Introduction
Pityriasis rosea (PR) is a common and self-limiting papulosquamous skin condition. It presents with the most distinguishable sign of a larger “herald” patch with a collarette of scale at the margins, followed by smaller, finer, bilateral erythematous scaly plaques. The lesions commonly occur over the trunk and extremities in a blaschkolinear “Christmas tree” distribution. PR is most encountered as a springtime eruption and typically affects children and adults (age, 10-35 years), with a peak in adolescence. The estimated prevalence of PR in the United States was found to be 0.3% to 3% at dermatologic centers and evenly distributed among both sexes as 0.13% in females and 0.14% in males.1
Upon diagnosis of PR, patients may be informed that the condition is self-limiting, noncontagious, and unlikely to recur. A typical course develops and resolves on average within 6 to 8 weeks without therapy.2, 3, 4 However, the cutaneous eruption has been reported to last for 3 to 6 months.1,5,6 For some patients, the “watchful waiting” approach may not be sufficient due to symptomology and psychosocial distress associated with the appearance of lesions. Symptomatic treatments for symptoms such as pruritus include topical corticosteroids, oral antihistamines, and anti-itch lotions. Alternative potential treatments include UV-B phototherapy; antivirals, such as acyclovir; and macrolide antibiotics,3,4,7 with only modest success in clearing the eruption. Of the macrolides, a triple-blinded study on erythromycin 250 mg 4 times daily for 2 weeks resulted in remission after 2 weeks in 65% of patients. Gastrointestinal upset was observed in 10% of patients. 4 A bilateral comparison study of UV-B in patients with PR notes clearance on the treated half of the body after an average of 4.7 to 6.8 treatments, with notable adverse effect in some patients of slight tenderness and dryness of the skin.7
Seasonal variations and clustering in close communities suggest an infectious agent as the inciting factor for PR. Although human herpesviruses (HHVs) (HHV-6 and HHV-7) have often been implicated in a causal relationship with PR,8 the exact cause remains unknown. Additionally, PR-like eruptions have been reported with COVID-19,9 vaccinations (ie, bacille Calmette-Guerin vaccine, influenza, diphtheria, smallpox, hepatitis B, and COVID-19), and medications (ie, gold, barbiturates, captopril, and clonidine).10
There are case reports of successful treatment of PR with acyclovir based on the theory of the pathogenic involvement of HHV-6 and HHV-7, particularly when administered within the first week of symptom onset.11 There are additional but fewer reports of using valacyclovir (known pharmaceutically as Valacyclovir), which is the prodrug of acyclovir.12 The efficacy of valacyclovir as treatment for PR has so far been reported in 1 case series of 3 patients: while watchful waiting led to worsening symptoms, treatment with valacyclovir 1 g 3 times daily for 1 week led to resolution of the condition within 2 to 3 weeks.13 The authors have proposed valacyclovir as an alternative therapeutic option due to its less frequent dosing and a more favorable safety profile when compared with those of acyclovir. The dosing schedule of valacyclovir used in this series was adapted from the established treatment regimen for herpes zoster infection (shingles), which consists of oral valacyclovir 1 g 3 times daily for 7 days, which is the standard dose without renal adjustment.
Materials and methods
A retrospective chart review was conducted on 9 patient cases from 2 clinical dermatology sites, 1 academic center and 1 private practice, both in New Jersey. The patients are adult patients aged 22 to 72 years who were evaluated between the years 2018 and 2022. The patients were then seen on follow-up weeks to months after treatment, and improvement in the appearance of rash and symptoms were documented along with any medication adverse events or intolerance. Clinical photographs were taken to monitor the progress.
Response to treatment was characterized in 3 tiers: (1) “none” was defined as lack of response or persistence of lesions and symptoms after full treatment course, (2) “significant improvement” was defined as improvement in the appearance and symptomology of primary lesions but a lack of complete clearance, and (3) “resolved” was defined as clearance of primary lesions with absence of residual symptoms and with either absence or presence of mild postinflammatory secondary changes.
Case presentation
For a summary of cases, please refer to Table I.
Table I.
Clinical characteristics of patients with pityriasis rosea who were treated with oral valacyclovir
| Age (y) | Sex | Diagnosis | Time of symptom onset prior to initial presentation | Timing of follow-up | Status of PR eruption | Side effects from valacyclovir | Confounding treatments |
|---|---|---|---|---|---|---|---|
| 28 | F | Clinical PR | 2 wk | 3 wk | Resolved | None | None |
| 22 | F | Clinical PR | 3 d | 6 wk | Resolved | None | Triamcinolone 0.1% cream |
| 32 | F | Clinical PR | 2 wk | 10 d | Resolved | None | None |
| 24 | M | Clinical inverse PR | 2 wk | 2 wk | Significant improvement | None | None |
| 72 | M | Clinical PR | 4 wk | 4 wk | Resolved | None | None |
| 27 | M | Clinical PR | 3 d | 2 wk | Significant improvement | None | Triamcinolone 0.1% cream |
| 67 | F | Clinical PR | 1 wk | 2 wk | Resolved | None | Triamcinolone 0.1% cream |
| 35 | F | Clinical PR | 2 wk | 2 wk | Significant improvement | None | Triamcinolone 0.1% cream |
| 29 | F | Biopsy-proven PR | 12 wk | 2 wk | Significant improvement | None | None; flare was thought to be secondary to COVID-19 vaccine |
PR, Pityriasis rosea.
Case 1
A 28-year-old woman presented with a 2-week history of a rash diagnosed clinically as PR. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was prescribed no other topical or oral treatments. On follow-up 3 weeks after the initial presentation, her rash was resolved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 5 weeks.
Case 2
A 22-year-old woman presented with a 3-day history of a rash diagnosed clinically as PR. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was also prescribed topical triamcinolone 0.1% cream. On follow-up 6 weeks after the initial presentation, her rash was resolved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 6 weeks and 3 days.
Case 3
A 32-year-old woman presented with a 2-week history of a rash diagnosed clinically as PR. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was prescribed no other topical or oral treatments. On follow-up 10 days after the initial presentation, her rash was resolved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 3 weeks and 3 days.
Case 4
A 24-year-old man presented with a 2-week history of a rash diagnosed clinically as PR. He was started on valacyclovir 1 g 3 times daily by mouth for 7 days. He was prescribed no other topical or oral treatments. On follow-up 2 weeks after the initial presentation, his rash was significantly improved. He reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 4 weeks.
Case 5
A 72-year-old man presented with a 4-week history of a rash diagnosed clinically as PR. He was started on valacyclovir 1 g 3 times daily by mouth for 7 days. He was prescribed no other topical or oral treatments. On follow-up 4 weeks after the initial presentation, his rash was resolved. He reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 8 weeks.
Case 6
A 27-year-old man presented with a 3-day history of a rash diagnosed clinically as PR. He was started on valacyclovir 1 g 3 times daily by mouth for 7 days. He was also prescribed topical triamcinolone 0.1% cream. On follow-up 2 weeks after the initial presentation, his rash was significantly improved. He reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 2 weeks and 3 days.
Case 7
A 67-year-old woman presented with a 1-week history of a rash diagnosed clinically as PR. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was also prescribed topical triamcinolone 0.1% cream. On follow-up 2 weeks after the initial presentation, her rash was resolved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 3 weeks.
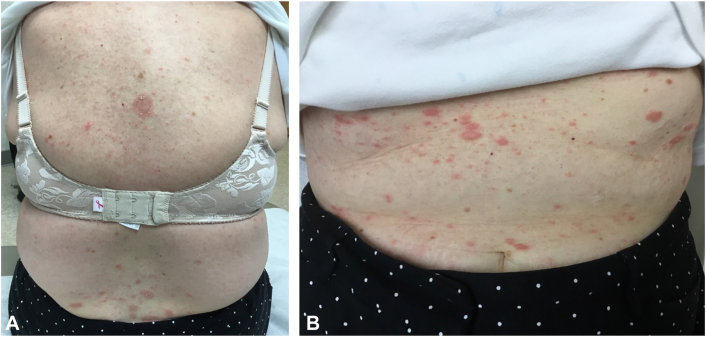
Please refer to Figs 1 and 2 for before-and-after clinical photographs.
Fig 1.
Initial presentation of patient 7 with a herald patch and round erythematous thin scaly plaques on the (A) posterior aspect of the trunk and (B) anterior aspect of the trunk.
Fig 2.
Patient 7 2 weeks after initiating valacyclovir therapy (3 weeks total time elapsed after rash onset) with resolution and only mild postinflammatory changes present on the (A) posterior aspect of the trunk and (B) anterior aspect of the trunk.
Case 8
A 35-year-old woman presented with a 2-week history of a rash diagnosed clinically as PR. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was also prescribed topical triamcinolone 0.1% cream. On follow-up 2 weeks after the initial presentation, her rash was significantly improved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 4 weeks.
Case 9
A 29-year-old woman presented with a 12-week history of a rash that started days after her COVID-19 booster vaccination. Diagnosis of PR was made clinically and with histopathologic evaluation. She was started on valacyclovir 1 g 3 times daily by mouth for 7 days. She was prescribed no other topical or oral treatments. On follow-up 2 weeks after the initial presentation, her rash was greatly improved. She reported no side effects from valacyclovir. The total time from onset to follow-up after treatment was 14 weeks.
Discussion
PR is a common, acute, and relatively benign papulosquamous dermatosis that typically self-resolves in 6 to 8 weeks without intervention. Prolonged duration of this rash has been reported to be up to 6 months. Due to the self-limiting nature of the condition, there is no standard of care for the active treatment of PR. Common therapeutics, such as topical steroids and oral antihistamines, are targeted to symptomology if present. Nonetheless, the watchful waiting approach may not be acceptable to some patients due to the psychosocial implications of the appearance of the rash, which may be confused with an infectious dermatosis, such as a dermatophytid, by the layperson. As with many dermatologic conditions, the patient’s quality of life must be considered, and some cases may necessitate active intervention.4
The proposed link between HHV-6/HHV-7 and PR has led to the suggested use of antivirals for treatment.14 The use of acyclovir for treatment of PR began appearing in the literature shortly after the HHV association was established, with mixed data in efficacy. In the authors’ opinion, the use of valacyclovir poses certain significant advantages over the use of acyclovir. Unlike acyclovir, which is dosed 3 to 5 times daily depending on indication, valacyclovir is offered orally in 500- and 1000-mg tablets and is typically taken 1 to 3 times daily. Valacyclovir is a generic medication associated with a relatively low out-of-pocket cost to patients, as listed on a coupon-based website with a cost as low as $6.76 for 21 1-g tablets. In the authors’ experience, it has been covered by various insurance companies for this rash without requirement of prior authorization.
It is noted that valacyclovir is the prodrug of acyclovir resulting in similar safety profiles. Some common side effects of valacyclovir include headache, dizziness, nausea, vomiting, joint pain, and rash (valacyclovir). Severe rash, such as Stevens-Johnson syndrome–toxic epidermal necrolysis spectrum, has been described in 1 case report after treatment with acyclovir, but, to our knowledge, there are no reports to date of this severe cutaneous adverse reaction after valacyclovir use.15 No patient in our case series reported adverse effects or side effects from taking valacyclovir for the specified dose and duration. No patient had to discontinue the week-long course due to medication side effects. No patient required renal dosing adjustment.
All patients in this case series reported either significant improvement (44.4%) or total resolution (55.6%) with the proposed treatment, suggesting that valacyclovir may be considered a reasonable treatment option for PR. Six of 9 patients treated with valacyclovir had complete resolution or significant improvement of their rash in a duration of <6 weeks, which is shorter than the cited duration of 6 weeks to 6 months with spontaneous resolution. Of 3 patients who were monitored with a rash for longer than 6 weeks, patient 9 suffered with the eruption for 12 weeks before evaluation in our clinic. Despite flaring after COVID-19 vaccination and failing potent topical corticosteroid treatment, her rash and quality of life were greatly improved at her 2-week follow-up.
Conclusion
Limitations to this case series include lack of matched control or comparison group, small sample size, and lack of histopathologic confirmation of diagnosis for all patients. The authors do note that there remains uncertainty as to whether the eruption cleared spontaneously or from the valacyclovir. It is believed that clearance of the PR eruption in 6 of our 9 cases in <6 weeks is a promising metric, especially when considering the safe and cost-effective nature of a short course of oral valacyclovir. Future work that would ideally include randomized control trials to assess the comparable effectiveness among valacyclovir, acyclovir, and placebo for PR as well as studies with larger sample sizes is needed.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Reviewed by and exemption obtained from Rutgers IRB (Pro 2022001389).
References
- 1.González L.M., Allen R., Janniger C.K., Schwartz R.A. Pityriasis rosea: an important papulosquamous disorder. Int J Dermatol. 2005;44(9):757–764. doi: 10.1111/j.1365-4632.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- 2.Drago F., Ciccarese G., Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4(8):800–801. doi: 10.1016/j.jdcr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung A.K.C., Lam J.M., Leong K.F., Hon K.L. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17(3):201–211. doi: 10.2174/1573396316666200923161330. [DOI] [PubMed] [Google Scholar]
- 4.Chuh A., Zawar V., Sciallis G., Kempf W. A position statement on the management of patients with pityriasis rosea. J Eur Acad Dermatol Venereol. 2016;30(10):1670–1681. doi: 10.1111/jdv.13826. [DOI] [PubMed] [Google Scholar]
- 5.Parsons J.M. Pityriasis rosea update: 1986. J Am Acad Dermatol. 1986;15(2 Pt 1):159–167. doi: 10.1016/s0190-9622(86)70151-5. [DOI] [PubMed] [Google Scholar]
- 6.Pedrazini M.C., da Silva M.H. Pityriasis rosea-like cutaneous eruption as a possible dermatological manifestation after Oxford-AstraZeneca vaccine: case report and brief literature review. Dermatol Ther. 2021;34(6) doi: 10.1111/dth.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valkova S., Trashlieva M., Christova P. UVB phototherapy for pityriasis rosea. J Eur Acad Dermatol Venereol. 2004;18(1):111–112. doi: 10.1111/j.1468-3083.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 8.Drago F., Ciccarese G., Rebora A., Broccolo F., Parodi A. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232(4):431–437. doi: 10.1159/000445375. [DOI] [PubMed] [Google Scholar]
- 9.Ehsani A.H., Nasimi M., Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(9):e436–e437. doi: 10.1111/jdv.16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litchman G., Nair P.A., Le J.K. StatPearls [Internet] StatPearls Publishing; 2021. Pityriasis rosea. [Google Scholar]
- 11.Chang H.C., Sung C.W., Lin M.H. The efficacy of oral acyclovir during early course of pityriasis rosea: a systematic review and meta-analysis. J Dermatolog Treat. 2019;30(3):288–293. doi: 10.1080/09546634.2018.1508820. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Biotechnology Information. Valacyclovir. National Center for Biotechnology Information; 2022. [Google Scholar]
- 13.Tzur L., Yang F.S.C., Deverapalli S. The use of antivirals in severe or recalcitrant cases of pityriasis rosea: a case series. JAAD Case Rep. 2022;28:100–103. doi: 10.1016/j.jdcr.2022.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe T., Kawamura T., Jacob S.E., et al. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol. 2002;119(4):793–797. doi: 10.1046/j.1523-1747.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 15.Sen S.S., Sil A., Chakraborty U., Chandra A. Stevens-Johnson syndrome-toxic epidermal necrolysis: a fatal cutaneous adverse reaction to oral acyclovir. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-238555. [DOI] [PMC free article] [PubMed] [Google Scholar]