Abstract
Objective:
Obesity and dysmetabolism are major risk factors for atrial fibrillation (AF). Fasting and post-load levels of glucose and non-esterified fatty acids (NEFA) reflect different facets of metabolic regulation. We sought to study their respective contributions to AF risk concurrently.
Methods:
We assessed levels of fasting and post-load glucose and NEFA in the Cardiovascular Health Study to identify associations with AF incidence, and secondarily with ECG parameters of AF risk available at baseline. Linear and Cox regressions were performed.
Results:
The study included 1,876 participants (age 77.7±4.4). During median follow-up of 11.4 years, 717 cases of incident AF occurred. After adjustment for potential confounders, post-load glucose showed an association with incident AF (HR per SD increment of post-load glucose=1.11 [95% CI=1.02, 1.21], p=0.017). Both glucose measures, but not NEFA, were positively associated with higher P wave terminal force in V1 (PTFV1); the association remained significant only for post-load glucose when the two measures were entered together (β per SD increment=138 μV*ms [95% CI=15, 260], p=0.028). Exploratory analyses showed significant interaction by sex for fasting NEFA (pinteraction=0.044) and post-load glucose (pinteraction=0.015) relative to AF, with relationships stronger in women. For post-load glucose, the association with incident AF was observed among women, but not men.
Conclusions:
Among older adults, post-load glucose was positively associated with incident AF, with consistent findings for PTFV1. In exploratory analyses, the relationship with AF appeared specific to women. These findings require further study, but suggest that interventions to address post-prandial dysglycemia late in life might reduce AF.
Keywords: atrial fibrillation, non-esterified fatty acids, glucose, metabolism, epidemiology
Introduction
Atrial fibrillation (AF) has been categorized as epidemic in Western nations.1 Given the rising tide of obesity throughout the world and its demonstrated contribution to AF, it is expected that this epidemic will continue and expand.1 A better understanding of associated metabolic dysregulation could help to identify novel modifiable risk factors that might be targeted for AF prevention.
Abnormal glucose metabolism is especially common among older adults in the U.S., with some 50% and 33% experiencing prediabetes and diabetes, respectively.2 While diabetes3 and metabolic syndrome4,5 are established risk factors for development of AF in middle-aged and older adults, glycemic measures themselves have not shown a clear relationship with incident AF in general population samples6 or cohorts free of diabetes.7 Further, most evaluations of dysglycemia and AF have centered on fasting glucose or glycated hemoglobin and have not focused on adults late in life, the group at highest risk of both disorders.1,2
Like glucose, non-esterified fatty acids (NEFA) are a key cellular fuel, and the predominant substrate for powering myocardial bioenergetics.8 Elevated levels of fasting NEFA have been associated with incident AF,9 insulin resistance and diabetes,10,11 and mortality.12 Less is known about the impact of post-load NEFA levels, which exhibit less decline in the setting of obesity and insulin resistance.13 The relationship of post-load NEFA with AF remains unstudied in any age group.
To fill these knowledge gaps, we added fasting and post-load NEFA to existing fasting and post-load glucose measures to a late follow-up visit of Cardiovascular Health Study (CHS). The primary objective of the present study was to determine the relative contributions of fasting and post-load NEFA, alongside fasting and post-load glucose, to incident AF late in life. In secondary analyses, we also evaluated the relationships of these NEFA and glucose measures to baseline ECG parameters known to be associated with AF.
Methods
Study Population
CHS is a prospective investigation of risk factors for CVD among adults ≥65 recruited from 4 U.S. locations.14 Random Medicare eligibility lists stratified by age and sex were used to identify potential participants.14 An initial recruitment wave enrolled 5,201 individuals in 1989–90; it was followed by a second wave in 1992–93, which enrolled 687 predominantly African-American individuals. Participants had to be able to give consent, reside in their respective region for the upcoming 3 years, and not be wheelchair dependent, institutionalized, or currently receiving treatment for cancer. CHS was approved by the Institutional Review Boards of participating centers.
CHS examinations involved medical history, physical examination, blood collection, and diagnostic testing. The present NEFA study included individuals with available serum and eligible for a 2-hour oral glucose tolerance test.
Glucose and NEFA Measurement
For OGTT, participants receiving glucose-lowering medication for diabetes were excluded. Serum was collected following an 8-hour overnight fast and 2 hours after ingestion of 75-gram dextrose at the 1996–1997 exam. Specimens were frozen at −80°C 70 minutes after collection and shipped to the CHS Central Laboratory (Burlington, VT) for long-term storage at this temperature. Glucose measurements were performed with the Kodak Ektachem 700 analyzer (Rochester, NY). Measurement of total NEFA was performed in 2019 using the Wako enzymatic method on specimens that were never thawed previously. The intra-assay coefficient of variation was 5%.
ECG Parameter Assessment
Twelve-lead electrocardiography performed at the 1996–97 visit was used to assess the maximal P wave duration and the wave terminal force in V1 (PTFV1).15 The P wave was measured in all 12 leads and the longest value taken as the maximal P wave duration. PTFV1 was calculated by multiplying the duration (ms) and amplitude (μV) of the negative terminal deflection of the P wave in lead V1.
AF Ascertainment
The primary outcome was incident AF or atrial flutter (both referred to as AF throughout). Annual electrocardiograms from 1989–90 to 1998–99 and ICD-9 codes from inpatient or outpatient records throughout the follow-up period (ending December 2014) were used for AF ascertainment.
Covariates
Covariates were largely determined at the 1996–97 examination. Age, sex, race, smoking status, alcohol consumption, and physical activity were assessed by self-report. Heavy alcohol consumption was defined in men as >14 drinks/week and in women as >7 drinks/week. Weight, height, waist circumference and blood pressure were measured using standardized procedures. Use of estrogen replacement and antihypertensive therapy was determined through medication inventory. Estimated glomerular filtration rate (eGFR) was derived from serum cystatin C. Laboratory measures were quantitated at the Central Laboratory. Diabetes was defined as fasting glucose ≥126 mg/dL or use of anti-diabetic medication up to and including the 1996–97 examination.16 Prevalent coronary heart disease (CHD) was assessed at study enrollment by participant report of physician diagnosis of angina, myocardial infarction, or coronary revascularization, validated by evidence from the baseline examination and record reviews. Incident CHD, similarly defined, was adjudicated by the CHS Events Committee. Prevalent stroke was based on participant report of physician diagnosis and validation. Prevalent heart failure (HF) was assessed using self-report, confirmed by use of HF medications, physician questionnaires, and medical record review. The proportion of missing data for all individual covariates was ≤5%; participants with missing covariate values were excluded from relevant analyses.
Statistical Analysis
Baseline characteristics were summarized using standard descriptive statistics. Because of the heavy tails of glucose and NEFA, all analyses were conducted after removal of extreme outliers >99th percentile of the distributions, as done previously.17 Incidence rates of AF were calculated using quasi-Poisson regression with offset.
Linear regression models were fit to examine the cross-sectional associations of fasting and post-load glucose, as well as fasting and post-load NEFAs, with the secondary ECG outcome measures. Cox proportional hazards models were fit to evaluate the relationships of glucose and NEFA measures with incident AF. Covariates for sequential models were selected based on prior associations or underlying biology, mirroring our approach in earlier work.17 Model 1 adjusted for age, sex, race, and clinic. Model 2 (main model) accounted additionally for height, weight, physical activity, smoking, alcohol use, estrogen replacement therapy, serum albumin, and eGFR. Model 3 explored the impact of adding covariates putatively in the causal pathway, namely, systolic blood pressure, antihypertensive medication use, prevalent CHD, prevalent HF, and prevalent stroke. We also explored the associations of ECG parameters with incident AF, with addition of fasting and post-load glucose to Model 3 covariates as the main model. Restricted cubic splines were examined to assess for departures from linearity. Since relationships were approximately linear, we report associations per SD increment in exposure levels. Proportional hazards assumptions were checked using Schoenfeld residuals; no meaningful violations were observed. Additional analyses assessed whether glucose measures were independently associated with the outcomes of interest by including them simultaneously in the models. In sensitivity analyses, we (i) explored whether risk estimates for AF in Model 1 differed when run in the subset with available covariates for Model 2 (after exclusion of n=56 participants with missing covariate data); and (ii) examined the impact of additionally adjusting for waist circumference and the inflammatory mediator C-reactive protein. We assessed for effect modification by sex and BMI by including appropriate cross-product terms in the main model.
All analyses were conducted with R (http://www.r-project.org). A two-sided p<0.05 was considered statistically significant. We did not adjust for multiple testing.
Participant and Public Involvement
Participants were not involved in the design of CHS.
Results
A flow chart describing participant selection is shown in Figure 1. Among the 4,413 individuals who participated in the 1996–97 examination, 677 were excluded for prevalent or unclear AF status. We restricted the study sample to participants who had all four primary exposure variables available. This left 1,876 individuals in the study, of whom 19 or 26 participants with outlier values, depending on the analysis, were were removed from the final analytical sample (Figure 1). Compared with excluded participants, those included had a lower cardiovascular risk profile.
Figure 1.
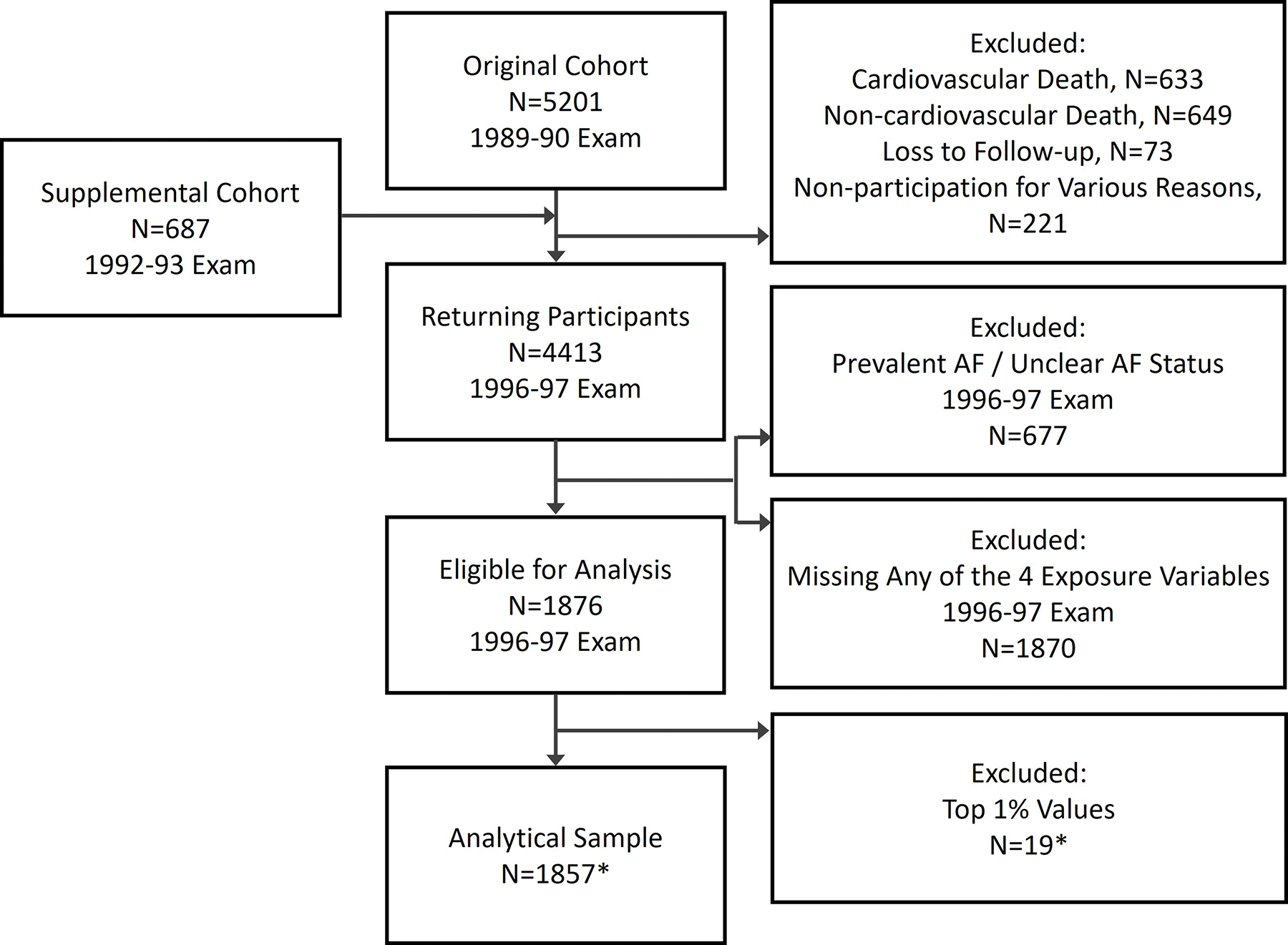
Flowchart of participant selection for the study. *Analytical sample of 1857 differs for each exposure measure (fasting glucose, postload glucose, fasting NEFA and postload NEFA), since the 19 participants excluded for each measure only partly overlap. For analyses adjusting concurrently for fasting and postload glucose, 26 participants with outlier values were excluded. AF, atrial fibrillation; NEFA, nonesterified fatty acids.
The characteristics of the study sample are presented in Table 1. Participants were, on average, late into their eighth decade, almost two-thirds were women, and one-seventh were Black. They were generally overweight, with few cardiovascular comorbidities. The prevalence of diabetes was low, by design; approprimately one-third of participants had impaired fasting glucose and slightly more had impaired glucose tolerance.
Table 1.
Baseline characteristics
| Characteristics* | Overall Cohort (n=1,876) |
|---|---|
| Age, yrs | 77.7 ± 4.4 |
| Female, n (%) | 2,721 (61.7) |
| Black race, n (%) | 265 (14.1) |
| Clinic, n (%) | |
| Forsyth County, NC | 432 (23.0) |
| Sacramento County, CA | 541 (28.8) |
| Washington County, MD | 391 (20.8) |
| Allegheny County, PA | 512 (27.3) |
| BMI, kg/m2 | 26.8 ± 4.5 |
| Height, m | 1.63 ± 0.1 |
| Weight, kg | 71.6 ± 13.9 |
| Waist circumference, cm | 96.6 ± 12.7 |
| Physical activity, n (%) | |
| None/Mild | 408 (22.0) |
| Moderate | 934 (50.3) |
| Strenuous | 514 (27.7) |
| Smoking, n (%) | |
| Current | 149 (8.1) |
| Former | 785 (42.5) |
| Never | 912 (49.4) |
| Heavy alcohol consumption, n (%) | 155 (8.3) |
| Estrogen replacement therapy, n (%) | 214 (11.4) |
| Systolic blood pressure, mmHg | 137 ± 20 |
| Blood pressure medication use, n (%) | 944 (50.4) |
| Diabetes†, n (%) | 50 (2.7) |
| Coronary heart disease, n (%) | 344 (18.3) |
| Heart failure, n (%) | 79 (4.2) |
| Stroke, n (%) | 86 (4.6) |
| Serum albumin, g/dL | 3.8 ± 0.3 |
| eGFR, mL/min/1.73 m2 | 72.7 ± 18.6 |
| CRP, mg/L | 2.3 (1.0, 4.7) |
| Fasting glucose, mg/dL | 90 (96, 103) |
| Post-load glucose, mg/dL | 130 (104, 165) |
| Impaired fasting glucose, n (%) | 606 (32.3) |
| Impaired glucose tolerance, n (%) | 786 (41.9) |
| Fasting NEFA, mmol/L | 0.4 (0.3, 0.5) |
| Post-load NEFA, mmol/L | 0.05 (0.04, 0.07) |
| Maximal P wave duration, ms | 112 ± 13 |
| P wave terminal force in V1, μV*ms | 2496 (1320, 3710) |
Mean ± standard deviation or (when not normally distributed) median and interquartile range for continuous variables and number (percent) for categorical variables.
Based on fasting glucose or anti-diabetic medication use up to and including the 1996–97 examination.
BMI = body mass index, CRP = C-reactive protein, eGFR = estimated glomerular filtration rate, NEFA = non-esterified fatty acids.
Assessment of cross-sectional relationships of glucose and NEFA measures with ECG parameters is presented in Table 2. There were no significant associations with maximal P wave duration. Both fasting and post-load glucose levels did exhibit significant associations with greater PTFV1 in the minimal and main models. In the main model, every SD increment in fasting glucose (15 mg/dL) was associated with a 165 μV*ms greater PTFV1, while every SD increment in post-load glucose (52 mg/dL) related to a 171 μV*ms greater PTFV1. These associations were attenuated but remained significant after adjustment for covariates that could also act at least partly as intermediates (Model 3). Upon inclusion of fasting and post-load glucose together in the main model, the association for post-load glucose remained significant (β per SD=138 [95% CI=15, 260], p=0.028) but that for fasting glucose did not (β per SD=102 [95% CI=−51, 255], p=0.191). Corresponding associations after further adjustment (Model 3) were attenuated, but remained significant for post-load glucose (β per SD=127 [95% CI=5, 249], p=0.043) and non-significant for fasting glucose (β per SD=89 [95% CI=−64, 242], p=0.257).
Table 2.
Cross-sectional associations with electrocardiographic parameters
| Maximal p wave duration (ms) | ||||
| Glucose | Fasting | Post-load | ||
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Model 1 | 0.91 (0.12, 1.70) | 0.024 | 0.57 (−0.10, 1.23) | 0.094 |
| Model 2 | 0.22 (−0.60, 1.04) | 0.602 | 0.31 (−0.36, 0.98) | 0.366 |
| Model 3 | 0.02 (−0.80, 0.84) | 0.957 | 0.16 (−0.51, 0.82) | 0.646 |
| NEFA | Fasting | Post-load | ||
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Model 1 | −0.01 (−0.69, 0.66) | 0.971 | 0.79 (−0.04, 1.61) | 0.061 |
| Model 2 | −0.14 (−0.82, 0.55) | 0.692 | 0.22 (−0.61, 1.05) | 0.607 |
| Model 3 | −0.28 (0.96, 0.40) | 0.418 | 0.19 (−0.63, 1.00) | 0.655 |
| P wave terminal force in V1 (μV*ms) | ||||
| Glucose | Fasting | Post-load | ||
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Model 1 | 186.15 (63.41, 308.89) | 0.003 | 172.00 (69.77, 274.23) | <0.001 |
| Model 2 | 164.95 (34.94, 294.96) | 0.013 | 171.48 (65.71, 277.26) | 0.002 |
| Model 3 | 144.89 (14.46, 275.32) | 0.030 | 157.13 (51.63, 262.64) | 0.004 |
| NEFA | Fasting | Post-load | ||
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Model 1 | 55.22 (−49.30, 159.73) | 0.301 | −21.08 (−148.49, 106.32) | 0.746 |
| Model 2 | 50.19 (−58.30, 158.67) | 0.365 | −39.01 (−169.89, 91.87) | 0.559 |
| Model 3 | 37.45 (−70.74, 145.64) | 0.498 | −39.41 (−169.51, 90.70) | 0.553 |
During median follow-up of 11.4 years, 717 cases of incident AF occurred, an average incidence rate of 4% per year. Of all glucose and NEFA measures, only post-load glucose showed a significant association with incident AF (Figure 2). After adjustment for potential confounders in the main model, each SD increment in post-load glucose was significantly associated with an 11% higher relative hazard for AF. With inclusion of putative intermediate variables (Model 3), the association was attenuated to a 9% higher relative hazard for AF and became marginally non-significant. Sensitivity analyses that compared Model 1 associations in the analytical study sample for glucose or NEFA with those in the subset with available main-model covariates showed indistinguishable results (Table S1). Likewise, additional adjustment for waist circumference and C-reactive protein did not materially alter the findings (Table S1). Concurrent adjustment for fasting and post-load measures of glucose in the main model showed a similar association for post-load glucose with AF (HR per SD=1.12 [95% CI=1.01, 1.24], p=0.029), which was attenuated and became marginally non-significant after inclusion of putative intermediates (HR per SD=1.10 [95% CI=0.99, 1.21], p=0.079).
Figure 2.
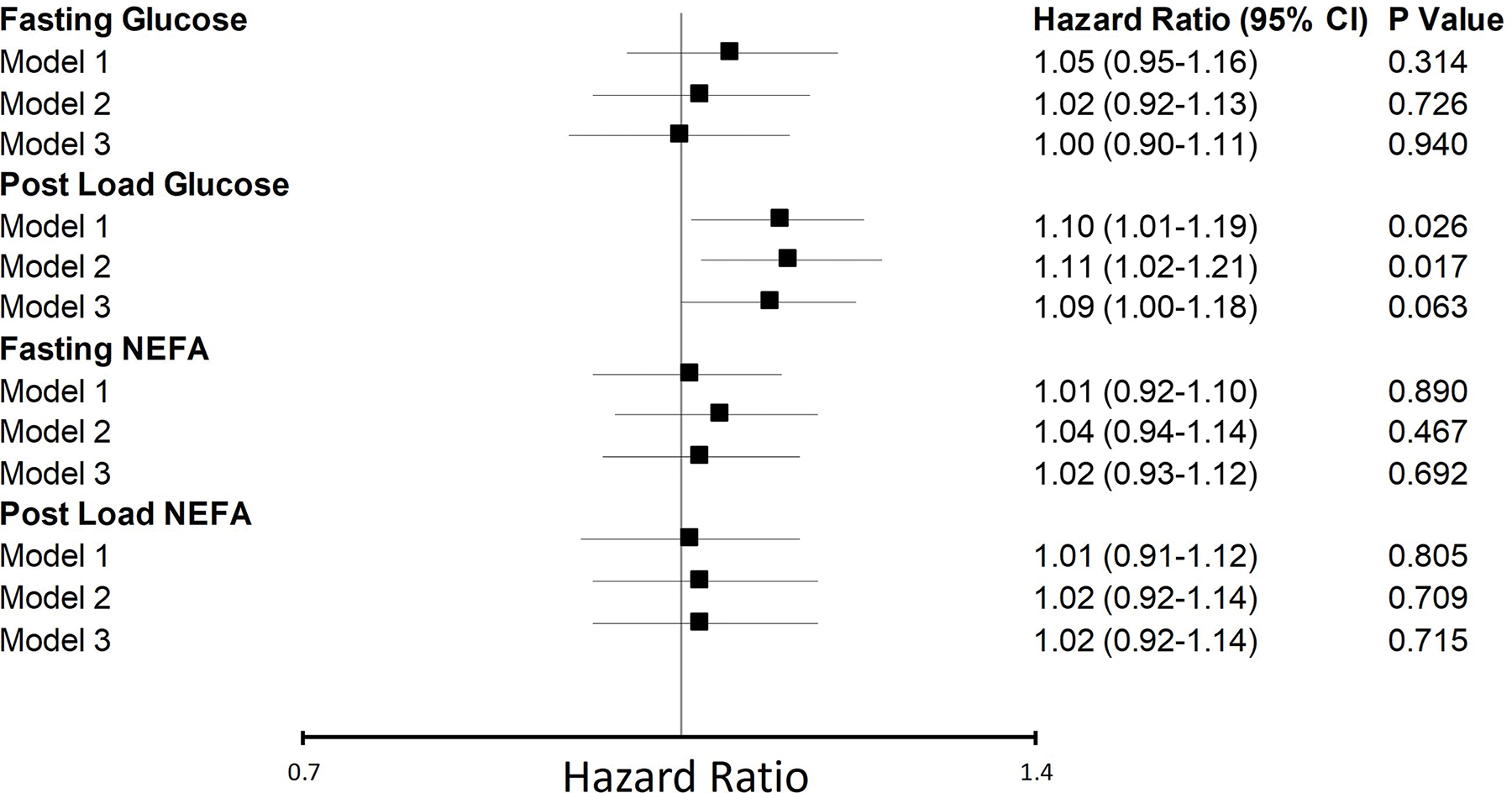
Associations of glucose and NEFA with incident atrial fibrillation. Standard deviations (SD) for glucose and NEFA measures are as follows: fasting glucose, SD=15 mg/dL; postload glucose, SD=52 mg/dL; fasting NEFA, SD=0.2 mmol/L; postload NEFA, SD=0.05 mmol/L. Model 1: adjusted for age, sex, race and clinic. Model 2 (main model): adjusted for variables in model 1, as well as height, weight, physical activity, smoking, alcohol use, oestrogen replacement therapy, serum albumin and eGFR. Model 3: adjusted for variables in model 2, as well as systolic blood pressure, antihypertensive medication use, prevalent coronary heart disease, prevalent heart failure and prevalent stroke. NEFA, non-esterified fatty acid, CI, confidence interval.
In exploratory analyses, there was evidence of interaction of fasting NEFA by sex in their association with incident AF (pinteraction=0.044). In particular, there was a tendency toward a positive association in women that was not observed in men (Table S2). There was also a significant interaction between post-load glucose and sex (pinteraction=0.015), such that a positive relationship of post-load glucose with AF was observed in women, but not men (Table S2). By contrast, no significant interaction by BMI was observed for any of the glucose or NEFA measures (all pinteraction≥0.399).
Additional exploratory analyses showed that both maximal P wave duration and PTFV1 were independently associated with incident AF (HR per SD=1.09 [95% CI=1.01, 1.18], p=0.038, and HR per SD=1.10 [95% CI=1.02, 1.19], p=0.019, respectively), validating their use as intermediate AF phenotypes in this population.
Discussion
In this study of older adults, post-load, but not fasting, glucose was significantly associated with higher incidence of AF, yet neither post-load nor fasting NEFA showed significant associations with this outcome (Figure 3). Exploratory analyses revealed effect modification of fasting NEFA and post-load glucose by sex, such that the associations with incident AF were stronger, and in the case of post-load glucose, only significant, in women. In secondary analyses, fasting and post-load glucose, but not fasting or post-load NEFA, were significantly associated with greater PTFV1 on ECG, though no glucose or NEFA measures were associated with maximal P wave duration. When both fasting and post-load glucose were modeled simultaneously, however, only the association of PTFV1 with post-load glucose remained significant.
Figure 3.
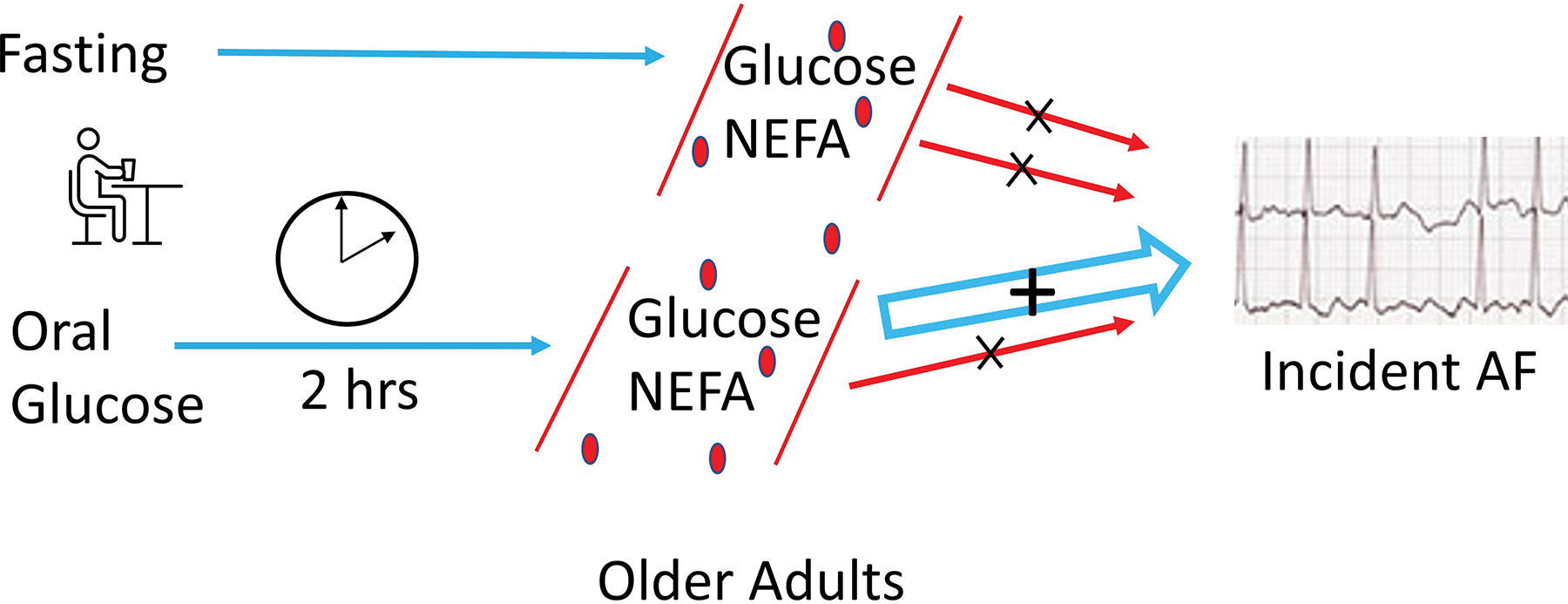
Graphic summary of study design and results. Among older adults, postload glucose was positively associated with incident AF. Associations were not detected for fasting glucose or NEFA nor postload NEFA. AF, atrial fibrillation; NEFA, non-esterified fatty acids; hrs, hours.
The associations of diabetes and the metabolic syndrome with incident AF are well documented.3–5 Yet available studies have not found consistent associations between glycemic measures and new-onset AF in the setting of low diabetes prevalence or its exclusion.6,7,18 Such studies have primarily evaluated fasting glucose and glycated hemoglobin, for which Mendelian randomization analysis failed to detect evidence of causality.19 In a clinical trial assessing interventions for adults with pre-diabetes (median age, 63), only fasting glucose was associated with incident AF.7 Previous work from CHS mostly involving participants at study entry (1989–90 exam; mean age, 72.6) found only a borderline association of post-load glucose with new-onset AF, and that only in a minimally adjusted model; no association with fasting glucose was observed.20 The present study focused on CHS participants attending the 1996–97 exam (mean age, 77.7) instead, observing a significant association of post-load glucose with AF incidence after main-model adjustment.
Existing data on the association of circulating NEFA with incident AF have been confined to fasting levels. In a prior CHS analysis, fasting NEFA measured at the 1992–93 exam were positively associated with onset of AF.9 A salient difference between the two analyses is that 15.2% of the earlier cohort had prevalent diabetes, whereas the corresponding prevalence in our sample was only 2.7%.
The current results are consistent with earlier CHS analyses documenting that post-load glucose is more strongly associated with atherosclerotic CVD, HF and mortality than fasting glucose.17,21 That previous reports7,20 failed to detect a similar association of post-load glucose with AF may well relate to the greater prominence of post-prandial glycemic derangements documented with progressively older age.22 The finding that only post-load glucose was positively related to PTFV1 upon concurrent adjustment further supports the primacy of post-load glucose for risk of this arrhythmia. PTFV1, like maximal P wave duration, has been linked to AF incidence,23 a finding replicated in our sample. It is unclear why a glucose association was not seen for maximal P wave duration, though the fact that both short and long P wave duration are associated with AF24 may disadvantage this measure.
The basis for the observed association with AF may relate to post-load glucose levels acting as a marker of more adverse homeostatic dysregulation or themselves inducing greater cellular damage. Whereas fasting glucose is chiefly a consequence of hepatic insulin resistance, post-load glucose is primarily determined by skeletal muscle insulin resistance and impaired pancreatic β-cell secretory capacity.22 Declining skeletal muscle and pancreatic β-cell health could signal more profound homeostatic disruption, greater systemic inflammation and oxidative stress, and heightened impairments in cardiovascular structure and function.25–27 On the other hand, post-load or post-prandial glucose excursions could be directly deleterious. Fluctuations in glucose levels after meals have been associated with activation of oxidative stress,28 which could foster the development of AF.26
The observation of a stronger association of post-load glucose with AF in female participants could reflect the lower baseline risk of AF in women or owe to metabolic derangements being of greater relative consequence in women. That a similar impact of female sex was not seen for post-load dysglycemia and atherosclerotic CVD, HF or mortality17,21 would argue against a more pronounced metabolic perturbation in older women compared with men. Given the exploratory nature of this analysis, the finding of effect modification by sex warrants additional study.
The lack of association for circulating NEFA with incident AF runs counter to previous findings in CHS. Perhaps only in the setting of more pronounced metabolic dysregulation, as seen with the substantially higher prevalence of diabetes in the earlier study,9 are serum NEFA a marker or contributor to AF risk. Alternatively, other aspects of the aging process, changes in participants’ dietary habits over the course of the study, or natural variation in NEFA levels over time may have weakened the association in question. Post-load NEFA could not be evaluated alongside fasting NEFA for the prior CHS analysis of AF. Yet, in a previous assessment from the 1996–97 exam, post-load NEFA, and not fasting NEFA, showed an association with incident diabetes.29 Given that total NEFA comprise multiple individual NEFA, fasting levels of which have been shown to bear directionally opposite associations with AF,30 the extent to which variation in specific NEFA could differentially influence these outcomes merits further study. Last, although there was evidence of effect modification by sex for fasting NEFA suggesting the presence of an association in women, no such interaction was detected in our previous work on AF. This exploratory finding will require future confirmation.
Our study has several limitations. As in any observational study, the possibility of residual confounding cannot be excluded. The current study can identify associations, but cannot establish causality. Participants had to survive to attend a follow-up examination and, by design, were free of treated diabetes. Both limit generalizability to the broader population of older adults. Further, because diabetes would have been preferentially diagnosed from fasting glucose, the sample may have been skewed toward a greater prevalence of post-load dysglycemia. Participants with glucose and NEFA levels at the upper extreme were excluded because of scant observations; the present results may not apply to such levels. AF ascertainment was via intermittent ECG (for the initial 2 years) and diagnosis codes, and information on type of AF (paroxysmal vs. persistent) was not available. This ascertainment approach is highly specific but insensitive, which may have biased our findings toward the null hypothesis. The one-time baseline assessment of fasting and post-load glucose and NEFA measures, as well as ECG parameters, also may have led to an under-estimation of associations given these measures’ intra-individual variability. Two-hour NEFA were assessed in response to an oral glucose load; the findings could differ in response to a regular meal.
In conclusion, post-load glucose, but not fasting glucose or NEFA measures, was positively associated with incident AF in this study of older adults, a finding corroborated by the more robust association of post-load than fasting glucose with PTFV1. Further research is necessary to understand why post-load glucose bears a particular association with AF in advanced old age, and whether this is indeed preferential to women, as a means of identifying potentially intervenable pathways in this high risk segment of the population.
Supplementary Material
Key Questions.
1. What is already known about this subject?
Diabetes and metabolic syndrome are established risk factors for the development of AF in middle-aged and older adults, with most evaluations of dysglycemia focusing on fasting glucose or glycated hemoglobin. Similarly, elevated levels of fasting NEFAs have been associated with incident AF, while little is known about the impact of post-load NEFA levels.
2. What does this study add?
This study of older adults is the first to examine the impact on AF incidence of fasting and post-load glucose and NEFA together. Post-load glucose was associated with a more than 10% higher risk of AF per SD increment; fasting glucose and non-esterified fatty acid measures showed no association. Exploratory analyses found evidence of effect modification by sex, such that an increased risk of AF was observed in women, but not in men.
3. How might this impact on clinical practice?
While additional research is necessary to determine mechanisms, further explore the sex-specific relationship observed in women, and confirm results within the context of a randomized controlled trial, these findings potentially reveal new targets for AF intervention. The current study suggests that therapies to improve post-prandial or post-load dysglycemia could impact AF prevention in advanced old age – the population at highest risk of AF.
Acknowledgements and Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 and R01AG053325 from the National Institute on Aging, and K24 HL135413 from NHBLI.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
J.R.K. reports stock ownership in Abbott, Bristol-Myers Squibb, Johnson & Johnson, Medtronic, Merck and Pfizer. The remaining authors have no relevant disclosures related to this work.
Footnotes
Ethics Statement
This study complied with the Declaration of Helsinki. All participants provided written informed consent. CHS was approved by the Institutional Review Boards of the Coordinating Center (University of Washington CR00004872) and field centers (University of Pittsburgh CR19040035-004, Johns Hopkins University 11007/CR811, Wake Forest University BG00-497, and University of California, Davis 300401-16).
References
- 1.Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NAM. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin Proc. 2016;91:1778–810. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- 3.Huxley RR, Filion KB, Konety S, Alonso A. Meta-Analysis of Cohort and Case–Control Studies of Type 2 Diabetes Mellitus and Risk of Atrial Fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159:850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huxley RR, Alonso A, Lopez FL, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart Br Card Soc. 2012;98:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latini R, Staszewsky L, Sun J-L, et al. Incidence of atrial fibrillation in a population with impaired glucose tolerance: the contribution of glucose metabolism and other risk factors. A post hoc analysis of the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research trial. Am Heart J. 2013;166:935–940.e1. [DOI] [PubMed] [Google Scholar]
- 8.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol Rev. 2010;90:207–58. [DOI] [PubMed] [Google Scholar]
- 9.Khawaja O, Bartz TM, Ix JH, et al. Plasma Free Fatty Acids and Risk of Atrial Fibrillation (From the Cardiovascular Health Study). Am J Cardiol. 2012;110:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman RN, Ader M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol Metab. 2000;11:351–6. [DOI] [PubMed] [Google Scholar]
- 11.Pankow JS, Duncan BB, Schmidt MI, et al. , Atherosclerosis Risk in Communities Study. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. [DOI] [PubMed] [Google Scholar]
- 12.Miedema MD, Maziarz M, Biggs ML, et al. Plasma Free Fatty Acids, Fatty Acid-binding Protein 4, and Mortality in Older Adults (From the Cardiovascular Health Study). Am J Cardiol. 2014;114:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol. 2008;295:R395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. [DOI] [PubMed] [Google Scholar]
- 15.German DM, Kabir MM, Dewland TA, Henrikson CA, Tereshchenko LG . Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. 2015;21:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kizer JR, Arnold AM, Benkeser D, et al. Total and high-molecular-weight adiponectin and risk of incident diabetes in older people. Diabetes Care. 2012;35:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oesterle A, Buzkova P, Pellegrini C, et al. Fasting and Post-Load Glucose and Non-Esterified Fatty Acids and Risk of Heart Failure and its Subtypes in Older Adults. J Gerontol A Biol Sci Med Sci. 2022;glac229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harati H, Zanetti D, Rao A, et al. No evidence of a causal association of type 2 diabetes and glucose metabolism with atrial fibrillation. Diabetologia. 2019;62:800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg PK, Biggs ML, Kaplan R, Kizer JR, Heckbert SR, Mukamal KJ. Fasting and Post-Glucose Load Measures of Insulin Resistance and Risk of Incident Atrial Fibrillation: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis NMCD. 2018;28:716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brutsaert EF, Shitole S, Biggs ML, et al. Relations of Postload and Fasting Glucose With Incident Cardiovascular Disease and Mortality Late in Life: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2016;71:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair A, Dunning T, Rodriguez-Mañas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol. 2015;3:275–85. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Zheng Z, Wu B, et al. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: A meta-analysis. Ann Noninvasive Electrocardiol Off J Int Soc Holter Noninvasive Electrocardiol Inc. 2020;25:e12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen JB, Kühl JT, Pietersen A, et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–95. [DOI] [PubMed] [Google Scholar]
- 25.Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seals DR, Brunt VE, Rossman MJ. Keynote lecture: strategies for optimal cardiovascular aging. Am J Physiol Heart Circ Physiol. 2018;315:H183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo G, Watterson S, Zhang S-D, et al. The role of senescence in the pathogenesis of atrial fibrillation: A target process for health improvement and drug development. Ageing Res Rev. 2021;69:101363. [DOI] [PubMed] [Google Scholar]
- 28.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. [DOI] [PubMed] [Google Scholar]
- 29.Shitole SG, Biggs ML, Ix JH, et al. Fasting and Postload Nonesterified Fatty Acids and Glucose Dysregulation in Older Adults. Am J Epidemiol. 2022;191:1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrini CN, Buzkova P, Lichtenstein AH, et al. Individual non-esterified fatty acids and incident atrial fibrillation late in life. Heart. 2021;107:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


