Abstract
The inferior mesenteric artery (IMA) has often been overlooked in favor of the celiac or superior mesenteric artery in arterial mesenteric ischemia, owing to the typically robust visceral collateral networks. In the present report, we have described a case series of patients in whom “salvage” revascularization of the IMA was performed after attempted celiac or superior mesenteric artery revascularization had been unsuccessful. The restored IMA inflow had resolved the symptoms for three patients. However, sole IMA revascularization was insufficient to reverse the course for two other patients with severe acute-on-chronic mesenteric ischemia. The IMA should be considered for salvage revascularization in the appropriate clinical scenario.
Keywords: Inferior, Mesenteric artery, Mesenteric ischemia, Mesenteric vascular insufficiency, Outcome assessment (health care), Thrombosis
Arterial mesenteric ischemia consists of a spectrum of clinical presentations, with heterogeneous visceral malperfusion and different temporal presentations resulting in variable degrees of bowel loss and associated morbidity. It can result from thrombosis, embolus, atherosclerotic steno-occlusive disease, or rarer causes such as nonocclusive mesenteric ischemia or vasculitis. It is precipitated by limitations in arterial inflow primarily via the celiac axis, superior mesenteric artery (SMA), and inferior mesenteric artery (IMA).
Given the extensive collateral pathways for visceral arterial perfusion and the usual predominance of the celiac artery and SMA, the IMA has frequently been overlooked as a crucial inflow route for the viscera and even the spinal cord. Iatrogenic embolization of the IMA to prevent type II endoleak in the setting of endovascular aortic aneurysm repair has even been considered for routine practice, whether before endovascular aortic aneurysm repair or postoperatively because of aneurysm sac progression.1 Ligation of the IMA has also been commonly performed with evidence of collateralization during open aortic surgery, with low rates of resultant intestinal ischemia.2 However, in other settings, the importance of the IMA can become evident when its sacrifice could precipitate mesenteric ischemia and, even, intra-abdominal catastrophe, especially for cases of prior right hemicolectomy.1,3,4 Further establishing its importance, a case report found the IMA to be a major contributor to the blood supply of the entire pelvis and segments of the lower extremities in the presence of extensive aortoiliac occlusive disease.5 In such cases, the IMA will have increased importance, typically in the presence of severe stenosis or chronic occlusion of the celiac artery and/or SMA.
Thus, although most cases of chronic or acute mesenteric ischemia have been attributable to the celiac artery and SMA,6, 7, 8 IMA revascularization could also be considered for successful treatment of mesenteric ischemia.7,9, 10, 11, 12, 13 In the present report, we have described the cases of five patients who had undergone IMA revascularization.
Methods
Study population
We identified patients who had undergone visceral angioplasty, stenting, direct thromboendarterectomy, or other open repair at the Massachusetts General Hospital between January 2010 and December 2020 using the Current Procedural Terminology (codes 37236, 37205, 35471, 35251, 35221, 34151, 35341, 35281, 37207, and 37799), resulting in 652 procedures. All operative reports of these patients were reviewed for the visceral revascularization procedures performed. The inclusion criteria were patients who had undergone revascularization of the IMA without concomitant celiac artery or SMA intervention, designated as “salvage” IMA revascularization. We included four patients who had undergone endovascular IMA revascularization and a fifth patient who had undergone open combined aortic and IMA endarterectomy (in total, <1% of the original cohort). Two patients had undergone celiac artery or SMA revascularization at other points during their hospital course. We were unable to capture IMA revascularization occurring with misapplication of the Current Procedural Terminology codes other than those used for the initial query. The institutional review board of the Massachusetts General Hospital approved the present study and waived the requirement for direct patient informed consent.
Medical record review
The patient records were reviewed for demographic factors, medical comorbidities, prescribed medications, and surgical history. A history of intervention on the mesenteric arteries was determined from the medical record review, including open surgical and endovascular procedures. The presenting symptoms and signs were ascertained from the admission documents. The laboratory values such as cell counts and lactate levels were noted within the preceding 24 hours before any procedure was performed. The anatomic characteristics of aortoiliac calcification, visceral arterial stenosis, visceral arterial occlusion, and stigmata of bowel inflammation or compromise were ascertained via am independent review of the imaging studies. The interventions and perioperative and long-term outcomes were reviewed using all the inpatient and outpatient records through July 2022. The surgical and endovascular procedures related to mesenteric ischemia were included (eg, bowel resection, aortovisceral bypass, angioplasty, stenting). Patency of the revascularization procedures was assessed via medical record review and an independent review of the duplex ultrasound and/or computed tomography findings postoperatively, when available.
Description of cases
Between 2010 and 2020, four patients had undergone endovascular salvage revascularization and one patient had undergone open salvage revascularization of the IMA at the Massachusetts General Hospital (Table). Four patients were women, and the age range was 47 to 84 years. Of the five patients, four had a history of smoking and one was a current smoker. Four patients had a diagnosis of peripheral arterial disease and one a diagnosis of atrial fibrillation. None of the five patients were receiving chronic anticoagulation therapy, although two were receiving antiplatelet monotherapy and one, dual antiplatelet therapy.
Table.
Perioperative and long-term outcomes
| Pt. No. | IMA intervention | Small bowel resection | ICU stay | LOS, days | 30-Day mortality | 30-Day readmission | Primary patency, months | Follow-up, months |
|---|---|---|---|---|---|---|---|---|
| 1 | Bare metal stent | Yes | Yes | 34 | No | No | 12a | 14 |
| 2 | Bare metal stent | No | No | 2 | No | No | 12a | 12 |
| 3 | Bare metal stent | Yes | Yes | 17 | Yes | No | – | – |
| 4 | Bare metal stent | No | Yes | 9 | No | No | 0.27 | 17 |
| 5 | Endarterectomy + Dacron patch | No | Yes | 14 | No | No | 3a | 3 |
ICU, Intensive care unit; IMA, inferior mesenteric artery; LOS, length of stay; Pt. No., patient number.
Lost to follow-up after stated period.
Patient 1
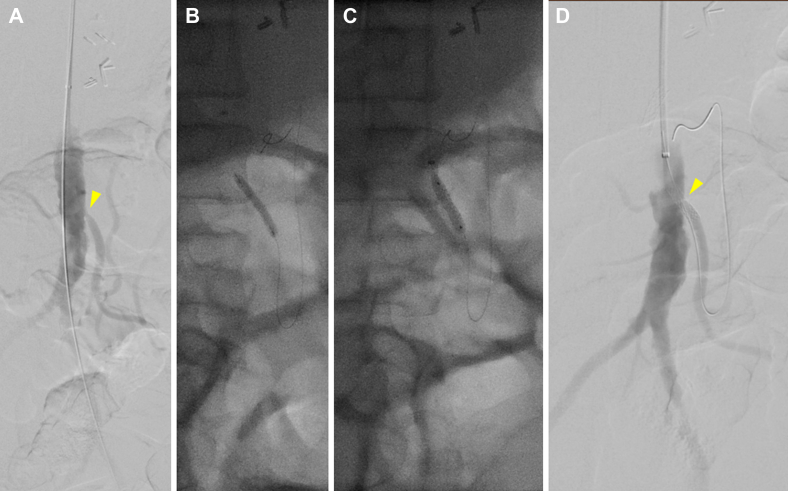
A 47-year-old female patient with multiple prior lower extremity bypasses and chronic mesenteric ischemia, with stenting of the SMA 1 year earlier, had presented to an outside institution with severe sudden-onset abdominal pain. Computed tomography (CT) demonstrated small bowel pneumatosis with portal venous gas, and the patient underwent urgent exploration, including ileocecectomy with primary ileocolic anastomosis. Postoperatively, the patient’s diet was advanced; however, on postoperative day (POD) 12, abdominal pain and distention had developed. CT angiography (CTA) demonstrated celiac artery and SMA occlusion with IMA stenosis. The patient was transferred to our institution, and heparin was administered for anticoagulation. The patient was then taken to the operating room for aortoceliac bypass. However, the graft had repeatedly thrombosed intraoperatively. Thus, empirically, the heparin was transitioned to argatroban, facilitating closure with a patent bypass. The patient was not able to tolerate an oral (per os [PO]) diet postoperatively. A CT scan demonstrated patency of the bypass but persistent stigmata of mesenteric ischemia. The patient underwent mesenteric angiography via a left brachial approach on POD 10 of 22, demonstrating a patent bypass with high-grade ostial IMA stenosis. Despite the patent celiac graft, the viscera remained poorly perfused. The IMA was stented with a bare metal stent after initial percutaneous transluminal angioplasty had resulted in a focal dissection and significant elastic recoil, with significant improvement seen on completion angiography (Fig 1). Postoperatively, the patient improved, the diet was advanced without consequences, and the remainder of the hospital course was unremarkable. The patient was discharged with warfarin anticoagulation therapy and was noted to have a patent bypass and IMA on duplex ultrasound 1 year postoperatively, after which point the patient was lost to follow-up.
Fig 1.
A, Preoperative flush aortogram of patient 1 demonstrating severe inferior mesenteric artery (IMA) stenosis (arrowhead) with steep angulation accommodated by a brachial approach. The aortoceliac bypass is not visible. B, Balloon angioplasty. C, Subsequent stent placement. D, Completion angiogram showing improved IMA stenosis (arrowhead).
Patient 2
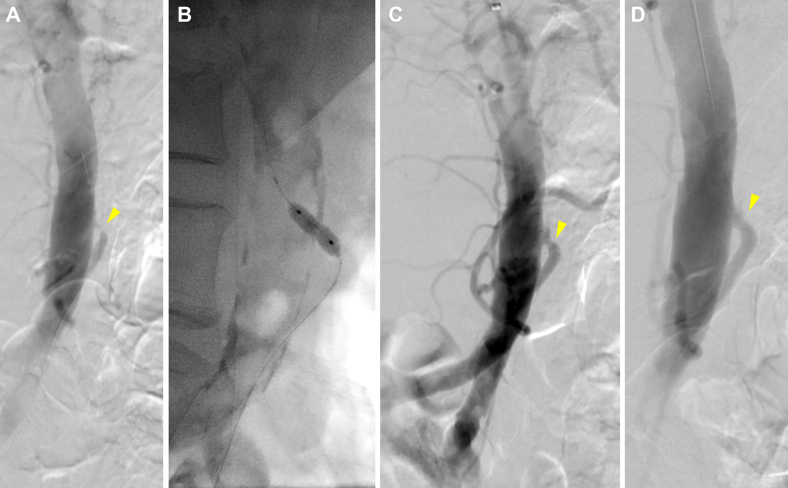
An 83-year-old female patient had presented with a 5-year history of postprandial abdominal pain and a 40-lb weight loss. She underwent elective mesenteric angiography via a left brachial approach, which demonstrated a chronically occluded common celiomesenteric trunk and high-grade focal ostial IMA stenosis. The celiomesenteric trunk occlusion could not be crossed; thus, the IMA was selectively catheterized and stented with a bare metal stent. After stenting, the IMA was noted on flush aortography to briskly fill a prominent meandering mesenteric artery, which filled the celiomesenteric trunk in a retrograde fashion (Fig 2). The patient received a loading dose of clopidogrel and was discharged on POD 2 with a prescription for dual antiplatelet therapy for 6 months. A patent IMA was noted through 1 year of follow-up.
Fig 2.
A, Preoperative flush aortogram of patient 2 showing occlusion of known celiomesenteric trunk with severe proximal inferior mesenteric artery (IMA) stenosis (arrowhead). B, Balloon angioplasty. C, Angiogram showing residual waist and modest improvement. D, Completion angiogram after stenting showing improved IMA flow.
Patient 3
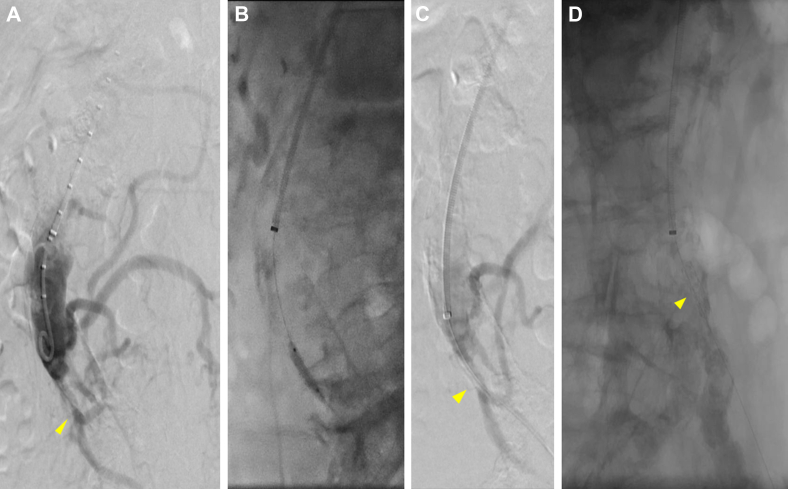
A 77-year-old male patient with chronic postprandial abdominal pain and weight loss had presented with a 10-day history of acute exacerbation of pain with additional nausea. He had a lactate level of 3.5 mmol/L, and CTA demonstrated calcified occlusions of the celiac artery and SMA with high-grade IMA stenosis and a chronic-appearing infrarenal aortic occlusion. The patient was resuscitated and underwent urgent mesenteric angiography via a left brachial approach, which again demonstrated the celiac artery and SMA occlusions. The IMA was stenotic but patent, providing collateral flow to the mesentery and bilateral lower extremities. The IMA was stented with significant angiographic improvement seen (Fig 3).
Fig 3.
A, Preoperative flush aortogram of patient 3 showing severe inferior mesenteric artery (IMA) stenosis (arrowhead) and distal filling of a prominent meandering mesenteric artery. B, Balloon angioplasty. C, Residual stenosis (arrowhead). D, Stent placement without visible stenosis (arrowhead).
After initial improvement, the patient had developed recurrent abdominal pain and worsening leukocytosis on POD 2, with CT demonstrating gas in the mesentery of the terminal ileum. On exploration, murky ascites was encountered, and the distal ileum was circumferentially necrotic. The distal ileum was resected, and the bowel was left in discontinuity, with a jejunoileal anastomosis performed 2 days later. He again showed improvement, was weaned from the vasopressors and extubated. However, he had developed recurrent abdominal pain with a PO diet challenge. CTA demonstrated a patent IMA stent; however, the patient subsequently developed a recurrent pressor requirement, prompting reexploration. The entire luminal gastrointestinal tract appeared viable, and the IMA was found to be pulsatile; thus, the patient was returned to the intensive care unit. After subsequent improvement and extubation, the patient aspirated, leading to reintubation and an increasing pressor requirement. Further escalation in care was not deemed to be within the patient’s goals. A later terminal extubation failed, and he died.
Patient 4
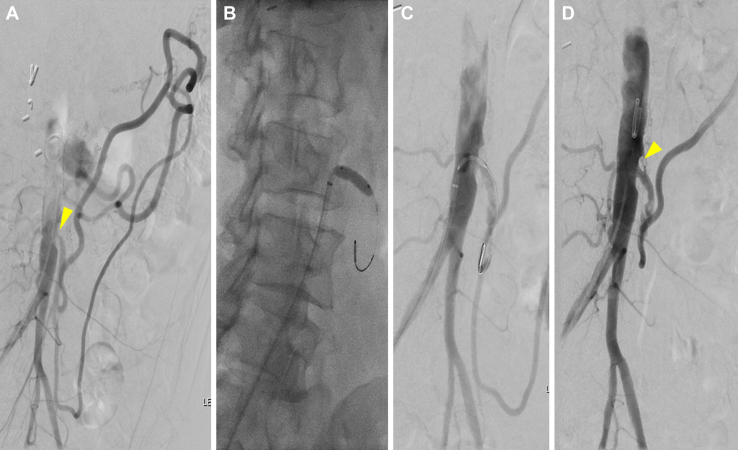
A 57-year-old female patient had presented with a 6-month history of postprandial abdominal pain, a 25-lb weight loss, and food fear. CTA revealed long-segment celiac artery and SMA occlusions with ostial IMA stenosis. Because of a nearly orthogonal takeoff of the IMA, the patient was brought electively for mesenteric angiography via a common femoral approach, which demonstrated robust retrograde filling of the SMA via a large meandering mesenteric artery. The IMA was selected and underwent angioplasty and stenting with a bare metal stent (Fig 4). However, on POD 1, the patient had developed severe abdominal pain and vomiting after a PO diet challenge. She underwent urgent aortoceliac and aorto-SMA bypass with a bifurcated graft. The IMA was found to be patent, with a water hammer pulse distal to the stent. Therefore, dissection of the meandering mesenteric artery was suspected. The patient’s postoperative course was unremarkable, and the patient was discharged on POD 7 after surveillance CTA had demonstrated patent aortic–visceral bypasses but with occlusion of the IMA stent. The patient had not had a return of mesenteric ischemia symptoms through 18 months of follow-up.
Fig 4.
A, Preoperative flush aortogram of patient 4 showing severe stenosis (arrowhead) in the inferior mesenteric artery (IMA), with the IMA feeding a robust collateral network to the viscera and lower extremities. B, Balloon angioplasty showing residual waist. C, Stent placement with improved stenosis (arrowhead) and flow to the meandering mesenteric artery.
Patient 5
A 68-year-old female patient had presented with a 1-year history of crampy postprandial abdominal pain and food fear, followed by an acute-on-chronic exacerbation with a 4-day history of a sudden increase in abdominal pain and hematochezia. At an outside facility, CTA revealed flush occlusions of the celiac artery and SMA with high-grade IMA stenosis, followed by post-stenotic dilatation. The IMA fed a prominent meandering mesenteric artery. She was transferred to our institution and promptly underwent mesenteric angiography. However, the celiac artery, SMA, and IMA could not be selectively catheterized because of severe calcific ostial disease. The patient received preoperative optimization and then underwent open aortic and IMA endarterectomy. The IMA was selected for open revascularization because the celiac and mesenteric arteries were both occluded without a palpable pulse noted on abdominal exploration. Bulky calcific plaque was found throughout the visceral aorta, precluding a clamp site that would have enabled minimization of the ischemic time, which was a priority given the degree to which the patient’s viscera appeared to depend on the IMA. After patch angioplasty of the IMA, the patient was returned to the intensive care unit. The remainder of the patient’s course was unremarkable, including tolerance of a PO diet on POD 4 and discharge on POD 5. The IMA was found to be patent on follow-up duplex ultrasound 3 months later, after which, the patient was lost to follow-up.
Discussion
Although >90% of cases of chronic mesenteric ischemia have been ascribed to atherosclerotic steno-occlusive disease, primarily in the SMA or celiac artery,3 the IMA can play an important role in the occurrence of mesenteric ischemia. The IMA can be sacrificed with impunity in a variety of situations, including some open and endovascular abdominal aortic aneurysm repairs; however, the IMA could have increased importance in situations of celiac artery and SMA disease or severe aortoiliac occlusive disease.1,2,4,5,14, 15, 16 Rarely, the IMA will perfuse the entire viscera via inflow through its two primary SMA collateral pathways, the marginal artery of Drummond, and the paracolic arc of Riolan. Additional visceral perfusion can proceed via the SMA–celiac artery collateral pathways, including the pancreaticoduodenal and gastroduodenal arteries. Several centers have demonstrated successful IMA revascularization, which will then perfuse the viscera via these pathways.5, 6, 7,9, 10, 11, 12, 13,17
Most currently available analyses of mesenteric ischemia treatment have been restricted to the celiac artery and SMA. In addition to case reports of IMA revascularization,9,10 some case series have been described by Sarac et al,7 Turba et al,12 and Wohlauer et al,13 all of which had described cases of chronic mesenteric ischemia. We have presented a case series of five patients with both chronic and acute-on-chronic mesenteric ischemia who had undergone “salvage” IMA revascularization, when concomitant celiac artery and SMA occlusions had precluded successful reperfusion of the viscera via those arteries. Our series included one patient for whom endovascular modalities had failed at revascularization of all three mesenteric arteries and open IMA endarterectomy had provided satisfactory results.
Especially given that atherosclerotic mesenteric ischemia will classically occur when significant lesions are present in two of the three mesenteric arteries,18 it is not surprising that treatment of mesenteric ischemia in the present series of patients had required salvage IMA revascularization. Some of our patients had short perioperative courses and successful resolution of their symptoms, although others had had protracted courses with significant morbidity. The results from our patients with chronic symptoms correspond well to those from the vailable literature on IMA revascularization for chronic mesenteric ischemia.7,12,13
Our results suggest that IMA revascularization should be considered when celiac artery and SMA revascularization cannot be achieved, whether as a bridge to open reconstruction or as definitive management. IMA revascularization should also be considered as a concomitant procedure when the celiac artery or SMA has been successfully treated, given its contribution to collateral flow. Furthermore, open revascularization of the IMA could be appropriate in rare clinical scenarios when aortoceliac or aorto-SMA bypass has been precluded by anatomic factors. For patients with acute or acute-on-chronic mesenteric ischemia, however, the often severe physiologic insult sustained by the viscera could be irrecoverable despite salvage IMA revascularization, just as with celiac artery or SMA revascularization. The variable course of our patients who had undergone IMA-specific revascularization highlights the tenuous nature of mesenteric perfusion when such severe disease is present that two to three mesenteric vessels have become occluded or stenosed.
A few technical points regarding IMA revascularization are worth highlighting. First, as was common in our series, left brachial access can enable easier selective catheterization of the IMA because of its traditionally steep aortic takeoff angle. Femoral access can also be successful and can be facilitated with reverse angle catheters. One patient in our series had had a more right-angle IMA takeoff and had undergone femoral access. The IMA also has earlier branches than the SMA, and the shorter proximal stump can make reliable sheath access difficult and require shorter stents. Difficulty in maintaining purchase in the IMA makes predilation angioplasty especially useful before stent placement. However, care is required to avoid dissection in the IMA, especially in cases of severe SMA disease when dissection of the IMA could compromise collateral flow via the meandering mesenteric artery. In the present series, balloon expandable stents were uniformly used for the IMA lesions and can be especially useful given the ostial location of most atherosclerotic IMA lesions.
The findings from our series should be interpreted in light of several limitations. We performed a retrospective review of a series of five cases at a quaternary referral center. Thus, it could be difficult to generalize our findings to a wider population. Operative decision-making and choice of revascularization modality, whether balloon angioplasty, endarterectomy, or stenting, was determined from the anatomic and clinical considerations, including operating surgeon discretion. Additionally, long-term follow-up details were limited in the present case series; thus, comparative judgments regarding freedom from symptoms after IMA revascularization would be difficult.
Conclusions
The IMA has often been ignored or overlooked in favor of the celiac artery and SMA in the setting of mesenteric ischemia. However, the findings from the present series of mesenteric ischemia have demonstrated the important potential benefit of IMA revascularization, especially in the setting of challenging stenoses or occlusions of the celiac artery and SMA. In such salvage situations, IMA revascularization should be considered in the appropriate clinical scenario.
From the Eastern Vascular Society
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Axelrod D.J., Lookstein R.A., Guller J., Nowakowski F., Ellozy S., Carrroccio A., et al. Inferior mesenteric artery embolization before endovascular aneurysm repair: technique and initial results. J Vasc Interv Radiol. 2004;15:1263–1267. doi: 10.1097/01.RVI.0000141342.42484.90. [DOI] [PubMed] [Google Scholar]
- 2.Bjorck M., Bergqvist D., Troeng T. Incidence and clinical presentation of bowel ischaemia after aortoiliac surgery—2930 operations from a population-based registry in Sweden. Eur J Vasc Endovasc Surg. 1996;12:139–144. doi: 10.1016/s1078-5884(96)80098-0. [DOI] [PubMed] [Google Scholar]
- 3.Shaw R.S., Green T.H. Massive mesenteric infarction following inferior mesenteric-artery ligation in resection of the colon for carcinoma. N Engl J Med. 1953;248:890–891. doi: 10.1056/NEJM195305212482103. [DOI] [PubMed] [Google Scholar]
- 4.Ward T.J., Cohen S., Fischman A.M., Kim E., Nowakowski F., Ellozy S., et al. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol. 2013;24:49–55. doi: 10.1016/j.jvir.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Alsafi A., Jenkins M.P., Hamady M.S. Use of stent grafts to preserve a large inferior mesenteric artery during endovascular aortic aneurysm repair. J Vasc Interv Radiol. 2019;30:1980–1981. doi: 10.1016/j.jvir.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Park W.M., Cherry K.J., Chua H.K., Clark R., Jenkins G., Harmsen W., et al. Current results of open revascularization for chronic mesenteric ischemia: a standard for comparison. J Vasc Surg. 2002;35:853–859. doi: 10.1067/mva.2002.123753. [DOI] [PubMed] [Google Scholar]
- 7.Sarac T.P., Altinel O., Kashyap V., Bena J., Lyden S., Srivastava S., et al. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008;47:485–491. doi: 10.1016/j.jvs.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz E., Tatou E., Favier-Blavoux C., Bouchot O., Cognet F., Cercueil J., et al. Endovascular treatment as first choice in chronic intestinal ischemia. Ann Vasc Surg. 2002;16:693–699. doi: 10.1007/s10016-001-0321-3. [DOI] [PubMed] [Google Scholar]
- 9.Brandão D., Koullias G.J., Caparrelli D.J., Diethrich E.B. Inferior mesenteric artery stenting: a solution for chronic mesenteric ischemia. Perspect Vasc Surg Endovasc Ther. 2009;21:186–189. doi: 10.1177/1531003509351096. [DOI] [PubMed] [Google Scholar]
- 10.Shah T., Singh M., Bhuriya R., Kovacs D., Khosla S. Rare case of “wandering artery of Drummond” as a result of chronic triple mesenteric vessel occlusion treated by isolated angioplasty and stenting of the inferior mesenteric artery. Int J Angiol. 2013;22:245–250. doi: 10.1055/s-0033-1348879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tasse J.C., Arslan B., Turba U.C. Isolated stenosis of the inferior mesenteric artery: to treat or not to treat? Tech Vasc Interv Radiol. 2015;18:51–55. doi: 10.1053/j.tvir.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Turba U.C., Saad W.E., Arslan B., Sabri S., Trotter S., Anjle J., et al. Chronic mesenteric ischaemia: 28-year experience of endovascular treatment. Eur Radiol. 2012;22:1372–1384. doi: 10.1007/s00330-011-2376-z. [DOI] [PubMed] [Google Scholar]
- 13.Wohlauer M., Kobeiter H., Desgranges P., Becquemin J.P., Cochennec F. Inferior mesenteric artery stenting as a novel treatment for chronic mesenteric ischemia in patients with an occluded superior mesenteric artery and celiac trunk. Eur J Vasc Endovasc Surg. 2014;27:e21–e23. doi: 10.1016/j.ejvsextra.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manunga J.M., Cragg A., Garberich R., Urbach J., Skeik N., Alexander J., et al. Preoperative inferior mesenteric artery embolization: a valid method to reduce the rate of type II endoleak after EVAR? Ann Vasc Surg. 2017;39:40–47. doi: 10.1016/j.avsg.2016.05.106. [DOI] [PubMed] [Google Scholar]
- 15.Samura M., Morikage N., Mizoguchi T., Takeuchi Y., Ueda K., Harada T., et al. Identification of anatomical risk factors for type II endoleak to guide selective inferior mesenteric artery embolization. Ann Vasc Surg. 2018;48:166–173. doi: 10.1016/j.avsg.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Nevala T., Biancari F., Manninen H., Matsi P., Makinen K., Ylonen K., et al. Inferior mesenteric artery embolization before endovascular repair of an abdominal aortic aneurysm: effect on type II endoleak and aneurysm shrinkage. J Vasc Interv Radiol. 2010;21:181–185. doi: 10.1016/j.jvir.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Aschenbach R., Bergert H., Kerl M., Zangos S., Neumeister A., Schlosser A., et al. Stenting of stenotic mesenteric arteries for symptomatic chronic mesenteric ischemia. Vasa. 2012;41:425–431. doi: 10.1024/0301-1526/a000232. [DOI] [PubMed] [Google Scholar]
- 18.Mikkelsen W.P. Intestinal angina: its surgical significance. Am J Surg. 1957;94:262–269. doi: 10.1016/0002-9610(57)90654-2. [DOI] [PubMed] [Google Scholar]