Abstract
Cancer of unknown primary (CUP) is a well-studied entity with guidelines available for the management of patients with CUP. The peritoneum represents one of the metastatic sites in CUP, and peritoneal metastases (PM) could present as CUP. PM of unknown origin remains a poorly studied clinical entity. There is only one series of 15 cases, one population-based study, and few other case reports on this subject. Studies on CUP, in general, cover some common tumour histological types like adenocarcinomas and squamous carcinomas. Some of these tumours may have a good prognosis though majority have high-grade disease with a poor long-term outcome. Some of the histological tumour types commonly seen in the clinical scenario of PM like mucinous carcinoma have not been studied. In this review, we divide PM into five histological types—adenocarcinomas, serous carcinomas, mucinous carcinomas, sarcomas and other rare varieties. We provide algorithms to identify the primary tumour site using immunohistochemistry when imaging, and endoscopy fails to establish the primary tumour site. The role of molecular diagnostic tests for PM or unknown origin is also discussed. Current literature on site-specific systemic therapy based on gene expression profiling does not show a clear benefit of this approach over empirical systemic therapies.
Keywords: Peritoneal metastases, Cancer of unknown primary (CUP), Pathology, Immunohistochemistry, Molecular diagnosis
Introduction
Definitive surgical treatment for peritoneal metastases was introduced approximately 40 years ago [1]. The combination of cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC) significantly prolongs the survival in selected patients compared to systemic chemotherapy alone and is curative for some of these patients with peritoneal metastases (PM) [2]. The indications for surgical treatment depend on the primary tumour site, disease extent, response to systemic chemotherapy and the patient’s ability to withstand the surgical procedure [3]. PM may present synchronously or metachronously. In a large majority of the patients with isolated PM, diagnostic tests are very sensitive and specific for diagnosing/detecting the primary tumour. If diagnostic tests that include serum tumour markers, cross section imaging, functional imaging and endoscopy fail to reveal a primary site in a patient with PM, a biopsy with histopathological evaluation and immunohistochemistry (IHC) will reveal the primary. Nevertheless, in some rare cases, the primary remains unknown despite an extensive and exhaustive work-up.
Sugarbaker first reported the benefit of performing cytoreductive surgery and HIPEC in 15 patients with PM with undetermined primary site [4]. In this series, there were 6 patients with a poorly differentiated adenocarcinoma, 4 with adenocarcinoma and 4 with mucinous adenocarcinoma. In the last 20 years, cross-sectional imaging has improved, and it has been demonstrated that most cases of mucinous carcinoma peritonei or pseudomyxoma peritonei arise from a primary appendiceal mucinous neoplasm which may not be visible if small or in the presence of extensive peritoneal disease [5]. In a report from the Eidhoven cancer registry, 9643 patients were diagnosed with cancer of unknown primary (CUP) from 1984 to 2011. One thousand fifty-one (11%) of these patients were diagnosed with PM, of whom 606 (58%) had PM as the only metastatic site and 445 (42%) patients also had other metastases [6]. The median survival of patients with PM of unknown origin was 42 days. Few patients (4%) received surgical treatment according to the updated results of this study [7].
Broadly, PM could be divided into the following histological types—adenocarcinomas, serous carcinomas, mucinous carcinomas, mesotheliomas, sarcomas and other rare tumours. Either it is a common histology with an occult primary or an uncommon histology that needs to be accurately diagnosed. Molecular testing is required to establish the diagnosis of some very rare peritoneal tumour with known underlying genetic mutations [8]. In addition, molecular tests have been used to reveal the primary in CUP [9]. Studies have shown that CUP in general has a poorer prognosis compared to other tumours and a unique molecular profile too [9]. In this manuscript, we provide a diagnostic approach to patients with PM of unknown origin with a focus of histopathological evaluation. The role of molecular diagnostic tests in this setting is also reviewed and elaborated.
Definition
Metastatic cancer of unknown primary (CUP) is defined as histologically confirmed malignancy, for which no primary site is found despite an extensive diagnostic work-up [10]. Similarly, peritoneal metastases may occur in the absence of a known, identified primary malignancy.
Pathogenesis
There are two theories explaining the development of CUP. The first one hypothesised that CUP does not undergo type 1 progression ( that is from a premalignant lesion to a malignant one) but instead it follows a type 2 progression without forming a primary site. The second hypothesised that CUP follows the parallel progression model, where metastases can arise early in the development of a malignant process [11, 12].
There are some histological types that have a favourable outcome with treatment and are considered to be tumours with a good prognosis like primary peritoneal serous carcinoma, and there are others that have a poor prognosis like adenocarcinoma having the marker profile of colonic origin [13].
Preliminary Work-up
A detailed clinical history and physical examination are an essential part of the diagnostic work-up. Age itself no longer precludes the occurrence of common cancers like colorectal, ovarian or stomach cancer. Rare tumours like germ cell tumours and desmoplastic small round cell tumors (DSRCTs) are present in the younger age group [14]. Serous carcinomas are a rare entity in males [15]. A history of a hysterectomy performed several years ago precludes the development of PM from endometrial stromal sarcomas. Morcellation of fibroids similarly is known to give rise to peritoneal leiomyomatosis and leiomyosarcomas [16]. In rare situations, even when there is a history of pervious malignancy, PM may not be secondary but due to a primary tumour arising from the peritoneum.
There are very few specific clinical findings in patients with PM that point towards the primary tumour site. For e.g., a urachal primary could present with mucin discharge in urine [17]. The finding of PM may be incidental during investigations performed for non-specific symptoms or the presentation may be of advanced disease with ascites and its ensuing problems. Some tumours like peritoneal leimyomatosis do not form ascites. In most other histologies, there could be ascites with debilitation. It is not uncommon for patients with PM to present with bilateral ovarian masses that represent an ovarian primary or are secondary to another malignancy. The features of an ovarian primary and metastases to the ovary may or may not been distinguished on imaging. Mucinous carcinomas often present with ovarian masses and the clinical picture of PMP. The primary may be too small to be detected on imaging, and IHC is necessary to establish the origin.
Upper and lower gastrointestinal endoscopy and whole body imaging or functional imaging should be performed to complete the work-up and rule out extra-peritoneal metastases. There is no pattern of peritoneal distribution that can point towards a particular diagnosis. Location of lymph nodes may help in the identification of the primary site. The alteration in the tumour marker levels could give some clue about the primary site but are seldom diagnostic. Germ cell markers are diagnostic but may not be elevated in dedifferentiated tumours that could be the underlying cause for PM of unknown origin.
The general condition may be well preserved in many patients even in the presence of extensive disease.
Histopathological Evaluation
Pathological evaluation of biopsy specimens or surgical specimens is the gold standard for establishing the diagnosis. IHC is a vital component of routine pathological evaluation. Molecular tests may be performed to confirm the diagnosis of rare tumours with known genetic alterations or to determine the diagnosis in cases where the histopathological evaluation is inconclusive and are discussed later in this manuscript.
It must be borne in mind that the pathological evaluation should not be performed in isolation, but keeping in mind the clinical history and other clinical findings. The other challenge is that in most instances, the diagnosis has to be made on a tissue sample that has been obtained by performing a transabdominal or laparoscopic biopsy and may be inadequate. Good co-ordination between the surgeons and pathologist is essential. Laparoscopic biopsy where possible is better as it allows better sampling. The morphology of the peritoneal deposits and disease extent can also be evaluated. Fluid samples could be paucicellular and non-representative of the tumour, and this histopathological evaluation is best performed on biopsy specimens. Moreover, appendix can be explored and removed, as it can be the primary site in mucinous PM. An ovarian mass, if small could also be resected to establish the diagnosis.
In this manuscript, we have broadly divided the tumours into 5 groups—adenocarcinomas, serous carcinomas, mucinous carcinomas, sarcomas and uncommon histologies. These groups are not mutually exclusive. This is just for arriving at a diagnosis. Serous carcinomas are a type of adenocarcinomas but since the peritoneum is a common site of spread and origin, both and they also constitute tumours that are commonly treated with surgery, we have described them separately from the adenocarcinomas.
Adenocarcinomas
Metastatic adenocarcinoma is perhaps, the most common histological diagnosis in PM. Though the exact incidence is not known, in majority of the cases, the underlying primary is in the colorectum, stomach and ovaries. In male patients, the commonest differentials would be colorectal, gastric and pancreaticobiliary primaries, whereas in females, it would be ovarian cancer, colorectal and gastric cancer [18]. Other less common primaries presenting with isolated PM are endometrial adenocarcinoma, small bowel adenocarcinoma, cervical adenocarcinoma, pancreaticobiliary adenocarcinomas and metastatic breast carcinoma [18]. In majority of the cases, the primary site is evident on imaging. Another diagnosis that should be kept in mind is diffuse malignant peritoneal mesothelioma (DMPM). It is not uncommon for less experienced pathologists to misdiagnose a mesothelioma as an adenocarcinoma. Very high-grade tumours could also be confused with an adenocarcinoma.
Histopathological Findings
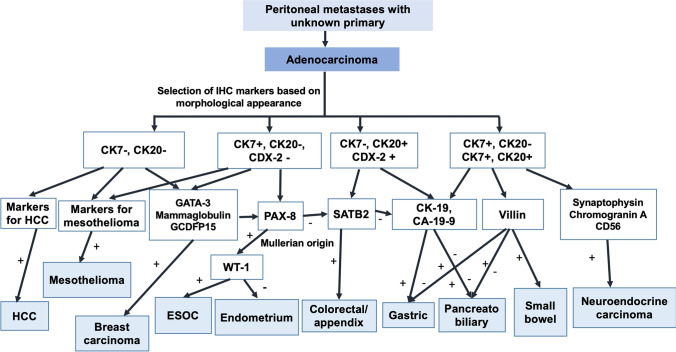
Histologically, it may not always be possible to distinguish between a gastric and colorectal cancer (CRC) or these tumors from other primaries. When the primary tumour is not seen on imaging or endoscopy, IHC should be performed to confirm the origin. The algorithm of using IHC to determine the primary in patients with adenocarcinoma is provided in Fig. 1.
Fig. 1.
Algorithm to determine the primary site in peritoneal metastases of unknown origin with the pathological diagnosis of adenocarcinoma. Abbreviations: HCC, hepatocellular carcinoma; ESOC, epithelial serous ovarian carcinoma
Another common differential diagnosis is endometrioid adenocarcinoma of the ovary which constitutes 10% of epithelial ovarian carcinoma (EOC). Ovarian endometrioid carcinomas are usually positive for CK7, oestrogen receptor (ER), CA125 and PAX8 and negative for CK20, CEA and CDX2 while the converse immunophenotype is seen in metastatic colorectal adenocarcinomas [19–21]. There are two specific markers for ovarian cancer that should be considered to establish the diagnosis of an ovarian primary—PAX-8 and WT-1. Paired-box 8 (PAX8) that is a sensitive marker for tumours of the thyroid, kidney and thymus and tumours derived from the Müllerian ducts. It is expressed by nearly 95% of EOCs [22, 23]. Uncommonly, some high-grade tumours may not express PAX-8, and it may be difficult to differentiate at times, an endometrioid adenocarcinoma from other ovarian tumours. WTI is a useful marker for distinguishing endometrioid adenocarcinoma from the other more common serous subtype [24]. In the normal mature ovary, WT1 is expressed in the ovarian surface epithelium and in stromal and granulosa cells [25]. In the tumour-bearing ovary, WT1 is characteristic of the serous subtype being rarely found in the others [25].
WT1 can, thus, be useful in the differential diagnosis of primary serous ovarian tumours with non-specific morphological features. It also helps to exclude other primary tumours of uterine, breast, pancreaticobiliary or gastrointestinal origin, exhibiting similar morphologic phenotype [25, 26]. The endometrioid variety rarely expresses WTI and has a heterogeneous WT1 expression. WT1 positivity implies that the tumour is either arising from the ovary or fallopian tube or peritoneum, while WT1 negativity indicates an ovarian tumour with origin in endometriosis foci [25]. WT1 positivity is also a marker for DMPM. Though an experienced pathologist will diagnose a DMPM on morphology alone and confirm the diagnosis with appropriate IHC markers, very high-grade tumours can cause confusion, and appropriate IHC markers should be used to rule out a DMPM as discussed in the next section on serous carcinomas.
CDX2 is used to establish a colorectal/lower gastrointestinal origin.
CRC needs to be distinguished not just from ovarian primaries but also from gastric and pancreaticobiliary tumours which may not always be clear on morphology alone. The typical IHC profile of CRC is an expression of CK20 and CDX2 and lack of expression of CK7 [27]. However, CDX2 and CK20 have been shown to be positive in up to 21% each of gastric cancers and 14% and 21% of ovarian mucinous adenocarcinomas, respectively. CK7 expression is seen in up to 50% of the gastric and mucinous ovarian carcinomas. This combination of CK20 and CDX2, in one study, was more helpful in differentiating colorectal from pancreatic adenocarcinoma, which was only 2% CDX2 positive, 15% CK20 positive and predominantly CK7 positive (94%), and with only 3% of colorectal adenocarcinoma being CK7 positive [27]. Pancreatic tumours also express CEA and CA-19–9.
Another confirmatory marker for CRC is SATB2 [28]. SATB2 is expressed in the epithelium of the lower gastrointestinal tract and is seen in only a few malignancies including colorectal/appendiceal adenocarcinomas, tumours of osteoblastic differentiation and renal/urothelial carcinomas [29]. SATB2 as a solitary marker is reported to have a sensitivity of 93% and specificity of 77% but when combined with CK20 and CK7 expression, the sensitivity becomes 83% and specificity 100% [30, 31]. The main application of SATB2 is to distinguish adenocarcinomas of colorectal origin from those of gastric and pancreatic origin in which the expression is low [32, 33]. The expression of this marker is low even in lung and gynaecological adenocarcinomas which form the other differential diagnosis [34]. Pancreatic ductal carcinomas are also positive for CK8, CK17, CK18, CK19, CEA, CA19-9, Dupan-2, MUC1, MUC4 and MUC5AC [35–38].
Distinction from Breast Carcinomas
Breast carcinoma can be a rare differential diagnosis of adenocarcinoma of the peritoneum. Invasive ductal and lobular carcinomas are morphologically different from gastrointestinal and ovarian primaries. PAX-8 and CA-125 are positive in endometrioid carcinomas and negative in breast cancer though CA-125 could be positive [39, 40]. Markers useful but not specific for breast cancer are GCDFP15, mammoglobin and GATA3 (usually negative in endometrioid carcinomas and positive in breast carcinomas) [41, 42]. A proportion of endometrioid adenocarcinomas may be mammoglobin positive [42].
Other Uncommon Primary Sites
In case of suspicion, TTF-1 can be used to rule out lung cancer. A high-grade neuroendocrine carcinoma (NEC) can give the appearance of an adenocarcinoma and can be ruled out using chromogranin A, synaptophysin and the Ki-67 proliferation index [43]. A NEC must be ruled out in poorly differentiated adenocarcinomas and poorly differentiated carcinomas. The other marker that is positive in all neuroendocrine neoplasms (NENs) is CD56 [44]. PM are usually part of widespread disease in these patients and are seen in over 15% of the patients [45]. The 2019 WHO classification divides NENs originating from different organs in the body into well-differentiated neuroendocrine tumours (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) in all sites where these neoplasms arise [46]. Though the diagnosis is made on histopathology in most cases, this division is based on their molecular differences. Mutations in MEN1, DAXX and ATRX are entity-defining for well-differentiated NETs, whereas NECs usually have TP53 or RB1 mutations. In some cases, these mutations can be of diagnostic benefit.
NETs arising from the distal small bowel have a greater propensity for producing PM and lymph node metastases [47]. Some peculiar features of PM arising from these tumours are the small size of deposits (< 5 mm) and mesenteric deposits along the blood vessels [48–51].
Carcinoids from the foregut and midgut are generally positive for chromogranin A and CD56, while those from the hindgut are usually negative [52–54]. Hindgut carcinoids on the other hand often express prostatic acid phosphatase [55]. A less helpful marker is CDX-2, which although positive for most CRC has an immunoreactivity of about 40% in well-differentiated carcinoids but has a reported 80% expression rate in poorly differentiated carcinoids [53, 56–58].
Hepatocellular carcinoma and small bowel adenocarcinoma are other rare differential diagnosis. Small bowel tumours constitute 1–3% of all the gastrointestinal malignancies [59, 60]. Adenocarcinomas constitute 30–45% of all small bowel tumours [61, 62]. Small bowel adenocarcinoma (SBA) is known to have a poor prognosis with a median overall survival ranging from 12 to 20 months [63, 64]. Half of these tumours are CK7 positive, unlike normal small intestinal mucosa which is CK7 negative and colorectal adenocarcinomas which are CK7 negative and CK20 positive [65]. SBA is also positive for CK20, CDX-2 and villin [65].
The markers specific for hepatocellular carcinoma (HCC) are Glyican-3, CD34, AFP, CD 10, CEA and HepPar-1 [66]. HCC includes variant-fibrolamellar HCC and intrahepatic cholangiocarcinomas. These tumours express only a limited number of keratin markers, namely CK8 and CK18 and thus most metastatic carcinomas can be excluded as they generally express a larger variety of keratin markers such as CK5/6, CK7, CK14 or CK20 in comparison to HCC [67].
Many times the marker profiles overlap or do not give a clear pointer towards the primary. It is important to correlate the histology findings with the IHC and not draw inference from only one of the two tests.
Serous Carcinomas
Serous carcinomas are the commonest variety of EOCs that have a predilection for peritoneal spread. Often the ovarian primaries are small in size and even inconspicuous. It has been shown that majority of the serous carcinomas arise from the fallopian tubes. The other less common sites of origin are the endometrium, cervix and the peritoneum itself [15]. The other differentials of a serous histology are peritoneal mesothelioma and breast cancer.
Histopathological Findings
The histological features of high-grade serous carcinomas are diagnostic and consist of branching papillary fronds, slit-like fenestrations, glandular complexity, moderate to marked nuclear atypia with marked pleomorphism, prominent nucleoli, stratification, frequent mitoses and stromal invasion (irregular or destructive infiltration by small glands or sheets of cells) [68]. Psammoma bodies are common. The stroma may be fibrous, oedematous, myxoid or desmoplastic. In comparison, low-grade tumours have extensive papillary features with many psammoma bodies, papillae, glands, cysts or irregular nests of cells with uniform round to oval nuclei and evenly distributed chromatin. The nuclear features are variable. The mitotic count is less than 10 per high-power field [68]. The cells lie in a variable amount of fibrous stroma. Some of the ovarian tumours have clear cell features and are considered clear-cell variants of serous carcinoma.
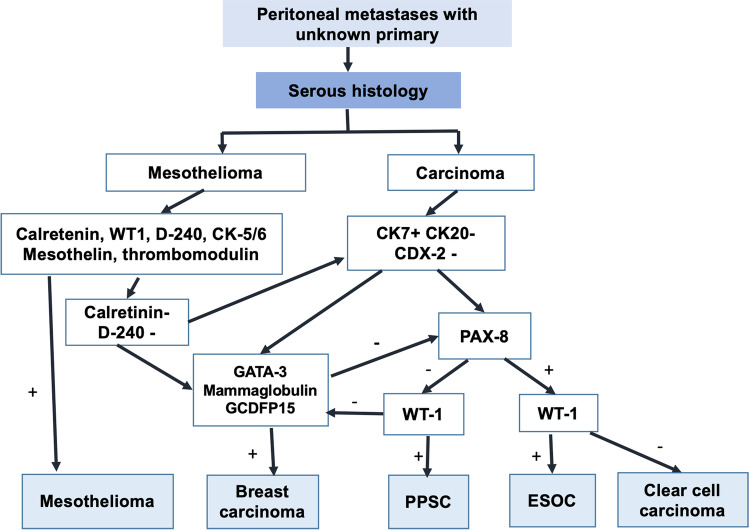
When the ovarian primary is not evident or the ovaries have been removed before, IHC is required to establish the site of origin. Another presentation could be of a pelvic mass with PM and the ovarian origin is not clear. As discussed above, PAX-8 is used to establish Mullerian origin and is negative in primary peritoneal serous carcinomas. WTI is positive in present majority of the ovarian serous carcinomas.
WT1 is in contrast expressed in less than a third of the endometrial serous tumours which are a less common source of peritoneal serous carcinomas compared to the ovaries [69]. However, in cases when both entities are WT1 positive, further investigations are needed to determine the primary site of origin [70]. The p53 expression can be similar in both the tumours. There may a situation in which both primaries co-exist. Making the distinction is important as endometrial serous carcinoma is a rare tumour, and the outcomes with serous carcinoma of the endometrium are inferior to those obtained for serous ovarian carcinoma. It is believed that some of the primary peritoneal serous carcinomas originate from a latent endometrial serous carcinoma [71–73].
WT1 differentiates serous ovarian carcinomas exhibiting similar morphology to that of pure clear cell ovarian carcinoma, as WT1 is negative in the latter [74]. Low-grade serous carcinomas usually present with large ovarian masses that infiltrate the adjacent peritoneum and viscera and are an uncommon cause of PM with unknown primary.
A common non-gynaecological malignancy that needs to be ruled out is diffuse malignant peritoneal mesothelioma (DMPM). Though it is a rare tumour, it is a peritoneal disease and thus, may be seen more often in a peritoneal surface malignancy unit than other common cancers like breast cancer that present rarely with isolated peritoneal disease. Though histological features can point towards the diagnosis of DMPM, IHC is essential to establish the diagnosis and comprises of both positive and negative markers [75]. DMPM arises from a single-cell line but has a spectrum of cytoarchitectural features that make it unique and often difficult to diagnose. The spectrum includes tumours that are entirely of epithelial or mesenchymal (sarcomatoid) type to a range of biphasic and intermediate forms [76]. The epithelial subtype is characterised by cuboidal or flattened epithelial-like malignant mesothelial cells with ample cytoplasm with distinct cellular membranes, and a relatively uniform, granular to vesicular nuclei. A pathognomonic positive calretinin, cytokeratins 5/6, WT-1, thrombomodulin and mesothelin stain, accompanied by a negative B72.3, CEA, CD 15, Leu-M1 and BER-EP4 immunostain are highly suggestive of DMPM [76]. Calretinin, WT1, CK5/6, D2-40, epithelial membrane antigen (EMA) and mesothelin are generally immunoreactive in DMPM but can also be positive in gynaecologic and non-gynaecologic adenocarcinoma [77].
There are some extremely well-differentiated papillary mesotheliomas that need to be distinguished from benign mesothelial proliferation.Another differential diagnosis is breast carcinoma. Metastatic breast carcinomas of ductal type can mimic a papillary serous or endometrioid ovarian cancer. The finding of a pelvic mass and/or disseminated peritoneal disease is not uncommon in a patient with a history of breast cancer and usually represents a new malignancy of ovarian origin. Yet, the rare possibility of metastatic breast disease needs to be considered and ruled out. As mentioned above, PAX-8, CA-125 and WT-1 are positive in serous carcinomas and negative in breast cancer though WT-1 and CA-125 could be positive [40, 41]. Markers useful but not specific for breast cancer are GCDFP15, mammoglobin and GATA3 (usually negative in serous carcinomas and positive in breast carcinomas) [42, 43]. An algorithm for determining the primary site in peritoneal metastases with serous histology is provided in Fig. 2.
Fig. 2.
Algorithm to determine the primary site in peritoneal metastases of unknown origin with the pathological diagnosis of serous carcinoma. Abbreviations: PPSC, primary peritoneal serous carcinoma; ESOC, epithelial serous ovarian carcinoma
Mucinous Carcinomas
Mucinous peritoneal metastases commonly arise from appendiceal tumours, colorectal tumours and ovarian tumours. Other primary sites include the pancreas, urachus and cervix. The term pseudomyxoma peritonei (PMP) is reserved for patients with mucinous ascites and the characteristic pattern of redistribution. In rare situations, high-grade mucinous carcinoma peritonei may be present without any apparent primary [78]. Either the primary has been removed during a prior surgical procedure and the diagnosis missed or it is a true case of peritoneal carcinomatosis with unknown primary. It is not known if mucinous tumours can arise de novo from the peritoneum.
Histopathological Findings
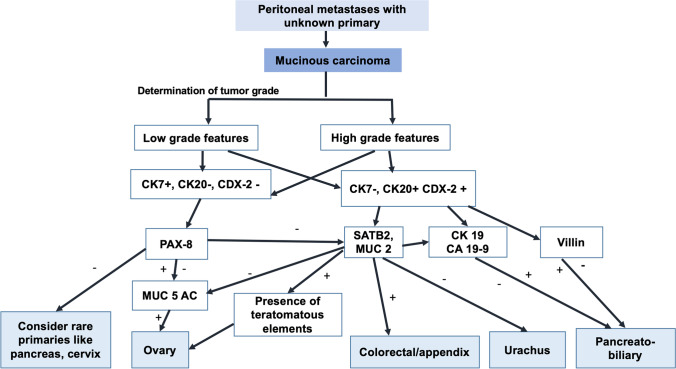
When mucinous ovarian tumours and PM are present, a lower gastrointestinal primary is always ruled out. Mucinous ovarian tumours can be borderline or malignant. Mucinous tumours of the intestinal type can arise de novo from the ovary. These tumours may not always be CK-7 positive and CK-20 negative like the other ovarian epithelial tumours. Focal and at times diffuse positivity is seen for CEA, CDX2 and CA19.9 as well [79]. As mentioned above, CDX2 will be expressed by appendiceal and colorectal primaries and not by ovarian primary tumour, but can vary. SATB2 is the confirmatory test for colorectal origin. Benign and malignant ovarian mucinous tumours associated with mature cystic teratomas may show massive mucin secretion, goblet cells, carcinoid-like patterns, pseudomyxoma ovarii and peritonei and signet ring cells characteristic of a gastrointestinal phenotype. These tumours express markers like CDX2, HepPar-1 and villin, and also have the cytokeratin 7–negative/cytokeratin 20–positive profile [80]. All these features would point towards a teratoid origin for this mucinous component, which should be differentiated from a metastasis from a gastrointestinal primary tumour. Demonstration of teratomatous foci may be difficult in rare cases when they are small and escape sampling or become overgrown by the mucinous neoplasm [80].
Urachal primary tumours have similar expression to the colorectal primaries. They are diffusely positive for CK-20, CDX-2, MUC-2 and MUC-5 AC and CK-7 expression is variable [81].
Tumour Grade
Mucinous PM arising from the appendix and ovary can be high grade or low grade. With the other primary sites, the tumours usually have a high grade.
Rare Differentials
Rarely, a metastatic cervical adenocarcinoma of usual type (HPV related) in the ovary may mimic a primary ovarian mucinous or endometrioid neoplasm [82]. Diffuse p16 immuno-reactivity in such cases may be useful in suggesting a metastatic cervical adenocarcinoma. These tumours can present with mucinous PM.
Some rare situations presenting that mimic mucinous PM have been enlisted by Carr et al. Malignant mesotheliomas in rare situations can have intracellular mucinous material rich in hyaluronic acid giving the appearance of signet ring cells [83]. These cells stain positive with mucin stains but can be distinguished as mesotheliomas when appropriate markers are used. Claudin-4 expression is seen in carcinomas and not mesotheliomas and can be used to make the distinction [84, 85]. Myxoid change occurring in endometriosis and papillary mesothelioma can mimic mucinous PM [86, 87]. An algorithm for determining the primary site in mucinous PM is provided in Fig. 3.
Fig. 3.
Algorithm to determine the primary site in peritoneal metastases of unknown origin with the pathological diagnosis of mucinous carcinoma/mucinous adenocarcinoma
Sarcomas
After the lungs and bones, the peritoneum is a common site of spread from soft tissue sarcomas. Nearly 30% of the sarcomas are intraabdominal disease. The commonest sarcomas metastasizing to the peritoneum are retroperitoneal liposacromas, uterine leimyosarcomas and low- and high-grade endometrial stromal sarcomas [88]. Low-grade endometrial stromal sarcoma can arise from the ovaries and the peritoneum itself [89].
PM from sarcomas can be present at the time of diagnosis but usually occur in the recurrent setting and could be due to tumour spillage during surgery. Some rare tumours like epitheloid leiomyosarcomas and gastrointestinal stromal tumours can arise from the omentum or peritoneum itself. In most cases, the primary site is apparent or there is a history of treatment of the primary tumour. The peritoneal sarcomas still require a search for a primary site before attributing the origin to the peritoneum. Peritoneal sarcomatosis with unknown primary has not been described.
Histopathological Features
Each of the sarcomas has distinct histological features and IHC and molecular marker profile that is well defined. The problem arises when the diagnosis has to be made on a small sample usually obtained through a trucut biopsy or when the tumours have poor differentiation.
Endometrial Stromal Sarcomas
Endometrial stromal sarcoma (ESS) has been divided into low and high grades in the World Health Organization (WHO) 2014 classification. High-grade sarcomas are defined by the presence of a recurrent chromosomal translocation –t(10;17) (q22; p13) resulting in YWHAE-NUTM2A or YWHAE-NUTM2B genetic fusions (collectively referred to as YWHAE-NUTM2) [90].
These rearrangements are mutually exclusive with the JAZF1/SUZ12/EPC1/PHF1 genetic rearrangements seen in low-grade endometrial stromal sarcomas.
This histopathological features of ESS have been described elsewhere [91–94]. Low-grade ESS show positive staining for CD10, ER and PR irrespective of the genotypes, and the staining pattern is generally diffuse in adequately fixed tumour samples [95–97]. There may be focal patchy staining for KIT, smooth muscle actin, caldesmon and/or desmin, with smooth muscle marker staining being more extensive in JAZF1 LGESS showing smooth muscle differentiation. The ki-67 proliferation index (< 5%) is low, nuclear cyclin D1 expression is typically weak and focal (< 5%) and DOG1 expression is absent [98–102]. High-grade ESS on the other hand has characteristic diffusely positive staining for cyclin D1 and is negative for CD10, ER and PR receptors. There is strong cytoplasmic c-KIT staining. Areas of low-grade ESS are seen in YWHAE-NUTM2 ESS. The term undifferentiated uterine sarcoma (UUS) is now used for tumours which were previously classified as endometrial undifferentiated sarcomas, and they can arise from smooth muscles as well. UUS is a diagnosis of exclusion and often has tumour necrosis [89].
On IHC, it can be positive for CD10 and hormone receptors; hence, it is important to not regard CD10 as evidence of endometrial stromal differentiation [89]. It may show very focal positive staining for smooth muscle actin, but the presence of positive staining for more than one smooth muscle markers should raise the suspicion for leiomyosarcoma or malignant PEComa [89].
Leiomyosarcomas
Leiomyosarcomas have a combination of diffuse moderate-to-severe nuclear atypia, greater than10 mitotic figures per 10 high-power fields (HPF) and presence of (coagulative) tumour-cell necrosis. The presence of any two of these features is essential for the diagnosis of a uterine leiomyosarcoma [103]. IHC is sometimes necessary to confirm the smooth-muscle nature and has been described in details in literature [104–113]. The diagnosis should be kept in mind when there is a history of prior treatment of uterine fibroids.
Extrauterine Leiomyosarcomas and Rare Omental Tumours
Leiomyosarcomas arising from the greater omentum have been reported [114–116]. The embryologic origin of these tumours is variable because of the different tissues that can be found in the omentum, namely vessels, lymphatics and fat [117].
Reported primary tumours of the omentum include leiomyosarcoma, fibrosarcoma, hemangiopericytoma, spindle cell sarcoma, liposarcoma, leiomyoma, lipoma, desmoid tumour, fibroma and mesothelioma [118, 119]. They derive from different elements in the greater omentum which is composed mainly of fat but contains various tissues such as vessels and lymphatics.
In a review by Branes et al., the median age of patients with leiomyosarcoma of the omentum in the cases published in the literature was 51 years [117]. The tumour was slightly more common among males (16 patients, 59.2%) and females (11 patients, 40.7%).
Pathological findings are the same as that in uterine leiomyosarcomas. The epithelioid variety has been seen more commonly in the omentum [120, 121]. Like the uterine LMS, these tumours can express CD117 without an underlying genetic mutation and, hence, targeted therapies specific for this mutation are ineffective [122].
Other Rare Tumours
Carcinosarcoma
Carcinosarcomas are a group of rare and aggressive malignancies that comprise of a mixture or carcinomatous and sarcomatous elements and can arise from various primary sites.
Initially, it was believed that one cell type gave rise to the other, it is the carcinomatous element that gives rise to the sarcomatous counterpart. The more modern theory is that both arise from the same precursor [123–126].
Carcinosarcomas have also been called a stable disruption of the epithelial-mesenchymal transition. The common sites of origin of carcinosarcomas are the uterus and adnexa, lung breast and head and neck sites.
In the Mullerian system, the uterus is the commonest site for the development of these tumours, followed by the vagina, cervix, adnexa and in very rare cases, they arise de novo from the peritoneum [127–131].
The carcinomatous and sarcomatous components may be intermittently mixed or be seen as two distinct components [132]. The epithelial component is often a high-grade carcinoma such as papillary serous carcinoma (66%) or endometrioid carcinoma (42%) [132]. Other uncommon histological subtypes include squamous cell carcinoma, basaloid squamous carcinoma, adenocarcinoma, adenosquamous carcinoma, adenobasal carcinoma, adenocystic carcinoma or an undifferentiated carcinoma [128]. The mesenchymal element may be (a) homologous, containing cells native to the uterus including stromal sarcoma, fibrosarcoma, undifferentiated sarcoma or leiomyosarcoma (2%) or (b) heterologous with mixed components including rhabdomyosarcoma (18%), chondrosarcoma (10%), osteosarcoma (5%) or liposarcoma (1%). One-third of carcinosarcomas have two or more sarcomatous elements, with high-grade stromal sarcoma being the most common type [133].
Carcinosarcomas are diagnosed based on morphological features alone, and immunohistochemistry is used for confirmation. Commonly expressed epithelial markers are epithelial membrane antigen and pancytokeratin. The commonly expressed stromal markers are desmin in areas of smooth muscle differentiation and S100 in areas with chondroid or lipomatous differentiation.
In carcinosarcoma of the ovary, the mesenchymal component may comprise of native ovarian stroma and its homologous malignant counterpart like undifferentiated sarcoma, leiomyosarcoma or endometrial stromal sarcoma [134, 135]. When the mesenchymal component is non-native of ovarian stroma, the sarcomatous elements may have component derived from skeletal muscle, bone or cartilage and is characteristically heterogenous.
Malignant Melanoma
Very limited information is available on the exact incidence of PM secondary to malignant melanoma. There are case reports on PM secondary to uterine, cutaneous and anorectal melanomas [136]. In one population-based registry, malignant melanoma was the underlying primary in 10% of the patients with extra-abdominal primaries as a source of PM [137]. Though the diagnosis of malignant melanoma should not be difficult for an experienced pathologist, an uncommon site of the origin could lead to confusion with other high-grade malignancies [138]. IHC should be confirmatory in such situations.
Molecular Diagnostics
During the last few decades, molecular biology has been added to armamentarium of diagnostic pathology. Molecular testing can have two goals:
To establish the site of origin and select the best suited systemic therapy which could result in a better response compared to empirical therapy
To identify druggable mutations [9]
Both NCCN and ESMO guidelines recommend molecular testing in patients with CUP [139, 140]. With the approval of tissue agnostic therapies, MSI testing is recommended by the NCCN guidelines for all patients with CUP [139]. Tissue-of-origin testing uses microarray technology or RT-PCR to compare the gene expression in the tumour tissue with that of a panel of different tissue types [9]. One 2000-gene, approved by the FDA for CUP, compares the RNA expression of the tumour with a panel of 15 different tissue types with established RNA profiles to determine the similarities and has been clinically validated [141, 142]. There is no specific assay for PM and such assays have been tested for specific histologies only that are more common at other metastatic sites like adenocarcinomas and sarcomas. RT-PCR assays, some of which have a panel of nearly 100 genes are also used to determine the probable primary tumour site [143–146]. In CUP, molecular tests have an accuracy of over 80% for correctly identifying the primary site. Once again, RT-PCR assays specific for PM, are not known.
Next generation sequencing (NGS) panels have been used to study the molecular profile of CUP. Both tissue samples and liquid biopsies have been used for NGS. In studies published so far, > 85% of the patients have one or more oncogenic driver mutation, and no two patients with two or more driver mutations had a similar molecular profile [147–153]. The most common alterations found in patients with CUP are in the TP53 gene (37–55% of cases), KRAS (18–20%), PIK3CA (9–15.4%), ARID1A (~ 11%) and EGFR (~ 6–17%) genes [147, 151–153]. Some of these mutations are druggable like EGFR and could improve outcomes over empirical systemic therapy alone though robust evidence for this is lacking. A list of the most common genetic alterations seen in some of the most common tumours causing PM is provided in Table 1.
Table 1.
Genetic mutations and biomarkers in common primary tumors giving rise to peritoneal metastases
| Primary tumour | Druggable mutations* | Non-druggable mutations |
|---|---|---|
| Serous ovarian carcinoma [154] | BRCA 1 and 2; PD-1; PDL-1; ATM | p53 |
| Gastric cancer [155, 156] | HER2, PD-L1, NTRK, ERBB2 | |
| Colorectal cancer[154] | HER2, PI3KCA, NTRK, ALK, ROS1, VEGF, dMMR, BRAF | |
| Diffuse malignant peritoneal mesothelioma [157, 158] | PI3KCA, AKT, mTOR, PDL-1, ALK | BAP-1 |
| Mucinous appendiceal tumours and PMP [159] | GNAS, MUC-2; MUC5B; MUC5AC | p53 |
| Pancreatic cancer [154] | KRAS, BRCA 1–2, EGFR | p53 |
| Endometrial cancer [160] | POLE, dMMR, PDL-1 | P53 |
| Breast cancer [154] | HER-2, BRCA 1–2, PIK3CA, ERα | |
| Biliary tract cancers [161] | FGFR, IDH, NTRK, d-MMR | |
| Tissue agnostic targets [9] | NTRK, High TMB, BRAF V600E, dMMR |
*Includes genetic mutations that are directly targeted by a drug and other alterations that are predictive of response to a particular targeted therapy (e.g. PDL-1, MSI-H); only the most frequent mutations are listed here
BRCA, breast cancer gene; PD-1, programmed cell death protein 1; PDL-1, programmed death ligand 1; ATM, ataxia telangectasia mutated; HER-2, human epidermal growth factor receptor-2; NTRK, neurotrophic tyrosine kinase receptor; ERBB2, erythroblastic oncogene B; PI3KCA, phosphatidylinositol-4,5 -bisphosphate 3-kinase catalytic subunit alpha; ALK, anaplastic lymphoma kinase; ROS-1, ROS protooncogene 1, receptor tyrosine kinase; VEGF, vascular endothelial growth factor; dMMR, deficiency in mismatch repair genes; BRAF, v-raf murine sarcoma viral oncogene homolog B1; AKT, protein kinase B; mTOR, mammalian target of rapamycin; GNAS, guanine nucleotide binding protein, alpha stimulating; MUC, oligomeric mucus/gel-forming; KRAS, Kirsten rat sarcoma viral oncogene homolog; EGFR, epidermal growth factor receptor; POLE, polymerase epsilon catalytic subunit; ERα, estrogen receptor α, FGFR, fibroblast growth factor receptor; IDH, isocitrate dehydrogenase; TMB, tumour mutational burden
Based on the available literature, most patients with PM of unknown origin seem to have advanced disease and few are eligible for surgical treatment. Molecular testing could help select the best systemic therapy for these patients though there are conflicting results from studies on CUP in general. Some studies have shown an improvement in survival when site-specific therapies are administered compared to empirical treatment while others have not reported a difference in survival. In a phase II trial including 130 patients, there was no survival advantage of site-specific systemic therapy based on gene expression profiling over conventional empirical therapy [162]. Another phase III trial did not show a benefit in PFS of site-specific therapy compared to empirical therapy alone. Both these studies had a large proportion of patients with a gene expression profile (GEP showing a hepatobiliary or pancreatic origin. Second, the site of metastases has not been specified [145]. Lymph node metastases alone constituted around 40% of the patients. It is unclear whether these results should be extrapolated to patients with isolated PM or even other metastatic sites. The differential response to systemic therapies according to the metastatic site has been demonstrated [163] PM have a worse molecular profile compared to other metastatic sites both in gastric cancer and colorectal cancer, and it has been shown that the more aggressive primary tumours give rise to PM [156, 164, 165]. Thus, PM of unknown origin should be investigated as a distinct clinical entity.
Conclusions
Peritoneal metastases can present with an occult primary. Careful evaluation of the clinical details, histopathological and immunohistochemistry evaluation can lead to a diagnosis in most cases. Awareness about the common and uncommon tumours giving rise to PM can facilitate the diagnostic process. Molecular diagnostic tests can help in identifying the site of origin and also identify druggable mutations. More collaborative research is needed for PM of unknown origin to understand the biology of these tumours, role of surgery, response to different therapies and survival.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Cancer. 2010;116:5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 2.Rajeev R, Turaga KK. Hyperthermic intraperitoneal chemotherapy and cytoreductive surgery in the management of peritoneal carcinomatosis. Cancer Control. 2016;23(1):36–46. doi: 10.1177/107327481602300107. [DOI] [PubMed] [Google Scholar]
- 3.Domenico S, Paul HS. Theoretical considerations for optimal cytoreductive surgery plus hyperthermic perioperative chemotherapy. J Gastrointest Dig Syst. 2015;5:359. doi: 10.4172/2161-069X.1000359. [DOI] [Google Scholar]
- 4.Sebbag G, Shmookler BM, Chang D, Sugarbaker PH. Peritoneal carcinomatosis from an unknown primary site. Management of 15 patients. Tumori. 2001;87(2):67–73. doi: 10.1177/030089160108700201. [DOI] [PubMed] [Google Scholar]
- 5.Morera-Ocon FJ, Navarro-Campoy C. History of pseudomyxoma peritonei from its origin to the first decades of the twenty-first century. World J Gastrointest Surg. 2019;11(9):358–364. doi: 10.4240/wjgs.v11.i9.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomassen I, Verhoeven RH, van Gestel YR, van de Wouw AJ, Lemmens VE, de Hingh IH. Population-based incidence, treatment and survival of patients with peritoneal metastases of unknown origin. Eur J Cancer. 2014;50(1):50–56. doi: 10.1016/j.ejca.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rijken A, Loef C, van de Wouw YAJ, et al. Updated incidence, treatment and survival of a nationwide cohort of patients with peritoneal metastases of unknown origin. Indian J Surg Oncol. 2022 doi: 10.1007/s13193-022-01567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt A. Parikh L, Mishra S, Glehen O (2020) Approach to a patient with peritoneal metastases with unknown primary site: focus on histopathological evaluation. In: Glehen O, Bhatt A (eds) Pathology of peritoneal metastases. Springer, Singapore. 10.1007/978-981-15-3773-8_11
- 9.Kato S, Alsafar A, Walavalkar V, Hainsworth J, Kurzrock R. Cancer of unknown primary in the molecular era. Trends Cancer. 2021;7(5):465–477. doi: 10.1016/j.trecan.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlidis N, Fizazi K. Cancer of unknown primary (CUP) Crit Rev Oncol Hematol. 2005;54:243–250. doi: 10.1016/j.critrevonc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Frost P. Unknown primary tumours: an example of accelerated (type 2) tumor progression. Basic Life Sci. 1991;57:233–237. doi: 10.1007/978-1-4684-5994-4_20. [DOI] [PubMed] [Google Scholar]
- 12.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 13.Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375–82. doi: 10.1016/j.jare.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes-Jordan A, Green H, Lin H, Owusu-Agyemang P, Mejia R, Okhuysen-Cawley R, Cortes J, Fitzgerald NE, McAleer MF, Herzog C, Huh WW, Anderson P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Surg Oncol. 2015;22(5):1726–1732. doi: 10.1245/s10434-014-4289-y. [DOI] [PubMed] [Google Scholar]
- 15.Hatano Y, Hatano K, Tamada M, Morishige KI, Tomita H, Yanai H, Hara A. A comprehensive review of ovarian serous carcinom. Adv Anat Path. 2019;26(5):329–339. doi: 10.1097/PAP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ. Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS One. 2012;7(11):e50058. doi: 10.1371/journal.pone.0050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palial KK, Yang B, Charlesworth PJS, Lewis CE, Browning L, Verrill C. A rare case of a urachal mucinous cystic tumour of low malignant potential. Cancer Stud Mol Med Open J. 2018;4(1):5–9. doi: 10.17140/CSMMOJ-4-122. [DOI] [Google Scholar]
- 18.Cortés-Guiral D, Hübner M, Alyami M, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers. 2021;7:91. doi: 10.1038/s41572-021-00326-6. [DOI] [PubMed] [Google Scholar]
- 19.McCluggage WG. Recent advances in immunohistochemistry in the diagnosis of ovarian neoplasms. J Clin Pathol. 2000;53:558–560. doi: 10.1136/jcp.53.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCluggage WG. Recent advances in immunohistochemistry in gynaecological pathology. Histopathology. 2002;46:309–326. doi: 10.1046/j.1365-2559.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 21.McCluggage WG, Young RH. Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol. 2005;22:3–32. doi: 10.1053/j.semdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan A, Steven SS, Hamilton C, Anjana K, Coffey D, Krishnan B, Truong LD. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–764. doi: 10.1038/modpathol.2011.3. [DOI] [PubMed] [Google Scholar]
- 23.Chai H, Ren Q, Fan Q, Ye L, Du G, Du H, Cheng Z. PAX8 is a potential marker for the diagnosis of primary epithelial ovarian cancer. Oncol Lett. 2017;14:5871–5875. doi: 10.3892/ol.2017.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makrigiannakis A, Amin K, Coukos G, Tilly JL, Coutifaris C. Regulated expression and potential roles of p53 and Wilms’ tumor suppressor gene (WT1) during follicular development in the human ovary. J Clin Endocrinol Metab. 2000;85(1):449–459. doi: 10.1210/jcem.85.1.6246. [DOI] [PubMed] [Google Scholar]
- 25.Bárcena C, Oliva E. WT1 expression in the female genital tract. Adv Anat Pathol. 2011;18(6):454–465. doi: 10.1097/PAP.0b013e318234aaed. [DOI] [PubMed] [Google Scholar]
- 26.Liliac L, Carcangiu ML, Canevari S, Căruntu ID, Ciobanu Apostol DG, Danciu M, Onofriescu M, Amălinei C. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Rom J Morphol Embryol. 2013;54(1):17–27. [PubMed] [Google Scholar]
- 27.Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11(10):3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 28.FitzPatrick DR, Carr IM, McLaren L, et al. Identification of SATB2 as the cleft palate gene on 2q32–q33. Hum Mol Genet. 2003;12(19):2491–2501. doi: 10.1093/hmg/ddg248. [DOI] [PubMed] [Google Scholar]
- 29.Conner JR, Hornick JL. Metastatic carcinoma of unknown primary: diagnostic approach using immunohistochemistry. Adv Anat Pathol. 2015;22(3):149–167. doi: 10.1097/pap.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson K, de Wit M, Brennan DJ, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35(7):937–948. doi: 10.1097/PAS.0b013e31821c3dae. [DOI] [PubMed] [Google Scholar]
- 31.Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: results of a pathology-based clinical prospective study. Am J Clin Pathol. 2014;141(5):630–638. doi: 10.1309/AJCPWW2URZ9JKQJU. [DOI] [PubMed] [Google Scholar]
- 32.Lin F, Shi J, Zhu S, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch Pathol Lab Med. 2014;138(8):1015–1026. doi: 10.5858/arpa.2013-0452-oa. [DOI] [PubMed] [Google Scholar]
- 33.Brandler TC, Jelloul F, Soto D, Das K, Rosen L, Bhuiya TA. Young investigator challenge: cadherin-17 and SATB2 in cytology specimens: do these new immunostains help in differentiating metastatic colorectal adenocarcinoma from adenocarcinomas of other origins? Cancer Cytopathol. 2015;123(12):706–713. doi: 10.1002/cncy.21644. [DOI] [PubMed] [Google Scholar]
- 34.Berg KB, Schaeffer DF. SATB2 as an immunohistochemical marker for colorectal adenocarcinoma: a concise review of benefits and pitfalls. Arch Pathol Lab Med. 2017;141(10):1428–1433. doi: 10.5858/arpa.2016-0243-RS. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein NS, Bassi D. Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol. 2001;115:695–702. doi: 10.1309/1NCM-46QX-3B5T-7XHR. [DOI] [PubMed] [Google Scholar]
- 36.Park SY, Kim HS, Hong EK, et al. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol. 2002;33:1078–1085. doi: 10.1053/hupa.2002.129422. [DOI] [PubMed] [Google Scholar]
- 37.Ji H, Isacson C, Seidman JD, et al. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Chhieng DC, Benson E, Eltoum I, et al. MUC1 and MUC2 expression in pancreatic ductal carcinoma obtained by fine-needle aspiration. Cancer. 2003;99:365–371. doi: 10.1002/cncr.11857. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32:1566–1571. doi: 10.1097/PAS.0b013e31816d71ad. [DOI] [PubMed] [Google Scholar]
- 40.Tornos C, Soslow R, Chen S, et al. Expression of WT1, CA125, and GCDFP-15 as useful markers in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol. 2005;29:1482–1489. doi: 10.1097/01.pas.0000176429.88702.36. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Shi J, Wilkerson ML, et al. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138:57–64. doi: 10.1309/AJCP5UAFMSA9ZQBZ. [DOI] [PubMed] [Google Scholar]
- 42.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–113. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Tovar J, Alonso HN, Morales CV, Lobo ME, Sanjuanbenito DA, Martinez ME. Peritoneal carcinomatosis secondary to carcinoid tumour. Clin Transl Oncol. 2007;9:804–805. doi: 10.1007/s12094-007-0143-z. [DOI] [PubMed] [Google Scholar]
- 44.Mertz H, Vyberg M, Paulsen SM, et al. Immunohistochemical detection of neuroendocrine markers in tumors of the lungs and gastrointestinal tract. Appl Immunohistochem. 1998;6:175–180. doi: 10.1097/00022744-199812000-00001. [DOI] [Google Scholar]
- 45.Vasseur B, Cadiot G, Zins M, et al. Peritoneal carcinomatosis in patients with digestive endocrine tumors. Cancer. 1996;78:1686–1692. doi: 10.1002/(SICI)1097-0142(19961015)78:8<1686::AID-CNCR8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 46.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinik AI, Thompson N, Eckhauser F, Moattari R. Clinical features of carcinoid syndrome and the use of somatostatin analogue in its management. Acta Oncol. 1989;28:389–402. doi: 10.3109/02841868909111212. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez RS, Liu EH, Alvarez JR, Ayers GD, Washington MK, Shi C. Should mesenteric tumor deposits be included in staging of well differentiated small intestine neuroendocrine tumors? Mod Pathol. 2014;27:1288–95. doi: 10.1038/modpathol.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. Role of imaging in the preoperative staging of small bowel neuroendocrine tumors. J Am Coll Surg. 2010;211:620–627. doi: 10.1016/j.jamcollsurg.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Søreide O, Berstad T, Bakka A, Schrumpf E, Hanssen LE, Engh V, Bergan A, Flatmark A. Surgical treatment as a principle in patients with advanced abdominal carcinoid tumors. Surgery. 1992;111:48–54. [PubMed] [Google Scholar]
- 51.Elias D, Lefevre JH, Duvillard P, Goéré D, Dromain C, Dumont F, Baudin E. Hepatic metastases from neuroendocrine tumors with a thin slice CT scan and pathological examination: they are many more than you think. Ann Surg. 2010;251:307–10. doi: 10.1097/SLA.0b013e3181bdf8cf. [DOI] [PubMed] [Google Scholar]
- 52.Kimura N, Pilichowska M, Okamoto H, et al. Immunohistochemical expression of chromogranins A and B, prohormone convertases 2 and 3, and amidating enzyme in carcinoid tumors and pancreatic endocrine tumors. Mod Pathol. 2000;13:140–146. doi: 10.1038/modpathol.3880026. [DOI] [PubMed] [Google Scholar]
- 53.Al-Khafaji B, Noffsinger AE, Miller MA, et al. Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Hum Pathol. 1998;29:992–9. doi: 10.1016/S0046-8177(98)90206-4. [DOI] [PubMed] [Google Scholar]
- 54.Fahrenkamp AG, Wibbeke C, Winde G, et al. Immunohistochemical distribution of chromogranins A and B and secretogranin II in neuroendocrine tumours of the gastrointestinal tract. Virchows Arch. 1995;426:361–367. doi: 10.1007/BF00191345. [DOI] [PubMed] [Google Scholar]
- 55.Sobin LH, Hjermstad BM, Sesterhenn IA, et al. Prostatic acid phosphatase activity in carcinoid tumors. Cancer. 1986;58:136–138. doi: 10.1002/1097-0142(19860701)58:1<136::AID-CNCR2820580124>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 56.Barbareschi M, Roldo C, Zamboni G, et al. CDX-2 homeobox gene product expression in neuroendocrine tumors: its role as a marker of intestinal neuroendocrine tumors. Am J Surg Pathol. 2004;28:1169–1176. doi: 10.1097/01.pas.0000131531.75602.b9. [DOI] [PubMed] [Google Scholar]
- 57.La Rosa S, Rigoli E, Uccella S, et al. CDX2 as a marker of intestinal EC-cells and related well-differentiated endocrine tumors. Virchows Arch. 2004;445:248–254. doi: 10.1007/s00428-004-1080-7. [DOI] [PubMed] [Google Scholar]
- 58.Jaffee IM, Rahmani M, Singhal MG, et al. Expression of the intestinal transcription factor CDX2 in carcinoid tumors is a marker of midgut origin. Arch Pathol Lab Med. 2006;130:1522–1526. doi: 10.5858/2006-130-1522-EOTITF. [DOI] [PubMed] [Google Scholar]
- 59.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–26. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 60.Locher C, Malka D, Boige V, et al. Combination chemotherapy in advanced small bowel adenocarcinoma. Oncology. 2005;69:290–294. doi: 10.1159/000089678. [DOI] [PubMed] [Google Scholar]
- 61.Overman MJ, Kopetz S, Wen S, et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038–2045. doi: 10.1002/cncr.23822. [DOI] [PubMed] [Google Scholar]
- 62.Talamonti MS, Goetz LH, Rao S, et al. Primary cancers of the small bowel: analysis of prognostic factors and results of surgical management. Arch Surg. 2002;137:564–570. doi: 10.1001/archsurg.137.5.564. [DOI] [PubMed] [Google Scholar]
- 63.North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg. 2000;66:46–51. doi: 10.1177/000313480006600110. [DOI] [PubMed] [Google Scholar]
- 64.Frost DB, Mercado PD, Tyrell JS. Small bowel cancer: a 30-year review. Ann Surg Oncol. 1994;1:290–295. doi: 10.1007/BF02303567. [DOI] [PubMed] [Google Scholar]
- 65.Chen ZM, Ritter JH, Wang HL. Differential expression of alpha-methylacyl coenzyme A racemase in adenocarcinomas of the small and large intestines. Am J Surg Pathol. 2005;29:890–896. doi: 10.1097/01.pas.0000167364.90899.59. [DOI] [PubMed] [Google Scholar]
- 66.Wong H, Chu P. Immunohistochemical features of the gastrointestinal tract tumors. J Gastrointest Oncol. 2012;3(3):262–284. doi: 10.3978/j.issn.2078-6891.2012.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson DE, Herndier BG, Medeiros LJ, et al. The diagnostic utility of the keratin profiles of hepatocellular carcinoma and cholangiocarcinoma. Am J Surg Pathol. 1988;12:187–197. doi: 10.1097/00000478-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Ehdaivand S. (2019) Serous carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovarytumorserouscarcinoma.html. Accessed 1 Nov 2019
- 69.Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004;44(2):109–115. doi: 10.1111/j.1365-2559.2004.01787.x. [DOI] [PubMed] [Google Scholar]
- 70.Sumathi VP, Al-Hussaini M, Connolly LE, Fullerton L, McCluggage WG. Endometrial stromal neoplasms are immunoreactive with WT-1 antibody. Int J Gynecol Pathol. 2004;23(3):241–247. doi: 10.1097/01.pgp.0000130051.04396.13. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu M, Toki T, Takagi Y, et al. Immunohistochemical detection of the Wilms’ tumor gene (WT1) in epithelial ovarian tumors. Int J Gynecol Pathol. 2000;19:158–163. doi: 10.1097/00004347-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 72.McCluggage WG. WT1 is of value in ascertaining the site of origin of serous carcinomas within the female genital tract. Int J Gynecol Pathol. 2004;23:97–99. doi: 10.1097/00004347-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Chen W, Husain A, Nelson GS, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol. 2017;36:128–139. doi: 10.1097/PGP.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 74.McCluggage WG. Immunohistochemical markers as a diagnostic aid in ovarian pathology. Diagn Histopathol. 2008;14(8):335–351. doi: 10.1016/j.mpdhp.2008.06.008. [DOI] [Google Scholar]
- 75.Davidson B. New diagnostic and molecular characteristics of malignant mesothelioma. Ultrastruct Pathol. 2008;32:227–240. doi: 10.1080/01913120802454298. [DOI] [PubMed] [Google Scholar]
- 76.Battifora H, McCaughey WTE. Tumors of the serosal membranes. Washington DC: Armed Forces Institute of Pathology; 1994. [Google Scholar]
- 77.Husain AN, Colby T, Ordonez N et al (2013) International Mesothelioma Interest Group. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 137(5):647–667 [DOI] [PubMed]
- 78.Delhorme JB, Severac F, Averous G, Glehen O, Passot G, Bakrin N, Marchal F, Pocard M, Lo Dico R, Eveno C, Carrere S, Sgarbura O, Quenet F, Ferron G, Goéré D, Brigand C, French National Network of Peritoneal Surface Malignancies (RENAPE) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendicular and extra-appendicular origin. Br J Surg. 2018;105(6):668–676. doi: 10.1002/bjs.10716. [DOI] [PubMed] [Google Scholar]
- 79.Bhatt A, Mishra S, Parikh L et al (2019) Essentials for pathological evaluation of peritoneal surface malignancies and synoptic reporting of cytoreductive surgery specimens—a review and evidence-based guide. Indian J Surg Oncol 332. 10.1007/s13193-019-00897-7 [DOI] [PMC free article] [PubMed]
- 80.Vang R, Gown AM, Barry TS, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130–1139. doi: 10.1097/01.pas.0000213281.43036.bb. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Ishibashi H, Hirano M, Takeshita K, Mizumoto A, Ichinose M, Nishino E, Kashu I, Yamamoto Y, Sugarbaker PH, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei arising from urachus. Ann Surg Oncol. 2015;22(8):2799–2805. doi: 10.1245/s10434-014-4336-8. [DOI] [PubMed] [Google Scholar]
- 82.Ronnett BM, Yemelyanova AV, Vang R, et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumours and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008;32:1835–1853. doi: 10.1097/PAS.0b013e3181758831. [DOI] [PubMed] [Google Scholar]
- 83.Cook DS, Attanoos RL, Jalloh SS, Gibbs AR. 'Mucin-positive' epithelial mesothelioma of the peritoneum: an unusual diagnostic pitfall. Histopathology. 2000;37:33–36. doi: 10.1046/j.1365-2559.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 84.Facchetti F, Lonardi S, Gentili F, et al. Claudin 4 identifies a wide spectruof epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Arch. 2007;451:669–680. doi: 10.1007/s00428-007-0448-x. [DOI] [PubMed] [Google Scholar]
- 85.Facchetti F, Gentili F, Lonardi S, Bercich L, Santin A. Claudin-4 in mesothelioma diagnosis. Histopathology. 2007;51:261–263. doi: 10.1111/j.1365-2559.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 86.McCluggage WG, Kirk SJ. Pregnancy associated endometriosis with pronounced stromal myxoid change. J Clin Pathol. 2000;53:241–242. doi: 10.1136/jcp.53.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diaz L, Okonkwo A, Solans EP, Bedrossian C, Rao MS. Extensive myxoid change in well differentiated papillary mesothelioma of the pelvic peritoneum. Ann Diagn Pathol. 2002;6:164–167. doi: 10.1053/adpa.2002.33902. [DOI] [PubMed] [Google Scholar]
- 88.Bhatt A, Ramakrishnan AS (2018) Rare indications for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. In: Bhatt A. (eds) Management of peritoneal metastases- cytoreductive surgery, HIPEC and beyond. Springer, Singapore
- 89.Lee CH, Nucci MR. Endometrial stromal sarcoma–the new genetic paradigm. Histopathology. 2015;67(1):1–19. doi: 10.1111/his.12594. [DOI] [PubMed] [Google Scholar]
- 90.Lee CH, Ou WB, Marino-Enriquez A, et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci U S A. 2012;109:929–934. doi: 10.1073/pnas.1115528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang KL, Crabtree GS, Lim-Tan SK, et al. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415–438. 2. doi: 10.1097/00000478-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 92.Evans HL. Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer. 1982;50:2170–2182. doi: 10.1002/1097-0142(19821115)50:10<2170::AID-CNCR2820501033>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 93.Hendrickson MR, Tavassoli FA, Kempson RL (2003) Mesenchymal tumours and related lesions. In: . World HealthOrganization Classification of Tumours Pathology and Genetics of Tumours of the Breast and Female Genital Organ Lyon, France: IARC Press
- 94.Norris HJ, Taylor HB. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966;19:755–766. doi: 10.1002/1097-0142(196606)19:6<755::AID-CNCR2820190604>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 95.Chu PG, Arber DA, Weiss LM, et al. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001;14:465–471. doi: 10.1038/modpathol.3880335. [DOI] [PubMed] [Google Scholar]
- 96.McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology. 2001;39:273–278. doi: 10.1046/j.1365-2559.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 97.Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–653. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 98.Lee CH, Ali RH, Rouzbahman M, et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with YWHAE-FAM22 rearrangement. Am J Surg Pathol. 2012;36:1562–1570. doi: 10.1097/PAS.0b013e31825fa931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein WM, Kurman RJ. Lack of expression of c-kit protein (CD117) in mesenchymal tumors of the uterus and ovary. Int J Gynecol Pathol. 2003;22:181–184. doi: 10.1097/00004347-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 100.Nakayama M, Mitsuhashi T, Shimizu Y, et al. Immunohistochemical evaluation of KIT expression in sarcomas of the gynecologic region. Int J Gynecol Pathol. 2006;25(70–76):59. doi: 10.1097/01.pgp.0000183047.45459.36. [DOI] [PubMed] [Google Scholar]
- 101.Caudell JJ, Deavers MT, Slomovitz BM, et al. Imatinib mesylate (gleevec)–targeted kinases are expressed in uterine sarcomas. Appl Immunohistochem Mol Morphol. 2005;13:167–170. doi: 10.1097/01.pai.0000129057.38941.a1. [DOI] [PubMed] [Google Scholar]
- 102.Lee CH, Liang CW, Espinosa I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathology-the GIST of it. Adv Anat Pathol. 2010;17:222–232. doi: 10.1097/PAP.0b013e3181d973c2. [DOI] [PubMed] [Google Scholar]
- 103.Hendrickson MR, Tavassoli FA, Kempson RL, et al. Mesenchymal tumours and related lesions. In: Tavassoli FA, Devilee P, et al., editors. World Health Organization classification of tumours: pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 236–243. [Google Scholar]
- 104.Oliva E, Young RH, Amin MB, et al. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002;26:403–412. doi: 10.1097/00000478-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 105.Rizeq MN, van de Rijn M, Hendrickson MR, et al. A comparative immunohistochemical study of uterine smooth muscle neoplasms with emphasis on the epithelioid variant. Hum Pathol. 1994;25:671–677. doi: 10.1016/0046-8177(94)90300-X. [DOI] [PubMed] [Google Scholar]
- 106.Bodner-Adler B, Bodner K, Czerwenka K, et al. Expression of p16 protein in patients with uterine smooth muscle tumors: an immunohistochemical analysis. Gynecol Oncol. 2005;96:62–66. doi: 10.1016/j.ygyno.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 107.Atkins KA, Arronte N, Darus CJ, et al. The use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32(98–102):39. doi: 10.1097/PAS.0b013e3181574d1e. [DOI] [PubMed] [Google Scholar]
- 108.O’Neill CJ, McBride HA, Connolly LE, et al. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50(851–858):41. doi: 10.1111/j.1365-2559.2007.02699.x. [DOI] [PubMed] [Google Scholar]
- 109.de Vos S, Wilczynski SP, Fleischhacker M, et al. p53 alterations in uterine leiomyosarcomas versus leiomyomas. Gynecol Oncol. 1994;54(205–208):43. doi: 10.1006/gyno.1994.1194. [DOI] [PubMed] [Google Scholar]
- 110.Blom R, Guerrieri C, Stal O, et al. Leiomyosarcoma of the uterus: a clinicopathologic, DNA flow cytometric, p53, and mdm-2 analysis of 49 cases. Gynecol Oncol. 1998;68(54–61):42. doi: 10.1006/gyno.1997.4889. [DOI] [PubMed] [Google Scholar]
- 111.Jeffers MD, Farquharson MA, Richmond JA, et al. p53 immunoreactivity and mutation of the p53 gene in smooth muscle tumours of the uterine corpus. J Pathol. 1995;177:65–70. doi: 10.1002/path.1711770111. [DOI] [PubMed] [Google Scholar]
- 112.Hall KL, Teneriello MG, Taylor RR, et al. Analysis of Ki-ras, p53, and MDM2 genes in uterine leiomyomas and leiomyosarcomas. Gynecol Oncol. 1997;65(330–335):44. doi: 10.1006/gyno.1997.4653. [DOI] [PubMed] [Google Scholar]
- 113.Lasota J, Jasinski M, Sarlomo-Rikala M, et al. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanimura TC, Nohara M, et al. Primary leiomyosarcoma of the omentum. Kurume Med J. 1980;27:101–105. doi: 10.2739/kurumemedj.27.101. [DOI] [PubMed] [Google Scholar]
- 115.Tsurumi H, Okada S, Koshino Y, Oyama M, Higaki H, Shimokawa K, Yamauchi O, Moriwaki H, Muto Y. A case of leiomyoblastoma (epithelioid leiomyosarcoma) of the greater omentum Gastroenterol. Jpn. 1991;26(3):370–375. doi: 10.1007/BF02781927. [DOI] [PubMed] [Google Scholar]
- 116.Mahon DE, Carp NZ, Goldhahn RT, et al. Primary leiomyosarcoma of the greater omentum: case report and review of the literature Am. Surg. 1993;59:160–163. [PubMed] [Google Scholar]
- 117.Brañes A, Bustamante C, Valbuena J, Pimentel F, Quezada N. Primary leiomyosarcoma of the greater omentum: a case report. Int J Surg Case Rep. 2016;28:317–320. doi: 10.1016/j.ijscr.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishida H, Ishida J. Primary tumours of the greater omentum. Eur Radiol. 1998;8(9):1598–1601. doi: 10.1007/s003300050594. [DOI] [PubMed] [Google Scholar]
- 119.Scwartz RW, Reames M, McGrath PC, et al. Primary solid neoplasms of the greater omentum. Surgery. 1991;109:543–549. [PubMed] [Google Scholar]
- 120.Stout AP, Hendry J, Purdie FJ. Primary solid tumours of the greater omentum. Cancer. 1963;16:231–243. doi: 10.1002/1097-0142(196302)16:2<231::AID-CNCR2820160214>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 121.Fattar S, Morton PCG, Schulman A, et al. Radiological diagnosis of primary greater omental mass lesion Clin. Radiol. 1981;32:325–330. doi: 10.1016/s0009-9260(81)80054-2. [DOI] [PubMed] [Google Scholar]
- 122.Weinberger HA, Ahmed MS. Mesenchymal solid tumors of the omentum. Surgery. 1997;82:754–759. [PubMed] [Google Scholar]
- 123.Virchow R (1863) Die Krankhaften Geschwülste. Berlin, Germany, Springer, Google Scholar
- 124.Ewing J. Neoplatic diseases. Philadelphia, PA: W.B. Saunders; 1919. [Google Scholar]
- 125.Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP, Black J et al (2016) Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc Natl Acad Sci U S A 113(43):12238–12243. 10.1073/pnas.1614120113 [DOI] [PMC free article] [PubMed]
- 126.Wada H, Enomoto T, Fujita M, et al. Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumours. Cancer Res. 1997;57:5379–5385. [PubMed] [Google Scholar]
- 127.Banik T, Halder D, Gupta N, Dey P Malignant mixed Mullerian tumor of the uterus: diagnosis of a case by fine-needle aspiration cytology and review of literature. Diagnostic Cytopathology. In press [DOI] [PubMed]
- 128.Ahuja A, Safaya R, Prakash G, Kumar L, Shukla NK. Primary mixed Mullerian tumor of the vagina—a case report with review of the literature. Pathol Res Pract. 2011;207(4):253–255. doi: 10.1016/j.prp.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Sharma NK, Sorosky JI, Bender D, Fletcher MS, Sood AK. Malignant mixed Mullerian tumor (MMMT) of the cervix. Gynecol Oncol. 2005;97(2):442–445. doi: 10.1016/j.ygyno.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 130.Duman BB, Kara IO, Gunaldi M, Ercolak V. Malignant mixed Mullerian tumor of the ovary with two cases and review of the literature. Arch Gynecol Obstet. 2011;283(6):1363–1368. doi: 10.1007/s00404-011-1845-6. [DOI] [PubMed] [Google Scholar]
- 131.Shen YM, Xie YP, Xu L, et al. Malignant mixed Mullerian tumor of the fallopian tube: report of two cases and review of literature. Arch Gynecol Obstet. 2010;281(6):1023–1028. doi: 10.1007/s00404-009-1331-6. [DOI] [PubMed] [Google Scholar]
- 132.Brown L. Pathology of uterine malignancies. Clin Oncol. 2008;20(6):433–447. doi: 10.1016/j.clon.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 133.El-Nashar SA, Mariani A. Uterine carcinosarcoma. Clin Obstet Gynecol. 2011;54(2):292–304. doi: 10.1097/GRF.0b013e31821ac635. [DOI] [PubMed] [Google Scholar]
- 134.Mok JE, Kim YM, Jung MH, Kim KR, Kim DY, Kim JH, et al. Malignant mixed Mullerian tumors of the ovary: experience with cytoreductive surgery and platinum-based combination chemotherapy. Int J Gynecol Cancer. 2006;16:101–105. doi: 10.1111/j.1525-1438.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 135.Boucher D, Tetu B. Morphologic prognostic factors of malignant mixed Mullerian tumors of the ovary: a clinicopathologic study of 15 cases. Int J Gynecol Pathol. 1994;13(1):22–28. doi: 10.1097/00004347-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 136.McBride M, Calhoun S. Peritoneal carcinomatosis arising from primary anorectal melanoma. J Radiol Case Rep. 2019;13(4):28–37. doi: 10.3941/jrcr.v13i4.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Flanagan M, et al. Peritoneal metastases from extra-abdominal cancer — a population-based study. Eur J Surg Oncol. 2018;44:1811–1817. doi: 10.1016/j.ejso.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 138.Lee ES, Ahn JH, Lee TS, Jeon HW. Metastatic malignant melanoma with peritoneal seeding in a young woman: a case report. Obstet Gynecol Sci. 2014;57(3):240–3. doi: 10.5468/ogs.2014.57.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in oncology: occult primary (cancer of unknown primary [CUP]). NCCN. Available at http://www.nccn.org/professionals/physician_gls/pdf/occult.pdf. Version 1.2022 — September 2, 2021; Accessed: 18 Jun 2022
- 140.Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v133–v138. doi: 10.1093/annonc/mdv305. [DOI] [PubMed] [Google Scholar]
- 141.Bridgewater J, et al. Gene expression profiling may improve diagnosis in patients with carcinoma of unknown primary. Br J Cancer. 2008;98:1425–1430. doi: 10.1038/sj.bjc.6604315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pillai R, et al. Validation and reproducibility of a microar- ray-based gene expression test for tumor identification in formalin-fixed, paraffin-embedded specimens. J Mol Diagn. 2011;13:48–56. doi: 10.1016/j.jmoldx.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Varadhachary GR, et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442–4448. doi: 10.1200/JCO.2007.14.4378. [DOI] [PubMed] [Google Scholar]
- 144.Hainsworth JD, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31:217–223. doi: 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- 145.Fizazi K, et al. LBA15_PR a phase III trial of empiric chemotherapy with cisplatin and gemcitabine or systemic treatment tailored by molecular gene expression analysis in patients with carcinomas of an unknown primary (CUP) site (GEFCAPI 04) Ann. Oncol. 2019;30:mdz394. doi: 10.1093/annonc/mdz394. [DOI] [Google Scholar]
- 146.Greco FA, et al. Molecular profiling in unknown primary cancer: accuracy of tissue of origin prediction. Oncologist. 2010;15:500–506. doi: 10.1634/theoncologist.2009-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ross JS, et al. Comprehensive genomic profiling of carcinoma of unknown primary site: new routes to targeted therapies. JAMA Oncol. 2015;1:40–49. doi: 10.1001/jamaoncol.2014.216. [DOI] [PubMed] [Google Scholar]
- 148.Tothill RW, et al. Massively-parallel sequencing assists the diagnosis and guided treatment of cancers of unknown primary. J Pathol. 2013;231:413–423. doi: 10.1002/path.4251. [DOI] [PubMed] [Google Scholar]
- 149.Gatalica Z, et al. Comprehensive analysis of cancers of unknown primary for the biomarkers of response to immune checkpoint blockade therapy. Eur J Cancer. 2018;94:179–186. doi: 10.1016/j.ejca.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 150.Loffler H, et al. Molecular driver alterations and their clinical relevance in cancer of unknown primary site. Oncotarget. 2016;7:44322–44329. doi: 10.18632/oncotarget.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gatalica Z, et al. Comprehensive tumor profiling identifies numerous biomarkers of drug response in cancers of unknown primary site: analysis of 1806 cases. Oncotarget. 2014;5:12440–12447. doi: 10.18632/oncotarget.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Varghese AM, et al. Clinical and molecular characterization of patients with cancer of unknown primary in the modern era. Ann Oncol. 2017;28:3015–3021. doi: 10.1093/annonc/mdx545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kato S, et al. Utility of genomic analysis in circulating tumor DNA from patients with carcinoma of unknown primary. Cancer Res. 2017;77:4238–4246. doi: 10.1158/0008-5472.CAN-17-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Danesi R, Fogli S, Indraccolo S, Del Re M, Dei Tos AP, Leoncini L, Antonuzzo L, Bonanno L, Guarneri V, Pierini A, Amunni G, Conte P. Druggable targets meet oncogenic drivers: opportunities and limitations of target-based classification of tumors and the role of Molecular Tumor Boards. ESMO Open. 2021;6(2):100040. doi: 10.1016/j.esmoop.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tanaka Y, Chiwaki F, Kojima S, et al. Multi-omic profiling of peritoneal metastases in gastric cancer identifies molecular subtypes and therapeutic vulnerabilities. Nat Cancer. 2021;2:962–977. doi: 10.1038/s43018-021-00240-6. [DOI] [PubMed] [Google Scholar]
- 156.Wang R, Song S, Harada K, GhazanfariAmlashi F, Badgwell B, Pizzi MP, et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut. 2020;69(1):18–31. doi: 10.1136/gutjnl-2018-318070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Joseph NM, Chen YY, Nasr A, et al. Genomic profiling of malignant peritoneal mesothelioma reveals recurrent alterations in epigenetic regulatory genes BAP1, SETD2, and DDX3X. Mod Pathol. 2017;30:246–254. doi: 10.1038/modpathol.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deraco M. et al. (2020) Peritoneal mesothelioma: disease biology and patterns of peritoneal dissemination. In: Glehen O., Bhatt A. (eds) Pathology of peritoneal metastases. Springer, Singapore. 10.1007/978-981-15-3773-8_6
- 159.Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J Rare Dis. 2014;9:71. doi: 10.1186/1750-1172-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19(9):510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boilève A, Hilmi M, Delaye M, Tijeras-Raballand A, Neuzillet C. Biomarkers in hepatobiliary cancers: what is useful in clinical practice? Cancers (Basel) 2021;13(11):2708. doi: 10.3390/cancers13112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hayashi H, et al. Randomized phase II trial comparing site-specific treatment based on gene expression profiling with carboplatin and paclitaxel for patients with cancer of unknown primary site. J Clin Oncol. 2019;37:570–579. doi: 10.1200/JCO.18.00771. [DOI] [PubMed] [Google Scholar]
- 163.Franko, J, Shi, Q, Meyers, JP et al. (17 more authors) (2016) Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 17(12). pp. 1709–1719. ISSN 1470–2045. 10.1016/S1470-2045(16)30500-9 [DOI] [PubMed]
- 164.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ubink I, van Eden WJ, Snaebjornsson P, Kok NFM, van Kuik J, van Grevenstein WMU, Laclé MM, Sanders J, Fijneman RJA, Elias SG, Borel Rinkes IHM, Aalbers AGJ, Kranenburg O. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br J Surg. 2018;105(2):e204–e211. doi: 10.1002/bjs.10788. [DOI] [PubMed] [Google Scholar]