Take Home Message
We found good evidence to suggest that prostate-specific membrane antigen targeting helps identify prostate cancer during surgery. The oncological benefits have yet to be investigated further.
Keywords: Fluorescence-guided surgery, Image-guided surgery, Prostate cancer, Prostate-specific membrane antigen, Radioguided surgery
Abstract
Context
Identifying malignant tissue and leaving adjacent structures undisturbed constitute an ongoing challenge in prostate cancer (PCa) surgery. Image and radioguided surgical technologies targeting the prostate-specific membrane antigen (PSMA) receptor may facilitate identification and removal of diseased tissue.
Objective
To perform a systematic review of the clinical studies on PSMA-targeted surgery.
Evidence acquisition
The MEDLINE (OvidSP), Embase.com, and Cochrane Library databases were searched. Identified reports were critically appraised according to the Idea, Development, Exploration, Assessment, Long-term framework criteria. The risk of bias (RoB) was assessed as per the Risk Of Bias In Non-randomized Studies—of Interventions tool. The strengths and limitations of the techniques and corresponding oncological outcomes were extracted as areas of interest. Data were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Evidence synthesis
In total, 29 reports were selected, including eight prospective studies, 12 retrospective analyses, and nine case reports, all with a high or an unclear RoB. In 72.4% of studies, PSMA targeting was achieved via radioguided surgery (RGS), predominantly using 99mTc-PSMA-I&S (66.7%). Hybrid approaches that complement RGS with optical guidance are emerging. The majority of studies retrieved were pilot studies with a short follow-up. In 13 reports, salvage lymph node surgery was discussed (44.8%). In 12 more recent reports (41.4%), PSMA targeting was studied in primary PCa surgery (50.0% lymph nodes and 50.0% surgical margins), and four studied both primary and salvage surgery (13.8%). Overall, specificity was higher than sensitivity (median 98.9% and 84.8%, respectively). Oncological outcomes were discussed only in reports on the use of 99mTc-PSMA-I&S in salvage surgery (median follow-up of 17.2 mo). A decline in prostate-specific antigen level of >90% ranged from 22.0% to 100.0%, and biochemical recurrence ranged from 50.0% to 61.8% of patients.
Conclusions
In PSMA-targeted surgery, most studies address salvage PSMA-RGS using 99mTc-PSMA-I&S. Available evidence suggests that the specificity of intraoperative PSMA targeting is higher than the sensitivity. The studies that included follow-up did not yet objectify a clear oncological benefit. Lacking solid outcome data, PSMA-targeted surgery remains investigational.
Patient summary
In this paper, we review recent advances in prostate–specific membrane antigen (PSMA)-targeted surgery, which is used to help identify and remove prostate cancer. We found good evidence to suggest that PSMA targeting helps identify prostate cancer during surgery. The oncological benefits have yet to be investigated further.
1. Introduction
During both primary and salvage prostate cancer (PCa) surgery, identifying the target PCa tissue among the surrounding healthy tissue provides a key challenge [1]. Patients are significantly more likely to have biochemical recurrence (BCR) and undergo adjuvant or early salvage cancer treatment when tumor-containing tissue remains in situ following PCa surgery. In general, surgeons rely on experience, anatomical knowledge, and the ability to correctly interpret preoperative imaging to resect PCa tissue [2]. The use of intraoperative imaging and radioguidance helps better distinguish between cancerous and healthy tissue during surgery [3], [4].
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein that is highly overexpressed in PCa cells and is used as the target for positron emission tomography (PET) imaging [5]. Owing to its high specificity, it is more accurate for nodal staging than magnetic resonance imaging (MRI), abdominal contrast-enhanced computed tomography (CT), or choline PET/CT, making its use increasingly common in staging of primary and recurrent PCa [5], [6]. However, the technique is less reliable for identifying small lymph node metastases (micro metastases <3 mm), and the PSMA-PET tracers are typically excreted by the kidneys, making it difficult to locate the primary cancer site [5], [7].
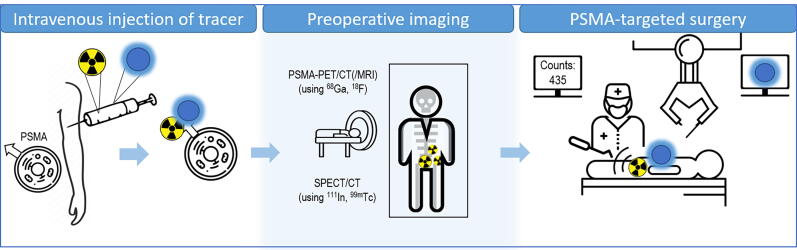
PSMA targeting has been proposed to extend beyond cancer diagnosis and into surgical guidance [7]. Multiple groups have explored a variety of tracer designs to realize this application, leading to the development of, for example, 99mTc-PSMA–targeted radiotracers [8], [9]—tracers that support noninvasive single photon emission computed tomography (SPECT)/CT, providing a surgical roadmap, as well as allow for intraoperative image guidance (Fig. 1) [5].
Fig. 1.
Schematic overview of clinical implementation of prostate-specific membrane antigen (PSMA)-guided surgery. Preoperative imaging can be either PSMA positron emission tomography (PET)/CT or single photon emission computed tomography (SPECT)/CT. CT = computed tomography; MRI = magnetic resonance imaging.
As of today, different PSMA-targeting surgical approaches have been used in PCa patients. In order to provide a comprehensive overview of the techniques and initial outcome data, a systematic review of the available clinical literature was conducted.
2. Evidence acquisition
2.1. Protocol registration and search strategy
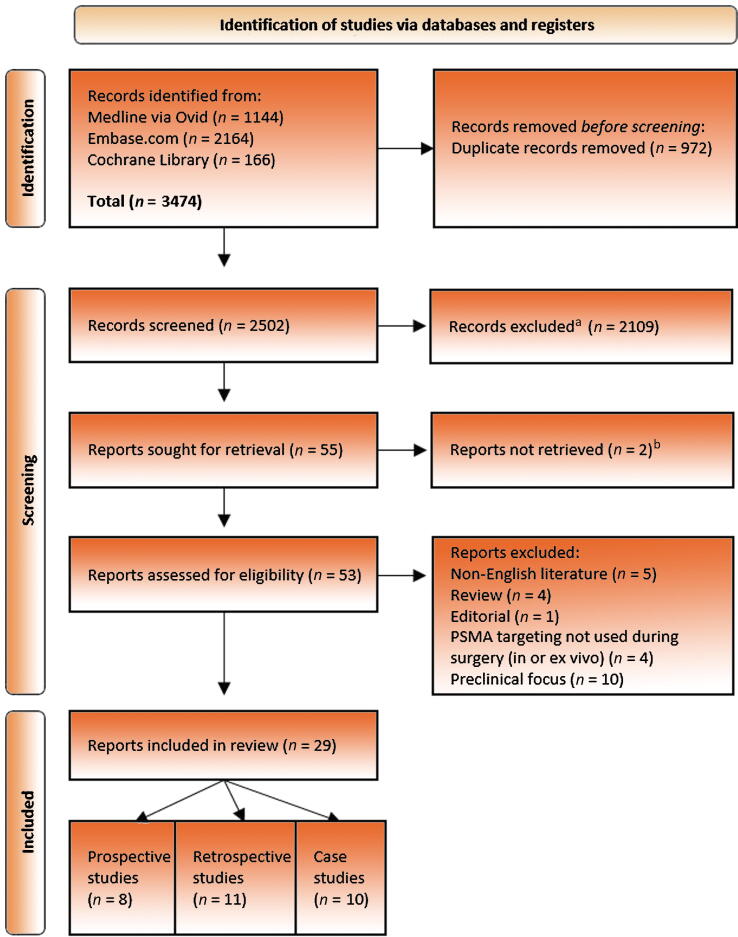
The protocol was registered on PROSPERO (CRD42022304195) in January 2022. A systematic web search was conducted using MEDLINE (OvidSP), Embase.com, and Cochrane Library (Fig. 2). The search was last updated in August 2022. The search was executed with the help of an expert information specialist (S.v.d.M.) and checked by a second information specialist. Search terms can be found in the Supplementary material. Conference abstracts from Embase.com were removed based on their indexed publication type. Citation chasing was done by one person. No other methods to acquire additional reports and no other limits were used. The results were deduplicated in EndNote 20 using the method of Bramer et al. [10]. After removal of duplicates, two authors (A.C.B. and S.K.) screened all abstracts and reviewed the full-text reports for eligibility using Rayyan software [11]. Discrepancies were resolved through consensus or by consultation with a third author (G.M.). Data collection focused on demographics and surgical and oncological outcomes, and was collected by two authors in a prespecified form in Excel (Microsoft Corporation, Redmond, WA, USA). The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [12].
Fig. 2.
PRISMA flowchart for literature search and selection. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses; PSMA = prostate-specific membrane antigen. aNonautomated. Records excluded by the author using Rayyan. bOnly abstract available.
2.2. Inclusion and exclusion criteria
As the first reports on the subject of PSMA targeting in surgery became available in 2015, the studies included in this review date from 2015 to 2022. Our review incorporated research that assessed the effectiveness of intraoperative PSMA-targeted surgical guidance in directing the surgeon toward the targeted tissue in vivo or that could confirm the target through an ex vivo analysis at the back table. Surgical procedures assisted by only preoperative PSMA PET for decision-making without intraoperative or back-table guidance were excluded. Only studies that were registered with a study protocol were considered prospective. Given the dynamic growth of PSMA-targeted surgery activities, case reports were included as well. Only original English-language literature full-text reports were considered. Pure preclinical work was excluded.
2.3. Assessment of risks of bias
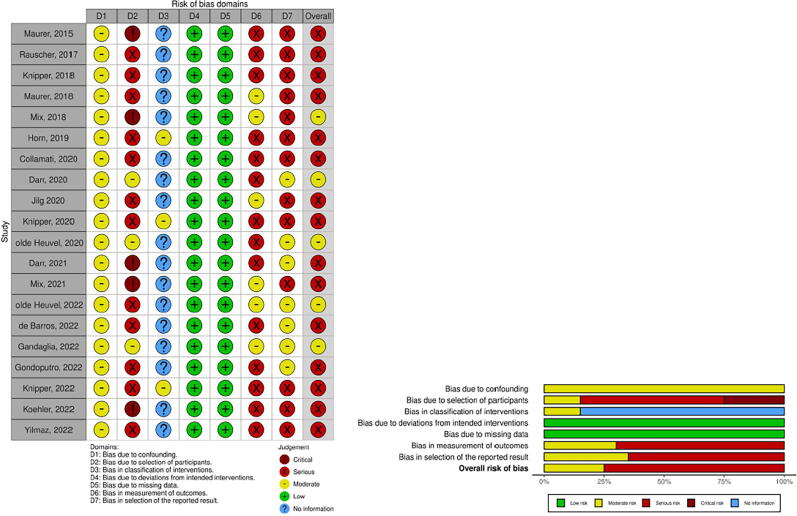
The studies were defined according to the Idea, Development, Exploration, Assessment, Long-term (IDEAL) framework (Table 1) [13]. The risk of bias was assessed through the Risk Of Bias In Non-randomized Studies—of Interventions(ROBINS-I) tool (Fig. 3) [14]. As the bias of case reports is not contributory, these were excluded from the assessment of the risk of bias.
Table 1.
IDEAL framework and surgical outcomes
| Focus on lymph nodes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Author (year) | IDEAL framework stage | Patients (patients treated with PSMA GS) | PSMA agent, administration, type of guidance | Type of surgery | Modality used | In or ex vivo | Total targets identified |
Sensitivity of surgical intervention | Specificity of surgical intervention | Metastasis size (mm) at histopathology | |||
| n (n) | PSMA PET/CT (/MRI) |
SPECT/CT | Intraoperatively/ex vivo | Tumor + at histopathology/total removed | % (95% CI) | % (95% CI) | Median (IQR) | |||||||
| [29] | Maurer (2015) | 1 | 5 | 111In-PSMA-I&T, IV, R | Open RP + ePLND sLND |
γ-probe declipse SPECT | In and ex vivo | 13 | – | 15 | 15/NR | NR | NR | 2.0–12.0 a |
| [33] | Rauscher (2017) | 2a | 31 |
111In-PSMA-I&T, IV, R |
Open sLND | γ-probe | In and ex vivo | NR | – | 54 FP = 6 FN = 4 |
52/145 | 92.3 (83.2–96.7) | 93.5 (81.7–97.9) | NR |
| [4] | Knipper (2019) | 2a | 42 (13) | 99mTc-PSMA-I&S, IV, R | Open sLND | γ-probe | In and ex vivo | 2.31b (1–6)a | NR | NR | 5b (1–15)a/range 2–53 | NR | NR | NR |
| [30] | Maurer (2018) | 2a | 31 | 99mTc-PSMA-I&S, IV, R | Open sLND | γ-probe | In and ex vivo | 44 | 25 | 46 FP = 0 FN = 12 |
58/132 | 83.6 (70.9–91.5) | 100 (–) | 12.0 (3.0–25.0) a |
| [31] | Mix (2018) | 2a | 6 | 111In-PSMA-617, IV, R | Open RP + ePLND sLND |
γ-probe HGD |
In and ex vivo | NR | NR | 35 FP = 2 FN = 3 |
38/318 single samples | 92.1 (–) | 98.8 (–) | NR |
| [24] | Horn (2019) | 2b | 121 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | Open sLND | γ-probe | In and ex vivo | 175 | NR | 180 | 214/median 11 | NR | NR | 3.0 (of regions) |
| [15] | Collamati (2020) | 1 | 7 | 68Ga-PSMA-11, IV, R | RA RP + ePLND | β-probe | Ex vivo | LNs: 4 | – | LNs: 4 FP = 1 |
LNs: 3/NR | NR | NR | Smallest identified node 7 mm |
| [25] | Jilg (2020) | 2 | 23 (21) | 111In-PSMA-617, IV, R | Open RP + ePLND sLND |
γ-probe HGD |
In and ex vivo | 87 | NR | γ-Probe: 72 HGD: 79 |
104/864 | γ-Probe: 62.1 (–) HGD: 71.2 (–) |
γ-Probe: 96.3 (–) HGD: 96.9 (–) |
NR |
| [32] | Mix (2021) | 1 | 6 | 99mTc-PSMA-I&S, IV, R | Open RP sLND |
γ-probe | In and ex vivo | NR | NR | 118 | 154/516 | 76.6 (0.69–0.83) | 94.0 (0.91–0.97) | NR |
| [17] | de Barros (2022) | 2a | 20 | 99mTc-PSMA-I&S, IV, R | RA sLND | γ-probe | In and ex vivo | 21 | 13 | 19 FN = 3 |
21/21 | 86.0 (–) | 100 (–) | 8.4 (3.9–15.0) |
| [19] | Gondoputro (2022) | 2a | 12 | 99mTc-PSMA-I&S, IV, R | RA RP + ePLND | γ-probe CT-guided hookwire |
In and ex vivo | 11 | 4 | 18 FP = 2 FN = 5 |
22/74 | In vivo 76.0 (53.0–92.0) Ex vivo 76.0 (53.0–92.0) |
In vivo 69.0 (55.0–81.0) Ex vivo 96.0 (87.0–99.0) |
9.0 (6.3–11.2) Smallest <1 mm |
| [27] | Knipper (2022) | 2b | 364 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | Open sLND | γ-probe | In and ex vivo | 364 | NR | 364 FP = 21 |
343/NR | NR | NR | NR |
| [23] | Yılmaz (2022) | 1 | 15 | 99mTc-PSMA-I&S, IV, R | RA RP + ePLND | γ-probe | In and ex vivo | NR | NR | 18 | 18/297 | 100 (–) | 100 (–) | NR |
| [18] | Gandaglia (2022) | 1 | 12 | 99mTc-PSMA-I&S, IV, R | RA RP + ePLND | γ-probe | In and ex vivo | 2 | 2 | 5 FP = 1 FN = 4 |
4/96 specimens | 50.0 | 99.0 | NR |
| [28] | Koehler (2023) | 1 | 9 |
99mTc-MIP-1404, IV, R |
Open sLND | γ-probe | In and ex vivo | 19 | 12 | 21 | 24/154 | 87.5 | 100.0 | 6 (2-4.5) |
| Case reports | ||||||||||||||
| [41] | Schottelius (2015) | 1 | 1 | 111In-PSMA-I&T, IV, R | Open sLND | γ-probe | In and ex vivo | NR | NR | NR | NR/NR | NR | NR | NR |
| [39] | Maurer (2016) | 1 | 1 | 111In-PSMA-I&T, IV, R | Open sLND | γ-probe | In and ex vivo | 1 | – | 1 | 1/NR | NR | NR | NR |
| [40] | Robu (2017) | 1 | 2 (1) | 99mTc-PSMA-I&S, IV, R | Open RP + ePLND | γ-probe | In and ex vivo | 1 | – | 1 | 1/NR | NR | NR | NR |
| [38] | Kratzik (2018) | 1 | 1 | 99mTc-PSMA-I&S, IV, R | Open sLND left sided | γ-probe | In and ex vivo | 1 | 1 | 1 | 1/NR | NR | NR | NR |
| [35] | Darr (2020) | 1 | 1 | 68Ga-PSMA-11, IV, O | Open sLND | CLI | Ex vivo | 1 | – | 1 | 2/17 | NR | NR | – |
| [42] | van Leeuwen (2019) | 1 | 1 | 99mTc-PSMA-I&S, IV, R | RA sLND | γ-probe | In and ex vivo | 1 | 1 | 1 | 1/NR | NR | NR | NR |
| [34] | Aras (2021) | 1 | 10 (2) | 18F-BF3-Cy3-ACUPA, IV, O | Open RP + ePLND | Solis 525C LED illuminator with CMOS camera | Ex vivo | NR | NR | 4 | 2/NR | NR | NR | NR |
| [37] | Erfani (2022) | 1 | 1 |
99mTc-PSMA IV, R |
Open sLND | γ-probe | In and ex vivo | 2 | 2 | 2 | 2/8 | NR | NR | NR |
| Author, year | Number of patients | PSMA agent, administration | Type of surgery | Modality used | Surgical margins | Sensitivity | Specificity | |||||||
| PSM intraoperatively/ex vivo | PSM at histopathology | % (95% CI) | % (95% CI) | |||||||||||
| Focus on prostate/local recurrence | ||||||||||||||
| [26] | Knipper (2021) | 2b | 40 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | Open sLND | γ-probe | In and ex vivo | NR | NR | NR | NR | NR | NR | NR |
| [22] | Darr (2020) | 1 | 10 | 68Ga-PSMA-11, IV, O | Open RP | CLI | Ex vivo | 2 | 3 | NR | NR | NA | ||
| [20] | olde Heuvel (2020) | 1 | 5 | 68Ga-PSMA-11, IV, O | RA RP | CLI | Ex vivo | 5 FP = 2 FN = 0 |
3 | NR | NR | NA | ||
| [21] | olde Heuvel (2022) | 1 | 15 | 68Ga-PSMA-11, IV, O | RA RP | CLI | Ex vivo | 6 hotspots FP = NR FN = 4 hotspots |
10 hotspots | NR | NR | NA | ||
| [16] | Darr (2021) | 1 | 7 | 68Ga-PSMA-11, IV, O | Open RP | CLI + 550 nm OF | Ex vivo | 3 | 3 | NR | NR | NA | ||
| Case reports | ||||||||||||||
| [36] | Eder (2021) | 1 | 1 | 68Ga-PSMA-914, IV, O | RA RP | NR | In and ex vivo | 1 | – | 1 | NR | NR | NR | NA |
CI =confidence interval; CLI = Cerenkov luminescence imaging; CMOS = complementary metal oxide semiconductor; CT = computed tomography; ePLND = extended pelvic lymph node dissection; FN = false negative; FP = false positive; Ga = gallium; HGD = high-purity germanium detector; IDEAL = Idea, Development, Exploration, Assessment, Long term; I&S = imaging and surgery; I&T = imaging and therapy; In = indium; IQR = interquartile range; IV = intravenous; LN = lymph node; MRI = magnetic resonance imaging; NA = not applicable; NR = not reported; O = optical guidance; OF = optical short-pass filter; PET = positron emission tomography; PSM = positive surgical margin; PSMA = prostate-specific membrane antigen; R = radioguidance; RA = robot assisted; RP = radical prostatectomy; SD = standard deviation; sLND = salvage lymph node dissection; SPECT = single photon emission computed tomography.
∼Maximum.
Range.
Mean (±SD).
Fig. 3.
Bias table according to Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I). (Case reports were excluded from this bias analysis.)
2.4. Data analysis and objectives
Owing to the high heterogeneity found among the included studies in terms of different procedures, different reporting, and different definitions of outcomes, a meta-analysis was not possible. A comprehensive narrative synthesis of the included studies was performed. Descriptive statistics were used to summarize baseline characteristic data.
3. Evidence synthesis
3.1. Study quality and baseline features
We included 29 reports on PSMA-targeted surgery (Fig. 2), of which eight were prospective studies [15], [16], [17], [18], [19], [20], [21], [22], 12 retrospective analyses [4], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], and nine case reports [34], [35], [36], [37], [38], [39], [40], [41], [42] Generally, a small number of patients were included: in eight of 29 (28%) reports ≥20 patients were included, of which two (retrospective) studies included >100 patients (7%). The remaining 72% of studies can be considered small-scale pilots or first-in-man reports (Fig. 4). In the primary setting (∼41% of reports), patients included were at an intermediate or a high risk with or without nodal involvement. In the salvage setting (∼45% of reports), the included patients had recurrent disease on PSMA PET/CT (in local recurrence a maximum of one lesion and in nodal recurrence a maximum of five) and were eligible for surgery. The maximum standard uptake values of the PSMA-PET/CT scans were not taken into account. A detailed description of patients’ demographics can be found in Supplementary Table 1.
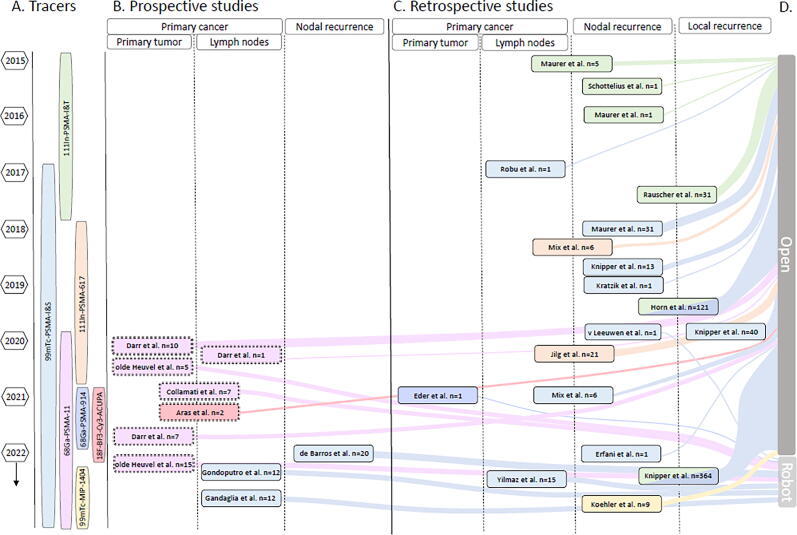
Fig. 4.
An overview of all reviewed literature. (A) All tracers chronologically aligned with year(s) of publication. (B) An overview of prospective studies. The color matches the studied tracer in A. Position on the y axis indicates the year of publication. Position on the x axis indicates the type of prostate cancer studied. (C) Overview of studies that retrospectively analyzed the data. The color matches the studied tracer in A. Position on the y axis indicates the year of publication. Position on the x axis indicates the type of prostate cancer studied. (D) Sankey diagram entwined in Figure 4B and C showing the distribution between open and robot-assisted surgical procedures by the number of patients. The color matches the tracer that was studied. PSMA = prostate-specific membrane antigen, The double outline ‘=’ means only ex vivo measurements were performed; the solid outline ‘—’ means in and ex vivo measurements were performed.
Seventeen studies (59%; including ten case reports) were considered stage 1 according to the IDEAL framework: proof of concept or first in man. These studies describe the clinical use of novel tracers or an adapted version of a previously studied tracer. Nine were considered exploring stage (stage 2a; 31%) and three were in developmental stage (stage 2b; 10%). None of the studies could be considered to be at stage ≥3 (assessment and long term; see Table 1) [13].
3.2. Preoperative features
Surgical resections were always guided by preoperative imaging roadmaps acquired >24 h prior to surgery (PSMA PET/CT 97% and [additional] PET/MRI 7% [28], [40]). In general, the guidance provided by PSMA PET/CT was considered leading even in cases where a SPECT/CT scan was done (see below). Fifteen (52%) of the studies reported on the outcomes of PSMA PET/CT at lesion level. Out of 473 tumor-positive lesions at histopathology, PSMA PET/CT identified 382 (81%). The smallest lesion identified on PSMA PET was 3 mm in size [4].
For the most commonly used tracers (99mTc-PSMA-I&S and 111In-PSMA-I&T), the mean injection time to surgery was around 24 h (range 16–28 h), with a median injected activity varying between 541 and 638 MBq and between 140 and 150 MBq, respectively. Studies used 68Ga-PSMA-11 injected between 76 and 127 MBq [16], [20]. A detailed description of the timing and injected activity of all tracers can be found in Supplementary Table 2.
When 111In- or 99mTc-labeled PSMA tracers were used, an additional preoperative SPECT/CT scan was performed within 24 h before surgery (in 43% of the procedures with 111In and in 93% with 99mTc). Of the studies that included SPECT/CT, only seven reported their findings, indicating that 60 out of 133 histopathologically confirmed tumor-positive lesions (45%) could be identified by SPECT/CT. The only studies that described all lesions being identified on SPECT/CT were case reports with a maximum of two lesions on the PSMA PET/CT [37], [38], [42].
3.3. Radioguidance
Clinically, PSMA guidance is employed in two main approaches: radioguidance and optical guidance. The former is most frequently described (22/29 reports [76%]; ranging from one to 364 patients) and makes use of gamma/beta-emitting radioisotopes. Clinical use of gamma-emitting radioisotopes started with the use of 111In-PSMA-I&T, followed not shortly by studies using 99mTc-PSMA-I&S and 111In-PSMA-617 [29], [31], [41]. In all these reports, guidance was facilitated by real-time tracing using a gamma probe. The design of the gamma probe varied depending on the surgical approach. A hand-held gamma probe for open surgery was used in 17/29 studies (59%; ranging from one to 364 patients). One study reported the combined use with a probe-based freehand SPECT scan [29]. The development of a DROP-IN gamma probe design enabled the performance of the first robotic PSMA-targeted surgery, a technique that was used in 5/29 studies (17%; ranging from one to 20 patients). In one study, a robotic DROP-IN beta probe has been employed ex vivo to confirm the presence of 68Ga-PSMA-11 in the prostate and nodal tissue [15].
Since 2018, all groups originally reporting the use of 111In-PSMA tracers (gamma emissions 171 kilo electron Volt [keV] and 245 keV; t1/2 = 2.8 d) converted to the use of 99mTc-PSMA-I&S; the 141 keV gamma emission and 6 h half-life of 99mTc are more compatible with the everyday clinical workflow and suffer less from background signals (details on tracer properties can be found in Supplementary Fig. 1 and Supplementary Table 3) [40]. Figure 4 shows that in 2020 111In-PSMA-617 ceases to be reported on and that new 99mTc-based tracers such as 99mTc-MIP-1404 are still being introduced.
3.4. Optical guidance
A different approach of PSMA guidance relies on converting the beta-emission of tracers such as 68Ga-PSMA-11 into a secondary optical signal (Cerenkov Luminescence; λem max < 450 nm) that has a very limited degree of tissue penetration (<3 mm) but can be recorded in a back-table (ex vivo) dark-room environment via highly sensitive optical detectors [43]—a strategy that was used in five of 29 (17%) studies reported (37 patients).
An alternative approach to combining beta emissions and optical guidance makes the use of the so-called hybrid (radioactive and fluorescently labeled) PSMA tracers [34], [36]—a strategy that was used in 2/29 (7%) studies (three patients). Eder et al. [36] reported the use of a PSMA-11–derived hybrid molecule tracer, PSMA-914 (68Ga-PSMA-914), using fluorescence imaging [44], while Aras et al. [34] used 18F-BF3-Cy3-ACUPA in an ex vivo setting. Unfortunately, the dyes 800 CW (λem max = 789 nm [45]) and Cy3 (λem max = 565 nm) are not optimally compatible with the da Vinci Firefly endoscope (Intuitive Surgical Inc., Sunnyvale, CA, USA), which is designed for the detection of indocyanine green (λem max = 820 nm; Supplementary Table 2 and Supplementary Fig. 1). As a result, the fluorescence of 18F-BF3-Cy3-ACUPA was imaged only ex vivo, for neither compound was investigated if radioguidance and optical guidance could complement each other.
3.5. Tumor-to-background values
A comparison between tracers or modalities was difficult as the in vivo as well as ex vivo reporting of tumor-to-background ratio (TBR) was not consistent, and different cutoffs to consider a lesion positive were used [18], [28]. Mostly this was defined as at least twice the background, with different definitions of the background (fatty tissue, lymph nodes, and psoas muscle). TBR ranged from 2.1 to 8.0 for 99mTc-PSMA-I&S (in vivo analysis, DROP-IN gamma probe, background: different types of tissue near the lesion) and from 10.0 to 30.0 for 99mTc-MIP-1404 (ex vivo analysis, hand-held gamma probe, background: fatty tissue) [17], [28].
3.6. Surgical outcomes
As illustrated in Figure 4, 69% of the reports studied open (tracers used in vivo: 111In-PSMA-I&T, 99mTc-PSMA-I&S, and 99mTc-MIP-1404; ex vivo: 68Ga-PSMA-11 and 18F-BF3-Cy3-ACUPA) and 31% robot-assisted (tracers used in vivo: 99mTc-PSMA-I&S and 68Ga-PSMA-914; ex vivo: 68Ga-PSMA-11) surgical interventions. Six studies (21%) focused only on the identification of margins in primary cancer (tracers used in vivo: 68Ga-PSMA-914; ex vivo: 68Ga-PSMA-11) or local recurrence (in vivo: 99mTc-PSMA-I&S), two (7%) on both prostate and lymph nodes (tracers used ex vivo: 68Ga-PSMA-11 and 18F-BF3-Cy3-ACUPA), and 21 (72%) on identifying tumor-positive lymph nodes during primary or salvage lymph node dissection (LND; tracers used in vivo: 111In-PSMA-617, 111In-PSMA-I&T, 99mTc-PSMA-I&S, and 99mTc-MIP-1404).
In nodal surgery, the accuracy of identifying tumor-containing lymph nodes was reported in 11 studies (38%): salvage LND in seven and primary LND in four (tracers used in vivo and ex vivo: 111In-PSMA-617, 111In-PSMA-I&T, 99mTc-PSMA-I&S, and 99mTc-MIP-1404; tracers used only ex vivo: 68Ga-PSMA-11). Generally, specificity (median 96.3, interquartile range [IQR] 93.8–99.4) was higher than sensitivity (median 80.1, IQR 76–92.1). Gondoputro et al. [19] reported the only discrepancy with in vivo specificity of 69% (sensitivity of 76%; 99mTc-PSMA-I&S; robotic procedure). A notable outlier was sensitivity of 50%, reported by Gandaglia et al. [18], in primary LND using 99mTc-PSMA-I&S. The influence of the tracers and the approach (open/robotic) on the accuracy could not be assessed given the different preoperative patient characteristics, differences in timing of injection, differences in location of the lesions, and differences in reporting.
In vivo identified PCa lesions ranged between <1 and 25 mm in size for 99mTc-PSMA-I&S, where the smallest lesion (<1 mm) was identified during robot-assisted surgery using a DROP-IN gamma probe [19]. For 111In-PSMA-I&T, the range was 2––12 mm. Four studies (14%) report identifying tumor-positive lesions via 99mTc-PSMA-I&S that did not show on preoperative PET [18], [19], [30]. False negatives, when reported, were lesions <5 mm [17], [18], [19]. An overview of the surgical outcomes can be found in Table 1.
In prostate-focused surgery, the accuracy of identifying positive surgical margins in vivo by radioguided surgery (RGS) was described only in one patient by Gondoputro et al. [19]. They successfully removed residual cancerous tissue but advised caution due to potential urinary contamination. The ex vivo evaluation of 68Ga-PSMA-11 with the DROP-IN beta probe correctly identified positive surgical margins in 40% (seven patients) [15]. Ex vivo Cerenkov imaging of 68Ga-PSMA-11 yielded 60–83% (37 patients) agreement with histopathology [16], [20], [21], [22]. Eder et al. [36] and Aras et al. [34] describe the visibility of the fluorescent component of the tracer (in and ex vivo, respectively) but did not quantify their results.
3.7. (Oncological) outcomes
Between the studies, the evidence varied as shown in Table 1; no study exceeded IDEAL framework stage 2b “development.”
Follow-up data after PSMA-targeted salvage surgery were analyzed retrospectively in ten studies (34%) reporting a median follow-up of 1–25.7 mo and were provided only for 99mTc-PSMA-I&S and 111In-PSMA-I&T. Nothing has yet been reported on the difference in oncological outcomes between these tracers.
A postoperative prostate-specific antigen (PSA) decline was reported in six of the 22 reports addressing salvage lymph node surgery [4], [23], [27], [30]. The PSA decline ranged between 67% and 100% of the patients, and a decline of >90% was reported in 22–100% (Table 2), indicating variations in treatment effectiveness. Knipper et al. [4] were the first to look at the difference in PSA decline after conventional salvage LND versus the addition of PSMA-targeted radioguidance (99mTc-PSMA-I&S, 42 patients) and found a significantly better outcome for the latter. However, long term follow-up data are missing.
Table 2.
Follow-up
| Ref | Author, year | Patients (patients treated with PSMA GS) | PSMA agent, administration, type of guidance | Duration (mo) | Additional treatment after PSMA GS needed | PSA progression | BCR/BCRFS | Survival | Complications (Clavien-Dindo) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (n) | Median (IQR) | n (%) | PSA at FU: median (IQR) cBR/decline: n (%) |
n (%) BCRFS (mo) (95% CI) |
n (%) | I/II n (%) |
III n (%) |
IV n (%) |
V n (%) |
|||
| Focus on lymph nodes | ||||||||||||
| [29] | Maurer (2015) | 5 | 111In-PSMA-I&T, IV, R | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [33] | Rauscher (2017) | 31 | 111In-PSMA-I&T, IV, R | 11.1 (2.7–19.4)a | 10 (33.0) after median of 4.1 mo | cBR: 2 (22.0) with FT 15 (75.0) without FT Decline >50%: 23 (76.7) Decline >90%: 16 (53.3) |
NR | NR | 6 (20.0) | 4 (13.0) | 0 (0.0) | 0 (0.0) |
| [4] | Knipper (2019) | 42 (13) | 99mTc-PSMA-I&S, IV, R | NR | NR | 0.069 (<0.01–3.3) a ng/ml Decline >50% (92.0) Decline >90% (53.0) |
NR | NR | NR | NR | NR | NR |
| [30] | Maurer (2018) | 31 | 99mTc-PSMA-I&S, IV, R | 13.8 (–) | 11 (35.0) after median of 3.7 mo | Decline >50%: 24 (80.0) Decline >90%: 17 (57.0) |
BCR: 17 (55.0) after median of 1.9 mo | NR | 12 (38.7) | 1 (3.2) | 0 (0.0) | 0 (0.0) |
| [31] | Mix (2018) | 6 | 111In-PSMA-617, IV, R | 24 (–) | NR |
at FU: 0.51 (0.03–4.85) |
NR | NR | NR | NR | NR | NR |
| [24] | Horn (2019) | 121 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | NR | 39 (32.2) after median of 4.6 mo | cBR: 77 (66%) Decline >50%: 88 (77.0) Decline >90%: 55 (48.0) |
41.8% BCRFS of at least 12 mo No significant difference between tracers |
NR | 29 (24.0) | 11 (9.0) | 0 (0.0) | 1 (1.0) |
| [15] | Collamati (2020) | 7 | 68Ga-PSMA-11, IV, R | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [25] | Jilg (2020) | 23 (21) | 111In-PSMA-617 IV, R | 25.7 (–) | 10 (43.5) | NR | 14/23 clinical progression | NR | NR | NR | NR | NR |
| [32] | Mix (2021) | 6 | 99mTc-PSMA-I&S, IV, R | 19.4 (17.0–22.7) | 6 (100.0) | At FU: 1.45 (0.15–17.1) | NR | AWD 6 (100.0) | NR | NR | NR | NR |
| [17] | de Barros (2022) | 20 | 99mTc-PSMA-I&S, IV, R | 15.0b | NR | Decline >50% (67.0) Decline >90% (22.0) |
BCR: 14/18 (88.0) | Overall 18/19 (94.7) | 5 (26.3) | 0 (0.0) | 0 (0.0) | 1 (5.3) |
| [19] | Gondoputro (2022) | 12 | 99mTc-PSMA-I&S, IV, R | 13 (4–22) | 6 (50.0) | pPSA 5/12 (42.0) | BCR: 2/12 (16.7) | Overall 12/12 (100.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [27] | Knipper (2022) | 364 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | No BCR: 10.8 (1.2–25.1) No treatment: 10.3 (2.3–24.0) |
121 (33.2) | cBR: 165 (45.3) | BCR: 225 (61.8) BCRFS: 7.8 (5.4–10.5) |
NR | 94 (25.6) | 23 (6.3) | 1 (0.28) | 0 (0.0) |
| [23] | Yılmaz (2022) | 15 | 99mTc-PSMA-I&S, IV, R | 23.5 c (14–30) a | 5 (33.3) | Decline >90% (100.0) | At 2.5 yr FU, BCRFS rate 86.7% | Overall 15/15 (100.0) | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [18] | Gandaglia (2022) | 12 | 99mTc-PSMA-I&S, IV, R | 1 | 3 (25.0) | pPSA 3/12 (25.0) | NR | Overall 12/12 (100.0) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) |
| [28] | Koehler (2023) | 9 | 99mTc-MIP-1404, IV, R | NR | NR | cBR: 5 (56.0) | NR | NR | NR | NR | NR | NR |
| Case reports | ||||||||||||
| [41] | Schottelius (2015) | 1 | 111In-PSMA-I&T, IV, R | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [39] | Maurer (2016) | 1 | 111In-PSMA-I&T, IV, R | NR | 0 (0.0) | <0.07 1 (100.0) |
NR | Overall 1 (100.0) | NR | NR | NR | NR |
| [40] | Robu (2017) | 2 (1) | 99mTc-PSMA-I&S, IV, R | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [38] | Kratzik (2018) | 1 | 99mTc-PSMA-I&S, IV, R | 1 | NR | <0.01 1 (100.0) |
NR | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [35] | Darr (2020) | 1 | 68Ga-PSMA-11, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [42] | van Leeuwen (2019) | 1 | 99mTc-PSMA-I&S, IV, R | NR | NR | <0.03 | NR | NR | NR | NR | NR | NR |
| [34] | Aras (2021) | 10 (2) |
18F-BF3-Cy3-ACUPA IV, O |
NR | NR | NR | NR | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| [37] | Erfani (2022) | 1 | 99mTc-PSMA, IV, R | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Focus on prostate/local recurrence | ||||||||||||
| [26] | Knipper (2021) | 40 | 111In-PSMA-I&T, 99mTc-PSMA-I&S, IV, R | 24.4 (11.8–41.9) | 12 (30.0) | cBR: 31 (77.5) | BCR: 22 (55.0) 23.7 (9.8–not reached) |
4 (10.0) | 3 (7.5) | 0 (0.0) | 0 (0.0) | |
| [22] | Darr (2020) | 10 | 68Ga-PSMA-11, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [20] | olde Heuvel (2020) | 5 | 68Ga-PSMA-11, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [21] | olde Heuvel (2022) | 15 | 68Ga-PSMA-11, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [16] | Darr (2021) | 7 | 68Ga-PSMA-11, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Case reports | ||||||||||||
| [36] | Eder (2021) | 1 | 68Ga-PSMA-914, IV, O | NR | NR | NR | NR | NR | NR | NR | NR | NR |
AWD = alive with disease; BCR = biochemical recurrence, defined as PSA >0.2 ng/ml; BCRFS = BCR-free survival; cBR = complete biochemical response (PSA <0.2 ng/ml); CI = confidence interval; FT = further treatment; FU = follow-up; Ga = gallium; GS = Gleason score; I&S = imaging and surgery; I&T = imaging and therapy; In = indium; IQR = interquartile Range; IV = intravenous; NR = not reported; O = optical guidance; pPSA = precursor PSA; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; R = radioguidance; SD = standard deviation.
Range.
Maximum.
Mean (±SD).
BCR was reported in five of 22 studies regarding salvage surgery, ranging from 50% to 61.8% of the patients with follow-ups ranging from 10.3 to 25.7 mo. One study looked at BCR in the primary setting and found a BCR of 16.7% within a median time of 13 mo (IQR 4–22) [19]. Horn et al. [24] concluded from their BCR-free survival (BCRFS) data that patients with low preoperative PSA and a single lesion on preoperative PSMA PET benefitted most from PSMA-targeted surgery. A range of 0–100% of the patient population was in need of additional treatment after PSMA-targeted surgery within a median time ranging from 1 to 25.7 mo.
Complications were classified according to Clavien-Dindo [46]. Seven of the ten studies that reported on complications report on patients with a grade I/II complication, with a percentage ranging from 8.3% to 38.7%. Five studies observed complications of grade 3 (percentage ranging from 3% to 25%), and only three patients from the total population of all studies experienced a complication of grade ≥IV [17], [24], [27]. None of the complications were ascribed to the tracer or image guidance procedure. The overall survival was again mentioned only in studies using 99mTc-PSMA-I&S and 111In-PSMA-I&T, and was 94.7–100% after a median follow-up ranging from 1 to 23.5 mo. Details concerning follow-up and outcomes can be found in Table 2.
3.8. Discussion
This systematic review summarized the existing literature on PSMA-targeted surgery in PCa patients. PSMA targeting provides a promising strategy to identify PCa both pre- and intraoperatively, and it seems that we have only just begun to find out what this technique can offer. Currently, the most widely implemented approach is PSMA-RGS using 99mTc-PSMA-I&S (∼50% of the studies on this topic and the study with the largest number of patients [n = 364]). Studies that used a conventional open surgical approach were the majority (69%) in comparison with those using robot-assisted surgery (31%).
Most studies present data on the value of PSMA-RGS in men with nodal recurrence (salvage surgery). One study suggested a benefit of PSMA-RGS versus conventional salvage LND [4]. Sensitivity for detecting nodal metastases during salvage surgery was dependent on the size and location of the lesion and ranged widely from 50% to 100% (eight trials). One of the most critical factors is selection of patients. Men with lower preoperative PSA and one lesion on imaging were most likely to benefit from RGS with a complete biochemical response rate of 45–66% in the largest series.
PSMA-PET imaging is known to be less dependable in identifying lesions under 3 mm [6], [7] This corresponds to our findings for intraoperative detection, where the median size of metastases found in this review ranged from 2 to 9 mm. Smaller nodes (<3 mm) were most frequently missed [17], [19]. The level of reporting of the correlation between RGS findings and histology in studies varied, with some studies reporting at the nodal level and others at the patient level. Moreover, whether an ex vivo analysis corresponded to intraoperative findings was not reported in all studies. This makes it difficult to compare detection rates among studies.
Tracer choice may also impact detection accuracy, a choice usually based on pharmacokinetics and pharmacodynamics as well as the sensitivity and specificity of the tracer. The studies with the largest patient groups (ranging from one to 364) included in these trials received RGS facilitated by 99mTc-PSMA-I&S. The properties of 99mTc-PSMA-I&S are well known, but this cannot be said for many of the other tracers, and comparative studies are lacking [47]. As 99mTc-PSMA-I&S has successfully been used in both the primary and the salvage setting, it is currently the favored tracer.
The oncological benefit of PSMA-targeted surgery was studied mainly using a biochemical response as an endpoint. Looking at all the data combined, there was a large variation in PSA decline. Knipper et al. [26] showed that addition of PSMA guidance to conventional salvage LND improved PSA decline, suggesting that PSMA-RGS may improve outcome in men with recurrent nodal disease when compared with conventional surgery. Around 50% of salvage RGS patients were BCR free at a median follow-up of 13.2 mo. Hereby, a low preoperative PSA level and a single lesion on preoperative PSMA PET yielded better BCRFS after salvage RGS [24].
In six studies, PSMA-RGS was used in primary LND. Sensitivity and specificity for the detection of nodal metastases was comparable with the salvage setting, but considering the short reported follow-up, no other oncological outcome data are available. Optical cancer detection by Cerenkov or fluorescence imaging has been studied only in the primary setting. No study has proved the oncological value of PSMA-targeted surgical margin imaging. PSMA tracers that solely rely on fluorescence are in development [48], [49]. However, preclinical or first-in-man data may not immediately translate to patient care. In fact, our search worryingly suggests that thus far only two optical (hybrid) tracers mentioned in preclinical reviews (68Ga-PSMA-914 and 18F-BF3-Cy3-ACUPA) were tested clinically in two case series [8], [9], [50].
This review is limited by the retrospective nature of the majority of the included studies with an unclear overlap in numbers of patients. The included studies had a high or an unclear risk of bias, were noncomparative, lacked a standardized way of reporting outcomes, and had short follow-ups. Furthermore, the sample sizes were generally small. A direct comparison of studies is also hampered by overlapping patient populations in several studies. Only one prospective first-in-man study that presented the proof-of-concept data contained ≥20 patients so far. Based on the results from this review, it is essential that further clinical trials are conducted based on standardized methodology and proper study endpoints. In addition, consensus on what PSMA-targeted surgery should provide for wider clinical implementation is desirable.
4. Conclusions
Consolidation of the existing literature on PSMA-targeted guidance during surgery in PCa indicates that the most common technique used is radioactive gamma-tracing in the open salvage setting. Techniques for use in robotic surgery and the addition of optical detection possibilities are in the pipeline. Intraoperative PSMA targeting has been proved to be technically sound, but no clear oncological benefit has yet been objectified. Lacking solid outcome data, currently PSMA-targeted surgical guidance should be considered an experimental treatment. Randomized controlled studies may be considered after consensus on the optimal surgical approaches and most valid clinical endpoints.
Author contributions: Henk G. van der Poel had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Berrens, Knipper, Marra, F.W.B. van Leeuwen, van der Poel.
Acquisition of data: Berrens, Knipper, van der Mierden.
Analysis and interpretation of data: Berrens, Knipper, Marra.
Drafting of the manuscript: Berrens, Knipper, F.W.B. van Leeuwen, van der Poel.
Critical revision of the manuscript for important intellectual content: Berrens, Knipper, Marra, P.J. van Leeuwen, van der Mierden, Donswijk, Maurer, F.W.B. van Leeuwen, van der Poel.
Statistical analysis: None.
Obtaining funding: F.W.B. van Leeuwen.
Administrative, technical, or material support: None.
Supervision: F.W.B. van Leeuwen, van der Poel.
Other: None.
Financial disclosures: Henk G. van der Poel certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: Fijs W.B van Leeuwen was financially supported by an Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) -Toegepaste en Technische Wetenschappen (TTW)-Vici (TTW BGT16141) grant and Koningin Wilhelmina Fonds voor de Nederlandse Kankerbestrijding (KWF)- Publiek Private Samenwerkingen (PPS) grant (no. 2022-PPS-14852).
Acknowledgement: Special thanks to Maarten van Meerbeek for the input on the figures and tables regarding tracer properties.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.05.014.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Eastham J.A., Scardino P.T., Kattan M.W. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008;179:2207–2211. doi: 10.1016/j.juro.2008.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R., Li T., Ye L., Lin L., Wei Y. The evidence behind robot-assisted abdominopelvic surgery. Ann Intern Med. 2022;175:W22. doi: 10.7326/L21-0781. [DOI] [PubMed] [Google Scholar]
- 3.Dell’Oglio P., Mottrie A., Mazzone E. Robot-assisted radical prostatectomy vs. open radical prostatectomy: Latest evidences on perioperative, functional and oncological outcomes. Curr Opin Urol. 2020;30:73–78. doi: 10.1097/MOU.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 4.Knipper S., Tilki D., Mansholt J., et al. Metastases-yield and prostate-specific antigen kinetics following salvage lymph node dissection for prostate cancer: a comparison between conventional surgical approach and prostate-specific membrane antigen-radioguided surgery. Eur Urol Focus. 2019;5:50–53. doi: 10.1016/j.euf.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Perera M., Papa N., Roberts M., et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–417. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Uprimny C., Kroiss A.S., Decristoforo C., et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–949. doi: 10.1007/s00259-017-3631-6. [DOI] [PubMed] [Google Scholar]
- 7.Mottet N., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Hensbergen A.W., Van Willigen D.M., Van Beurden F., et al. Image-guided surgery: are we getting the most out of small-molecule prostate-specific-membrane-antigen-targeted tracers? Bioconjug Chem. 2020;31:375–395. doi: 10.1021/acs.bioconjchem.9b00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derks Y.H.W., Löwik D.W.P.M., Sedelaar J.P.M., et al. PSMA-targeting agents for radio- and fluorescence guided prostate cancer surgery. Theranostics. 2019;9:6824–6839. doi: 10.7150/thno.36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bramer W.M., Giustini D., De Jonge G.B., Holland L., Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104:240–243. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Med Flum. 2021;57:444–465. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ergina P.L., Barkun J.S., McCulloch P., Cook J.A., Altman D.G., IDEAL Group IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. 2013;346 doi: 10.1136/bmj.f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collamati F., van Oosterom M.N., De Simoni M., et al. A DROP-IN beta probe for robot-assisted 68Ga-PSMA radioguided surgery: first ex vivo technology evaluation using prostate cancer specimens. EJNMMI Res. 2020;10:92. doi: 10.1186/s13550-020-00682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darr C., Costa P.F., Kesch C., et al. Prostate specific membrane antigen-radio guided surgery using Cerenkov luminescence imaging—utilization of a short-pass filter to reduce technical pitfalls. Transl Androl Urol. 2021;10:3972–3985. doi: 10.21037/tau-20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Barros H.A., van Oosterom M.N., Donswijk M.L., et al. Robot-assisted prostate-specific membrane antigen–radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol. 2022;82:97–105. doi: 10.1016/j.eururo.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Gandaglia G., Mazzone E., Stabile A., et al. Prostate-specific membrane antigen radioguided surgery to detect nodal metastases in primary prostate cancer patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection: results of a planned interim analysis of a prospective phase 2 study. Eur Urol. 2022;82:411–418. doi: 10.1016/j.eururo.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Gondoputro W., Scheltema M.J., Blazevski A., et al. Robot-assisted prostate-specific membrane antigen-radioguided surgery in primary diagnosed prostate cancer. J Nucl Med. 2022;63:1659–1664. doi: 10.2967/jnumed.121.263743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.olde Heuvel J., de Wit-van der Veen B.J., van der Poel H.G., et al. 68Ga-PSMA Cerenkov luminescence imaging in primary prostate cancer: first-in-man series. Eur J Nucl Med Mol Imaging. 2020;47:2624–2632. doi: 10.1007/s00259-020-04783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.olde Heuvel J., De Wit-Van Der Veen B.J., Van Der Poel H.G., et al. Cerenkov luminescence imaging in prostate cancer: not the only light that shines. J Nucl Med. 2022;63:29–35. doi: 10.2967/jnumed.120.260034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darr C., Harke N.N., Radtke J.P., et al. Intraoperative 68Ga-PSMA Cerenkov luminescence imaging for surgical margins in radical prostatectomy: a feasibility study. J Nucl Med. 2020;61:1500–1506. doi: 10.2967/jnumed.119.240424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yılmaz B., Şahin S., Ergül N., et al. 99mTc-PSMA targeted robot-assisted radioguided surgery during radical prostatectomy and extended lymph node dissection of prostate cancer patients. Ann Nucl Med. 2022;36:597–609. doi: 10.1007/s12149-022-01741-9. [DOI] [PubMed] [Google Scholar]
- 24.Horn T., Krönke M., Rauscher I., et al. Single lesion on prostate-specific membrane antigen-ligand positron emission tomography and low prostate-specific antigen are prognostic factors for a favorable biochemical response to prostate-specific membrane antigen-targeted radioguided surgery in recurrent prostate cancer. Eur Urol. 2019;76:517–523. doi: 10.1016/j.eururo.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Jilg C.A., Reichel K., Stoykow C., et al. Results from extended lymphadenectomies with [111In]PSMA-617 for intraoperative detection of PSMA-PET/CT-positive nodal metastatic prostate cancer. EJNMMI Res. 2020;10:17. doi: 10.1186/s13550-020-0598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knipper S., Ascalone L., Ziegler B., et al. Salvage surgery in patients with local recurrence after radical prostatectomy. Eur Urol. 2021;79:537–544. doi: 10.1016/j.eururo.2020.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Knipper S., Mehdi Irai M., Simon R., et al. Cohort study of oligorecurrent prostate cancer patients: oncological outcomes of patients treated with salvage lymph node dissection via prostate-specific membrane antigen–radioguided surgery. Eur Urol. 2023;83:62–69. doi: 10.1016/j.eururo.2022.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Koehler D., Sauer M., Klutmann S., et al. Feasibility of 99m Tc-MIP-1404 for SPECT/CT imaging and subsequent PSMA-radioguided surgery in early biochemical recurrent prostate cancer: a case series of 9 patients. J Nucl Med. 2023;64:59–62. doi: 10.2967/jnumed.122.263892. [DOI] [PubMed] [Google Scholar]
- 29.Maurer T., Weirich G., Schottelius M., et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol. 2015;68:530–534. doi: 10.1016/j.eururo.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Maurer T., Robu S., Schottelius M., et al. 99m Technetium-based prostate-specific membrane antigen–radioguided surgery in recurrent prostate cancer. Eur Urol. 2019;75:659–666. doi: 10.1016/j.eururo.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Mix M., Reichel K., Stoykow C., et al. Performance of 111In-labelled PSMA ligand in patients with nodal metastatic prostate cancer: correlation between tracer uptake and histopathology from lymphadenectomy. Eur J Nucl Med Mol Imaging. 2018;45:2062–2070. doi: 10.1007/s00259-018-4094-0. [DOI] [PubMed] [Google Scholar]
- 32.Mix M., Schultze-Seemann W., von Büren M., et al. 99mTc-labelled PSMA ligand for radio-guided surgery in nodal metastatic prostate cancer: proof of principle. EJNMMI Res. 2021;11:22. doi: 10.1186/s13550-021-00762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauscher I., Düwel C., Wirtz M., et al. Value of 111In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: correlation with histopathology and clinical follow-up. BJU Int. 2017;120:40–47. doi: 10.1111/bju.13713. [DOI] [PubMed] [Google Scholar]
- 34.Aras O., Demirdag C., Kommidi H., et al. Small molecule, multimodal, [18F]-PET and fluorescence imaging agent targeting prostate-specific membrane antigen: first-in-human study. Clin Genitourin Cancer. 2021;19:405–416. doi: 10.1016/j.clgc.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darr C., Krafft U., Fendler W.P., et al. First-in-man intraoperative Cerenkov luminescence imaging for oligometastatic prostate cancer using 68Ga-PSMA-11. Eur J Nucl Med Mol Imaging. 2020;47:3194–3195. doi: 10.1007/s00259-020-04778-y. [DOI] [PubMed] [Google Scholar]
- 36.Eder A., Omrane M., Stadlbauer S., et al. The PSMA-11-derived hybrid molecule PSMA-914 specifically identifies prostate cancer by preoperative PET/CT and intraoperative fluorescence imaging. Eur J Nucl Med Mol Imaging. 2021;48:2057–2058. doi: 10.1007/s00259-020-05184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erfani S., Sadeghi R., Aghaee A., Ghorbani H.R., Roshanravan V. Prostate-specific membrane antigen radioguided surgery for salvage pelvic lymph node dissection in a man with prostate cancer. Clin Nucl Med. 2022;47:E174–E176. doi: 10.1097/RLU.0000000000003944. [DOI] [PubMed] [Google Scholar]
- 38.Kratzik C., Dorudi S., Schatzl M., Sinzinger H. Tc-99m-PSMA imaging allows successful radioguided surgery in recurrent prostate cancer. Hell J Nucl Med. 2018;21:202–204. doi: 10.1967/s002449910906. [DOI] [PubMed] [Google Scholar]
- 39.Maurer T., Schwamborn K., Schottelius M., et al. PSMA theranostics using PET and subsequent radioguided surgery in recurrent prostate cancer. Clin Genitourin Cancer. 2016;14:e549–e552. doi: 10.1016/j.clgc.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Robu S., Schottelius M., Eiber M., et al. Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med. 2017;58:235–242. doi: 10.2967/jnumed.116.178939. [DOI] [PubMed] [Google Scholar]
- 41.Schottelius M., Wirtz M., Eiber M., Maurer T., Wester H.J. [111In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5:68. doi: 10.1186/s13550-015-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Leeuwen F.W.B., Van Oosterom M.N., Meershoek P., et al. Minimal-invasive robot-assisted image-guided resection of prostate-specific membrane antigen-positive lymph nodes in recurrent prostate cancer. Clin Nucl Med. 2019;44:580–581. doi: 10.1097/RLU.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 43.Chin P.T.K., Welling M.M., Meskers S.C.J., Valdes Olmos R.A., Tanke H., Van Leeuwen F.W.B. Optical imaging as an expansion of nuclear medicine: Cerenkov-based luminescence vs fluorescence-based luminescence. Eur J Nucl Med Mol Imaging. 2013;40:1283–1291. doi: 10.1007/s00259-013-2408-9. [DOI] [PubMed] [Google Scholar]
- 44.Meershoek P., KleinJan G.H., van Willigen D.M., et al. Multi-wavelength fluorescence imaging with a da Vinci Firefly—a technical look behind the scenes. J Robot Surg. 2021;15:751–760. doi: 10.1007/s11701-020-01170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Y., Liu Y., Chen Z., Gu N., Wang J. Manufacture of IRDye800CW-coupled Fe3O4 nanoparticles and their applications in cell labeling and in vivo imaging. J Nanobiotechnol. 2010;8:25. doi: 10.1186/1477-3155-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clavien P.A., Barkun J., De Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 47.Aalbersberg E.A., van Andel L., Geluk-Jonker M.M., Beijnen J.H., Stokkel M.P.M., Hendrikx J.J.M.A. Automated synthesis and quality control of [99mTc]Tc-PSMA for radioguided surgery (in a [68Ga]Ga-PSMA workflow) EJNMMI Radiopharm Chem. 2020;5:10. doi: 10.1186/s41181-020-00095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen H., Antaris A., Van den Berg N., et al. Pd54-11 Results of the phase 1 safety and efficacy prostate specific membrane antigen (PSMA) targeting fluorophore for image guided surgery in patients undergoing robotic prostatectomy. J Urol. 2022;207(Suppl 5):e920. doi: 10.1097/ju.0000000000002631.11. [DOI] [Google Scholar]
- 49.Kularatne S.A., Thomas M., Myers C.H., et al. Evaluation of novel prostate-specific membrane antigen-targeted near-infrared imaging agent for fluorescence-guided surgery of prostate cancer. Clin Cancer Res. 2019;25:177–187. doi: 10.1158/1078-0432.CCR-18-0803. [DOI] [PubMed] [Google Scholar]
- 50.Derks Y.H.W., van Lith S.A.M., Amatdjais-Groenen H.I.V., et al. Theranostic PSMA ligands with optimized backbones for intraoperative multimodal imaging and photodynamic therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2022;49:2425–2435. doi: 10.1007/s00259-022-05685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.