Abstract
As social micropredators, myxobacteria are studied for their abilities to prey on bacteria and fungi. However, their predation of oomycetes has received little attention. Here, we show that Archangium sp. AC19 secretes a carbohydrate-active enzyme (CAZyme) cocktail during predation on oomycetes Phytophthora. These enzymes include three specialized β-1,3-glucanases (AcGlu13.1, –13.2 and –13.3) that act as a cooperative consortium to target β-1,3-glucans of Phytophthora. However, the CAZymes showed no hydrolytic effects on fungal cells, even though fungi contain β-1,3-glucans. Heterologous expression of AcGlu13.1, –13.2 or –13.3 enzymes in Myxococcus xanthus DK1622, a model myxobacterium that antagonizes but does not predate on P. sojae, conferred a cooperative and mycophagous ability that stably maintains myxobacteria populations as a mixture of engineered strains. Comparative genomic analyses suggest that these CAZymes arose from adaptive evolution among Cystobacteriaceae myxobacteria for a specific prey killing behavior, whereby the presence of Phytophthora promotes growth of myxobacterial taxa by nutrient release and consumption. Our findings demonstrate that this lethal combination of CAZymes transforms a non-predatory myxobacterium into a predator with the ability to feed on Phytophthora, and provides new insights for understanding predator-prey interactions. In summary, our work extends the repertoire of myxobacteria predatory strategies and their evolution, and suggests that these CAZymes can be engineered as a functional consortium into strains for biocontrol of Phytophothora diseases and hence crop protection.
Subject terms: Microbiology, Molecular biology
Introduction
Predation plays a major role in determining community structures [1]. As micropredators, myxobacteria are classified as keystone taxa in the microbial food web and frequently determines community’s organization [2–4]. Myxobacterial predation is commonly considered as a cooperative behavior, but solitary predation by individual cells also leads to prey lysis [5]. During predation, secondary metabolites, lytic enzymes and outer membrane vesicles (OMVs) are secreted as lethal cocktails to extracellularly digest bacterial and fungal prey for consumption, as primarily studied in the genera Myxococcus and Corallococcus [6, 7]. As important soil microbial community members, oomycetes form a diverse group of fungus-like eukaryotes, and plant-pathogenic oomycetes cause devastating diseases [8]. However, mechanisms involved in myxobacterial predation of oomycetes remain underexplored.
The cell wall of microbes, including plant pathogens, constitutes a protective shield from external aggression, and represents a critical barrier between prey microbes and predatory myxobacteria [9]. In order to break through this barrier, myxobacteria evolved a diverse set of predation strategies. These include the ability to disrupt the cell envelope by antimicrobial metabolites and cell wall degrading enzymes (CWDEs). Particular examples include antibiotic TA (myxovirescin) against prey bacteria and β-1,6-glucanase against fungi prey [7, 10]. These contact-independent killing factors exert long-range diffusion and lytic activity against target cells. However, contact-dependent prey lysis is also observed through a Tad-like apparatus and a type III secretion-like system [5, 11, 12]. These combined findings raise the question whether myxobacteria deploy similar strategies in mediating predation of oomycetes by targeting their cell wall.
Phytophthora, a genus of oomycetes, contains various destructive plant pathogenic species, including P. sojae, the agent of soybean root and stem rot. As opposed to fungi, the major composition of oomycetes cell walls consists essentially of cellulose and β-1,3-glucans, while chitin and β-1,6-glucans occur in small amounts in the cell walls of some oomycetes [13, 14]. Considering the wide distribution of cellulose in plant cell walls and the potential damaging effects of cellulases, the utilization of carbohydrate-active enzymes (CAZymes) like β-1,3-glucanases, which mediate plant- or microbe-oomycetes interactions, have received considerable attention as defense mechanisms [15, 16]. Since oomycetes cell wall compositions are complex and dynamic [17], it is commonly assumed that enzymatic complexes, like the lytic mixture used in protoplasts preparations, exhibit efficient extracellular decomposition of cell walls [18, 19]. Recently, we reported the role of a solitary β-1,6-glucanase, named GluM, in the decomposition of fungal cell walls by a myxobacterium [7]. By extension, myxobacterial predation of oomycetes likely also relies on CAZyme-like CWDEs.
Myxobacteria prey on bacterial and fungal pathogens and serve as biological control agents of plant diseases [3, 20, 21]. In this study, we show that some members of myxobacteria control soybean Phytophthora stem rot. We started by investigating secreted factors that could interact with oomycetes by genomic sequence and secretory proteome analysis. We report that three specialized CAZymes (β-1,3-glucanases AcGlu13.1, -13.2 and -13.3) from Archangium sp. AC19 (Cystobacteraceae) are essential for cooperative cell wall decomposition of oomycetes Phytophthora during predation. CAZymes degrade complex carbohydrates and provide a competitive advantage by allowing nutrition utilization, host invasion or immunity response [22, 23]. Diverse glucoside hydrolases are abundantly found in myxobacterial genomes, however AC19 and other myxobacteria are not able to utilize hydrolyzed glycans or their sugar products for growth, raising questions about the functional roles of CAZymes in myxobacteria taxa. Here, we propose the function of this consortium of CAZymes allowed AC19 to adapt and prey on oomycetes. Our discovery of this specialized weapon adds to the knowledge of predator-prey interactions, and opens a new avenue for understanding the ecological consequences of myxobacterial adaptive evolution.
Materials and methods
Growth conditions
Archangium sp. strain AC19 and other myxobacterial isolates were cultured in VY/4 (0.25% yeast cells (Angel, China) and 0.1% CaCl2, pH 7.2, 0.5 mg/L vitamin B12 when necessary, w/v), and VY/4 containing 1.5% agar was used for growth on solid media at 30 °C. The model M. xanthus strain DK1622 and related transformants were cultured on CTT [24] or VY/4 at 30 °C. P. sojae wild-type strain P6497 and its GFP-labeled transformant were used as models of plant-pathogenic oomycetes, which were cultured on V8 plates at 25 °C [25]. Liquid V8 medium was used to collect the mycelium of Phytophthora supplied with Rye sucrose when necessary [26]. All strains (Tables S1 and S2), plasmids and primers (Table S3) used in this study are listed in supplementary materials.
Myxobacteria-oomycetes confrontation assays
Co-cultures of Phytophthora, used as a prey, with different myxobacteria as potential predators were carried out on solid VY/4 medium supplemented with Rye sucrose agar (RSA) and V8 when necessary [26]. These assays were done on 4-mm-diameter agar discs that were removed from the edge of an actively growing prey strain, and transferred to the center of VY/4 or mixed medium (Methods in Supplementary Materials) for 2–3 days (time to attain a colony diameter of 2 cm), after which the myxobacteria were inoculated around the Phytophthora colonies. Swarming growth of myxobacteria was observed by light microscopy. For co-cultural assays in liquid media, the prepared Phytophthora or Magnaporthe oryzae Guy11 mycelium were transferred into TC media (TPM buffer (10 mM Tris-HCl, 8 mM MgS04·7H2O, 1 mM potassium phosphate, pH 7.6), 0.1% casitone, 20 mL) with the same wet weight (50 mg), and the myxobacteria were added to a final concentration of 1×105 cells/mL, and co-cultured at 30 °C for 2–3 days. Detailed observations of the mycelium were realized by scanning electron microscope (SEM, Hitachi SU8010). For qPCR, the total DNA or RNA from solid or liquid conditions was isolated by liquid nitrogen treatment (HiScript III All-in-one RT SuperMix Perfect, Vazyme Biotech Co., Ltd, China) following the manufacturer’s instructions.
Biocontrol assay
To determine the anti-oomycete activity of AC19, soybean cultivar (Hefeng 47) was used as the test plant. Briefly, soybean seeds were surface-sterilized and grown in plastic pots (12 cm diameter and 12 cm height) containing vermiculite at 25 °C with 80% relative humidity under a photoperiod of 16 h-light/8 h-dark for about two weeks. To perform the hypocotyl inoculation tests, the hypocotyls of two-week-old soybean seedlings were incised with a small wound and inoculated with 4-mm-diameter agar discs of P. sojae. The treatments were designed as follows: (1) Control setup of soybean seedlings prepared without any treatment; soybean seedlings incubated with: (2) P6497, (3) mixture of P6497 and AC19 (1 mL, 1 × 106 cells/mL), (4) AC19 (1 mL, 1 × 106 cells/mL), (5) mixture of P6497 and pesticide (mixture of hymexazol and metalaxy, 2,000 × diluent). The agar discs were placed in an AC19 suspension (1 × 106 cells/mL) at 28 °C for 20 min, or sterile water before inoculation. The plants were incubated in a humid environment for 2–3 days. Disease severity in seedlings, including the control efficiency and disease index, were analyzed according to a five scores ranking system: 0, healthy state with no apparent discoloration; 1, less than 25% discoloration; 2, 25–50% discoloration; 3, 50–75% discoloration; 4, more than 75% discoloration of the neck, or dead plants [27]. Each treatment included six replicates with five soybeans plants per pot. The disease severity index was calculated as (number of plants × disease index rate)/(total number of plants × 4). Control efficacy was calculated from the values of disease index, which was estimated as (control-treatment)/control × 100% [27].
Growth inhibition assay
To determine the effects of the extracellular secretion on the growth of P. sojae, Archangium sp. AC19 was cultured in VY/4 liquid media at 180 rpm and 30 °C for 3–4 days, and the supernatant was collected after centrifugation at 12,000 rpm for 20 min at 4 °C. About 10 L supernatant of a culture (SUP) was prepared for further purification of the cell wall hydrolase. The heat-inactive SUP (TSUP) was prepared by placing the SUP in a boiling water bath for 10 min, while low molecular weight substances of SUP (SUF) was prepared by ultrafiltration (molecular cut-off 10 kDa) and used as a control. For the growth inhibition assay, agar discs (0.1 × 0.1 cm) were removed from the edge of actively growing P. sojae, and transferred to the center of 96-well plates containing 90 μL liquid V8 medium and 10 μL SUP or other enzymes in each well with different concentrations and combinations. The growing P. sojae were observed with a stereomicroscope (Nikon SMZ-10) after 24 h incubation at 25 °C, and qPCR analysis was performed to quantify the biomass of P. sojae from different treatments with 6-wells as a repetition. The mycelial morphology was observed by differential interference contrast (DIC) microscopy or SEM when necessary.
Glucoside hydrolase activity assay
The β-1,3-glucanase activity assay was performed using laminarin and pachymaran as substrates, and the amount of released reducing sugar was quantified by the dinitrosalicylic (DNS) acid method [28]. Briefly, reaction mixture comprising 1 mL of 0.5% (w/v) substrate and 10 µL of appropriately diluted enzyme in 50 mM Tris-HCl buffer (pH 7.0) or 50 mM PBS buffer (6.0 or 8.0) was incubated at 60 °C for 20 min. Then the released reducing sugars were determined using the DNS method via the absorption at 540 nm (A540) as described [28]. One unit of β-1,3-glucanase and other hydrolase activity is defined as the amount of enzyme that releases 1 µmol of reducing sugar per minute under the described assay conditions. The protein concentration was determined by the Bradford method using bovine serum albumin (BSA) as the standard [29]. To identified the activity of β-1,6-glucanase, cellulase and amylase, pustulan, CMC-Na and starch (0.5%, w/v) were used as substrates, and the activity assay was done as described above.
Purification, identification and heterologous expression of cell wall hydrolases from Archangium sp. AC19
The SUP of an AC19 culture was prepared as described above. For purification of cell wall hydrolases, 10 L SUP was fractionated at 80% saturation ammonium sulfate in cold condition, followed by centrifugation at 15,000 × g and 4 °C for 20 min, and the pellet was then dissolved in 20 mL of 50 mM Tris-HCl buffer (pH 7.0). The cell wall hydrolases were purified from the dissolved pellet using the prepared cell wall of P. sojae (methods in Supplementary Materials) as an affinity matrix. Briefly, the dissolved solution was mixed with cell wall (20 mg/mL, wet weight) at 4 °C for 2 h with continuous gentle stirring. The cell wall and corresponding supernatant was harvested by low speed centrifugation at 5,000 g and 4 °C for 10 min, and the insoluble sediment was washed 6 times in 50 mM Tris-HCl buffer (pH 7.0, pre-cooled to 4 °C). The binding enzymes were then released from the cell wall-matrix by re-suspending the cell wall in 10 mL Tris-HCl buffer (50 mM, pH 7.0) and incubated at 45 °C for 6 h to allow cell wall enzymolysis, and the supernatant was collected by centrifugation at 12,000 g and 4 °C for 20 min, and subject to ammonium sulfate precipitation (80% saturation) in cold condition. After centrifugation (15,000 × g for 20 min at 4 °C), the resulting pellet was resuspended in 1 mL Tris-HCl buffer (50 mM, pH 7.0) and dialyzed in the same solution at 4 °C for 12 h. The resulting enzyme solution (lytic factors) was analyzed by SDS-PAGE and protein bands identified by NanoLC-ESI-MS/MS. Heterologous expression of identified cell wall hydrolases was done in E. coli BL21 (DE3) with pET29a (+) as the expression vector. Recombinant proteins were purified using a HisSep Ni-NTA MagBreds His (Cat. 20561ES08; Yeasen, Shanghai, China) (Methods in Supplementary Materials).
To characterize hydrolytic activity, laminarin, pachyman, pustulan, CMC-Na and starch were used as substrates, and activity assays of SUP, SUP-S and lytic factors were done as described above. To determine the effects of SUP solutions on the mycelium integrity, the freshly prepared mycelium (5 mg, wet weight) of P. sojae P6497 (methods in Supplementary Materials) was mixed with SUP, SUP-S and lytic factor (0.1 mL) in 1 mL Tris-HCl buffer (50 mM, pH 7.0), followed by incubation at 30 °C for 30 min. The mycelial morphology was observed using DIC microscopy (Zeiss Axio Observer A1).
Assays for binding affinity and hydrolysis pattern
In the binding assay, the purified recombinant AcGlu13.1, -13.2 and -13.3 proteins (1 mg/mL) were mixed with cell walls of P. sojae (50 mg/mL, wet weight), M. oryzae Guy11 (50 mg/mL, wet weight) or MCC (microcrystalline cellulose, 0.2 mg/mL) at 4 °C for 2 h with continuous gentle stirring. BSA (bovine serum albumin) was used as a negative control. The amounts of recombinant proteins remaining in the supernatant or co-precipitated with substrates were quantified by the Bradford method and SDS-PAGE. Fluorescent labeling of AcGlu13.1, -13.2 and -13.3 for MST measurements was done using Lightning-Link (R-PE) Kit (703-0015; Innova Biosciences, Expedeon, San Diego, CA, USA) according to the manufacturer’s protocol. Labeled proteins were used at a concentration of 20 nM with the MST power set to high at a laser intensity of 40%. Binding affinities were measured through a series of 16 successive 29 dilutions of the ligand stock solutions (laminarin) dissolved in the respective assay buffers, and the Kd Fit function of Nano Temper Analysis Software (Version 1.5.41) was used to fit the curve and calculate the value of Kd [30].
For determination of the hydrolysis pattern, the crude cell walls of P. sojae with binding recombinant proteins and SUP, as described above, were re-suspended in 50 mM Tris-HCl buffer (optimum pH) and incubated at 45 °C for 6 h for substrate hydrolysis. The oligosaccharide composition was analyzed by TLC on silica gel 60 plates (Merck, Germany) using n-butanol-methanol-H2O (8:4:3, v/v/v) as the solvent system, and HPLC using a Cosmosil Sugar-D column and a RID detector (Nacalai Tesque Co., Japan) [7]. The substrate specificity of recombinant proteins was performed using laminarin and pachymaran (5 mg/mL) as the substrate, and hydrolysis products were analyzed by TLC. General properties of AcGlu13.1, -13.2 and -13.3, including optimal pH and temperature, were investigated using laminarin as the substrate (methods in Supplementary Materials).
Assays for cell viability and pathogenicity
The freshly prepared GFP-labeled P. sojae or M. oryzae mycelium (5 mg, wet weight) was mixed with AcGlu13.1, -13.2 and -13.3 (0.01 mg/mL) in Tris-HCl buffer (50 mM, pH 7.0), followed by incubation at 30 °C for 30 min. The buffer-treated mycelium was used as control. Fluorescence detection was performed using a Zeiss LSM 710 confocal laser scanning microscope (Carl Zeiss, Jena, Germany) [12]. The pathogenicity phenotypes of P. sojae were determined by inoculation of hypocotyls of etiolated soybean seedlings [31]. 4-mm-diameter agar discs were removed from the edge of an actively growing P. sojae P6497, followed by placing into 0.01 mg/mL AcGlu13.1, -13.2 and -13.3 protein solutions and combinations of GluC.1 + 2 + 3 (0.03 mg/mL), or buffer (50 mM Tris-HCl) at 30 °C for 30 min. Then the agar discs were removed from the protein solution and inoculated onto the hypocotyls of etiolated soybean seedlings (susceptible Williams cultivar), followed by incubation in the dark at 25 °C. Disease symptoms were scored 36 h after infection, the lesion lengths were measured, and the biomass of infected P. sojae was determined by qPCR.
Assays for synergistic decomposition of oomycetes Phytophthora cell walls
The synergistic effects of AcGlu13.1, -13.2 and -13.3 on the growth of P. sojae P6497 was determined by different combinations of the three enzymes: AcGlu13.1, -13.2, -13.3 with a final concentration of 0.1–5 μg/mL for each enzyme; GluC.1 + 2: AcGlu13.1 + AcGlu13.2; GluC.1 + 3: AcGlu13.1 + AcGlu13.3; GluC.2 + 3: AcGlu13.2 + AcGlu13.3 (0.2-10 μg/mL, equal mass mixture); GluC.1 + 2 + 3: AcGlu13.1 + AcGlu13.2 + AcGlu13.3 (0.3-15 μg/mL, equal mass mixture). P. sojae growth was determined in 96-well plates as described above, and the biomass of P6497 was qualified by qPCR for contrastive analysis.
The synergistic decomposition of crude P. sojae cell walls was determined with AcGlu13.1, -13.2, -13.3 and combination GluC.1 + 2 + 3 consisted of the three enzymes. Single enzyme (0.01 mg/mL and 1 mg/mL) or GluC.1 + 2 + 3 with a final concentration of 0.03 and 3 mg/mL (equal mass mixture) was mixed with mycelium (200 mg/mL, wet weight) and incubated at 45 °C for different reaction times. The amount of released reducing sugar was quantified by the DNS acid method [28].
Cooperative predation test of M. xanthus strains
Heterologous expression of AcGlu13.1, -13.2 and -13.3 in M. xanthus DK1622 under the pilA promoter produced the corresponding derivative strains CL1008, CL1009 and CL1010 [24]. Vector construction and transformation methods are described in Supplementary Methods. These M. xanthus strains were used in a series of predation tests. P. sojae was cultured on VY/4 medium at 25 °C for 2–3 days until the colony diameters reached approximately 2 cm, after which M. xanthus strains were inoculated around the P. sojae colonies. The swarming growth of myxobacteria at the contact zone with P. sojae mycelium was observed by light microscopy (Olympus CX31). The cooperative predation by myxobacteria was determined by construction of synthetic bacterial community with combination of CL1008, CL1009 and CL1010. The single strain cultures were adjusted to a final density of 1 × 109 cells/mL, ConS.1-1 was an equal volume mixture of strains with a final concentration of 3 × 109 cells/mL. ConS.1-3 and ConS.1–5 was a mixture of CL1008, CL1009 and CL1010 with mixing ratio of 1:1:1 and 1:5:5, respectively, and the final concentrations of ConS.1-3 and ConS.1–5 were 1 × 109 cells/mL and 2.2 × 109 cells/mL, respectively. Each inoculated site contained 3 μL, and total DNA from 16 sites (1.5 cm × 1.5 cm for each site) on the solid plates was isolated. The biomass of P. sojae P6497 and myxobacteria during co-culture was qualified by qPCR.
Feeding experiment in soil
To determine the growth performance of myxobacteria in the presence of P. sojae P6497 in soil, non-sterile soil was mixed with P6497 mycelium at a final density of 0.5 mg/g (wet weight of mycelium/soil), after which myxobacteria were added followed by uniform soil mixing. Treatments were designed as follows: AC19, DK1622 or CL1008 were used at a final concentration of ~1 × 104 cells/g, respectively; DK1622 + CL1008 or DK1622 + AC19 mixed at ratios of 1:1, at final concentrations of 2 × 104 cells/g, respectively. The soil pots (200 g) were incubated at 30 °C, where the non-sterile soil and P6497-containing soil, without any treatment, were used as controls. Soil was collected and DNA extracted at different time intervals (0, 3, 5, 7 and 10 day), and the biomass of AC19, DK1622, CL1008 and P6497 was quantified by qPCR. Characteristic primer that designed from acGlu13.1 was used for biomass quantification of CL1008. Three independent biological replicates were carried out with a total of six pots per treatment.
Statistics and reproducibility
All experiments were repeated at least three times where similar results were found. Data represent mean ± SE of all biological replicates. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Duncan’s test as a multiple comparison test using SPSS 20.0 (IBM Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically high significant (**), value of p < 0.01 was considered statistically highest significant (***). All gels, blots, and micrographs were repeated for at least three times independently with similar results.
Results
Archangium sp. AC19 preys on Phytophthora and protects soybeans from stem rot disease
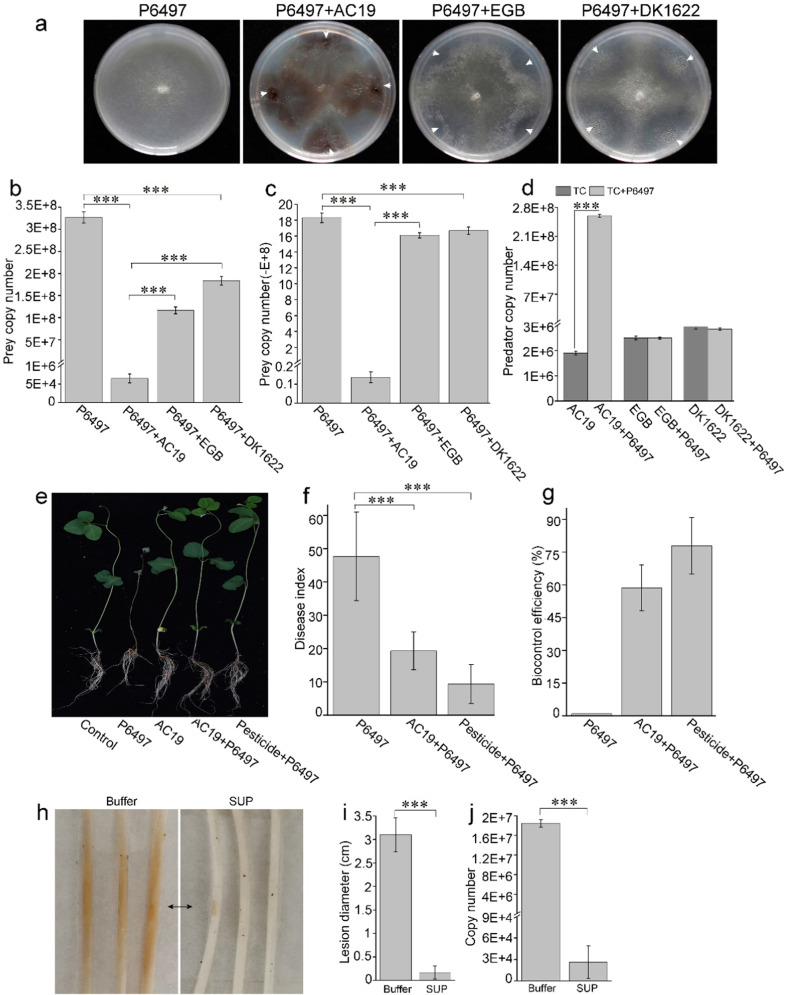
As mobile predators, myxobacteria prey on a wide variety of bacteria and fungi [32]. To investigate potential interactions with oomycetes, we tested the action of various myxobacteria on P. sojae P6497, a model species for the study of oomycetes plant pathogen [33]. We co-cultured 66 different myxobacterial isolates against P6497 on solid VY/4 media. Most isolates demonstrated various degrees of inhibitory activities against P. sojae (Fig. S1). An isolate (designate as AC19), belonging to genus of Archangium by sequence analysis of four housekeeping genes (16 S rRNA, fusA, clpX and lepA) (Fig. S2), showed strong predatory activity against P6497 during co-cultivation. AC19 was able to feed and grow on P. sojae in plate assays, resulting in dilapidated P6497 colonies. This was distinct from growth inhibition of P6497 by Corallococcus sp. EGB and M. xanthus DK1622, which produced a zone of growth inhibition, apparently caused by diffusible factors (Fig. 1a). To enumerate the viability of P. sojae from the dilapidated colonies, qPCR analysis was performed. Results showed that the biomass of P6497 was reduced by about four orders of magnitude by AC19 (Fig. 1b). Decreased biomass of P6497 (two orders of magnitude) were also observed from co-culture with AC19 in liquid conditions (Fig. 1c). Whereas co-cultures of EGB and DK1622 only resulted in slight P6497 biomass reduction in both solid (Fig. 1b) and liquid conditions (Fig. 1c). Importantly, AC19 biomass increased by two orders of magnitude, in liquid media, a clear indication of predation, whereas the biomasses of EGB or DK1622 remained unchanged (Fig. 1d). These results were consistent with the prey growth inhibition findings from plate assays. Additionally, growth promotion of AC19 from prey-killing was also observed from co-cultures of P. capsici, P. nicotianae and P. infestans on solid (Fig. S3a) and in liquid (Fig. S3b, c), indicating that AC19 has broad-spectrum anti-Phytophthora properties. These results show that AC19 kills and consumes P. sojae during myxobacteria-prey interactions.
Fig. 1. Biocontrol of P. sojae by Archangium sp. AC19.
a Co-cultures of P. sojae P6497 against AC19, EGB or DK1622 on VY/4 plates. P6497 was cultured on VY/4 plates for 2–3 days (time to attain a colony diameter of 2 cm), and then myxobacteria were spotted near Phytophthora colonies and gown at 30 °C for 3 days. White arrow heads indicate the inoculation sites of myxobacteria. b Quantitative analysis of the biomass of P6497 from the solid plates by qPCR. P6497 was cultivated separately or co-cultured with indicated myxobacteria on VY/4 plates. Genomic DNAs were harvested from plates and P6497 biomass quantified. Biomass analysis of P6497 (c) and myxobacteria (d) was quantified from liquid TC media (TPM buffer, 0.1% casitone, pH 7.2) by qPCR. Indicated myxobacterial strains (1 × 105 cells/mL, final concentration) were incubated alone or with P6497 (50 mg, wet weight) in 20 mL TC media at 30 °C for 2 days. e Effects of P6497 infection in conjunction with AC19. Disease index (f) and biocontrol efficiency (g). All soybean seedlings were incised with a small wound and inoculated with 4-mm-diameter agar discs of P6497 with or without AC19 or pesticide except for the control sample. h–j Biocontrol efficacy of SUP in soybean hypocotyl infection assays. The agar discs (4-mm-diameter) of P6497 were pretreated with SUP (0.02 mg/mL) at 30 °C for 20 min before incubation. Representative photographs (h), average lesion diameters (brown color; three representative samples; black arrow indicates inoculation sites) (i) and quantitative biomass of P6497 (j) with standard errors shown. The biomass was measured by qPCR of genomic DNA with standard curves. Error bars denote ± standard error of the mean (SD), and asterisks (***) indicate statistically significant differences (p < 0.01) to control based on Student’s t test.
To determine whether AC19 inhibited the growth of P. sojae on soybean plants, we conducted biocontrol experiments in a growth chamber. As shown in Fig. 1e-g, AC19 exhibited prominent biocontrol efficacy (60%) in soybean hypocotyl infection assays compared with chemical pesticide (80%), which produced healthy plants with significant reduction in P. sojae lesions (Fig. 1e and Fig. S4). The interactions between AC19 and P6497 were further examined by time-lapse video microscopy, which showed that when approaching the mycelia of P. sojae P6497, AC19 attached to and moved along the mycelia (Movie S1). This finding indicated that AC19 uses the mycelia as a dispersal network that promotes efficient predation. To assess activity of the supernatant fraction (SUP), a piece of mycelium of P. sojae was first inoculated onto the hypocotyls of etiolated soybean seedlings. Symptoms became visible after 36 h on plants pretreated with buffer, whereas the SUP protected soybean from disease development (Fig. 1h). P. sojae lesion length (Fig. 1i) and biomass (Fig. 1j) in the inoculated soybean plants was quantified and revealed that SUP significantly reduced P. sojae growth and inhibited infection. These results show that AC19 is an effective biocontrol agent of P. sojae that includes secreted substance(s).
Secretome analysis reveals CAZymes acting on P. sojae cell wall
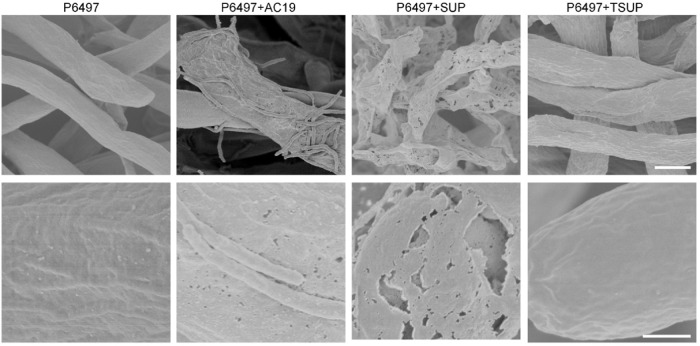
We sought to identify molecules involved in AC19 predation, with a focus on CAZymes in the SUP fraction. The SUP, thermally inactivated SUP (TSUP) and SUP ultrafiltrate (SUF; cutoff <10 kDa) samples were tested for inhibition on P. sojae P6497 growth. In contrast to SUP, TSUP and SUF exhibited no obvious effects on growth of P6497 (Fig. S5). Additionally, the morphology of the P6497 mycelial cell wall was examined after AC19 and SUP treatments. SEM observations showed that cell wall integrity of P6497 treated with TSUP remained intact compared with perforated structures after treatments with AC19 and SUP (Fig. 2). Hence, we concluded that proteins, i.e. cell wall hydrolases from AC19, were involved in the predation and digestion of Phytophthora.
Fig. 2. Cell wall decomposition of P. sojae by AC19 and SUP.
SEM analysis of the cell walls of P. sojae P6497 after co-culture withAC19, SUP or TSUP treatments. SUP, cell-free culture supernatants of AC19; TSUP, thermally inactivated SUP; Scale bars: top, 5 μm; bottom, 1 μm.
Considering the insolubility of β-glucan in the P. sojae cell wall, we hypothesized that cell wall hydrolases from AC19 contained carbohydrate-binding domains that contributed toward efficient cell wall hydrolysis. Using P. sojae cell wall as an affinity matrix, cell wall binding and lytic factors were absorbed from culture supernatants of AC19. Consistent with our hypothesis, absorption led to substantial loss of cell wall decomposition activity in the SUP fraction (Fig. S6a). Binding factors were then released by incubation at 45 °C for 6 h, by allowing cell wall material to be hydrolyzed by the absorbed proteins. Compared to the SUP fraction, these released factors in turn showed a strong lytic activity towards P. sojae (Fig. S6). Substrate specificity tests showed that a putative β-1,3-glucanases and β-1,6-glucanases were significantly enriched to the absorbed proteins. However, β-1,4-glucanases were detected in SUP without enrichment (Table S4). These data suggest that SUP contains CAZymes-like CWDEs that exhibits efficient cell wall binding and decomposition activity.
We examined the occurrence, diversity, functional classification of genes encoding CAZymes from the AC19 genome. Of 9,999 gene sequences, 501 encoded CAZyme sequences (5.01%), where carbohydrate binding and glycosidic bond hydrolyase-affiliated sequences contributed 54.5% (273 out of 501) (Fig. S7). Using quantitative proteomics, we next identified 186 proteins in the SUP by MS/MS and database searching, where 22 proteins (11.8% of 186 proteins) were predicted to be hydrolases with carbohydrate-binding or fascin-like module or lectin from functional domain predictions, representing 8.06% (22 out of 273) of the CAZymes described above. Among them, 12 proteins (6.5% of the total) contained N-terminal signal peptides (SP) for secretion, consistent with their extracellular localization (Table S5). To assess activity, these proteins were cloned, heterologously expressed in E. coli and purified, and their glycoside hydrolase activities were characterized. These CAZymes contain predicted β-1,3-glucanase (6/12), β-1,4-glucanase (3/12) and chitinase (3/12) domains, which was consistent with P. sojae cell wall composition. The impact of these proteins on mycelial morphology and cell integrity was assessed with a GFP-labeled P. sojae. Here enzymatic treatments from several β-1,3-glucanases resulted in significant leakage of fluorescence (Table S5).
Exploration of the core secretome with P. sojae cell wall-binding capacity
To further screen for the CAZymes in the SUP fraction (Fig. 2 and Table S5), monosaccharide composition of the released soluble saccharides from absorbed proteins hydrolysis was analyzed. Results showed that the released saccharides were mainly composed of glucose (Fig. S8), indicating the presence of β-glucanases. We checked gene expression levels of the identified CAZymes during the co-culture of AC19 and P. sojae P6497 by qRT-PCR. The expression levels of three genes (ID: 1003498; 1003497 and 1009265) were significantly upregulated by 138.0, 53.4 and 44.7 folds upon co-culture with P. sojae (Fig. S9). We therefore suspected these core members of the secretome (termed AcGlu13.1 for 1009265, AcGlu13.2 for 1003498 and AcGlu13.3 for 1003497) play important roles in AC19 predatory activity against P. sojae. Sequence analysis revealed that AcGlu13.1 and AcGlu13.2 contain SP motifs and glycoside hydrolase (GH) catalytic domains associated with the GH55 and GH30 families, respectively, while AcGlu13.3 contains a SP and a fascin-like module (Fig. S10), which was characterized as a novel member of a carbohydrate-binding module (CBM) family [34]. Phylogenetic and BLASTx analysis indicated that AcGlu13.1, -13.2 and -13.3, as well as their orthologs from the genera of Myxococcaceae and Cystobacteraceae (Fig. S11), contain conserved key residues in the catalytic region (Fig. S12), but share low identities (less than 30%) to the identified glycoside hydrolases from CAZy database (Fig. S13).
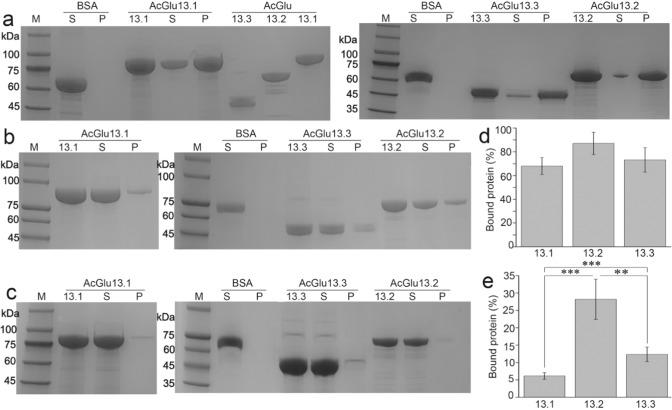
Enzyme assays showed that the specific activities of AcGlu13.1, -13.2 and -13.3 were 339.8, 31.9 and 757.3 U/mg, respectively, with laminarin as substrate (Table S6). Since our tests found that the P. sojae cell wall was an efficient binding matrix for purification of the β-1,3-glucanases, we deduced that these β-1,3-glucanases possess high substrate affinity. To verify this and test for specificity, enzyme binding to insoluble P. sojae cell wall material (mainly β-1,3-glucan and cellulose), MCC (cellulose) and soluble laminarin (branched β-1,3-glucan) was tested by pull-down and MST assays. AcGlu13.1, -13.2 and -13.3 bound with high affinity to the P. sojae cell wall (Fig. 3a, d) and moderate affinity to MCC (Fig. 3b, e). However negligible affinity to the M. oryzae cell wall was detected (Fig. 3c). AcGlu13.2 did not bind laminarin, while AcGlu13.1 and AcGlu13.3 bound with rather low affinities (Kd of 0.63 and 0.59 mM) (Fig. S14a). We therefore concluded that AcGlu13.1 and AcGlu13.3 bind to β-1,3-glucans and β-1,4-glucans in P. sojae cell wall, while AcGlu13.2 may specifically bind β-1,4-linked and/or β-1,4/1,3 branched chains, as fungal cell walls are composed of β-1,3/1,6-branched glucans without β-1,4-glucan linkages [14]. The specific binding ability of AcGlu13.1, -13.2 and -13.3 to the cell wall of P. sojae P6497 likely contributes to their efficient cell wall lytic activity.
Fig. 3. Binding affinity of AcGlu13.1, -13.2 and A-13.3 to cell wall material.
SDS-PAGE analysis of adsorbed recombinant enzymes from pull-down assays with P. sojae cell wall (a), 50 mg/mL, (wet weight) and MCC (b), (0.2 mg/mL) and M. oryzae cell wall (c), 50 mg/mL, (wet weight) as substrates. BSA (bovine serum albumin) was used as a negative control. The protein concentration of AcGlu13.1, –13.2 and –13.3 was 1 mg/mL. The corresponding proportions of the bound proteins in each mixture of P. sojae cell wall (d) and MCC (e) were quantified using the Bradford assay. The proportion of bound protein (%) from M. oryzae cell wall was not calculated as the extremely low binding affinity. M, protein marker; S, supernatant; P, pellet.
Multiple sequence analysis revealing the possible origin of predatory enzymes
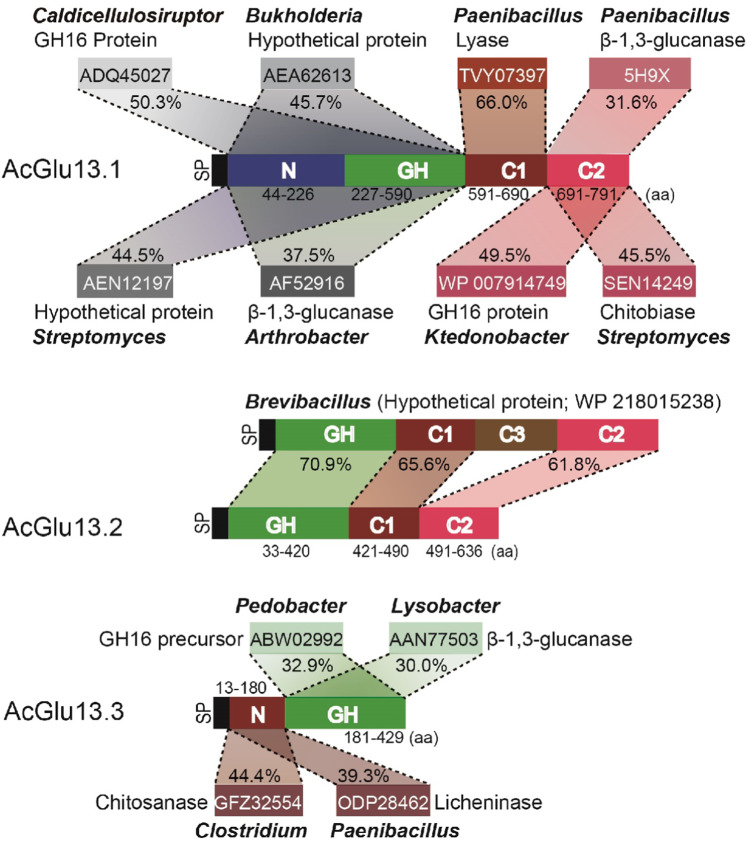
Since AcGlu13.1, –13.2 and –13.3 and orthologues had a relatively limited distribution in myxobacteria, and bacteria are well known to acquire genes from their environment by various strategies [35], we searched for homologous sequences in diverse microbes. The GH catalytic domains of AcGlu13.1 and AcGlu13.3 had their closest orthologues in Caldicellulosiruptor, Burkholderia, Streptomyces, Arthrobacter, Pedobacter and Lysobacter (identity between 37.5% and 50.3%). Additionally, the C1 and C2 domains in AcGlu13.1 share 66% and 49.5% sequences identities to a Paenibacillus lyase (TVY07397.1) and Ktedonobacter glycoside hydrolase (WP 007914749.1), respectively, as well as 45.5% sequences identity to a Streptomyces chitobiase (SEN14249) (Fig. 4 and Fig. S15a, b). Furthermore, the N-domain in AcGlu13.3 shares 44.4% and 39.3% sequence identities to a Clostridium chitosanase (GFZ32554.1) and Paenibacillus licheninase (ODP28462.1), respectively (Fig. 4 and Fig. S15c). Curiously, a hypothetical protein (WP 218015238.1) from Brevibacillus shares 64.6% sequences identity to AcGlu13.2 with similar GH, C1 and C2 domains, but homology to the C3 domain (140 amino acids) was absent (Fig. 4 and Fig. S16). Taken together, as all these domains of AcGlu13.1, -13.2, and -13.3 have their closest orthologues in distant soil bacterial species (identity between (37.5% and 70.9%)), we suggest that AC19 obtained AcGlu13.1 and AcGlu13.3 by horizontal gene transfer (HGT) or predation and domain swaps from other microbes, while AcGlu13.2 was similarly acquired by HGT, for example, from a Brevibacillus-like microbe, where the C3 domain was lost [36]. In turn, certain myxobacteria evolved as efficient predators of oomycetes.
Fig. 4. Homologous sequence analysis of the core CAZymes from AC19 secretory SUP.
AcGlu13.1, -13.2 and -13.3 each contain multiple domains that share their highest identity with proteins from indicated bacteria. SP represents signal peptide; N represents N-terminal domain; GH represents catalytic domain; C1, C2 and C3 represent the C-terminal domain; Amino acid (aa) numbers represent the locations of different domains.
Unique modes of action of core CAZymes enable efficient anti-Phytophthora activity
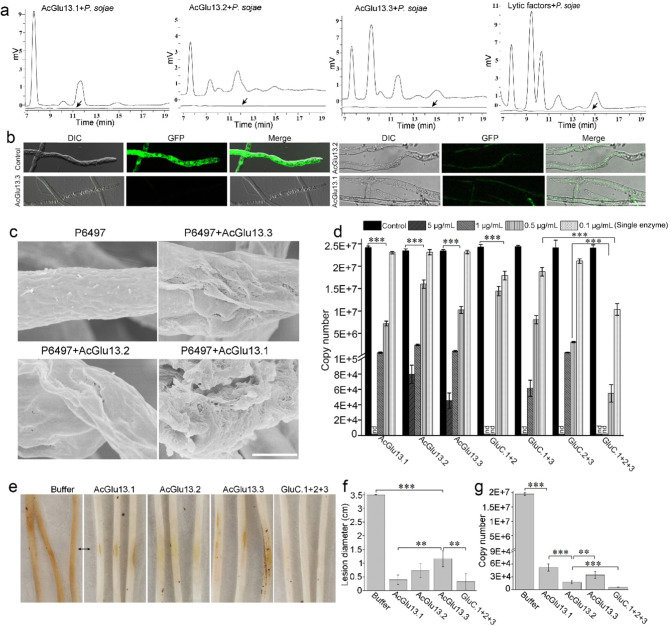
To clarify the modes of action on Phytophthora cell walls, we characterized the three core CAZymes by their ability to hydrolyze the P. sojae cell wall. This analysis found they released oligosaccharides and glucose (Fig. 5a), again demonstrating that AcGlu13.1, –13.2 and –13.3 altered the integrity of the P. sojae cell wall. Furthermore, HPLC analysis showed that AcGlu13.2 and AcGlu13.3 exhibit similar product profiles from P. sojae cell wall hydrolysis, while AcGlu13.1 showed a different product profile (Fig. 5a). AcGlu13.2 showed endo-hydrolytic activity toward branched β-1,3-glucan (laminarin), and no activity was observed toward pachymaran, whose profile was different from AcGlu13.1 and AcGlu13.3 (Fig. S17). These combined results show that AcGlu13.1, -13.2 and A-13.3 exhibit overlapping and yet distinct hydrolytic characteristics.
Fig. 5. AcGlu13.1, -13.2 and -13.3 degrade cell walls of P. sojae and reduce virulence.
a HPLC analysis of the released oligosaccharide from enzymatic hydrolysis of P. sojae cell wall material (200 mg/mL). The heat-inactive enzyme from the linear efflux curve was used as control (bottom flat lines, black arrow). b Microscopic analysis of enzyme treated GFP-labeled P. sojae mycelia. The reaction systems were done with purified AcGlu13.1, –13.2 and –13.3 (0.01 mg/mL) and P. sojae mycelia expressing cytoplasmic GFP, at 30 °C for 30 min. Cell envelop disruption was detect by loss of the GFP signal and the mycelial morphology was observed by DIC microscopy, scale bar: 25 μm. c SEM analysis of the cell walls of P. sojae P6497 after AcGlu13.1, –13.2 and –13.3 treatments (10 μg/mL). Scale bar, 2 μm. d Quantification of P. sojae biomass after AcGlu13.1, –13.2 and –13.3 treatments by qPCR in 96-well plates. The growth state of P. sojae was observed by light-microscope (Fig. S18a). The concentration of AcGlu13.1, –13.2 and –13.3 was 5, 1, 0.5 and 0.1 µg/mL, GluC.1 + 2: combinations of AcGlu13.1 and AcGlu13.2 (equal mass mixing); GluC.1 + 3: combines AcGlu13.1 and AcGlu13.3 (equal mass mixing); GluC.2 + 3: combines AcGlu13.2 and AcGlu13.3 (equal mass mixing); GluC.1 + 2 + 3: combines AcGlu13.1, -13.2 and -13.3 (equal mass mixing); nd, not detected. e–g Effects of enzymatic treatments on soybean hypocotyl infection. The agar discs (4-mm-diameter) of P6497 was pretreated by single enzyme (0.01 mg/mL) and GluC.1 + 2 + 3 (0.03 mg/mL) at 30 °C for 20 min before incubation. Representative photographs (e), average lesion diameters (f) and quantitative biomass of P6497 (g) with standard errors were analyzed. A value of p < 0.05 was considered statistically significant (**), value of p < 0.01 was considered statistically highest significant (***).
The impact on mycelial morphology of P. sojae treated with the three core CAZymes was next examined. Enzymatic treatments caused the leakage of cytoplasmic contents as indicated by the release of the GFP (Fig. 5b). Similarly, perforation of the mycelial structure was observed upon AcGlu13.1, -13.2 and -13.3 treatments (Fig. 5c), which paralleled the results from AC19 and SUP treatments (Fig. 2). AcGlu13.1 treatment resulted in exfoliated surface layers of the cell wall compared with perforated structures caused by AcGlu13.2 and AcGlu13.3, suggesting that AcGlu13.1 displays a distinct degradation activity.
We explored the effect of the three CAZymes on P. sojae growth. Amongst various concentrations, the 0.5 µg/mL minimum concentration of enzymes were sufficient to suppress mycelial growth (Fig. 5d and Fig. S18a). Otherwise, inhibiting activity toward P. sojae growth was negligible from single enzyme treatments at a concentration of 0.1 µg/mL and weak activity with enzyme pairs at 0.2 µg/mL. Strikingly, when AcGlu13.1, –13.2 and –13.3 (GluC.1 + 2 + 3) were mixed, growth inhibition of P. sojae was dramatically (58.3% reduction) increased at 0.1 µg/mL for each enzyme, compared with single enzyme use, and to a lesser extent compared with dual enzyme pairs (Fig. 5d). A cooperative-like growth inhibition was also observed from GluC.1 + 2 + 3 with assigned concentration of 0.5 µg/mL for each enzyme (Fig. 5d). These results show that the three-enzyme complex displays synergistic interactions on inhibiting P. sojae growth.
In the soybean hypocotyl infection assay, AcGlu13.1, -13.2, -13.3 and GluC.1 + 2 + 3 all exhibited prominent inhibition against P. sojae infections (Fig. 5e). Here, after treatment of P. sojae with enzyme at a concentration of 10 μg/mL, a significant reduction in lesion length (Fig. 5f) and biomass (Fig. 5g) was observed after 36 h on plants. Determination of P. sojae biomass in the inoculated soybean plants, done by quantitative genomic PCR, revealed that GluC.1 + 2 + 3 treatment reduced the biomass of P. sojae by four orders of magnitude compared to the control, and outperformed the single treatments of AcGlu13.1, –13.2 and –13.3 (Fig. 5g). Moreover, these enzymes also exhibited inhibitory activity against the oomycetes P. capsici, P. nicotianae and P. infestans (Fig. S18b, c), suggesting a broad-spectrum anti-Phytophthora activity. These findings suggest these enzymes are efficient CAZymes against diverse members of Phytophthora.
Heterologous glucanases expression endows predatory behavior on oomycetes by a laboratory strain
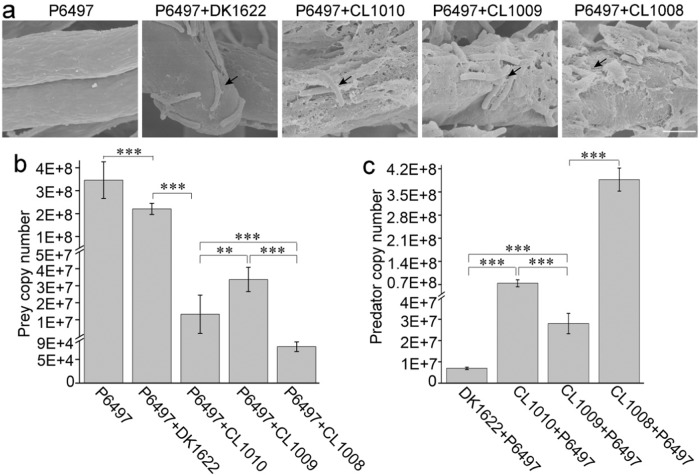
Because of failed attempts to genetically manipulate AC19, the model strain M. xanthus DK1622 was tested as a surrogate to probe the role of the discovered enzymes in predation of oomycetes. This was possible because DK1622 showed poor inhibitory activity against P. sojae growth (Fig. 1a–c). We therefore heterologously expressed AcGlu13.1, -13.2 or -13.3 in DK1622 by using the site-specific integration vector pSWU19 (Fig. S19a), generating strains CL1008 (AcGlu13.1), CL1009 (AcGlu13.2) and CL1010 (AcGlu13.3), which had similar growth rates to WT (Fig. S19b). Co-culture assay of M. xanthus and P. sojae was performed on VY/4 medium, and their interactions was further examined by SEM. Here a large number of M. xanthus cells attached to the surface of P. sojae mycelia. When treated with DK1622, the mycelial cell wall integrity of P6497 remained intact (Fig. 6a), and fruiting bodies formation from the co-culture assay indicated that DK1622 cannot acquire enough nutrients (Fig. S19c). While in contrast, perforated and decomposed structures were seen after treatments with CL1008, C1009 or CL1010, at the sites of myxobacterial attachment (Fig. 6a). Hence, combing with SEM observation, we deduced that these engineered strains gain predatory ability as they could forage over P. sojae mycelia by gliding movements along the mycelium (Fig. S19c).
Fig. 6. Essential roles of AcGlu13.1, -13.2 and -13.3 in the predation of P. sojae by a heterologous myxobacterium.
a SEM analysis of the cell walls of P. sojae P6497 after co-culture with DK1622, CL1008 (acGlu13.1), CL1009 (acGlu13.2) or CL1010 (acGlu13.3). Mycelial cell wall integrity was intact when co-cultured with DK1622, compared with decomposed surfaces at the contact sites by the derivative M. xanthus cells. Black arrows indicate attached myxobacteria. Representative SEM images shown; scale bar, 2 μm. qPCR analysis of the biomass of P6497 (b) and M. xanthus strains (3 μL, 1 × 109 cells/mL) (c) from co-culture assays on VY/4 plates. All myxobacteria (3 μL for each site) were inoculated next to a colony of P6497 and incubated for 3 days. Total DNA was isolated from a mixture of 16 sites (1.5 cm × 1.5 cm) and biomass determined from three independent experiments. The growth state of P. sojae P6497 was observed by light-microscope (Fig. S15c).
To quantify their predatory behavior, qPCR was used to measure biomass of strains during co-culture. The results showed that the biomass of P6497 (3.5×108 copy number) was reduced to 3.4 × 107 copy number and 1.3 × 107 copy number from predation by CL1009 and CL1010, respectively, and four orders of magnitude reduction by CL1008 (reduced to 7.7 × 104 copy number) was also identified. In contrast, the relative biomass of P6497 only decreased by 65% when incubated with the parent strain DK1622 (reduced to 2.2 × 108 copy number) (Fig. 6b). These results were consistent with co-cultural assays where DK1622 initiated development in the absence of sufficient predation and hence lack of nutrition (Fig. S19c). In addition, the predation of P6497 significantly supported the growth of CL1008, C1009 and C1010 by increasing their biomass of about one or two orders of magnitude, while DK1622 showed little change (Fig. 6c).
In prior studies we showed that DK1622 bearing a β-1,6-glucanase (aka Oar protein) was able to prey on the fungus M. oryzae [7]. This result suggests that M. xanthus strains harboring the β-1,3-glucanases AcGlu13.1, -13.2, and -13.3 may enhance the predatory ability toward fungi, considering the nature of fungal cell wall composition. To test this hypothesis, mycelial morphology of M. oryzae was examined from enzymatic treatments and co-culture assays. We found that the addition of these enzymes (Fig. S20a), or expression of them in DK1622 derived strains (Fig. S20b, c), exhibited no obvious effects on mycelial morphology and growth of M. oryzae Guy11 compared to controls. Nevertheless, these results were consistent with the binding results (Fig. 3c). In contrast, co-cultures of AC19 resulted in obvious biomass reduction of M. oryzae Guy11 (Fig. S20c), suggesting that AcGlu13.1, -13.2 and -13.3 were narrow spectrum weapons for predation of oomycetes Phytophthora by AC19. Together, these results indicate that AC19 evolved the ability to prey on oomycetes and fungi by use of different weapons, and heterologous expression of AcGlu13.1, –13.2 and –13.3 in M. xanthus provided a specific predatory advantage towards oomycetes.
Three CAZymes in Myxococcus constitute a cooperative cell wall degrading consortium
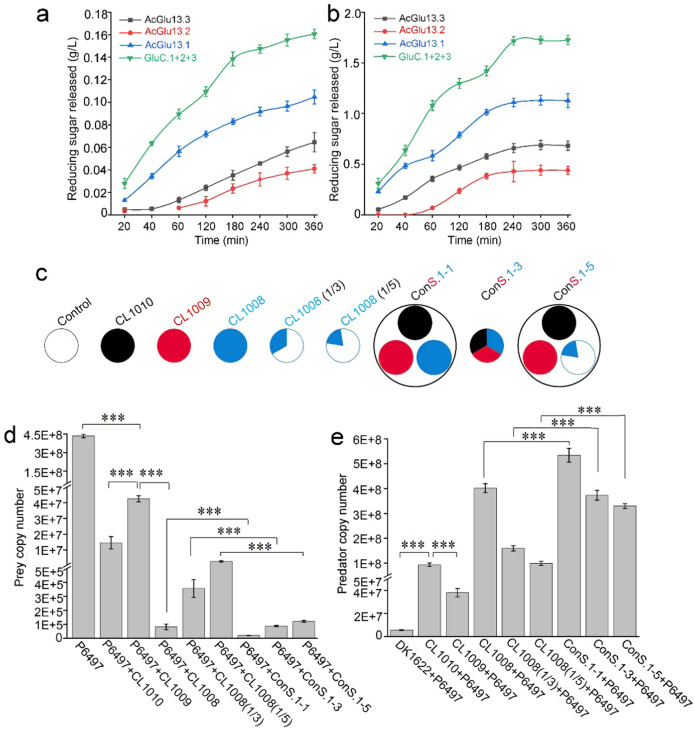
We hypothesized that the three CAZymes AcGlu13.1 and -13.2 and -13.3, expressed in Myxococcus constitute a multi-enzyme consortium governing cell-wall hydrolysis in a synergistic or cooperative manner. To test this, we first assayed the in vitro decomposition of P. sojae cell wall. We found that the three-enzyme consortium was more active on P. sojae cell wall degradation over a 360 min period, as assessed by efficient release of reducing sugars, as compared with single enzyme treatments. This enhanced hydrolysis was also observed over a range of enzyme-to-substrate ratios (Figs. 7a, 1:20,000; Figs. 7b, 1:200, mg/mg). As mentioned above, these enzymes exhibit a high binding affinity toward the P. sojae cell wall (Fig. 3a, Fig. S14b). Together, these data show that AcGlu13.1, –13.2 and –13.3 can degrade the P. sojae cell wall in an additive or even synergistic action, meaning that the enzymes in combination had more potent activity than individual enzymes.
Fig. 7. Cooperative predation of P. sojae by M. xanthus engineered with a multi-enzyme system.
a, b Reducing sugar production by purified AcGlu13.1, -13.2, -13.3 enzymes and a consortium mixture against the P. sojae cell wall. The hydrolysis mixtures contained mycelium (wet weight, 200 mg/mL) and single enzymes (0.01 mg/mL), (a); 1 mg/mL, (b) and were incubated at 45 °C and assayed at different times. The consortium was a combination of the three enzymes (equal mass mixture) with a final concentration of 0.03 (a) and 3 mg/mL (b). (c) Experimental scheme of three synthetic communities (ConS) based on the different combinations of strains. The circle denotes the population of each strain with equal proportion and the color schemes correlate with the enzymes used in (a) and (c). Community cells were re-suspended to uniform suspensions (3 μL) before incubated with P. sojae on agar plates. Quantitative biomass of P. sojae P6497 (d) and M. xanthus (e) from co-culture assays on VY/4 solid plates by qPCR. All M. xanthus strains (3 μL for each incubation site) were inoculated next to the colony of P6497, and DNA was isolated from a mixture of 16 sites (1.5 cm × 1.5 cm) and biomass determined from three independent experiments. The growth state of P6497 was observed by light-microscope (Fig. S21).
We tested for in vivo predation of P. sojae by synthetic communities consisting of different combinations of strains CL1008, CL1009 and CL1010 (Fig. 7c and Fig. S21). These co-culture assays were again done on VY/4 agar plates. The prey biomass of P. sojae was reduced by four orders of magnitude (reduced to 2.1 × 104 copy number) upon predation of ConS.1-1 (three equal number strain mixture) compared to the non-inoculated control (4.3 × 108 copy number), and outperformed the sum of three single treatments (reduced to 8.1 × 104 copy number) (Fig. 7d). Consistent with this, the corresponding results of predator biomass increased with the ConS.1-1 consortium (Fig. 7e), suggesting a cooperative interaction between CL1008, CL1009 and CL1010 in the synthetic community during P. sojae predation. We also observed that CL1008 displayed robust activity in prey-killing compared with CL1009 and CL1010, which was consistent with our earlier results (Fig. 6b, c). Therefore, to address assay sensitivity, we changed the predator synthetic mixture by reducing the number of CL1008 cells, generating ConS.1-3 (one-third cell number for all three strains) and ConS.1–5 (one-fifth cell number of CL1008 versus CL1009 and CL1010), and the results were compared to monocultures of CL1008 (1/3) and CL1008 (1/5), respectively (Fig. 7c schema). qPCR results showed that CL1008 (1/3) at lower population densities exhibited a proportional drop in predatory ability compared with CL1008 (Fig. 7d, e). In contrast, ConS.1-3 retained the ability to prey on P. sojae compared with CL1008 (1/3). For example, prey biomass was reduced by four orders of magnitude (reduced to 8.7 × 104 copy number) by ConS.1-3 predation compared to the non-inoculated control (4.3 × 108 copy number), while upon predation of strain CL1008 (1/3), the prey biomass was reduced to 3.6 × 105 copy number (Fig. 7d), indicating that the ConS.1-3 community exhibits a cooperative predatory behavior.
We asked whether ConS.1–5, with a lower CL1008 population, can efficiently prey on P. sojae compared to individual cultures. Quantification of P. sojae biomass from the co-culture showed that P. sojae biomass was reduced by three orders of magnitude (reduced to 1.2 × 105 copy number) upon predation of ConS.1–5 compared with CL1008 (1/5) (reduced to 2.7×106 copy number), which was lower than the sum of three single treatments (reduced to 3.3 × 105 copy number) (Fig. 7d). Meanwhile, the biomass of ConS.1–5 proportionately increased by nearly two orders of magnitude (increased to 3.3 × 108 copy number) from the predation of P. sojae P6497 as compared to DK1622 (5.5 × 106 copy number), and also outperformed the sum of three single treatments (increased to 2.3 × 108 copy number) (Fig. 7e). These results show that a decrease in CL1008 cell numbers causes a proportional decline in predatory ability, whereas, the synthetic community harboring the core multi-enzyme consortium maintains efficient community-wide predatory behavior.
Multi-enzyme consortium arises from adaptive evolution within the Cystobacteraceae taxa
As microbe interactions alter the evolution of resource utilization [37], we investigated whether the oligosaccharides released from hydrolysis of the P. sojae cell wall by the tripartite enzyme consortium, provide nutrients for AC19. We found that AC19 was not able to utilize glucose and oligosaccharides, released from cell wall degradation, for growth (Fig. S22). Although sugars and oligosaccharides are often used as carbon/energy sources by many bacterial species, myxobacterial species frequently cannot utilize them [38]. However, considering that myxobacteria dedicate a remarkable proportion of their genomes to glycoside hydrolases (~5% of all genes encoded, whereas most organisms dedicate 1–3% of their genes to CAZymes) [22], we hypothesized that this tripartite enzyme consortium evolved as a specific weapon to attack and degrade Phytophthora and other oomycetes, but not for sugar utilization.
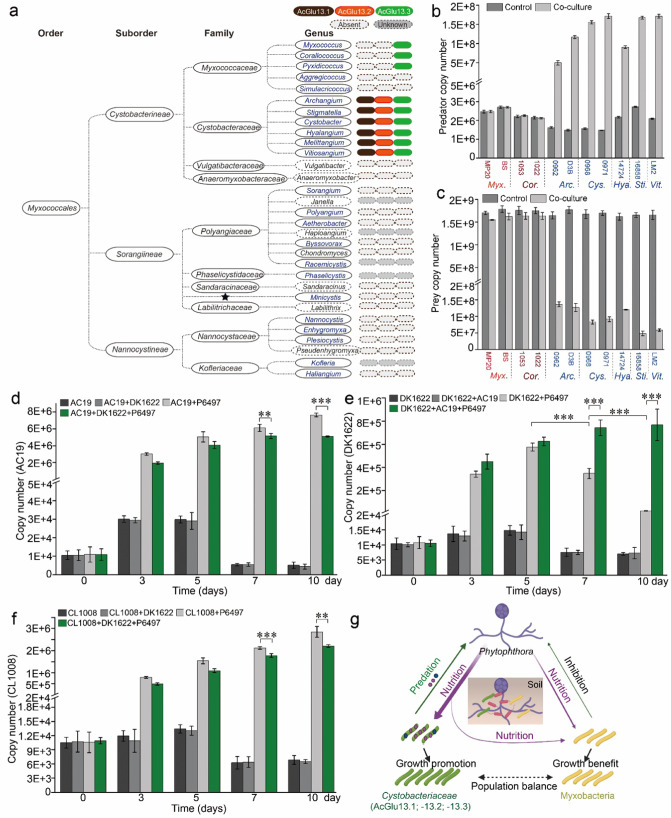
To assess the prevalence of the glucoside hydrolases in the order Myxococcales, we searched for AcGlu13.1, -13.2 and -13.3 orthologs from a wide variety of natural Myxococcales isolates from in-house and public databases with identified species classification and reference genomes. Although recent reports suggest Myxococcota be classified as an independent phylum, here we decided to use the classic and commonly used classification of the order Myxococcales [39, 40]. Co-occurrence of AcGlu13.1, –13.2 and –13.3 and their orthologs were widely found in the family of Cystobacteraceae, including the genera of Archangium (A. violaceum and A. primigenium), Stigmatella (S. aurantiaca, S. hybrid and S. erecta), Cystobacter (C. fuscus, C. ferrugineus and C. gracilis), Hyalangium (H. minutum), Melittangium (M. boletus) and Vitiosangium. However, only AcGlu13.3 and its orthologs were found in some genera of the Myxococcaceae family, while AcGlu13.1 and AcGlu13.2 orthologs were absent (Fig. 8a, Fig. S11). For example, Corallococcus sp. strain EGB bearing an AcGlu13.3 homolog LamC, which we identified as a bacterial and fungal predator [7, 34], only exhibited inhibitory interactions against P. sojae (Fig. 1a, c). Most other myxobacteria lacked AcGlu13.1-13.2-13.3 (Fig. 8a). The predominance of these three enzymes across broad genera belonging to Cystobacteraceae natural isolates, suggests that this multi-enzyme consortium arose from adaptive evolution during their interactions with oomycetes in nature. To investigate this possibility, we selected 11 myxobacterial isolates encompassing representative members of the Myxococcaceae and Cystobacteraceae families (Melittangium isolate not available), for their predatory ability toward P. sojae. Co-culture assays and qPCR analysis showed that all the myxobacterial isolates from Cystobacteraceae possess the ability to feed on P. sojae P6497, as determined by an increase in their biomasses by 1–2 orders of magnitude from prey-killing, with a corresponding decrease in P. sojae biomass (Fig. 8b, c). By contrast, isolates from Myxococcaceae exhibit growth inhibition against P6497, but the biomass of these myxobacteria did not increase, indicating prey were not lysed and consumed. These results provided an evolutionary scenario leading to an adapted specialized cell wall-decomposing enzyme system, which increased certain Cystobacteraceae taxa to predate on oomycetes Phytophthora.
Fig. 8. Predation of P. sojae among Cystobacteraceae is correlated with the presence of the specialized glucanase consortium.
a Identification of AcGlu13.1-AcGlu13.2-AcGlu13.3 orthologs across the order Myxococcales. Genera that harbor or lack orthologs are indicated, dark gray ovals are genera with unavailable genome, ★ undesignated family affiliation, blue font documented predatory myxobacteria with ability to feed on bacteria and/or fungi. Co-culture assay of P. sojae P6497 with myxobacteria isolates across the families Cystobacteraceae and Myxococcaceae. Quantitative biomass of myxobacteria (b) and P6497 (c) from co-culture assays by qPCR. P6497 (50 mg, wet weight) was incubated with myxobacteria (1 × 105 cells/mL, final concentration) in 20 mL TC media at 30 °C for 3 days. Soil feeding experiments with mixtures of P6497 and AC19, DK1622 and CL1008 in non-sterile soil. Quantitative biomass of AC19 (d), DK1622 (e), CL1008 (f) from different culture mixtures and times (day 0, 3, 5, 7 and 10) by qPCR. (g) Suggested model of nutritional interaction between predatory (green) and non-predatory (yellow) myxobacteria taxa on Phytophthora (purple). Experimental scheme of the soil pot experiment was designed based on different mixtures of strains (Fig. S23a). Arrow intensities indicate relative impacts.
To explore oomycetes predation by myxobacteria that contained or lacked the three specialized CAZymes in a relatively natural environment, we designed soil feeding experiments. Here, samples were collected at day 0, 3, 5, 7 and 10 for DNA extraction to measure changes in the abundance of P. sojae and myxobacteria during the co-incubation by qPCR (Fig. S23a). All treatments reduced the abundance of P. sojae to varying degrees, and treatment with P6497 + AC19 had the most significant effect (Fig. S23b). As expect, the biomasses of AC19 increased by two orders of magnitude from prey-killing within 3 days (Fig. 8d). Whereas, the biomass of DK1622 increased at day 3 and 5, but decreased at day 7 and 10 when mixed with P6497 (Fig. 8e), indicating limited nutrient release. In contrast, CL1008 equipped with AcGlu13.1, maintained growth promotion from day 3 through 10 (CL1008 + P6497; Fig. 8f). Moreover, the biomass of DK1622 from treatment of DK1622 + AC19 + P6497 continuously increased (Fig. 8e). However, the biomass of AC19 from treatment of AC19 + DK1622 + P6497 was lower than that of AC19 + P6497 (Fig. 8d), and similarly, a lower biomass of CL1008 was also observed from comparison of CL1008 + DK1622 + P6497 to CL1008 + P6497 (Fig. 8f), indicating nutrients were diverted from the top predator strains. These findings demonstrate that growth of DK1622 benefits from the co-existence of another myxobacteria that can prey on P6497 by presumably acquiring released nutrients. Therefore, in diverse soil populations some myxobacteria may benefit from the predatory activity of other myxobacteria, thus helping to maintain the stability of the overall myxobacteria population (Fig. 8g).
Discussion
Various species of bacterial predators have evolved versatile strategies to prey on other microbes as nutrient sources [36]. Myxobacteria are ubiquitous micropredators that possess the capability to feed on a broad range of soil bacteria and fungi [7, 21, 41], and continue to show promise as biocontrol agents for controlling of plant diseases [3]. Myxobacterial predation is thought to be a multifactorial process involving prey sensing, motility and secreted factors to realize prey-killing and digestion. During predator-prey interactions, antibiotics, lytic enzymes and OMVs containing hydrolases, are implicated in lysing bacterial and fungal cells [41]. Although, oomycetes and myxobacteria are diverse taxa with global distributions, their interactions were previously unknown. In this study, we show that an integrated cell wall decomposition system from Archangium sp. AC19 contributes to binding and cooperative hydrolysis of oomycetes Phytophothora during predation. Our findings indicate this specialized CAZymes system arose from adaptive evolution, which created new predator-prey interactions between myxobacteria and Phytophothora. Together, our findings provide a broader understanding of the ecological significance of myxobacteria predation in soil environments.
As keystone taxa in the soil microbial food web [4], predatory myxobacteria interact with other species via different cellular mechanisms. Recently, the Kil and type III-like secretion systems were shown to mediate cell contact-dependent killing of bacteria by M. xanthus [7, 11, 12], and the outer membrane β-1,6-glucanase GluM from Corallococcus sp. EGB, supports predation against fungi [7]. Here, the three β-1,3-glucanases constitute a secreted hydrolytic consortium involved in cell wall degradation and predation of oomycetes. The expression of the three enzymes is dramatically induced when AC19 is co-cultured with P6497 (Fig. S9), indicating they sense oomycetes as prey. To the best of our knowledge, this is the first report describing myxobacteria ability to prey on Phytophothora. Importantly, Phytophothora are economically significant pathogens [16], and therefore a myxobacterial biocontrol agent that affects these crop pathogens has high ecological and commercial value.
We discovered an enzyme consortium consisting of the three CAZymes that participates in predator-prey interactions and exhibits a specific characteristic of acting on the Phytophthora cell wall. Unlike the fungal chitin and β-1,6-glucans, which are only found in a few oomycetes groups [14], the oomycete cell wall primarily contains β-1,3-glucans and cellulose. Although oomycetes and fungi share β-1,3-glucans as critical components of their cell walls, the enzyme consortium displays low affinity and activity towards M. oryzae cell walls. We deduced that the fine structural organization, such as side chain modifications and their dynamic structures, leads to inefficiencies of these related β-1,3-glucanases on those targets [14]. Since this enzyme consortium also binds cellulose (Fig. 3b), the cross-linking of cellulose and β-1,3-glucans might promote their hydrolytic activity on oomycetes cell walls in a synergistic manner. Moreover, the inability of AcGlu13.1, -13.2, and -13.3 to promote predation toward fungi (Fig. S20), further indicates this consortium is a specific weapon against Phytophothora related organisms. Although the three β-1,3-glucanases exhibit no effects on fungi, AC19 nevertheless efficiently predates on fungi (M. oryzae, Fig. S20c). This, and other findings, led to our hypothesis that adaptive evolution in Cystobacteraceae endows them with the specialized cell-wall decomposition system that confers mycophagous ability toward oomycetes Phytophthora. In turn, this increases their predatory fitness and access to nutrients.
In a broader context, the evolution and acquisition of particular CAZymes provide certain microbes and fungi with competitive advantages. For instance, gut microflora and oceanic marine fungi have establish ecological niches by using CAZymes capable of degrading specific polysaccharides [22]. Other CAZymes are involved in carbon cycling, toxin or drug inactivation or host immune responses by structural modification or enzymatic breakdown [22, 42]. In myxobacterial genomes diverse glucoside hydrolases are also abundantly found. However, co-culturing of AC19 with carbohydrate released from P. sojae failed to stimulate its growth, but did stimulate E. coli growth [43] (Fig. S22). Taken together, this indicates that these CAZymes (AcGlu13.1, -13.2, and -13.3) instead evolved as predatory tools to attack oomycetes related to Phytophthora, and that this adaptive evolution conferred mycophagous ability that stably maintains myxobacteria populations in soil by prey lysis and nutrient release (Fig. 8g).
Predatory behavior of myxobacteria has long been viewed as example of bacterial cooperativity [44, 45]. That is, like fruiting-body development, predatory killing of prey cells by myxobacteria involves density-dependent cooperation, where a threshold number of cells are needed to secret effective levels of digestive enzymes for predation (commonly called “wolf pack” predation) [45]. However, evidence supporting this hypothesis is limited, and a number of mechanistic features of predation instead suggest selfishness behaviors during feeding [32, 46]. In this study, we constructed synthetic myxobacterial communities where each strain harbored one of the three CAZymes and when mixed as a three-strain consortium, maximum predation fitness was observed compared to monocultures (Fig. 7). As expected, we also found that when the density of CL1008 cells was decreased there was a corresponding decrease in predation (Fig. 7c, d). These results thus show a correlation between myxobacteria density and predation. Moreover, we demonstrated that growth of M. xanthus DK1622 benefits from co-existence with a predatory myxobacterium of P. sojae by presumed access to liberated nutrients (Fig. 8g). These results show that cooperative behavior can exist within synthetic communities. Whether such cooperative behaviors exists among wild myxobacteria is an intriguing but unexplored idea [46].
In summary, our findings highlight the importance of a cooperative team of CAZymes in the predator-prey interactions between myxobacteria and oomycetes. This system, among others, allows myxobacteria to act as a broad spectrum keystone predatory taxa in diverse soil ecosystems [4]. Moreover, our results offer new insights into the evolution of prey specificity by myxobacterial lineages, while the precise evolutionary history and detailed functions of the consortium needs further investigation.
Supplementary information
Acknowledgements
We thank Professor Daolong Dou and Suomeng Dong (Nanjing Agricultural University) for generously providing Phytophthora strains, Assistant Professor Danyu Shen (Nanjing Agricultural University) and Junjun Wu (Jiangnan University) for genome analysis, and Assistant Professor Xu Han (Tianjin Institute of Industrial Biotechnology Chinese Academy of Sciences) for protein structure predictions. This work was supported by the National Natural Science Foundation of China (32070027 to ZL, 32170123 to ZC and 32270066 to ZL), the Key Research & Development Plan of Jiangsu Province (BE2020340 to ZC), the National Science and Technology Major Project (2020ZX08009-04B to ZC), the National Key Research and Development Program of China (2021YFC2103600 to ZC and ZL) and the Fundamental Research Funds for the Central Universities (KYZZ2022001 to ZL). We also thank Mr. Gang Hu and Chun Qin (Electron Microscope Experimental Center, College of Life Sciences, Nanjing Agricultural University) for their help with SEM.
Author contributions
Z.L., L.Z., and Z.C. designed the experiments; L.Z. performed the majority of the experiments and data interpretation. JHW and CD expressed enzymes and performed pot experiments. ML, JYW, JH and XL performed binding and infection assays; LL, CX and LLZ. helped with experiments. XY and YH analyzed the data; JJW, YL and DW contributed in myxobacterial isolates, evolutionary analysis and manuscript edits. LZ, ZKL and ZC wrote and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhoukun Li, Email: zkl@njau.edu.cn.
Zhongli Cui, Email: czl@njau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-023-01423-y.
References
- 1.Hungate BA, Marks JC, Power ME, Schwartz E, van Groenigen KJ, Blazewicz SJ, et al. The functional significance of bacterial predators. mBio. 2021;12:e00466–00421. doi: 10.1128/mBio.00466-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Li S, Li W, Jiang D, Han K, Wu Z, et al. Myxobacterial community is a predominant and highly diverse bacterial group in soil niches. Env Microbiol Rep. 2014;6:45–56. doi: 10.1111/1758-2229.12107. [DOI] [PubMed] [Google Scholar]
- 3.Ye X, Li Z, Luo X, Wang W, Li Y, Li R, et al. A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community. Microbiome. 2020;8:49. doi: 10.1186/s40168-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petters S, Groß V, Söllinger A, Pichler M, Reinhard A, Bengtsson M, et al. The soil microbial food web revisited: Predatory myxobacteria as keystone taxa? ISME J. 2021;15:2665–75. doi: 10.1038/s41396-021-00958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Wang Y, Lu H, Liu Q, Wang C, Hu W, et al. Dynamics of solitary predation by Myxococcus xanthus on Escherichia coli observed at the single-cell level. Appl Environ Microbiol. 2020;86:e02286–02219. doi: 10.1128/AEM.02286-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. Myxobacteria: moving, killing, feeding, and surviving together. Front Microbiol. 2016;7:781. doi: 10.3389/fmicb.2016.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Ye X, Liu M, Xia C, Zhang L, Luo X, et al. A novel outer membrane β-1, 6-glucanase is deployed in the predation of fungi by myxobacteria. ISME J. 2019;13:2223–35. doi: 10.1038/s41396-019-0424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamoun S. Molecular genetics of pathogenic oomycetes. Eukaryot Cell. 2003;2:191–9. doi: 10.1128/EC.2.2.191-199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berleman JE, Kirby JR. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol Rev. 2009;33:942–57. doi: 10.1111/j.1574-6976.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Wei X, Ebright R, Wall D. Antibiotic production by myxobacteria plays a role in predation. J Bacteriol. 2011;193:4626–33. doi: 10.1128/JB.05052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seef S, Herrou J, de Boissier P, My L, Brasseur G, Robert D, et al. A Tad-like apparatus is required for contact-dependent prey killing in predatory social bacteria. Elife. 2021;10:e72409. doi: 10.7554/eLife.72409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiery S, Turowski P, Berleman JE, Kaimer C. The predatory soil bacterium Myxococcus xanthus combines a Tad-and an atypical type 3-like protein secretion system to kill bacterial cells. Cell Rep. 2022;40:111340. doi: 10.1016/j.celrep.2022.111340. [DOI] [PubMed] [Google Scholar]
- 13.Grenville-Briggs LJ, Anderson VL, Fugelstad J, Avrova AO, Bouzenzana J, Williams A, et al. Cellulose synthesis in Phytophthora infestans is required for normal appressorium formation and successful infection of potato. Plant Cell. 2008;20:720–38. doi: 10.1105/tpc.107.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fesel PH, Zuccaro A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet Biol. 2016;90:53–60. doi: 10.1016/j.fgb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Tonón C, Daleo G, Oliva C. An acidic β-1, 3 glucanase from potato tubers appears to be patatin. Plant Physiol Bioch. 2001;39:849–54. doi: 10.1016/S0981-9428(01)01311-0. [DOI] [Google Scholar]
- 16.Wang Y, Tyler BM, Wang Y. Defense and counterdefense during plant-pathogenic oomycete infection. Annu Rev Microbiol. 2019;73:667–96. doi: 10.1146/annurev-micro-020518-120022. [DOI] [PubMed] [Google Scholar]
- 17.Clavaud C, Aimanianda V, Latge J-P Organization of fungal, oomycete and lichen (1, 3)-β-glucans. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides: Elsevier; 2009. p. 387-424.
- 18.Liu N, Li H, Chevrette MG, Zhang L, Cao L, Zhou H, et al. Functional metagenomics reveals abundant polysaccharide-degrading gene clusters and cellobiose utilization pathways within gut microbiota of a wood-feeding higher termite. ISME J. 2019;13:104–17. doi: 10.1038/s41396-018-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng H, Wan C, Zhang Z, Chen H, Li Z, Jiang H, et al. Specific interaction of an RNA-binding protein with the 3′-UTR of its target mRNA is critical to oomycete sexual reproduction. PLoS Pathog. 2021;17:e1010001. doi: 10.1371/journal.ppat.1010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Ye X, Chen P, Ji K, Zhou J, Wang F, et al. Antifungal potential of Corallococcus sp. strain EGB against plant pathogenic fungi. Biol Control. 2017;110:10–7. doi: 10.1016/j.biocontrol.2017.04.001. [DOI] [Google Scholar]
- 21.Li Z, Wang T, Luo X, Li X, Xia C, Zhao Y, et al. Biocontrol potential of Myxococcus sp. strain BS against bacterial soft rot of calla lily caused by Pectobacterium carotovorum. Biol Control. 2018;126:36–44. doi: 10.1016/j.biocontrol.2018.07.004. [DOI] [Google Scholar]
- 22.Wardman JF, Bains RK, Rahfeld P, Withers SG. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat Rev Microbiol. 2022;20:542–56. [DOI] [PubMed]
- 23.Rakoff-Nahoum S, Coyne M, Comstock L. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr Biol. 2014;24:40–9. doi: 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Y, Zhang Z, Liu Y, Zhou XW, Anwar MN, Li Z, et al. A nuclease‐toxin and immunity system for kin discrimination in Myxococcus xanthus. Environ Microbiol. 2018;20:2552–67. doi: 10.1111/1462-2920.14282. [DOI] [PubMed] [Google Scholar]
- 25.Gao C, Xu H, Huang J, Sun B, Zhang F, Savage Z, et al. Pathogen manipulation of chloroplast function triggers a light-dependent immune recognition. Proc Natl Acad Sci USA. 2020;117:9613–20. doi: 10.1073/pnas.2002759117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Gu L, Zhang Y, Yan T, Kong G, Kong L, et al. An oomycete plant pathogen reprograms host pre-mRNA splicing to subvert immunity. Nat Commun. 2017;8:1–15. doi: 10.1038/s41467-017-02233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao K, Kinkel LL, Samac DA. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control. 2002;23:285–95. doi: 10.1006/bcon.2001.1015. [DOI] [Google Scholar]
- 28.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem. 1976;72:248–54. [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Wawra S, Fesel P, Widmer H, Neumann U, Lahrmann U, Becker S, et al. FGB1 and WSC3 are in planta‐induced β‐glucan‐binding fungal lectins with different functions. N. Phytol. 2019;222:1493–506. doi: 10.1111/nph.15711. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z, Song T, Zhu L, Ye W, Wang Y, Shao Y, et al. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell. 2015;27:2057–72. doi: 10.1105/tpc.15.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furness E, Whitworth DE, Zwarycz A Predatory interactions between myxobacteria and their prey. The Ecology of Predation at The Microscale: Springer. p. 2020;1-36.
- 33.Tyler BM. Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol. 2007;8:1–8. doi: 10.1111/j.1364-3703.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Li Z, Wu J, Li L, Li D, Ye X, et al. Functional analysis of a novel β-(1, 3)-glucanase from Corallococcus sp. strain EGB containing a fascin-like module. Appl Environ Microbiol. 2017;83:e01016–17. doi: 10.1128/AEM.01016-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. The microbial pan-genome. Curr Opin Genet Dev. 2005;15:589–94. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Pérez J, Moraleda‐Muñoz A, Marcos‐Torres FJ, Muñoz‐Dorado J. Bacterial predation: 75 years and counting! Environ Microbiol. 2016;18:766–79. doi: 10.1111/1462-2920.13171. [DOI] [PubMed] [Google Scholar]
- 37.Evans R, Beckerman AP, Wright RC, McQueen-Mason S, Bruce NC, Brockhurst MA. Eco-evolutionary dynamics set the tempo and trajectory of metabolic evolution in multispecies communities. Curr Biol. 2020;30:4984–8.e4. doi: 10.1016/j.cub.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bretscher AP, Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978;133:763–8. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Wang J, Wu S, Zhang Z, Li Y. Global geographic diversity and distribution of the Myxobacteria. Microbiol Spectr. 2021;9:e00012–21. doi: 10.1128/spectrum.00012-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waite DW, Chuvochina M, Pelikan C, Parks DH, Yilmaz P, Wagner M, et al. Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities. Int J Syst Evol Microbiol. 2020;70:5972–6016. doi: 10.1099/ijsem.0.004213. [DOI] [PubMed] [Google Scholar]
- 41.Thiery S, Kaimer C. The predation strategy of Myxococcus xanthus. Front Microbiol. 2020;11:2. doi: 10.3389/fmicb.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–5. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Irschik H, Reichenbach H. An unusual pattern of carbohydrate utilization in Corallococcus (Myxococcus) coralloides (Myxobacterales) Arch Microbiol. 1985;142:40–4. doi: 10.1007/BF00409234. [DOI] [Google Scholar]
- 44.Cao P, Dey A, Vassallo CN, Wall D. How myxobacteria cooperate. J Mol Biol. 2015;427:3709–21. doi: 10.1016/j.jmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg E, Keller KH, Dworkin M. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol. 1977;129:770–7. doi: 10.1128/jb.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall RC, Whitworth DE. Is “Wolf‐Pack” predation by antimicrobial bacteria cooperative? Cell behaviour and predatory mechanisms indicate profound selfishness, even when working alongside kin. BioEssays. 2019;41:1800247. doi: 10.1002/bies.201800247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.