Abstract
Purpose of Review
This review summarizes recent evidence published since a previous review in 2018 on the association between egg consumption and risk of cardiovascular disease (CVD) mortality, CVD incidence, and CVD risk factors.
Recent Findings
No recent randomized controlled trials were identified. Evidence from observational studies is mixed, with studies reporting either an increased risk or no association of highest egg consumption with CVD mortality, and a similar spread of increased risk, decreased risk, or no association between egg intake and total CVD incidence. Most studies reported a reduced risk or no association between egg consumption and CVD risk factors. Included studies reported low and high egg intake as between 0 and 1.9 eggs/week and 2 and ≥14 eggs/week, respectively. Ethnicity may influence the risk of CVD with egg consumption, likely due to differences in how eggs are consumed in the diet rather than eggs themselves.
Summary
Recent findings are inconsistent regarding the possible relationship between egg consumption and CVD mortality and morbidity. Dietary guidance should focus on improving the overall quality of the diet to promote cardiovascular health.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11883-023-01109-y.
Keywords: Egg consumption, Cardiovascular disease, Mortality, Stroke, Heart disease, Lipids, Blood pressure, Hypertension
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality globally [1]. Early studies, which indicated that elevated serum cholesterol was associated with an increased risk of heart disease [2, 3], led to the American Heart Association (AHA) recommending limiting dietary cholesterol to less than 300 mg/day with specific recommendations to restrict egg consumption, which are high in cholesterol, to a maximum of three eggs per week [4]. A later analysis from the Framingham study found no association between egg intake and blood cholesterol or heart disease [5], and in 2002, the AHA removed its advice to limit egg intake while retaining its recommendation to consume less than 300 mg/day of dietary cholesterol [6]. During this time, findings surfaced indicating that increased intake of dietary cholesterol was associated with decreased synthesis of endogenous cholesterol [7], and in 2013, the AHA announced that “there is insufficient evidence to determine whether lowering dietary cholesterol reduces low-density lipoproteins (LDL) cholesterol” [8]. As a result, the 2015–2020 Dietary Guidelines for Americans removed the recommendation of setting a limit to the maximum intake of 300 mg/day of cholesterol. However, the controversy around the impact of consuming foods high in cholesterol, including eggs, on CVD risk remains.
A possible explanation for the controversy is that foods high in cholesterol are also typically high in saturated fat [9], which is well documented to increase LDL cholesterol and CVD risk [10]. Thus, it is difficult to determine the independent effects of dietary cholesterol on the blood lipid profile. Based on nutrient profiles from Australian food databases, eggs are high in cholesterol but low in saturated fat, with the average large whole egg (50 g) containing 244 mg of cholesterol but only 1.2 g of saturated fat [11]. However, eggs, like any individual food, are not consumed in isolation, but as part of an overall diet, which can influence total cholesterol and saturated fat intake. For example, in the American diet, intakes of cholesterol and saturated fat increase in parallel, which may be due to eggs being frequently consumed with bacon or sausage which are high in saturated fat [12]. Previous reviews considering egg consumption and blood lipids have reported contradictory findings, with a 2018 review of randomized controlled trials (RCT) finding that consuming eggs does not adversely affect the blood lipid profile [13]. However, in contrast, a subsequent meta-analysis of RCTs reported a positive relationship between changes in dietary cholesterol (all sources, including eggs) and changes in LDL cholesterol, after controlling for saturated fat [14], and so, the relationship remains unclear.
When the relationship between egg consumption and CVD risk has been reviewed, there have also been mixed findings. The majority of systematic reviews and meta-analyses observed no association between egg consumption and CVD risk [15–20], but a small number of studies identified an increased risk [21, 22], particularly in people with diabetes [17, 18, 23]. These inconsistencies in study findings continue to fuel the controversy around the impact of egg consumption on CVD risk.
This review aims to update current evidence identified from a systematic search and narrative review of observational studies and RCTs from 2019 onwards to explore the association between egg consumption and CVD risk, specifically, CVD mortality, incidence, and risk factors in adults. In addition, confounding factors that may play a role in the associations found will be discussed.
Methods
Search Strategy
An electronic search of PubMed, Web of Science, and Embase was conducted from January 1, 2019, to September 14, 2022, to identify the potentially eligible studies recent studies with the following search strategy: “[(egg OR eggs) AND (cardiovascular diseases OR cardiovascular OR coronary heart disease OR CHD OR CVD OR stroke OR myocardial infarction OR ischemic heart disease OR ischemic stroke OR hemorrhagic stroke) AND (randomized control trial OR RCT OR cohort OR prospective OR longitudinal OR follow-up OR case-cohort OR nested case control).” Studies were selected if they met the following inclusion criteria: (i) they were conducted on human adults; (ii) evaluated associations between egg intake and risk of CVD (fatal and nonfatal); (iii) evaluated associations between egg intake and CVD risk factors (e.g., hypertension, blood lipid profile, body fat mass). All references were evaluated by two independent reviewers (all by ESC, half each by SC and AMC) with an independent reviewer (either SC or AMC) resolving any disagreement.
Data Extraction
Data were extracted by two independent reviewers (ESC and SC) from each identified study using a standardized extraction form. The following information was collected: (i) author names; (ii) year of publication; (iii) study cohort name and country; (iv) sample size, sex, and age (mean or range) of participants; (v) study aim and design; (vi) follow-up period; (vii) exposure type/dose/frequency; (viii) CVD outcome measures; (ix) CVD results; (x) covariates used in adjustments.
Results
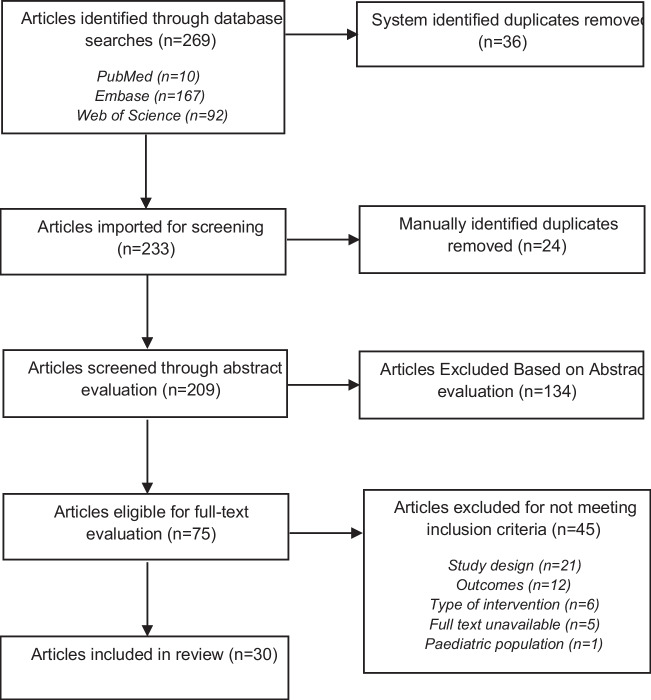
Out of 269 references published during the four-year search period, 209 abstracts were screened, 75 were eligible for full-text screening, and 30 studies (35 data sets) were identified to review (all observational, no RCTs) (See Fig. 1). From the 30 studies, 13 were conducted with data from populations living in the USA [24, 25••, 26–29, 30•, 31–34, 35•, 36], eight from China [15, 35•, 37, 38••, 39–42], eight from Europe [43–50], one from Iran [51], and one was a multinational cohort study [52•].
Fig. 1.
Flow Diagram of search strategy and included articles
The majority (> 50%) of studies controlled for age, gender, education, smoking status, alcohol consumption, physical activity, energy intake, BMI, hypertension, and diabetes. Two studies (7%) controlled for saturated fat intake [25••, 30•], eleven studies (37%) controlled for vegetable intake [28, 29, 36, 38••, 39, 41, 42, 48–50, 52•], six studies (20%) controlled for meat intake [26, 38••, 39, 41, 50, 52•] (two (6%) for processed meat [26, 50]), three (10%) for Mediterranean diet score [28, 44, 46], three (10%) for dietary approaches to stop hypertension (DASH) score [31–33], and six (20%) for diet quality [24, 26, 34, 35•, 37, 51]. Three (10%) studies which were across multiple countries controlled for center or region but not ethnicity [45, 48, 52•].
Eggs and CVD Mortality
In the past 4 years, 11 observational studies [15, 24, 27, 29, 30•, 34, 35•, 43, 44, 46, 52•] have examined the associations between eggs and CVD mortality (See Supplementary Table 1). Of these, six publications reported on CVD mortality [24, 29, 30•, 34, 43, 46], and all except one [43] reported a higher risk of CVD mortality with the highest egg intake vs. the lowest egg intake (hazard ratio (HR) ranging from 1.14–1.75). Four out of five studies reporting a higher risk were from the USA [24, 29, 30•, 34] with the remaining study from Italy [46]. One of these five studies [30•] was a substitution study that reported a reduced risk of CVD mortality (total, heart disease, and stroke [males only]) when 3% of energy from plant protein was substituted for egg protein (HR range 0.72–0.76). In one study [24], the significant association was lost after adjusting for dietary cholesterol, and no association was found for egg white consumption. Five publications [15, 27, 35•, 44, 52•] reported no association between eggs and CVD mortality (one reported on heart disease mortality only [27]). One study [35•], after grouping cardiometabolic subtypes (coronary heart disease (CHD), stroke, and diabetes) together, found that the highest egg intake in the White American cohort was associated with higher cardiometabolic mortality, reduced risk was found with moderate egg intake in the Chinese cohort, and no association was found in the Black American cohort.
Eggs and CVD Incidence (Non-Fatal CVD)
Fifteen observational studies reported on egg consumption and CVD incidence (non-fatal CVD) in the last 4 years [25••, 26, 28, 31–34, 38••, 39, 45, 48–51, 52•] (See Supplementary Table 1). Eight reported on total CVD incidence [25••, 26, 28, 34, 38••, 39, 51, 52•] with four studies finding an increased incidence (HR ranging from 1.06–1.39) with the highest (≥ 7 eggs/week) vs. lowest egg intake (< 1 egg/week) [34], high (> 6 eggs/week) and low intake (< 3 eggs/week) [38••], or with a 0.5 egg/day continuous intake [25••], noting that in this last study, significance was lost after adjusting for dietary cholesterol. The fourth study was a substitution study that reported that substituting eggs with fish, nuts, legumes, or whole grains was associated with 2–3% lower relative risks for incident CVD when the substitution amount was one serving per week and 15–21% lower relative risks when the substitution amount was one serving per day [26]. Two studies found a decreased incidence (HR ranging from 0.78–0.89) [39, 52•] (data from the PURE study [52•]), and three found no association [28, 51, 52•] (data from the ONTARGET/TRANSCEND study [52•]).
Five studies reported on egg intake and stroke incidence with three finding no association [49, 51, 52•] and two finding an increased risk (HR ranging from 1.07–1.34). One study reported an increased risk of total stroke and ischemic stroke with high and low egg intake (> 6 and < 3 eggs/week for total stroke, and > 6 and < 1 egg/week for ischemic stroke) and an increased risk of hemorrhagic stroke with low egg intake (< 3 eggs/week) [38••]. The other study found an increased risk of 7% for total stroke and 25% for hemorrhagic stroke with 20 g egg/day continuous intake (total: 95% CI 1.01–1.14, P trend = 0.031, hemorrhagic: 95% CI 1.09–1.43, P trend = 0.002) but no association for ischemic stroke [45].
Individual studies investigating heart disease reported on the incidence of ischemic heart disease, coronary heart disease, myocardial infarction, or venous thromboembolism. Two studies reported on ischemic heart disease, one reporting an increased association with highest (≥ 7 eggs/week) vs. lowest egg intake (< 1 egg/week) [34] and the other showing a 7% decreased risk for continuous 20 g egg/day intake (95% CI 0.88–0.99, P trend = 0.023) [48]. Coronary heart disease incidence was reported in three studies, two finding no association [33, 51] except in a sub-analysis where an increased risk of 30% (95% CI 1.03–1.56) was found in the older cohort [33], and one finding an increased risk with highest (> 6 eggs/week) vs. moderate egg consumption (3 < 6 eggs/week) [38••]. Three studies reported on myocardial infarction incidence, one reported an 11–13% increased risk [32], one found no association [51], and one study with two data sets found a 17% reduced risk (data from the PURE study) and no association (data from the ONTARGET/TRANSCEND study) [52•]. No association was found for the incidence of heart disease [52•] or the incidence of venous thromboembolism [50].
Eggs and CVD Risk Factors
Nine observational studies [15, 36, 37, 40, 42, 47, 49, 52•, 53] were identified in the last 4 years that examined the associations between egg consumption and CVD risk factors including lipid and lipoprotein concentrations, blood pressure (including the presence of hypertension), and adiposity (See Supplementary Table 1). Four publications examined the association between eggs and lipid profile [15, 36, 37, 52•]. Two studies found no association [36, 52•], one study found a decrease in lipid profile (with no association for high-density lipoprotein (HDL) cholesterol) [15], and one study found an increase in total cholesterol (TC) and LDL cholesterol but a decrease in triglycerides (TG) and an increase in HDL cholesterol [37]. One study [40] reported on the association between eggs and lipoprotein particle concentrations and found reduced risk [40].
Four studies examined eggs and blood pressure [15, 36, 49, 52•]. Two studies found reduced systolic blood pressure [15, 52•] (data set from the PURE study [52•]), and three studies found reduced diastolic blood pressure [15, 49, 52•] (data set from the PURE study [52•]) with the highest egg intake (> 45 g/day or ≥ 7 eggs/week). In contrast, one study reported increased systolic and diastolic blood pressure (data set from ONTARGET/TRANSCEND study [52•]). One study found no association between eggs and systolic blood pressure [49], and one study found no association for both diastolic and systolic blood pressure [36]. Two studies reported on the association between eggs and hypertension [42, 47]. No association was found for the highest (≥ 7 eggs/week) vs. the lowest (< 1 egg/week) egg intake, but an increased risk was found for lower egg intakes (2–6.9 eggs/week) [47]. A decreased risk was noted in a substitution study where eggs were substituted for meat [42].
Three studies described the associations between eggs and adiposity (BMI, body fat, waist circumference) [15, 36, 41]. One study [41] completed analysis by sex and found a decreased risk of adiposity for females with the highest (approximately 50 g/day) vs. lowest egg intake (0 g/day) but no association for males, and two studies found no association with eggs and waist circumference [15, 36].
Ethnicity
The majority of research included in this review was from cohort data collected in three geographical regions, with population ethnicity influencing the direction of associations between egg consumption and CVD outcomes. Thirteen publications were from studies conducted in populations residing in the USA [24, 25••, 26–29, 30•, 31–34, 35•, 36]; twelve studies reported on eggs and non-fatal and fatal CVD [24, 25••, 26–29, 30•, 31–34, 35•] and one on risk factors [36], with seven identifying an increased risk [24, 25••, 26, 29, 30•, 31, 34], five indicating no association [27, 28, 32, 33, 36] and one indicating an increased risk (White Americans) or no association (Black Americans) [35•]. Eight studies used data from cohorts residing in China [15, 35•, 37, 38••, 39–42] with four studies reporting on eggs and non-fatal and fatal CVD [15, 35•, 38••, 39] and five reporting on eggs and CVD risk factors [15, 37, 40–42]. In contrast to the US findings, only one of these studies reported an increased risk [38••], six showed reduced risk [35•, 37, 39–42], and one found no association [15]. Eight studies used cohort data from Europe reporting on eggs and non-fatal and fatal CVD as well as CVD risk factors [43–50]. Three confirmed an increased risk [45–47], one found a reduced risk [43], and four found no association [44, 48–50]. One study from Iran found no association with non-fatal CVD [51]. A final study including cohort data from 21 countries also found no association between fatal CVD and CVD risk factors [52•].
Subgroups
Eleven studies conducted subgroup analyses on eggs and CVD mortality, incidence, and/or risk factors [25••, 31, 32, 34, 38••, 39, 46–49, 52•]. Six studies evaluated sex differences: six for men [25••, 31, 38••, 39, 48, 49] and four for women [31, 38••, 39, 48]. All but two studies found no association or no difference to the overall study outcomes. One study, which reported no association in the total cohort, found a reduced risk of IHD in males [48], and another study that reported a reduced risk of total CVD and hypertension in the total cohort found no association for males but confirmed reduced risk for females [39]. Eight studies assessed BMI in a sub-analysis [31, 32, 34, 38••, 39, 46–48] with the majority (seven studies) reporting no association or no difference to the overall study findings. One study reported an increased risk of MI incidence for people with a BMI over 25 kg/m2 but found no association in the total cohort [32]. Nine completed sub-analyses for type 2 diabetes, and all but two found no association [25••, 31, 32, 34, 38••, 46–48]. The two studies [31] reporting an increased risk of CVD incidence in persons with type 2 diabetes (one specific to ischemic stroke [31]) also reported increased risk in their overall findings.
Discussion
Eggs are a highly nutritious food; they offer a complete source of protein, containing all essential amino acids and a complement of vitamins and minerals [54]. However, the high cholesterol content in eggs makes them a food of concern, which has led to a plethora of studies over the last few decades investigating egg consumption and the risk of CVD.
In this narrative review (with studies identified by a systematic search), we summarized recent evidence from studies published from 2019 to September 2022 with regard to the impact of egg consumption on CVD risk. The studies were all observational, and the findings were mixed. For CVD mortality, an equal number of studies reported an increased risk or no association with the highest egg intake. This was similar to earlier reviews which also found mixed results. A meta-analysis of 39 observational studies including nearly 2 million individuals found no association between the highest intake of eggs and CVD mortality [55], and similar findings were presented in another meta-analysis of 24 observational studies of over 11 million individuals that found no association between highest intake of eggs and CVD mortality [56]. However, contrasting findings were reported in a meta-analysis of 19 observational studies that found a nonlinear dose–response association between egg consumption and CVD mortality, although the certainty of the evidence for these observations was rated as very low [57]. In this latter review, the majority (80%) of studies reporting an increased risk were studied from US populations. Similarly, in a meta-analysis which found no association in the total population, an increased risk was reported in a sub-analysis by ethnicity in the American population [56].
It is difficult to study food in isolation without considering the effect of foods consumed in the whole diet and the nutrients they collectively contribute. In Western populations, eggs are typically consumed with meat (often processed) which is high in saturated fat, while in Asian cultures, eggs are frequently consumed in meals with vegetables [27]. It is also possible that Western populations consume more cholesterol from other sources (e.g., red meat, full-fat dairy, and discretionary foods), as compared to people from Asian cultures [12, 58, 59]. In the American diet for example, due to dietary patterns, dietary cholesterol and saturated fat increase in parallel, e.g., eggs with bacon and sausage [12]. Cooking methods are also different, with higher temperatures and longer cooking time leading to oxidative damage to vitamins [60]. Furthermore, while nutrition databases have an average for the cholesterol content of eggs, this commonly differs by country and can be influenced by multiple factors including feed composition [61–63], housing systems (free range vs. caged) [64], and age of the laying hens [65]. These factors may contribute to the observed country differences. Very few studies in this review controlled for saturated fat; no studies have considered cooking methods, whether eggs were from free-range or caged chickens, or the feed they were provided; and only 37% controlled for vegetable intake, 20% controlled for red meat, and 6% controlled for processed meat which may confound associations.
Regarding CVD incidence (non-fatal), the findings of this review were also mixed. Almost an equal spread of increase, decrease, and no association between egg intake and total CVD incidence was found. Of the eight studies reporting an increased risk of a CVD event, six (75%) were from the USA, and in two of these studies, the populations were majority (92%) male. Therefore, similar to the mortality findings, there is a potential effect of ethnicity, sex, and dietary patterns. Although not consistently so, studies reporting increased incidence of CVD were often from older cohorts or studies with longer duration and hence an aging cohort, both of which would predispose to an increased risk of CVD incidence. Similar mixed findings have been reported in earlier review studies. A meta-analysis of 17 observational studies found no association with CHD or stroke and an increased risk of heart failure with high compared to low egg consumption [16]. In a meta-analysis of nine observation studies, eating one egg daily was not associated with an increased risk of ischemic heart disease (IHD) and was associated with a small reduction in stroke [15]. Similarly, a meta-analysis of 21 observational studies also found no association between egg consumption and CVD risk and found beneficial effects toward stroke risk [66]. An umbrella review of seven systematic reviews and 15 meta-analyses concluded that increased egg consumption is not associated with CVD risk in the general population [67].
In this review, most studies assessing egg consumption and CVD risk factors found a reduced risk or no association. Interestingly, the majority (56%) of studies were from Chinese cohorts, and only one study was from the USA. However, due to the exploratory nature of observational studies, these studies are unable to establish a causal relationship between egg intake and CVD risk factors. Randomized controlled trials are required to provide evidence of causation, and as there were no RCTs during the defined period of this review, our discussion will focus on review studies of earlier RCTs that examined eggs and CVD risk factors. A systematic review of 23 RCTs found nonsignificant effects of increasing the consumption of eggs on risk markers for CVD [13]. In a meta-analysis of 28 RCTs, high egg consumption increased total cholesterol, LDL, and HDL cholesterol, but not LDL-to-HDL or TC-to-HDL ratios or triglycerides compared with low egg control diets [68]. In another meta-analysis of eight RCTs comparing greater than four eggs/week to less than 4 eggs/week, egg consumption was not associated with differences in blood lipid profile or blood pressure [69] and in two meta-analyses looking at the effects of different food groups on hypertension, regular egg consumption was associated with a lower risk of hypertension [70, 71].
In summary, recent data on the associations between egg consumption and risk of CVD mortality, incidence, and risk factors is mixed. With observational data, it is difficult to assess the relationship of any individual food independently of a dietary pattern. The risk associations reported in the reviewed observational studies are likely to be attributed to the dietary pattern accompanying high egg intake (e.g., eating eggs with bacon and sausage or as part of a meal with vegetables) and/or other risk factors present in people with high egg consumption. For example, in Asian cultures, egg consumption is positively associated with socioeconomic status and physical activity, inversely associated with smoking, and generally correlated with other aspects of a healthy dietary pattern (e.g., higher intake of fiber, vegetables, and fruit) [15]. In the USA, egg consumption is correlated with low physical activity, smoking, and dietary patterns high in saturated fat (e.g., full-fat dairy, red meat, and processed meat) [72]. Very few of the studies reviewed adjusted analyses for dietary confounders like meat, processed meat, vegetables, and saturated fat, and this could have impacted outcomes. Therefore, the suggestion that egg consumption by itself promotes the risk and development of CVD is questionable compared with the overall complexity of the dietary pattern, physical activity, and genetic predisposition. There is, however, evidence suggesting higher egg consumption could be associated with a higher risk of CVD in people with diabetes [17, 18, 73], but not all studies confirm these findings, specifically after adjusting for background diet [74]; further studies are needed. The best evidence for CVD prevention supports adopting a change in overall dietary pattern [75], and therefore, dietary guidance to reduce CVD risk should focus on implementing a healthy dietary pattern rather than removing a single food, such as eggs.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Declarations
Conflict of Interest
JDB is currently funded by the American Egg Board’s Egg Nutrition Center. They have also received grant support from the Almond Board of California, the Almond Board of Australia, the International Nut and Dried Fruit Council, and Dried Fruit Australia. AMC is currently funded by the American Egg Board’s Egg Nutrition Center. AMC is the immediate past president of the Nutrition Society of Australia. They have also received grant support from the Almond Board of California, the Almond Board of Australia, the International Nut and Dried Fruit Council, and Dried Fruit Australia. And they have been a consultant for Nuts for Life. AMH is currently funded by the American Egg Board’s Egg Nutrition Center. They have also received grant support from the Almond Board of California, the Almond Board of Australia, the International Nut and Dried Fruit Council, and the Dried Fruit Australia. SC and ESC declare that they have no conflicts of interest or funding to disclose.
Human and Animal Rights and Informed Consent
The article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.World Health Organization. The top 10 causes of death. [https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death Accessed 29 November 2022]
- 2.Dietschy JM, Siperstein MD. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967;8(2):97–104. doi: 10.1016/S0022-2275(20)38921-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JD, Lindsey CA, Dietschy JM. Influence of dietary cholesterol on cholesterol metabolism. Ann N Y Acad Sci. 1968;149(2):808–821. doi: 10.1111/j.1749-6632.1968.tb53837.x. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association: Diet and heart disease. American Heart Association Dallas, TX, USA. In.; 1968.
- 5.Dawber TR, Nickerson RJ, Brand FN, Pool J. Eggs, serum cholesterol, and coronary heart disease. Am J Clin Nutr. 1982;36(4):617–625. doi: 10.1093/ajcn/36.4.617. [DOI] [PubMed] [Google Scholar]
- 6.McNamara DJ. The fifty year rehabilitation of the egg. Nutrients. 2015;7(10):8716–8722. doi: 10.3390/nu7105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones PJ, Pappu AS, Hatcher L, Li ZC, Illingworth DR, Connor WE. Dietary cholesterol feeding suppresses human cholesterol synthesis measured by deuterium incorporation and urinary mevalonic acid levels. Arterioscler Thromb Vasc Biol. 1996;16(10):1222–1228. doi: 10.1161/01.ATV.16.10.1222. [DOI] [PubMed] [Google Scholar]
- 8.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Soliman GA. Dietary cholesterol and the lack of evidence in cardiovascular disease. Nutrients. 2018;10(6). [DOI] [PMC free article] [PubMed]
- 10.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001;20(1):5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 11.Food Standards Australia New Zealand. AUSNUT 2011–13 – Australian food composition database. Canberra: FSANZ. 2014;Available at www.foodstandards.gov.au. In
- 12.Xu Z, McClure ST, Appel LJ. Dietary cholesterol intake and sources among U.S adults: results from National Health and Nutrition Examination Surveys (NHANES), 2001-2014. Nutrients. 2018;10(6). [DOI] [PMC free article] [PubMed]
- 13.Geiker NRW, Larsen ML, Dyerberg J, Stender S, Astrup A. Egg consumption, cardiovascular diseases and type 2 diabetes. Eur J Clin Nutr. 2018;72(1):44–56. doi: 10.1038/ejcn.2017.153. [DOI] [PubMed] [Google Scholar]
- 14.Vincent MJ, Allen B, Palacios OM, Haber LT, Maki KC. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am J Clin Nutr. 2019;109(1):7–16. doi: 10.1093/ajcn/nqy273. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Lam TH, Jiang CQ, et al. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank cohort study and meta-analyses. Eur J Nutr. 2019;58(2):785–796. doi: 10.1007/s00394-018-1692-3. [DOI] [PubMed] [Google Scholar]
- 16.Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–1090. doi: 10.1080/10408398.2017.1392288. [DOI] [PubMed] [Google Scholar]
- 17.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(1):146–159. doi: 10.3945/ajcn.112.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. 2013;346:e8539. doi: 10.1136/bmj.e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander DD, Miller PE, Vargas AJ, Weed DL, Cohen SS. Meta-analysis of egg consumption and risk of coronary heart disease and stroke. J Am Coll Nutr. 2016;35(8):704–716. doi: 10.1080/07315724.2016.1152928. [DOI] [PubMed] [Google Scholar]
- 20.Qin C, Lv J, Guo Y, et al. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart. 2018;104(21):1756–1763. doi: 10.1136/heartjnl-2017-312651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khawaja O, Singh H, Luni F, et al. Egg consumption and incidence of heart failure: a meta-analysis of prospective cohort studies. Front Nutr. 2017;4:10. doi: 10.3389/fnut.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missimer A, DiMarco DM, Andersen CJ, Murillo AG, Vergara-Jimenez M, Fernandez ML. Consuming two eggs per day, as compared to an oatmeal breakfast, decreases plasma ghrelin while maintaining the LDL/HDL ratio. Nutrients. 2017;9(2). [DOI] [PMC free article] [PubMed]
- 23.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis. 2013;229(2):524–530. doi: 10.1016/j.atherosclerosis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang P, Wu F, Mao L, et al. Egg and cholesterol consumption and mortality from cardiovascular and different causes in the United States: a populationbased cohort study. PLoS Med. 2021;18(2) (no pagination)(e1003508). [DOI] [PMC free article] [PubMed]
- 25.Zhong VW, Van Horn L, Cornelis MC, et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321(11):1081–1095. doi: 10.1001/jama.2019.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong VW, Allen NB, Greenland P, et al. Protein foods from animal sources, incident cardiovascular disease and all-cause mortality: a substitution analysis. Int J Epidemiol. 2021;50(1):223–233. doi: 10.1093/ije/dyaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia PF, Pan XF, Chen C, Wang Y, Ye Y, Pan A. Dietary intakes of eggs and cholesterol in relation to all-cause and heart disease mortality: a prospective cohort study. J Am Heart Assoc. 2020;9(10) (no pagination)(e015743). [DOI] [PMC free article] [PubMed]
- 28.Wang M, Wang Z, Lee Y, et al. Dietary meat, trimethylamine N-oxide-related metabolites, and incident cardiovascular disease among older adults: the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2022;42(9):E273–E288. doi: 10.1161/ATVBAHA.121.316533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Liu B, Snetselaar LG, et al. Association of major dietary protein sources with all-cause and cause-specific mortality: prospective cohort study. J Am Heart Assoc. 2021;10(5):1–24. doi: 10.1161/JAHA.119.015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Liao LM, Weinstein SJ, Sinha R, Graubard BI, Albanes D. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern Med. 2020;180(9):1173–1184. doi: 10.1001/jamainternmed.2020.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Ramady O, Latifi AN, Treu T, et al. Egg consumption and risk of acute stroke in the million veteran program. Clin Nutr ESPEN. 2022;50:178–182. doi: 10.1016/j.clnesp.2022.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djousse L, Ho YL, Nguyen XMT, et al. Egg consumption and risk of coronary artery disease in the million veteran program. Clin Nutr. 2020;39(9):2842–2847. doi: 10.1016/j.clnu.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djousse L, Zhou G, McClelland RL, et al. Egg consumption, overall diet quality, and risk of type 2 diabetes and coronary heart disease: a pooling project of US prospective cohorts. Clin Nutr. 2021;40(5):2475–2482. doi: 10.1016/j.clnu.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen GC, Chen LH, Mossavar-Rahmani Y, et al. Dietary cholesterol and egg intake in relation to incident cardiovascular disease and all-cause and cause-specific mortality in postmenopausal women. Am J Clin Nutr. 2021;113(4):948–959. doi: 10.1093/ajcn/nqaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan XF, Yang JJ, Lipworth LP, et al. Cholesterol and egg intakes with cardiometabolic and all-cause mortality among Chinese and low-income Black and White Americans. Nutrients. 2021;13(6):15. doi: 10.3390/nu13062094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riseberg E, Lopez-Cepero A, Mangano KM, Tucker KL, Mattei J. Specific dietary protein sources are associated with cardiometabolic risk factors in the Boston Puerto Rican health study. J Acad Nutr Diet. 2022;122(2):298-+. [DOI] [PMC free article] [PubMed]
- 37.Zhang X, Liu F, Li J, et al. Longitudinal association of egg consumption habits with blood lipids among Chinese adults: results from the prediction for atherosclerotic cardiovascular disease risk in China project. Chin Med J. 2022;135(6):747–749. doi: 10.1097/CM9.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia X, Liu FC, Yang XL, et al. Associations of egg consumption with incident cardiovascular disease and all-cause mortality. Sci China Life Sci. 2020;63(9):1317–1327. doi: 10.1007/s11427-020-1656-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Wang L, Liu LJ, et al. Longitudinal association of egg intake frequency with cardiovascular disease in Chinese adults. Nutr Metab Carbiovasc Dis. 2022;32(4):908–917. doi: 10.1016/j.numecd.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Pan L, Chen L, Lv J et al. Association of egg consumption, metabolic markers, and risk of cardiovascular diseases. medRxiv. 2021;07. [DOI] [PMC free article] [PubMed]
- 41.Liu RR, Zhao YL, Li Q, Dang SN, Yan H. Body fat mass, fat distribution and egg consumption: a population-based study in Chinese adults egg consumption and body fat in rural Chinese. J Am Coll Nutr. 2020;39(6):528–536. doi: 10.1080/07315724.2019.1700200. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Zhao JK, Wang JP, Wang T. Association between animal source foods consumption and risk of hypertension: a cohort study. Eur J Nutr. 2021;60(5):2469–2483. doi: 10.1007/s00394-020-02423-w. [DOI] [PubMed] [Google Scholar]
- 43.Zupo R, Sardone R, Donghia R, et al. Traditional Dietary patterns and risk of mortality in a longitudinal cohort of the Salus in Apulia study. Nutrients. 2020;12(4):15. doi: 10.3390/nu12041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamora-Ros R, Cayssials V, Cleries R, et al. Moderate egg consumption and all-cause and specific-cause mortality in the Spanish European prospective into cancer and nutrition (EPIC-Spain) study. Eur J Nutr. 2019;58(5):2003–2010. doi: 10.1007/s00394-018-1754-6. [DOI] [PubMed] [Google Scholar]
- 45.Tong TYN, Appleby PN, Key TJ, et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur Heart J. 2020;41(28):2632–2640. doi: 10.1093/eurheartj/ehaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruggiero E, Di Castelnuovo A, Costanzo S, et al. Egg consumption and risk of all-cause and cause-specific mortality in an Italian adult population. Eur J Nutr. 2021;60(7):3691–3702. doi: 10.1007/s00394-021-02536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macdonald CJ, Madika AL, Bonnet F, Fagherazzi G, Lajous M, Boutron-Ruault MC. Cholesterol and egg intakes, and risk of hypertension in a large prospective cohort of french women. Nutrients. 2020;12(5) (no pagination)(1350). [DOI] [PMC free article] [PubMed]
- 48.Key TJ, Appleby PN, Bradbury KE, et al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease: a prospective study of 7198 incident cases among 409 885 participants in the pan-European EPIC cohort. Circulation. 2019;139(25):2835–2845. doi: 10.1161/CIRCULATIONAHA.118.038813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdollahi AM, Virtanen HEK, Voutilainen S, et al. Egg consumption, cholesterol intake, and risk of incident stroke in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2019;110(1):169–176. doi: 10.1093/ajcn/nqz066. [DOI] [PubMed] [Google Scholar]
- 50.Kunutsor SK, Laukkanen JA, Virtanen JK. Egg and cholesterol intake, apolipoprotein E4 phenotype and risk of venous thromboembolism: findings from a prospective cohort study. Br J Nutr. 2022. [DOI] [PMC free article] [PubMed]
- 51.Mohammadifard N, Taheri M, Haghighatdoost F et al. Egg consumption and risk of cardiovascular events among Iranians: results from Isfahan Cohort Study (ICS). Eur J Clin Nutr. 2022. [DOI] [PubMed]
- 52.Dehghan M, Mente A, Rangarajan S, et al. Association of egg intake with blood lipids, cardiovascular disease, and mortality in 177,000 people in 50 countries. Am J Clin Nutr. 2020;111(4):795–803. doi: 10.1093/ajcn/nqz348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, Xue Y, Wang Y, et al. Association between dietary patterns and dyslipidemia in adults from the Henan rural cohort study. Asia Pac J Clin Nutr. 2020;29(2):299–308. doi: 10.6133/apjcn.202007_29(2).0013. [DOI] [PubMed] [Google Scholar]
- 54.Song WO, Kerver JM. Nutritional contribution of eggs to American diets. J Am Coll Nutr. 2000;19(5 Suppl):556s–562s. doi: 10.1080/07315724.2000.10718980. [DOI] [PubMed] [Google Scholar]
- 55.Godos J, Micek A, Brzostek T, et al. Egg consumption and cardiovascular risk: a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. 2021;60(4):1833–1862. doi: 10.1007/s00394-020-02345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma W, Zhang Y, Pan L et al. Association of egg consumption with risk of all-cause and cardiovascular disease mortality: a systematic review and dose-response meta-analysis of observational studies. J Nutr. 2022;07. [DOI] [PubMed]
- 57.Yang PF, Wang CR, Hao FB, et al. Egg consumption and risks of all-cause and cause-specific mortality: a dose-response meta-analysis of prospective cohort studies. Nutr Rev. 2022;80(7):1739–1754. doi: 10.1093/nutrit/nuac002. [DOI] [PubMed] [Google Scholar]
- 58.Su C, Jia X, Wang Z, Wang H, Zhang B. Trends in dietary cholesterol intake among Chinese adults: a longitudinal study from the China Health and Nutrition Survey, 1991–2011. BMJ Open. 2015;5(6):e007532. doi: 10.1136/bmjopen-2014-007532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carson JAS, Lichtenstein AH, Anderson CAM, et al. Dietary cholesterol and cardiovascular risk: a science advisory from the American Heart Association. Circulation. 2020;141(3):e39–e53. doi: 10.1161/CIR.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 60.Tang D, Wang R, He X, et al. Comparison of the edible quality of liquid egg with different cooking methods and their antioxidant activity after in vitro digestion. Food Res Int. 2021;140:110013. doi: 10.1016/j.foodres.2020.110013. [DOI] [PubMed] [Google Scholar]
- 61.Sarlak S, Tabeidian SA, Toghyani M, Shahraki ADF, Goli M, Habibian M. Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol Trace Elem Res. 2021;199(5):1986–1999. doi: 10.1007/s12011-020-02284-8. [DOI] [PubMed] [Google Scholar]
- 62.Zheng M, Mao P, Tian X, Guo Q, Meng L. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult Sci. 2019;98(5):2250–2259. doi: 10.3382/ps/pey550. [DOI] [PubMed] [Google Scholar]
- 63.Pliego AB, Tavakoli M, Khusro A, et al. Beneficial and adverse effects of medicinal plants as feed supplements in poultry nutrition: a review. Anim Biotechnol. 2022;33(2):369–391. doi: 10.1080/10495398.2020.1798973. [DOI] [PubMed] [Google Scholar]
- 64.Islam Z, Sultan A, Khan S, Alhidary IA, Abdelrahman MM, Khan RU. Impact of varying housing systems on egg quality characteristics, fatty acid profile, and cholesterol content of Rhode Island Red × Fyoumi laying hens. Trop Anim Health Prod. 2021;53(5):456. doi: 10.1007/s11250-021-02913-x. [DOI] [PubMed] [Google Scholar]
- 65.Anderson KE. Comparison of fatty acid, cholesterol, and vitamin A and E composition in eggs from hens housed in conventional cage and range production facilities. Poult Sci. 2011;90(7):1600–1608. doi: 10.3382/ps.2010-01289. [DOI] [PubMed] [Google Scholar]
- 66.Marventano S, Godos J, Tieri M, et al. Egg consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr. 2020;71(3):325–331. doi: 10.1080/09637486.2019.1648388. [DOI] [PubMed] [Google Scholar]
- 67.Mah E, Chen CO, Liska DJ. The effect of egg consumption on cardiometabolic health outcomes: an umbrella review. Public Health Nutr. 2020;23(5):935–955. doi: 10.1017/S1368980019002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rouhani MH, Rashidi-Pourfard N, Salehi-Abargouei A, Karimi M, Haghighatdoost F. Effects of egg consumption on blood lipids: a systematic review and meta-analysis of randomized clinical trials. J Am Coll Nutr. 2018;37(2):99–110. doi: 10.1080/07315724.2017.1366878. [DOI] [PubMed] [Google Scholar]
- 69.Wang MX, Wong CH, Kim JE. Impact of whole egg intake on blood pressure, lipids and lipoproteins in middle-aged and older population: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2019;29(7):653–664. doi: 10.1016/j.numecd.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhang DZ. Red meat, poultry, and egg consumption with the risk of hypertension: a meta-analysis of prospective cohort studies. J Hum Hypertens. 2018;32(7):507–517. doi: 10.1038/s41371-018-0068-8. [DOI] [PubMed] [Google Scholar]
- 71.Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. doi: 10.3945/an.117.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vu T-HT, Van Horn L, Daviglus ML, et al. Association between egg intake and blood pressure in the USA: the International Study on Macro/Micronutrients and Blood Pressure (INTERMAP) Public Health Nutr. 2021;24(18):6272–6280. doi: 10.1017/S1368980021002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li MY, Chen JH, Chen C, Kang YN. Association between egg consumption and cholesterol concentration: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(7):1–15. doi: 10.3390/nu12071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard C, Cristall L, Fleming E, et al. Impact of egg consumption on cardiovascular risk factors in individuals with type 2 diabetes and at risk for developing diabetes: a systematic review of randomized nutritional intervention studies. Can J Diabetes. 2017;41(4):453–463. doi: 10.1016/j.jcjd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Pallazola VA, Davis DM, Whelton SP, et al. A clinician’s guide to healthy eating for cardiovascular disease prevention. Mayo Clin Proc Innov Qual Outcomes. 2019;3(3):251–267. doi: 10.1016/j.mayocpiqo.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.