Abstract
Introduction
Alteration of autonomic function is the main pathophysiology of most types of syncope, including syncope due to orthostatic hypotension and neurally mediated syncope or reflex syncope. The aim of this study was to investigate the difference in autonomic dysfunction assessed between each type of syncope and to evaluate the association between the severity of autonomic dysfunction and the recurrence of syncope.
Methodology
Three hundred and six participants, including 195 syncope and 109 healthy control participants, were recruited to this retrospective cohort study. Autonomic function was initially assessed by the Thai version of the Composite Autonomic Symptom Score 31 (COMPASS 31), a self-administered questionnaire.
Result
According to one hundred and ninety-five syncope participants, twenty-three participants had syncope due to orthostatic hypotension, 61 had reflex syncope, 79 had presyncope, and 32 had unclassified syncope. Participants in the syncope due to orthostatic hypotension and reflex syncope groups had significantly higher COMPASS 31 scores than the control and presyncope groups, of which the syncope due to orthostatic hypotension group had the highest score. The cutoff score of 32.9 for COMPASS 31 had a sensitivity of 50.0% and a specificity of 81.9% to predict the recurrence of syncope.
Conclusion
The degree of autonomic dysfunction, which was assessed by COMPASS 31, could vary depending on the syncope type. The COMPASS 31, which is an easy-to-use self-administered questionnaire utilized for the assessment of autonomic symptoms and function, was a helpful tool for classifying some types of syncope and predicting the recurrence of syncope, which could lead to appropriate further management.
Keywords: Autonomic nervous system, Dysautonomia, Syncope, Syncope due to orthostatic hypotension, Vasovagal syncope
1. Introduction
Syncope is defined as a brief onset, transient loss of consciousness for a short duration (not exceeding 2 min) as a result of cerebral hypoperfusion, with full recovery occurring spontaneously [1]. Syncope is a common clinical problem that affects up to 35% of the general population in their lifetime and accounts for 1%–5% of emergency department visits [[2], [3], [4]]. Moreover, syncope had a recurrence rate of up to 22% at 2 years’ follow-up [5]. Diagnostic tools and management differ based on the type and cause of syncope. Thus, to effectively manage patients with syncope, it is necessary to identify the cause of the syncope. However, the diagnostic accuracy for syncope in routine practice, which may not be according to the standard guidelines, is only 65%. Moreover, diagnostic accuracy for the two common causes of syncope, which are reflex syncope and syncope due to orthostatic hypotension (OH), is 78% and 57%, respectively [6].
The diagnosis of syncope is primarily based on history-taking, physical examination, including supine and standing blood pressure measurements, and electrocardiography. Moreover, further investigations should be considered in patients who have an uncertain diagnosis or in high-risk patients [1]. There are three main groups of syncope, including syncope due to OH, neurally mediated syncope or reflex syncope, and cardiogenic syncope. Alteration of autonomic function is responsible for the pathophysiology of most types of syncope, including primary and secondary autonomic failure in syncope due to OH and a sudden temporary change in autonomic response that causes the abrupt cessation or decreased reflex control of blood pressure in reflex syncope [1,[7], [8], [9]]. Identifying the autonomic dysfunction in these patients could help clinicians understand more about the causes of syncope and choose suitable care or additional investigation. However, there is no information about the autonomic function status in each type of syncope, as well as the effect of autonomic dysfunction on the clinical manifestation, prognosis, or recurrence of syncope.
Although the composite autonomic severity score from autonomic function testing, developed and refined primarily at the Mayo Clinic, is the gold standard test for assessing autonomic function, it is inconvenient and time-consuming because it comprises several tests and resources may be limited to perform the assessment [10,11]. The Composite Autonomic Symptom Scale 31 (COMPASS 31), which is a self-administered questionnaire used for assessing autonomic symptoms and function, is accessible and useful. Moreover, it is easier to administer than the gold standard test [12]. COMPASS 31 has been used as a screening tool for autonomic dysfunction in many diseases, including multiple sclerosis [13], Parkinson's disease [14], small fiber polyneuropathy [15], diabetic neuropathy [16,17], and scleroderma [18], and has been translated into many languages, including the Thai language [19]. The Thai version of the COMPASS 31 questionnaire, which has content and language equivalence, has been tested for validity and reliability [19]. However, no similar study has been conducted in patients with syncope and no cutoff has been provided for diagnosing autonomic dysfunction in these patients.
This study aimed to explore the difference in autonomic dysfunction between each type of syncope and to determine the association between the severity of autonomic dysfunction and the prognosis or recurrence of syncope.
2. Method
2.1. Study population
A total of 195 patients who had a history of syncope or presyncope and visited the King Chulalongkorn Memorial Hospital (Bangkok, Thailand) between January 1, 2021, and February 1, 2022, were enrolled in a retrospective cohort study and had been asked to complete the Thai version of COMPASS 31 (Thai-COMPASS 31) after the patients gave their informed consent. The inclusion criteria were a provisional diagnosis of syncope, age ≥18 years, and the ability to read and write the Thai language. Patients were excluded from the study if they refused to provide consent or were unable to complete the questionnaire. Patients whose syncope event occurred as a consequence of cardiogenic abnormalities, including arrhythmia, bradycardia (sinus node dysfunction and atrioventricular conduction system disease), tachycardia (supraventricular and ventricular), and structural defects, were excluded from the study. Patients were categorized into four groups (syncope due to OH, neurally mediated syncope or reflex syncope, unclassified syncope, and presyncope) based on the type of syncope. The type of syncope was defined according to the 2018 ESC guidelines for the diagnosis and management of syncope. Unclassified syncope was established for patients whose provisional diagnosis could not be assigned to a specific type of syncope according to 2018 ESC guidelines [6]. The presyncope group was defined as those who only had presyncope signs and symptoms that mostly appeared before the unconscious episode but were not followed by loss of consciousness [1]. The recurrence of syncope in this study was defined as the occurrence of episode of syncope that happen after the first episode. The study followed all relevant ethical principles, and the institutional committee of the faculty of medicine, Chulalongkorn University, Thailand on human research approved the protocol, which had been performed in compliance with the international guidelines for human research protection as Declaration of Helsinki.
2.2. Healthy control population
The study recruited 109 participants from patients who visited the King Chulalongkorn Memorial Hospital (Bangkok, Thailand) and online volunteers between January 1, 2021, and July 30, 2022, using the voluntary response method. The inclusion criteria were the absence of a history of syncope or presyncope and the ability to read and write the Thai language. Patients were excluded from the study if they refused to provide consent or were unable to complete the questionnaire. Participants were asked to complete the Thai version of COMPASS 31 questionnaire.
2.3. Initial assessment of autonomic dysfunction
The Thai-COMPASS 31 was used as a screening tool for autonomic function status [19]. It consists of 31 questions that are classified into six domains: orthostatic intolerance (four items), vasomotor (three items), secretomotor (four items), gastrointestinal (twelve items), bladder (three items), and pupillomotor (three items). All patients were requested to answer the questionnaire, which took 5–10 min to complete. The Thai-COMPASS 31 total score, which is the sum of the weighted scores of each domain, ranges between 0 and 100. The maximum score for each domain after being weighted are as follows: 40 points for orthostatic intolerance, 5 points for vasomotor, 15 points for secretomotor, 25 points for gastrointestinal, 10 points for bladder, and 5 points for pupillomotor. After informed consent was obtained from the participants, they were asked to complete the Thai-COMPASS 31 at their convenience in a private room. Participants were given the opportunity to freely answer the questionnaire.
2.4. Clinical data collection
We collected the demographic and clinical data of all participants from the King Chulalongkorn Memorial Hospital database and from the direct responses of the patients. The collected data included date of birth, sex, medical history regarding the syncope event (date of the first diagnosis of syncope, type of syncope, number of syncope episodes, and frequency of syncope recurrence), other medical history, comorbidities, and current medication. Medications that affect the autonomic nervous system, including antidepressants (selective serotonin reuptake inhibitors [SSRIs] or serotonin and norepinephrine reuptake inhibitors [SNRIs]), beta blockers, angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers, alpha-1 blockers, antihistamines, cholinesterase inhibitors, and central antihypertensives (clonidine, guanfacine, alpha-methyl-DOPA), were considered in the data analysis because patients who take these medications have been shown to have significantly worse results in the COMPASS 31 questionnaire when compared to patients not taking such drugs [20].
2.5. Statistical analysis
All data were analyzed with GraphPad Prism version 9.3.1 (GraphPad software, San Diego, California USA) and STATA version 17 (StataCorp LP, College Station, Texas). The distribution of data was assessed by the D'Agostino and Pearson test. Numerical data was presented as mean ± SD or median (interquartile range [IQR]) for continuous variables and as frequency for categorical variables. A Fisher's exact test was used to compare the difference in demographic data between the control participants and syncope patients. For each syncope group (presyncope, syncope due to OH, reflex syncope, and unclassified syncope) and the control group, the total Thai-COMPASS 31 score and each domain score were compared using the nonparametric Kruskal-Wallis test. The Mann-Whitney U test and unpaired t-test were used to compare the scores between the syncope due to OH and control, reflex syncope and control, and syncope due to OH and reflex syncope groups. A p-value of <0.05 indicated statistical significance. Logistic regression was used to estimate the probability of syncope recurrence. We used both Youden's index and Liu's index to determine an optimal cutoff point for predicting syncope recurrence. To account for the discordance between these two indexes, we used the Akaike information criterion (AIC) and Bayesian information criterion (BIC) to determine which index was better at providing an optimal cutoff value. Furthermore, the appropriate index also depended on the sensitivity and specificity for clinical purposes.
3. Result
3.1. Participants
We recruited a total of 304 participants to the study. Of 195 participants in the syncope group, 23 (11.8%) had syncope due to OH, 61 (31.28%) had reflex syncope, 79 (40.51%) had presyncope, and 32 (16.41%) had unclassified syncope. Among the 23 participants in the syncope due to OH group, 15 had known causes of OH, including 7 participants with volume depletion, 6 with drug induced OH, and 2 with anemia. The demographic data of all the participants are shown in Table 1. There was no significant difference in comorbidities and medication use except for underlying diabetes mellitus (p = 0.006) and use of SSRI/SNRI, beta blockers, and antihistamine (p = 0.023, 0.012, and 0.011, respectively) between each syncope group. The participants with reflex syncope were significantly younger than those with syncope due to OH.
Table 1.
Demographic data of participants.
| Control (109) | Syncope due to OH (23) | Reflex syncope (61) | Presyncope (79) | Unclassified syncope (32) | p-value | |
|---|---|---|---|---|---|---|
| Age, years | 52.14 ± 20.61 | 61.30 ± 20.77 | 48.69 ± 19.63 | 56.68 ± 15.92 | 58.31 ± 17.63 | 0.001* |
| Gender, (female/male) | 68/41 | 13/10 | 33/28 | 41/38 | 23/9 | 0.290 |
| Comorbidities | ||||||
| Diabetes mellitus | 12 | 4 | 5 | 2 | 8 | 0.006* |
| Parkinson disease | 1 | 2 | 1 | 0 | 1 | 0.057 |
| Depression | 0 | 0 | 1 | 0 | 0 | 0.406 |
| Othersa | 0 | 0 | 0 | 0 | 0 | NA |
| Medication | ||||||
| SSRI, SNRI | 0 | 1 | 4 | 0 | 1 | 0.023* |
| Beta blockers | 7 | 7 | 7 | 6 | 4 | 0.012* |
| ACE inhibitors | 4 | 3 | 4 | 3 | 2 | 0.412 |
| Calcium channel blockers | 10 | 4 | 5 | 7 | 5 | 0.579 |
| Alpha-1 blockers | 0 | 3 | 3 | 4 | 0 | 0.011* |
| Antihistamine | 3 | 1 | 2 | 3 | 1 | 0.993 |
| Cholinesterase inhibitors | 0 | 0 | 0 | 0 | 0 | NA |
| Central antihypertensives | 0 | 0 | 0 | 0 | 0 | NA |
* p-values <0.05 are considered significant.
Other comorbidities: multiple sclerosis, multiple system atrophy, primary amyloidosis, Lewy body dementia, fibromyalgia, small fiber polyneuropathy, and postural tachycardia syndrome.
3.2. The total COMPASS 31 score and domain scores between the syncope groups
The mean COMPASS 31 score was 12.12 ± 9.38, 24.35 ± 15.52, 26.18 ± 16.89, 16.07 ± 12.03, and 19.28 ± 12.68 for the control, syncope due to OH, reflex syncope, presyncope, and unclassified syncope groups, respectively. As syncope due to OH from known causes such as drug-induced OH, volume depletion, and anemia, which can experience amelioration or recovery, might be distinct from the other syncope types whose etiology is due to autonomic dysfunction, we excluded the 15 participants who had an identifiable cause of syncope from the syncope due to OH group. After excluding these 15 participants, the mean total COMPASS 31 score of this group rose to 33.63 ± 15.69, which was the highest score among all the groups. There was a statistically significant difference in the total score and in the orthostatic intolerance, gastrointestinal domain, and bladder domain scores of COMPASS 31 between the groups (p < 0.001, <0.001, 0.001, and 0.007, respectively). The total and domain scores of COMPASS 31 according to the groups are shown in Table 2. The total and domain scores of COMPASS 31 for each group are provided in Supplementary Tables 1–6.
Table 2.
The total and domain COMPASS 31 scores based on the groups of participants.
| Group | Orthostatic intolerance | Vasomotor | Secretomotor | Gastrointestinal | Bladder | Pupillomotor | Total |
|---|---|---|---|---|---|---|---|
| Control (n = 109) | 0.00 (0.00, 8.00) | 0.00 (0.00, 0.00) | 2.14 (0.00, 4.29) | 2.68 (0.89, 4.91) | 0.00 (0.00, 1.11) | 1.00 (0.00, 2.00) | 10.22 (4.92, 16.99) |
| Syncope due to OH (n = 8) | 24.00 (11.00, 27.00) | 0.00 (0.00, 0.00) | 4.29 (1.07, 8.04) | 3.57 (2.68, 7.14) | 2.22 (0.00, 5.00) | 1.33 (0.00, 2.15) | 33.83 (22.47, 41.54) |
| Reflex syncope (n = 61) | 12.00 (8.00, 20.00) | 0.00 (0.00, 0.00) | 2.14 (0.00, 6.43) | 5.36 (2.68, 8.93) | 0.00 (0.00, 2.78) | 0.67 (0.00, 1.67) | 23.36 (11.34, 38.51) |
| Presyncope (n = 79) | 8.00 (0.00, 12.00) | 0.00 (0.00, 0.00) | 2.14 (0.00, 6.43) | 3.57 (0.00, 6.25) | 0.00 (0.00, 1.11) | 0.00 (0.00, 1.67) | 14.56 (6.123, 27.18) |
| Unclassified syncope (n = 32) | 4.00 (0.00,16.00) | 0.00 (0.00, 0.00) | 4.29 (0.00, 6.43) | 5.36 (2.01, 6.92) | 0.00 (0.00, 2.22) | 1.00 (0.00, 1.92) | 16.21 (8.705, 32.23) |
| p-value | <0.001** | 0.073 | 0.235 | 0.001* | 0.007* | 0.276 | <0.001** |
* p-values <0.05 are considered significant; ** p-values <0.001 are considered highly significant, the number in brackets is interquartile range.
3.3. The syncope due to OH and reflex syncope groups had higher COMPASS 31 scores than the control and presyncope groups
To distinguish between the two common types of syncope, including syncope due to OH and reflex syncope, and the control group, we compared the COMPASS 31 scores. There was no significant difference in age, sex, and underlying diseases between these two syncope groups and the control group (p = 0.475, 0.304, and >0.05 respectively), but significant differences were noted regarding the use of SSRI, beta blockers, and alpha-1 blockers, which were higher in the syncope groups (p = 0.015, 0.034, and 0.006, respectively) (Table 1). Participants who had syncope due to OH had higher total COMPASS 31 scores (33.83 [IQR 22.47–41.54] vs 10.22 [IQR 4.92–16.99], p < 0.001) and domain scores for orthostatic intolerance (24.00 [IQR 11.00–27.00] vs 0.00 [IQR 0.00–8.00], p < 0.001) and bladder (2.22 [IQR 0.00–5.00] vs 0.00 [IQR 0.00–1.11], p = 0.007) than the control group (Table 2). Similarly, we found that participants who had reflex syncope had higher total COMPASS 31 scores (median 23.36 [IQR 11.34–38.51] vs 10.22 [IQR 4.92–16.99], p < 0.001) and domain scores for orthostatic intolerance (12.00 [IQR 8.00–20.00] vs 0.00 [IQR 0.00–8.00], p < 0.001), vasomotor (00.00 [IQR 0.00–0.00] vs 0.00 [IQR 0.00–0.00], p = 0.039), gastrointestinal (5.36 [IQR 2.68–8.93] vs 2.68 [IQR 0.89–4.91], p < 0.001), and bladder (2.22 [IQR 0.00–2.78] vs 0.00 [IQR 0.00–1.11], p = 0.002) than the control group (Table 2).
To determine whether participants who had only presyncopal symptoms and no loss of consciousness tended to have autonomic dysfunction similar to participants with syncope due to OH and reflex syncope, we compared the COMPASS 31 scores of these participants. Participants who had syncope due to OH had higher total COMPASS 31 scores (33.83 [IQR 22.47–41.54] vs 14.56 [IQR 6.123–27.18], p = 0.001) and domain scores for orthostatic intolerance (24.00 [IQR 11.00–27.00] vs 8.00 [IQR 0.00–12.00], p < 0.001) and bladder (2.22 [IQR 0.00–5.00] vs 0.00 [IQR 0.00–1.11], p = 0.048) than the presyncope group (Table 2). Participants with reflex syncope also had higher total COMPASS 31 scores (23.36 [IQR 11.34–38.51] vs 14.56 [IQR 6.123–27.18], p < 0.001) and domain scores for orthostatic intolerance (12.00 [IQR 8.00–20.00] vs 8.00 [IQR 0.00–12.00], p < 0.001), vasomotor (00.00 [IQR 0.00–0.00] vs 0.00 [IQR 0.00–0.00], p = 0.013), and gastrointestinal (5.36 [IQR 2.68–8.93] vs 3.57 [IQR (0.00, 6.25)], p = 0.003) than the presyncope group (Table 2). However, there was no significant difference in the total COMPASS 31 and domain scores between the syncope due to OH and reflex syncope groups (p = 0.196, 0.089, 0.676, 0.435, 0.684, 0.267, and 0.531 for total score, orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, bladder, and pupillomotor, respectively).
3.4. Comparison between participants with syncope due to OH and those with known causes of OH
Participants with syncope due to OH had higher total COMPASS 31 scores (33.63 ± 15.69 vs 19.40 ± 13.41, p = 0.033) and domain scores for orthostatic intolerance domain than participants who had known causes of OH (24.00 [IQR 11.00–27.00] vs 12.00 [IQR 0.00–20.00], p = 0.038).
3.5. Usefulness of the COMPASS 31 score for predicting the recurrence of syncope
We divided the 101 participants who had syncope into two groups based on the recurrence of syncope: the recurrence (n = 40) and non-recurrence (n = 61) groups. The total numbers of episodes of syncope in each group are shown in Table 3. Among participants in the recurrence group, two had syncope due to OH, 28 had reflex syncope, and 10 had unclassified syncope. The diagnostic potential of the COMPASS 31 score for predicting the recurrence of syncope was analyzed by receiver operating characteristic (ROC) curve analysis. To determine the optimal cutoff score, we used Youden's index, which is the point that maximizes the sum of the sensitivity and specificity, and Liu's index, which maximizes the product of the sensitivity and specificity. The results of the ROC curve analysis are shown in Fig. 1. For Youden's index, the cutoff score of 36.8 for COMPASS 31 had a sensitivity of 45.0% and a specificity of 90.2% for predicting the recurrence of syncope, with positive and negative predictive values of 75.0% and 71.4%, respectively (AIC = 123.13, BIC = 128.36). For Liu's index, the cutoff value of 32.9 for COMPASS 31 had a sensitivity of 50.0% and a specificity of 82.0%, with positive and negative predictive values of 64.5% and 71.4%, respectively (AIC = 128.08, BIC = 133.31). Moreover, the cutoff score of 18 for the orthostatic intolerance domain had a sensitivity of 47.5% and a specificity of 87.3%.
Table 3.
The total numbers of episodes of syncope compared between groups of syncope.
| Syncope due to OH (8) | Reflex syncope (61) | Unclassified syncope (32) | p-value | |
|---|---|---|---|---|
| Total episodes of syncope | 0.63 | |||
| 1 episode | 6 (75.00%) | 33 (54.10%) | 22 (68.75%) | |
| 2 episodes | 2 (25.00%) | 14 (22.95%) | 5 (15.62%) | |
| 3 episodes | 0 (0.00%) | 1 (1.64%) | 2 (6.25%) | |
| 4 episodes | 0 (0.00%) | 3 (4.92%) | 1 (3.12%) | |
| More than 4 episodes | 0 (0.00%) | 10 (16.39%) | 2 (6.25%) |
Fig. 1.
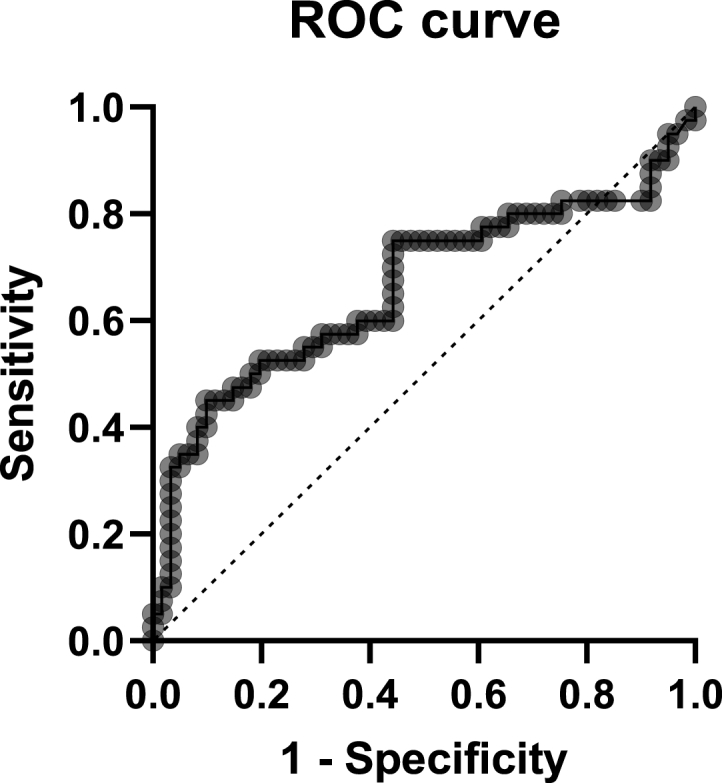
Receiver operating characteristic curve analysis of COMPASS 31 for predicting the recurrence of syncope.
4. Discussion
Considering its pathophysiology, syncope is classified into three main groups, including syncope due to OH, reflex syncope, and cardiogenic syncope. Autonomic nervous system disorders are responsible for the mechanism underling the majority of syncope types, especially syncope due to OH and reflex syncope. The current study suggests that each syncope type may have a different degree of autonomic dysfunction based on the COMPASS 31 score, a screening tool for autonomic dysfunction. Participants who had syncope due to OH had the highest severity of autonomic dysfunction assessed by the Thai version of the COMPASS 31 questionnaire, with a mean score of 33.6. Both the total score and domain scores for orthostatic intolerance and bladder were also higher than in the control and presyncope groups. Moreover, these participants had a higher COMPASS 31 score than participants who had syncope from drug-induced OH and volume depletion. This is consistent with reports that indicate that the main mechanism of syncope due to OH is autonomic dysfunction [1,9,21].
The autonomic response that triggers hemodynamic instability is abruptly altered in reflex syncope, leading to a sudden decrease in sympathetic function, with a simultaneous increase in parasympathetic response. In contrast to the persistent process in syncope due to OH from autonomic failure, the autonomic function in reflex syncope usually deteriorates just when the triggers occur [8,22,23]. As such, participants with reflex syncope are less likely to have sustained autonomic dysfunction. However, the study showed that the reflex syncope group had higher COMPASS 31 scores compared to the presyncope and control groups. We propose three explanations for this result. First, participants who had reflex syncope may have had some degree of autonomic dysfunction. This hypothesis is compatible with the study by Lee et al. [24] which indicated that patients with vasovagal syncope had various severities of autonomic dysfunction as assessed by the composite autonomic severity score. Second, participants who had syncope from autonomic dysfunction may have been misdiagnosed with reflex syncope due to insufficient investigation or an uncertain diagnosis. Third, patients with reflex syncope may have a fluctuation of autonomic function status that can occasionally produce autonomic symptoms that lead to a high COMPASS 31 score. However, there was no difference in the COMPASS 31 total and domain scores between the syncope due to OH and reflex syncope groups.
In the present study, the COMPASS 31 score was used to predict the recurrence of syncope. We preferred a cutoff score of 32.9 for COMPASS 31, which had a sensitivity of 50.0% and a specificity of 81.9% to predict the recurrence of syncope, to a cutoff score of 36.8 because of the higher sensitivity, as it can be used to screen more patients who are at risk of recurrent syncope. Furthermore, the domain score for orthostatic intolerance was also useful for predicting the recurrence of syncope with a cutoff score of 18, with a sensitivity of 47.5% and a specificity of 87.3%.
In terms of clinical application, the COMPASS 31 questionnaire can be applied in the initial assessment of patients with syncope for further investigation and proper management, especially in settings with limited resources. Patients with a high COMPASS 31 score should be closely monitored for the recurrence of syncope and thoroughly evaluated for underlying autonomic dysfunction. Moreover, the COMPASS 31 questionnaire is a practical and accessible tool that can be used to evaluate syncope patients in any setting. The COMPASS 31 questionnaire also has some minor drawbacks, including ambiguous questions in some domains that required further explanation from the researcher and some culturally and contextually specific questions that resulted in some points being misunderstood. However, most patients were able to complete the questionnaire without any problems after proper instructions from the interviewers. This issue is common in all questionnaires in terms of generalizability. This study has some limitations. First, because it was a retrospective cohort study, our assessment of autonomic function could have been slightly different from real time assessments during the syncope episode. Therefore, further study is required to examine the development of autonomic dysfunction in these patients over time through longitudinal follow-up, during which the degree of autonomic dysfunction could change. Another limitation is that there are fewer persons in the syncope due to OH group than in the other groups. However, even with a small sample size, we found that the COMPASS 31 was a useful tool for screening autonomic function in this group by its high specificity.
5. Conclusion
In conclusion, the COMPASS 31 questionnaire, which is an easy-to-use self-administered questionnaire utilized for the assessment of autonomic symptoms and function, was a helpful tool for classifying some types of syncope and predicting the recurrence of syncope, which could lead to appropriate further management. Furthermore, the reflex syncope group had higher COMPASS 31 scores than the control and presyncope groups, which raises the question of whether the reflex syncope participants encountered autonomic dysfunction akin to syncope due to OH. Future research should involve prospective cohort studies that follow all the participants from their presentation to the emergency department in order to monitor syncope recurrence throughout the same period and to assess their autonomic function status during any syncopal episode. Moreover, further study on the diagnosis of autonomic dysfunction by the COMPASS score should be performed. The gold standard of autonomic function test, such as HRV or Ewing's batter of autonomic function tests, are the reference in making the diagnosis autonomic dysfunction patients.
Author contribution statement
Nithit Singtokum: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Ronpichai Chokesuwattanaskul; Jakkrit Amornvit: Conceived and designed the experiments, Analyzed and interpreted the data, Wrote the paper.
Stephen Kerr: Analyzed and interpreted the data,.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17035.
Contributor Information
Jakkrit Amornvit, Email: jakkrit.a@chula.ac.th.
Stephen Kerr, Email: stephen.k@chula.ac.th.
Ronpichai Chokesuwattanaskul, Email: drronpichai@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brignole M., Moya A., de Lange F.J., Deharo J.-C., Elliott P.M., Fanciulli A., et al. ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 2.da Silva R.M. Syncope: epidemiology, etiology, and prognosis. Front. Physiol. 2014;5:471. doi: 10.3389/fphys.2014.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jameson J.L., Kasper D.L., Longo D.L., Fauci A.S., Hauser S.L., Loscalzo J. McGraw-hill education; New York: 2018. Harrison's Principles of Internal Medicine. [Google Scholar]
- 4.Probst M.A., Kanzaria H.K., Gbedemah M., Richardson L.D., Sun B.C. National trends in resource utilization associated with ED visits for syncope. The American journal of emergency medicine. 2015;33:998–1001. doi: 10.1016/j.ajem.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solbiati M., Casazza G., Dipaola F., Rusconi A.M., Cernuschi G., Barbic F., et al. Syncope recurrence and mortality: a systematic review. EP Europace. 2014;17:300–308. doi: 10.1093/europace/euu327. [DOI] [PubMed] [Google Scholar]
- 6.Van Wijnen V.K., Gans R.O., Wieling W., Ter Maaten J.C., Harms M.P. Diagnostic accuracy of evaluation of suspected syncope in the emergency department: usual practice vs. ESC guidelines. BMC Emerg. Med. 2020;20:1–9. doi: 10.1186/s12873-020-00344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saklani P., Krahn A., Klein G. Syncope. Circulation. 2013;127:1330–1339. doi: 10.1161/CIRCULATIONAHA.112.138396. [DOI] [PubMed] [Google Scholar]
- 8.Adkisson W.O., Benditt D.G. Pathophysiology of reflex syncope: a review. J. Cardiovasc. Electrophysiol. 2017;28:1088–1097. doi: 10.1111/jce.13266. [DOI] [PubMed] [Google Scholar]
- 9.Chisholm P., Anpalahan M. Orthostatic hypotension: pathophysiology, assessment, treatment and the paradox of supine hypertension. Intern. Med. J. 2017;47:370–379. doi: 10.1111/imj.13171. [DOI] [PubMed] [Google Scholar]
- 10.Assessment: clinical autonomic testing report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 1996;46:873–880. [PubMed] [Google Scholar]
- 11.Low P.A., editor. Composite Autonomic Scoring Scale for Laboratory Quantification of Generalized Autonomic Failure. Mayo Clin Proc. Elsevier; 1993. [DOI] [PubMed] [Google Scholar]
- 12.Sletten D.M., Suarez G.A., Low P.A., Mandrekar J., Singer W. Compass 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin. Proc. 2012;87:1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foschi M., Giannini G., Merli E., Mancinelli L., Zenesini C., Viti B., et al. Frequency and characteristics of dysautonomic symptoms in multiple sclerosis: a cross-sectional double-center study with the validated Italian version of the Composite Autonomic Symptom Score-31. Neurol. Sci. 2021;42:1395–1403. doi: 10.1007/s10072-020-04620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y., Seok J.M., Park J., Kim K.-H., Min J.-H., Cho J.W., et al. The composite autonomic symptom scale 31 is a useful screening tool for patients with Parkinsonism. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treister R., O'Neil K., Downs H.M., Oaklander A.L. Validation of the composite autonomic symptom scale 31 (COMPASS‐31) in patients with and without small fiber polyneuropathy. Eur. J. Neurol. 2015;22:1124–1130. doi: 10.1111/ene.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco C., Di Gennaro F., D'Amato C., Morganti R., Corradini D., Sun A., et al. Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet. Med. 2017;34:834–838. doi: 10.1111/dme.13310. [DOI] [PubMed] [Google Scholar]
- 17.Singh R., Arbaz M., Rai N.K., Joshi R. Diagnostic accuracy of composite autonomic symptom scale 31 (COMPASS-31) in early detection of autonomic dysfunction in type 2 diabetes mellitus. Diabetes, Metab. Syndrome Obes. Targets Ther. 2019;12:1735. doi: 10.2147/DMSO.S214085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler B.L., Russell J.W., Hummers L.K., McMahan Z.H. Symptoms of autonomic dysfunction in systemic sclerosis assessed by the COMPASS-31 questionnaire. J. Rheumatol. 2018;45:1145–1152. doi: 10.3899/jrheum.170868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taychagumpoo Chamaiporn. Chulalongkorn University; Bangkok: 2019. Validity and Realiability of Thai Composite Autonomic Symptom Score 31 (Thai-COMPASS 31) for Autonomic Beuropathy in Neuromuscular Outpatient Clinic at King Chulalongkorn Memorial Hospital [Thesis] [Google Scholar]
- 20.Ruška B., Pavičić T., Pavlović I., Junaković A., Adamec I., Crnošija L., et al. Performance of the COMPASS-31 questionnaire with regard to autonomic nervous system testing results and medication use: a prospective study in a real-life setting. Neurol. Sci. 2018;39:2079–2084. doi: 10.1007/s10072-018-3542-8. [DOI] [PubMed] [Google Scholar]
- 21.Ricci F., De Caterina R., Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J. Am. Coll. Cardiol. 2015;66:848–860. doi: 10.1016/j.jacc.2015.06.1084. [DOI] [PubMed] [Google Scholar]
- 22.Mosqueda-Garcia R., Furlan R., Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation. 2000;102:2898–2906. doi: 10.1161/01.CIR.102.23.2898. [DOI] [PubMed] [Google Scholar]
- 23.Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011;21:69–72. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.E., Lee D.W. Vasovagal syncope with mild versus moderate autonomic dysfunction: a 13-year single-center experience. Clin. Exp. Pediatr. 2022;65:47. doi: 10.3345/cep.2021.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


