Abstract
Background:
A variety of dermatoses have been reported in the growing number of patients treated with immune-checkpoint inhibitors (ICIs), but the current understanding of cutaneous immune-related adverse events (irAEs) is limited.
Objective:
To determine the cumulative incidence, distribution, and risk factors of cutaneous irAEs after ICI initiation.
Methods:
This was a retrospective cohort study of patients in a national insurance claims database including cancer patients treated with ICIs and matched controls.
Results:
The study included 8637 ICI patients and 8637 matched controls. The overall incidence of cutaneous irAEs was 25.1%, with a median onset time of 113 days. The ICI group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous irAEs.
Limitations:
Retrospective design without access to patient chart data.
Conclusions:
This study identifies cutaneous irAEs in a real-world clinical setting and highlights patient groups that are particularly at risk. The results can aid dermatologists at the bedside in the diagnosis of cutaneous irAEs and in formulating management recommendations to referring oncologists regarding the continuation of ICI therapy.
Keywords: Cutaneous, dermatologic, drug reactions, immune-checkpoint inhibitors, immune-related adverse events, immunotherapy
INTRODUCTION
The treatment of cancer has changed with the development of novel therapies, including anticytotoxic T-lymphocyte antigen-4 (anti-CTLA-4), anti-programmed cell death protein-1, and anti-programmed cell death ligand-1 antibodies. These therapies have demonstrated improved overall survival and durable response rates for multiple advanced malignancies.1-4 Immune checkpoint inhibitors (ICIs) are becoming standard therapy for an increasing number of advanced malignancies, with 36.1% of patients with these cancers eligible for ICI therapy in 2019.5
Immune-related adverse events (irAEs) affect more than half of treated patients.3,6 These toxicities can affect any organ through the increased activation of the immune system elicited by ICIs.3,6,7 Additionally, irAEs can result in therapy discontinuation or require treatment with systemic immunosuppression, which poses a risk of reduced ICI efficacy.7-10 As the use of ICIs increases, so will the incidence of irAEs, underscoring the importance of research into their incidence, type, and severity.
Among irAEs, cutaneous toxicities are reported most frequently.3,4,6,11-18 However, the majority of available literature on irAEs is derived from clinical trial experience with limited dermatologic pheno-typing, small observational studies, and case reports. Real-world irAE studies are limited by the relatively recent introduction of ICI therapy and small sample sizes at single treatment sites.3,13-16,18-23 We aimed to characterize the epidemiology, timing, and risk factors for the development of cutaneous diagnoses following ICI initiation in the first population-level analysis in the United States.
METHODS
Study cohort
Using deidentified claims data from Aetna, a national health insurance plan, we identified cancer patients by cancer type, cutaneous diagnoses (defined by International Classification of Diseases-9 and -10 codes), and ICI and systemic immunosuppressant use as defined by the Healthcare Common Procedure Coding System and the National Drug Code (Supplemental Tables 1-8 available via Mendeley at https://data.mendeley.com/datasets/8f3tfgbdjx/1). Cutaneous diagnoses included in this study were derived from published literature and expert opinion. Combination therapy was defined as claims for both anti-CTLA-4 and either anti-programmed cell death protein-1 or anti-programmed cell death ligand-1 therapies.
The data set for our analysis included deidentified medical claims, pharmacy claims, enrollment, and demographic data (including the region of residence, median income, and unemployment rate, as determined by zip code information from enrollment data combined with the 2010 United States census) for members between January 3, 2011, and December 31, 2019.24 Age-, sex-, primary cancer type-, index year-, and Charlson comorbidity index grade-matched controls were selected using 1:1 exact matching.25 To ensure similar follow-up times in the ICI and control groups, the index year was defined as the year of therapy initiation for ICI patients and the year of primary malignancy diagnosis for non-ICI cancer control patients. Charlson comorbidity index scores were graded by severity as mild (1-2), moderate (3-4), and severe (≥5).26
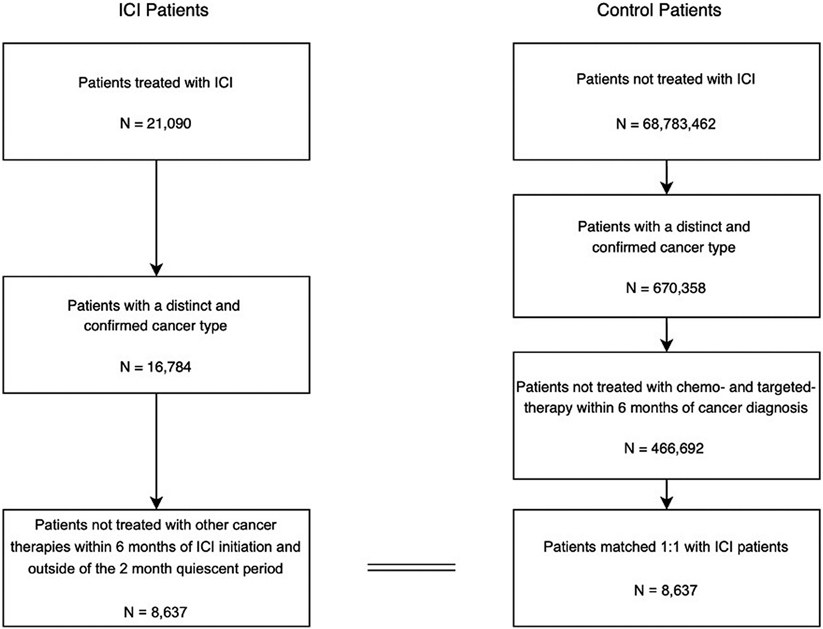
Patients with immunotherapy treatment within the first 2 months of database enrollment were excluded from the study, as they could have started treatment on their previous insurance plan. Patients receiving conventional chemotherapy or targeted therapies within 6 months of ICI initiation (for the study group) and cancer diagnosis (for the control group) were also excluded from the analysis. These patients were excluded to isolate the risk to ICI therapy. The flow diagram for this study is presented in Fig 1. Patients were censored on the date of insurance expiration. To avoid identification of systemic immunosuppression unrelated to the cutaneous diagnosis, the window for use was limited to 7 days following a the new cutaneous diagnosis. The Harvard Medical School Institutional Review Board granted approval for the use of the deidentified claims database.
Fig 1.
Study flow diagram.
Outcomes of the study
The primary outcomes included the cumulative incidence and distribution of cutaneous diagnoses after ICI initiation. We also aimed to identify risk factors for the development of cutaneous diagnoses of interest, defined as cutaneous diagnoses with an incidence rate ratio (IRR) > 1 for the ICI cohort compared with the control group and adjusted P value < .05 after multiple comparison correction. The secondary outcome was the rate of systemic immunosuppression use (as a proxy for high-grade cutaneous irAEs) following a cutaneous diagnosis in the ICI group.
Statistical analysis
To compare groups, we used Pearson’s chi-squared test or Fisher exact test for categorical variables and t-test or Kruskal-Wallis test for continuous variables. The time to development of any of the cutaneous events was calculated from the date of administration of the first ICI treatment for the ICI group and cancer diagnosis for the control group. Only new dermatologic diagnoses following treatment initiation for the ICI group or cancer diagnosis for the control group were considered in the analyses.
IRRs along with the 95% confidence intervals (CIs) and P values were reported. Because of low counts, the Haldane-Anscombe correction was used in the calculation of IRRs. P values were adjusted using the Benjamini-Hochberg correction for multiple comparisons, with significant differences defined as an adjusted P value < .05. Multivariable logistic regression was performed to analyze predictors of cutaneous diagnoses of interest in ICI recipients. Variables included in the regression were ICI target, cancer type, age, sex, Charlson comorbidity index, and measures of socioeconomic status as determined by zip code data. All analyses were conducted in R version 3.6.3 (R Statistical Software).
RESULTS
Study population
We identified 8637 patients who received ICI therapy and 8637 matched controls. Demographic and baseline characteristics for the study and control populations are presented in Table I. There was no difference in the median follow-up time between the 2 groups (1.9 years vs 1.9 years, P = .07). Melanoma (26.6%), lung cancer (40.0%), and renal cell carcinoma (12.3%) were the most represented malignancies. In the ICI group, 6595 (76.4%) received PD-1 inhibitors, 532 (6.2%) received PD-L1 inhibitors, 766 (8.9%) received anti-CTLA-4 therapy (ipilimumab), and 744 (8.6%) received combination therapy.
Table I.
Demographic and baseline characteristics of immune checkpoint inhibitor therapy and control groups
| Characteristic | Control patients | ICI patients | P value |
|---|---|---|---|
| N = 8637 | N = 8637 | ||
| Age (years) (mean [SD]) | 67.5 (11.8) | 67.5 (11.8) | 1 |
| Follow-up time (years) (median [IQR]) | 1.9 (0.9-3.2) | 1.9 (0.9-3.2) | .07 |
| Treatment duration (months) (median [IQR]) | - | 2.0 (5.0) | |
| Cancer type (%) | 1 | ||
| Bladder cancer | 487 (5.6) | 487 (5.6) | |
| Breast cancer | 254 (2.9) | 254 (2.9) | |
| Cervical cancer | 52 (0.6) | 52 (0.6) | |
| Colorectal carcinoma | 224 (2.6) | 224 (2.6) | |
| Gastric cancer | 71 (0.8) | 71 (0.8) | |
| Head and neck squamous cell carcinoma | 293 (3.4) | 293 (3.4) | |
| Hodgkin disease | 63 (0.7) | 63 (0.7) | |
| Liver cancer | 262 (3.0) | 262 (3.0) | |
| Lung cancer | 3454 (40.0) | 3454 (40.0) | |
| Melanoma | 2299 (26.6) | 2299 (26.6) | |
| Prostate cancer | 119 (1.4) | 119 (1.4) | |
| Renal cell carcinoma | 1059 (12.3) | 1059 (12.3) | |
| Sex = male (%) | 5113 (59.2) | 5113 (59.2) | 1 |
| CCI group (%) | 1 | ||
| Mild (1-2) | 210 (2.4) | 210 (2.4) | |
| Moderate (2-4) | 398 (4.6) | 398 (4.6) | |
| Severe (>5) | 8029 (93.0) | 8029 (93.0) | |
| Region (%) | <.001 | ||
| Midwest | 1580 (21.3) | 1563 (20.8) | |
| Northeast | 1438 (19.4) | 1347 (17.9) | |
| South | 3487 (47.0) | 3476 (46.2) | |
| West | 921 (12.4) | 1142 (15.2) | |
| Median income ($) (median [IQR]) | 56,856 (44,011-76,006) | 58,750 (45,722.5-76,813) | <.001 |
| Unemployment rate (%) (median [IQR]) | 5.6% (4.3%-7.0%) | 5.5% (4.3%-6.9%) | .02 |
CCI, Charlson comorbidity index; ICI, immune-checkpoint inhibitors; IQR, interquartile range; SD, standard deviation.
Incidence
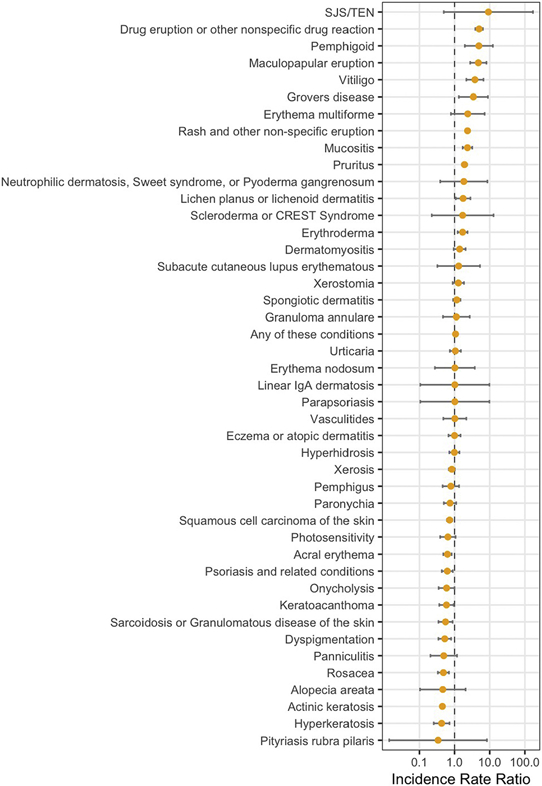
Patients in the 2 groups were at a similar risk of developing any cutaneous diagnosis (IRR 1.07; 95% CI, 1.01-1.13; P = .07; Fig 2 and Supplemental Table 9). Only 10 (23.3%) of these diagnoses occurred more frequently in ICI recipients (referred to as cutaneous diagnoses of interest), and 8 diagnoses were less frequent in the ICI cohort. The incidence of any cutaneous diagnosis among patients in the ICI group was 25.1% (Table II). Among the cutaneous diagnoses of interest, rash and other nonspecific eruption as well as drug eruption or other nonspecific drug reaction had incidences of 9.0% and 4.2%, respectively. A maculopapular eruption was observed in 0.9% of the patients in the ICI group. Pruritus (4.8%) was the second most frequent diagnosis. Vitiligo was diagnosed in 0.7% of ICI recipients, with 89.5% of these diagnoses in patients with melanoma. Lichen planus (0.5%), mucositis (1.5%), erythroderma (1.1%), pemphigoid (0.3%), and Grover disease (0.2%) were also identified as cutaneous diagnoses of interest.
Fig 2.
Cutaneous immune-related adverse events. Incidence rate ratios for cutaneous diagnoses previously reported as immune-related adverse events for immune-checkpoint inhibitor group compared to the control group.
Table II.
Incidence, timing, and systemic immunosuppression use within 7 days following cutaneous diagnosis in patients treated with immune-checkpoint inhibitors
| Cutaneous diagnosis | Developed diagnosis, n (%) |
Median time of onset (IQR) (days) |
Received systemic immunosuppression, n (%) |
|---|---|---|---|
| Any of the following* | 2171 (25.1%) | 113.0 (42.0-254.0) | 109 (5.0%) |
| Rash and other nonspecific eruption † | 779 (9.0%) | 121.0 (42.0-259.0) | 44 (5.6%) |
| Pruritus † | 416 (4.8%) | 139.0 (56.0-328.8) | 16 (3.8%) |
| Drug eruption or other nonspecific drug reaction † | 359 (4.2%) | 133.0 (45.5-277.5) | 20 (5.6%) |
| Actinic keratosis | 335 (3.9%) | 215.0 (82.0-442.0) | 4 (1.2%) |
| Squamous cell carcinoma of the skin | 207 (2.4%) | 197.0 (80.5-406.5) | 9 (4.3%) |
| Xerosis | 180 (2.1%) | 215.5 (85.0-481.0) | 3 (1.7%) |
| Spongiotic dermatitis | 145 (1.7%) | 175.0 (90.0-388.0) | 8 (5.5%) |
| Mucositis † | 128 (1.5%) | 144.0 (64.0-346.3) | 4 (3.1%) |
| Erythroderma † | 98 (1.1%) | 212.0 (64.3-393.3) | 2 (2.0%) |
| Acral erythema | 83 (1.0%) | 273.0 (123.5-473.0) | 1 (1.2%) |
| Maculopapular eruption † | 76 (0.9%) | 187.0 (98.0-310.5) | 4 (5.3%) |
| Hyperhidrosis | 68 (0.8%) | 260.0 (97.0-449.3) | 2 (2.9%) |
| Xerostomia | 63 (0.7%) | 170.0 (59.5-290.5) | 4 (6.3%) |
| Dermatomyositis | 59 (0.7%) | 113.0 (52.5-215.5) | 2 (3.4%) |
| Urticaria | 59 (0.7%) | 225.0 (73.5-429.0) | 4 (6.8%) |
| Vitiligo † | 57 (0.7%) | 294.0 (196.0-489.0) | 1 (1.8%) |
| Eczema or atopic dermatitis | 48 (0.6%) | 206.5 (77.3-377.8) | 0 (0.0%) |
| Psoriasis and related conditions | 47 (0.5%) | 220.0 (91.0-399.0) | 2 (4.3%) |
| Lichen planus † | 45 (0.5%) | 213.0 (124.0-354.0) | 1 (2.2%) |
| Rosacea | 42 (0.5%) | 320.0 (116.5-575.3) | 1 (2.4%) |
| Paronychia | 40 (0.5%) | 219.5 (92.5-345.8) | 0 (0.0%) |
| Dyspigmentation | 33 (0.4%) | 321.0 (199.0-545.0) | 0 (0.0%) |
| Keratoacanthoma | 27 (0.3%) | 244.0 (165.0-442.5) | 1 (3.7%) |
| Sarcoidosis or granulomatous disease of the skin | 27 (0.3%) | 341.0 (102.5-467.5) | 2 (7.4%) |
| Pemphigoid † | 26 (0.3%) | 294.0 (231.0-530.0) | 2 (7.7%) |
| Photosensitivity | 24 (0.3%) | 374.5 (107.5-453.0) | 0 (0.0%) |
| Pemphigus | 23 (0.3%) | 191.0 (123.0-387.5) | 1 (4.3%) |
| Onycholysis | 22 (0.3%) | 275.5 (134.8-450.5) | 1 (4.5%) |
| Hyperkeratosis | 20 (0.2%) | 249.0 (194.0-659.3) | 0 (0.0%) |
| Grover disease † | 18 (0.2%) | 266.5 (112.0-384.3) | 0 (0.0%) |
| Vasculitides | 13 (0.2%) | 118.0 (87.0-226.0) | 1 (7.7%) |
| Erythema multiforme | 10 (0.1%) | 106.0 (53.0-130.0) | 0 (0.0%) |
| Granuloma annulare | 10 (0.1%) | 223.5 (178.8-273.8) | 1 (10.0%) |
| Panniculitis | 7 (0.1%) | 413.0 (205.5-699.0) | 0 (0.0%) |
| Erythema nodosum | 4 (0.0%) | 382.5 (299.3-647.0) | 1 (25.0%) |
| Neutrophilic dermatosis, Sweets disease, or pyoderma gangrenosum | 4 (0.0%) | 257.0 (134.5-500.8) | 0 (0.0%) |
| SJS/TEN | 4 (0.0%) | 84.0 (29.8-219.0) | 0 (0.0%) |
| Subacute cutaneous lupus erythematosus | 4 (0.0%) | 81.0 (48.0-148.3) | 0 (0.0%) |
| Alopecia areata | 2 (0.0%) | 242.5 (233.8-251.2) | 0 (0.0%) |
| Scleroderma or CREST syndrome | 2 (0.0%) | 266.0 (188.0-344.0) | 0 (0.0%) |
| Linear IgA dermatosis | 1 (0.0%) | 183.0 (183.0-183.0) | 0 (0.0%) |
| Parapsoriasis | 1 (0.0%) | 55.0 (55.0-55.0) | 0 (0.0%) |
CREST, Calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia; IgA, immunoglobulin-A; IQR, interquartile range; IRR, incidence rate ratio; SJS/TEN, Stevens-Johnson syndrome/toxic epidermal necrolysis.
Patients with more than 1 cutaneous immune-related adverse event are counted only once.
Cutaneous diagnoses of interest were defined as dermatologic diagnoses with an IRR >1 for the ICI cohort compared with the control group and adjusted P value < .05 after multiple comparison correction.
Timing and distribution
The median time to onset for any cutaneous diagnosis was 113 (interquartile range [IQR] 42.0-254.0) days into treatment with ICIs (Table II). The distribution of all cutaneous diagnoses and cutaneous diagnoses of interest, respectively, are depicted in Supplemental Fig 1, A and B. For the cutaneous diagnoses of interest in the ICI group, 17.6% occurred in the first month, 63.1% by 6 months, and 84.6% by 1 year after therapy initiation.
Risk factors
Patients treated with ipilimumab monotherapy were less likely to experience a cutaneous diagnosis of interest when compared to treatment with pembrolizumab monotherapy (odds ratio [OR] 0.78; 95% CI, 0.62-0.98; P < .05), after adjusting for demographics, medical comorbidities, and primary tumor type (Table III). Combination therapy increased this risk (OR 1.53; 95% CI, 1.25-1.88; P < .001). Compared to lung cancer, melanoma and renal cell carcinoma were also identified as independent risk factors for developing cutaneous diagnosis of interest (OR 2.47, 95% CI 2.11-2.89, P < .001; and OR 1.65, 95% CI 1.36-2.00, P < .001, respectively).
Table III.
Multivariable logistic regression for patients treated with immune-checkpoint inhibitor therapy with odds ratio for defining event to be of any of the cutaneous diagnoses of interest
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| ICI | |||
| Pembrolizumab | ref* | ref* | ref* |
| Atezolizumab | 0.92 | 0.69-1.22 | .58 |
| Avelumab | 0.88 | 0.05-5.26 | .91 |
| Ipilimumab | 0.78 | 0.62-0.98 | <.05 |
| Nivolumab | 0.98 | 0.85-1.13 | .80 |
| Combination | 1.53 | 1.25-1.88 | <.001 |
| Age | 0.99 | 0.99-1.00 | <.05 |
| Cancer type | |||
| Lung cancer | ref* | ref* | ref* |
| Melanoma | 2.47 | 2.11-2.89 | <.001 |
| Renal cell carcinoma | 1.65 | 1.36-2.00 | <.001 |
| Other cancer | 0.98 | 0.82-1.16 | .79 |
| Sex = male | 0.93 | 0.82-1.04 | .21 |
| CCI | 0.99 | 0.97-1.01 | .29 |
| Median income ($1000s) | 1.00 | 1.00-1.00 | .11 |
| Unemployment rate (%) | 0.99 | 0.96-1.02 | .49 |
CCI, Charlson comorbidity index; CI, confidence interval; ICI, immune-checkpoint inhibitors; OR, odds ratio.
Ref = reference.
Use of systemic immunosuppression
Overall, 5.0% of patients in the ICI group received systemic immunosuppression within 7 days of cutaneous diagnosis (Table II). Some of the most common conditions in which systemic immunosuppression was used included drug eruption and other nonspecific drug reactions (5.6%), rash and other nonspecific eruptions (5.6%), maculopapular eruption (5.3%), and pemphigoid (7.7%).
DISCUSSION
ICI therapy has revolutionized the treatment of advanced cancer. Given the novelty of this therapeutic class, current real-world epidemiology, clinical understanding, and treatment patterns of their adverse events remain limited. The present study provides the first population-level and largest analysis of cutaneous eruptions in patients treated with ICIs in the United States.
The findings of this study are of particular clinical relevance to dermatologists evaluating ICI recipients with new cutaneous eruptions in the setting of ICI therapy. Dermatologists should have a high degree of suspicion for cutaneous irAEs in patients presenting with 1 of the diagnoses identified to have a higher frequency in this patient population. This knowledge can have a profound impact on diagnosis and management recommendations a dermatologist makes to oncology regarding ICI continuation. Furthermore, we found that in the real-world setting, cutaneous diagnoses are made later and a higher percentage of affected patients are treated with systemic immunosuppression than reported in clinical trials.3,6,27 These results suggest that dermatologists can work with oncologists to facilitate early evaluation of these vulnerable patients, which may prevent their progression to a higher grade toxicity, subsequent systemic immunosuppressive treatment, and other organ involvement.3,9,28
Maculopapular eruption, pruritus, and lichen planus are among the most commonly reported cutaneous irAEs, and were all identified as cutaneous diagnoses of interest in our study.3,6,7,11,13,14,22,29,30 However, maculopapular eruption and lichen planus were more rare in our ICI cohort than in prior studies, likely because many of these eruptions were coded under the 2 broad rash and drug-eruption diagnoses. Difficulties in recognizing and characterizing cutaneous irAEs by nondermatology providers have been reported and can further explain the lower incidence of specific diagnoses.31 Vitiligo, another commonly reported irAE, was identified primarily in patients with melanoma, as has been reported in prior literature.6 Among the more rarely reported irAEs, mucositis, erythroderma, pemphigoid, and Grover disease were also identified as cutaneous diagnoses of interest in our study.3,6,10,11,17,18,22,27,29,30,32-34
The lower incidence of 8 previously reported cutaneous diagnoses raises the possibility that reports of these diagnoses in ICI recipients may be due to causes unrelated to ICI therapy, including incidental diagnoses, paraneoplastic syndromes, and drug eruptions from coadministrated medications for the treatment of unrelated comorbidities.35-37 Specifically, the lower or similar incidence of cutaneous neoplasms (actinic keratosis, squamous cell carcinoma, and keratoacanthoma) observed in our ICI study population helps correct potential observational bias in previous reports.6,38,39 Because ICIs reconstitute the host’s overall immune response, they are likely to treat any cutaneous neoplasms that may arise in these patients. Moreover, due to this mechanism, PD-1 inhibition is now an approved treatment for advanced squamous cell carcinoma of the skin.40
Dermatologists also should be aware that the overall incidence of any cutaneous diagnosis among patients treated with ICIs is likely lower than previous estimates, which suggested that over a third of patients develop cutaneous irAEs.6 The prior estimate was derived primarily from studies of patients with melanoma, identified as an independent risk factor in our study. The incidence of rash and pruritus was lower (both 10%) in a meta-analysis of patients with lung cancer treated with ICIs, which was the most common cancer type in our study.41 It is also important to note that our analysis was limited to new cutaneous diagnoses. Thus, we could not determine the incidence of flares of preexisting conditions, like psoriasis. Furthermore, most cutaneous irAEs are low grade and might go unreported outside of clinical trials, as patients might not seek medical care, or when they do, providers might not record them in patient charts.3,6,8,10,22,27,42 As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population.
The median time to first cutaneous diagnosis in the ICI cohort was 113 days, which was a considerable delay compared to widely published ranges of 21 to 42 days.3,13,14,16,27,43 With the extended follow up in our study, a higher number of delayed reactions might have been identified.6,15,21 However, this finding is more likely a reflection of the real-world setting of this study, where patients present later due to a lack of urgency or access outside of clinical trials. Two studies of patients referred for dermatologic evaluation reported a median time to presentation of around 120 days, which is more consistent with our estimates.10,20 Additionally, as previously reported, pemphigoid diagnoses occurred many months (294 days) following ICI initiation.6,10,44 Patients should be educated about delayed presentations that would warrant evaluation, even after they complete their treatment course.
We further identified risk factors for the development of cutaneous diagnoses of interest to aid dermatologists evaluating new eruptions in ICI recipients. Patients with underlying melanoma and renal cell carcinoma were more likely to develop these diagnoses than patients with lung cancer. In melanoma, the increased incidence of vitiligo can partially explain the additional risk of irAEs observed in this population. Combination therapy carried the greatest risk in our study as has been widely reported.3,6 In contrast to prior studies, patients receiving ipilimumab monotherapy were less likely to develop a cutaneous diagnosis of interest compared to PD-1 monotherapy in our analysis. Because cutaneous irAEs associated with ipilimumab monotherapy are primarily derived from the treatment of melanoma,6,7,13,17 and melanoma is an independent risk factor for cutaneous toxicities, it is possible that these prior studies did not appropriately consider malignancy type as a confounder, overestimating the rate of cutaneous irAEs in ipilimumab monotherapy.34,45,46 It is important for dermatologists to be aware of these risk factors, as early identification and treatment of high-risk patients may prevent complications and reliance on systemic immunosuppression.3,9,28
The use of systemic immunosuppression may blunt antitumor efficacy of ICIs and is generally reserved for patients presenting with high-grade toxicities.7-9 Still, 5.0% of ICI recipients received systemic immunosuppression in the present study, which is on the high end of the 1%-5% incidence for high-grade cutaneous irAEs previously reported in clinical trials.3,6,27 Our findings could be the result of delayed diagnosis in nonclinical trial settings, as discussed above, allowing more time for progression to a higher grade. Additionally, there may also be greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols.47 For example, in a retrospective study of ICI patients referred for dermatologic evaluation, 46% and 13% were diagnosed with grade 2 and grade 3-4 dermatologic toxicities, respectively, and 20% of all patients were treated with systemic immunosuppression.10 Additionally, a higher incidence of high-grade reactions has been reported in patients with bullous pemphigoid; a trend that is also observed in our study.3,6,22,29,32,44
There are several limitations to this study, including its retrospective design and inability to query individual patient charts for details, such as pathology reports and underlying cancer stage due to the nature of the claims database, although there is no prior evidence that cancer stage is associated with cutaneous irAEs. Patients who were lost to follow up and some delayed events might not have been captured. Additionally, some previously reported dermatologic diagnoses were identified by broad diagnostic codes, presenting a challenge to characterizing the underlying adverse event. However, this limitation is unlikely to have differently impacted the study and control populations.
CONCLUSION
ICI therapy was associated with a higher incidence of only 10 dermatologic diagnoses and a lower incidence of cutaneous neoplasms. The median time to onset was approximately 4 months following ICI initiation and 1 in 20 patients received systemic immunosuppression. These findings can assist dermatologists at the bedside in early diagnosis and management of cutaneous irAEs.
Supplementary Material
CAPSULE SUMMARY.
A quarter of patients treated with immune-checkpoint inhibitor therapy developed cutaneous toxicities.
Pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, and Grover disease were seen with higher frequency in the setting of immune-checkpoint inhibitor therapy.
Funding sources:
Supported by the National Institutes of Health grants 5T32GM007309 and F30HL142131 awarded to Dr Wongvibulsin and T32GM007753 and F30CA224588 awarded to Dr Kalinich, and Dr Yu is supported in part by the Blavatnik Center for Computational Biomedicine Award. Funding sources for this project had no role in the design and conduct of the study.
The authors thank Susanne Churchill, Erica Meyer, and Nathan Palmer of the Department of Biomedical Informatics at Harvard Medical School for providing assistance with claims data access. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used:
- anti-CTLA-4
anti-cytotoxic T-lymphocyte antigen-4
- CI
confidence interval
- ICI
immune checkpoint inhibitor
- irAE
immune-related adverse event
- IRR
incidence rate ratio
- IQR
interquartile range
- OR
odds ratio
Footnotes
Conflicts of interest
None disclosed.
IRB approval status: The Harvard Medical School Institutional Review Board granted approval for the use of the deidentified claims database.
REFERENCES
- 1.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisler A, Phillips G, Barrios D, et al. CME Part II: immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassel JC, Heinzerling L, Aberle J, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. [DOI] [PubMed] [Google Scholar]
- 5.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibaud V Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. [DOI] [PubMed] [Google Scholar]
- 7.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamal S, Hudson M, Fifi-Mah A, Ye C. Immune-related adverse events associated with cancer immunotherapy: a review for the practicing rheumatologist. J Rheumatol. 2020;47(2):166–175. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. 2019;37(30):2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habre M, Habre SB, Kourie HR. Dermatologic adverse events of checkpoint inhibitors: what an oncologist should know. Immunotherapy. 2016;8(12):1437–1446. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Chang J, Mendenhall M, Cherry G, Goldman JW, Kulkarni RP. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinical features and management. Ther Adv Med Oncol. 2018;10, 1758834017751634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254–263. [DOI] [PubMed] [Google Scholar]
- 15.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber JS, Dummer R, De Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–1682. [DOI] [PubMed] [Google Scholar]
- 17.Inno A, Metro G, Bironzo P, et al. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103(5):405–421. [DOI] [PubMed] [Google Scholar]
- 18.Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44(2):158–176. [DOI] [PubMed] [Google Scholar]
- 19.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. [DOI] [PubMed] [Google Scholar]
- 20.Wang LL, Patel G, Chiesa-Fuxench ZC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol. 2018;154(9):1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57(6):664–669. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Yu K, Palmer N, Fox K, Kou SC, Kohane IS. Autoimmune effects of lung cancer immunotherapy revealed by data-driven analysis on a nationwide cohort. J Clin Pharm Ther. 2020;107(2):388–396. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, Fong A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 26.Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. [DOI] [PubMed] [Google Scholar]
- 28.Rudzki JD. Management of adverse events related to checkpoint inhibition therapy. Memo. 2018;11(2):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelaki A, Lampropoulou DI, Aravantinos G. Immune-related dermatologic toxicities: to make a long story short. Cutan Ocul Toxicol. 2020;39(1):10–12. [DOI] [PubMed] [Google Scholar]
- 31.Hsiehchen D, Watters MK, Lu R, Xie Y, Gerber DE. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open. 2019;2(9):e1911519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anedda J, Atzori L, Rongioletti F, Pilloni L. Nivolumab bullous pemphigoid: case description and literature review. J Clin Exp Pathol. 2019;9(1):1–3. [Google Scholar]
- 33.Lomax AJ, Lim J, Cheng R, et al. Immune toxicity with checkpoint inhibition for metastatic melanoma: case series and clinical management. J Skin Cancer. 2018;2018: 9602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaunitz GJ, Loss M, Rizvi H, et al. Cutaneous eruptions in patients receiving immune checkpoint blockade: clinicopathologic analysis of the non-lichenoid histologic pattern. Am J Surg Pathol. 2017;41(10):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva JA, Mesquita KD, Igreja AC, et al. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cadmus SD, Pearlstein MV, Pearlstein KA, Googe PB, Jolly PS. Paraneoplastic psoriasis in a patient with prostate cancer. JAAD Case Rep. 2018;4(3):220–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan YC, Shammout S, Cobley J, Hayes M, Akhtar M, Powell J. Metastatic prostate cancer presenting as subacute cutaneous lupus erythematosus. Australas J Dermatol. 2020;61(1):e113–e114. [DOI] [PubMed] [Google Scholar]
- 38.Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455–461.e1. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhari S, Leon A, Levin E, Neuhaus I, Liao W. Case report of multiple keratoacanthomas and squamous cell carcinomas in a patient eeceiving pembrolizumab. J Drugs Dermatol. 2017;16(5):513. [PubMed] [Google Scholar]
- 40.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos Garrett NF, da Costa AC, Damiani G, Vasques CI. Patients with lung cancer undergoing immune checkpoint inhibitors: a meta-analysis of dermatological toxicities. Crit Rev Oncol Hematol. 2020;152:102983. [DOI] [PubMed] [Google Scholar]
- 42.Basch E Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3(8):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber JS. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist. Am Soc Clin Oncol Educ B. 2012;32:174–177. [DOI] [PubMed] [Google Scholar]
- 44.Molina GE, Reynolds KL, Chen ST. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: a cross-sectional study. Br J Dermatol. 2020;183(6):1126–1128. [DOI] [PubMed] [Google Scholar]
- 45.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 46.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Comprehensive Cancer Network. Management of toxicities. Clin Pract Guidel Oncol. Accessed October 9, 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.