Abstract
Background:
Recently, arterial stiffness has been associated with cerebral small vessel disease (SVD), brain atrophy and vascular dementia. Arterial stiffness is assessed via pulse wave velocity (PWV) measurement and is strongly dependent on arterial blood pressure. While circadian blood pressure fluctuations are important determinants of end-organ damage, the role of 24-h PWV variability is yet unclear.
Objectives:
We here investigated the association between PWV and its circadian changes on brain morphology and cognitive function in community-dwelling individuals.
Design:
Single-centre, prospective, community-based follow-up study.
Methods:
The study cohort comprised elderly community-based participants of the Austrian Stroke Prevention Family Study which was started in 2006. Patients with any history of cerebrovascular disease or dementia were excluded. The study consists of 84 participants who underwent ambulatory 24-h PWV measurement. White matter hyperintensity volume and brain volume were evaluated by 3-Tesla magnetic resonance imaging (MRI). A subgroup of patients was evaluated for cognitive function using an extensive neuropsychological test battery.
Results:
PWV was significantly related to reduced total brain volume (p = 0.013), which was independent of blood pressure and blood pressure variability. We found no association between PWV with markers of cerebral SVD or impaired cognitive functioning. Only night-time PWV values were associated with global brain atrophy (p = 0.005).
Conclusions:
This study shows a relationship of arterial stiffness and reduced total brain volume. Elevations in PWV during night-time are of greater importance than day-time measures.
Keywords: arterial stiffness, brain atrophy, cerebral small vessel disease, cognitive function, pulse wave velocity
Introduction
In recent studies, arterial stiffness has been related to cerebral small vessel disease (SVD), brain volume and cognitive function. With increasing age, the elasticity of the aortic wall is reduced, resulting in an increased aortic pulse wave transmitted to the cerebral microvasculature and consecutive brain tissue damage. 1 This process is accelerated by exposure to vascular risk factors such as arterial hypertension, diabetes mellitus and cardiac disease.2,3
Arterial stiffness has been related to higher burden of white matter hyperintensities (WMHs).4,5 In addition, recent studies investigated a possible association between arterial stiffness and microstructural changes of the white matter captured by diffusion tensor imaging (DTI) techniques. Arterial stiffness related to lower fractional anisotropy and to higher mean diffusivity, suggesting that increased arterial stiffness exerts widespread detrimental effects on microstructural integrity of the white matter.6–8
Current literature also indicates an association of arterial stiffness and reduced brain volume as well as impaired cognitive abilities, including executive function and processing speed.2,9,10
The gold standard of measurement of arterial stiffness is assessment of carotid-femoral pulse wave velocity (PWV). 11 New technological approaches of PWV measurements, however, have been developed. These include pulse wave analysis which can easily be combined with a blood pressure measurement device allowing repetitive evaluation in an ambulatory 24-h setting.12,13 As arterial stiffness is highly depending on arterial blood pressure, a circadian rhythm of PWV is assumed.14,15 Data on circadian changes of PWV and its associations to cerebral structural damage and impairment of cognitive function, however, are lacking.
Therefore, this study aims to investigate the associations between PWV and its circadian fluctuations and focal SVD-related brain lesions, the peak width of skeletonized mean diffusivity (PSMD), brain volume and cognitive functioning.
Methods
Study design and patient data assessment
The study cohort consists of participants of the Austrian Stroke Prevention Family Study (ASPS-Fam) which was started as a single-centre, prospective, community-based follow-up study in 2006. The aim was to investigate the effect of vascular risk factors on cerebral morphology and cognitive function in an elderly cohort (age 50–75 years) and their first-degree relatives. Patients with a history of cerebrovascular events or dementia were excluded.16,17
In this study, all patients of the ASPS-Fam cohort who underwent 3-Telsa magnetic resonance imaging (MRI) brain imaging and 24-h blood pressure measurement with pulse wave analysis were included (Figure 1). Since 2011, PWV measurement was consecutively administered as part of the study protocol and all patients were included with the exception of 18 individuals who did not undergo brain MRI. There were no significant differences between patients with PWV measurement and the remainder of Austrian Stroke Prevention Family Study participants regarding demographics and vascular risk factors. In addition, a subgroup of the cohort underwent neuropsychological testing. All diagnostic tests were taken on the same day in one ambulatory visit.
Figure 1.
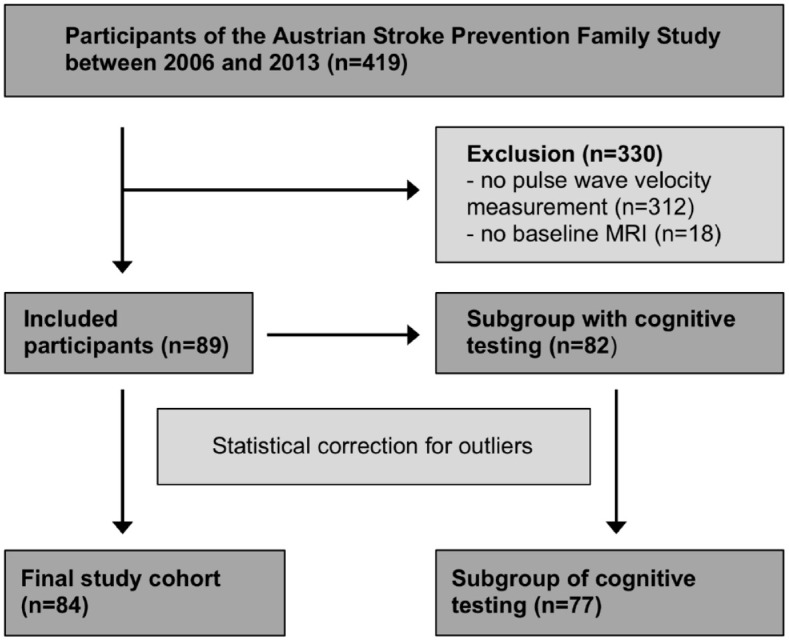
Flow diagram of patient selection and subgroup analysis.
Data comprised demographics, vascular risk factors and current medication. Vascular risk factors such as arterial hypertension, diabetes mellitus and cardiac disease were defined according to recent guideline recommendations18–20 or if respective medication was already prescribed. Blood pressure variability was calculated using the standard deviation of the 24-h systolic blood pressure measurements.
Analysis of PWV
All patients underwent pulse wave analysis which was included in a 24-h blood pressure measurement. The PWV was assessed using the Mobil-o-Graph (I.E.M. Stolberg, Germany), an oscillometric ambulatory blood pressure monitoring device. 21 Following diastolic blood pressure measurement, the device records the pulse waveform over 10 s. Thus, its integrated software (HMS CS 5.1) generates an estimated aortic pulse wave form and calculates the aortic PWV via its own ARCSolver algorithm. 22
All measurements during 24 h were summed up and divided by the number of measurements to create an average 24-h PWV value. Pragmatically, for the average day-time PWV value only measurements between 6:00 a.m. and 10:00 p.m. and for the average night-time PWV value measurements between 10:00 p.m. and 6:00 a.m. were included, respectively.
Brain imaging
MRI investigations were performed on a 3-Tesla scanner. Apart from standard T1 and T2 sequences, the study protocol included an axial fluid-attenuated inversion recovery sequence (repetition time = 10.000 ms, echo time = 69 ms, slice thickness = 3 mm), DTI (repetition time = 4000–5000 ms, echo time = 28, slice thickness = 5 mm, acquisition matrix = 128 × 128), magnetization transfer imaging (MTI, repetition time = 40 ms, echo time = 7.38 ms, flip angle = 15°, slice thickness = 3 mm, resolution = 0.86 × 0.86 mm2) and a high-resolution T1-weighted 3D sequence (MPRAGE, repetition time = 1900 ms, echo time = 2.19 ms, flip angle = 9°, isotropic resolution = 1 mm).
Peak width of Skeletonized Mean Diffusivity (PSMD) was generated from the DTI data using fractional anisotropy maps and histogram analysis of the mean diffusivity within the voxels of the white matter skeleton [FMRIB Software Library (FSL)] according to a script initially described by Baykara et al. 7 (freely available online at http://www.psmd-marker.com/). PSMD is thus defined as the difference between the 95th and the 5th percentile of mean diffusivity levels distribution of all integrated voxels.
WMH volume (cm3) was measured using the IDL programme (Exelis Visual Information Solutions, USA), in which single lesions were identified and outlined. Every single lesion was segmented and multiplied by slice thickness to calculate the total WMH volume (FSLMATHS, FSL).
Total brain volume was assessed using the software SIENAX (FSL 6.0) which extracts brain and skull images and compares them with standardized samples. Thus, segmentation according to tissue type was performed in order to create the volume of basal ganglia, neocortical tissue and white matter volume apart from total brain volume. An adjustment for intracranial volume was performed.
Cognitive function
A subgroup of the study cohort underwent neuropsychological testing (Figure 1) using a predefined neuropsychological test battery which comprised tests evaluating executive function, visuopractical skills, memory and learning abilities. 17 Executive function was assessed using the Wisconsin Card Sorting Test, the Trail Making Test (part B) and the Digit Span Backwards Test. Visuopractical skills were evaluated with the Purdue Pegboard Test. 23 Memory and learning were tested using Bäumler’s learning and memory test. This test battery consists of six subcategories which evaluate verbal, figurative and visual memory.
Summary measures of cognitive functions were calculated by converting test results to z scores and computing the average scores within each cognitive domain. We additionally calculated a measure of global cognitive ability from all cognitive domains. 24 All tests administered to the study participants are known to be sensitive to detect even subtle cognitive impairment and we used summary measures of cognitive function in the analyses rather than the results of individual tests to reduce the likelihood for ceiling effects.
Statistical analysis
The IBM SPSS Statistics (version 26) was used for statistical analysis. The number of study participants was corrected for outliers according to the parameter brain volume. Outliers were defined as variables outside the range of the standard deviation multiplied by 2.5.
Nominal parameters are shown in absolute numbers and percentages. Continuous parameters were tested for normal distribution using the Shapiro–Wilk test. If normally distributed, they are presented using mean and standard deviation. The median and interquartile range are given for non-normally distributed data. Univariate group comparisons were done by either two-sample independent t test (parametric data) or the Wilcoxon test (nonparametric data).
To identify variables that correlate to PWV, Spearman rank correlation was used. A multivariable mixed linear model was used to evaluate the relationship between PWV and imaging parameters. This analysis was adjusted for age, sex, vascular risk factors and family structure. Afterwards, false discovery rate correction (FDR) 25 was performed. Statistical significance was defined as a probability value below 0.05.
Results
A total of 84 participants (median age 72 ± 14 years) were included in the study (Figure 1). There were 43 women and 41 men. Baseline characteristics, including vascular risk factors and imaging parameters, are presented in Table 1. The most common vascular risk factors were smoking (50%) and arterial hypertension (73%). Ongoing treatment for arterial hypertension was taken by 21% of participants (n = 18). The use of antihypertensive medication was not related to PWV in multivariate analysis corrected for age and sex (p = 0.804) and was identical for night- and day-time values (p > 0.8, respectively).
Table 1.
Demographics, vascular risk factors and imaging measures and their correlation to pulse wave velocity (24-h measurement).
| All patients (N = 84) | Pulse wave velocity 24 h (Spearman correlation, rs) |
p | |
|---|---|---|---|
| Demographics | |||
| Age, years, median (IQR) | 72 (14.3) | 0.942 | <0.001 |
| Women, n (%) | 43 (51.2) | −0.024 | 0.831 |
| Education, years, median (IQR) | 10 (3.0) | −0.178 | 0.106 |
| Risk factors | |||
| Hypertension, n (%) | 61 (72.6) | 0.393 | <0.001 |
| Diabetes mellitus, n (%) | 11 (13.1) | −0.006 | 0.958 |
| Smoking, n (%) | 42 (50.0) | 0.036 | 0.751 |
| Cardiac disease, n (%) | 25 (29.8) | 0.282 | 0.009 |
| Systolic blood pressure, mmHg, mean ± SD | 122.7 ± 9.8 | 0.293 | 0.007 |
| Systolic blood pressure variability, mmHg, mean ± SD | 15.8 ± 3.5 | 0.274 | 0.012 |
| MRI parameters (cm3) | |||
| White matter hyperintensity volume, median (IQR) | 5.2 (8.0) | 0.472 | <0.001 |
| Peak width of skeletonized mean diffusivity mmHg, mean ± SD | 3.1 × 10–4 ± 0.4 × 10–4 | 0.452 | <0.001 |
| Total brain volume, median (IQR) | 1091.2 (156.8) | −0.617 | <0.001 |
| Basal ganglia volume, median (IQR) | 530.0 (69.6) | −0.593 | <0.001 |
| Neocortical volume, median (IQR) | 428.9 (55.0) | −0.553 | <0.001 |
| White matter volume, median (IQR) | 557.8 (74.0) | −0.503 | <0.001 |
| Cognitive testing (Z scores) | |||
| Executive function, median (IQR) | 0.219 (0.85) | −0.303 | 0.007 |
| Visuopractical skills, median (IQR) | 0.133 (1.19) | −0.564 | <0.001 |
| Memory and learning, mean ± SD | 0.027 ± 0.92 | −0.397 | <0.001 |
| Global cognitive performance, mean ± SD | 0.025 ± 0.92 | −0.491 | <0.001 |
IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation.
The 24-h measurement of PWV [median = 10 m/s, interquartile range (IQR) = 3 m/s] revealed higher values during day-time than during the night-time (median = 10 m/s, IQR = 3 m/s versus median = 9 m/s, IQR = 2 m/s, p < 0.001, Figure 2).
Figure 2.
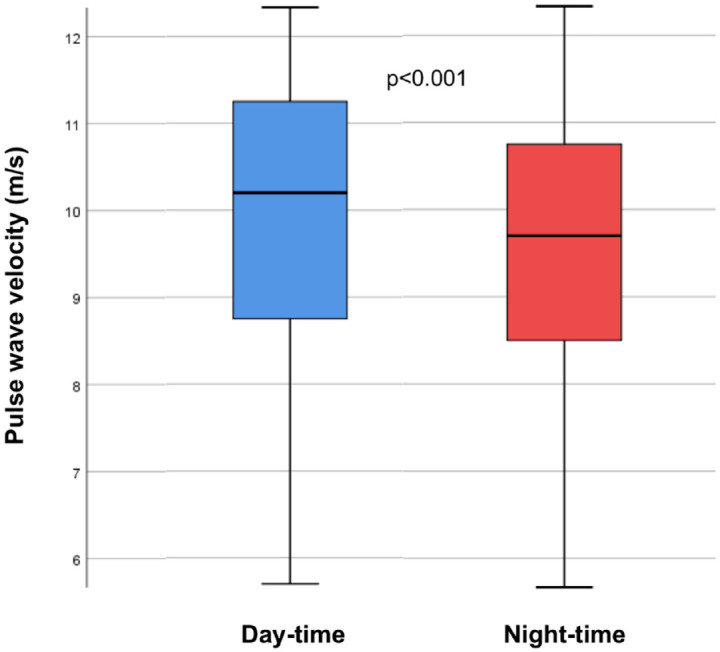
Difference of pulse wave velocity during day-time and night-time measurements.
Cerebral SVD markers
In univariate analysis, PWV was significantly related to WMH volume (rs = 0.472, p < 0.001) and PSMD (rs = 0.452, p < 0.001). Associations were seen for day-time and night-time PWV. The associations were no longer significant when correcting for age and vascular risk factors (Table 2).
Table 2.
Multivariable linear regression model relating PWV to imaging parameters during 24-h measurement. a .
| β coefficient | SE | p | |
|---|---|---|---|
| PWV 24 h | |||
| White matter hyperintensity volume | −0.142 | 0.260 | 0.587 |
| Peak width of skeletonized mean diffusivity | 7.06 × 10−6 | 1.07 × 10−5 | 0.444 |
| Total brain volume | −36.871 | 14.787 | 0.015 b |
| Basal ganglia volume | −17.188 | 8.129 | 0.038 |
| Neocortical volume | −9.802 | 7.404 | 0.189 |
| White matter volume | −19.682 | 8.743 | 0.027 |
| PWV day-time | |||
| White matter hyperintensity volume | −0.261 | 0.199 | 0.194 |
| Peak width of skeletonized mean diffusivity | −5.76 × 10−8 | 8.11 × 10−6 | 0.995 |
| Total brain volume | −17.460 | 11.918 | 0.147 |
| Basal ganglia volume | −6.073 | 6.536 | 0.356 |
| Neocortical volume | 1.064 | 5.884 | 0.857 |
| White matter volume | −11.386 | 6.975 | 0.107 |
| PWV night-time | |||
| White matter hyperintensity volume | 0.048 | 0.211 | 0.820 |
| Peak width of skeletonized mean diffusivity | 1.01 × 10−5 | 7.01 × 10−6 | 0.228 |
| Total brain volume | −32.300 | 11.493 | 0.006 b |
| Basal ganglia volume | −15.143 | 6.344 | 0.019 b |
| Neocortical volume | −10.500 | 5.752 | 0.069 |
| White matter volume | −17.156 | 6.811 | 0.014 b |
PWV, pulse wave velocity.
Analysis is adjusted for age, sex, systolic blood pressure, diabetes mellitus and cardiac disease.
Remained significant after false discovery rate correction (FDR).
Brain volume
As can be seen in Table 1, in univariate analysis PWV was correlated to total brain volume (rs = –0.617, p < 0.001), white matter volume, neocortical grey matter volume and basal ganglia volume After correction for possible confounders, including age, sex, systolic blood pressure and major vascular risk factors, 24-h PWV related significantly to total brain volume, white matter und basal ganglia volume (Table 2). Night-time PWV related to all segmented brain volumes, but there were no associations with PWV measured during day-time (Table 2). The association between 24-h PWV and total brain volume as well as the associations between night-time PVW and total brain volume, white matter and basal ganglia volumes remained significant after FDR correction for multiple comparisons.
Cognitive impairment
A total of 77 patients underwent neuropsychological testing to evaluate cognitive function. PWV was related to executive function, visuopractical skills, memory/learning and the global cognitive performance in univariate analysis (Table 1), but these associations were no longer significant when correcting for possible confounders, including age and vascular risk factors.
Discussion
This community-based cohort study of elderly individuals shows a significant relationship between increased PWV and reduced brain volume. While night- and day-time PWV correlated with reduced cortical and white matter volume, only night-time PWV reached statistical significance. The associations between PWV and brain volume were independent of vascular risk factors, including arterial hypertension and its variations. Our data confirm previous studies on PVW being a marker of increased risk for brain atrophy.2,9 The finding that night-time elevations are of higher relevance is a novel finding. After correction for potential confounders, we failed to show a relationship between PWV and focal cerebral SVD-related changes and cognitive function.
This contrasts a previous investigation of Van Sloten et al. who reported PWV elevations to relate to increased WMH burden in a meta-analysis of 23 studies. 5 We cannot exclude that the sample size of our investigation was too small to detect a weak association of PWV and WMHs. It is also of note that the previous meta-analysis also included patients with cerebrovascular and Alzheimer’s disease who are likely to have larger WMH loads, while these conditions were considered exclusion criteria in our study on community-dwelling persons. 26
We also failed to show an association with DTI-detected microstructural white matter changes measured by PSMD.7,8 DTI measures are considered to have higher sensitivity for detection of SVD-related brain changes than WMH volume. 6
Previous data on the association between arterial stiffness and brain volume are inconsistent. While the community-based ‘Reykjavik’ study did not find a significant association of PWV and global brain atrophy, 4 Palta and co-workers corroborate our findings by showing an inverse relationship between PWV and total brain volume. 2 Similar results come from the Framingham study which also reported an inverse relationship cross-sectionally but failed to determine an association with progression of brain atrophy over an observational period of 6 years. 9
The pathophysiological processes underlying the association between PWV and brain atrophy are incompletely understood. Some authors suggested that increasing PWV leads to remodelling in the cerebral microvasculature of the whole brain resulting in reduced blood flow and loss of brain volume.10,27 The lack of an association with vascular changes in our investigation is rather against this view. Another explanation is exacerbation of age-related neurodegenerative processes. A longitudinal study by Hughes et al. 28 demonstrated a relationship between increased arterial stiffness and the progression of beta amyloid plaques in 81 nondemented elderly patients. This association was independent of sex, hypertension and age.28,29 Nonetheless, at this point mechanistic explanations on the relationship between arterial stiffness and brain atrophy are speculative.
Recently published studies investigating arterial stiffness and its influence on brain structure and function rely on single PWV measurements. To the best of our knowledge, we here present the first study investigating the relationship of 24-h PWV, structural brain damage and cognitive function under consideration of day-time and night-time values. A higher importance of night-time measures is also supported by a study of Aissopou et al., 30 who demonstrated an association between 24-h PWV and a reduced diameter of retinal vessels with the relationship being closest for night-time measurements. 30
PWV is highly dependent on arterial blood pressure. Circadian changes of systolic blood pressure could have been responsible for our study findings. We, however, corrected our analysis for systolic blood pressure which indicates an influence of the circadian changes of PWV on brain volume being independent of blood pressure and its variations.
The limitations of this study are its cross-sectional design and the relatively small number of study participants. Strengths are the thorough diagnostic work-up, including assessment of the circadian fluctuations of PWV in 24-h measurement, and quantitative MRI evaluation of structural and microstructural changes. The finding of our study that increased PWV particularly during night-time relates to lower total brain volume might be of clinical relevance. It is known that life style modifications such as weight reduction, physical exercise and smoking cessation can reduce arterial stiffness.31,32 On the contrary, antihypertensive drugs, especially angiotensin-converting enzyme (ACE) inhibitors, are able to significantly decrease arterial stiffness. 33 Respective prescriptions preferably in the evening could reduce night-time PWV and consequently lower the risk of global brain atrophy and its progression. In our study cohort, only 21% of included study participants received antihypertensive medication. We found no significant effect of antihypertensive drugs on PWV in our study cohort. As the number of respective patients in the analysis might have been too small to detect significant associations, these results should be interpreted with caution.
These assumptions warrant further longitudinal research on the exact mechanisms underlying the association of PWV and its circadian changes on brain structure and function. Preventive measures may ultimately be derived.
Conclusion
We showed a relationship between increased PWV representing a measure of arterial stiffness and reduced brain volume. The association was independent of arterial blood pressure but also of blood pressure variability. PWV elevations during night-time are of greater importance than day-time measures. We found no association between PWV and cognitive impairment, but larger samples with longitudinal assessment are needed to ultimately determine the clinical implications of alterations in PWV and its circadian rhythm.
Acknowledgments
The study received financial support from the Austrian Alzheimer Society.
Footnotes
ORCID iDs: Melanie Haidegger  https://orcid.org/0000-0003-0180-7917
https://orcid.org/0000-0003-0180-7917
Markus Kneihsl  https://orcid.org/0000-0002-6334-9432
https://orcid.org/0000-0002-6334-9432
Simon Fandler-Höfler  https://orcid.org/0000-0001-9043-0378
https://orcid.org/0000-0001-9043-0378
Thomas Gattringer  https://orcid.org/0000-0002-6065-6576
https://orcid.org/0000-0002-6065-6576
Contributor Information
Melanie Haidegger, Department of Neurology, Medical University of Graz, Graz, Austria.
Simon Lindenbeck, Department of Neurology, Medical University of Graz, Graz, Austria.
Edith Hofer, Department of Neurology, Medical University of Graz, Graz, Austria; Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, Graz, Austria.
Christina Rodler, Department of Neurology, Medical University of Graz, Graz, Austria.
Robert Zweiker, Division of Cardiology, Department of Internal Medicine, Medical University of Graz, Graz, Austria.
Sabine Perl, Division of Cardiology, Department of Internal Medicine, Medical University of Graz, Graz, Austria.
Lukas Pirpamer, Department of Neurology, Medical University of Graz, Graz, Austria.
Markus Kneihsl, Department of Neurology, Medical University of Graz, Graz, Austria.
Simon Fandler-Höfler, Department of Neurology, Medical University of Graz, Graz, Austria.
Thomas Gattringer, Department of Neurology, Medical University of Graz, Graz, Austria; Division of Neuroradiology, Vascular and Interventional Radiology, Department of Radiology, Medical University of Graz, Graz, Austria.
Christian Enzinger, Department of Neurology, Medical University of Graz, Graz, Austria.
Reinhold Schmidt, Department of Neurology, Medical University of Graz, Auenbruggerplatz 22, 8036 Graz, Austria.
Declarations
Ethics approval and consent to participate: This study was approved by the ethics committee of the Medical University of Graz, Austria (Approval number: 17-088 ex 05/06). Written informed consent was obtained from all study participants.
Consent for publication: Not applicable.
Author contributions: Melanie Haidegger: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Simon Lindenbeck: Data curation; Formal analysis; Methodology; Writing – review & editing.
Edith Hofer: Data curation; Formal analysis; Visualization; Writing – review & editing.
Christina Rodler: Conceptualization; Formal analysis; Writing – review & editing.
Robert Zweiker: Methodology; Writing – review & editing.
Sabine Perl: Methodology; Writing – review & editing.
Lukas Pirpamer: Formal analysis; Methodology; Software; Writing – review & editing.
Markus Kneihsl: Conceptualization; Methodology; Writing – review & editing.
Simon Fandler-Höfler: Formal analysis; Investigation; Writing – review & editing.
Thomas Gattringer: Conceptualization; Methodology; Writing – review & editing.
Christian Enzinger: Conceptualization; Formal analysis; Writing – review & editing.
Reinhold Schmidt: Conceptualization; Investigation; Project administration; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: The data acquired for this study are available from the corresponding author upon reasonable request.
References
- 1.Cooper LL, Mitchell GF. Aortic stiffness, cerebrovascular dysfunction, and memory. Pulse 2016; 4: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palta P, Sharrett AR, Wei J, et al. Central arterial stiffness is associated with structural brain damage and poorer cognitive performance: the ARIC study. J Am Heart Assoc 2019; 8: e011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araghi M, Shipley MJ, Wilkinson IB, et al. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur J Epidemiol 2020; 35: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility – Reykjavik study. Brain 2011; 134: 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Sloten TT, Protogerou AD, Henry RM, et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev 2015; 53: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Palta P, Meyer ML, et al. Aortic stiffness and white matter microstructural integrity assessed by diffusion tensor imaging: the ARIC-NCS. J Am Heart Assoc 2020; 9: e014868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms: novel SVD imaging marker. Ann Neurol 2016; 80: 581–592. [DOI] [PubMed] [Google Scholar]
- 8.Deary IJ, Ritchie SJ, Muñoz Maniega S, et al. Brain peak width of skeletonized mean diffusivity (PSMD) and cognitive function in later life. Front Psychiatry 2019; 10: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 2013; 81: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng L, Vinters HV, Mack WJ, et al. Differential effects of ischemic vascular disease and Alzheimer’s disease on brain atrophy and cognition. J Cereb Blood Flow Metab 2016; 36: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson IB, Mäki-Petäjä KM, Mitchell GF. Uses of arterial stiffness in clinical practice. Arterioscler Thromb Vasc Biol 2020; 40: 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omboni S, Posokhov IN, Kotovskaya YV, et al. Twenty-four-hour ambulatory pulse wave analysis in hypertension management: current evidence and perspectives. Curr Hypertens Rep 2016; 18: 72. [DOI] [PubMed] [Google Scholar]
- 13.Omboni S, Posokhov IN, Rogoza AN. Evaluation of 24-hour arterial stiffness indices and central hemodynamics in healthy normotensive subjects versus treated or untreated hypertensive patients: a feasibility study. Int J Hypertens 2015; 2015: 601812–601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsova TY, Korneva VA, Bryantseva EN, et al. The 24-hour pulse wave velocity, aortic augmentation index, and central blood pressure in normotensive volunteers. Vasc Health Risk Manag 2014; 10: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spronck B, Heusinkveld MH, Vanmolkot FH, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 2015; 33: 330–338. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt R, Lechner H, Fazekas F, et al. Assessment of Cerebrovascular risk profiles in healthy persons: definition of research goals and the Austrian stroke prevention study (ASPS). Neuroepidemiology 1994; 13: 308–313. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt R, Fazekas F, Kapeller P, et al. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 1999; 53: 132–139. [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Mancia G, Spiering W, et al. ESC scientific document group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain JJ, Rhinehart AS, Shaefer CF, et al. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann Intern Med 2016; 164: 542–552. [DOI] [PubMed] [Google Scholar]
- 20.ROSE GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 1962; 27: 645–658. [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W, Tölle M, Zidek W, et al. Validation of the mobil-O-graph: 24 h-blood pressure measurement device. Blood Press Monit 2010; 15: 225–228. [DOI] [PubMed] [Google Scholar]
- 22.Weber T, Wassertheurer S, Rammer M, et al. Validation of a Brachial Cuff-based method for estimating central systolic blood pressure. Hypertension 2011; 58: 825–832. [DOI] [PubMed] [Google Scholar]
- 23.Tiffin J, Asher EJ. The Purdue pegboard: norms and studies of reliability and validity. J Appl Psychol 1948; 32: 234–247. [DOI] [PubMed] [Google Scholar]
- 24.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci 2010; 11: 201–211. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000; 25: 60–83. [Google Scholar]
- 26.Frey BM, Petersen M, Mayer C, et al. Characterization of white matter hyperintensities in large-scale MRI-studies. Front Neurol 2019; 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazzouri AZ, Yaffe K. Arterial stiffness and cognitive function in the elderly. J Alzheimers Dis 2014; 42: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial stiffness and β-amyloid progression in nondemented elderly adults. JAMA Neurol 2014; 71: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roseborough A, Ramirez J, Black SE, et al. Associations between amyloid β and white matter hyperintensities: a systematic review. Alzheimers Dement 2017; 13: 1154–1167. [DOI] [PubMed] [Google Scholar]
- 30.Aissopou EK, Argyris AA, Nasothimiou EG, et al. Ambulatory aortic stiffness is associated with narrow retinal arteriolar caliber in hypertensives: the SAFAR study. Am J Hypertens 2016; 29: 626–633. [DOI] [PubMed] [Google Scholar]
- 31.Ashor AW, Lara J, Siervo M, et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014; 9: e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu-Jie W, Hui-Liang L, Bing L, et al. Impact of smoking and smoking cessation on arterial stiffness in healthy participants. Angiology 2013; 64: 273–280. [DOI] [PubMed] [Google Scholar]
- 33.Boutouyrie P, Lacolley P, Briet M, et al. Pharmacological modulation of arterial stiffness. Drugs 2011; 71: 1689–1701. [DOI] [PubMed] [Google Scholar]


