Abstract
Background.
Mast cells are initiators and main effectors of allergic inflammation, together with eosinophils, with whom they can interact in a physical and soluble cross-talk with marked pro-inflammatory features, the Allergic Effector Unit. The pro-resolution role of mast cells, alone or in co-culture with eosinophils, has not been characterized yet.
Objectives.
We aimed to investigate select pro-resolution pathways in mast cells in vitro and in vivo in allergic inflammation.
Methods.
In vitro, we employed human and murine mast cells and analyzed release of resolvin D1 and expression of 15-Lipoxygenase after IgE-mediated activation. We performed co-culture of IgE-activated mast cells with peripheral blood eosinophils and investigated 15-Lipoxygenase expression and Resolvin D1 release. In vivo, we performed Ovalbumin/Alum and Ovalbumin/S. aureus enterotoxin B allergic peritonitis model in Wild Type mice following a MC “overshoot” protocol.
Results.
We found that IgE-activated mast cells release significant amounts of resolvin D1 30 min after activation, while 15-Lipoxygenase expression remained unchanged. Resolvin D1 release was found to be decreased in IgE-activated mast cells co-cultured with peripheral blood eosinophils for 30 min. In vivo, mast cell-overshoot mice exhibited a trend of reduced inflammation, together with increased peritoneal resolvin D1 release.
Conclusions.
Mast cells can actively contribute to resolution of allergic inflammation by releasing resolvin D1.
Keywords: Allergic inflammation, Allergic peritonitis, Eosinophils, Mast Cells, Resolution, Resolvin D1
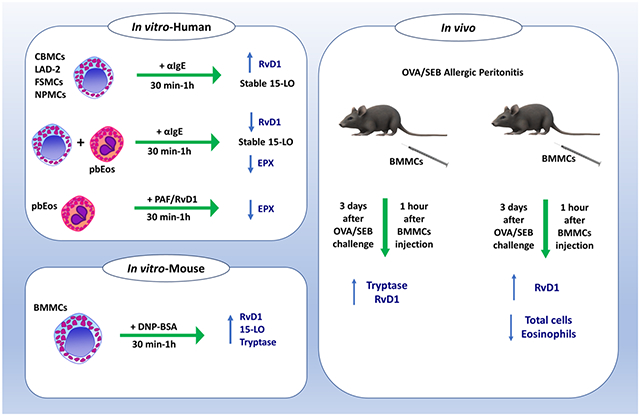
Graphical Abstract
INTRODUCTION
Mast cells (MCs) are crucial initiators of allergic inflammation (AI)[1], principally orchestrating the early phase of the response. After activation and subsequent degranulation, MC-derived mediators facilitate the recruitment and activation of eosinophils (Eos)[2] that are mostly involved in the late phase response. We have previously coined the term Allergic Effector Unit (AEU) to describe the physical and functional cross-talk of MCs and Eos that elicits marked pro-inflammatory short- and long-term outcomes[3,4].
Following the late phase, innate resolution mechanisms are initiated and drive the system back to homeostasis. Resolution of inflammation is defined as the period between the infiltration of inflammatory cells at the site of damage and their clearance[5]. In acute inflammatory events the resolution process is normally successful, whereas in case of failure, chronic inflammation prevails, as seen in asthma and atopic dermatitis.[6] The innate resolution in AI involves an active process commonly regulated by leukocyte-derived lipid molecules, collectively known as specialized pro-resolving lipid mediators (SPMs) and by their receptors[7]. These include the D-series resolvins (RvDs) produced by the enzyme 15-lipoxygenase (15-LO) from the lipid precursor docosahexaenoic acid (DHA)[5,8,9]. Other SPMs, such as lipoxins, are produced via the 5-LO pathway, which is also and mainly responsible for the biosynthesis of cysteinyl leukotrienes from arachidonic acid[10]. Functions of resolvins include induction of phagocytosis and apoptosis, inhibition of inflammatory cell infiltration and promotion of wound healing in AI[8].
Despite the vast literature describing the pro-inflammatory properties of MCs in AI, limited information exists regarding their possible role as a source of SPMs underlying the resolution of AI. In this regard, a previous report showed that human cord blood-derived MCs (CBMCs) express 15-LO[11]. However, the production of resolvins by MCs alone or in the AEU arrangement, either in vivo or in vitro has not been reported to date.
In the present work, we aimed to characterize the production and release of RvD1 from MCs in a first effort to explore their potential involvement in the resolution of AI. Considering the species- and type-related molecular and functional heterogeneity in human and rodent MC biology[12-14], we employed a number of relevant experimental systems of both human and rodent origin. Namely, we investigated in vitro whether human MCs, alone and in co-culture with Eos, and mouse MCs can produce RvD1 after immunoglobulin (Ig) E-mediated activation. The expression levels of 15-LO were analyzed in order to assess the putative modulation of the RvD1 biosynthetic pathway. We also employed mouse bone marrow-derived MCs (BMMCs), which are of the “mucosal” type, as opposed to the “connective tissue” type mouse MCs that exhibit different functional characteristics[13]. In order to dissect the contribution of MCs towards resolution of AI in vivo, we analyzed inflammatory cell infiltration and RvD1 peritoneal levels in a wild type (WT) mouse model of ovalbumin (OVA)/Staphylococcus aureus enterotoxin B (SEB)-induced allergic peritonitis (AP). In this model, we adopted an overshoot strategy which involved the injection of MCs in numbers higher than the ones normally found in the peritoneal cavity.
Our findings demonstrate for the first time that both human and mouse MCs produce RvD1 upon IgE-mediated activation, yet without detectable alterations in 15-LO expression. Of note, human Eos exposed to PAF released eosinophil peroxidase but did not release RvD1. In agreement with the in vitro data, the in vivo injection of MCs resulted in reduction of cell infiltration and augmentation of RvD1 peritoneal levels. These results point to the participation of MCs in the resolution of AI by producing and releasing RvD1.
MATERIALS AND METHODS
Cells
Cord blood-derived mast cells (CBMCs) were obtained by culturing umbilical cord blood mononuclear cells. Briefly, fresh cord blood was diluted with Hank's balanced salt solution, loaded onto Ficoll-Paque, and centrifuged (350 × g for 25 min). Mononuclear cells were washed twice with Hank's balanced salt solution and resuspended in 100 ml minimal essential medium alpha (MEM-α) containing 10% (vol/vol) fetal calf/bovine serum (FCS/FBS), penicillin (100 U ml−1), streptomycin (100 μg ml−1), ribonucleosides/deoxyribonucleosides, and stem cell factor (100 ng ml−1) (a gift from Amgen) (CBMC complete medium). Culture medium was replaced weekly. CBMC were used after 6– 8 weeks of culture, when >97% were positive for toluidine blue staining. LAD-2 cells were cultured and maintained as previously described [34]. FSMCs and nasal polyps MCs were obtained as previously described [35,36].
Peripheral blood eosinophils (pbEos) were purified from peripheral blood of asymptomatic, mildly atopic volunteers (5–10% blood eosinophilia) not taking any drug. Isolation was performed by negative selection using micro-magnetic beads (anti-CD16 and anti-CD3 Ab) (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) and the MACS system. Eosinophils collected at a purity of >98% (Kimura staining), and a viability of >98 % (trypan blue staining), were re-suspended (1×106 cells/ml) in culture media consisting of RPMI-1640 supplemented with heat-inactivated foetal bovine serum (10%), penicillin-streptomycin solution (100 U/ml) (Biological Industries, Beit Haemek, Israel) and GM-CSF (20 ng/ml; Peprotech, Rocky Hill, NJ, USA).
Bone marrow-derived mast cells (BMMCs) were obtained from bone marrows of 7-8-week old C57BL/6 mice. In brief, BM was obtained from the femurs of the mice after dissection in sterile conditions. Dispersed cells were cultured for 4 weeks to obtain mature BMMCs. Prior to experimentation, BMMCs were assessed for 95% viability (by Trypan blue exclusion) and for 90% maturity by acidic toluidine blue staining and expression of characteristic surface markers (cKit and FcεRI) by flow cytometry.
Peritoneal macrophages (MΦ) were purified from purified from the peritoneal cavity of wild type (WT) mice challenged with either PBS, ovalbumin and Staphylococcus aureus enterotoxin B (SEB) (OVA/SEB) or OVA/SEB plus bone marrow-derived mast cells (OVA/SEB-BMMCs) 6 days after challenge. Lavages were incubated at 37°C for 3 h to let cells adhere. After incubation, cells were washed 3 times with warm PBS to remove non-adherent cells. Adherent cells (MΦ) were cultured o.n. or for 24 h with RPMI containing 10% (vol/vol) fetal calf/bovine serum (FCS/FBS), penicillin (100 U ml−1), streptomycin (100 μg ml−1). After incubation, cells were harvested with a cell scraper and counted in a hemacytometer. Cell suspensions were centrifuged and supernatants were collected and stored at −80° before analysis.
All plasticwares were purchased from ThermoFisher Scientific (MA, USA).
Cell treatment and activation.
CBMCs (1 x 106 cells/ml) were sensitized with recombinant hSCF (100 ng/ml; Peprotech Asia, Rehovot, Israel), recombinant hIL-4 (10 ng/ml, Peprotech Asia) and human myeloma IgE (0.3 μg/ml; Calbiochem, Merck, Burlington, Massachussets, USA) for 3 days. LAD-2 cells (1 x 106 cells/ml) were sensitized overnight with human myeloma IgE (100 ng/ml; Calbiochem). For RvD1 production experiments, CBMCs and pbEos (1X105 cells/well of each type) were incubated in Tyrode’s Buffer (TB) containing 0.1% gelatin (TG), 1.8 mM CaCl2, 0.9 mM MgCl2 (TG++) with 20 μM docosahexaenoic acid (DHA) (Cayman Chemical, Ann Arbor, Michigan, USA) for 30 min at 37°C prior activation as described below. Non-activated controls were incubated with TG++ buffer alone. CBMCs and pbEos were pre-incubated at 37°C for 30 min to initiate the cross-talk between the cells. CBMC, LAD-2, FSMCs and NPMCs activation was performed by adding 5 μg/ml mouse anti-human IgE (Dako, Agilent, Santa Clara, CA, USA). FSMCs and NPMCs were directly activated, since it was previously found that these cells do not need to be sensitized, as they already have IgE antibodies bound to the FcεRI receptor[37]. pbEos (1 x 105 cells/well) were primed with 50 ng/ml GM-CSF then activated with 10−6M PAF. Non-IgE-mediated activation was performed with 10 μg/ml SEB (Sigma-Aldrich). Cells were activated for different time points as indicated at 37°C. Supernatants were collected after centrifugation 250 g, 5 min, 4°C and immediately used for mediators’ release assessment.
MC mediator release assessment.
Tryptase release levels in MCs supernatants were evaluated by a chromogenic assay, as described in prior studies [38]. For release, CBMC supernatants were incubated with 25mM N-p-tosyl-gly-pro-lys-p-nitroanilide (Sigma) at 37°C until colour development. Absorbance at 410 nm was read and followed every 5-10 min. Percentage of release was calculated as following: % release = (O.D. supernatants/O.D. supernatants + O.D. lysates) x 100.
RvD1 levels were detected via a specific RvD1 ELISA kit (detection range: 3.3-2000 pg/ml) (Cayman Chemical, Ann Arbor, MI, USA) as per manufacturer’s instructions. Samples were diluted before the assay and the values obtained were multiplied by the dilution factor.
Eosinophils peroxidase release assay
Eosinophil peroxidase (EPX) levels in pbEos supernatants were detected via a chromogenic assay as previously described with slight modifications [38]. Briefly, pbEos were seeded and activated on a 96-U-shaped wells plate previously coated with 2.5% BSA, to prevent binding of EPX to the plate itself. On another BSA-coated plate, the supernatants were transferred and the EPX standard curve was prepared (range 7.8-32000 pg/ml). Supernatants and standards were incubated with the OPD substrate solution (Cat.# 34006, ThermoFisher Scientific, Waltham, MA, USA), prepared according to the manufacturer’s instructions, for 5-15 min until color development. The reaction was blocked by addition of H2SO4 2M and absorbance was read at 495 nm.
Mice
Wild type (WT) C57BL/6 mice were obtained from Harlan Laboratories (Rehovot, Israel) and maintained in-house. In all experiments, gender and age-matched mice were used and housed under specific pathogen-free conditions.
RT-PCR
Total RNA was extracted from cell pellets via Quick-RNA Miniprep kit (ZymoResearch, CA, USA) according to manufacturer’s instructions. RNA concentration was assessed via Nanodrop ND-1000 (ThermoFisher Scientific). cDNA was prepared from total RNA via qScript cDNA Synthesis Kit (Quantabio, MA, USA) following manufacturer’s protocol. RT-PCR was performed on cDNA with specific primers for human 15-LO (Fw: 5’- CAGCCTAGGCAACGTGGTGAAACC-3’; Rv: 5’-CCTCCTGGGTCGTCTCTGTCCTCA-3’), human 5-LO (Fw: 5’- GGCAGCAGGGCCATCTTCAT-3’; Rv: 5’-GTTGAAGCCGGTCGACAAGG-3’), murine 15-LO (Fw: 5’-AAAGAGGACGCCTGGTTCTG-3’; Rv: 5’-TGTCCTCTCGAAATCGCTGG-3’), murine 5-LO (Fw: 5’-GCGAGTGACAGGGTCAAGAA-3’; Fw: 5’-CCAGCGGTAACATGGGAACT-3’), Annexin A1 (Fw: 5’- AGAAGGTAGAGATAAAGACACT-3’; Rv: 5’- AGCTAAAACAACCTCCTCAA-3’), (Sigma-Aldrich, Rehovot, Israel). Gel pictures were acquired via BioRad Chemidoc XRS (BioRad, CA, USA) and analysed via Image Lab software (BioRad).
Flow cytometry staining.
Cells (1-3 x 105) were washed with FC (flow cytometry) buffer (PBS + 0.1% BSA) and resuspended in Blocking Buffer (FC buffer + 2.5% goat serum). For intracellular staining, samples were washed in PBS and resuspended in fixation buffer (PBS + 4% PFA) and incubated at 37°C for 10 min in the dark. Cells were washed with PBS and resuspended in 90% methanol for permeabilization for 30 min on ice. For 15-LO expression, CBMCs were incubated with mouse anti-human APC 15-LO (Cat.# bs-6505R, Bioss Antibodies Inc., Woburn, Massachussets, USA). CBMCs and pbEos were incubated with either FITC mouse anti-human FcεRIα (Cat.# 334607, BioLegend, San Diego, CA, USA) or PE mouse anti-human CCR3 (Cat.# FAB155P, R&D biosystems, Minneapolis, MN, USA). All antibodies were incubated at 4°C for 30 min and matching isotype controls were used for each antibody.
For in vivo experiments, MCs were stained with APC rat anti mouse CD117 (cKit) and PE rat anti mouse FcεRIα (Cat.# 105812 and 134307 respectively, Biolegend), Eos were stained with PE rat anti-mouse Siglec-F (Cat.# 562068, BD biosciences, San Jose, CA, USA) and APC-anti mouse CCR3 (Cat.# 144511, Biolegend). Each antibody was matched by its isotype control. Cells were acquired with BD LSR II (BD Bioscience) (20,000 events/second) and analyzed with FlowJo software. Cells were gated according to physical parameters and to the specific staining used.
Allergic peritonitis model: OVA/SEB “overshoot” protocol
C57BL (7–9-wk-old) WT mice were subcutaneously sensitized with 100 μg OVA (Sigma-Aldrich) and 1 μg SEB (Sigma-Aldrich) in 200 μl of PBS on day-14 and day -7. On day 0, mice were challenged intraperitoneally with 10 μg OVA and 0.1 μg SEB in 200 μl of PBS. Two groups of OVA-challenged mice received 2 x 106 BMMCs 3 days after challenge. Mice were euthanized 2 and 6 days after challenge. For the in vivo MC activation assessment, mice were euthanized 1h after BMMC injection. After euthanasia, the peritoneal cavity was washed with 3 ml of cold PBS + 3% FCS, and total cell numbers were counted by Trypan blue exclusion. Lavages were centrifuged (5 min, 4°C, 300xg), and supernatants were collected and stored (−80°C) for assessment of tryptase/cytokines/RvD1 release. Cells were resuspended in FC buffer (PBS with 2% FCS, 0.5% BSA) for FC staining.
Statistical analysis
Data are expressed as mean ± SEM. Statistical comparisons between experimental groups were performed using one-way/two-way ANOVA and post-hoc Tukey/Bonferroni multiple comparison test. For less than three experimental groups, Student’s two-tailed t-test was employed. Data were analyzed with Microsoft Excel (Microsoft, Washington, USA). A two-tailed ‘p’ value of less than 0.05 was considered statistically significant for all analyses.
Study approval
Cord blood and nasal polyps was obtained according to the Institutional Helsinki Committee guidelines of Hadassah Hospital, and its use was approved by the committee. All blood samples for Eos purification were collected following ethical approval of the Hadassah-Hebrew University Human Experimentation Helsinki Committee. Written informed consent was obtained according to its guidelines.
All mouse experiments were approved by the Animal Experimentation Ethics Committee of the Hebrew University of Jerusalem and performed in accordance with the guidelines of the committee.
RESULTS
Human MCs produce RvD1.
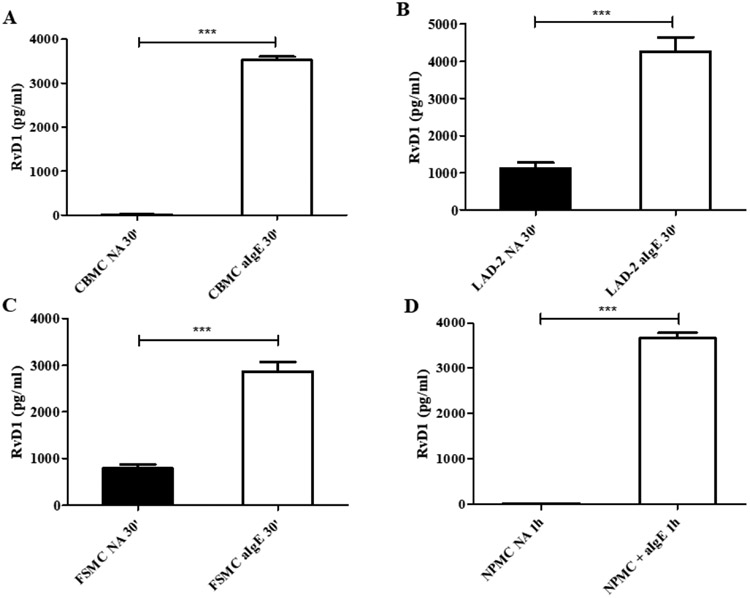
In order to investigate whether the resolution machinery in human MCs is induced by cell activation, we determined RvD1 production and release in IgE-activated CBMCs, laboratory of allergic diseases 2 (LAD-2) MCs, foreskin-derived MCs (FSMCs) and nasal polyp-derived MCs (NPMCs). The results provide first evidence that human MCs can be effectors of resolution in AI, since DHA-preincubated CBMCs (Figure 1A), LAD-2 (Figure 1B) and FSMCs (Figure 1C) were capable to produce and release high amounts of RvD1 at 30 min after IgE-mediated activation. Importantly, MCs from nasal polyps (NPMCs) of allergic patients also released high concentrations of RvD1 1h after activation (Figure 1D). Cell activation in every experiment was assessed by determining the release of tryptase as indicator of MC activation (Figure S1). To examine whether MCs produce and release RvD1 specifically after IgE-mediated activation, we employed the IgE-independent activator SEB. SEB-mediated activation did not trigger RvD1 release from any of the studied MC types (Figure S2A-D). Of note, RvD1 levels remained high up to 72h after IgE-mediated activation (Figure S3). It is noteworthy that RvD1 levels did not change over time, implying that this SPM is either continuously synthesized or it remains stable under the investigated culture conditions.
Figure 1.
Human mast cells release resolvin D1. Resolvin D1 (RvD1) production from preincubated with 20 μM docosahexaenoic acid cord blood-derived mast cells (CBMCs) (A), laboratory of allergic diseases 2 (LAD-2) cells (B), foreskin-derived mast cells (FSMCs) (C) and nasal polyp-derived mast cells (NPMCs) (D) was determined 30 min (30’) or 1h after immunoglobulin E-mediated activation (aIgE) and in non-activated (NA) cells (***p<0.01). Data are expressed as mean ± SEM of three independent experiments.
Expression of 15-LO is not altered upon MC activation, while expression of 5-LO is upregulated after activation.
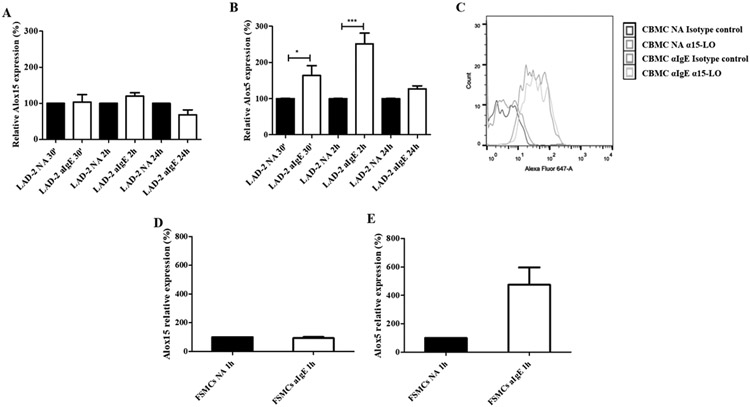
We analyzed the expression of 15-LO in activated and non-activated MCs. Our results showed that 15-LO expression did not change significantly in IgE-activated CBMCs and LAD-2 cells. The levels of the arachidonate 15-lipoxygenase gene (Alox15) mRNA, encoding human 15-LO, remained constant for up to 24 h in both activated and resting LAD-2 cells (Figure 2A). On the contrary, in these cells, the levels of Alox5 mRNA significantly increased 2 h after activation and returned to basal values within 24 h (Figure 2B). Interestingly, 15-LO levels did not change significantly either in CBMCs 30 min after IgE-mediated activation (Figure 2C) or in FSMCs, as shown by the Alox15 mRNA levels (Figure 2D). SEB did not elicit 15-LO expression in FSMCs 1h after activation (Figure S4A). In FSMCs, in contrast to 15-LO, 5-LO expression was significantly increased 1 h after IgE-mediated activation (Figure 2E), but only slightly increased after SEB-mediated activation (Figure S4B), indicating that FSMCs were activated by SEB. This suggests that, independently of their phenotype, human MCs have an innate pro-resolving capacity, specifically after the allergic type of activation.
Figure 2. 15-lipoxygenase expression in mast cells is not modulated after cell activation.
RT-PCR analysis of Alox15 (A) and Alox5 (B) expression in immunoglobulin E-activated (αIgE) and not-activated (NA) laboratory of allergic diseases 2 (LAD-2) cells, preincubated with 20 μM docosahexaenoic acid (DHA), 30 min, 1.5 and 24 h after activation. (*p<0.05, ***p<0.001). (C) Representative histogram of 15-lipoxygenase protein expression in cord blood-derived mast cells (CBMCs) preincubated with 20 μM docosahexaenoic acid (DHA) 30 min after IgE-mediated activation. RT-PCR analysis of Alox15 (D) and Alox5 (E) expression in IgE-activated and not-activated foreskin-derived mast cells (FSMCs), preincubated with 20 μM docosahexaenoic acid (DHA), 1h after activation. Data are expressed as mean ± SEM of three independent experiments.
MC-Eos co-culture modulates RvD1 production but not 15-LO expression.
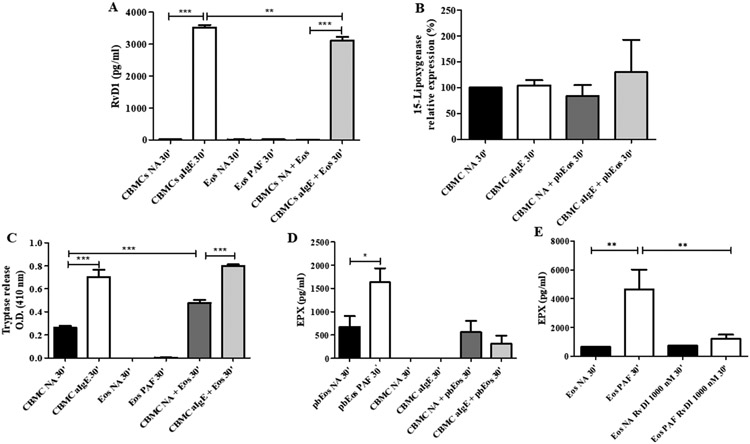
We next examined whether the AEU influences the pro-resolution potential of MCs. Therefore, we co-cultured CBMCs and peripheral blood Eos (pbEos) and analyzed 15-LO expression and RvD1 production 30 min after IgE-mediated activation. We found that RvD1 release was significantly decreased in the IgE-activated CBMCs/pbEos co-culture, while 15-LO expression in MCs was slightly but not significantly increased 30 min after co-culture of MCs with pbEos (Figure 3A-B). MC activation was confirmed by the significantly increased tryptase release in the CBMC-pbEos co-culture, which was further enhanced upon IgE-dependent MC activation (Figure 3C), in agreement to previously published data[4].
Figure 3. Mast cell-eosinophil co-culture modulates resolvin D1 production but not 15-lipoxygenase expression in mast cells.
(A) Resolvin D1 (RvD1) production (n=3), (B) Intracellular flow cytometry staining of 15-lipoxygenase, (C) tryptase release (n=3), and (D) eosinophil peroxidase (EPX) release from immunoglobulin E-activated (aIgE) cord blood-derived mast cells (CBMCs) and their respective non activated (NA) counterparts, alone and in co-culture with NA peripheral blood eosinophils (pbEos). (E) Eosinophil peroxidase (EPX) release from peripheral blood eosinophils (Eos) after 1h incubation with 1000 nM resolvin D1 (RvD1). Activation of pbEos was assessed following incubation in the presence of 10−6 M platelet activating factor (PAF). All measurements were performed 30 min after cell activation. Data are expressed as mean ± SEM of three independent experiments. (*p<0.05, **p<0.01; ***p<0.001).
To assess Eos activation, we analyzed the levels of eosinophil peroxidase (EPX) in the mono- and co-culture supernatants. We found that EPX release remained very low when Eos were co-cultured with IgE-activated or non-activated MCs (Figure 3D). Of note, EPX levels were significantly lower when pbEos were co-cultured with IgE-activated CBMCs for 24h (Figure S5). Notably, MCs and Eos viability remained stable up to 72h after initiation of the co-culture, similarly to what we have previously reported[3].
To test whether this effect was due to RvD1, we incubated pbEos with exogenous RvD1. Indeed, EPX release from Eos activated with platelet activating factor (PAF) was significantly reduced after treatment with RvD1 (Figure 3E).
Release of RvD1 from BMMC is increased by FcεRI cross-linking, while expression of 15-LO is not modulated by cell activation.
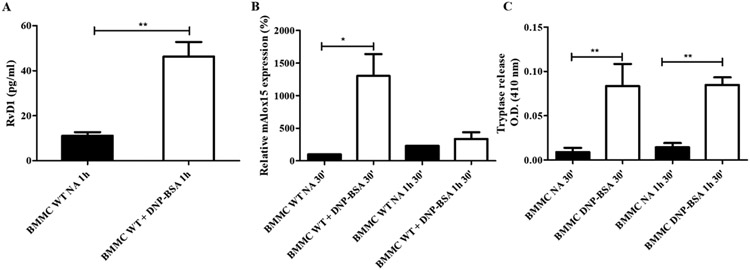
We aimed to investigate whether murine BMMC proresolution machinery is modulated by cell activation similarly to their human counterparts. We are the first to show that murine BMMCs of the mucosal phenotype release RvD1 immediately after IgE-mediated activation (Figure 4A). Expression of 15-LO was not significantly affected by IgE-mediated activation at any of the time points examined (Figure 4B). Cell activation was confirmed by analysis of mouse tryptase release (Figure 4C). Interestingly, IgE-activated BMMCs produced less RvD1 than human MCs. Collectively, these data indicate a comparable innate potential for the mucosal type and the connective tissue type MCs to resolve AI.
Figure 4. Release of resolvin D1 from bone marrow-derived mast cells is increased by FcεRI cross-linking, while expression of 15-lipoxygenase is not modulated by cell activation.
Resolvin D1 (RvD1) release (A), 15-lipoxygenase (Alox15) expression (B) and tryptase release (C) from IgE-activated (DNP-BSA) and not-activated (NA) bone marrow-derived mast cells 1h after activation (BMMCs) (*p<0.05; **p<0.01) Data are expressed as mean ± SEM of three independent experiments.
MC overshoot in an OVA/SEB AP murine model shows a trend of reduced inflammation and significant increase of RvD1.
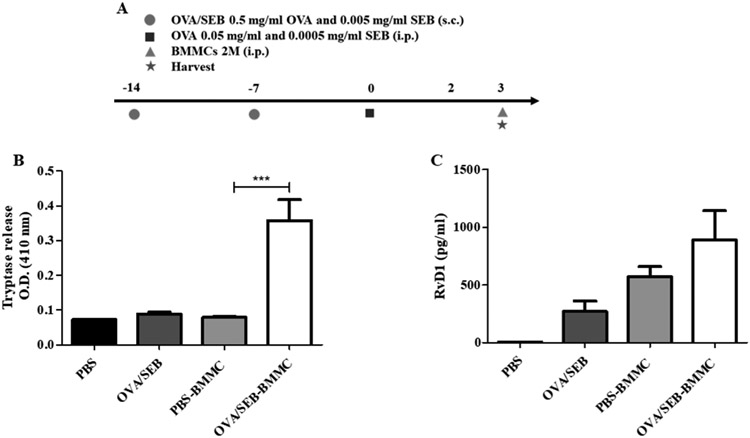
In order to dissect the role of MCs in the resolution of AI in vivo, we used a murine AP model employing OVA/SEB sensitization and challenge. This model that we have recently developed is MC-dependent and has similarities to the human pathology due to the presence of SEB as adjuvant[15]. We employed a strategy we termed overshoot, involving injection of higher-than-physiological MC numbers in the peritoneal cavity after the peak of inflammation. This way, we aimed to induce a pro-resolution phenotype in injected BMMCs due to the post-peak microenvironment in the peritoneal cavity. Our first question was whether the injected BMMCs would be activated by the microenvironment created in the peritoneal cavity immediately after the peak of inflammation at 48h, when the system is already initiating resolution. Therefore, we injected 2 million BMMCs in the peritoneal cavity of OVA/SEB AP mice 3 days after challenge (Figure 5A). We found that, 1 h after injection, OVA/SEB-BMMC-injected mice presented with increased tryptase peritoneal levels compared to the phosphate buffered saline (PBS)-challenged control mice (Figure 5B). This demonstrates that the injected BMMCs were activated by the peritoneal microenvironment. Remarkably, tryptase release was accompanied by a non-significant RvD1 release in OVA/SEB-BMMC-injected mice (Figure 5C). This might indicate that, after the peak of inflammation, MCs are activated by the peritoneal microenvironment to release RvD1. Next, we analyzed the course of inflammation in the overshoot OVA/SEB AP model (Figure 6A). OVA/SEB-BMMCs mice showed slightly but not significantly reduced numbers of total cells (Figure 6B) and Eos (Figure 6C). MC numbers did not significantly change throughout the course of AP but OVA/SEB-BMMCs mice retained high numbers of BMMCs in the peritoneal cavity (Figure 6D). In order to investigate further the pro-resolution features in this model, we evaluated RvD1 content in the peritoneal lavage. We found that RvD1 levels were significantly increased in OVA/SEB-challenged mice 2 days after challenge, and in PBS-challenged mice injected with BMMCs 6 days after challenge (Figure 6E). Interestingly, OVA/SEB-BMMCs mice displayed a strong and significant increase in RvD1 peritoneal levels 6 days after challenge in comparison to not-BMMC-injected mice (Figure 6E). Of note, macrophages (MΦ) purified from OVA/SEB-BMMC mice did not release high levels of RvD1 (Figure S6).
Figure 5. Bone marrow-derived mast cells release tryptase and resolvin D1 1h after intraperitoneal injection in ovalbumin/Staphylococcus aureus enterotoxin B-challenged mice.
(A) Schematic representation of the model. Tryptase (B) and resolvin D1 (RvD1) (C) levels in the peritoneal lavages of wild type (WT) mice challenged with either PBS, ovalbumin and Staphylococcus aureus enterotoxin B (SEB) (OVA/SEB) or OVA/SEB plus bone marrow-derived mast cells (OVA/SEB-BMMCs) 1h after injection of BMMCs and 3d after OVA/SEB challenge (n=4 mice/group, ***p<0.001). Data are expressed as mean ± SEM.
Figure 6. Mast cell overshoot mice show a trend of reduced inflammation in an ovalbumin/Staphylococcus aureus enterotoxin B-induced allergic peritonitis model.
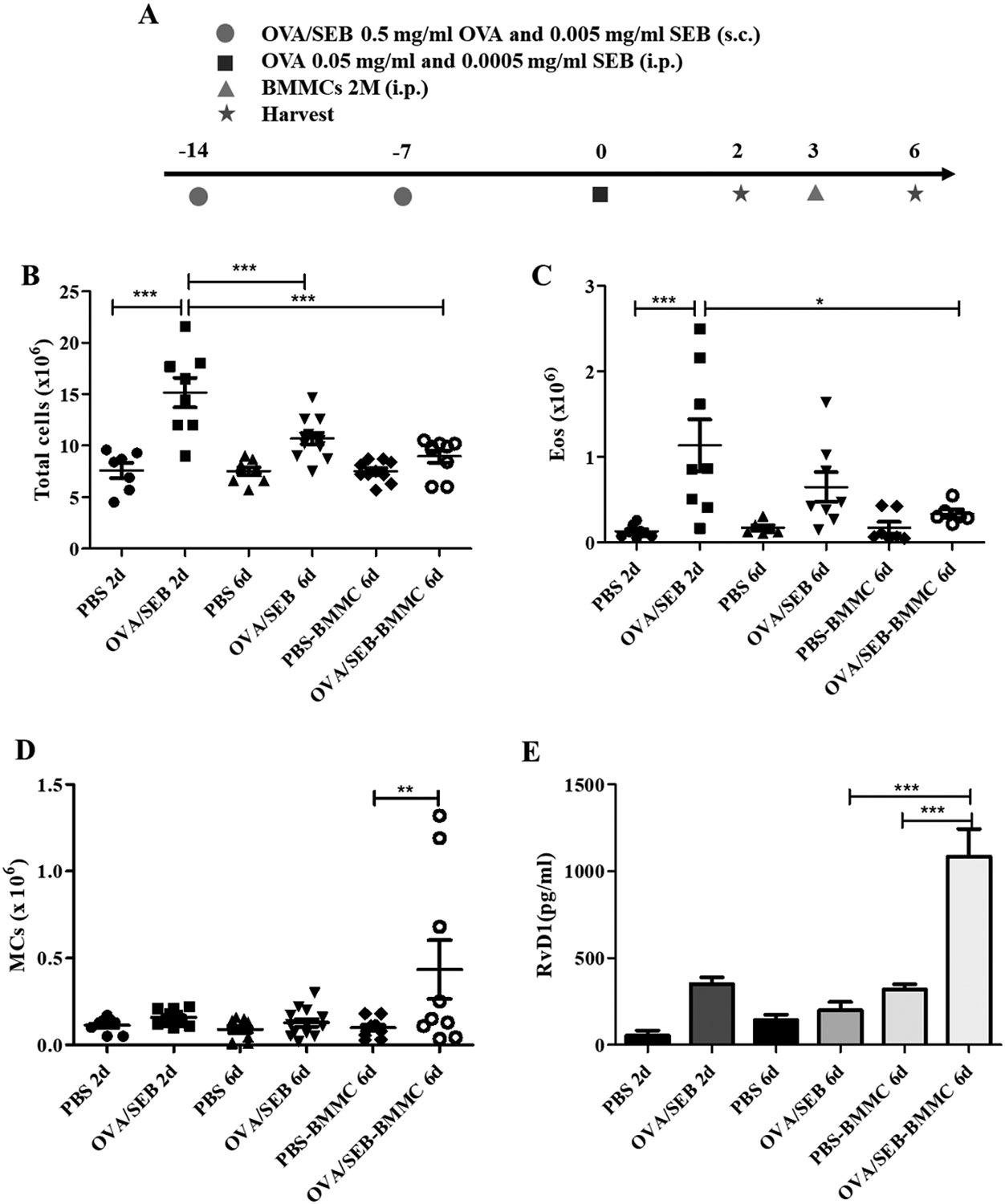
(A) Schematic representation of the model. Total cells (B), eosinophils (Eos) (C), mast cells (MCs) numbers (D) and resolvin D1 (RvD1) levels (E) in the peritoneal lavages of wild type (WT) mice challenged with either PBS, ovalbumin and Staphylococcus aureus enterotoxin B (SEB) (OVA/SEB) or OVA/SEB plus bone marrow-derived mast cells (OVA/SEB-BMMCs)). Data are expressed as mean ± SEM of three independent experiments (3-6 mice/group/experiment, **p<0.01, ***p<0.001).
DISCUSSION
The role of MCs as initiators of AI has been extensively described over the years. However, despite the evidence of MCs expressing 15-LO that is involved in resolvin biosynthesis, the possibility of MCs participating in the resolution of AI has never been investigated before. So far, in this regard, it is known that human MCs can release IL-10 after IgE-mediated activation[16,17]. Our aim was to assess whether MCs have the potential to intervene in the resolution of AI by analyzing the production of the SPM RvD1 in several in vitro and in vivo AI systems. Our findings show for the first time that human MCs can produce RvD1 after IgE-mediated activation. So far, the production of RvD1 has been reported in human polymorphonuclear leukocytes[18] and macrophages[19], and its role has been described in mouse allergic airway inflammation models[20]. Notably, in this paper we demonstrate that IgE-mediated activation dramatically modulates RvD1 release from different MC types, including CBMCs, LAD-2, FSMCs and NPMCs, in the short time frame investigated. Interestingly, this effect is possibly specific for IgE-mediated activation, since the non-IgE-dependent activation induced by SEB failed to elicit RvD1 production and release comparable to that observed in the allergic-type activation. This may be due to the different signal transduction pathways triggered in MCs by SEB, which engages, for instance, toll-like receptor 2 and cluster of differentiation (CD) 48[21]. Preliminary results showed that CBMCs activated with either papain (Puzzovio PG, Shamri R, Levi-Schaffer F, unpublished results) or compound 48/80 (Puzzovio PG, Levi-Schaffer F, unpublished data) released very low amounts of RvD1.
The enzyme responsible for the initiation of RvD1 synthesis from the essential fatty acid DHA is 15-LO[22]. Previous reports have shown that 15-LO is bound to the cytosolic membrane and its translocation and activation are calcium-dependent[23]. Moreover, expression of 15-LO in human CBMCs was found to increase 120 h after IL-4 treatment at the mRNA and protein level [11]. In this report we are the first to show that 15-LO expression does not change significantly after IgE-mediated activation, neither in LAD-2 nor in CBMCs or FSMCs. This is in agreement with the literature, since expression of 15-LO was not found to increase shortly after activation of human MCs[11]. Therefore, it can be suggested that, soon after IgE-mediated activation, 15-LO activity is modulated but its expression remains unaltered. This may explain the high production of RvD1 in activated MCs despite 15-LO unchanged expression.
Our next question was whether MC pro-resolution potential could be affected by the cross-talk with Eos in the AEU. We have previously described that MCs and Eos in co-culture display enhanced activation features, such as release and expression of pro-inflammatory mediators and cytokines, chemotactic properties[3,4] and increased survival[3]. This work is the first to report that, when incubated with Eos, human CBMCs do not show significant increase in 15-LO expression, regardless of their activation. However, upon IgE-mediated activation, MCs release significantly less RvD1 compared to the activated CBMCs that are not in co-culture with Eos. We hypothesize that, since the AEU is mainly pro-inflammatory in the time frame examined, MC pro-resolution potential is dampened by the cross-talk with Eos and that RvD1 production is reduced in favor of pro-phlogistic mediators. However, Eos co-cultured with either IgE-activated or non-activated MCs showed reduced EPX release in comparison to PAF-activated Eos. This indicates that MCs are able to reduce Eos activation in the AEU, possibly due to the released RvD1. Indeed, we found that exogenous RvD1 can significantly reduce EPX release in vitro. This is likely due to RvD1 engagement of its receptor, ALX/FPR2, present on the surface of human Eos[24].
Human Eos have been shown to express 15-LO[25] and to produce RvE3 from the precursor 18-hydroxyeicosapentaenoic acid via the 15-LO pathway[26]. Additionally, human Eos were found to be major producers of the pro-resolving mediator protectin D1, and that in severe asthmatic patients its synthesis is dysregulated, contributing to the symptoms of severe asthma[27]. Our findings point out that human Eos produce only traces of RvD1, even after PAF-mediated activation. Altogether, this might indicate the possibility that MCs, by releasing RvD1 already shortly after IgE-mediated activation, control the intensity of the initial response in an autocrine fashion and by reducing Eos activation. This, in turn, may help the subsequent steps of resolution, in which natural killer (NK) cells kill Eos and macrophages eliminate the dead cells. Indeed, previously published data argue for the recruitment of NK cells in AI sites and for their intervention in Eos clearance, NK cell cytotoxic activity being increased by RvE1[28]. Moreover, the involvement of NK cells in resolution was found to be disrupted in severe asthma[29]. In MΦ, previous reports showed that RvD1 induces their switch towards the M2 phenotype, resulting in increased anti-inflammatory and efferocytotic properties[30]. Therefore, there are several possible roles for MC produced RvD1 to regulate the functions of the effector cells in allergic responses.
The findings of in vitro human studies on the pro-resolving character of MCs were supported by the evidence obtained from mouse and in vivo studies. Interestingly, activated BMMCs behaved similarly to human MCs, releasing RvD1 and not modulating 15-LO after IgE-mediated activation. Of note, BMMCs produce lower amounts of RvD1 than IgE-activated human MCs. This is the first time that mouse MCs are shown to produce RvD1 after allergic type activation, since until recently it has only been reported how MCs react to exogenous SPMs[31].
In order to dissect the contribution of MCs towards resolution of AI in vivo, we employed the overshoot strategy in an OVA/SEB-induced AP model we recently developed in our laboratory[15]. This model is MC-dependent, Th2-skewed and closer to the human pathology due to the presence of SEB and not involving alum[15]. Indeed, alum was reported to activate the immune system in vivo by inducing a Th2 response independently of MC activation[32]. Our data show that, after injection in the peritoneal cavity, mouse MCs release both pro-inflammatory (tryptase) and pro-resolution (RvD1) mediators 1h after OVA/SEB challenge. Activation of BMMCs in the peritoneal cavity was possibly due to the persistence of IgE antibodies against OVA and SEB in the peritoneal inflammatory milieu. In addition, we observed a trend of reduced inflammation at the resolution time points (6 days after challenge) in both total cells and Eos numbers. Interestingly, MCs were retained in high numbers in the peritoneal cavity of OVA/SEB-BMMCs mice at resolution time points. Importantly, we report for the first time that RvD1 in the peritoneal cavity showed a time-dependent release. We previously published similar kinetics for lipoxin A4 (LXA4) release, which was found to be dysregulated in human severe asthma[33], in the peritoneum of OVA/alum-challenged BALB/c mice, with LXA4 concentrations peaking 72h after challenge and decreasing thereafter[9]. Noteworthy, as shown in Figure 6E, OVA/SEB-BMMCs mice showed a significant up-modulation of RvD1 release in comparison to mice that did not receive BMMCs. We inferred that the increase in RvD1 peritoneal levels is possibly due to the injected BMMCs releasing the SPM and not to MΦ, due to the low RvD1 amounts that peritoneal MΦ released. This might also point out that the ability of MΦ to produce and release RvD1 is not affected by MCs.
Collectively, the in vivo data indicate that mouse MCs, similarly to their human counterparts, have the potential to participate in the resolution of AI in vivo by releasing RvD1 already after their activation. Importantly, this release is up-regulated in an inflammatory microenvironment, in which injected MCs are able to persist.
In conclusion, by employing several in vitro and in vivo approaches we demonstrated that MCs have the potential to produce RvD1, thus participating in the resolution of AI. This property can be influenced by modifications in the inflammatory microenvironment during the course of AI. The orchestration of both pro-inflammatory and pro-resolving signals by MCs is a novel concept that deserves careful consideration as it could lead to a better understanding of MC homeostatic potential and to new therapeutic strategies in allergic reactions.
Supplementary Material
Highlights.
Human and mouse mast cells release resolvin D1 shortly after IgE-mediated activation.
Resolvin D1 production from mast cells is reduced in the Allergic Effector Unit.
In vivo, mast cell overshoot results in a trend of reduced inflammation.
ACKNOWLEDGEMENTS
The Authors would like to thank Ms. Alexandra Eliassaf (The Core Research Facility, Faculty of Medicine, The Hebrew University of Jerusalem) for advice on the FC analysis and technical help and dr. Revital Shamri for her assistance in RvD1-related experiments. E.T. is a Lady Davis Visiting Professor at the Hebrew University of Jerusalem, Israel.
FUNDING SOURCES
This study was funded by grants from the United States-Israel Binational Science foundation BSF 2015045 (FLS, BDL), Rosetrees Trust UK (F.L.S.) and U.S. National Institutes of Health grants R01HL122531 and P01GM095467 (B.D.L.). F.L.S. is affiliated with the Adolph and Klara Brettler Center for Molecular Pharmacology and Therapeutics at the School of Pharmacy of The Hebrew University of Jerusalem.
ABBREVIATIONS
- 5-LO
5-Lipoxygenase
- 15-LO
15-Lipoxygenase
- AD
atopic dermatitis
- AEU
Allergic Effector Unit
- AI
allergic inflammation
- AP
allergic peritonitis
- BMMCs
Bone Marrow-derived Mast Cells
- CBMCs
Cord Blood-derived Mast Cells
- Eos
Eosinophils
- FC
flow cytometry
- FSMCs
Foreskin-derived Mast Cells
- i.p.
intraperitoneal
- LTC4
leukotriene C4
- LXA4
Lipoxin A4
- MCs
Mast Cells
- o.n.
(overnight)
- PAF
platelet activating factor
- pb
peripheral blood
- RvD1
Resolvin D1
- s.c.
subcutaneous
- SCF
Stem Cell Factor
- SEB
Staphylococcus aureus enterotoxin B
- SPMs
specialized pro-resolving lipid mediators
- WT
Wild Type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS STATEMENT
The authors have declared that no conflict of interest exists.
CREDIT AUTHOR STATEMENT
Pier Giorgio Puzzovio: Conceptualization, Investigation, Methodology, Validation, Formal Analysis, Writing-Original Draft, Visualization. Hadas Pahima: Investigation, Methodology. Tresa George: Investigation. David Mankuta: Resources. Ron Eliashar: Resources. Ekaterini Tiligada: Conceptualization, Writing- Review & Editing. Bruce D. Levy: Conceptualization, Writing-Review & Editing, Supervision, Funding Acquisition. Francesca Levi-Schaffer: Conceptualization, Resources, Writing-Review & Editing, Supervision, Project Administration, Funding Acquisition.
REFERENCES
- [1].Robida PA, Puzzovio PG, Pahima H, Levi-Schaffer F, Bochner BS, Human eosinophils and mast cells: Birds of a feather flock together, Immunol. Rev 282 (2018) 151–167. 10.1111/imr.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Galli SJ, Tsai M, Piliponsky AM, The development of allergic inflammation., Nature. 454 (2008) 445–54. 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, Mankuta D, Eliashar R, Zabucchi G, Levi-Schaffer F, Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro., Allergy. 66 (2011) 376–85. 10.1111/j.1398-9995.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- [4].Elishmereni M, Bachelet I, Nissim Ben-Efraim AH, Mankuta D, Levi-Schaffer F, Interacting mast cells and eosinophils acquire an enhanced activation state in vitro., Allergy. 68 (2013) 171–9. 10.1111/all.12059. [DOI] [PubMed] [Google Scholar]
- [5].Fullerton JN, Gilroy DW, Resolution of inflammation: a new therapeutic frontier, Nat. Rev. Drug Discov 15 (2016) 551–567. 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- [6].Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM, Resolution o Inflammation: What Controls Its Onset?, Front. Immunol 7 (2016). 10.3389/fimmu.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Serhan CN, Levy BD, Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators, J. Clin. Invest 128 (2018) 2657–2669. 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Serhan CN, Chiang N, Dalli J, Levy BD, Lipid mediators in the resolution of inflammation., Cold Spring Harb. Perspect. Biol 7 (2014) a016311. 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karra L, Singh Gangwar R, Shamri R, Puzzovio PG, Cohen-Mor S, Levy BD, Levi-Schaffer F, Leukocyte CD300a Contributes to the Resolution of Murine Allergic Inflammation., J. Immunol. (2018). 10.4049/jimmunol.1801000. [DOI] [PubMed] [Google Scholar]
- [10].Miyata J, Fukunaga K, Kawashima Y, Ohara O, Kawana A, Asano K, Arita M, Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases, Prostaglandins Other Lipid Mediat. 150 (2020) 106477. 10.1016/j.prostaglandins.2020.106477. [DOI] [PubMed] [Google Scholar]
- [11].Gulliksson M, Brunnström A, Johannesson M, Backman L, Nilsson G, Harvima I, Dahlén B, Kumlin M, Claesson H-E, Expression of 15-lipoxygenase type-1 in human mast cells., Biochim. Biophys. Acta 1771 (2007) 1156–65. 10.1016/j.bbalip.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [12].Levi-Schaffer F, Gibbs BF, Hallgren J, Pucillo C, Redegeld F, Siebenhaar F, Vitte J, Mezouar S, Michel M, Puzzovio PG, Maurer M, Selected recent advances in understanding the role of human mast cells in health and disease, J. Allergy Clin. Immunol (2022). 10.1016/j.jaci.2022.01.030. [DOI] [PubMed] [Google Scholar]
- [13].Dwyer DF, Barrett NA, Austen KF, Immunological Genome Project Consortium, Expression profiling of constitutive mast cells reveals a unique identity within the immune system., Nat. Immunol 17 (2016) 878–87. 10.1038/ni.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Frossi B, Mion F, Sibilano R, Danelli L, Pucillo CEM, Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity?, Immunol. Rev 282 (2018) 35–46. 10.1111/imr.12636. [DOI] [PubMed] [Google Scholar]
- [15].Pahima H, Puzzovio PG, Levi-Schaffer F, A novel mast cell-dependent allergic peritonitis model, Clin. Exp. Immunol (2021) cei.13619. 10.1111/cei.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Royer B, Varadaradjalou S, Saas P, Gabiot A-C, Kantelip B, Féger F, Guillosson J-J, Kantelip J-P, Arock M, Autocrine regulation of cord blood–derived human mast cell activation by IL-10☆, J. Allergy Clin. Immunol 108 (2001) 80–86. 10.1067/mai.2001.115753. [DOI] [PubMed] [Google Scholar]
- [17].Puzzovio PG, Brüggemann TR, Pahima H, Mankuta D, Levy BD, Levi-Schaffer F, Cromolyn Sodium differentially regulates human mast cell and mouse leukocyte responses to control allergic inflammation, Pharmacol. Res 178 (2022) 106172. 10.1016/j.phrs.2022.106172. [DOI] [PubMed] [Google Scholar]
- [18].Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L, Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals., J. Exp. Med 196 (2002) 1025–37. 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dalli J, Serhan CN, Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators., Blood. 120 (2012) e60–72. 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD, Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses., J. Immunol 189 (2012) 1983–91. 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rocha-de-Souza CM, Berent-Maoz B, Mankuta D, Moses AE, Levi-Schaffer F, Human Mast Cell Activation by Staphylococcus aureus: Interleukin-8 and Tumor Necrosis Factor Alpha Release and the Role of Toll-Like Receptor 2 and CD48 Molecules, Infect. Immun 76 (2008) 4489–4497. 10.1128/IAI.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN, Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation., J. Biol. Chem 282 (2007) 9323–34. 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- [23].Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kühn H, Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme., Blood. 91 (1998) 64–74. http://www.ncbi.nlm.nih.gov/pubmed/9414270. [PubMed] [Google Scholar]
- [24].Planagumá A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD, Airway Lipoxin A 4 Generation and Lipoxin A 4 Receptor Expression Are Decreased in Severe Asthma, Am. J. Respir. Crit. Care Med 178 (2008) 574–582. 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nadel JA, Conrad DJ, Ueki IF, Schuster A, Sigal E, Immunocytochemical localization of arachidonate 15-lipoxygenase in erythrocytes, leukocytes, and airway cells., J. Clin. Invest 87 (1991) 1139–45. 10.1172/JCI115110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Isobe Y, Kato T, Arita M, Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation., Front. Immunol 3 (2012) 270. 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K, Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma, J. Allergy Clin. Immunol 131 (2013) 353–360.e2. 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- [28].Haworth O, Cernadas M, Levy BD, NK Cells Are Effectors for Resolvin E1 in the Timely Resolution of Allergic Airway Inflammation, J. Immunol 186 (2011) 6129–6135. 10.4049/jimmunol.1004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duvall MG, Barnig C, Cernadas M, Ricklefs I, Krishnamoorthy N, Grossman NL, Bhakta NR, V Fahy J, Bleecker ER, Castro M, Erzurum SC, Gaston BM, Jarjour NN, Mauger DT, Wenzel SE, Comhair SA, Coverstone AM, Fajt ML, Hastie AT, Johansson MW, Peters MC, Phillips BR, Israel E, Levy BD, and B.I.S.A.R.P.-3 I. National Heart, Lung, Natural killer cell-mediated inflammation resolution is disabled in severe asthma., Sci. Immunol 2 (2017). 10.1126/sciimmunol.aam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schmid M, Gemperle C, Rimann N, Hersberger M, Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32, J. Immunol 196 (2016) 3429–3437. 10.4049/jimmunol.1501701. [DOI] [PubMed] [Google Scholar]
- [31].Hagemann PM, Nsiah-Dosu S, Hundt JE, Hartmann K, Orinska Z, Modulation of Mast Cell Reactivity by Lipids: The Neglected Side of Allergic Diseases, Front. Immunol 10 (2019). 10.3389/fimmu.2019.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miki H, Nakahashi-Oda C, Sumida T, Shibuya A, Involvement of CD300a Phosphatidylserine Immunoreceptor in Aluminum Salt Adjuvant-Induced Th2 Responses., J. Immunol 194 (2015) 5069–76. 10.4049/jimmunol.1402915. [DOI] [PubMed] [Google Scholar]
- [33].Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, Bleecker ER, Castro M, Erzurum SC, Fahy JV, Gaston BM, Denlinger LC, Mauger DT, Wenzel SE, Comhair SA, Coverstone AM, Fajt ML, Hastie AT, Johansson MW, Peters MC, Phillips BR, Levy BD, ALX receptor ligands define a biochemical endotype for severe asthma, JCI Insight. 2 (2017). 10.1172/jci.insight.93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rådinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM, Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood., Curr. Protoc. Immunol. Chapter 7 (2010) Unit 7.37. 10.1002/0471142735.im0737s90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Benyon RC, Lowman MA, Church MK, Human skin mast cells: their dispersion, purification, and secretory characterization., J. Immunol. 138 (1987) 861–7. http://www.ncbi.nlm.nih.gov/pubmed/2433332. [PubMed] [Google Scholar]
- [36].Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F, The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells., J. Immunol 175 (2005) 7989–95. http://www.ncbi.nlm.nih.gov/pubmed/16339535. [DOI] [PubMed] [Google Scholar]
- [37].Babina M, Guhl S, Stärke A, Kirchhof L, Zuberbier T, Henz BM, Comparative cytokine profile of human skin mast cells from two compartments-strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming, J. Leukoc. Biol 75 (2004) 244–252. 10.1189/jlb.0403157. [DOI] [PubMed] [Google Scholar]
- [38].Gangwar RS, Pahima H, Puzzovio PG, Levi-Schaffer F, Update on Eosinophil Interaction with Mast Cells: The Allergic Effector Unit, in: 2021: pp. 221–242. 10.1007/978-l-0716-1095-4_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.