Abstract
Substance P (SP) is a tachykinin expressed by various cells in the nervous and immune systems. SP is predominantly released by neurons and exerts its biological and immunological effects through the neurokinin receptors, primarily the neurokinin-1 receptor (NK1R). SP is essential for maintaining ocular surface homeostasis, and its reduced levels in disorders like diabetic neuropathy disrupt the corneal tissue. It also plays an essential role in promoting corneal wound healing by promoting the migration of keratocytes. In this review, we briefly discuss the structure, expression, and function of SP and its principal receptor NK1R. In addition, SP induces pro-inflammatory effects through autocrine or paracrine action on the immune cells in various ocular surface pathologies, including dry eye disease, herpes simplex virus keratitis, and Pseudomonas keratitis. We provide an in-depth review of the pathogenic role of SP in various ocular surface diseases and several new approaches developed to counter the immune-mediated effects of SP either through modulating its production or blocking its target receptor.
1. Introduction
Substance P (SP) is an undecapeptide member of the tachykinin family, discovered by Von Euler and Gaddum in 1931 from the equine brain and intestinal tissue extracts [1–3]. It is produced by an array of cells, including neurons, astrocytes, microglia, epithelial and endothelial cells, and immune cells, such as T-cells, dendritic cells (DCs), and eosinophils [4–10]. SP exerts its biological effects through the class I (rhodopsin-like) family of G-protein coupled neurokinin receptors known as neurokinin 1 receptor (NK1R), neurokinin 2 receptor (NK2R), and neurokinin 3 receptor (NK3R) [11]. It exhibits the highest affinity for NK1R, which occurs in two isoforms and is expressed by neurons, immune cells, corneal epithelium, and keratocytes [12].
On the ocular surface, SP is predominantly produced by the innervating ophthalmic branch fibers of the trigeminal ganglion (TG) [13,14]. SP and its metabolites have been documented in the tears of healthy individuals [15]. In patients suffering from corneal hypoesthesia and diabetic keratopathy, SP levels in tears are significantly reduced, thereby disrupting the ocular surface homeostasis [16–18]. The topical application of SP–derived peptide (FGLM peptide) promotes healing in cases of neurotrophic keratopathy [2,19,20]. The current evidence also propounds SP’s role in corneal healing by promoting migration and proliferation of epithelial cells [21–23]. Moreover, SP induces proinflammatory effects causing miosis, intraocular inflammation, and conjunctival hyperemia on ocular application [24,25].
In this comprehensive review article, we outline the structure of SP and its functions at the ocular surface in inflammation and tissue homeostasis. Furthermore, we provide an overview of the structure and expression of the neurokinin receptors through which SP exerts its biological effects and the development of novel therapeutics by modulating its expression.
2. Structure, expression, and function of Substance P
SP is encoded by the Tac1 (pre-protachkinin-A) gene, which also encodes neuropeptide K, neurokinin A, and neuropeptide-gamma [26,27]. Located on chromosome 7 in humans, TAC1 consists of seven exons and six introns that can be alternatively spliced into four different mRNA variants, including the coding sequence for SP [28–30]. The eleven amino acid sequence forming the building blocks of SP are – “H-Arg1--Pro2-Lys3-Pro4-Gln5-Gln6-Phe7-Phe8-Gly9-Leu10-Met11-NH2” - consisting of two positively charged and six non-polar amino acid residues [31]. The amphiphilic properties of SP are attributed to polar, positively charged N-terminal and non-polar, uncharged C-terminus, thus allowing it to interact with the phospholipid bilayer of the plasma membrane [31,32].
SP has been isolated from the central and peripheral nervous system as it is expressed by neurons, astrocytes, and microglia [8,9,33]. Although categorized as a neuropeptide, SP is abundantly produced in various non-neuronal tissues, including the bone and lymphoid organs [34,35]. Moreover, its expression in various immune cells such as T cells, macrophages, DCs, and mast cells is well characterized, suggesting its critical role in mediating the crosstalk between the nervous and immune system [5,36–40]. SP is also expressed by epithelial cells, endothelial cells, and immunomodulatory mesenchymal stem cells [4,10,41]. Due to its ubiquitous expression by numerous cell types throughout the body, SP mediates diverse cell-specific physiological and pathological functions [42].
The earliest and most widely documented function of SP is its role in pain perception and neurogenic inflammation (Fig. 1). The pain sensation promotes its release from the peripheral nociceptive nerve fibers into the synaptic cleft of the dorsal horn, triggering an excitatory postsynaptic potential to induce central sensitization [43,44]. Furthermore, elevated SP levels have been reported in an array of painful corneal pathology models such as DED, infectious keratitis, and intrastromal suture placement, suggesting its role in corneal nociception [45–49]. It has been demonstrated that mice lacking SP exhibited significantly less corneal nociception. Additionally, NK1R antagonism also leads to reduced nocifensive behavior in healthy mice [50]. Despite the consensus regarding SP’s role as a pain signaling molecule, several recent studies have indicated that it also exhibits anti-nociceptive properties in the peripheral nervous system [51,52]. Lin and colleagues reported the anti-nociceptive effect of SP against chronic mechanical hyperalgesia in muscles by attenuating acid-sensing ion channel 3-induced inward current [53].
Fig. 1.
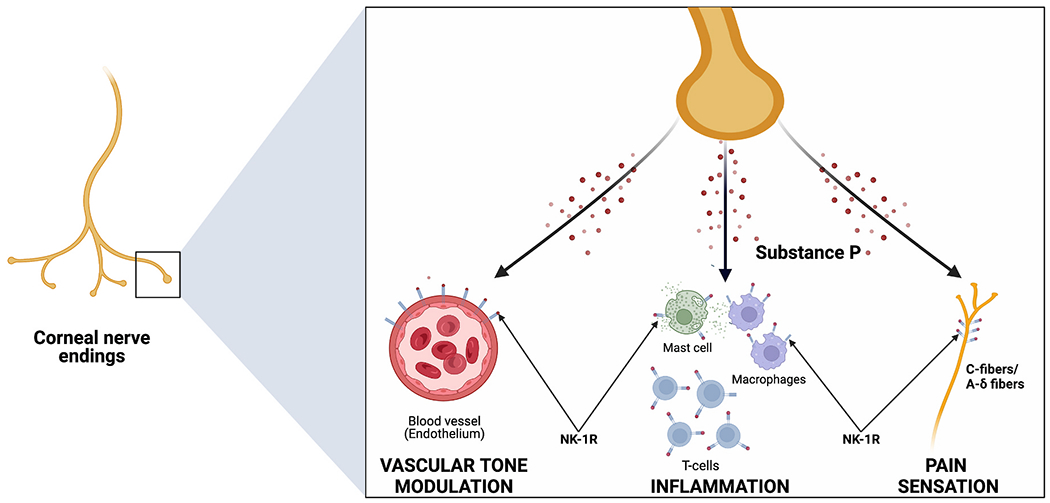
Substance P is primarily released by the corneal nerve endings and acts primarily via the neurokinin-1 receptor (NK1R). (1) It causes vasodilation and increased permeability of the microvasculature directly, and indirectly through degranulation of mast cells. (2) It has pro-inflammatory effects through activation of T-lymphocytes and macrophages. (3) SP also plays in essential role in pain transmission to the CNS via Aδ and C fibers.
Apart from relaying pain signals, SP also induces an immune response and acts as a critical mediator in neuro-immune communication [31,54,55]. It enhances lymphocytic proliferation by upregulating IL-2 expression and directly stimulates immunoglobulin production [56–58]. It enhances the proliferation of bone marrow stromal cells through upregulation of the Wnt signaling pathway [59]. Furthermore, it promotes hematopoiesis via induction of IL-1 and stem cell factors in the bone marrow stroma and the peripheral blood [56,60]. SP-induced production of chemokines and adhesion molecules stimulates immune cell recruitment, further amplifying the inflammatory responses. Several studies have shown that SP upregulates the chemotactic cytokines such as CCL4, CXCL2, MCP-1, CCL5, and IL-8, which recruit monocytes, and lymphocytes to the site of inflammation [61–65]. Moreover, NK1R-deficient mice showed aberrant neutrophil chemotactic response to exogenous IL-1β [66]. The upregulation of adhesion molecules, MAC-1, and its ligand ICAM-1 on NK1R+ DCs induced by SP further facilitates the directional infiltration of macrophages into lymph nodes [67–69].
SP activates eosinophils and cause their degranulation and superoxide release [70]. In a model of atopic dermatitis, Raap and colleagues observed a biphasic response to SP due to expression of both NK1R (few) and NK2R (abundant) in eosinophils [71]. They demonstrated the protective role of SP in preventing eosinophil apoptosis by inducing Ca2+ influx. SP also induces Ca2+ influx and superoxide production by binding to NK1R expressed by neutrophils promoting inflammation [72,73]. It promotes neutrophil chemotaxis to the inflamed tissue by increased production of chemotactic cytokines mentioned previously and induces phagocytic activity [74]. In addition to enhancing proliferation and stimulating the recruitment of immune cells, SP mediates a variety of their functions. It induces superoxide production by neutrophils and amplifies their phagocytic activity [72,74]. In mice, macrophages following SP stimulation resulted in higher secretion of bioactive IL-12, which induces SP expression by macrophages, further underlining the role of SP in perpetuating immune response [75,76]. Similarly, NK1R agonist treatment resulted in IL-12 secretion by bone marrow-derived DCs, which when injected into mice induced type I immunity [69].
Beyond its immunological role, SP exerts a pro-angiogenic effect directly by inducing nitric oxide production in the endothelial cells and indirectly through induction of mast cell degranulation and secretion of various pro-angiogenic factors such as vascular endothelial growth factors (VEGF) and TNF-α [40,77–80]. SP-induced angiogenesis facilitates the trafficking of immune cells to the inflamed tissues. SP plays a role in vasodilation in part by inducing histamine release from the mast cells through NK1R dependent and independent pathways (via direct G protein–mediated activation) [39,40,81,82]. Activated mast cells also secrete tryptase, which activates protease-activated receptors-2 on neurons to release SP, further perpetuating neurogenic inflammation [83].
3. Structure, expression, and signaling of NK1R
Substance P exerts various biological functions by binding to the tachykinin receptors, namely NK1R, NK2R, and NK3R, belonging to the class I (rhodopsin-like) G-protein coupled receptor family [84]. SP interacts with all three receptor types but preferentially binds to NK1R [85,86]. Encoded by TAC1R, NK1R is found on chromosome 2 in humans and consists of seven hydrophobic transmembrane domains, three extracellular and three intracellular loops. Two isoforms of NK1R are found in neuronal and immune cells – full-length and truncated NK1R (NK1R-T) [87]. The full-length NK1R consists of 407 amino acid residues and one C-terminal intracellular domain, whereas NK1R-T is composed of 311 amino acids and lacks the C-terminal intracellular domain [88–90].
Full-length NK1R is expressed primarily in the central nervous system except the cerebellum [91]. In contrast, NK1R-T is predominantly found in peripheral tissues, including the heart, lung, prostate and spleen, and immune cells such as human monocytes, macrophages and colonic epithelial cells [90,92,93]. The truncated isoform does not interact with β-arrestin, an essential adapter protein in SP signaling desensitization, resulting in impaired desensitization and endocytosis of the SP-NK1R complex [94]. Moreover, NK1R-T has a ten-fold lower affinity and elicits a weaker electrophysiologic response to SP than the full-length isoform [88]. The two isoforms also exhibit contrasting signaling properties; SP stimulation triggers phosphorylation of PKCδ in cells expressing NK1R but inhibits phosphorylation of PKCδ in cells expressing NK1R-T [86]. The speed of ERK activation (peak within 1–2 min) is significantly faster upon binding to full-length NK1R compared to truncated NK1R binding (peak within 20–30 min). These findings suggest that carboxyl terminus in full form of NK1R plays a crucial role in activating downstream signaling pathways.
SP exerts varied functions upon binding to the two isoforms expressed by the immune cells. SP induced NK1R-T stimulation does not activate NF-κB, resulting in decreased mRNA expression of IL-8 [89,94]. On the contrary, stimulation of NK1R-T expressed on human peripheral blood monocytes enhances CCL5-induced calcium mobilization but does not mobilize intracellular calcium [65]. Moreover, a recent study reported that monocytes that exclusively express NK1R-T and are incapable of triggering calcium mobilization start expressing the full form of NK1R after differentiating into macrophages and exhibiting SP-induced calcium response [90].
Upon activation of NK1R, G-protein coupled receptor kinases (GRKs) translocate from the cytosol to the plasma membrane and phosphorylate the carboxyl-terminal domain of NK1R bound to SP [95]. β-arrestins concurrently translocate to the plasma membrane to interact with the phosphorylated NK1R [96]. The SP/NK1R-β-arrestin complex is internalized by an endosome which subsequently undergoes acidification [97]. Once dissociated from NK1R, SP is degraded by endothelin-converting enzyme-1 (ECE-1), freeing the receptor to be recycled back to the cell surface [98]. This process of de- and re-sensitization is tightly regulated primarily by the concentration of SP. At low SP concentrations (<1 nM), NK1R is minimally phosphorylated and internalized but rapidly dissociates from β-arrestin to be recycled back to the cell surface, whereas high SP concentrations (>10 nM) result in extensive phosphorylation and internalization of NK1R with prolonged association with β-arrestin [66]. Moreover, following prolonged stimulation with SP, NK1R is ubiquitinated and ultimately degraded by lysosomes [99].
Depending on the G protein subtype associated with the NK receptors, SP can induce different signaling pathways and result in diverse physiological and pathological changes. The stimulation of the Gq subunit activates phospholipase Cβ (PLCβ), which causes the formation of inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 increases the cytosolic calcium levels regulating the phosphoinositol 3-kinase-mediated activation of the anti-apoptotic molecule Akt [100]. Moreover, the calcium mobilization and DAG trigger protein kinase C (PKC) to initiate the NF-κB-mediated production of pro-inflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α) [101,102]. Signaling through the Gs subunit of the NK receptor activates adenylate cyclase, which increases the intracellular concentration of cyclic adenosine monophosphate (cAMP) and stimulates protein kinase A (PKA) [103–105]. PKA, like PKC, signals mitogen-activated protein kinase (MAPK) to activate extracellular signal-regulated kinases (ERK) 1 and 2, which translocate into the nucleus and mediate the expression of various cytokines [106]. NK1R signaling also transactivates the epidermal growth factor receptor (EGFR), which activates the p38/MAPK pathway and extracellular ERK 1 and 2 to promote cell proliferation [64,107,108].
4. Role of Substance P and NK1R in ocular surface physiology
The cornea is one of the most densely innervated tissues in the human body, with a surfeit of SP producing sensory nerves [10,109]. In addition to sensory function, corneal nerves play an important role in the blink reflex, wound healing, tear production and secretion, and trophic factors such as SP [15]. Aside from the trigeminal sensory neurons, corneal epithelial cells, stromal keratocytes, and immune cells secrete SP at the ocular surface [5,10]. Due to the constitutive SP expression at the ocular surface and its presence in normal human tears, several studies have explored its role in preserving tissue homeostasis [45,110]. SP release from the corneal nerve endings has been shown to mediate tear reflex [15]. Furthermore, the evidence in the literature highlights the critical role played by SP in maintaining the corneal epithelial integrity through the upregulation of tight junction proteins, zonula occludens-1 and E-cadherin [111,112]. SP also contributes to the maintenance of corneal epithelial architecture through regulating regeneration of the corneal epithelium. SP enhances insulin-like growth factor-1 (IGF-1), fibronectin, and IL-6 to promote corneal epithelial migration [113,114]. Additionally, SP modulates epithelial cell attachment to the extracellular matrix to promote wound healing. Specifically, SP and IGF-1 upregulate α5β1 integrin (a fibronectin receptor/integrin), inducing tyrosine phosphorylation of focal adhesion kinase and paxillin in corneal epithelial cells, thereby promoting their attachment to extracellular matrix proteins [115,116]. In fact, several studies have demonstrated the therapeutic efficacy of SP and IGF-1 in promoting corneal wound healing in patients with neurotrophic and anhidrotic keratopathy, herpetic keratitis, and Riley-Day syndrome [19,20,117]. In another study, SP prevents hyperosmotic stress-induced apoptosis of corneal epithelial cells through Akt activation [118]. Moreover, naïve NK1R−/− mice show excessive exfoliation of the apical corneal epithelial cells, suggesting SP expression is critical to maintaining the corneal epithelial integrity [119].
Beyond modulating neurogenic inflammation, SP plays a critical role in maintaining immune homeostasis at the ocular surface by regulating both pro-and anti-inflammatory cytokines and maintaining the epithelial barrier against infections. It upregulates the production of IFN-γ from natural killer cells to exert a protective effect against invading bacteria. Concomitantly, SP also downregulates the mTOR pathway, resulting in increased expression of proinflammatory cytokines IL-12p40 and IL-23 and decreased expression of IL-10 [120,121]. Contrary to its pro-inflammatory function in innate immunity, SP is reported to induce the loss of immune balance by affecting the adaptive immune response at the ocular surface. Paunicka and colleagues demonstrated that severing corneal nerves in one eye induced SP secretion in both eyes, resulting in the loss of corneal immune privilege. Consequently, regulatory T cells were suppressed, and the mice showed higher allograft rejection in both eyes [122]. Literature suggests that SP plays multi-faceted roles on the ocular surface by either promoting or disrupting the ocular surface physiology; therefore, future studies are warranted to better understand the mechanisms responsible for the evident dichotomy in SP functions at the ocular surface.
5. Role of substance P and Neurokinin 1 receptor in ocular surface disease pathogenesis
a. Herpes Simplex Keratitis
In the preclinical stages of Herpes Simplex Keratitis (HSK), the neuronal damage leads to a progressive reduction in SP levels [123]. SP levels increase in the clinical stages of the disease, typically peaking at 14 days post-infection [124,125]. (Fig. 2) Moreover, higher levels of SP are observed in severe HSK cases due to the excessive release of SP by extensively damaged nerves. There is a concurrent pro-inflammatory effect is driven by SP binding to NK1R, primarily due to activation of NF-κB signaling pathway, causing increased expression of inflammatory cytokines (IL-6 and IFN-γ) and chemokines [such as CCL3, CXCL2, macrophage inflammatory protein-2 (MIP-2) MIP-1α, and monocyte chemoattractant protein-1 (MCP-1)] generating an influx of immune cells into the inflamed tissue [119,124,126–128].
Fig. 2.
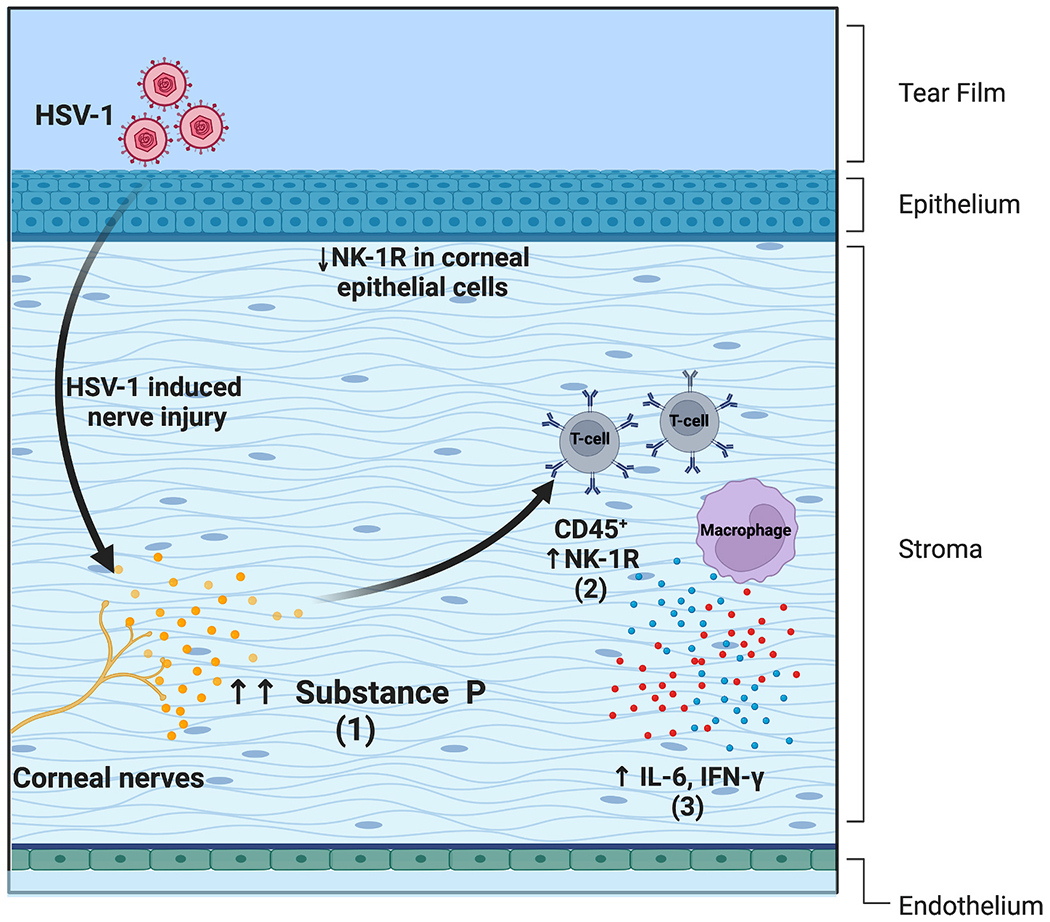
(1) An increase in SP levels is observed in severe cases of herpes simplex keratitis due to excessive release by damaged nerves. (2) In HSV-1 infected corneas, non-immune cells (CD45−) exhibit reduced cell surface expression of NK1R post-infection and immune cells (CD45+ T-cells and macrophages) show an increase in the NK1R expression. (3) There is also a concurrent SP mediated increase in levels of pro-inflammatory cytokines (IL-6 and IFN-γ).
The non-immune cells (CD45−) exhibit reduced cell surface and increased intracellular expression of NK1R at 15 days post-infection. In contrast, immune cells (CD45+) isolated from the cornea increase the NK1R expression, underlining the possible immune crosstalk between corneal nerves and immune cells in viral keratitis. As such, subconjunctival treatment of clinical HSK with Spantide I, an NK1 antagonist, substantially attenuated corneal opacity and angiogenesis. Specifically, the study reports a significant reduction in the percentage of helper T-cells (CD45+CD4+) and neutrophils (CD45+Ly6G+CD11b+) on day 16-day post-infection, indicating the pro-inflammatory role of corneal SP in severe HSK development.
Interestingly, corneal opacity and angiogenesis are aggravated in NK1R−/− mice in early stages of HSK, contradicting the previous hypothesis that the absence of NK1R reduces disease severity [128]. Gaddipati and colleagues reported early development of severe HSK in infected NK1R−/− mice compared to infected C57BL/6J (B6) mice controls [119]. A significantly higher viral load and elevated levels of chemoattractant chemokines such as CXCL1, CXCL2, and CCL2 were observed in NK1R−/− mice. The rapid post-infection pathological changes in NK1R−/− mice, such as epithelial sloughing, reduced central antigen-presenting cells, higher viral load, cytokines, and chemokines levels, and increased mature neutrophils and Th1 cells, underline the critical role of NK1R in maintaining ocular surface homeostasis and reduces the susceptibility to develop severe HSK upon ocular HSV-1 infection. The use of Spantide I (an antagonist of NK1) is effective in significantly reducing the percentage of T-cells (CD45+CD4+) and neutrophils (CD45+Ly6G+CD11b+) at 16 days post-infection, indicating that the absence of SP-NK1 signaling is more detrimental than the antagonism of NK1R [124].
b. Pseudomonas Keratitis
Pseudomonas aeruginosa is Gram-negative bacteria and is one of the most common causes of keratitis, particularly in cases associated with contact lens use [129]. Recent studies have outlined the role of SP in Pseudomonas keratitis in murine models. Lighvani and colleagues outlined the role of SP in the induction of immune response resulting in increased IFN-γ production by NK cells via NK1R interactions [130]. The role of SP in Pseudomonas keratitis were confirmed by blocking the effects through Spantide I application, which resulted in significantly reduced corneal IFN-γ and IL-18 levels and corneal perforation. Moreover, Spantide I application significantly reduced type I cytokines and enhanced IL-10 production in mice with Pseudomonas keratitis, thereby improving the disease outcomes [46]. SP promoted mRNA expression of growth factors - hepatocyte growth factor and fibroblast growth factor-7 [131]. Zhou and colleagues reported better disease outcomes by delaying the apoptosis of polymorphonuclear cells (PMN) by blocking SP interaction with NK1R and improved outcomes in susceptible C57BL/6 mice [48].
c. Dry Eye Disease
DED is a multifactorial ocular surface disorder with an estimated prevalence of 6.8% among the US adult population [132]. It can present as a stand-alone disease or an ocular manifestation of immune-mediated systemic disorders such as graft-versus-host disease, Sjögren syndrome, and rheumatoid arthritis [133–136]. Moreover, DED patients have compromised corneal sub-basal nerves, more pronounced in aqueous-deficient DED [137]. DED is characterized by an inflammatory process, most notably a Th17 mediated inflammation that suggests an autoimmune pathology [122,138]. With its pro-inflammatory effects, SP plays a role in the pathogenesis of several autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease [7,120,139,140].
Over the years, several groups including ours have studied the role of SP in the auto-immune component of dry eye disease (DED), which is primarily mediated by Th17 cells [2]. Significantly higher levels of SP are observed in tears of DED patients and the cornea, conjunctiva, and draining lymph nodes (dLNs) of DED murine models in comparison to their healthy counterparts. Elevated levels of SP in cornea and trigeminal ganglion have also been reported with DED symptoms in a menopause model using ovariectomized rats [141]. The high levels of SP trigger a neurogenic inflammatory response, which leads to the release of various pro-inflammatory cytokines in the ocular surface milieu [42]. The pro-inflammatory cytokines promote the maturation of antigen-presenting cells at the ocular surface, which migrate to dLNs and prime naïve T cells to generate CD4+ helper T cells, including Th17 cells [142,143]. (Fig. 3) In DED, an increase in the frequency of the NK1R-expressing regulatory T-cells (Tregs) is observed; however, these Tregs show aberrant expression of immunomodulatory markers such as CTLA-4, PD-1, and cytokines TGF-β and IL-10. Moreover, these NK1R+Tregs had a weaker suppressive function against T effector cells, indicating a certain degree of dysfunctionality.
Fig. 3.
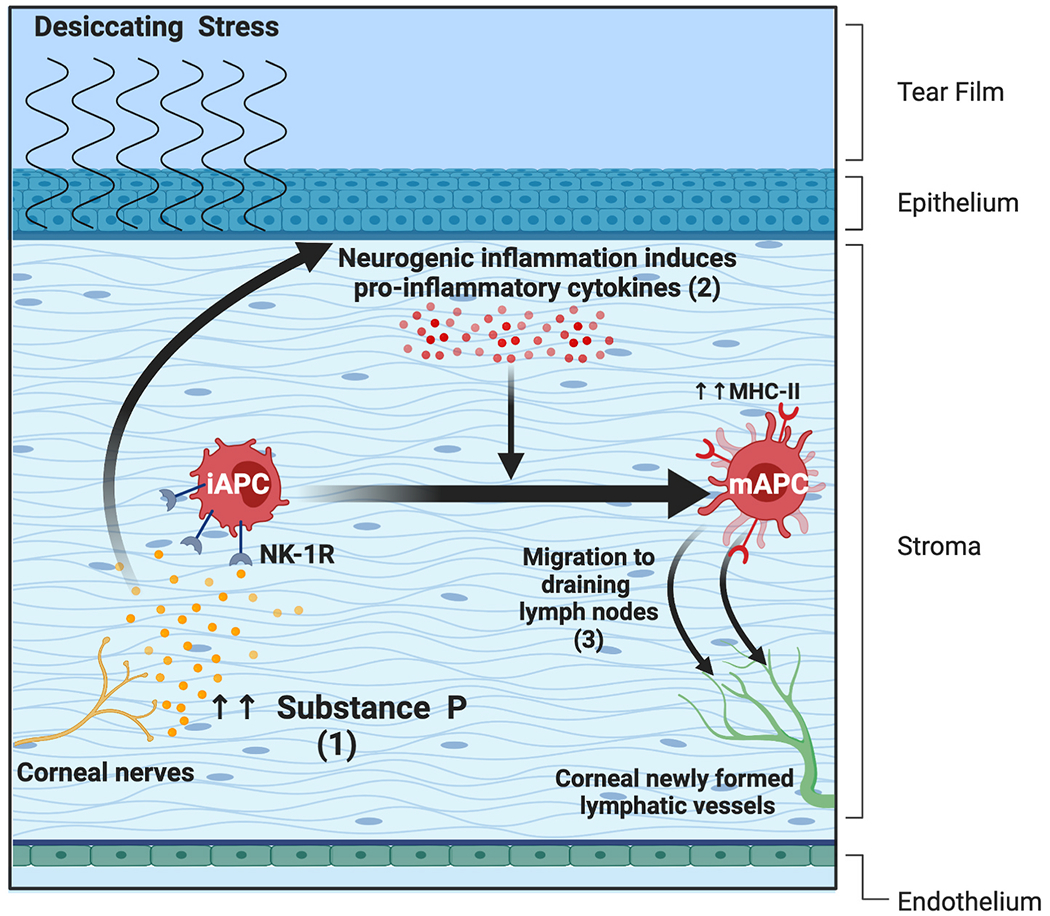
(1) Elevated levels of SP trigger a neurogenic inflammatory response, which leads to the release of pro-inflammatory cytokines in the ocular surface microenvironment. (2) The pro-inflammatory cytokines promote the maturation of antigen-presenting cells (APCs) at the ocular surface. (3) These APCs migrate to dLNs and prime naïve T cells to generate CD4+ helper T cells, including Th17 cells.
Activation of heat receptors transient receptor potential cation channel (subfamily V member 1/subfamily A member 1) (TRPV1/TRPA1) and cold receptor transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8) induce SP to be released on the ocular surface and cause pain [144,145]. A recent report using an extra orbital lacrimal gland excision murine model of DED demonstrated cold nociception is mediated by SP release, and both TRPV1 and TRPM8 contribute to this process despite the insensitivity of TRPV1 channels to cooling. Furthermore, the cold-induced release of SP via the expression of TRPV1 in TRPM8+ neurons. Given the low frequency of TRPM8 terminals, abnormal SP expression, and hypersensitive nerves in mice following nerve severing surgery, He and colleagues concluded that delayed corneal surgery-induced dry eye-like pain (DELP) mainly occurs due to high SP levels, low frequency of TRPM8 terminals, and hypersensitive nerves [145,146]. Similarly, complications of DED and DELP following refractive surgery in patients have been attributed to surgery-induced corneal denervation and neuroinflammation [147–157].
Moreover, refractive surgery decreases tear production, tear film quality, and blinking reflex, which are the primary underlying factors in the pathogenesis of DED [158]. It has been validated that surgical incisions and laser exposure activate stromal keratocytes (to promote wound healing) and trigger neurogenic inflammation [159]. A clinical cross-sectional study revealed tear SP levels positively correlate with the severity of dry eye symptoms and negatively correlate with corneal sensitivity following laser-assisted in-situ keratomileusis (LASIK). Liu and colleagues compared the tear proteomic and neuromediator profiles following small incision lenticule extraction (SMILE) versus LASIK. Their results also showed that SP level was significantly increased postoperatively in the LASIK group, with only a moderate increase in SMILE patients [147,159].
c. Corneal Wound Healing and neurotrophic keratopathy
Substance P produced in the corneal epithelial cells and stromal keratocytes augments IL-8 production in the corneal cells expressing NK1R [160]. Sloniecka and colleagues elucidate the role of IL-8 in promoting keratocyte migration [12]. (Fig. 4) Thus, SP-NK1R interaction appears to promote corneal wound healing. Furthermore, SP reverts the wound healing delay in diabetic corneal epitheliopathy [21]. In an alkali-burn model, the accelerated wound healing properties of SP have been attributed to the mobilization of bone marrow CD29+ stromal cells into circulation and to the site of corneal injury [60].
Fig. 4.
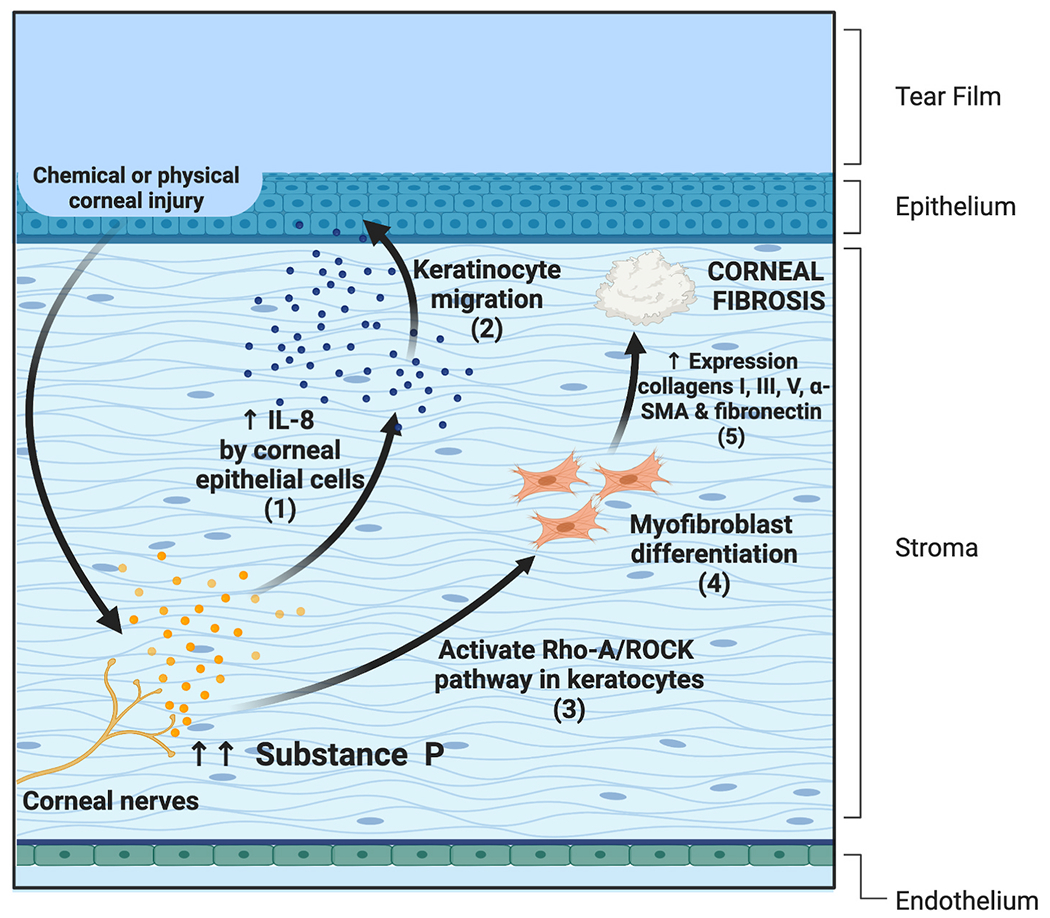
(1) SP promotes corneal would healing by inducing IL-8 production in corneal epithelial cells and (2) promoting keratocyte migration. (3) SP promotes fibrotic changes in the cornea through NK1R mediated activation of the RhoA/ROCK pathway which causes (4) myofibroblast differentiation and (5) an increase in the production of collagen I, III, and V, lumican, α-Sooth Muscle Actin (SMA), fibronectin, and increases corneal fibroblasts contraction.
Recent studies have shown promising outcomes on SP application in combination with insulin growth factor-1 (IGF-1) [161,162]. In a study utilizing ex vivo cultures, SP and IGF-1 promoted corneal epithelial cell migration [163]. This effect occurred in a dose-dependent manner which was primarily mediated by NK1R and through protein kinase C and p38 MAPK activation pathway [115,164,165]. In murine models of neurotrophic keratopathy, the application of SP and IGF-1 recapitulates the corneal barrier function [166,167]. The efficacy of this combination has also been used to significantly accelerate the epithelial healing rate after photorefractive keratectomy in rabbits [168]. However, a recent report revealed the role of SP in promoting fibrotic changes in the cornea through NK1R mediated activation of the RhoA/ROCK pathway, thereby increasing the production of collagen I, III, and V, lumican, α-Sooth Muscle Actin (SMA), fibronectin, and increases corneal fibroblasts contraction, an undesirable function in corneal wound healing [12].
Topical treatment with SP failed to promote corneal epithelial wound healing in an injury-induced model in rabbits [169]. In contrast, the truncated synthetic form of SP, known as FGLM, promotes corneal epithelial cell migration when used in combination with IGF-1 in a rabbit corneal injury model [163]. Similarly, four amino-acid long peptides (SSSR) combined with IGF-1 and FGLM have shown promising outcomes [170]. Applying truncated synthetic forms of SP negates the unwanted adverse effects typically associated with SP, such as miosis and angiogenesis [171]. The encouraging outcomes in preclinical studies have laid the foundation to study the efficacy of SP (in combination with IGF-1) and its synthetic forms for treating diseases with an underlying epithelial defect. In 2007, Nishida et al. showed the efficacy of topical treatment with FGLM-amide and IGF-1 to resurface persistent epithelial wounds in 11 patients with neurotrophic keratopathy [ 172 ]. A year later, Yamada et al. reported similar success with FGLM-amide and SSSR containing eye drops in a larger patient set with 25 individuals [173]. In 2009, Chikamoto et al. conducted a randomized clinical trial with 29 diabetic patients. The same eye drop was shown to be effective in preventing superficial punctate keratopathy after cataract surgery [174].
d. Conjunctival hyperemia
The inflammatory response due to dry eye and allergic antigens mediates vasodilation through the dense innervation of the ocular surface by the trigeminal sensory system [175–179]. The conjunctival insults detected by afferent sensory neurons and relayed to the central nervous system led to an efferent sympathetic/parasympathetic response at the ocular surface, causing local release of neuromediators, including SP and calcitonin gene-related peptide (CGRP). In allergy, elevated SP levels have been reported in patients with allergic conjunctivitis [180]. Further studies in a guinea pig ovalbumin-induced allergy model elucidated SP’s role as a mediator of allergic conjunctivitis, causing conjunctival vasodilation and hyperpermeability through NK1R receptors on blood vessels [181].
SP is a known vasodilator and has been shown to cause plasma extravasation in the conjunctiva [182]. SP can cause further vasodilation of the conjunctival microvasculature through enhanced mast cell degranulation and release of TNF-α [183]. Earlier, the mast cell degranulation by SP was considered G-protein mediated activation-dependent instead of NK1R mediated response [39,40]. However, recent studies have reported high expression of NK1R by mast cells. Moreover, studies show a bidirectional signal between mast cells and SP-expressing nerves [81,82]. SP released from sensory nerve endings also acts on mast cells to synthesize TNF-α, which plays a role in vasodilation [80].
6. Modulating Substance P production
The most commonly performed ophthalmic procedures to correct visual acuity - LASIK and PRK- cause severe corneal neuropathic pain [150–153]. In LASIK, epithelial nerve bundles and superficial stromal nerves are excised by a microkeratome, while during PRK, epithelium removal causes inadvertent damage to interspersed epithelial nerves. These procedures injure the corneal nerves and interrupt the transmission of the sensory neurons to the TG and central nervous system, ultimately producing pathologic pain sensation [184]. SP is present either in dorsal root ganglia (DRG) or TG and then packed into vesicles, moved to central and peripheral processes through axonal transport, mediating nociceptive transmission [184,185]. This normal process is interrupted after nerve damage and restoring the physiological condition and consequent basal nociception is challenging. Thus, SP synthesis and transport have been the focus of research in the past two decades.
A deeper understanding of the SP synthesis through the endogenous expression of the Tac1 gene and the associated regulatory elements is being utilized for finding novel therapeutic alternatives for pain-related and behavioral diseases. At the genomic level, the Tac1 gene is driven by the Tac1 promoter (Tac1prom) in the sensory neurons, and its activity is modulated by numerous different stimuli such as nonspecific noxious stimulation LPS (bacterial infection), membrane depolarization potassium-induced, glucocorticoid receptors, and capsaicin [50,130,186–193]. Although the regulatory elements that modulate SP production in corneal nerves are yet to be fully elucidated, corneal SP secretion is augmented on nerve injury, which shows a deleterious effect on Tregs, leading to loss of immune privilege after corneal transplantation [122]. A recent study showed that induction of ocular inflammation by noxious stimulation with benzalkonium chloride triggered SP expression in the TG and persisted even two months post-inflammation resolution [194].
7. Modulation of NK1R activity: clinical implications
a. Corneal neovascularization
The development of corneal neovascularization is associated with significant visual impairment and affects the outcomes of corneal transplantation [195–197]. Although this process is mediated by multiple chemical factors, such as reduction in Pigment Epithelium Derived Factor (PEDF) expression, reports suggest that SP also plays an essential role [198,199].
Detectable amounts of SP are synthesized in the epithelial cells and nerves of the healthy cornea, but vesicle sequestration permits the normal tissue to remain avascular [200]. However, insults to the cornea trigger the release of SP from the nerves, resulting in inflammation and neovascularization [201]. Through its action on NK1R, SP promotes endothelial cell proliferation and migration through the nitric oxide activation pathway combined with fibroblast growth factor-β [77,202]. Indirectly, SP promotes infiltration of leukocytes in the cornea and induces a pro-angiogenic phenotype shift in most leukocyte populations. Specifically, SP favors vessel dilation, leukocyte diapedesis, and chemotaxis [12,63]. Through NK1R, SP stimulates VEGF synthesis in mast cells, superoxide and chemokine production in neutrophils, and IL-12 production in macrophages and DCs [191–193]. Additionally, SP binds to NK2R and induces the release of oxygen radicals. Interestingly, once the inflammation has ensued, SP is produced by activated leukocytes, promoting an autocrine, pro-angiogenic loop [122,130].
The role of SP via NK1R is clinically relevant as lymphangiogenesis plays a critical role in corneal graft rejection [203]. In a patient cohort with corneal neovascularization, SP concentration was significantly elevated in the tear fluid and clinically correlated with the extent of neo-vessel formation [204]. Multiple in vivo preclinical models and clinical studies have suggested a central role of SP in promoting both hemangiogenesis and lymphangiogenesis [49,205,206]. Expectedly, SP knocked down animals exhibited reduced corneal hem-and lymphangiogenesis after inducing inflammatory injuries [204]. This effect was attributed to NK1R activation, as the topical application of selective NK1R antagonist Lanepitant effectively inhibited corneal neovascularization. Interestingly, the anti-angiogenic effect of NK1R blocking was also maintained in pre-established corneal neovascularization, which is notoriously more resistant to treatment [49, 207]. Altogether, the therapeutic modulation of NK1R can be targeted to prevent or curtail corneal neovascularization, prevent consequential corneal opacity, and maintain the corneal immune privilege [208].
b. Dry eye disease
In the desiccating stress murine model of DED, SP promotes the maturation of corneal antigen-presenting cells, consequently activating effector Th17 cells and contributing to the dysfunction of the immunosuppressive function of Tregs [45,143]. These findings have been further confirmed through NK1R antagonism suggesting potential new therapies for DED. The role of SP in inducing nociception and neurogenic inflammation has been widely studied [209,210]. Neurogenic inflammation has been implicated in the development and chronicity of DED [211–213]. Moreover, pain and ocular discomfort are two cardinal symptoms of DED, and evidence suggests stimulation of the lacrimal functional unit in the absence of tear film can result in neurogenic inflammation [211,213–215]. Furthermore, the inflammatory process associated with the DED can modulate the excitability of the small-diameter fibers, leading to the antidromic release of SP and enhancing the immune environment [212].
In a recently published study, we observed topical treatment of DED mice with NK1R antagonists CP-99,994 and L-733,060 suppress acquisition of major histocompatibility complex class II by antigen-presenting cells at the ocular surface and subsequently reduce the generation and activity of Th17 cell in dLNs [45]. The significant amelioration of DED in mice confirmed these changes at the cellular level. In another study from our group, we reported the efficacy of Spantide I (NK1R antagonist) in attenuating the DED immune response [143]. Specifically, we observed that the addition of Spantide I to the in vitro cultures prevented SP-mediated reduction in Treg cell frequencies and their suppressive function. On systemically treating DED mice with Spantide I, we observed that Treg function was restored, pathogenic Th17 response was suppressed, and the animals had significantly lower corneal fluorescein staining scores than the controls.
8. Conclusions
The ubiquitous expression of SP and its receptors highlights their therapeutic potential for various ocular surface diseases ranging from infectious to neurotropic keratitis; however, its functional dichotomy poses a major challenge. As outlined in this review, on one hand, SP promotes corneal epithelial wound healing by promoting cell migration and at the same time causes poor clinical outcome by amplifying the inflammatory response at the ocular surface after chemical or mechanical trauma and infections. Additionally, the evidence in the literature clearly shows a direct correlation between SP expression and severity of ocular surface inflammation. The preliminary evidence suggests that SP promotes epithelial wound healing at low concentrations and short time, whereas a prolonged exposure to significantly elevated levels induces inflammation and angiogenesis. A deeper understanding of the time and concentration dependent impact of SP on the ocular surface is essential for developing therapeutics targeting it.
NK1R receptor blockers (Lanepitant and Fosaprepitant) have been shown to be efficacious in inhibiting angiogenesis and inflammation in animal models and do not have toxic effects to the ocular surface or corneal nerves. However, their therapeutic application remains limited, primarily due to the gaps in knowledge about the factors modulating the NK1R expression levels in different cell types and diseases despite the scientific insight into the structure and functions of SP and neurokinin receptors. Moreover, the current clinical and experimental studies on the effects of SP solely rely on its functions via NK1R. Several groups, including ours, are working on developing insights into the physiological and pathological response generated by SP via the other two receptors - NK2R and NK3R. Due to its high neuronal density, the cornea is a simple yet powerful model for developing a deeper understanding of the mechanisms and role of SP in pain induction, immunology, and angiogenesis. Thus, more studies in the near future utilizing ocular surface disease can shed light on therapeutic applications of SP and targeting its receptors.
Acknowledgements
All the figures included in this manuscript were prepared by Rohan Bir Singh, using Biorender.com.
Funding Sources
This work was supported by National Institutes of Health grants R01EY20889 (RD), K08EY031759 (THD), and core grant P30EY003790.
Footnotes
Financial disclosures
Reza Dana: Kala Pharmaceuticals (Watertown, MA) consultant, Aramis Biosciences (Boston, MA) equity, Claris Biotherapeutics (Boston, MA) equity; The following authors have no financial disclosures: Rohan Bir Singh, Wonkyung Cho, Amirreza Naderi, Gustavo Ortiz, Aytan Musayeva, Thomas H. Dohlman, Yihe Chen, Giulio Ferrari.
Conflict of interest
Massachusetts Eye and Ear owns intellectual property rights related to anti-inflammation by targeting Substance P in ocular surface diseases
All authors attest that they meet the current ICMJE criteria for authorship.
References
- [1].Pernow B. Substance P-A putative mediator of antidromic vasodilation. Gen Pharmacol 1983;14. 10.1016/0306-3623(83)90055-l. [DOI] [PubMed] [Google Scholar]
- [2].Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res 2013;117:118–25. 10.1016/j.exer.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Euler Us v, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol 1931;72. 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Milner P, Bodin P, Guiducci S, Del Rosso A, Kahaleh MB, Matucci-Cerinic M, et al. Regulation of substance P mRNA expression in human dermal microvascular endothelial cells. Clin Exp Rheumatol 2004;22. [PubMed] [Google Scholar]
- [5].Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol 1998;86. 10.1016/S0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- [6].Marriott I, Bost KL. IL-4 and IFN-γ up-regulate substance P receptor expression in murine peritoneal macrophages. J Immunol 2000;165. 10.4049/jimmunol.165.1.182. [DOI] [PubMed] [Google Scholar]
- [7].Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine Schistosomiasis mansoni produce substance P. J Immunol 1988:141. [PubMed] [Google Scholar]
- [8].Barker R, Larner A. Substance P and multiple sclerosis. Med Hypotheses 1992;37. 10.1016/0306-9877(92)90011-Z. [DOI] [PubMed] [Google Scholar]
- [9].Lai JP, Zhan GX, Campbell DE, Douglas SD, Ho WZ. Detection of substance P and its receptor in human fetal microglia. Neuroscience 2000;101. 10.1016/S0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- [10].Watanabe M, Nakayasu K, Iwatsu M, Kanai A. Endogenous substance P in corneal epithelial cells and keratocytes. Jpn J Ophthalmol 2002. 10.1016/S0021-5155(02)00617-2. [DOI] [PubMed] [Google Scholar]
- [11].Suvas S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 2017;199:1543–52. 10.4049/jimmunol.1601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sloniecka M, Roux SLE, Zhou Q, Danielson P. Substance p enhances keratocyte migration and neutrophil recruitment through interleukin-8. Mol Pharmacol 2016;89:215–25. 10.1124/mol.115.101014. [DOI] [PubMed] [Google Scholar]
- [13].Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol 2010;94:784–9. 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- [14].He J, Bazan HEP. Neuroanatomy and neurochemistry of mouse cornea. Investig Ophthalmol Vis Sci 2016;57. 10.1167/iovs.15-18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kovács I, Ludány A, Koszegi T, Fehér J, Kovács B, Szolcsányi J, et al. Substance P released from sensory nerve endings influences tear secretion and goblet cell function in the rat. Neuropeptides 2005;39. 10.1016/j.npep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [16].Yamada M, Ogata M, Kawai M, Mashima Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am J Ophthalmol 2000;129:671–2. [DOI] [PubMed] [Google Scholar]
- [17].Varnell RJ, Freeman JY, Maitchouk D, Beuerman RW, Gebhardt BM. Detection of substance P in human tears by laser desorption mass spectrometry and immunoassay. Curr Eye Res 1997;16. 10.1076/ceyr.16.9.960.5040. [DOI] [PubMed] [Google Scholar]
- [18].Tummanapalli SS, Willcox MDP, Issar T, Yan A, Pisarcikova J, Kwai N, et al. Tear film substance P: a potential biomarker for diabetic peripheral neuropathy. Ocul Surf 2019;17. 10.1016/j.jtos.2019.08.010. [DOI] [PubMed] [Google Scholar]
- [19].Brown SM, Lamberts DW, Reid TW, Nishida T, Murphy CJ. Neurotrophic and anhidrotic keratopathy treated with substance P and insulinlike growth factor I. Arch Ophthalmol 1997;115. 10.1001/archopht.1997.01100160096021. [DOI] [PubMed] [Google Scholar]
- [20].Chikama TI, Fukuda K, Morishige N, Nishida T. Treatment of neurotrophic keratopathy with substance-P-derived peptide (FGLM) and insulin-like growth factor I. Lancet 1998;351. 10.1016/S0140-6736(98)24024-4. [DOI] [PubMed] [Google Scholar]
- [21].Yang L, Di G, Qi X, Qu M, Wang Y, Duan H, et al. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014;63. 10.2337/dbl4-0163. [DOI] [PubMed] [Google Scholar]
- [22].Reid TW, Murphy CJ, Iwahashi CK, Foster BA, Mannis MJ. Stimulation of epithelial cell growth by the neuropeptide substance P. J Cell Biochem 1993;52. 10.1002/jcb.240520411. [DOI] [PubMed] [Google Scholar]
- [23].Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol 2008;92:896–900. 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- [24].Tsuji F, Hamada M, Shirasawa E. Tachykinins as enhancers of prostaglandin E2-induced intraocular inflammation. Ocul Immunol Inflamm 1998;6. 10.1076/ocii.6.1.19.8080. [DOI] [PubMed] [Google Scholar]
- [25].Anderson JA, Hernandez E, Duzman E, Malfroy B. Recombinant enkephalinase effectively inhibits substance p-induced miosis in the rabbit Eye cup model. Curr Eye Res 1990;9. 10.3109/02713689008999594. [DOI] [PubMed] [Google Scholar]
- [26].Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature 1983;306. 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- [27].Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci U S A 1987;84. 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carter MS, Krause JE. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide γ. J Neurosci 1990;10:2203–14. 10.1523/jneurosci.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Krause JE, Takeda Y, Hershey AD. Structure, functions, and mechanisms of substance preceptor action. J Invest Dermatol 1992;98:S2. 10.111l/1523-1747.epl2462082. [DOI] [PubMed] [Google Scholar]
- [30].Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev 2002;54. 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- [31].Chang MM, Leeman SE, Niall HD. Amino-acid sequence of substance P. Nat New Biol 1971;232:86–7. [DOI] [PubMed] [Google Scholar]
- [32].Bignardi C. Substance P self-aggregation revised: a chromatographic and mass spectrometry analysis. J Chromatogr Separ Tech 2012. 10.4172/2157-7064.1000140. 03. [DOI] [Google Scholar]
- [33].Hökfelt T, Johansson O, Ljungdahl Å, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature 1980;284. 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- [34].Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 2008;36:309–18. 10.1016/j.exphem.2007.ll.007. [DOI] [PubMed] [Google Scholar]
- [35].Li FXZ, Xu F, Lin X, Wu F, Zhong JY, Wang Y, et al. The role of substance P in the regulation of bone and cartilage metabolic activity. Front Endocrinol 2020;11:77. 10.3389/fendo.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 1997. [PubMed] [Google Scholar]
- [37].Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol 2001;114:131–41. [DOI] [PubMed] [Google Scholar]
- [38].Lambrecht BN, Germonpré PR, Everaert EG, Carro-Muino I, De Veerman M, De Felipe C, et al. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol 1999. . [DOI] [PubMed] [Google Scholar]
- [39].Columbo M, Horowitz EM, Kagey-Sobotka A, Lichtenstein LM. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopathol 1996;81:68–73. 10.1006/din.1996.0159. [DOI] [PubMed] [Google Scholar]
- [40].Repke H, Bienert M. Structural requirements for mast cell triggering by substance P-like peptides. Agents Actions 1988;23:207–10. 10.1007/BF02142542. [DOI] [PubMed] [Google Scholar]
- [41].Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye J-H, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can Be induced to produce the neurotransmitter substance P by interleukin-1α. Stem Cell 2005;23. 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- [42].Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Neuropeptide substance P and the immune response. Cell Mol Life Sci 2016;73:4249–64. 10.1007/s00018-016-2293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].De Koninck Y, Henry JL. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A 1991;88:11344–8. 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev 1993;73:229–308. 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- [45].Yu M, Lee SM, Lee H, Amouzegar A, Nakao T, Chen Y, et al. Neurokinin-1 receptor antagonism ameliorates dry eye disease by inhibiting antigen-presenting cell maturation and T helper 17 cell activation. Am J Pathol 2020;190:125–33. 10.1016/j.ajpath.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hazlett LD, McClellan SA, Barrett RP, Liu J, Zhang Y, Lighvani S. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci 2007;48:797–807. 10.1167/iovs.06-0882. [DOI] [PubMed] [Google Scholar]
- [47].McClellan SA, Zhang Y, Barrett RP, Hazlett LD. Substance P promotes susceptibility to pseudomonas aeruginosa keratitis in resistant mice: anti-inflammatory mediators downregulated. Investig Ophthalmol Vis Sci 2008;49. 10.1167/iovs.07-1369. [DOI] [PubMed] [Google Scholar]
- [48].Zhou Z, Barrett RP, McClellan SA, Zhang Y, Szliter EA, Van Rooijen N, et al. Substance P delays apoptosis, enhancing keratitis after Pseudomonas aeruginosa infection. Investig Ophthalmol Vis Sci 2008;49. 10.1167/iovs.08-1906. [DOI] [PubMed] [Google Scholar]
- [49].Bignami F, Giacomini C, Lorusso A, Aramini A, Rama P, Ferrari G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Invest Ophthalmol Vis Sci 2014;55. 10.1167/iovs.14-14553. [DOI] [PubMed] [Google Scholar]
- [50].Vitar RML, Barbariga M, Fonteyne P, Bignami F, Rama P, Ferrari G. Modulating ocular surface pain through neurokinin-1 receptor blockade. Investig Ophthalmol Vis Sci 2021;62. 10.1167/IOVS.62.3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chang CT, Jiang BY, Chen CC. Ion channels involved in substance P-mediated nociception and antinociception. Int J Mol Sci 2019;20. 10.3390/ijms20071596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang Y, Lei J, Jha RK, Kiven S, Gupta K. Substance P modulates electroacupuncture analgesia in humanized mice with sickle cell disease. J Pain Res 2019,12. 10.2147/JPR.S210196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lin CCJ, Chen WN, Chen CJ, Lin YW, Zimmer A, Chen CC. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci U S A 2012;109. 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Herbert MK, Holzer P. Die neurogene entzündung. I. Grundlegende mechanismen, physiologie und pharmakologie. Anasthesiol Intensivmed Notfallmedizin Schmerztherapie 2002;37. 10.1055/s-2002-32233. [DOI] [PubMed] [Google Scholar]
- [55].Saria A. Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol 1984;82. 10.1111/j.1476-5381.1984.tbl6461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 1995;86. 10.1182/blood.v86.2.482.bloodjournal862482. [DOI] [PubMed] [Google Scholar]
- [57].Pascual DW, Xu-Amano J, Kiyono H, Mcghee JR, Bost KL. Substance P acts directly upon cloned B lymphoma cells to enhance IgA and IgM production. J Immunol 1991;146. [PubMed] [Google Scholar]
- [58].Eglezos A, Andrews PV, Boyd RL, Helme RD. Effects of capsaicin treatment on immunoglobulin secretion in the rat: further evidence for involvement of tachykinin-containing afferent nerves. J Neuroimmunol 1990;26:131–8. 10.1016/0165-5728(90)90084-Z. [DOI] [PubMed] [Google Scholar]
- [59].Mei G, Xia L, Zhou J, Zhang Y, Tuo Y, Fu S, et al. Neuropeptide SP activates the WNT signal transduction pathway and enhances the proliferation of bone marrow stromal stem cells. Cell Biol Int 2013;37. 10.1002/cbin.10158. [DOI] [PubMed] [Google Scholar]
- [60].Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29 + stromal-like cells. Nat Med 2009;15. 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- [61].Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1β expression in human T lymphocytes. J Neuroimmunol 2002;131. 10.1016/S0165-5728(02)00277-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor: influence of agonist concentration and Rab GTPases. J Biol Chem 2004;279:30670–9. 10.1074/jbc.M402479200. [DOI] [PubMed] [Google Scholar]
- [63].Castellani ML, Vecchiet J, Salini V, Conti P, Theoharides TC, Caraffa A, et al. Stimulation of CCL2 (MCP-1) and CCL2 mRNA by substance P in LAD2 human mast cells. Transl Res 2009;154:27–33. 10.1016/j.trsl.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [64].Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-κB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol 2008;294. 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- [65].Chernova I, Lai J-P, Li H, Schwartz L, Tuluc F, Korchak HM, et al. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1R). J Leukoc Biol 2008;85:154–64. 10.1189/jlb.0408260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M. Impaired IL-1 beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br J Pharmacol 1998;124:1013–5. 10.1038/SJ.BJP.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-Biased immune responses. J Immunol 2007;178. 10.4049/jimmunol.178.11.7006. [DOI] [PubMed] [Google Scholar]
- [68].Takashima A. Harnessing DCs by substance P. Blood 2013;121. 10.1182/blood-2013-02-483354. [DOI] [PubMed] [Google Scholar]
- [69].Janelsins BM, Sumpter TL, Tkacheva OA, Rojas-Canales DM, Erdos G, Mathers AR, et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood 2013;121. 10.1182/blood-2012-07-446054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kroegel C, Giembycz MA, Barnes PJ. Characterization of eosinophil cell activation by peptides. Differential effects of substance P, melittin, and FMET-Leu-Phe. J Immunol 1990;145. [PubMed] [Google Scholar]
- [71].Raap M, Rüdrich U, Ständer S, Gehring M, Kapp A, Raap U. Substance P activates human eosinophils. Exp Dermatol 2015;24:557–9. 10.llll/exd.12717. [DOI] [PubMed] [Google Scholar]
- [72].Serra MC, Bazzoni F, Della Bianca V, Greskowiak M, Rossi F. Activation of human neutrophils by substance P. Effect on oxidative metabolism, exocytosis, cytosolic Ca2+ concentration and inositol phosphate formation. J Immunol 1988;141:2118–24. [PubMed] [Google Scholar]
- [73].Tanabe T, Otani H, Bao LH, Mikami Y, Yasukura T, Ninomiya T, et al. Intracellular signaling pathway of substance P-induced superoxide production in human neutrophils. Eur J Pharmacol 1996;299. 10.1016/0014-2999(95)00816-0. [DOI] [PubMed] [Google Scholar]
- [74].Bar-Shavit Z, Goldman R, Stabinsky Y, Gottlieb P, Fridkin M, Teichberg VI, et al. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun 1980;94:1445–51. 10.1016/0006-291X(80)905811. [DOI] [PubMed] [Google Scholar]
- [75].Arsenescu R, Blum AM, Metwali A, Elliott DE, Weinstock JV. IL-12 induction of mRNA encoding substance P in murine macrophages from the spleen and sites of inflammation. J Immunol 2005;174:3906–11. 10.4049/jimmunol.174.7.3906. [DOI] [PubMed] [Google Scholar]
- [76].Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol 1997;158:2334–9. [PubMed] [Google Scholar]
- [77].Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 1994;94:2036–44. 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi CA. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res 1990;40. 10.1016/0026-2862(90)90024-L. [DOI] [PubMed] [Google Scholar]
- [79].Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Potalivo G, Caraffa A, et al. Vascular endothelial growth factor (VEGF), mast cells and inflammation. Int J Immunopathol Pharmacol 2013;26. 10.1177/039463201302600206. [DOI] [PubMed] [Google Scholar]
- [80].Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol 1993;150:4478–85. [PubMed] [Google Scholar]
- [81].Okada T, Hirayama Y, Kishi S, Miyayasu K, Hiroi J, Fujii T. Functional neurokinin NK-1 receptor expression in rat peritoneal mast cells. Inflamm Res 1999;48:274–9. 10.1007/s000110050459. [DOI] [PubMed] [Google Scholar]
- [82].Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123:398–410. 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci 2001;22. 10.1016/S0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- [84].Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 2014;94:265–301. 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R, et al. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem 1989;264. 10.1016/s0021-9258(19)84619-7. [DOI] [PubMed] [Google Scholar]
- [86].Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci 2011;1217. 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Takeda Y, Chou KB, Takeda J, Sachais BS, Krause JE. Molecular cloning, structural characterization and functional expression of the human substance P receptor. Biochem Biophys Res Commun 1991;179. 10.1016/0006-291X(91)91704-G. [DOI] [PubMed] [Google Scholar]
- [88].Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol 1992;41:24–30. [PubMed] [Google Scholar]
- [89].Lai JP, Lai S, Tuluc F, Tansky MF, Kilpatrick LE, Leeman SE, et al. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci U S A 2008;105:12605–10. 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, et al. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci U S A 2006;103:7771–6. 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Caberlotto L, Hurd YL, Murdock P, Wahlin JP, Melotto S, Corsi M, et al. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci 2003;17:1736–46. 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- [92].Gillespie E, Leeman SE, Watts LA, Coukos JA, O’Brien MJ, Cerda SR, et al. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis-associated cancer. Proc Natl Acad Sci U S A 2011;108:17420–5. 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Goode T, O’Connell J, Ho WZ, O’Sullivan GC, Collins JK, Douglas SD, et al. Differential expression of neurokinin-1 receptor by human mucosal and peripheral lymphoid cells. Clin Diagn Lab Immunol 2000;7:371–6. 10.1128/CDLI.7.3.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].DeFea KA, Vaughn ZD, O’Bryan EM, Nishijima D, Déry O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A 2000;97:11086–91. 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nishimura K, Warabi K, Roush ED, Frederick J, Schwinn DA, Kwatra MM. Characterization of GRK2-catalyzed phosphorylation of the human substance P receptor in Sf9 membranes. Biochemistry 1998;37:1192–8. 10.1021/bi972302s. [DOI] [PubMed] [Google Scholar]
- [96].McConalogue K, Déry O, Lovett M, Wong H, Walsh JH, Grady EF, et al. Substance P-induced trafficking of β-arrestins: the role of β- arrestins in endocytosis of the neurokinin-1 receptor. J Biol Chem 1999;274:16257–68. 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- [97].Grady EF, Garland AM, Gamp PD, Lovett M, Payan DG, Bunnett NW. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Mol Biol Cell 1995;6:509–24. 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Roosterman D, Cottrell GS, Padilla BE, Muller L, Eckman CB, Bunnett NW, et al. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci U S A 2007;104:11838–43. 10.1073/pnas.0701910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, et al. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem 2006;281:27773–83. 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- [100].Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci U S A 2007;104. 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Garcia-Recio S, Gascón P. Biological and pharmacological aspects of the NK1-receptor. BioMed Res Int 2015;2015. 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Schwindinger WF, Robishaw JD. Heterotrimeric G-protein βγ-dimers in growth and differentiation. Oncogene 2001;20. 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- [103].Palanche T, Ilien B, Zoffmann S, Reck MP, Bucher B, Edelstein SJ, et al. The neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem 2001;276. 10.1074/jbc.Ml04363200. [DOI] [PubMed] [Google Scholar]
- [104].Blount P, Krause JE. Functional nonequivalence of structurally homologous domains of neurokinin-1 and neurokinin-2 type tachykinin receptors. J Biol Chem 1993:268. [PubMed] [Google Scholar]
- [105].Mistrova E, Kruzliak P, Chottova Dvorakova M. Role of substance P in the cardiovascular system. Neuropeptides 2016;58. 10.1016/j.npep.2015.12.005. [DOI] [PubMed] [Google Scholar]
- [106].Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer CV, et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci 2004;24. 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-α mediate substance p-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem 2004;279. 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- [108].Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem 2000;275. 10.1074/jbc.M003990200. [DOI] [PubMed] [Google Scholar]
- [109].Müller U, Vrensen GFJM, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Investig Ophthalmol Vis Sci 1997;38:985–94. [PubMed] [Google Scholar]
- [110].Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T. Substance P and its metabolites in normal human tears. Investig Ophthalmol Vis Sci 2002;43. [PubMed] [Google Scholar]
- [111].Ko JA, Yanai R, Nishida T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett 2009;583. 10.1016/j.febslet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- [112].Araki-Sasaki K, Aizawa S, Hiramoto M, Nakamura M, Iwase O, Nakata K, et al. Substance P-induced cadherin expression and its signal transduction in a cloned human corneal epithelial cell line. J Cell Physiol 2000;182. . [DOI] [PubMed] [Google Scholar]
- [113].Yamada N, Yanai R, Inui M, Nishida T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Investig Ophthalmol Vis Sci 2005;46. 10.1167/iovs.04-0775. [DOI] [PubMed] [Google Scholar]
- [114].Nishida T, Yamada N, Yanai R, Kimura K, Inui M. Sensitizing effects of substance P on corneal epithelial cells to IGF-1, IL-6 and fibronectin. Ocul Surf 2005;3:S98. 10.1016/S1542-0124(12)70512-8. [DOI] [PubMed] [Google Scholar]
- [115].Nakamura M, Chikama TI, Nishida T. Up-regulation of integrin α5 expression by combination of substance P and insulin-like growth factor-1 in rabbit corneal epithelial cells. Biochem Biophys Res Commun 1998;246. 10.1006/bbrc.1998.8704. [DOI] [PubMed] [Google Scholar]
- [116].Nakamura M, Nagano T, Chikama TI, Nishida T. Up-regulation of phosphorylation of focal adhesion kinase and paxillin by combination of substance P and IGF-1 in SV-40 transformed human corneal epithelial cells. Biochem Biophys Res Commun 1998;242. 10.1006/bbrc.1997.7899. [DOI] [PubMed] [Google Scholar]
- [117].Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf 2005;3:194–202. 10.1016/S1542-0124(12)70206-9. [DOI] [PubMed] [Google Scholar]
- [118].Yang L, Sui W, Li Y, Qi X, Wang Y, Zhou Q, et al. Substance P inhibits hyperosmotic stress-induced apoptosis in corneal epithelial cells through the mechanism of akt activation and reactive oxygen species scavenging via the neurokinin-1 receptor. PLoS One 2016;11. 10.1371/journal.pone.0149865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gaddipati S, Rao P, Jerome AD, Burugula BB, Gerard NP, Suvas S. Loss of neurokinin-1 receptor alters ocular surface homeostasis and promotes an early development of herpes stromal keratitis. J Immunol 2016;197. 10.4049/jimmunol.1600836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Foldenauer MEB, McClellan SA, Berger EA, Hazlett LD. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J Immunol 2013;190. 10.4049/jimmunol.1203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xu Q, Fitzsimmons B, Steinauer J, O’Neill A, Newton AC, Hua XY, et al. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J Neurosci 2011;31. 10.1523/JNEUROSCI.2139-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Paunicka KJ, Mellon J, Robertson D, Petroll M, Brown JR, Niederkorn JY. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant 2015. 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tullo AB, Keen P, Blyth WA, Hill TJ, Easty DL. Corneal sensitivity and substance P in experimental herpes simplex keratitis in mice. Investig Ophthalmol Vis Sci 1983;24. [PubMed] [Google Scholar]
- [124].Twardy BS, Channappanavar R, Suvas S. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Invest Ophthalmol Vis Sci 2011;52:8604–13. 10.1167/iovs.ll-8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Twardy B, Channappanavar R, Suvas S. Herpetic Stromal Keratitis (HSK) lesion severity correlates with the amount of pro-inflammatory neuropeptide Substance P in the cornea (42.21). J Immunol 2010:184. [Google Scholar]
- [126].Wang L, Wang R, Xu C, Zhou H. Pathogenesis of herpes stromal keratitis: immune inflammatory response mediated by inflammatory regulators. Front Immunol 2020;11:766. 10.3389/FIMMU.2020.00766/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Guo CJ, Lai JP, Luo HM, Douglas SD, Ho WZ. Substance P up-regulates macrophage inflammatory protein-1 β expression in human T lymphocytes. J Neuroimmunol 2002;131:160. 10.1016/S0165-5728(02)00277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol 2007;293. 10.1152/ajpcell.00060.2007. [DOI] [PubMed] [Google Scholar]
- [129].Singh RB, Das S, Chodosh J, Sharma N, Zegans ME, Kowalski RP, et al. Paradox of complex diversity: challenges in the diagnosis and management of bacterial keratitis. Prog Retin Eye Res 2021. 10.1016/J.PRETEYERES.2021.101028. [DOI] [PubMed] [Google Scholar]
- [130].Lighvani S, Huang X, Trivedi PP, Swanborg RH, Hazlett LD. Substance P regulates natural killer cell interferon-γ production and resistance to Pseudomonas aeruginosa infection. Eur J Immunol 2005;35:1567–75. 10.1002/eji.200425902. [DOI] [PubMed] [Google Scholar]
- [131].Foldenauer MEB, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Substance P affects growth factors in pseudomonas aeruginosa-infected mouse cornea. Cornea 2012;31. 10.1097/ICO.0b013e31824d6ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 Years and older. Am J Ophthalmol 2017. 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- [133].Shikari H, Antin JH, Dana R. Ocular graft-versus-host disease: a review. Surv Ophthalmol 2013;58:233–51. 10.1016/j.survophthal.2012.08.004. [DOI] [PubMed] [Google Scholar]
- [134].Brito-Zeron P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjogren syndrome. Nat Rev Dis Prim 2016;2:16047. 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- [135].Smolen JS, Aletaha D, Mclnnes IB. Rheumatoid arthritis. Lancet (London, England) 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- [136].Singh RB, Yung A, Coco G, Sinha S, Dohlman TH, Yin J, et al. Efficacy and retention of silicone punctal plugs for treatment of dry eye in patients with and without ocular graft-versus-host-disease. Ocul Surf 2020. 10.1016/j.jtos.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cox SM, Kheirkhah A, Aggarwal S, Abedi F, Cavalcanti BM, Cruzat A, et al. Alterations in corneal nerves in different subtypes of dry eye disease: an in vivo confocal microscopy study. Ocul Surf 2021;22. 10.1016/j.jtos.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 2012. 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Guo M, Jiang Z, Chen Y, Wang F, Wang Z. Inflammatory cytokines in midbrain periaqueductal gray contribute to diabetic induced pain hypersensitivity through phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Korean J Pain 2021;34. 10.3344/KJP.2021.34.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Keeble JE, Brain SD. A role for substance P in arthritis? Neurosci Lett 2004;361:176–9. 10.1016/j.neulet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- [141].Kumar V, Sur VP, Guha R, Konar A, Hazra S. Estrogen modulates corneal nociception and maintains corneal homeostasis in rat eye. Cornea 2018;37. 10.1097/IC0.0000000000001437. [DOI] [PubMed] [Google Scholar]
- [142].De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, Fang B, Zheng X, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2009;2:243–53. 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Taketani Y, Marmalidou A, Dohlman TH, Singh RB, Amouzegar A, Chauhan SK, et al. Restoration of regulatory T-cell function in dry eye disease by antagonizing substance P/Neurokinin-1 receptor. Am J Pathol 2020;190:1859–66. 10.1016/j.ajpath.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf 2004;2. 10.1016/S1542-0124(12)70112-X. [DOI] [PubMed] [Google Scholar]
- [145].Li F, Yang W, Jiang H, Guo C, Huang AJW, Hu H, et al. TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun 2019;10. 10.1038/s41467-019-13536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].He J, Pham TL, Kakazu AH, Bazan HEP. Remodeling of substance P sensory nerves and transient receptor potential Melastatin 8 (TRPM8) cold receptors after corneal experimental surgery. Invest Ophthalmol Vis Sci 2019;60:2449–60. 10.1167/iovs.18-26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Liu YC, Yam GHF, Lin MTY, Teo E, Koh SK, Deng L, et al. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J Adv Res 2021;29. 10.1016/j.jare.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016;100. 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: Manifestations, incidence, and predictive factors. J Cataract Refract Surg 2015;41. 10.1016/j.jcrs.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Raoof D, Pineda R. Dry eye after laser in-situ Keratomileusis. Semin Ophthalmol 2014;29. 10.3109/08820538.2014.962663. [DOI] [PubMed] [Google Scholar]
- [151].Solomon KD, Holzer MP, Sandoval HP, Vargas LG, Werner L, Vroman DT, et al. Refractive surgery survey 2001. J Cataract Refract Surg 2002;28. 10.1016/S0886-3350(01)01318-9. [DOI] [PubMed] [Google Scholar]
- [152].Levitt AE, Galor A, Weiss JS, Felix ER, Martin ER, Patin DJ, et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post-operative pain disorders. Mol Pain 2015;11. 10.1186/si2990-015-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Shtein RM Post-LASIK dry eye. Expet Rev Ophthalmol 2011;6. 10.1586/eop.ll.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg 2001;27. 10.1016/S0886-3350(00)00835-X. [DOI] [PubMed] [Google Scholar]
- [155].Murakami Y, Manche EE. Prospective, randomized comparison of self-reported postoperative dry eye and visual fluctuation in LASIK and photorefractive keratectomy. Ophthalmology 2012;119. 10.10l6/j.ophtha.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [156].Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf 2010;8. 10.1016/S1542-0124(12)70224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Denoyer A, Landman E, Trinh L, Faure JF, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology 2015;122. 10.1016/j.ophtha.2014.10.004. [DOI] [PubMed] [Google Scholar]
- [158].Yang L, Mehta J, Liu YC. Corneal neuromediator profiles following laser refractive surgery. Neural Regen Res 2021;16. 10.4103/1673-5374.308666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chao C, Golebiowski B, Zhao X, Chen S, Zhou S, Stapleton F. Long-term effects of LASIK on corneal innervation and tear neuropeptides and the associations with dry eye. J Refract Surg 2016;32:518–24. 10.3928/1081597X-20160603-01. [DOI] [PubMed] [Google Scholar]
- [160].Tran MT, Lausch RN, Oakes JE. Substance P differentially stimulates IL-8 synthesis in human corneal epithelial cells. Investig Ophthalmol Vis Sci 2000;41. [PubMed] [Google Scholar]
- [161].Di Zazzo A, Coassin M, Varacalli G, Galvagno E, De Vincentis A, Bonini S. Neurotrophic keratopathy: pros and cons of current treatments. Ocul Surf 2019;17. 10.1016/j.jtos.2019.09.002. [DOI] [PubMed] [Google Scholar]
- [162].Goins KM New insights into the diagnosis and treatment of neurotrophic keratopathy. Ocul Surf 2005;3. 10.1016/S1542-0124(12)70158-1. [DOI] [PubMed] [Google Scholar]
- [163].Nishida T, Nakamura M, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol 1996;169:159–66. . [DOI] [PubMed] [Google Scholar]
- [164].Ofuji K, Nakamura M, Nishida T. Signaling regulation for synergistic effects of substance P and insulin-like growth factor-1 or epidermal growth factor on corneal epithelial migration. Jpn J Ophthalmol 2000;44. 10.1016/S0021-5155(99)00168-9. [DOI] [PubMed] [Google Scholar]
- [165].Nakamura M, Chikama TI, Nishida T. Participation of p38 MAP kinase, but not p44/42 MAP kinase, in stimulation of corneal epithelial migration by substance P and IGF-1. Curr Eye Res 2005;30. 10.1080/02713680591006129. [DOI] [PubMed] [Google Scholar]
- [166].Nagano T, Nakamura M, Nakata K, Yamaguchi T, Takase K, Okahara A, et al. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Investig Ophthalmol Vis Sci 2003;44. 10.1167/iovs.03-0189. [DOI] [PubMed] [Google Scholar]
- [167].Nakamura M, Kawahara M, Nakata K, Nishida T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Investig Ophthalmol Vis Sci 2003;44. 10.1167/iovs.02-0868. [DOI] [PubMed] [Google Scholar]
- [168].Ghiasi Z, Gray T, Tran P, Dubielzig R, Murphy C, McCartney DL, et al. The effect of topical substance-p plus insulin-like growth factor-1 (IGF-1) on epithelial healing after photorefractive keratectomy in rabbits. Transl Vis Sci Technol 2018;7. 10.1167/tvst.7.l.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kingsley RE, Marfurt CF. Topical substance P and corneal epithelial wound closure in the rabbit. Investig Ophthalmol Vis Sci 1997;38. [PubMed] [Google Scholar]
- [170].Yamada N, Yanai R, Kawamoto K, Nagano T, Nakamura M, Inui M, et al. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Investig Ophthalmol Vis Sci 2006;47. 10.1167/iovs.05-1205. [DOI] [PubMed] [Google Scholar]
- [171].Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J 1999;46. 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- [172].Nishida T, Chikama TI, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance P-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol 2007;51. 10.1007/sl0384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- [173].Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol 2008;92:896–900. 10.1136/BJO.2007.130013. [DOI] [PubMed] [Google Scholar]
- [174].Chikamoto N, Chikama TI, Yamada N, Nishida T, Ishimitsu T, Kamiya A. Efficacy of substance P and insulin-like growth factor-1 peptides for preventing postsurgical superficial punctate keratopathy in diabetic patients. Jpn J Ophthalmol 2009;53:464–9. 10.1007/S10384-009-0693-4. [DOI] [PubMed] [Google Scholar]
- [175].Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 2018;10:17–28. [PMC free article] [PubMed] [Google Scholar]
- [176].McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol 2015;5:439–73. 10.1002/cphy.cl40014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf 2004;2:248–53. 10.1016/S1542-0124d2)70112-X. [DOI] [PubMed] [Google Scholar]
- [178].Singh RB, Liu L, Anchouche S, Yung A, Mittal SK, Blanco T, et al. Ocular redness – I: etiology, pathogenesis, and assessment of conjunctival hyperemia. Ocul Surf 2021;21. 10.1016/j.jtos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Singh RB, Liu L, Yung A, Anchouche S, Mittal SK, Blanco T, et al. Ocular redness – II: progress in development of therapeutics for the management of conjunctival hyperemia. Ocul Surf 2021;21:66–77. 10.1016/j.jtos.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin Exp Allergy 1997;27:372–8. [PubMed] [Google Scholar]
- [181].Yamaji M, Takada M, Fujiwara R, Ohishi H, Izushi K, Sugimoto Y, et al. Role of substance P in experimental allergic conjunctivitis in Guinea pigs. Methods Find Exp Clin Pharmacol 1997;19:637–43. [PubMed] [Google Scholar]
- [182].Figini M, Javdan P, Cioncolini F, Geppetti P. Involvement of tachykinins in plasma extravasation induced by bradykinin and low pH medium in the Guinea-pig conjunctiva. Br J Pharmacol 1995;115:128–32. 10.llll/J.l476-5381.1995.TB16329.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Suvas S Role of substance p neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 2017;199. 10.4049/jimmunol.1601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Seybold VS The role of peptides in central sensitization. Handb Exp Pharmacol 2009;194. 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- [185].Hökfelt T, Pernow B, Wahren J, Substance P. A pioneer amongst neuropeptides. J Intern Med 2001;249. 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- [186].Muraleedharan CK, McClellan SA, Barrett RP, Li C, Montenegro D, Carion T, et al. Inactivation of the miR-183/96/182 cluster decreases the severity of Pseudomonas aeruginosa-induced keratitis. Investig Ophthalmol Vis Sci 2016;57. 10.1167/iovs.16-19134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Huang HY, Lai YL. Lipopolysaccharide induces preprotachykinin gene expression. Am J Respir Cell Mol Biol 2003;29. 10.1165/rcmb.2002-01070C. [DOI] [PubMed] [Google Scholar]
- [188].Ng SW, Zhang H, Hegde A, Bhatia M. Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia. J Leukoc Biol 2008;83. 10.1189/jlb.0807575. [DOI] [PubMed] [Google Scholar]
- [189].Jara-Oseguera A, Simon S, Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol 2010;1. 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem 2010;114. 10.1111/j.14714159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Hay CW, Shanley L, Davidson S, Cowie P, Lear M, McGuffin P, et al. Functional effects of polymorphisms on glucocorticoid receptor modulation of human anxiogenic substance-P gene promoter activity in primary amygdala neurones. Psychoneuroendocrinology 2014;47. 10.1016/j.psyneuen.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Donaldson LF, McQueen DS, Seckl JR. Neuropeptide gene expression and capsaicin-sensitive primary afferents: maintenance and spread of adjuvant arthritis in the rat. J Physiol 1995;486. 10.1113/jphysiol.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Garrett NE, Cruwys SC, Kidd BL, Tomlinson DR. Effect of capsaicin on substance P and nerve growth factor in adjuvant arthritic rats. Neurosci Lett 1997;230. 10.1016/S0304-3940(97)00458-8. [DOI] [PubMed] [Google Scholar]
- [194].Byun YS, Mok JW, Chung SH, Kim HS, Joo CK. Ocular surface inflammation induces de novo expression of substance P in the trigeminal primary afferents with large cell bodies. Sci Rep 2020;10. 10.1038/s41598-020-72295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Uy HS, Chan PS, Ang RE. Topical bevacizumab and ocular surface neovascularization in patients with Stevens-Johnson syndrome. Cornea 2008;27:70–3. 10.1097/ICO.0b013e318158f6ad. [DOI] [PubMed] [Google Scholar]
- [196].Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin®) eye drops against corneal neovascularization. Graefe’s Arch Clin Exp Ophthalmol 2009;247:1375–82. 10.1007/s00417-009-1099-l. [DOI] [PubMed] [Google Scholar]
- [197].Cursiefen C, Bock F, Horn FK, Kruse FE, Seitz B, Borderie V, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization. Interim results of a randomized phase II trial. Ophthalmology 2009;116:1630–7. 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [198].Singh RB, Blanco T, Mittal SK, Taketani Y, Chauhan SK, Chen Y, et al. Pigment Epithelium-derived Factor secreted by corneal epithelial cells regulates dendritic cell maturation in dry eye disease. Ocul Surf 2020;18:460–9. 10.1016/j.jtos.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Singh RB, Blanco T, Mittal SK, Alemi H, Chauhan SK, Chen Y, et al. Pigment epithelium-derived factor enhances the suppressive phenotype of regulatory T-cells in a murine model of dry eye disease. Am J Pathol 2021. 10.1016/j.ajpath.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–42. 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- [201].Ferrari G, Hajrasouliha A, Sadrai Z, Ueno H, Chauhan S, Dana R. Nerves and neovessels inhibit each other in the cornea. Investig Ophthalmol Vis Sci 2013;54:813–20. 10.1167/iovs.ll-8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Ziche M, Morbidelli L, Geppetti P, Maggi CA, Dolara P. Substance induces migration of capillary endothelial cells: a novel NK-1 selective receptor mediated activity. Life Sci 1991;48. 10.1016/0024-3205(91)90417-A. [DOI] [PubMed] [Google Scholar]
- [203].Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003;22:273–81. 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- [204].Barbariga M, Fonteyne P, Ostadreza M, Bignami F, Rama P, Ferrari G. Substance p modulation of human and murine corneal neovascularization. Investig Ophthalmol Vis Sci 2018;59:1305–12. 10.1167/iovs.17-23198. [DOI] [PubMed] [Google Scholar]
- [205].Lee SJ, Im ST, Wu J, Cho CS, Jo DH, Chen Y, et al. Corneal lymphangiogenesis in dry eye disease is regulated by substance P/neurokinin-1 receptor system through controlling expression of vascular endothelial growth factor receptor 3. Ocul Surf 2021;22:72–9. 10.1016/J.JTOS.2021.07.003. [DOI] [PubMed] [Google Scholar]
- [206].Liu L, Dana R, Yin J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. Faseb J 2020;34:6229–43. 10.1096/fj.201903236R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bignami F, Lorusso A, Rama P, Ferrari G. Growth inhibition of formed corneal neovascularization following Fosaprepitant treatment. Acta Ophthalmol 2017;95:e641–8. 10.1111/aos.13304. [DOI] [PubMed] [Google Scholar]
- [208].Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology 2010;117. 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- [209].Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 2014;15. 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- [210].Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn-Schmiedeberg’s Arch Pharmacol 1979;310. 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- [211].Fakih D, Zhao Z, Nicolle P, Reboussin E, Joubert F, Luzu J, et al. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J Neuroinflammation 2019;16. 10.1186/sl2974-019-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Beuerman RW, Stern ME. Neurogenic inflammation: a first line of defense for the ocular surface. Ocul Surf 2005;3. 10.1016/S1542-0124(12)70256-2. S-203-S-206. [DOI] [PubMed] [Google Scholar]
- [213].Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 2004;78. 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [214].Wolpert LE, Snieder H, Jansonius NM, Utheim TP, Hammond CJ, Vehof J. Medication use and dry eye symptoms: a large, hypothesis-free, population-based study in The Netherlands. Ocul Surf 2021;22. 10.1016/j.jtos.2021.06.009. [DOI] [PubMed] [Google Scholar]
- [215].Okumura Y, Inomata T, Midorikawa-Inomata A, Sung J, Fujio K, Akasaki Y, et al. DryEyeRhythm: a reliable and valid smartphone application for the diagnosis assistance of dry eye. Ocul Surf 2022. 10.1016/J.JTOS.2022.04.005. [DOI] [PubMed] [Google Scholar]


