameters and pigment content | Superoxide dismutase, chlorophyll a & b and carotenoid | Ngoroyemoto et al. (2020) | ||
| True Algae Max (TAM) | Cucumber | Silaspiro [4.4]nona-1,3,6,8-tetraene, 3,8-bis (diethylboryl)-2,7-diethyl-1,4,6,9-tetraphenyl-, Milbemycin-oxime, and Nonadecane- | Foliar spray | Increase in yield and physical traits | – | Hassan et al. (2021) |
| Hot Pepper | Foliar spray | Increased chlorophyll, phenolic compounds and total nutrients | – | Ashour et al. (2021) | ||
| Polysaccharide-enriched extracts (PEE) | Tomato | Glucose, maltose and galactose and sulfate | Foliar spray | Improved growth and fruit nutritional quality | – | Mzibra et al. (2021) |
| Padina gymnospora | Tomato | n-Hexadecanoic acid, furfural, furan and octadecanoic acid | Seed treatment | Increased root development | Superoxide dismutase, peroxidase, phenylalanine ammonia lyase and phosphate transporter 4 | González et al. (2020) |
| Sargassum vulgare | Tomato |
Fucoidans, and fucosterol derivatives |
Foliar spray | Enhanced plant growth and increase in chlorophyll content | Peroxidase 64, glutathione S-transferase, cytochrome P 450 and ferredoxin | Ali et al. (2022) |
| Sweet pepper | ||||||
| Ulva lactuca | Tomato | Ulvans and glucuronan | Internodal injection | Increase in phenolic content in leaves and induction of salicylic acid in leaves | Phenylalanine ammonia lyase and salicylic acid | El Modafar et al. (2012) |
| Tomato | Foliar and soil drench application | Increased shoot length, root length and weight | – | Hernández-Herrera et al. (2014) | ||
| Maize | Xylose, Arabinose, Rhamnose, Glucose & Galactose | Seed priming | Increase in enzymatic activities, and protein content | Peroxidase, catalase and ascorbic acid | Hamouda et al. (2022b) | |
| Caulerpa sertularioides | Tomato | Compound not specified | Foliar and soil drench application | Increased shoot length, root length and weight | – | Hernández-Herrera et al. (2014) |
| Sargassum wightii | Green gram |
Fucoidans, fucoxanthin and β-carotene |
Foliar and root application | Increased root length, shoot length, early flowering and increased number of pods | – | Kumar et al. (2012) |
| Tulsi | Foliar spray | Enhanced overall growth and biochemical parameters | – | Uthirapandi et al. (2018) | ||
| Laminaria | Lettuce | Laminarin and laminin | Foliar spray | Increase in yield, growth and nitrate content | – | Di Mola et al. (2019) |
| Maize | Foliar spray | Stimulated root growth, morphology and plant nutrition | Esterase enzyme | Ertani et al. (2018) | ||
| Ecklonia maxima | Cabbage | Eckol and phloroglucinol | Foliar spray | Increase in photosynthetic pigments and growth parameters such as root and shoot length | Proline, α-amylase and myrosinase | Rengasamy et al. (2016) |
| Kappaphycus alvarezii | Wheat | Carrageenans and dieckol | Foliar spray | Increased nutrient content in grains and yield | – | Shah et al. (2013) |
| Maize | Foliar spray | Improved yield and growth | – | Trivedi et al. (2017) | ||
| Chaetomorpha antennina | Tomato | Amylopectin and flavonoids | Seed priming | Increase in pigment content, root and shoot length | Chlorophyll a & b, carotenoid, ascorbic acid and phenol | Muthu-Pandian Chanthini et al. (2019) |
| Gracilaria edulis | Wheat | Carrageenan, glycine and eckol | Foliar spray | Increased nutrient content in grains and yield | – | Shah et al. (2013) |
|
Ulva linza and Corallina officinalis |
Wheat | Compound not specified | Seed priming | Increase in germination percentage and growth parameters | Superoxide dismutase, catalase, peroxidase, glutathione peroxidase,glutathione reductase, glutathione S-transferases and ascorbate peroxidase | Hamouda et al. (2022a) |
| Ulva fasciata | Maize | Ulvans | Seed priming | Increase in enzymatic activities, and protein content | Peroxidase, catalase and ascorbic acid | Hamouda et al. (2022b) |
| Acanthophora spicifera, Gelidium robustum and Gracilaria parvispora | Mung bean | Compound not specified |
Seed priming |
Increase in root and shoot length | – | Di Filippo-Herrera et al. (2018) |
| Laminaria japonica | Broccoli | Laminarin and laminin | Irrigation | Increase in glucosinolae and phenolic compound of broccoli | – | Flores et al. (2021) |
| Caulerpa racemose | Tulsi | Flavonoids and β-carotene | Foliar spray | Enhanced overall growth and biochemical parameters | – | Uthirapandi et al. (2018) |
| Turbinaria ornata | Maize |
3-Acetonylcyclopentanone; 4- Methylthiomorpholine-1,1-Diox ide; Cholest-3-eno [3,4-d] Pyrimidine, 2-Chloro; 1, 8-Dibromooctan |
Soil drench | Increase in growth and yield | Dehydrogenase, phosphatase and protease | (Muniswami et al. 2021) |
| Radish | Foliar spray | Enhanced seed germination and increase in root and shoot length | – | Karthik et al. (2020) | ||
| Padina minor | Soybean | β-carotene and flavonoids | Foliar spray | Increase in growth parameters | – | Noli et al. (2021) |
|
Seasol (Durvillaea potatorum) |
Lettuce | trans-Zeatin, trans-zeatin riboside, their dihydro derivatives, isopentenyladenine and isopentenyladenosine | Foliar spray | Increase in root and shoot biomass | – | Yusuf et al. (2019) |
| Strawberry | Seed priming and soil drench | Increase in yield and root length | – | Mattner et al. (2018) |
“– “Not available. This table summarizes the list of seaweed extracts used to improve plant growth and development
Seasol a commercially available extract prepared from the seaweeds Duvillaea potatorum and A. nodosum increased the root length density and the yield (Mattner et al. 2018). Soybean treated with A. nodosum extract showed increased photosynthetic efficiency and chlorophyll content while also promoting the growth of the root system (do Rosário Rosa et al. 2021). Seaweed extract-treated tomato and pepper showed enhanced chlorophyll content (Ali et al. 2019). The extract obtained from Macrocystis pyrifera promoted the growth of tomato plants and increased the adventitious root formation in the mung bean (Briceño-Domínguez et al. 2014).
Finnie and Staden (1985) reported that Ecklonia maxmia seaweed promoted the in-vitro growth of tomato root. Both root elongation and root extension were significantly enhanced when applied (Finnie and van Staden 1985). Liquid seaweed extracts (LSEs) derived from seaweed such as Ulva lactuca, Caulerpaa sertularioides, P. gymnospora and Sargassum liebmannii enhanced germination response and increased the physiological traits of tomato. When compared to foliar spray, soil drench application was shown to be more effective (Hernández-Herrera et al. 2014). Application of U. fasciata and S. lacerifolium to soil boosted radish growth, improved germination percentage, and improved morphological and biochemical indices (Ahmed et al. 2020). The wheat seed primed with an aqueous extract of Ulva linza showed a substantial increase in physiological characteristics and mitotic index. New proteins were expressed in seedlings treated with an extract which might attribute to the activity of a bioactive substance in the extract (Hamouda et al. 2022a). Wine grape yield of several cultivars was increased by an average of 14.7% by foliar application of Durvillaea potatorum and A. nodosum (Arioli et al. 2021).
A study using A. nodosum extract in spinach showed enhanced biomass, protein content, chlorophyll and carotenoid content, flavonoids and antioxidant activity. The increase in biomass is associated with an increase in the expression of the Glutamine synthetase 1 (GS1) which is involved in nitrogen integration (Xu and Leskovar 2015). The increased expression of glutathione reductase (GR), and choline monooxygenase (CMO) was linked to increase in chlorophyll content. The upregulation of glutathione reductase (GR), thylakoid bound peroxidase (tAPX) and monodehydroascorbate reductase (MDHAR) was linked to the increase in chitinase activity, phenolics and flavonoids. These genes were discovered to be related to the phenylpropanoid and flavonoid pathways which are known to promote growth and improve overall nutrition (Fan et al. 2013). Flavonoids are secondary metabolite which is important for plant development. Chalcone isomerase (CHI), a key enzyme in the biosynthesis of flavanone precursors and phenylpropanoid plant defense compounds were also upregulated in response to seaweed extract treatment. The use of A. nodosum extracts improved the nitrogen use efficiency in barley plants by increasing the N content in barley shoot which was linked with the upregulation of root nitrate transporter (NRT1.1, NRT2.1, and NRT 1.5) (Goñi et al. 2021). Jannin et al. (2012) reported that A. nodosum application to Brassica napus increased the uptake of nitrogen and sulfate content in root and shoot. The expression of genes (BnNRT1.1 and BnNRT2.1) which encode nitrate transporter was shown to increase in roots of the treated plant (Jannin et al. 2012).The investigation of molecular responses in plant to A.nodosum extract treatment revealed that the increase in chlorophyll concentration was primarily due to downregulation of stay green protein (SRG) which was able to limit leaf protein degradation and protect chlorophyll degradation during leaf senescence. Senescence-associated cysteine protease was down-regulated whereas genes involved in photosynthesis, cell metabolism and S and N metabolism were up regulated (Rayorath et al. 2008; Jannin et al. 2012).
Cell wall undergo structural changes upon biotic and abiotic stress and they store carbohydrate for plant growth. Previously it was reported that Kelpak® can alter the allocation of carbon between cellulose synthesis and secondary cell wall by regulating sucrose synthase expression (Kocira et al. 2020). Sucrose is required for cell wall synthesis and several mechanisms contribute to cell wall synthesis (Decker and Kleczkowski 2019). In another study it was reported that with application of S. vulgare in tomato and sweet pepper cell-wall biosynthesis-related transcripts were upregulated. The MYB transcription are significantly upregulated and are related to secondary cell wall formation. The MYB are activated by SND1 (Secondary wall-associate NAC domain protein 1) and it activates the biosynthesis of lignin, cellulose and hemicellulose (Miedes et al. 2014; Decker and Kleczkowski 2019).
Phytohormones are necessary for plant growth and development. Thus, the alteration of the innate phytohormone pool by the seaweed-based biostimulants can have a major impact on plant development. Recent research has shown that seaweed and its components can influence the expression of genes involved in the biosynthesis of growth hormones such as NCED3 (Nine-cis-epoxycarotenoid dioxygenase3), ERF2 (Ethylene-responsive transcription factor 2), ICS2 (Isochorismate synthase 2) etc. The genes are upregulated in plants upon treatment of seaweed extracts (Ali et al. 2019).
When extracts are applied at the recommended levels, (do Rosário Rosa et al. 2021) the growth hormones present within the extract is insufficient to invoke physiological changes in plants (Górka and Wieczorek 2017). However, the components within the seaweed extract may induce innate pathways for the biosynthesis of growth hormones in plants. Aremu et al. (2015) isolated eckol and phloroglucinol from Ecklonia maxima and investigated their effects on Eucomis autumnails. It was observed that exogenous application of eckol and phloroglucinol caused increase in rise of auxin level which resulted in a 1.5-fold increase in the root length of the treated plant. The compounds influence auxin’s root-lengthening activity by preventing decarboxylation and acting as a cofactor which promotes the breakdown of the IAA oxidase (Aremu et al. 2015). It has been observed that biostimulants promote overall plant growth, physiological processes, agronomic aspects, and yield quality of crops (Yao et al. 2020).
Mitigating plant abiotic stress using seaweed-based biostimulants
Abiotic stress such as temperature extremes (heat and cold), drought, salinity, and heavy metals are key issues restricting agricultural growth and sustainability across the world. It negatively impacts growth and yield formation. These abiotic stresses are interrelated and can manifest osmotic stress, ion distribution failure, and plant cell homeostasis. Developing stress-tolerant plants through genetic engineering is a challenging task and has achieved limited success (Agarwal et al. 2012). As an early reaction to abiotic stress plants undergo internal oxidative stress by excess overproduction of reactive oxygen species (ROS). An imbalance is created between ROS production and the ability to detoxify and remove intermediate substances (Bencze et al. 2004). The increased production of ROS causes structural alteration to DNA and proteins, inhibition of antioxidant enzymes, and activation of programmed cell death (PCD) (Huang et al. 2019). To counteract the deleterious effects, plants use a wide range of defense mechanisms (Fig. 2). In order to scavenge the ROS, plants employ both enzymatic and nonenzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) and phenolic compounds (Gruszka et al. 2018; Yadav et al. 2019). Prominent results prove that seaweed can mitigate abiotic stress in plants (Table 2). For example, pre-treatment with A. nodosum based biostimulant had a favourable effect on Arabidopsis under drought-stressed conditions. It also altered the expression levels of genes implicated in ABA-responsive and antioxidant system pathways resulting in improved photosynthetic performance than untreated plants (Santaniello et al. 2017). The use of polysaccharides extracted from brown seaweed Lessonia nigrescens improved the salt tolerance level in wheat plants (Zou et al. 2019). The treatment minimized the oxidative damage in plants by increasing the antioxidant activity of SOD, CAT, and POD (peroxidase) enzymes by controlling the relative electric leakage and malondialdehyde content which are two parameters that determine membrane permeability and lipid peroxidation. Similarly, the use of U. rigida improved the salt tolerance in wheat plant and protected it from oxidative degradation, and increased the enzymatic activities in plants (Chernane et al. 2015). The application of polysaccharides extracted from Grateloupia filicina mitigated the tolerance of rice seedling to salinity stress and improved rice growth under salinity conditions (Liu et al. 2019). Milkweed seedling treated S. angustifolium extract can tolerate salinity up to 15 dS m−1 and the survival rate was increased by 69%. The treatment increased the growth parameters with a decrease in electrolyte leakage (Bahmani Jafarlou et al. 2022). Similarly, supplementation of S. muticum and Jania rubens provided salt stress amelioration in chickpea and enhanced activity of SOD and APX were related with improved growth parameters. Moreover, four key amino acids including serine, threonine, proline, and aspartic acid were identified from roots that contribute to stress reduction (Abdel Latef et al. 2017). Garcilaria dura extract applied to wheat elevated drought stress tolerance and increased the biomass and crop yield (Sharma et al. 2019). The foliar application of K. alvarezii significantly mitigated the water stress in maize with increased seed yield primarily by increasing all yield attributes, particularly cob length, the number of grains per cob, and length of grain fill. It also resulted in increased antioxidant activity and reduced reactive oxygen species levels, leading to a reduced degree of oxidative stress in treated plants. (Trivedi et al. 2018).
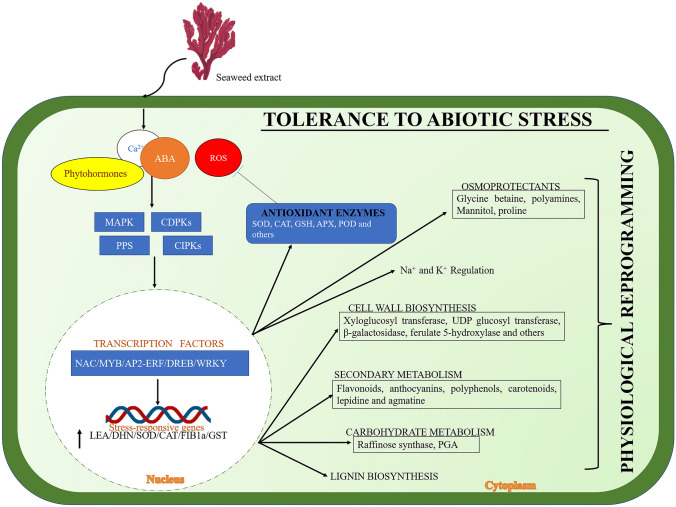
Fig. 2.
The schematic illustration highlights the application of seaweed extract on plants and the response of plants to abiotic stresses. Seaweed extract application to plants is perceived by a complex sensing mechanism in which various secondary messenger’s function (Abscisic acid (ABA), phytohormones, Ca2+, etc.,). The secondary messengers cause a myriad of downstream processes such as the activation of MAPKs (Mitogen-activated protein kinases), CIPKs (Calcineurin B-like proteins), PPs (Protein phosphatases) and CDPKs (Calcium-dependent protein kinases) activating the transcriptional factors and they regulate the stimulation of antioxidant enzymes thus scavenging ROS production. The other stress-inducible genes are involved in the alteration of cell wall biosynthesis, carbohydrate metabolism, secondary metabolites, and regulation of Na+ and K+ transporters thus altering the physiological adaption of plants to abiotic stress and enhancing the tolerance to abiotic stress. Note MAPK mitogen-activated protein kinase, CDPKs calcium-dependent protein kinases, PPs protein phosphatases, CIPKs calcineurin B-like protein (CBL)-CBL-interacting protein kinase, WRKY tryptophan arginine lysine tyrosine, MYB myeloblastosis, DREB dehydration responsive element-binding, AP2-APETALA2, EREB ethylene responsive element binding factor, LEA late embryogenesis abundant, DHN dehydrin, FIB1a fibrillarin1a, GST glutathione S-transferase, SOD superoxide dismutase, CAT catalase, GSH glutathione, APX ascorbate peroxidase, POD peroxidase, PGA-3 phosphoglyceric
Table 2.
Effect of different seaweed extracts in mitigating abiotic stress in plants
| Seaweed species (extract/commercial name) | Plant | Abiotic stress type | Response of plant’s trait | Genes/proteins/pathways involved | References |
|---|---|---|---|---|---|
| K. alvarezii | Tobacco | Salt stress | Increased photosynthesis, electron transfer and higher expression of stress-defence genes | Na+/H+ antiporter gene | Kumari et al. (2022) |
| Maize | Drought stress | Upregulation of gene coding enhancement of root growth seed development and antioxidant related enzymes | Auxin transporter like protein, root cap periphery 2 protein and profilin | Kumar et al. (2020) | |
| Ecklonia maxmia | Zucchini squash | Salt stress | Increased shoot biomass, chlorophyll content and nutritional status | – | Rouphael et al. (2016) |
| Kelpak® | Cowpea | Drought stress | Increase in photosynthetic pigments, shoot and nodule production | – | Voko et al. (2022) |
| A. nodosum | Soybean | Drought stress | Increase in relative water content, stomatal conductance and improved ROS-scavenging capacity | FIB1a, PIP1B, NYFA3, TP55 and BIP | Shukla et al. (2018) |
| Sugarcane | Drought stress | Enhanced water content, water retention capacity and improved sugar content | – | Chen et al. (2019) | |
| Maize | Cold stress | Increased activity of superoxide dismutase (SOD) in root and leaf tissue. Decrease in leaf damage and shoot inhibition | Antioxidant pathway | Bradáčová et al. (2016) | |
| Avocado | Salt stress | Increased concentration of Calcium (Ca) and Potassium (K) in leaves | – | Bonomelli et al. (2018) | |
| Tomato | Water stress | Reduction in stem water potential and ABA accumulation | ABA pathway | Campobenedetto et al. (2021) | |
| Tomato | Heat stress | Enhanced thermotolerance with changes in biochemical and gene transcription | HSP101.1, HSP70.9, HSP17.7 | Carmody et al. (2020) | |
| Spinach | Heat stress | Improved germination percentage, seedling vigour and antioxidant metabolism. Decrease in hydrogen peroxide (H2O2) and malondialdehyde content (MDA) | Antioxidant pathway | Neto et al. (2020) | |
| Arabidopsis | Cold stress | Upregulation of cold related genes, decrease in electrolyte leakage and decrease in chlorophyll damage | AtCHL1, AtCHL2, COR15A, RD29A, and CBF3 | Rayirath et al. (2009) | |
| Mustard greens | Drought stress | Increase in photosynthetic rate, total soluble sugars and antioxidant enzyme activity | – | Sujata et al. (2022) | |
| Arabidopsis | Drought stress | Better photosynthetic performance and increased expression levels of genes involved in antioxidant pathways | PIP1;2 and βCA1 | Santaniello et al. (2017) | |
| Laminaria digitata | Tomato | Water stress | Reduction in stem water potential and ABA accumulation | ABA pathway | Campobenedetto et al. (2021) |
| K. alvarezii | Maize | Water stress | Increase in cob length, number of grains per cob and length of grain fill. Increase of enzymes such as SOD, CAT and APX | Antioxidant pathway | Trivedi et al. (2018) |
| Gracilaria dura | Wheat | Drought stress |
Reduced loss of water, increase in chlorophyll, proline content and osmotic potential Increased accumulation of ABA |
Dehydrin, ERD2, HA1 and P5CS1 | Sharma et al. (2019) |
| Fucus spiralis | Wheat | Salt stress | Enhanced seed germination, growth parameters and activity of antioxidant enzymes | – | Chernane et al. (2015) |
| P. gymnospora | Tomato | Salt stress | Enhanced photosynthetic performance, increased antioxidant activity and reduced oxidative damage | – | Hernández-Herrera et al. (2022) |
| Dictyota dichotoma | Rice | Salt stress | Enhancement of seed germination | – | El-Katony et al. (2021) |
| Grateloupia filicina | Rice | Salt stress | Increase in germination potential, root and shoot length. Increase in proline content, SOD and POD | Antioxidant pathway | Liu et al. (2019) |
| Sargassum angustifolium | Milkweed | Salt stress | Increase in growth parameters such as root length, root and shoot dry weight. Increase in chlorophyll pigment and decreased electrolyte leakage | Antioxidant pathway | Bahmani Jafarlou et al. (2022) |
| Zostera marina | Tomato | Salt stress | Enhanced salt tolerance | Antioxidant pathway (APX, CAT, SOD and POX) | Vinoth et al. (2017) |
|
S. muticum Jania rubens |
Chickpea | Salt stress | Increased in Chl a, Chl b, and carotenoids. Increase in SOD, CAT, POD and APX activities | SnRKs (sucrose non-fermenting kinases) | Abdel Latef et al. (2017) |
| Lessonia nigrescens | Wheat | Salt stress | Increase in plant growth, increase in chlorophyll content and improved antioxidant activities | TaHKT2, TaSOS1 and TaNHX2 | Zou et al. (2019) |
“– “ Not available, This table summarizes the list of seaweed extracts used to mitigate abiotic stress in plants
PIP1B plasma membrane intrinsic protein 1B, NYFA3 nuclear factor Y subunit A, PIP1;2-Plasma membrane Intrinsic Protein 1;2, βCA1-β-Carbonic Anhydrase 1 HSP heat shock protein, TaHKT2 Triticum aestivum high affinity K+ transporter 2, TaSOS1 Triticum aestivum salt overly sensitive 1, TaNHX2- Triticum aestivum Na ( +)/ H( +) exchanger 1, AtCHL1 Chlorophyllase, COR15A cold regulated 15 A, RD29A response-to-dehydration 29A and CBF3 centromere DNA-binding protein complex 3
Seaweed extract could be another approach for dealing with temperatures that impede the growth and development of plants. Application of A. nodosum extract (ANE) to tomato seedling enhanced heat stress tolerance and showed significant improvement in flower development and fruit production. The constituents in ANE formulation were associated with an accumulation of soluble sugars, and increase in heat shock proteins (HSPs) gene transcription in heat exposed tomato flowers before fertilization (Carmody et al. 2020). Algafect a commercial seaweed extract (A. nodosum, Fucus spp., and Laminaria spp.) was applied to maize and it enhanced the low temperature tolerance of root zone during early growth. On application of seaweed extract, the SOD activity was increased in root and leaf tissue as it plays an important role in antioxidative stress defense (Bradáčová et al. 2016).
Seed priming has emerged as an essential approach for producing plants that are resistant to various stress and it is a simple and alternative practice compared to conventional methodologies (breeding or transgenics). Seaweed extract is successfully utilized as a priming agent to enhance stress tolerance in plants (Bhanuprakash and Yogeesha 2016). Priming wheat seeds with Hormophysa cuneiformis and Actinotrichia fragilis reduced salt stress and it also enhanced the growth and increased the level of antioxidant activity in plants (Latef et al. 2021).
Maize and black-eyed pea seeds primed with seaweed liquid extract (SLE) prepared from Ulva fasciata, Cystoseira compressa and Laurencia obtusa exhibited remarkable tolerance to salinity stress and increased the seedling morphological parameter (Hussein et al. 2021). The negative effects of salinity in tomato seedlings were attenuated by priming the seeds with Ulva lactuca methanol extract. The hydrogen peroxide concentration and antioxidant activity were decreased in plants treated with methanol extract and a high concentration of total phenol and soluble sugars were recorded in methanol extract suggesting these compounds might play a key role in mitigating salinity stress in plants (El et al. 2021). The use of natural plant biostimulants has been recommended to boost plant resilience to abiotic environmental stresses (Nephali et al. 2020; Deolu-Ajayi et al. 2022).
Mitigating plant biotic stress using seaweed-based biostimulants
The emergence of infectious plant pathogens has increased due to changes in climatic conditions and intensive agriculture (Anderson et al. 2004). Even though new technology, research, and products help agriculture in maintaining integrated management and farming practices, pathogens (primarily bacteria, viruses, and fungi) have decreased agricultural productivity inflicting economic damage on at least 10% world's food supply. To counteract the biotic stresses induced by pathogenic infection, plants activate different defense mechanisms. Mitigation of plant biotic stress has been successfully achieved by priming the plant molecular defense by treatment with different chemicals, natural substances, and phytohormones (Aranega-Bou et al. 2014). SEs and their bioactive compounds are successfully used as defense priming agents in plants to trigger an immune response in plants (Craigie 2010; Islam et al. 2020).
Many seaweed extracts have been reported to exhibit antagonistic activity both in-vitro and in the fields (Table 3). For example, Ulva lactuca reduced the fusarium wilt severity when sprayed onto leaves of common beans (de Borba et al. 2019). The use of Ochtodes secundiramea and Laurencia dendroidea extracts in papaya and banana efficiently inhibited Colletotrichum gloeosporioides, a fungal species that severely hampers fruits post-harvest (Machado et al. 2014). The cucumber plants sprayed with A. nodosum exhibited enhanced resistance against Phytophthora melonis by induction of defense enzymes and genes. Moreover, the plants showed a higher level of phenolic accumulation compared to the control (Abkhoo and Sabbagh 2015). Similarly, the use of A. nodosum in carrot and broccoli successfully controlled the foliar diseases caused by different fungal pathogens (Jayaraj et al. 2008; Mattner et al. 2014). The extracts of brown algae Cystoseria myriophylloides, Laminaria digitata and Fucus spiralis inhibited the growth of tomato pathogens Verticillium dahlia and Agrobacterium tumefaciens both in-vitro and in the greenhouse. It was also reported that defense enzyme polyphenol oxidase and peroxidase activity were substantially increased in comparison to control (Esserti et al. 2016). The extracts of Ulva lactuca, S. filipendula and Gelidium serrulatum suppressed the Alternaria solani and Xanthomonas campestris pv vesicatoria in tomato plants. The seaweed-extract treated plant showed reduced severity of diseases and induced salicylic acid and jasmonate signaling pathways (Ramkissoon et al. 2017). The root-knot diseases in eggplant and watermelon caused by fungi Fusarium solani were suppressed using extracts of Spatoglossum variabile, Stokeyia indica, and Melanothamnus afaqhusainii. The use of Padina pavonica benzene extract caused more mortality of Dsydercus cingulatus an economically important cotton pest (Sahayaraj and Kalidas 2011). In banana and tomato plants the root-knot infection caused by a nematode, (Meloidogyne spp.) was controlled by soil application of SEs such as Ulva lactuca, Ulva fasciata, and Stokeyia indica (El-Ansary and Hamouda 2014; Ghareeb et al. 2019).
Table 3.
Effect of different seaweed extracts on disease resistance in different plant species
| Seaweed species (extract/commercial name) | Plant | Diseases | Organism | Response of plant | Gene/proteins/pathways involved | References |
|---|---|---|---|---|---|---|
| Gelidium serrulatum | Tomato | Early blight bacterial spot |
Alternaria solani Xanthomonas campestris |
Induced expression of signalling pathways | Jasmonate signalling pathway | Ramkissoon et al. (2017) |
|
Kappaphycus sp. and Eucheuma sp. |
Rice | Fungal blast diseases | Magnaporthe oryzae | Increase in expression of various defense related genes and enzymes | PR-1 6 PAL 6 | Sahana et al. (2021) |
| A. nodosum (ANE) | Strawberry | Powdery mildew | Podosphaera aphanis | Increased the expression of defense-related enzymes and also induced secondary metabolism in plants | PAL and PPO | Bajpai et al. (2019) |
| Strawberry | Red spider infestation | Tetranychus urticae | Reduction in red spider mite incidence | NA | Hankins and Hockey (1990) | |
| Arabidopsis | Root-knot nematode | Meloidogyne javanica | Reduction in number of nematodes | – | Wu et al. (1998) | |
| Broccoli | Clubroot |
Plasmodiophora brassice |
Decrease of plasmodia in root hairs | – | Wite et al. (2015) | |
| Gracilaria confervoides | Cucumber | – |
Macrophomina phaseolina Rhizoctonia solani Fusarium solani |
NA | – | Soliman et al. (2018) |
| Acanthophora spicifera | Rice | Fungal blast | Pyricularia oryzae | Increase in expression of defence-related enzymes | PO and PAL | Flora et al. (2012) |
| U.lactuca | ||||||
| K.alvarezii sap | Tomato | Charcoal rot | Macrophomina phaseolina | Increase in expression of defence responsive genes and phytohormones | PR-1b1, PR-3 and PR-5 | Agarwal et al. (2015) |
| Durvillaea potatorum | Broccoli | Clubroot |
Plasmodiophora brassice |
Decrease of plasmodia in root hairs | – | Wite et al. (2015) |
| P.pavonia | Rice | Fungal blast | Pyricularai oryzae | Reduction in severity of diseases and increase expression of defense related genes | Flora et al. (2012) | |
| Rice | Fungal blast | Pyricularia oryzae | Increase in expression of defence-related enzymes | PO and PAL | Flora et al. (2012) | |
| S. fusiforme | Tomato | Powdery mildew, gray mould |
Phytophthora infestans Oidium spp |
Induced O2− production and hypersensitive cell death in tomato tissue | – | Sbaihat et al. (2015) |
| S. tenerrimum | Cotton | Red cotton pest | Dysdercus cingulatus | Reduced the nymphal development | – | Sahayaraj and Mary Jeeva (2012) |
| S. vulgare | Potato | Pythium leak | Pythium aphanidermatum | Reduced rot penetration in potato | – | Ammar et al. (2017) |
| Stokeyia indica |
Eggplant and Watermelon |
Root rotting diseases |
F.solani M.phaseolina |
Supression of root knot nematode | – | Baloch et al. (2013) |
| Ulva armoricana | Cucumber | Powdery mildew | Sphaerotheca fuliginea | Reduction in powdery mildew infeciton | – | Jaulneau et al. (2011) |
“– “Not available. This table summarizes the list of seaweed extracts used to mitigate biotic stress in plants
PR-1 pathogenesis-related protein 1, PAL 6-phenylalanine ammonia lyase 6, PAL phenylalanine ammonia lyase, PPO polyphenol oxidase and PO peroxidase
Plants activate their defense mechanism against pathogens by recognizing pathogen-associated molecular patterns (PAMP) (Göhre and Robatzek 2008). Seaweed-based elicitors act as PAMPs and the signaling pathways are activated by the phytohormones jasmonic acid (JA), ethylene (ET), and salicylic acid (SA) (Dumas et al. 2010). Elicitors or plant defense activators generally induce partial, broad-range systemic resistance, as indicated by a considerable reduction in disease symptoms caused by various pathogens. The bio-elicitors stimulate the plant’s defense mechanisms, influencing genetic reprogramming (Pršić and Ongena 2020). Seaweeds contain bioactive compounds such as carrageenans, fucans, laminarans and ulvans that have been used as elicitors to protect the plant against various diseases (Shukla et al. 2016). Carrageenans possess antiviral properties and elicit defensive responses in plants. For example, tomato plants treated with carrageenan significantly suppressed tomato chlorotic dwarf viroid replication and expression. Additionally, jasmonic acid-related genes AOS (allene oxide synthase) and LOX (lipoxygenase) were up-regulated during viroid infection in plants treated with carrageenan (Sangha et al. 2011, 2015). The к-carrageenan extracted from Hypnea musciformis enhanced the immunity in tobacco plants infected with Tobacco Mosaic Virus (TMV) and activated SA and JA/ET dependent pathways defense mechanisms (Ghannam et al. 2013). Priming plants with seaweed-based elicitors enhances the plant immunity against phytopathogens by inducing defense-related genes and enzymes. For example, the Arabidopsis thaliana seedling treated with A.nodosum seaweed extract boosted the plant's immunity against various bacterial pathogens and also activated the expression of defense-related genes such as WRKY30 (WRKY DNA binding protein), (cytochrome P450, family 71, subfamily A, polypeptide 12), and PR-1 (Pathogenesis-related protein-1) (Cook et al. 2018). Seaweed-based elicitors are environmentally friendly crop protection strategies available and are a developing paradigm in the prevention of plant diseases that activates the plant immune system prior to pathogen overcoming. It also activates the innate immunity of plants by upregulating the defense-related genes and hormones thus rescuing plants from biotic stress.
Mechanisms of action of seaweed extracts under biotic and abiotic stress conditions
The seaweed-based biostimulants obtained from complex extraction contain a wide range of bioactive compounds that in theory can generate multiple positive effects throughout plant development. This is because they trigger signaling pathways, resulting in physiological changes in plants. For example, overexpression of NtLTP4 (Nicotiana tabacum Lipid transfer protein 4) in Nicotiana tabacum increased tolerance to salt and drought stress. The NtLTP4 modulates transcription levels of salt-responsive genes NHX1 (Na+/H+antiporter) and HKT1 (high-affinity K+ transporter1) to reduce Na+ toxicity. (Xu et al. 2018). The tomato and pepper crops treated with seaweed extract were shown to increase the upregulation of gene transcripts Ga2Ox (Gibberellin 2-oxidase), IAA (Indole-3-acetic acid), and IPT (Isopentenyl transferase) which are involved in the production of phytohormones in plants (Ali et al. 2019).
The usage of seaweed extract modulates the level of ABA, commonly known as the plant’s stress hormone. Treatment of Arabidopsis with ethyl acetate extract of A.nodosum under salt stress increased the transcript accumulation of the SnRK2 (SNF1-related protein kinase 2) gene which activates the ABA signaling network (Jithesh et al. 2018). The bioactive components present in the ethyl acetate fraction of the A. nodosum extract enhance salt tolerance and regulate stress-related signal transduction. Shukla et al.(2018) reported that GmCYP707A1a and GmCYP707A3b genes which regulate ABA biosynthesis during dehydration and hydration cycles were overexpressed in soybean attributing to the application of seaweed extract and also induced drought resistance (Shukla et al. 2018). Similarly, the A. nodosum extract regulated GmFIB1a (Glycine max Fibrillin 1a) expression and protected photosystem ll. Proline induces osmotic stress-related genes in frost tolerance (Wei et al. 2022). The use of seaweed extract on A. thaliana accumulated proline by activation of proline biosynthesis and also mediated freezing tolerance (Nair et al. 2012). Similarly in another study, the use of S. angustifolium improved drought tolerance by enhancing the expression of P5CS (Pyrroline-5-carboxylate synthase) a crucial gene in the biosynthesis pathway of proline, and is overexpressed under stressful conditions (Shahriari et al. 2021). According to Ghamdi et al. (2018) the use of seaweed extract had a synergistic effect in reported Asparagus aethiopicus plants subjected to saline stress. There was a significant enhancement of morphological and physiological characteristics of the treated plant compared to the control. The experiment also revealed the salinity stress was mitigated by upregulation of genes ANN1 (Annexins D1), ANN2 (Annexins D2), PIP1 (plasma membrane intrinsic protein 1) and CHS (Chalcone Synthase) that are related to osmotic adjustment and flavonoid biosynthesis (Al-Ghamdi and Elansary 2018). Pre-treatment with seaweed extract induces partial stomatal closure which is linked to alteration in expression of genes associated with ABA-responsive and antioxidant system pathways. NCED3 (Nine-cis-epoxycarotenoid dioxygenase) genes implicated in the ABA-biosynthesis pathway was significantly expressed during dehydration and ABA-responsive gene RAB18 (responsive to ABA) and RD29A (Responsive to Desiccation) transcript were expressed in treated plants during drought stress (Santaniello et al. 2017). The bean (Phaseolus vulgaris) treated with A. nodosum was shown to increase drought tolerance by reducing ROS-induced MDA (Malondialdehyde) generation and increasing CAT (Catalase) activity (Eugenia Amaral Carvalho et al. 2018). The WRKY transcription factors are involved in the development and physiological processes of plants. They interact with MAPK (mitogen-activated protein kinase) and regulate plant function by acting downstream of multiple MAPKs. The application of K. alvarezii (K-sap) increased the expression of the TaMPK10 gene encoding MAP Kinase in wheat and increased the tolerance of plants under drought stress (Patel et al. 2018). Cold stress in Arabidopsis was mitigated by the use of seaweed extract which increased the chlorophyll content and lowered the expression of AtCLH1 and AtCLH2 (chlorophyll degradation genes) and also induced the expression of cold-responsive genes such as COR15A, RD29A, and CBF3 (Rayirath et al. 2009). In pepper plants, the salinity stress and oxidative damage were reduced by the application of seaweed extract which upregulated the antioxidant activity (SOD, CAT, and APX) (Elansary et al. 2017).
The application of seaweed extracts and their bioactive components activate the up-regulation of numerous defense-related genes, plant hormones, and defense enzymes and these factors interact and cross talk to build a complex network that aids in disease tolerance in plants. The seaweed polysaccharides induce oxidative burst and activate various signaling pathways such as SA, JA, and ET at a systemic level and this leads to activation of different defense-related players such as pathogenesis-related protein (PR) and enzymes like phenylalanine ammonia-lyase (PAL) and lipoxygenase (LOX) (Vera et al. 2011) (Fig. 3). For instance, the A. nodosum extract application enhanced resistance against Pseudomonas syringae pv. tomato DC3000. The extract activated the JA-dependent pathway by inducing the expression of PDF1.2 JA-related genes and the decrease in diseases symptoms on the leaves is linked to the enhanced expression of JA -related gene transcripts (Subramanian et al. 2011). The application of K-sap in tomato seedlings enhanced the concentration of hormones (ABA, IAA, SA, and zeatin) as well as pathogenesis-related genes PR-1b1, PR-3, and PR-5 during Macrophomina phaseolina infection (Agarwal et al. 2015). In pepper and tomato plants the foliar application of S. vulgare and Acanthophora spicifera reduced the severity of the pathogens Xanthomonas campestris pv. vesicatoria and Alternarai solani by activating genes (PR-1a, Pinll, and ETR-1) which are involved in defense signaling pathways (Ali et al. 2020). Cluzet et al.(2004) reported that the use of 500 µg mL−1 of Ulva spp extract in Medicago truncatula induced expression of genes related to defense signaling pathways, pathogenesis-related protein and cell wall protein (Cluzet et al. 2004). To reduce stress in plants seaweed extracts can be promising strategy and moreover the experiments on both biotic and abiotic stress have proven that seaweed based biostimulants are beneficial.
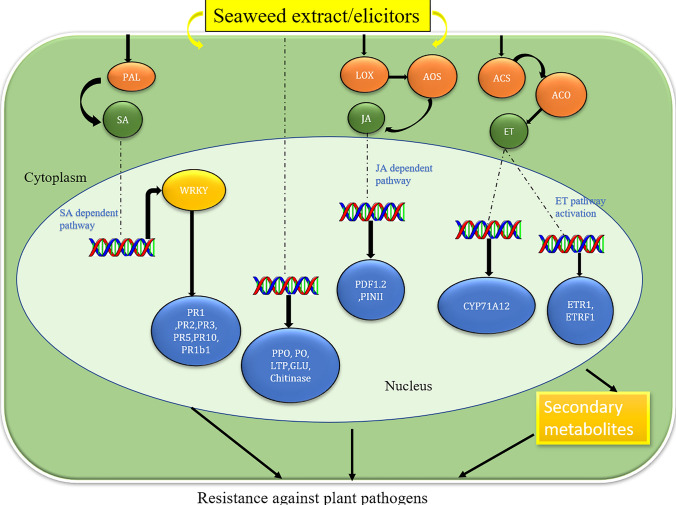
Fig. 3.
The schematic illustration highlights the plausible mechanism of action of seaweed extracts against fungal and bacterial disease control. Seaweed extract may directly affect pathogens or indirectly by eliciting plant defense machinery. Such elicitation occurs by activation of several enzymes (PAL, LOX and ACS) which leads to the activation of several metabolic pathways such as (SA, JA and ET) thus upregulating several defense genes which induce the biosynthesis of several secondary metabolites which are involved in diseases suppression. Note: ROS reactive oxygen species, SA salicylic acid, PAL phenylalanine ammonia lyase, LOX 5-lipoxygenase, AOS active oxygen species, JA jasmonic acid, ACC 1-aminocyclopropane-1-carboxylic acid, ACS-ACC synthase, ACO-ACC oxidase, ET ethylene, PR pathogenesis related protein, PPO polyphenol oxidase, PO prophenoloxidase, LTP lipid-transfer proteins, GLU glutamate, PDF1.2 Plant defensin1.2, PINII proteinase inhibitor II, CYP71A12 cytochrome P450, family 71, subfamily A, polypeptide 12, ETR1 Ethylene response 1
Conclusion
The utilization of seaweeds extracts and their bioactive compounds can influence the performance of crops. The advantages of seaweed products in agriculture are undeniable as they increase the uptake of nutrients, enhance both the morphological and physiological characteristics, and also mitigate stress in plants. It has been already reported that they can act as both biostimulants and bio-elicitors in plants. Moreover, the SEs are environment friendly and are also effective in inducing innate immunity in plants. SEs can be used in combination with other biofertilizers to gain maximum yield and reduce the use of chemical fertilizers. The chemical constituents of SEs must be studied and analysed to determine the mode of application, dosage, and duration of administration to different crops to obtain long-term benefits. To supply consistent and dependable products to end users, efficient production procedure based on an in-depth understanding of the molecular mechanisms by which these extracts elicit beneficial impacts in treated plants is essential. The composition of seaweed is complex and it is difficult to identify the bioactive molecule that induces specific effect in plants. The use of new approaches (high throughput sequencing, metabolomics and genomics) can unravel the mechanisms of action. The seaweed extracts have been utilized in research; they must be marketed in order to reach farmer’s level.
Acknowledgements
The authors are grateful to the management of the Vellore Institute of Technology for providing the necessary support to write this article. The authors acknowledge Dr. Sudhakaran R of Vellore Institute of Technology—aquaculture laboratory for his guidance in writing this review article.
Author contribution
BR prepared and revised the manuscript under the direction of VR.
Funding
The authors confirm that this review article was not written under the financial sponsorship of any funding agency.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there were no competing interests either financial or non-financial.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas M, Anwar J, Zafar-Ul-Hye M, et al. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae. 2020;6(2):28. doi: 10.3390/HORTICULTURAE6020028. [DOI] [Google Scholar]
- Abdel Latef AAH, Srivastava AK, Saber H, et al. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci Rep. 2017;7(1):12. doi: 10.1038/s41598-017-07692-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abkhoo J, Sabbagh SK. Control of Phytophthora melonis damping-off, induction of defense responses, and gene expression of cucumber treated with commercial extract from Ascophyllum nodosum. J Appl Phycol. 2015;28(2):1333–1342. doi: 10.1007/S10811-015-0693-3. [DOI] [Google Scholar]
- Agarwal PK, Shukla PS, Gupta K, Jha B. Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol. 2012;54(1):102–123. doi: 10.1007/S12033-012-9538-3. [DOI] [PubMed] [Google Scholar]
- Agarwal P, Patel K, Das AK, et al. Insights into the role of seaweed Kappaphycus alvarezii sap towards phytohormone signalling and regulating defence responsive genes in Lycopersicon esculentum. J Appl Phycol. 2015;28(4):2529–2537. doi: 10.1007/S10811-015-0784-1. [DOI] [Google Scholar]
- Ahmed DAEA, Gheda SF, Ismail GA. Efficacy of two seaweeds dry mass in bioremediation of heavy metal polluted soil and growth of radish (Raphanus sativus L.) plant. Environ Sci Pollut Res. 2020;28(10):12831–12846. doi: 10.1007/S11356-020-11289-8. [DOI] [PubMed] [Google Scholar]
- Al-Ghamdi AA, Elansary HO. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol Biochem. 2018;129:273–284. doi: 10.1016/J.PLAPHY.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Ali O, Ramsubhag A, Jayaraman J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE. 2019;14:e0216710. doi: 10.1371/JOURNAL.PONE.0216710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali O, Ramsubhag A, Jayaraman J. Phytoelicitor activity of Sargassum vulgare and Acanthophora spicifera extracts and their prospects for use in vegetable crops for sustainable crop production. J Appl Phycol. 2020;33(1):639–651. doi: 10.1007/S10811-020-02309-8. [DOI] [Google Scholar]
- Ali O, Ramsubhag A, Jayaraman J. Transcriptome-wide modulation by Sargassum vulgare and Acanthophora spicifera extracts results in a prime-triggered plant signalling cascade in tomato and sweet pepper. AoB PLANTS. 2022 doi: 10.1093/AOBPLA/PLAC046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar N, Jabnoun-Khiareddine H, Mejdoub-Trabelsi B, et al. Pythium leak control in potato using aqueous and organic extracts from the brown alga Sargassum vulgare (C. Agardh, 1820) Postharvest Biol Technol. 2017;130:81–93. doi: 10.1016/J.POSTHARVBIO.2017.04.010. [DOI] [Google Scholar]
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19(10):535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Aranega-Bou P, Lde la Leyva O, MI Finiti, et al. Priming of plant resistance by natural compounds Hexanoic acid as a model. Front Plant Sci. 2014;5:488. doi: 10.3389/FPLS.2014.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aremu AO, Masondo NA, Rengasamy KRR, et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta. 2015;241:1313–1324. doi: 10.1007/s00425-015-2256x. [DOI] [PubMed] [Google Scholar]
- Arioli T, Mattner SW, Hepworth G, et al. Effect of seaweed extract application on wine grape yield in Australia. J Appl Phycol. 2021;33:1883–1891. doi: 10.1007/S10811-021-02423-1. [DOI] [Google Scholar]
- Ashour M, Hassan SM, Elshobary ME, et al. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum) Plants. 2021;10:1045. doi: 10.3390/PLANTS10061045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani Jafarlou M, Pilehvar B, Modaresi M, Mohammadi M. Seaweed liquid extract as an alternative biostimulant for the amelioration of salt-stress effects in Calotropis procera (Aiton) W.T. J Plant Growth Regul. 2022;2022:1–16. doi: 10.1007/S00344-021-10566-1. [DOI] [Google Scholar]
- Bajpai S, Shukla PS, Asiedu S, et al. A Biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol J. 2019;35:406. doi: 10.5423/PPJ.OA.03.2019.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloch GN, Tariq S, Ehteshamul-Haque S, et al. Management of root diseases of eggplant and watermelon with the application of asafoetida and seaweeds. Researchgatenet. 2013;86:138–142. doi: 10.5073/JABFQ.2013.086.019. [DOI] [Google Scholar]
- Bencze S, Veisz O, Bedo Z. Effects of high atmospheric CO2 and heat stress on phytomass, yield and grain quality of winter wheat. Cereal Res Commun. 2004;32:75–82. doi: 10.1007/BF03543283. [DOI] [Google Scholar]
- Bhanuprakash K, Yogeesha HS. Seed priming for abiotic stress tolerance: an overview. Abiotic Stress Physiol Horticult Crops. 2016;103:117. doi: 10.1007/978-81-322-2725-0_6. [DOI] [Google Scholar]
- Bonomelli C, Celis V, Lombardi G, Mártiz J. Salt stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy. 2018;8:64. doi: 10.3390/AGRONOMY8050064. [DOI] [Google Scholar]
- Bradáčová K, Weber NF, Morad-Talab N, Asim M, Imran M, Weinmann M, Neumann G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem Biol Technol Agricult. 2016;3(1):1–10. doi: 10.1186/S40538-016-0069-1. [DOI] [Google Scholar]
- Briceño-Domínguez D, Hernández-Carmona G, Moyo M, et al. Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J Appl Phycol. 2014;26:2203–2210. doi: 10.1007/s10811-014-0237-2. [DOI] [Google Scholar]
- Caijiao C, Leshan H, Mengke Y, et al. Comparative studies on antioxidant, angiotensin-converting enzyme inhibitory and anticoagulant activities of the methanol extracts from two brown algae (Sargassum horneri and Sargassum thunbergii) Russ J Mar Biol. 2021;47(5):380–387. doi: 10.1134/S1063074021050035. [DOI] [Google Scholar]
- Campobenedetto C, Agliassa C, Mannino G, et al. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.) Agriculture. 2021;11:557. doi: 10.3390/AGRICULTURE11060557. [DOI] [Google Scholar]
- Caradonia F, Battaglia V, Righi L, et al. Plant Biostimulant regulatory framework: prospects in Europe and current situation at international level. J Plant Growth Regul. 2018;38(2):438–448. doi: 10.1007/S00344-018-9853-4. [DOI] [Google Scholar]
- Carmody N, Goñi O, Łangowski Ł, O’Connell S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front Plant Sci. 2020;11:807. doi: 10.3389/FPLS.2020.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MEA, de Camargo PR, Gaziola SA, Azevedo RA. Is seaweed extract an elicitor compound? Changing proline content in drought-stressed bean plants. Comunicata Scientiae. 2018;9(2):292–297. doi: 10.14295/cs.v9i2.2134. [DOI] [Google Scholar]
- Chen D, Huang Y, Shen D, Zhou W, Ao J, Jiang Y, Fahd R. Effects of seaweed extracts on promoting growth and improving stress resistance in sugarcane. Asian Agric Res. 2019;11(1812-2019-3316):69–76. [Google Scholar]
- Chernane H, Latique S, Mansori M, El Kaoua M. Salt stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. J Agric Vet Sci. 2015;8(3):36–44. [Google Scholar]
- Cluzet S, Torregrosa C, Jacquet C, et al. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004;27:917–928. doi: 10.1111/J.1365-3040.2004.01197.x. [DOI] [Google Scholar]
- Colla G, Rouphael Y. Biostimulants in horticulture. Sci Hortic. 2015;196:1–2. doi: 10.1016/J.SCIENTA.2015.10.044. [DOI] [Google Scholar]
- Cook J, Zhang J, Norrie J, et al. Seaweed extract (Stella Maris®) activates innate immune responses in arabidopsis thaliana and protects host against bacterial pathogens. Mar Drugs. 2018;16:2345. doi: 10.3390/MD16070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. 2010;23(3):371–393. doi: 10.1007/S10811-010-9560-4. [DOI] [Google Scholar]
- de Borba MC, de Freitas MB, Stadnik MJ. Ulvan enhances seedling emergence and reduces Fusarium wilt severity in common bean (Phaseolus vulgaris L.) Crop Prot. 2019;118:66–71. doi: 10.1016/J.CROPRO.2018.12.014. [DOI] [Google Scholar]
- De Corato U, Salimbeni R, De Pretis A, et al. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharvest Biol Technol. 2017;131:16–30. doi: 10.1016/J.POSTHARVBIO.2017.04.011. [DOI] [Google Scholar]
- de Sousa AM, Ayub RA, Viencz T, Botelho RV (2019) Fruit set and yield of apple trees cv. Gala treated with seaweed extract of ascophyllum nodosum and thidiazuron. Revista
- Decker D, Kleczkowski LA. UDP-sugar producing pyrophosphorylases: distinct and essential enzymes with overlapping substrate specificities, providing de novo precursors for glycosylation reactions. Front Plant Sci. 2019;9:1822. doi: 10.3389/fpls.2018.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deolu-Ajayi AO, van der Meer IM, van der Werf A, Karlova R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022;45:2537–2553. doi: 10.1111/PCE.14391. [DOI] [PubMed] [Google Scholar]
- dos Santos PLF, Zabotto AR, Jordão HWC, et al. Use of seaweed-based biostimulant (Ascophyllum nodosum) on ornamental sunflower seed germination and seedling growth. Ornam Hortic. 2019;25:231–237. doi: 10.1590/2447-536X.V25I3.2044. [DOI] [Google Scholar]
- Di Filippo-Herrera DA, Muñoz-Ochoa M, Hernández-Herrera RM, Hernández-Carmona G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J Appl Phycol. 2018;31(3):2025–2037. doi: 10.1007/S10811-018-1680-2. [DOI] [Google Scholar]
- Di Mola I, Cozzolino E, Ottaiano L, Giordano M, Rouphael Y, Colla G, Mori M. Effect of vegetal-and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy. 2019;9(10):571. doi: 10.3390/agronomy9100571. [DOI] [Google Scholar]
- do Rosário Rosa V, Farias dos Santos AL, Alves da Silva A, et al. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol Biochem. 2021;158:228–243. doi: 10.1016/J.PLAPHY.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Dookie M, Ali O, Ramsubhag A, Jayaraman J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci Hortic. 2021;276:109715. doi: 10.1016/J.SCIENTA.2020.109715. [DOI] [Google Scholar]
- Dumas B, Jaulneau V, Lafitte C, et al. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J Biomed Biotechnol. 2010 doi: 10.1155/2010/525291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziugieł T, Wadas W. Possibility of increasing early crop potato yield with foliar application ofseaweed extracts and humic acids. J Central Eur Agric. 2020;21:300–310. doi: 10.5513/JCEA01/21.2.2576. [DOI] [Google Scholar]
- El-Ansary MSM, Hamouda RA. Biocontrol of root-knot nematode infected banana plants by some marine algae. Russ J Mar Biol. 2014;40(2):140–146. doi: 10.1134/S1063074014020047. [DOI] [Google Scholar]
- El-Katony TM, Deyab MA, El-Adl MF, et al. Extracts of the brown alga Dictyota dichotoma (Hudson) J. V. Lamouroux alleviate salt stress in rice (Oryza sativa L.) during germination. J Plant Growth Regul. 2021;40:986–999. doi: 10.1007/s00344-020-10156-7. [DOI] [Google Scholar]
- El M, El BM, Barakate M, et al. Ulva lactuca extract and fractions as seed priming agents mitigate salinity stress in tomato seedlings. Plants. 2021;10:1104. doi: 10.3390/PLANTS10061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Modafar C, Elgadda M, El Boutachfaiti R, et al. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci Hortic. 2012;138:55–63. doi: 10.1016/J.SCIENTA.2012.02.011. [DOI] [Google Scholar]
- Elansary HO, Yessoufou K, Abdel-Hamid AME, et al. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front Plant Sci. 2017;8:830. doi: 10.3389/FPLS.2017.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertani A, Francioso O, Tinti A, et al. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. As biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front Plant Sci. 2018;9:428. doi: 10.3389/FPLS.2018.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esserti S, Smaili A, Rifai LA, et al. Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J Appl Phycol. 2016;29(2):1081–1093. doi: 10.1007/S10811-016-0996-Z. [DOI] [Google Scholar]
- Fan D, Hodges DM, Critchley AT, Prithiviraj B. A commercial extract of brown Macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun Soil Sci Plant Anal. 2013;44:1873–1884. doi: 10.1080/00103624.2013.790404. [DOI] [Google Scholar]
- Finnie JF, Van Staden J. Effect of seaweed concentrate and applied hormones on in vitro cultured tomato roots. J Plant Physiol. 1985;120(3):215–222. doi: 10.1016/S0176-1617(85)80108-5. [DOI] [Google Scholar]
- Flora G, Rani SMV. An approach towards control of blast by foliar application of seaweed concentrate. Sci Res Report. 2012;2(3):213–217. [Google Scholar]
- Flores P, Pedreño MA, Almagro L, et al. Increasing nutritional value of broccoli with seaweed extract and trilinolein. J Food Compos Anal. 2021;98:103834. doi: 10.1016/J.JFCA.2021.103834. [DOI] [Google Scholar]
- Fornes F, Sanchez-Perales M, Guardiola JL (2002) Effect of a seaweed extract on the productivity of'de Nules' clementine mandarin and navelina orange
- Ghannam A, Abbas A, Alek H, et al. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga. (hypnea Musciformis) 2013 doi: 10.1016/j.pmpp.2013.07.001. [DOI] [Google Scholar]
- Ghareeb RY, Adss IA, Bayoumi SR, El-Habashy DE. The nematicidal potentiality of some algal extracts and their role in enhancement the tomato defense genes against root knot-nematodes. Egypt J BiolPest Control. 2019;29:1–10. doi: 10.1186/S41938-019-0153-5. [DOI] [Google Scholar]
- Göhre V, Robatzek S. Breaking the barriers: microbial effector molecules subvert plant immunity. Annu Rev Phytopathol. 2008;46:189–215. doi: 10.1146/annurev.phyto46120407110050. [DOI] [PubMed] [Google Scholar]
- Gomathi R, Kohila S, Ramachandiran K. Evaluating the effect of seaweed formulations on the quality and yield of sugarcane. Madras Agric J. 2017;104(march 1–3):1. [Google Scholar]
- Goñi O, Łangowski Ł, Feeney E, et al. Reducing nitrogen input in barley crops while maintaining yields using an engineered biostimulant derived from ascophyllum nodosum to enhance nitrogen use efficiency. Front Plant Sci. 2021 doi: 10.3389/FPLS.2021.664682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-González MF, Ocampo-Alvarez H, Santacruz-Ruvalcaba F, et al. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular Mycorrhizal Fungi and seaweed extract. Front Plant Sci. 2020;11:999. doi: 10.3389/FPLS.2020.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górka B, Wieczorek PP. Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J Chromatogr B. 2017;1057:32–39. doi: 10.1016/J.JCHROMB.2017.04.048. [DOI] [PubMed] [Google Scholar]
- Gruszka D, Janeczko A, Dziurka M, et al. Non-enzymatic antioxidant accumulations in BR-deficient and BR-insensitive barley mutants under control and drought conditions. Physiol Plant. 2018;163:155–169. doi: 10.1111/PPL.12674. [DOI] [PubMed] [Google Scholar]
- Gupta S, Stirk WA, Plačková L, et al. Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (onion) J Plant Physiol. 2021;262:153437. doi: 10.1016/J.JPLPH.2021.153437. [DOI] [PubMed] [Google Scholar]
- Hamouda MM, Khalil S-AM, Gad D. Potential of seaweed extract on growth, physiological, cytological and biochemical parameters of wheat (Triticum aestivum L.) seedlings. J Soil Sci Plant Nutr. 2022;1:1–14. doi: 10.1007/S42729-022-00774-3. [DOI] [Google Scholar]
- Hamouda RA, Hussein MH, El-Naggar NEA, et al. Promoting effect of soluble polysaccharides extracted from Ulva spp. on Zea mays L. growth. Molecules. 2022;27:1394. doi: 10.3390/MOLECULES27041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins SD, Hockey HP (1990) The effect of a liquid seaweed extract from (Fucales, Phaeophyta) on the two-spotted red spider mite. In: Thirteenth international seaweed symposium, pp 555–559. 10.1007/978-94-009-2049-1_80
- Hassan SM, Ashour M, Sakai N, et al. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus) Agriculture. 2021;11:320. doi: 10.3390/AGRICULTURE11040320. [DOI] [Google Scholar]
- Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, et al. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.) J Appl Phycol. 2014;26:619–628. doi: 10.1007/s10811-013-0078-4. [DOI] [Google Scholar]
- Hernández-Herrera RM, Sánchez-Hernández CV, Palmeros-Suárez PA, et al. Seaweed extract improves growth and productivity of tomato plants under salinity stress. Agronomy. 2022;12:2495. doi: 10.3390/AGRONOMY12102495/S1. [DOI] [Google Scholar]
- Huang H, Ullah F, Zhou DX, et al. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. 2019;10:800. doi: 10.3389/FPLS.2019.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MH, Eltanahy E, Al Bakry AF, et al. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. J Appl Phycol. 2021;33(2):1273–1291. doi: 10.1007/S10811-020-02330-x. [DOI] [Google Scholar]
- Islam MT, Gan HM, Ziemann M, et al. Phaeophyceaean (Brown Algal) extracts activate plant defense systems in arabidopsis thaliana challenged with Phytophthora cinnamomi. Front Plant Sci. 2020;11:852. doi: 10.3389/FPLS.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannin L, Arkoun M, Etienne P, et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J Plant Growth Regul. 2012;32(1):31–52. doi: 10.1007/S00344-012-9273-9. [DOI] [Google Scholar]
- Jaulneau V, Lafitte C, Corio-Costet MF, et al. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur J Plant Pathol. 2011;131:393–401. doi: 10.1007/s10658-011-9816-0. [DOI] [Google Scholar]
- Jayaraj J, Wan A, Rahman M, Punja ZK. Seaweed extract reduces foliar fungal diseases on carrot. Crop Prot. 2008;27:1360–1366. doi: 10.1016/J.CROPRO.2008.05.005. [DOI] [Google Scholar]
- Jiménez-Escrig A, Cambrodón IG. Nutritional evaluation and physiological effects of edible seaweeds. Arch Latinoam Nutr. 1999;49:114–120. [PubMed] [Google Scholar]
- Jithesh MN, Shukla PS, Kant P, et al. Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J Plant Growth Regul. 2018;38(2):463–478. doi: 10.1007/S00344-018-9861-4. [DOI] [Google Scholar]
- Karthik T, Sarkar G, Babu S, et al. Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva reticulata. Biocatal Agric Biotechnol. 2020;28:101712. doi: 10.1016/J.BCAB.2020.101712. [DOI] [Google Scholar]
- Kauffman GL, Kneivel DP, Watschke TL. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007;47:261–267. doi: 10.2135/CROPSCI2006.03.0171. [DOI] [Google Scholar]
- Kim SK, editor. Handbook of marine macroalgae: biotechnology and applied phycology. New York: Wiley; 2011. [Google Scholar]
- Khan W, Rayirath UP, Subramanian S, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul. 2009;28(4):386–399. doi: 10.1007/S00344-009-9103-x. [DOI] [Google Scholar]
- Kocira S, Szparaga A, Findura P, Treder K. Modification of yield and fiber fractions biosynthesis in Phaseolus vulgaris L. by treatment with biostimulants containing amino acids and seaweed extract. Agronomy. 2020;10:1338. doi: 10.3390/AGRONOMY10091338. [DOI] [Google Scholar]
- Kumar NA, Vanlalzarzova B, Sridhar S, Baluswami M. Effect of liquid seaweed fertilizer of Sargassum wightii Grev on the growth and biochemical content of green gram (Vigna radiata (L.) R. Wilczek) Recent Res Sci Technol. 2012;4(4):40–45. [Google Scholar]
- Kumar R, Trivedi K, Anand KGV, et al. Science behind biostimulant action of seaweed extract on growth and crop yield: insights into transcriptional changes in roots of maize treated with Kappaphycus alvarezii seaweed extract under soil moisture stressed conditions. J Appl Phycol. 2020;32:599–613. doi: 10.1007/s10811-019-01938-y. [DOI] [Google Scholar]
- Kumari J, Haque MI, Jha RK, Rathore MS. The red seaweed Kappaphycus alvarezii antiporter gene (KaNa+/H+) confers abiotic stress tolerance in transgenic tobacco. Mol Biol Rep. 2022;2022:1–15. doi: 10.1007/S11033-022-07213-7. [DOI] [PubMed] [Google Scholar]
- Latef AAHA, Zaid A, Alwaleed EA. Influences of priming on selected physiological attributes and protein pattern responses of salinized wheat with extracts of Hormophysa cuneiformis and Actinotrichia fragilis. Agronomy. 2021;11:545. doi: 10.3390/AGRONOMY11030545. [DOI] [Google Scholar]
- Liu H, Chen X, Song L, et al. Polysaccharides from Grateloupia filicina enhance tolerance of rice seeds (Oryza sativa L.) under salt stress. Int J Biol Macromol. 2019;124:1197–1204. doi: 10.1016/J.IJBIOMAC.2018.11.270. [DOI] [PubMed] [Google Scholar]
- Lola-Luz T, Hennequart F, Gaffney M. Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleraceae) following application of commercial seaweed extracts of the brown seaweed, (Ascophyllum nodosum) Agric Food Sci. 2013;22:288–295. doi: 10.23986/AFSCI.7676. [DOI] [Google Scholar]
- Machado LP, Matsumoto ST, Jamal CM, et al. Chemical analysis and toxicity of seaweed extracts with inhibitory activity against tropical fruit anthracnose fungi. J Sci Food Agric. 2014;94:1739–1744. doi: 10.1002/JSFA.6483. [DOI] [PubMed] [Google Scholar]
- Mattner SW, Villalta ON, Wite D, et al. In vitro suppression of Sclerotinia minor by a seaweed extract from Durvillaea potatorum and Ascophyllum nodosum. Aust Plant Dis Notes. 2014;9:1–5. doi: 10.1007/S13314-014-0137-y. [DOI] [Google Scholar]
- Mattner SW, Milinkovic M, Arioli T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J Appl Phycol. 2018;30:2943–2951. doi: 10.1007/S10811-017-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak I, Górka B, Wieczorek PP, et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting. Plant Growth. 2016 doi: 10.1080/09670262.2015.1134813. [DOI] [Google Scholar]
- Miedes E, Vanholme R, Boerjan W, Molina A. The role of the secondary cell wall in plant resistance to pathogens. Front Plant Sci. 2014;5:358. doi: 10.3389/FPLS.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniswami DM, Buvaneshwari K, Fathima Rosa Mystica L, et al. Comparative assessment of different biofertilizers in maize (Zea mays L.) cultivation. Biomass Conv Bioref. 2021 doi: 10.1007/s13399-021-01543-5. [DOI] [Google Scholar]
- Muthu-Pandian Chanthini K, Senthil-Nathan S, Stanley-Raja V, et al. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill) Biocatal Agric Biotechnol. 2019;20:101190. doi: 10.1016/J.BCAB.2019.101190. [DOI] [Google Scholar]
- Mzibra A, Aasfar A, Khouloud M, et al. Improving growth, yield, and quality of tomato plants (Solanum lycopersicum L) by the application of moroccan seaweed-based biostimulants under greenhouse conditions. Agronomy. 2021;11:1373. doi: 10.3390/AGRONOMY11071373. [DOI] [Google Scholar]
- Nair P, Kandasamy S, Zhang J, et al. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012;13:1–23. doi: 10.1186/1471-2164-13-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephali L, Piater LA, Dubery IA, et al. Biostimulants for plant growth and mitigation of abiotic stresses: a metabolomics perspective. Metabolites. 2020;10:1–26. doi: 10.3390/METABO10120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto APDA, Oliveira GRF, da Mello SC, et al. Seed priming with seaweed extract mitigate heat stress in spinach: effect on germination, seedling growth and antioxidant capacity. Bragantia. 2020;79:502–511. doi: 10.1590/1678-4499.20200127. [DOI] [Google Scholar]
- Ngoroyemoto N, Kulkarni MG, Stirk WA, Gupta S, Finnie JF, van Staden J. Interactions between microorganisms and a seaweed-derived biostimulant on the growth and biochemical composition of Amaranthus hybridus L. Nat Product Commun. 2020;15(7):1934578. [Google Scholar]
- Noli ZA, Suwirmen A, Aliyyanti P. Effect of liquid seaweed extracts as biostimulant on vegetative growth of soybean. IOP Conf Ser Earth Environ Sci. 2021;759:012029. doi: 10.1088/1755-1315/759/1/012029. [DOI] [Google Scholar]
- Ozbay N, Demirkiran AR. Enhancement of growth in ornamental pepper (Capsicum annuum L.) plants with application of a commercial seaweed product, stimplex®. Appl Ecol Environ Res. 2019;17:4361–4375. doi: 10.15666/aeer/1702_43614375. [DOI] [Google Scholar]
- Patel K, Agarwal P, Agarwal PK. Kappaphycus alvarezii sap mitigates abiotic-induced stress in Triticum durum by modulating metabolic coordination and improves growth and yield. J Appl Phycol. 2018;30:2659–2673. doi: 10.1007/s10811-018-1423-4. [DOI] [Google Scholar]
- Parmar N, Singh KH, Sharma D, Singh L, Kumar P, Nanjundan J, Khan YJ, Chauhan DK, Thakur AK. Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3 Biotech. 2017;7(4):239. doi: 10.1007/s13205-017-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Correia F (2015) Introdução. Macroalgas Marinhas da Costa Portuguesa: Biodiversidade, ecologia e utilizações 342
- Prakash P, Mitra A, Nag R, Sunkar S. Effect of seaweed liquid fertilizer and humic acid formulation on the growth and nutritional quality of Abelmoschus esculentus. Asian J Crop Sci. 2018;10:48–52. doi: 10.3923/AJCS.2018.48.52. [DOI] [Google Scholar]
- Pramanick B, Brahmachari K, Mahapatra BS, et al. Growth, yield and quality improvement of potato tubers through the application of seaweed sap derived from the marine alga Kappaphycus alvarezii. J Appl Phycol. 2017;29(6):3253–3260. doi: 10.1007/S10811-017-1189-0. [DOI] [Google Scholar]
- Pršić J, Ongena M. Elicitors of plant immunity triggered by beneficial bacteria. Front Plant Sci. 2020;11:1675. doi: 10.3389/FPLS.2020.594530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Jagmohan S, Jayaraj P, et al. Effects of Ascophyllum nodosum extract on sweet pepper plants as an organic biostimulant in grow box home garden conditions. J Appl Phycol. 2021;34(1):647–657. doi: 10.1007/S10811-021-02611-Z. [DOI] [Google Scholar]
- Ramkissoon A, Ramsubhag A, Jayaraman J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J Appl Phycol. 2017;29(6):3235–3244. doi: 10.1007/S10811-017-11600. [DOI] [Google Scholar]
- Rayirath P, Benkel B, Mark Hodges D, et al (2009) Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana [DOI] [PubMed]
- Rayorath P, Jithesh MN, Farid A, et al. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J Appl Phycol. 2008;20:423–429. doi: 10.1007/s10811-007-9280-6. [DOI] [Google Scholar]
- Rengasamy KRR, Kulkarni MG, Stirk WA, Van Staden J. Eckol—a new plant growth stimulant from the brown seaweed Ecklonia maxima. J Appl Phycol. 2015;27:581–587. doi: 10.1007/S10811-014-0337-Z. [DOI] [Google Scholar]
- Rengasamy KRR, Kulkarni MG, Pendota SC, Van Staden J. Enhancing growth, phytochemical constituents and aphid resistance capacity in cabbage with foliar application of eckol—a biologically active phenolic molecule from brown seaweed. New Biotechnol. 2016;33:273–279. doi: 10.1016/J.NBT.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Rouphael Y, De Micco V, Arena C, et al. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J Appl Phycol. 2016;29(1):459–470. doi: 10.1007/S10811-016-0937-x. [DOI] [Google Scholar]
- Sahana BN, PrasannaKumar MK, Mahesh HB, et al. Biostimulants derived from red seaweed stimulate the plant defence mechanism in rice against Magnaporthe oryzae. J Appl Phycol. 2021;34(1):659–665. doi: 10.1007/S10811-021-02627-5. [DOI] [Google Scholar]
- Sahayaraj K, Kalidas S (2011) Evaluation of nymphicidal and ovicidal effect of a seaweed, Padina pavonica (Linn.)(Phaeophyceae) on cotton pest, Dysdercus cingulatus (Fab.)
- Sahayaraj K, Mary Jeeva Y. Nymphicidal and ovipositional efficacy of seaweed Sargassum tenerrimum (J. Agardh) against Dysdercus cingulatus (Fab.) (Pyrrhocoridae) Chilean J Agric Res. 2012;72:152–156. doi: 10.4067/S0718-58392012000100024. [DOI] [Google Scholar]
- Sangha JS, Kandasamy S, Khan W, et al. λ-carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar Drugs. 2015;13:2875–2889. doi: 10.3390/MD13052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha JS, Khan W, Ji X, et al. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana Resistance to Trichoplusia ni (Cabbage Looper) PLoS ONE. 2011;6:e26834. doi: 10.1371/JOURNAL.PONE.0026834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaniello A, Scartazza A, Gresta F, et al. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front Plant Sci. 2017;8:1362. doi: 10.3389/FPLS.2017.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbaihat L, Takeyama K, Koga T, et al. Induced resistance in solanum lycopersicum by algal elicitor extracted from sargassum fusiforme. Sci World J. 2015 doi: 10.1155/2015/870520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MT, Zodape ST, Chaudhary DR, Eswaran K, Chikara J. Seaweed sap as an alternative liquid fertilizer for yield and quality improvement of wheat. J Plant Nutr. 2013;36(2):192–200. doi: 10.1080/01904167.2012.737886. [DOI] [Google Scholar]
- Shahriari AG, Mohkami A, Niazi A, et al. Application of brown algae (Sargassum angustifolium) Extract for improvement of drought tolerance in Canola (Brassica napus L.) Iran J Biotechnol. 2021;19:e2775. doi: 10.30498/IJB.2021.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamya Arokia Rajan M, Thriunavukkarasu R, Joseph J, Aruni W. Effect of seaweed on seed germination and biochemical constituents of Capsicum annuum. Biocatal Agric Biotechnol. 2020;29:101761. doi: 10.1016/J.BCAB.2020.101761. [DOI] [Google Scholar]
- Sharma HSS, Fleming C, Selby C, et al. Plant biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol. 2013;26(1):465–490. doi: 10.1007/S10811-013-0101-9. [DOI] [Google Scholar]
- Sharma S, Chen C, Khatri K, et al. Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol Biochem. 2019;136:143–154. doi: 10.1016/J.PLAPHY.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Shukla PS, Borza T, Critchley AT, Prithiviraj B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front Mar Sci. 2016;3:81. doi: 10.3389/FMARS.2016.00081. [DOI] [Google Scholar]
- Shukla PS, Shotton K, Norman E, et al. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB PLANTS. 2018 doi: 10.1093/AOBPLA/PLX051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla PS, Mantin EG, Adil M, et al. Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci. 2019;10:655. doi: 10.3389/FPLS.2019.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Chaudhary B. Preliminary phycochemical analysis and in vitro antibacterial screening of Pithophora oedogonia (Mont.) Wittrock: a freshwater green alga forming mats in the water bodies. J Algal Biomass Utiliz. 2010;1:33–41. [Google Scholar]
- Singh S, Singh MK, Pal SK, et al. Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J Appl Phycol. 2015;28(3):2099–2112. doi: 10.1007/S10811-015-0680-8. [DOI] [Google Scholar]
- Soliman AS, Ahmed AY, Abdel-Ghafour SE, El-Sheekh MM, Sobhy HM. Antifungal bio-efficacy of the red algae Gracilaria confervoides extracts against three pathogenic fungi of cucumber plant. Middle East J Appl Sci. 2018;8(3):727–735. [Google Scholar]
- Stamatiadis S, Evangelou E, Jamois F, Yvin JC. Targeting Ascophyllum nodosum (L.) Le Jol. extract application at five growth stages of winter wheat. J Appl Phycol. 2021;33(3):1873–1882. doi: 10.1007/S10811-021-02417-Z. [DOI] [Google Scholar]
- Subramanian S, Sangha JS, Gray BA, et al. Extracts of the marine brown macroalga, Ascophyllum nodosum, induce jasmonic acid dependent systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Sclerotinia sclerotiorum. Eur J Plant Pathol. 2011;131(2):237–248. doi: 10.1007/S10658-011-9802-6. [DOI] [Google Scholar]
- Sujata GV, Baliyan V, et al. Alleviating drought stress in Brassica juncea (L.) Czern & Coss. by foliar application of biostimulants—orthosilicic acid and seaweed extract. Appl Biochem Biotechnol. 2022;2022:1–29. doi: 10.1007/S12010-022-04085-2. [DOI] [PubMed] [Google Scholar]
- Tandon S, Dubey A. Effects of Biozyme (Ascophyllum nodosum) biostimulant on growth and development of soybean [Glycine max (L. Merill] Commun Soil Sci Plant Anal. 2015;46(7):845–858. doi: 10.1080/00103624.2015.1011749. [DOI] [Google Scholar]
- Trivedi K, Vijay Anand KG, Kubavat D, et al. Crop stage selection is vital to elicit optimal response of maize to seaweed bio-stimulant application. J Appl Phycol. 2017;29(4):2135–2144. doi: 10.1007/S10811-017-1118-2. [DOI] [Google Scholar]
- Trivedi K, Vijay Anand KG, Vaghela P, Ghosh A. Differential growth, yield and biochemical responses of maize to the exogenous application of Kappaphycus alvarezii seaweed extract, at grain-filling stage under normal and drought conditions. Algal Res. 2018;35:236–244. doi: 10.1016/J.ALGAL.2018.08.027. [DOI] [Google Scholar]
- Uthirapandi V, Suriya S, Boomibalagan P, Eswaran S, Ramya SS, Vijayanand N, Kathiresan D. Biofertilizing potential of seaweed liquid extracts of marine macro algae on growth and biochemical parameters of Ocimum sanctum. J Pharmacogn Phytochem. 2018;7(3):3528–3532. [Google Scholar]
- Vera J, Castro J, Gonzalez A, Moenne A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar Drugs. 2011;9:2514–2525. doi: 10.3390/MD9122514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N, Sehrawat KD, Mundlia P, et al. Potential use of Ascophyllum nodosum as a biostimulant for improving the growth performance of Vigna aconitifolia (Jacq) Marechal. Plants (basel, Switzerland) 2021 doi: 10.3390/PLANTS10112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinoth S, Sundari GP, et al. Evaluation of seagrass liquid extract on salt stress alleviation in tomato plants. Asian J Plant Sci. 2017;16:172–183. doi: 10.3923/AJPS.2017.172.183. [DOI] [Google Scholar]
- Voko MP, Kulkarni MG, Ngoroyemoto N, et al. Vermicompost leachate, seaweed extract and smoke-water alleviate drought stress in cowpea by influencing phytochemicals, compatible solutes and photosynthetic pigments. Plant Growth Regul. 2022;97(2):327–342. doi: 10.1007/S10725-022-00815-Y. [DOI] [Google Scholar]
- Wei TL, Wang ZX, He YF, et al. Proline synthesis and catabolism-related genes synergistically regulate proline accumulation in response to abiotic stresses in grapevines. Sci Hortic. 2022;305:111373. doi: 10.1016/J.SCIENTA.2022.111373. [DOI] [Google Scholar]
- Wite D, Mattner SW, Porter IJ, Arioli T. The suppressive effect of a commercial extract from Durvillaea potatorum and Ascophyllum nodosum on infection of broccoli by Plasmodiophora brassicae. J Appl Phycol. 2015;27:2157–2161. doi: 10.1007/S10811-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Jenkins T, Blunden G, et al. Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J Appl Phycol. 1998;10(1):91–94. doi: 10.1023/A:1008067420092. [DOI] [Google Scholar]
- Xu C, Leskovar DI. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci Hortic. 2015;183:39–47. doi: 10.1016/J.SCIENTA.2014.12.004. [DOI] [Google Scholar]
- Xu Y, Zheng X, Song Y, et al. NtLTP4, a lipid transfer protein that enhances salt and drought stresses tolerance in Nicotiana tabacum. Sci Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-27274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav DS, Rai R, Mishra AK, et al. ROS production and its detoxification in early and late sown cultivars of wheat under future O3 concentration. Sci Total Environ. 2019;659:200–210. doi: 10.1016/J.SCITOTENV.2018.12.352. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wang X, Chen B, Zhang M, Ma J. Seaweed extract improved yields, leaf photosynthesis, ripening time, and net returns of tomato (Solanum lycopersicum Mill.) ACS Omega. 2020;5(8):4242–4249. doi: 10.1021/acsomega.9b04155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf R, Kristianse P, Warwick N. Effect of two seaweed products and equivalent mineral treatments on lettuce (Lactuca sativa L.) growth. J Agron. 2019;18:100–106. doi: 10.3923/JA.2019.100.106. [DOI] [Google Scholar]
- Zou P, Lu X, Zhao H, et al. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front Plant Sci. 2019;10:48. doi: 10.3389/FPLS.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Not applicable.