Abstract
In the era of potent P2Y12 inhibitors, according to current guidelines, treatment with glycoprotein IIb/IIIa inhibitors (GPIs) should be limited to bail-out and/or highly thrombotic situations. Similarly, the recommendation for aspiration thrombectomy (AT) is downgraded to very selective use. We examine the prevalence, and predictors of GPI and AT use in STEMI patients referred to primary percutaneous coronary intervention (PCI). Data on 116,873 consecutive STEMI patients referred to primary PCI in Poland between 2015 and 2020 were analyzed. GPIs were administered in 29.3%, AT was used in 11.6%, and combined treatment with both in 6.1%. There was a mild trend toward a decrease in GPI and AT usage during the analyzed years. On the contrary, there was a rapid growth of the ticagrelor/prasugrel usage rate from 6.5 to 48.1%. Occluded infarct-related artery at baseline and no-reflow during PCI were the strongest predictors of GPI administration (OR 2.3; 95% CI 2.22–2.38 and OR 3.47; 95% CI 3.13–3.84, respectively) and combined usage of GPI and AT (OR 4.4; 95% CI 4.08–4.8 and OR 3.49; 95% CI 3.08–3.95 respectively) in a multivariate logistic regression model. Similarly, the administration of ticagrelor/prasugrel was an independent predictor of both adjunctive treatment strategies. In STEMI patients in Poland, GPIs are selectively used in one in four patients during primary PCI, and the combined usage of GPI and AT is marginal. Despite the rapid growth in potent P2Y12 inhibitors usage in recent years, GPIs are selectively used at a stable rate during PCI in highly thrombotic lesions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-023-02811-z.
Keywords: Percutaneous coronary intervention, Registries, Glycoprotein IIb/IIIa inhibitors, Aspiration thrombectomy, Myocardial infarction, Antiplatelet therapy
Highlights
GPIs and aspiration thrombectomy are selectively used in contemporary STEMI patients during primary PCI in Poland, with GPIs administered in one in four patients and aspiration thrombectomy in one in ten patients.
The rapid growth in the use of potent P2Y12 inhibitors, such as ticagrelor and prasugrel, in Poland has not led to a significant reduction in the use of GPIs and aspiration thrombectomy during primary PCI in STEMI patients.
The target population for GPI administration and/or aspiration thrombectomy during primary PCI in STEMI is characterized by a higher ischemic risk profile, indicating that these strategies are being used selectively in patients with a greater need for aggressive treatment.
Introduction
Rapid platelet inhibition is a crucial part of reperfusion therapy with primary percutaneous coronary interventions (PCI) in patients with ST-elevation myocardial infarction (STEMI). Glycoprotein IIb/IIIa inhibitors (GPIs) were routinely used as adjunctive periprocedural pharmacotherapy in the era of clopidogrel [1, 2]. After introducing novel, potent P2Y12 inhibitors, ticagrelor and prasugrel, the role of GPIs remains discussed due to unclear benefits and safety of such combined therapy [3, 4]. According to current guidelines, their usage should be limited to bail-out and/or highly thrombotic situations [5]. GPIs can be either used in adjunct to oral P2Y12 inhibitors in patients pre-treated before PCI or as a bridging strategy in patients in which a P2Y12 inhibitor was not administered before PCI [6]. Similarly, the recommendation for the aspiration thrombectomy strategy for thrombotic lesions in STEMI was downgraded in current guidelines to very selective but not routine use [7]. On the other hand, the onset of action of potent oral P2Y12 inhibitors may be delayed in STEMI patients, especially in those with hemodynamic instability or concomitant opioid administration [7, 8]. These all make the landscape of the thrombus-containing lesion treatment strategy rather defensive, underlying the need for individual approach to STEMI patients. Data concerning the daily clinical practice of GPIs usage and the combined strategy of GPIs with aspiration thrombectomy are lacking.
Presented study aimed to examine the prevalence, procedural characteristics, and predictors of GPI administration in all-comers contemporary STEMI patients referred to primary PCI in Poland. Considering the procedural approach to thrombus-containing lesions we also analyzed data on the combined usage of GPIs and aspiration thrombectomy.
Methods
The presented analysis focused on consecutive STEMI patients referred to Primary PCI in Poland between 2015 and 2020. Data were prospectively collected and stored via electronic case report forms in the National PCI Registry (ORPKI) operated by the Jagiellonian University Medical College in Krakow and endorsed by the Polish Association of Cardiovascular Interventions of the Polish Cardiac Society. ORPKI is a national registry collecting data on all PCI procedures performed in Poland [9–11]. For this analysis data on 116,873 consecutive PCI procedures in STEMI were retrieved from the database. Patients’ demography, baseline clinical characteristics, angiography and PCI details, as well as periprocedural pharmacotherapy were analyzed. Information on GPI administration included whether the drug was administered and which one was given (abciximab, eptifibatide, or tirofiban), but did not include details on the method of bolus administration (intravenous or intracoronary) or infusion specifics. Two GPIs administration approaches were described: "adjunctive" therapy in patients pretreated early with P2Y12 inhibitors and "bridging" therapy in patients in which a P2Y12 inhibitor was not administered before PCI. Angiography was assessed by PCI operators but not by the core-lab. We focused on two strategies during procedures: (1) GPIs administration and (2) the combined usage of GPIs and aspiration thrombectomy. Results were also analyzed in the context of potent P2Y12 inhibitors periprocedural treatment (ticagrelor, prasugrel). Additionally, we analyzed the usage of both treatment strategies according to site STEMI volume.
Statistical analysis
Standard descriptive statistics were used in the analysis. Quantitative variables were described with median with interquartile range (for non-normal distribution of data). Normality was assessed by the Shapiro–Wilk test. Categorical variables were presented as percentages. Chi square test or Fisher Exact Test were used for comparing categorical data, as appropriate. Temporal trends were analyzed with Cochran-Armitage trend test. A logistic regression analysis was constructed to identify predictors of the use of GPIs and aspiration thrombectomy. Variables, including demography, clinical characteristics, and angiography parameters, were considered by calculating simple models. Final multiple models were constructed using a stepwise combined (forward/backward) technique with minimization of the Bayesian Information Criterion as a target, taking into account all variables with p-value from simple model < 0.2 or of clinical importance. Results are presented as odds ratios with an associated 95% confidence interval. The level of statistical significance was set at alpha value < 0.05. All statistical analyses were performed using the R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria, 2021) with package “rms” version 6.2–0 and JMP 15.2 (SAS Institute Inc., Cary, NC, USA, 2020).
Results
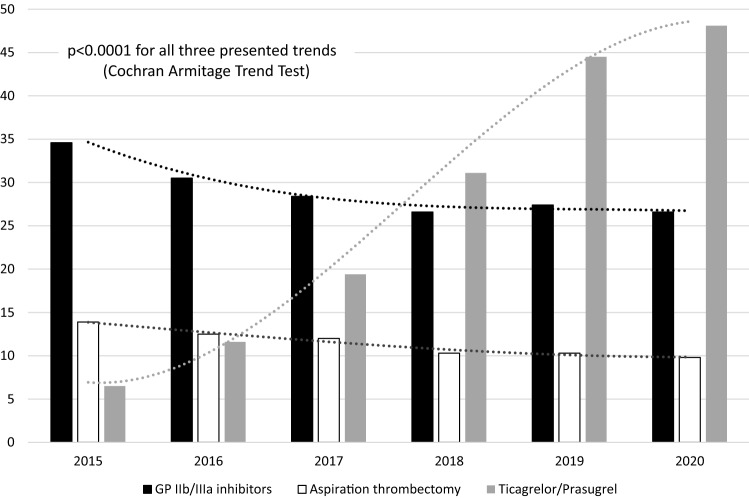
During six years, 116,873 consecutive patients in STEMI were treated with primary PCI. GPIs were administered in 29.3% of these patients (eptifibatide 23.6%, abciximab 5.3%, tirofiban 0.4%), aspiration thrombectomy was used in 11.6%, and combined treatment with GPIs and aspiration thrombectomy in 6.1%. There was a trend towards decrease in GPIs and aspiration thrombectomy usage during the analyzed years, which was mainly seen between 2015 to 2018 with a plateau beyond 2018 at the level of about 27% for GPIs and about 10% for aspiration thrombectomy. Conversely, there was rapid growth in ticagrelor/prasugrel usage rate from 6.5% in 2015 to 48.1% in 2020 (Fig. 1).
Fig. 1.
Temporal trends in glycoprotein (GP) IIb/IIIa inhibitors administration (black), aspiration thrombectomy (white) and ticagrelor or prasugrel administration (grey) in consecutive ST-segment elevation myocardial infarction patients referred to primary PCI in Poland between 2015 and 2020 (p < 0.0001 for all three trends)
GP IIb/IIIa inhibitors administration
Patients with periprocedural GPIs administration were younger, more often men, with history of previous PCI and smoking, and presented with cardiogenic shock on admission. They were less likely to have diabetes, chronic kidney disease and previous stroke (Table 1). In the GPIs group, we observed a higher rate of occluded infarct-related artery at baseline (more often left main or/and left anterior descending artery), PCI of bifurcation, no-reflow during PCI, use of aspiration thrombectomy and administration of ticagrelor/prasugrel (Table 1). Of the 34,208 GPIs treated patients, those drugs were administered as "adjunctive" therapy in 59.1% of patients or as "bridging" therapy in 40.9% of patients. When analyzed patients pretreated with P2Y12 inhibitors before PCI, GPIs were administered in 31% of those patients.
Table 1.
Demography, clinical and procedural characteristics according to glycoprotein IIb/IIIa inhibitors administration
| Glycoprotein IIb/IIIa inhibitor (+) n = 34,208 (29.3%) |
Glycoprotein IIb/IIIa inhibitor (−) n = 82,665 (70.7%) |
Total n = 116,873 | P | |
|---|---|---|---|---|
| Age (years) (Q1; Q3) | 63 (56; 70) | 66 (58; 75) | 65 (58; 73) | < 0.001 |
| Male gender (%) | 71.9 | 66.4 | 68.1 | < 0.001 |
| Arterial hypertension (%) | 58.8 | 58.2 | 58.4 | 0.07 |
| Diabetes mellitus (%) | 16.7 | 17.8 | 17.5 | < 0.001 |
| Chronic kidney disease (%) | 2.6 | 3.7 | 3.4 | < 0.001 |
| Smoking (%) | 34.1 | 28.7 | 30.3 | < 0.001 |
| COPD (%) | 2.1 | 2.1 | 2.1 | 0.63 |
| Previous MI (%) | 12.5 | 12.4 | 12.5 | 0.61 |
| Previous PCI (%) | 12.4 | 11.7 | 11.9 | < 0.001 |
| Previous CABG (%) | 1.7 | 1.8 | 1.7 | 0.33 |
| Previous stroke (%) | 2.4 | 3.5 | 3.2 | < 0.001 |
| Killip class on admission: | < 0.001 | |||
| I (%) | 79.0 | 84.6 | 82.9 | |
| II (%) | 11.9 | 9.4 | 10.1 | |
| III (%) | 4.2 | 2.7 | 3.2 | |
| IV (%) | 4.9 | 3.3 | 3.8 | |
| Cardiac arrest before admission (%) | 5.3 | 4.8 | 4.9 | 0.0016 |
| Femoral access (%) | 23.8 | 22.3 | 22.7 | < 0.001 |
| Angiography result | < 0.001 | |||
| Single vessel (%) | 45.6 | 45.3 | 45.4 | |
| Multivessel without LMCA (%) | 46.8 | 47.9 | 47.6 | |
| Multivessel with LMCA (%) | 7.2 | 6.6 | 6.8 | |
| LMCA only (%) | 0.4 | 0.2 | 0.2 | |
| Infarct related artery | < 0.001 | |||
| LMCA (%) | 3.2 | 2.1 | 2.5 | |
| LAD (%) | 43 | 40.2 | 41 | |
| Cx (%) | 13.0 | 14.0 | 13.7 | |
| RCA (%) | 41.4 | 38.9 | 39.6 | |
| Other (%) | 4.8 | 3.2 | ||
| Bifurcation (%) | 8.6 | 7.0 | 7.5 | < 0.001 |
| Aspiration thrombectomy (%) | 20.9 | 7.8 | 11.6 | < 0.001 |
| P2Y12 inhibitors | < 0.001 | |||
| Clopidogrel (%) | 59.9 | 64.7 | 63.3 | |
| Prasugrel (%) | 3.3 | 1.9 | 2.3 | |
| Ticagrelor (%) | 36.8 | 33.4 | 34.4 | |
| Administration before PCI (%) | 59.1 | 58.8 | 58.9 | 0.31 |
| Administration during PCI (%) | 40.9 | 41.2 | 41.1 | |
| TIMI flow before PCI | < 0.001 | |||
| 0 (%) | 72.6 | 52 | 58.1 | |
| 1 (%) | 11.7 | 16 | 14.8 | |
| 2 (%) | 9.6 | 16.8 | 14.7 | |
| 3 (%) | 6.1 | 15.2 | 12.4 | |
| TIMI flow after PCI | < 0.001 | |||
| 0 (%) | 1.8 | 2.5 | 2.3 | |
| 1 (%) | 2 | 1.4 | 1.5 | |
| 2 (%) | 6.8 | 3.9 | 4.8 | |
| 3 (%) | 89.4 | 92.2 | 91.4 | |
| Stent implantation (%) | 92.4 | 91.4 | 91.7 | < 0.001 |
| Drug eluting stents (%) | 89.8 | 88.7 | 89.1 | < 0.001 |
| Total amount of contrast (ml) (Q1: Q3) | 160 (130; 200) | 150 (120; 200) | 150 (120; 200) | < 0.001 |
| Total radiation dose (mGy) (Q1; Q3) | 796 (451; 1356) | 676 (390; 1144) | 707 (405; 1206) | < 0.001 |
| Time from pain onset to PCI (min) (Q1; Q3) | 230 (136; 480) | 245 (147;540) | 240 (143; 522) | < 0.001 |
| No-reflow (%) | 3.2 | 0.8 | 1.5 | < 0.001 |
| Cardiac arrest during procedure (%) | 2.5 | 1.4 | 1.8 | < 0.001 |
COPD chronic obstructive pulmonary disease, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, LMCA left main coronary artery, LAD left anterior descending artery, Cx circumflex artery, RCA right coronary artery, TIMI thrombolysis in myocardial infarction
In a multivariate logistic regression model, the occluded infarct-related artery at baseline and no-reflow during PCI were the strongest independent predictors of GPIs administration. Others positive predictors were male sex, smoking, cardiogenic shock on admission, the culprit within left main and/or left anterior descending coronary artery, previous PCI, bifurcation treatment, administration of ticagrelor/prasugrel, and aspiration thrombectomy usage. The negative predictors were age, chronic kidney disease, previous stroke, and cardiac arrest before admission. Results multivariate logistic regression analysis are presented in Table 2. Results of univariate logistic regression are presented in table S1 in supplementary information.
Table 2.
Multivariable logistic regression analyses for predicting glycoprotein IIb/IIIa inhibitors administration
| OR | 95% CI | P value | |
|---|---|---|---|
| Age (per 1 year) | 0.98 | 0.98–0.98 | < 0.001 |
| Sex (male) | 1.12 | 1.09–1.16 | < 0.001 |
| Previous stroke | 0.71 | 0.65–0.77 | < 0.001 |
| Previous PCI | 1.12 | 1.08–1.17 | < 0.001 |
| Smoking | 1.07 | 1.04–1.1 | < 0.001 |
| Chronic kidney disease | 0.78 | 0.72–0.85 | < 0.001 |
| Killip class IV on admission | 1.58 | 1.47–1.7 | < 0.001 |
| Ticagrelor or prasugrel | 1.08 | 1.05–1.11 | < 0.001 |
| Cardiac arrest before admission | 0.89 | 0.84–0.95 | < 0.001 |
| Aspiration thrombectomy during PCI | 2.58 | 2.49–2.68 | < 0.001 |
| TIMI 0 or 1 in baseline angiography | 2.3 | 2.22–2.38 | < 0.001 |
| IRA in LMCA and/or LAD | 1.2 | 1.17–1.23 | < 0.001 |
| PCI of bifurcation | 1.24 | 1.18–1.3 | < 0.001 |
| No-reflow during PCI | 3.47 | 3.13–3.84 | < 0.001 |
PCI percutaneous coronary intervention, IRA infarct-related artery, LMCA left main coronary artery, LAD left anterior descending artery, TIMI thrombolysis in myocardial infarction;
Combined usage of GP IIb/IIIa inhibitors and aspiration thrombectomy
Patients with the combined strategy with periprocedural GPIs administration and aspiration thrombectomy were younger, more often men, with a history of smoking, and presented with cardiogenic shock on admission. They were less likely to have diabetes mellitus, chronic kidney disease, and previous stroke (Table S2 in supplementary information). In angiography, we observed a higher rate of the occluded infarct-related artery in baseline angiography (more often the right coronary artery) and a higher rate of no-reflow during PCI. They more often received ticagrelor/prasugrel (Table S2 in supplementary information).
Similarly to GPIs administration analysis, the occluded infarct-related artery at baseline and no-reflow during PCI were the strongest independent predictors of combined usage of GP IIb/IIIa inhibitors and aspiration thrombectomy in a multivariate logistic regression model. Results of univariate and multivariate logistic regression analysis are presented in table S3 in supplementary information.
Results according to site STEMI volume
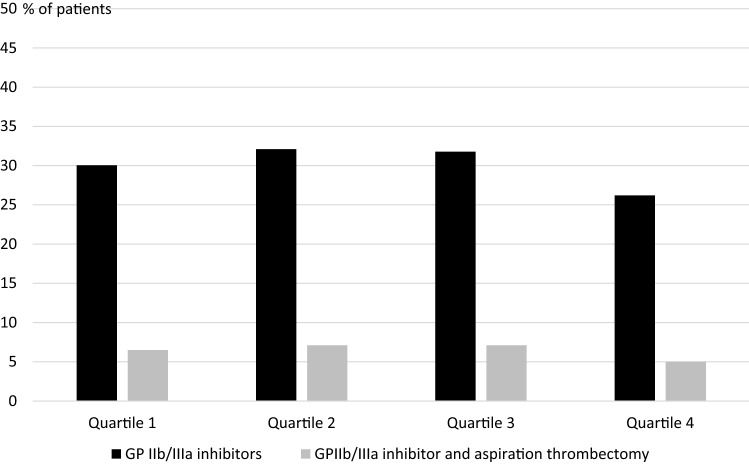
We analyzed the usage of both treatment strategies (rate of GPIs administration and combined treatment with of GPIs and aspiration thrombectomy) according to site STEMI volume (quartiles). Data according to site volume (primary PCI in STEMI) quartiles are presented in Fig. 2. No relationship between treatment strategies and site volume was confirmed.
Fig. 2.
Glycoprotein (GP) IIb/IIIa inhibitors administration (black) and combined strategy (GP IIb/IIIa inhibitors administration and aspiration thrombectomy) (grey) according to site ST-segment elevation myocardial infarction volume (quartiles)
Discussion
The most important findings from the presented analysis are: (1) in all-comers contemporary STEMI patients in Poland, GPIs and aspiration thrombectomy are selectively used in respectively one in four and one in ten patients during primary PCI in STEMI, (2) the combined treatment strategy with both GPIs and aspiration thrombectomy during primary PCI is marginal, (3) the use of those strategies was not particularly reduced by the rapid growth in potent P2Y12 inhibitors use in Poland in recent years, (4) target STEMI population for GPI administration is characterized by high ischemic risk.
Primary PCI of thrombotic lesions in patients with STEMI may be challenging due to the risk of no-reflow and distal embolization. Rapid platelet inhibition plays an important role during mechanical reperfusion [12, 13]. GPIs were routinely used as adjunctive periprocedural pharmacotherapy during primary PCI for many years [1]. Several reports have confirmed their beneficial effect in patients with STEMI [14, 15]. Worth noticing is that most older trials concerning GPIs were performed with clopidogrel. Nowadays, in the era of more potent P2Y12 inhibitors, ticagrelor and prasugrel, the role of GPIs is debatable mainly due to the lack of clear data on the benefits and bleedings risks of such an approach. According to current guidelines, their usage should be limited to bail-out and/or highly thrombotic situations [5]. These may influence everyday clinical practice and lower the rate of GPI usage, especially with high penetration of potent P2Y12 inhibitors. Over, the results of our analysis do not fully confirm these assumptions, as we observed only a mild trend towards decrease in use of GPIs through years 2015–2020, despite the pronounced increase in use of ticagrelor and prasugrel. Blanchart K et al. showed (based on prospective registry data) the GPI usage in 41% of STEMI patients treated with ticagrelor or prasugrel during primary PCI. Importantly, 52% of those patients have received GPI as a systematic strategy [16]. Similarly, in the analysis of the FAST-MI program, GPIs were used in about 40% of STEMI patients during PCI, with 54% of ticagrelor or prasugrel administration (including prehospital dual antiplatelet therapy) [17]. The main concern about treatment with GPI on top of potent P2Y12 inhibitors is elevated bleeding risk. However, to date, there is no dedicated clinical trial to assess such risk (and potential clinical benefit) in the contemporary STEMI cohort. In the above-mentioned registry with the 98% of radial approach there was no significant increase of bleeding in the GPI cohort [16]. Meta-analysis of PLATO, TRITON-TIMI 38, and CURRENT-OASIS 7 trials have shown a similar risk of bleedings with GPI and potent P2Y12 inhibitors compared to GPI and clopidogrel. The increase in bleeding risk with GPIs was present regardless of the type of P2Y12 inhibitor but relative risk was higher with clopidogrel than with ticagrelor or prasugrel. This suggests that GPIs treatment is the main factor of elevated bleeding risk, and administration of potent P2Y12 inhibitors may have no further influence on bleeding risk [18]. On the other hand, there is no clear evidence of the clinical benefit of GPIs administration on top of prasugrel and ticagrelor, and the benefit of novel P2Y12 inhibitors compared to clopidogrel in reducing the risk of ischemic events is independent of GPI use [16]. This underlines the role of the individual, tailored approach to identify patients who benefit from GPIs administration the most. According to our analysis, GPIs are administered in patients with the higher ischemic risk profile and relatively lower bleeding risk profile, with occluded infarct-related artery and/or as bail-out for no-reflow. Another possible approach selectively used in some centers is intracoronary GPI bolus not followed by infusion. The idea of this strategy is based on the benefit of rapid antiplatelet effect in STEMI without aggressive prolonged treatment (earlier offset of action) as a potential balanced approach to optimize bleeding and ischemic risks. Galli M et al. showed such approach as safe in selected patients at high ischemic risk both in those pretreated and in those naïve to P2Y12 inhibitors. [6]. In our study, GPIs were administered as "adjunctive" therapy to approximately 60% of patients receiving GPIs, a practice largely driven by the prevalent clinical strategy of initiating early dual antiplatelet therapy in STEMI patients before PCI. In those early treated with P2Y12 inhibitor, GPIs were administered in about one third what is slightly lower rate comparing to other studies [16, 17].
Aspiration thrombectomy is a tool designed for mechanical thrombus removal and to prevent distal embolization [19]. In initial reports, a beneficial effect of routine thrombectomy on angiographic and short-term clinical outcomes in STEMI patients was observed [20–23]. Those relatively small-scale studies were performed in centers with high experience in aspiration thrombectomy technique. However, further large-scale trials with routine aspiration thrombectomy in STEMI did not confirm any clinical benefit [24–26]. Moreover, the question was raised about the safety of routine use of thrombectomy before PCI taking into account the increased risk of stroke [27, 28]. Potential benefit of aspiration thrombectomy is multifactorial including optimal patients selection and adequate technique. Similarly to GPIs, recent recommendations advocate against the routine use of thrombectomy (class III for routine usage) [5]. In our registry, we observed only a mild trend toward decreased use of thrombectomy throughout the analyzed years. On the other hand, the combined use of both GPIs and thrombectomy applied to a small proportion of patients. It may be driven by guidelines and class III recommendation, which may be understood as contraindications but rather should be as very selective usage. In the registry data presented by Blanchart K et al. thrombectomy was used in 26% of patients, mostly as adjunctive therapy to GPIs [16]. In our analysis, no-reflow phenomenon and occluded infarct-related artery at baseline were the strongest predictors for this kind of pharmaco-mechanical approach to thrombus lesion and similarly to GPIs alone it was mainly used in patients with the higher ischemic risk profile. Some novel thrombectomy techniques are under evaluation to improve treatment results [29].
The presented study has several limitations. First, the observation is limited only to procedure time, and no longer follow-up was available. Analysis is based on registry data describing everyday clinical practice; thus, it is impossible to compare the outcomes of different strategies with GPIs and aspiration thrombectomy due to important differences in baseline characteristics. Because of the two above-mentioned limitations, there is no point in performing further statistical analysis for in-cathlab outcomes, even including propensity score matching. Data about the way of bolus administration (intravenous or intracoronary) and infusion details are unavailable. However, routine practice is to give infusion after bolus injection. Finally, angiograms were assessed by operators. No core lab analysis was performed in this registry.
Conclusions
The presented analysis shows that GPIs are used in primary PCI in STEMI in one in four patients in Poland, and combined use of GPIs and aspiration thrombectomy is marginal. An increase in ticagrelor and prasugrel use does not seem to impact rates of use of GPIs and thrombectomy in Poland. The target population for GPI and/or thrombectomy is characterized by high ischemic risk.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding
None.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors report no financial relationships or conflicts of interest regarding the content herein.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA. 2005;293:1759–1765. doi: 10.1001/jama.293.14.1759. [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Galli M, Collet JP, Kastrati A, O'Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–e1396. doi: 10.4244/EIJ-D-21-00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiviott SD, Braunwald E, McCabe CH, TRITON-TIMI 38 Investigators et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, ESC Scientific Document Group et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.Galli M, Migliaro S, Rodolico D, et al. Intracoronary bolus of glycoprotein IIb/IIIa inhibitor as bridging or adjunctive strategy to oral P2Y12 inhibitor load in the modern setting of ST-elevation myocardial infarction. Minerva Cardiol Angiol. 2022;70(6):697–705. doi: 10.23736/S2724-5683.21.05669-6. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos D, Xanthopoulou I, Gkizas V, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804. doi: 10.1161/CIRCINTERVENTIONS.112.972323. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim K, Christoph M, Schmeinck S, et al. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation. 2014;85:649–656. doi: 10.1016/j.resuscitation.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Rakowski T, Siudak Z, Dziewierz A, Plens K, Kleczyński P, Dudek D. Contemporary use of P2Y12 inhibitors in patients with ST-segment elevation myocardial infarction referred to primary percutaneous coronary interventions in Poland: Data from ORPKI national registry. J Thromb Thrombolysis. 2018;45:151–157. doi: 10.1007/s11239-017-1579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakowski T, De Luca G, Siudak Z, et al. Characteristics of patients presenting with myocardial infarction with non-obstructive coronary arteries (MINOCA) in Poland: data from the ORPKI national registry. J Thromb Thrombolysis. 2019;47:462–466. doi: 10.1007/s11239-018-1794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakowski T, Węgiel M, Siudak Z, et al. Prevalence and predictors of coronary artery perforation during percutaneous coronary interventions (from the ORPKI National Registry in Poland) Am J Cardiol. 2019;124:1186–1189. doi: 10.1016/j.amjcard.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Lesiak M, Komosa A. Dual antiplatelet therapy for reduction in mortality in patients with acute and chronic coronary syndromes. Postepy Kardiol Interwencyjnej. 2021;17:340–343. doi: 10.5114/aic.2021.112082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tubek S, Kuliczkowski W, Gąsior M, et al. Antiplatelets in acute coronary syndrome in Poland - from guidelines to clinical practice. Postepy Kardiol Interwencyjnej. 2021; 17 : 141 – 154 . doi: 10.5114/aic.2021.107492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber K, Holmes DR, Jr, van Hof AW, et al. Use of glycoprotein IIb/IIIa inhibitors in primary percutaneous coronary intervention: insights from the APEX-AMI trial. Eur Heart J. 2010;31:1708–1716. doi: 10.1093/eurheartj/ehq143. [DOI] [PubMed] [Google Scholar]
- 15.Montalescot G, Barragan P, Wittenberg O, ADMIRAL Investigators et al. Abciximab before direct angioplasty and stenting in myocardial infarction regarding acute and long-term follow-up platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895–1903. doi: 10.1056/NEJM200106213442503. [DOI] [PubMed] [Google Scholar]
- 16.Blanchart K, Heudel T, Ardouin P, et al. Glycoprotein IIb/IIIa inhibitors use in the setting of primary percutaneous coronary intervention for ST elevation myocardial infarction in patients pre-treated with newer P2Y12 inhibitors. Clin Cardiol. 2021;44:1080–1088. doi: 10.1002/clc.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danchin N, Puymirat E, Cayla G, FAST-MI Investigators et al. One-year survival after ST-segment-elevation myocardial infarction in relation with prehospital administration of dual antiplatelet therapy. Circ Cardiovasc Interv. 2018;11(9):e007241. doi: 10.1161/CIRCINTERVENTIONS.118.007241. [DOI] [PubMed] [Google Scholar]
- 18.Roule V, Agueznai M, Sabatier R, et al. Safety and efficacy of IIb/IIIa inhibitors in combination with highly active oral antiplatelet regimens in acute coronary syndromes: a meta-analysis of pivotal trials. Platelets. 2017;28:174–181. doi: 10.1080/09537104.2016.1218453. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 20.Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008;24:2989–3001. doi: 10.1093/eurheartj/ehn421. [DOI] [PubMed] [Google Scholar]
- 21.Dudek D, Mielecki W, Burzotta F, et al. Thrombus aspiration followed by direct stenting: a novel strategy of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Results of the Polish-Italian-Hungarian RAndomized ThrombEctomy Trial (PIHRATE Trial) Am Heart J. 2010;160:966–972. doi: 10.1016/j.ahj.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Sardella G, Mancone M, Bucciarelli-Ducci C, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 24.Lagerqvist B, Fröbert O, Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–1120. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- 25.Fröbert O, Lagerqvist B, Olivecrona GK, TASTE Trial et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 26.Jolly SS, Cairns JA, Yusuf S, TOTAL Investigators et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolly SS, Cairns JA, Yusuf S, TOTAL Investigators et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127–135. doi: 10.1016/S0140-6736(15)00448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud AN, Bavry AA, Elgendy IY. The risk for stroke with aspiration thrombectomy: procedure or patient related? Insights from a meta-analysis. JACC Cardiovasc Interv. 2016;9:1750–1752. doi: 10.1016/j.jcin.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Klaudel J, Surman D, Pawłowski K, Trenkner W. Stroke thrombectomy catheter for aspiration of refractory or inaccessible clot in acute myocardial infarction. Postepy Kardiol Interwencyjnej. 2022;18:65–69. doi: 10.5114/aic.2022.115299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.