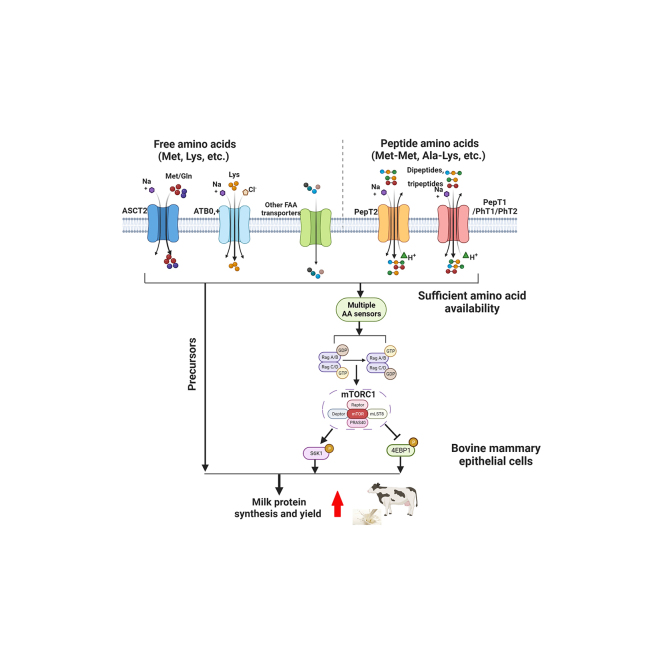
Graphical Abstract
Summary: Milk produced from the mammary glands of dairy animals has been recognized as a vital source of high-quality protein for human consumption. In addition to the common form, free amino acids (AA) absorbed from blood as predominant precursors for milk protein synthesis, peptide-bound AA transported by multiple transport systems into mammary epithelial cells also facilitate milk protein production. Here we summarized the expanding knowledge about how the 2 different AA transporter systems contribute to mammary AA utilization for milk protein synthesis as well as the major mTORC1-S6K1-4EBP1-mediated regulatory mechanism in the context of lactation. Such knowledge possibly provides insights into the feeding strategies of improving milk protein production in dairy animals.
Highlights
-
•
Free and peptide-bound AA serve as 2 major substrates for milk protein synthesis and participate in the regulation of this process as well.
-
•
The transport and utilization of AA into bovine mammary epithelial cells are mediated by a multiple transport system that is affected by several influential factors.
-
•
The peptide-bound AA transporters might render relatively high efficiency for milk protein synthesis compared with free AA transporters.
-
•
Free and peptide-bound AA and their corresponding transporters are predominantly involved in mTOR signaling to influence milk protein synthesis.
Abstract
Free and peptide-bound AA act as building blocks and key regulators of milk protein. To improve milk protein production, mammary epithelial cells of lactating mammals require extensive AA movement across the plasma membrane via multiple transport systems. Recent studies on bovine mammary cells/tissues have expanded the number of AA transporter systems identified and the knowledge on their contribution to AA utilization for milk protein synthesis and the regulatory machinery. However, in lactating cows, the exact intracellular location of mammary AA transporters and the extent of mammary net AA utilization for milk protein production remain unclear. This review highlights the existing knowledge on various characteristics, such as substrate specificity, kinetics, their effects on AA uptake and utilization, and regulatory mechanism, of recently examined bovine mammary free and peptide-bound AA transporters.
Lactating mammary glands (MG) require large amounts of AA to support milk protein synthesis. Free and peptide-bound AA are 2 substrate sources for milk protein synthesis and affect the process of AA uptake by the mammary epithelial cells (MEC) from the blood mediated by their corresponding AA transporters (AAT; Zhou et al., 2021). Furthermore, dairy researchers have observed that supplying sufficient and balanced AA plays a critical role in elevating milk protein concentration and yield (Wang et al., 2010; Haque et al., 2012; Nichols et al., 2019). Recent studies have promoted new AA transporter identification and in-depth elucidation of their unique properties and the regulatory mechanisms, advancing our knowledge in this field. This review highlights the progress in bovine mammary AA transporters research in terms of substrate specificity kinetics, AA utilization, and related regulatory mechanisms. The knowledge will expand our understanding of AA utilization and milk protein synthesis, possibly providing insights into how to achieve an optimal milk protein production in dairy animals.
The uptake of free AA by MEC mediated by their corresponding AAT requires energy for AA movement across the plasma membrane (Shennan and Peaker, 2000). The solute carrier family accounts for the majority of AAT, which are further classified into various groups (A, N, ASC, B, L, T, x−c, and y+) based on their substrate binding specificity and transport mechanism (Christensen, 1990; Kandasamy et al., 2018). Studies have enhanced our understanding of functions and mechanisms of multiple AAT in bovine MG tissue/MEC (Table 1; Baik et al., 2009; Bionaz and Loor, 2011; Nan et al., 2014; Lin et al., 2018a; Qi et al., 2018; Dai et al., 2020a). The efficiency of AAT transporting free AA into lactating MEC, across the plasma membrane, may be directly influenced by the substrate binding specificity of the transporters. A recent study using 13C-labeled AA flux approach reported that the efficiency of bovine mammary cell uptake and utilization of EAA varied in response to different EAA (Huang et al., 2021). As Met and Lys are proven to be the first 2 limiting AA for lactating dairy cows (Council, 2001), increasing Met (Dai et al., 2020a) and Lys (Lin et al., 2018a) availability in bovine MEC led to a dose-dependent increase in both casein production and total protein incorporation rate. This increase may have been caused by the elevation of transporter ASCT2 and ATB0,+ activities. Despite the difference in lactation stages, feeding rations and experimental designs in a meta-analysis study, dairy cows supplemented with rumen-protected Met (Chen et al., 2011; Wei et al., 2022) and Lys (Vyas and Erdman, 2009) displayed improved milk yield, milk protein content, milk N utilization efficiency, and milk fat content. In dairy cows, deficiency of either all 3 branched-chain AA (Leu, Ile, and Val) or Leu alone lowered the yield and percentage of milk protein, but no relationship was found between the deficiency of these branched-chain AA and the activity of mTORC1-mediated pathway (Doelman et al., 2015). In rat mammary cells, LAT1 (encoded by SLC7A5) has been identified as a specific Leu transporter that is Na+-independent (Km = 81 µM; Vmax = 56 pmol/106 cells per min) (Matsumoto et al., 2013). Moreover, compared with the positive control supplemented with EAA, in MAC-T (an immortalized bovine mammary cell line) and bovine mammary tissues, Leu deficiency alone led to the dephosphorylation rate of mTOR and rpS6. Re-supplementation of Leu restored the phosphorylation of mTOR, rpS6, as well as fractional protein synthesis rates (Appuhamy et al., 2012). To date, there is no clear evidence of LAT1 being a specific Leu transporter in bovine mammary cells. The aforementioned discussion indicates a certain linkage between precursors (the individual/combined AA) and their corresponding transporters that result in the increased milk protein production. In response to various AA in bovine MEC, specific AA transporter screening and manipulation of the activity and expression of these transporters would advance the knowledge of the accurate function and location of multiple free AAT.
Table 1.
Amino acid transport systems of bovine mammary gland
| System | Gene | Protein | Substrates | Properties | References |
|---|---|---|---|---|---|
| A | SLC38A2 | SNAT2 | l-Gln | Na+-dependent, low pH sensitive | Qi et al., 2018 |
| N | SLC38A3 | SNAT3 | l-Gln | Na+-coupled; H+-antiporter | Baik et al., 2009 |
| ASC | SLC1A4 | ASCT1 | Thr, l-Ala, l-Ser | High-affinity short-chain-amino acids | Nan et al., 2014 |
| B0 | SLC1A5 | ASCT2 | Neutral AA | Na+-dependent | Bionaz and Loor, 2011; Dai et al., 2020a |
| B0,+ | SLC6A14 | ATB0,+ | Neutral AA, cationic AA | Na+- and Cl−-dependent | Lin et al., 2018a |
| L | SLC7A5 | LAT1 | Neutral AA | Na+-independent | Lin et al., 2018b |
| SLC7A8 | LAT2 | Neutral AA | Na+-independent | Ding et al., 2019 | |
| T | SLC16A10 | TAT1 | l-Phe, l-Tyr, l-Trp | Na+- and Cl−-dependent, insensitive to pH | Baik et al., 2009 |
| x-AG | SLC1A1 | EAAT3 | l-Glu, d/l-Asp, l-Cys | Na+-dependent, K+-antiport | Bionaz and Loor, 2011 |
| SLC1A2 | EAAT2 | l-Glu, d/l-Asp | Dai et al., 2018 | ||
| SLC1A3 | EAAT1 | l-Glu, d/l-Asp | Xie et al., 2015 | ||
| y+ | SLC7A1 | CAT-1 | l-Arg, other cationic AA | Na+-dependent | Bionaz and Loor, 2011 |
| SLC7A2 | CAT-2 | l-Arg | Ding et al., 2019 | ||
| y+L | SLC7A7 | y+LAT1 | Neutral AA, cationic AA | Na+-dependent | Baik et al., 2009 |
| BETA | SLC6A1 | GAT1 | GABA | Cl−-dependent | Patel et al., 2019 |
| SLC6A6 | TAUT | Taurine, beta-alanine | Li et al., 2019 | ||
| Gly | SLC6A9 | GLYT1 | Gly, sarcosine | Na+- and Cl−-dependent | Baik et al., 2009 |
| SLC family | SLC15A1 | PepT1 | Oligopeptides | Proton electrochemical gradient-dependent, sensitive to pH | Wang et al., 2019 |
| SLC15A2 | PepT2 | Wang et al., 2018, 2020, 2022 | |||
| SLC15A4 | PhT1 | Wang et al., 2019 | |||
| SLC15A3 | PhT2 | Wang et al., 2018 |
With the exception to substrate effects, lactating dairy cows require mammary AAT to be coordinately regulated by other factors, such as hormones, blood flow, physiological conditions, and their potential interactions, to ultimately maximize their function (Weaver and Hernandez, 2016; Cant et al., 2018). To initiate lactation, the lactogenic hormones (such as prolactin, cortisol, growth hormone, insulin, and so on), working coordinately, are essential for the functional differentiation of MEC and lactogenesis (Weaver and Hernandez, 2016). Growth hormone and insulin, individually or the combination of hydrocortisone, insulin, and prolactin, supplemented to dairy cows in pregnancy or mid-lactation periods improved lactation performance, showing higher mammary AA uptake and milk protein synthesis (Menzies et al., 2009; Burgos et al., 2010; Sciascia et al., 2015). Measuring the differences between arteriovenous/venous AA concentrations and blood flow rate has been considered as a reliable indicator for determining the net uptake of AA by bovine MEC and their utilization for milk protein synthesis (Wang et al., 2010; Cant et al., 2018; Malacco et al., 2022; Smith et al., 2022). As the requirement for AA precursor changes during the lactation cycle, dairy cows during late gestation and early lactation initiate muscle mobilization to provide AA for body maintenance and colostrogenesis (McCabe and Boerman, 2020). Dairy cows experience a significant negative protein balance, especially during the first 30 d of lactation, with a peak increase in milk protein demand (Cardoso et al., 2021). Various physiological factors such as heat stress-induced hyperthermia (Gao et al., 2017) and mastitis (especially Staphylococcus aureus-induced; Abd Al-Bar Ahmed Al-Farha, 2022) heavily reduce milk yield, milk protein yield, and milk N utilization along with an overall reduction in blood concentrations and mammary uptake of EAA and NEAA in dairy cows. A close relationship was observed between lactogenic hormones and AA utilization efficiency for milk protein synthesis predominantly by promoting precursor availability. However, the influence of other factors and their mechanism of action remain unknown. Further studies are required to improve AA labeling/infusing techniques to dynamically record alterations in mammary protein metabolism and monitor the real-time changes in mammary AA transport, uptake, and utilization in different situations.
Peptide-bound AA have drawn increasing attention as new sources for milk protein synthesis owing to their more effective and energy-saving features than those of free AA. Supporting this notion, a study on rat mammary explants has shown most di- and tripeptides of Met, among 18 kinds of peptide-bound Met, significantly promoted 15% to 76% greater 3H-leucine incorporation into secreted protein synthesis than free Met (Wang et al., 1996). For dairy cows, it has been assumed the peptide-bound AA pool in blood may serve as a reserve-AA pool with a relatively high utilization rate for milk protein synthesis, thereby balancing the shortage of AA in the free AA pool (Tagari et al., 2008). Recent studies on dairy cows have focused on peptide-bound AA transport components and their impacts. There are at least 4 peptide-bound AAT encoded by the SLC15 family peptide transporter (PepT) 1 and PepT2 and peptide histidine transporter (PhT) 1 and PhT2 (Viennois et al., 2018). PepT1 is primarily expressed at the apical membrane of the intestine epithelial cells (Daniel and Kottra, 2004). Several studies have shown that peptide-bound AA (Met-Met and Phe-Phe) promoted αS1- and β-casein synthesis using bovine MG explants and MEC along with the elevation of PepT2 expression (Yang et al., 2015; Zhou et al., 2015; Wang et al., 2019). A similar elevation of PepT1, PepT2, and PhT1 expression in the MG tissues of lactating mice fed with Met-Met versus Met alone was observed, along with increased gluconeogenic AA concentrations in the maternal and fetal plasma and improved lactation performance (31% increased mammary alveoli abundance, 84% increased αS1-casein production, 34% increased milk yield, and 8.7% increased energy production; Chen et al., 2018, 2020). Furthermore, peptide-bound AA may have the potential to increase free release and utilization rate within lactating MG to promote lactogenesis and milk protein synthesis.
As PepT2 may be involved in the milk protein synthesis stimulated by peptide-bound AA than by free AA with relatively high efficiency, a recent study investigated PepT2 properties and the possible transport machinery. PepT2 knockdown caused a remarkable reduction in the bovine MEC uptake rate of a model peptide in the presence of Met-Met (Wang et al., 2019). PepT2 is presumed to have 12 transmembrane domains with a large extracellular loop containing 5 potential N-glycosylation sites between the ninth and tenth transmembrane domains (Wang et al., 2020), suggesting the potential role of glycosylation of PepT2 in its transportation function. As expected, inhibition of N-linked glycosylation by its inhibitor (tunicamycin) significantly lowered the uptake of a model dipeptide in Chinese hamster ovary cells transfected with the cloned bovine PepT2 plasmid. These observations imply that N-glycosylation is an essential mechanism in posttranslational modifications of protein synthesis in lactating bovine MG. Owing to strict stereoselectivity, PepT2 has a preference for peptide-bound AA containing l-AA rather than d-AA and for those N terminus-located acidic AA. Therefore, it is rational to observe that PepT2 participates in the uptake of a wide range of peptide-bound AA, such as Met-Met, Thr-Phe-Phe, and Gly-Sar, in bovine MEC (Zhou et al., 2011; Yang et al., 2015; Wang et al., 2018, 2019) or the intestine/stomach epithelial cells (Xu et al., 2019; Hou et al., 2022). Notably, the uptake efficiency of Met-Met is compromised due to competition from other dipeptides (Met-Lys, Lys-Lys, Gly-Met, Gly-Leu, and Met-Leu; Wang et al., 2018). However, whether PepT2 is the exclusive transporter for the aforementioned peptide-bound AA requires further investigation. To date, the accurate location of PepT2 and the other 3 peptide-bound AAT in bovine MEC remains unclear. In contrast, PepT1 has a special preference for charged anionic AA due to its proton-dependent transport mechanism (Steel et al., 1997). However, the relationship between PepT1 and milk protein synthesis in bovine MG remains unclear. Additionally, the expression levels of PepT1 and PhT1 were highly enhanced to a similar extent. Elevation in PepT2 expression in MG tissues was observed in pregnant mice with Met-Met supply compared with that in MG tissues of Met-deficient ones (Chen et al., 2018). Despite the elevated expression of PhT1 protein, the knockdown of PhT1 did not alter the model dipeptide and Met-Met uptake into bovine MEC (Wang et al., 2019). Previous work showed that absorption of large amounts of peptide-bound AA into the small intestine is required due to increased lactation demands during mid-lactation period in dairy cows because of the extensive presence of PepT1 in the gastrointestinal tract (Xu et al., 2018). Due to its conservative substrate recognition, studies related to the function and substrate selectivity of PhT2 in bovine MG are very limited and require further exploration.
In addition to their role in transport, AA/peptide transporters presumably function upstream of central mTOR signaling to allow AA availability and initiate responses that affect growth and health-related events. Mechanically, extracellular AA are the first transported into cells across plasma membrane by the corresponding transporters; then, mTORC1 identifies these AA inputs and promotes anabolism and cell growth using small GTPases (RAG-A/B, RAG-C/D), multiple regulatory members (FLIP1/2, GATOR1/2, SESTRIN2, and so on), and downstream substrates (S6K, 4E-BP1; Battaglioni et al., 2022). Given the conservative function mechanisms of mTOR (Saxton and Sabatini, 2017), AA/peptide transporters would probably act in an indirect manner to participate in AA-mediated mTORC1 activation and the subsequent milk protein synthesis in bovine MG. Indeed, the inhibition of free/peptide-bound AAT led to limited AA availability in bovine MEC, lowered casein expression, and inactivated mTOR signaling. For example, the GPNA-inhibited ASCT2 uptake of Met (Dai et al., 2020a) and BCH-prevented ATB0,+ uptake of Lys (Lin et al., 2018a) into bovine MEC, both resulting in reduced casein synthesis by attenuating mTORC1 activity via dephosphorylation of mTOR, S6K1, and 4E-BP1. Moreover, Met deficiency in bovine MEC initiates the general control non-derepressible 2 (GCN2) pathway by elevating activating transcription factor 4 expression and the phosphorylation rate of eukaryotic translation initiation factor 2A (Dai et al., 2020b). However, when primary bovine MEC respond to Met deficiency, no relationship between the activity of its transporter ASCT2 and activation/inactivation of the GCN2 pathway is observed. Being highly expressed and extensively located on the plasma membrane of lactating bovine MG (Lin et al., 2018b), LAT1 is also responsible for Met uptake, which can be blocked by mTOR inhibition by rapamycin, thus leading to the reduction in casein synthesis (Duan et al., 2017). As a sensor of extracellular EAA in mouse MEC, T1R1/T1R3 affects the expression of SLC1A5/SLC3A2/SLC7A5 transporters and participates in mTORC1-regulated milk protein synthesis (Wang et al., 2017). These findings suggest that AAT activity and expression can be controlled by AA availability, and in turn, these AAT are involved in mTORC1 signaling pathways to further regulate milk protein synthesis. Additionally, mammary peptide-bound AA studies in mice (Chen et al., 2018, 2020) and dairy cows (Wang et al., 2018, 2019) demonstrated that Met-Met dipeptide stimulated the expression of targeting transporters and the activation of the mTOR system, ultimately leading to enhancement of milk protein synthesis. Moreover, mTORC1 regulates PepT2 activity by regulating the Nedd4–2-mediated ubiquitination (Wang et al., 2022). Altogether, there exists a certain relation, even if indirect, between free/peptide-bound AAT and the mTORC1 system to coherently regulate milk protein synthesis in dairy cows. Studies investigating in-depth mechanisms underlying these free/peptide-bound AA or the relevant AA transport path-mediated milk protein synthesis are warranted.
The transport system of mammary free/bound AA acts as a main contributor for net mammary AA uptake, playing a crucial role in controlling milk protein production for lactating dairy cows. The functional machinery underlying free/bound AAT that mediates AA delivery and utilization for milk protein synthesis includes multiple factors, such as substrate specificities, circulation in the whole body and MG, hormones, and dairy herd health status, working in coordination. Abundant evidence indicates that various free/bound AA have the ability to regulate their corresponding transporters in a substrate-grade/concentration-dependent manner, in which mTOR signaling indirectly integrates these inputs to further impair milk protein synthesis and yield. The crosstalk between AA substrates and lactogenic hormones in milk protein synthesis has been highlighted. The interaction among the 4 influential factors in this process remains undetermined; additionally, sophisticated free/peptide-bound AA labeling and modeling techniques are required for the accurate and practical evaluation of mammary net free/peptide-bound AA uptake rate. Additionally, the functional and regulatory entities, including accurate intracellular location and kinetic properties, underlying free/peptide-bound AAT in bovine MEC require further in-depth investigation, and their method of recognizing mTOR needs to be evaluated using super-resolution microscopy and color-tagged labeling-transporter techniques.
Notes
This research was supported by grants from the earmarked fund for CARS36 (the China Agriculture Research System) and National Natural Science Foundations of China (31872989).
No human or animal subjects were used, so this analysis did not require approval by an Institutional Animal Care and Use Committee or Institutional Review Board.
The authors have not stated any conflicts of interest.
Footnotes
Presented as part of the Lactation Biology Symposium: Nutrient Transport in the Mammary Gland held at the ADSA Annual Meeting, June 2022.
References
- Abd Al-Bar Ahmed Al-Farha K.R.P. Assessment of milk yield and composition during bovine mastitis caused by a variety of pathogens. Rev. Electrón. Vet. 2022;23:219–226. [Google Scholar]
- Appuhamy J.A., Knoebel N.A., Nayananjalie W.D., Escobar J., Hanigan M.D. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2012;142:484–491. doi: 10.3945/jn.111.152595. 22298573. [DOI] [PubMed] [Google Scholar]
- Baik M., Etchebarne B., Bong J., VandeHaar M. Gene expression profiling of liver and mammary tissues of lactating dairy cows. Asian-Australas. J. Anim. Sci. 2009;22:871–884. doi: 10.5713/ajas.2009.90061. [DOI] [Google Scholar]
- Battaglioni S., Benjamin D., Wälchli M., Maier T., Hall M.N. mTOR substrate phosphorylation in growth control. Cell. 2022;185:1814–1836. doi: 10.1016/j.cell.2022.04.013. 35580586. [DOI] [PubMed] [Google Scholar]
- Bionaz M., Loor J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights. 2011;5:83–98. doi: 10.4137/BBI.S7003. 21698073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos S.A., Dai M., Cant J.P. Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J. Dairy Sci. 2010;93:153–161. doi: 10.3168/jds.2009-2444. 20059914. [DOI] [PubMed] [Google Scholar]
- Cant J.P., Kim J.J.M., Cieslar S.R.L., Doelman J. Symposium review: Amino acid uptake by the mammary glands: Where does the control lie? J. Dairy Sci. 2018;101:5655–5666. doi: 10.3168/jds.2017-13844. 29605320. [DOI] [PubMed] [Google Scholar]
- Cardoso F.F., Donkin S.S., Pereira M.N., Pereira R.A.N., Peconick A.P., Santos J.P., Silva R.B., Caproni V.R., Parys C., Danes M.A.C. Effect of protein level and methionine supplementation on dairy cows during the transition period. J. Dairy Sci. 2021;104:5467–5478. doi: 10.3168/jds.2020-19181. 33685687. [DOI] [PubMed] [Google Scholar]
- Chen Q., Dai W., Sun Y., Zhao F., Liu J., Liu H. Methionine partially replaced by methionyl-methionine dipeptide improves reproductive performance over methionine alone in methionine-deficient mice. Nutrients. 2018;10 doi: 10.3390/nu10091190. 30200399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zhao F.Q., Ren Y., Han J., Liu J., Li Y., Liu H. Parenterally delivered methionyl-methionine dipeptide during pregnancy enhances mammogenesis and lactation performance over free methionine by activating PI3K-AKT signaling in methionine-deficient mice. J. Nutr. 2020;150:1186–1195. doi: 10.1093/jn/nxaa005. 32006013. [DOI] [PubMed] [Google Scholar]
- Chen Z.H., Broderick G.A., Luchini N.D., Sloan B.K., Devillard E. Effect of feeding different sources of rumen-protected methionine on milk production and N-utilization in lactating dairy cows. J. Dairy Sci. 2011;94:1978–1988. doi: 10.3168/jds.2010-3578. 21426989. [DOI] [PubMed] [Google Scholar]
- Christensen H.N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. 2404290. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhao F., Liu J., Liu H. ASCT2 is involved in SARS-mediated β-casein synthesis of bovine mammary epithelial cells with methionine supply. J. Agric. Food Chem. 2020;68:13038–13045. doi: 10.1021/acs.jafc.9b03833. 31597423. [DOI] [PubMed] [Google Scholar]
- Dai W., Zhao F., Liu J., Liu H. Seryl-tRNA synthetase is involved in methionine stimulation of β-casein synthesis in bovine mammary epithelial cells. Br. J. Nutr. 2020;123:489–498. doi: 10.1017/S0007114519002885. 31711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W.T., Zou Y.X., White R.R., Liu J.X., Liu H.Y. Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct. Integr. Genomics. 2018;18:125–140. doi: 10.1007/s10142-017-0580-x. 29275436. [DOI] [PubMed] [Google Scholar]
- Daniel H., Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. 12905028. [DOI] [PubMed] [Google Scholar]
- Ding L., Shen Y., Wang Y., Zhou G., Zhang X., Wang M., Loor J.J., Chen L., Zhang J. Jugular arginine supplementation increases lactation performance and nitrogen utilization efficiency in lactating dairy cows. J. Anim. Sci. Biotechnol. 2019;10:3. doi: 10.1186/s40104-018-0311-8. 30680190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelman J., Kim J.J., Carson M., Metcalf J.A., Cant J.P. Branched-chain amino acid and lysine deficiencies exert different effects on mammary translational regulation. J. Dairy Sci. 2015;98:7846–7855. doi: 10.3168/jds.2015-9819. 26342977. [DOI] [PubMed] [Google Scholar]
- Duan X., Lin Y., Lv H., Yang Y., Jiao H., Hou X. Methionine induces LAT1 expression in dairy cow mammary gland by activating the mTORC1 signaling pathway. DNA Cell Biol. 2017;36:1126–1133. doi: 10.1089/dna.2017.3792. 29040000. [DOI] [PubMed] [Google Scholar]
- Gao S.T., Guo J., Quan S.Y., Nan X.M., Fernandez M.V.S., Baumgard L.H., Bu D.P. The effects of heat stress on protein metabolism in lactating Holstein cows. J. Dairy Sci. 2017;100:5040–5049. doi: 10.3168/jds.2016-11913. 28390717. [DOI] [PubMed] [Google Scholar]
- Haque M.N., Rulquin H., Andrade A., Faverdin P., Peyraud J.L., Lemosquet S. Milk protein synthesis in response to the provision of an “ideal” amino acid profile at 2 levels of metabolizable protein supply in dairy cows. J. Dairy Sci. 2012;95:5876–5887. doi: 10.3168/jds.2011-5230. 22884342. [DOI] [PubMed] [Google Scholar]
- Hou P., Wang C., Zhou M., Liu H. Properties and regulation of Gly-Sar uptake and transport in bovine intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. (Berl.) 2022;106:24–32. doi: 10.1111/jpn.13546. 33834547. [DOI] [PubMed] [Google Scholar]
- Huang X., Yoder P.S., Teixeira I., Hanigan M.D. Assessing amino acid uptake and metabolism in mammary glands of lactating dairy cows intravenously infused with methionine, lysine, and histidine or with leucine and isoleucine. J. Dairy Sci. 2021;104:3032–3051. doi: 10.3168/jds.2020-18169. 33455768. [DOI] [PubMed] [Google Scholar]
- Kandasamy P., Gyimesi G., Kanai Y., Hediger M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018;43:752–789. doi: 10.1016/j.tibs.2018.05.003. 30177408. [DOI] [PubMed] [Google Scholar]
- Li M., Xi P., Xu Y., Wang Z., Han X., Ren W., Phouthapane V., Miao J. Taurine attenuates Streptococcus uberis-induced bovine mammary epithelial cells inflammation via phosphoinositides/Ca(2+) signaling. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01825. 31447841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Li S., Zou Y., Zhao F.Q., Liu J., Liu H. Lysine stimulates protein synthesis by promoting the expression of ATB0,+ and activating the mTOR pathway in bovine mammary epithelial cells. J. Nutr. 2018;148:1426–1433. doi: 10.1093/jn/nxy140. 30184226. [DOI] [PubMed] [Google Scholar]
- Lin Y., Duan X., Lv H., Yang Y., Liu Y., Gao X., Hou X. The effects of L-type amino acid transporter 1 on milk protein synthesis in mammary glands of dairy cows. J. Dairy Sci. 2018;101:1687–1696. doi: 10.3168/jds.2017-13201. 29224866. [DOI] [PubMed] [Google Scholar]
- Malacco V.M.R., Beckett L., Hilger S., Doane P., Reis R.B., Donkin S.S. Effects of increased doses of lysine in a rumen-protected form on plasma amino acid concentration and lactational performance of dairy cows fed a lysine-deficient diet. J. Dairy Sci. 2022;105:3064–3077. doi: 10.3168/jds.2021-20823. 35151485. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Nakamura E., Nakamura H., Hirota M., San Gabriel A., Nakamura K., Chotechuang N., Wu G., Uneyama H., Torii K. Production of free glutamate in milk requires the leucine transporter LAT1. Am. J. Physiol. Cell Physiol. 2013;305:C623–C631. doi: 10.1152/ajpcell.00291.2012. 23804198. [DOI] [PubMed] [Google Scholar]
- McCabe C.J., Boerman J.P. Invited review: Quantifying protein mobilization in dairy cows during the transition period. Appl. Anim. Sci. 2020;36:389–396. doi: 10.15232/aas.2019-01929. [DOI] [Google Scholar]
- Menzies K.K., Lefèvre C., Macmillan K.L., Nicholas K.R. Insulin regulates milk protein synthesis at multiple levels in the bovine mammary gland. Funct. Integr. Genomics. 2009;9:197–217. doi: 10.1007/s10142-008-0103-x. 19107532. [DOI] [PubMed] [Google Scholar]
- Nan X., Bu D., Li X., Wang J., Wei H., Hu H., Zhou L., Loor J.J. Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells. Physiol. Genomics. 2014;46:268–275. doi: 10.1152/physiolgenomics.00119.2013. 24474444. [DOI] [PubMed] [Google Scholar]
- Nichols K., Bannink A., Dijkstra J. Energy and nitrogen balance of dairy cattle as affected by provision of different essential amino acid profiles at the same metabolizable protein supply. J. Dairy Sci. 2019;102:8963–8976. doi: 10.3168/jds.2019-16400. 31378498. [DOI] [PubMed] [Google Scholar]
- NRC . 7th ed. National Academies Press; 2001. Nutrient Requirements of Dairy Cattle. [PubMed] [Google Scholar]
- Patel O.V., Casey T., Plaut K. Profiling solute-carrier transporters in key metabolic tissues during the postpartum evolution of mammary epithelial cells from nonsecretory to secretory. Physiol. Genomics. 2019;51:539–552. doi: 10.1152/physiolgenomics.00058.2019. 31545931. [DOI] [PubMed] [Google Scholar]
- Qi H., Meng C., Jin X., Li X., Li P., Gao X. Methionine promotes milk protein and fat synthesis and cell proliferation via the SNAT2–PI3K signaling pathway in bovine mammary epithelial cells. J. Agric. Food Chem. 2018;66:11027–11033. doi: 10.1021/acs.jafc.8b04241. 30274521. [DOI] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. 28283069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia Q.L., Pacheco D., McCoard S.A. Administration of exogenous growth hormone is associated with changes in plasma and intracellular mammary amino acid profiles and abundance of the mammary gland amino acid transporter SLC3A2 in mid-lactation dairy cows. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134323. 26226162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shennan D.B., Peaker M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000;80:925–951. doi: 10.1152/physrev.2000.80.3.925. 10893427. [DOI] [PubMed] [Google Scholar]
- Smith M., Cronin S., Mateos J., Martinez del Olmo D., Valdez F., Gressley T.F. Comparison of plasma methionine response to 3 rumen-protected methionine products in lactating Holstein dairy cows. Appl. Anim. Sci. 2022;38:110–117. doi: 10.15232/aas.2021-02226. [DOI] [Google Scholar]
- Steel A., Nussberger S., Romero M.F., Boron W.F., Boyd C.A., Hediger M.A. Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J. Physiol. 1997;498:563–569. doi: 10.1113/jphysiol.1997.sp021883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagari H., Webb K., Jr., Theurer B., Huber T., DeYoung D., Cuneo P., Santos J.E., Simas J., Sadik M., Alio A., Lozano O., Delgado-Elorduy A., Nussio L., Bittar C.M., Santos F. Mammary uptake, portal-drained visceral flux, and hepatic metabolism of free and peptide-bound amino acids in cows fed steam-flaked or dry-rolled sorghum grain diets. J. Dairy Sci. 2008;91:679–697. doi: 10.3168/jds.2007-0629. 18218756. [DOI] [PubMed] [Google Scholar]
- Viennois E., Pujada A., Zen J., Merlin D. Function, regulation, and pathophysiological relevance of the POT superfamily, specifically PepT1 in inflammatory bowel disease. Compr. Physiol. 2018;8:731–760. doi: 10.1002/cphy.c170032. 29687900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas D., Erdman R.A. Meta-analysis of milk protein yield responses to lysine and methionine supplementation. J. Dairy Sci. 2009;92:5011–5018. doi: 10.3168/jds.2008-1769. 19762820. [DOI] [PubMed] [Google Scholar]
- Wang C., Liu H.Y., Wang Y.M., Yang Z.Q., Liu J.X., Wu Y.M., Yan T., Ye H.W. Effects of dietary supplementation of methionine and lysine on milk production and nitrogen utilization in dairy cows. J. Dairy Sci. 2010;93:3661–3670. doi: 10.3168/jds.2009-2750. 20655436. [DOI] [PubMed] [Google Scholar]
- Wang C., Sun Y., Zhao F.Q., Liu J., Liu H. Functional characterization of peptide transporters in bovine mammary epithelial cells. J. Agric. Food Chem. 2019;67:213–219. doi: 10.1021/acs.jafc.8b05637. 30525553. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhao F., Liu J., Liu H. Dipeptide (methionyl-methionine) transport and its effect on β-casein synthesis in bovine mammary epithelial cells. Cell. Physiol. Biochem. 2018;49:479–488. doi: 10.1159/000492987. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhao F., Liu J., Liu H. The ubiquitin ligase Nedd4–2 mediates the regulation of PepT2 by mTORC1 in bovine mammary epithelial cells. Anim. Nutr. 2022;10:12–18. doi: 10.1016/j.aninu.2021.11.008. (Zhongguo Xu Mu Shou Yi Xue Hui) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao F.Q., Liu J., Liu H. Short communication: The essential role of N-glycosylation in the transport activity of bovine peptide transporter 2. J. Dairy Sci. 2020;103:6679–6683. doi: 10.3168/jds.2019-16858. 32331895. [DOI] [PubMed] [Google Scholar]
- Wang S., Webb K.E., Jr., Akers M.R. Peptide-bound methionine can be a source of methionine for the synthesis of secreted proteins by mammary tissue explants from lactating mice. J. Nutr. 1996;126:1662–1672. doi: 10.1093/jn/126.6.1662. 8648441. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu J., Wu H., Fang X., Chen H., Zhang C. Amino acids regulate mTOR pathway and milk protein synthesis in a mouse mammary epithelial cell line is partly mediated by T1R1/T1R3. Eur. J. Nutr. 2017;56:2467–2474. doi: 10.1007/s00394-016-1282-1. 27539583. [DOI] [PubMed] [Google Scholar]
- Weaver S.R., Hernandez L.L. Autocrine-paracrine regulation of the mammary gland. J. Dairy Sci. 2016;99:842–853. doi: 10.3168/jds.2015-9828. 26299162. [DOI] [PubMed] [Google Scholar]
- Wei C., He T., Wan X., Liu S., Dong Y., Qu Y. Meta-analysis of rumen-protected methionine in milk production and composition of dairy cows. Animals (Basel) 2022;12 doi: 10.3390/ani12121505. 35739842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.L., Ye P.S., Zhang Y.S., Shen X.Z. Effect of high-concentrate diet on amino acid transporter expression and milk quality in Holstein dairy cows. Genet. Mol. Res. 2015;14:5246–5257. doi: 10.4238/2015.May.18.16. 26125719. [DOI] [PubMed] [Google Scholar]
- Xu Q., Liu H., Zhao F., Wu Y., Huang X., Liu Z., Liu J. Mechanism of peptide absorption in the isolated forestomach epithelial cells of dairy cows. J. Sci. Food Agric. 2019;99:100–108. doi: 10.1002/jsfa.9148. 29797328. [DOI] [PubMed] [Google Scholar]
- Xu Q., Liu Z., Liu H., Zhao F., Huang X., Wu Y., Liu J. Functional characterization of oligopeptide transporter 1 of dairy cows. J. Anim. Sci. Biotechnol. 2018;9:7. doi: 10.1186/s40104-017-0219-8. 29387385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.X., Wang C.H., Xu Q.B., Zhao F.Q., Liu J.X., Liu H.Y. Methionyl-methionine promotes α-s1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR-mediated signaling pathways. J. Nutr. 2015;145:1748–1753. doi: 10.3945/jn.114.208330. 26108540. [DOI] [PubMed] [Google Scholar]
- Zhou M., Xu L., Zhao F., Liu H. Regulation of milk protein synthesis by free and peptide-bound amino acids in dairy cows. Biology (Basel) 2021;10 doi: 10.3390/biology10101044. 34681143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.M., Wu Y.M., Liu H.Y., Liu J.X. Effects of phenylalanine and threonine oligopeptides on milk protein synthesis in cultured bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. (Berl.) 2015;99:215–220. doi: 10.1111/jpn.12246. 25199802. [DOI] [PubMed] [Google Scholar]
- Zhou M.M., Wu Y.M., Liu H.Y., Zhao K., Liu J.X. Effects of tripeptides and lactogenic hormones on oligopeptide transporter 2 in bovine mammary gland. J. Anim. Physiol. Anim. Nutr. (Berl.) 2011;95:781–789. doi: 10.1111/j.1439-0396.2010.01110.x. 21198960. [DOI] [PubMed] [Google Scholar]