Abstract
Background
Low back pain or sciatic pain because of lumbar intervertebral disc herniation (LDH) is caused by mechanical compression and/or an inflammatory component on the nerve root. However, it is difficult to define to what extent each component contributes to the pain. This study attempted to explore the effects of macrophage polarization on clinical symptoms in patients experiencing LDH after surgery, and investigated the association between macrophage cell percentages and clinical efficacy.
Methods
This study retrospectively harvested nucleus pulposus (NP) tissue samples from 117 patients. Clinical symptoms and efficacy using the visual analog scale (VAS) and Oswestry Disability Index (ODI) were evaluated at different time points preoperatively and postoperatively. CD68, CCR7, CD163, and CD206 were selected as macrophage phenotypic markers.
Results
Seventy‐six samples showed positive expression of macrophage markers in NP samples of patients with LDH, whereas 41 patients displayed negative results. No significant differences were detected between the two groups, involvement of several demographic data, and preoperative clinical findings. With respect to the macrophage‐positive group, no significant correlation was detected between the positive rate of the four markers and the VAS score or ODI after surgery. However, patients with NP samples positive for CD68 and CCR7 expression showed significantly lower VAS scores 1 week after surgery compared with those in the negative group. Moreover, the improvement in VAS score showed a strong positive correlation with CD68‐ and CCR7‐positive cell percentages.
Conclusions
Our results indicated that pro‐inflammatory M1 macrophages may be associated with the reduction of chronic pain after surgery. Therefore, these findings contribute to better personalized pharmacological interventions for patients with LDH, considering the heterogeneity of pain.
Keywords: inflammation, intervertebral disc, low back pain, osteoarthritis, spinal surgery
1. INTRODUCTION
Lumbar disc herniation (LDH) is defined as the displacement of the material of the spinal disc that consequently leads to mechanical compression of the nerve roots 1 and is considered a major contributor to low back pain or leg pain. 2 However, some or all of its many complex symptoms cannot be explained by the concept of neural compression. The intervertebral disc (IVD) is the largest avascular organ in the human body, and the broken immune‐privileged homeostasis of the nucleus pulposus (NP) tissue into the epidural space elicits an autoimmune reaction. 3 , 4 Some studies have reported that sciatic pain is induced by mild disc herniation, but with marked symptoms, indicating that inflammatory and immune changes can cause sciatic pain. 5 LDH specimens contain varying populations, such as NP, annulus fibrosus (AF), cartilaginous endplates (CEP), and bone tissues. 6 Only NP tissue is particularly isolated from the immune system of the host owing to its position between two CEP and within the dense collagen fibrous structure of the AF. 7 Therefore, it has been postulated that (i) both inflammation and the mechanical compression cause sciatic pain and in turn, (ii) the herniated NP tissue is the main cause of this. Unfortunately, to date, the source of sciatic pain induced by immune or inflammatory reactions is unknown.
Recently, inflammatory cells, such as macrophages, lymphocytes, and fibroblasts, were observed in surgically removed IVD specimens. 8 , 9 Especially, macrophages have been identified in a large number of patients with LDH, and these macrophages have not only been suggested to play a role in the resorption of herniated disc material but may, at least partially, play a role in the induction of an inflammatory response. 10 , 11 Macrophages play a key role in governing normal tissue homeostasis and regulating disease progression by transforming into dynamic phenotypes in many tissues, such as classically activated M1 phenotypes and alternatively activated M2a or M2c phenotypes. 10 M1 macrophages expressing inducible nitric oxide synthase, CCR7, or CD86 produce high levels of pro‐inflammatory factors such as interleukin (IL)‐1, IL‐1α, IL‐1β, IL‐6, IL‐8, IL‐12, IL‐18, IL‐23, IL‐27, tumor necrosis factor (TNF)‐α, and bone morphogenic protein 2, while M2 macrophages are further subdivided into M2a and M2c. M2a macrophages are often considered to be anti‐inflammatory and express arginase‐1 and CD206, and secrete IL‐10, CCL18, IL‐1Ra, IL‐10, and transforming growth factor‐beta (TGF‐β), while M2c macrophages, distinguished by the cell surface marker CD163, have been described as a remodeling phenotype because they secrete high levels of matrix metalloproteinase (MMP)‐7 and MMP‐8. 12 Over the past two decades, several studies have attempted to explore the association between M1‐related and/or M2‐related factors and clinical symptoms; however, the results remained controversial because these inflammatory cytokines were harvested from different tissues, such as the CSF, blood, and NP. 5
To date, little is known about whether macrophages contribute to the clinical outcomes of radicular pain after surgery. Therefore, we investigated the association between M1/M2 macrophage polarization and clinical symptoms or efficacy, including visual analog scale (VAS) and Oswestry Disability Index (ODI) scores, in patients with LDH. Based on previously published evidence, CD68, CCR7, CD206, and CD163 were selected as phenotypic markers to identify macrophages, 13 , 14 , 15 , 16 M1‐polarized macrophages, 8 , 11 , 17 , 18 , 19 M2a‐polarized macrophages, 17 , 20 , 21 , 22 , 23 and M2c‐polarized macrophages, 13 , 15 , 16 , 17 respectively. The aim of the present study was to explore whether macrophage polarization plays a role in patients of LDH experiencing sciatic symptoms after surgery.
2. MATERIALS AND METHODS
2.1. Inclusion and exclusion criteria for patients with LDH
This study was conducted at the Gaozhou People's Hospital, Department of Orthopedics, Guangdong, China, and was approved by the hospital's ethics committee (No. 2019‐026). Written informed consent was obtained from the individual(s) and/or minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article. The study was performed according to the amended declaration of Helsinki. We retrospectively selected patients with LDH according to the following inclusion criteria: (a) patients diagnosed with LDH who underwent percutaneous endoscopic lumbar discectomy (PELD) between January 2017 and January 2021; (b) available VAS score (0–10 points) and ODI index preoperatively and postoperatively at different time points collected using a cell phone; (c) available NP paraffin specimens and magnetic resonance images (MRI); and (d) informed consent provided by the patient allowing the use of tissue specimens for medical research purposes. The exclusion criteria were as follows: (a) LDH caused by a tumor, infection, or other disorders that could affect “normal” spinal aging and degeneration; (b) presence of medical conditions that could potentially affect macrophage infiltration and polarization, such as ankylosing spondylitis and rheumatoid arthritis; (c) previous treatment with steroid injection within 6 months prior to study initiation; (d) cases of recurrent surgery at the same disc level; (e) age > 75 years and Pfirrmann grades V for the diseased segment IVD. A total of 242 patients with LDH were included in the study, of whom 32 lacked MRI data, 58 were lost to follow‐up, and 35 were excluded owing to low‐quality paraffin samples. Figure 1 shows an MRI image, herniated NP tissue and HE staining, and a flow chart for the inclusion of patients with LDH in this study.
FIGURE 1.
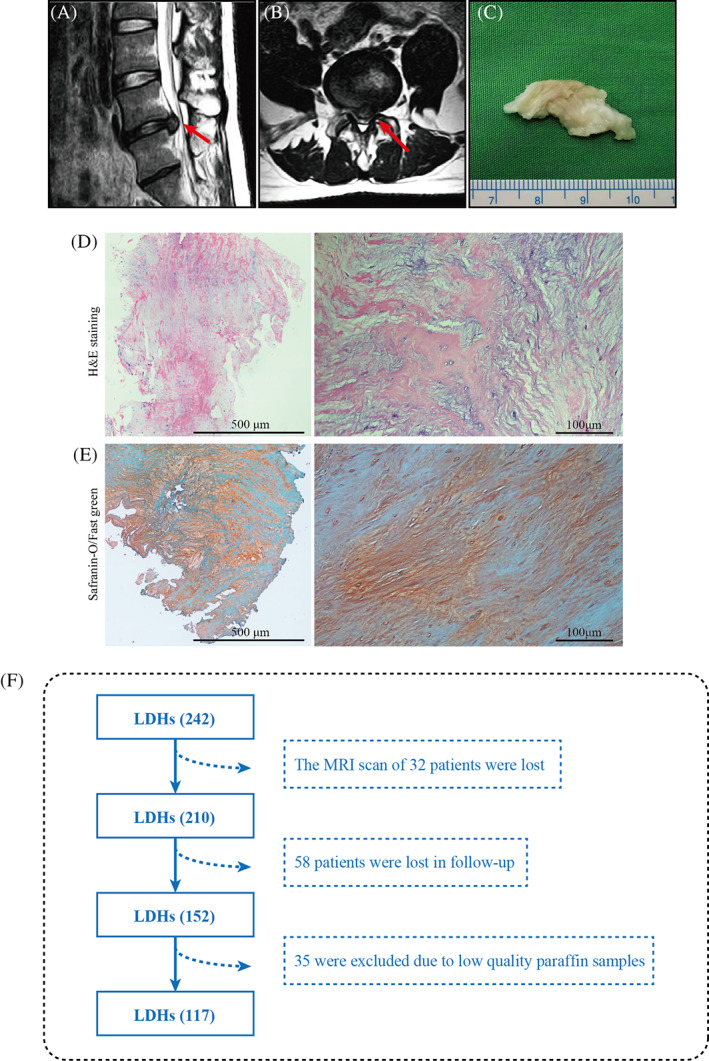
An image of the nucleus pulposus (NP) and a flow diagram of the selection of patients with lumbar disc hernia (LDH) in the study. Magnetic resonance image (MRI) of the sagittal (A) and horizontal position (B) of the LDH patient is shown, with the red arrow indicating the herniated NP. General view (C), hematoxylin and eosin (H&E) staining (D), and Safranine O/Fast Green (E) of the herniated NP tissue. (F) Flow diagram of the selection of patients with LDH in the study.
2.2. Operation procedure for patients with LDH
In this study, all the 117 patients included underwent PELD. They were placed in the prone position and operated on using the TESSYS technique under local anesthesia. C‐arm fluoroscopy was used to locate the puncture point above the iliac crest, approximately 9–12 cm from the midline. Next, after local infiltration of lidocaine, under the guidance of the C‐arm, a No. 30 needle was introduced into the side hole from the entry point, and the guide wire was inserted. Next, an 8‐mm cut in the area of the guide wire was made. The dilator was then inserted continuously and used to expand the bone hole appropriately. The working sleeve was pushed along the dilator, and the endoscope was inserted into the working channel and flushing system through the working sleeve. The endoscopic forceps were then used to identify and remove the herniated IVD material until the nerve root was fully decompressed. The working cannula was finally removed and the skin sutured.
2.3. Human NP specimen collection and Immunohistochemistry
Human NP tissues were harvested and washed with phosphate‐buffered saline (PBS) to remove blood. They were then fixed with 4% paraformaldehyde within 15 min. After 12 h, the NP tissues were washed six times with PBS and then dehydrated and embedded in paraffin. Finally, 5‐μm‐thick serial sections were cut and stored at 4°C for further study. For Histological staining, the sections were cut for both hematoxylin and eosin (H&E) and Safranin O/Fast Green (SOFG) staining. For immunohistochemical analysis, primary antibodies against, CD68 (1:100, ab213363), CCR7 (1:200, ab89064), CD163 (1:100, ab126756), and CD206 (1:200, ab64693) were used, and all were purchased from Abcam (Cambridge, UK). Sections were stained with horseradish peroxidase‐conjugated secondary antibodies (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA, USA), and 3,3‐diaminobenzidine was used to visualize the chromogen and hematoxylin used for counter‐staining. 24 After dehydration and clearance with dimethyl benzene, the sections were sealed with neutral gum, and images were obtained using a Leica inverted microscope (Leica Microsystems, Wetzlar, Germany). For positive marker expression analysis, ×20 magnification bright‐field microscopy was used for each specimen, and fields with unhealthy regions and macrophage accumulation were selected throughout the entire IVD section. Unhealthy regions were defined as areas with extensive damage and obvious defects in the organization of the extracellular matrix and cellular distribution patterns (i.e., granulation‐like tissue, tears, cracks, ruptures, cell clustering, sclerosis, and irregular contours or decreased endplate thickness). 9 If no cells or single cells tested positive for markers or unhealthy regions were in the field of vision, the microscope was moved to another chosen area. The total number of cells in each selected IVD area was counted by two authors (T. D. Q. and Z. T. L.) in consensus using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA). The number of positively stained NP macrophages was calculated as the mean value of five analyzed fields.
2.4. Sciatica Pain Score and ODI evaluation
Both the Sciatica Pain Score and the ODI evaluation were determined following a retrospective analysis by patients using their cell phones. The ODI is the most widely used outcome measure for functional outcomes in spine surgery and is considered the “gold standard” for the evaluation of functional outcomes of the lower back. 25 For each section, the total possible score was 5; the section score was 0 if the total statement was marked, 5 if the last statement was marked, and the score was calculated if the 10 sections were completed. The patients were also evaluated using VAS scores (0 = no pain; 10 = worst pain ever experienced) to assess the level of leg discomfort at different time points. Both VAS scores and ODI were recorded preoperatively (1 day before surgery) and at 7 days and 12 months postoperatively.
2.5. Statistical analysis
The values obtained are shown as the mean ± standard deviation and frequency distribution, as appropriate. The Mann–Whitney U test, a nonparametric test, was used to determine the statistical difference between groups. The chi‐squared test was used to evaluate frequency data. Spearman's nonparametric correlation analysis was used to evaluate the relationship between the percentage of positively stained cells and the clinical characteristics of the patients. Statistical analyses were performed using SPSS (version 19.0; IBM Corp., Armonk, NY, USA). The level of significance was set at p < 0.05.
3. RESULTS
3.1. Comparison of the clinical baseline data and characteristics between the macrophage (+) and macrophage (−) groups
Immunohistology results found that no positive cells or single positive cells were detected in 41 patients, classifying them as the macrophage‐negative group. In the macrophage‐positive expression group, 76 NP samples from patients with LDH positively exhibited at least one of the four markers. The macrophage markers were mostly detected around structural abnormal zones, such as cracks and tears. The positive cells displayed diverse cell morphologies, with some being similar to hypertrophic chondroid‐like cells (Figure 2). No difference was found between the macrophage‐positive (n = 76) and macrophage‐negative groups (n = 41) in terms of age, sex, duration of preoperative symptoms, smoking status, use of analgesics, Modic changes, high‐intensity zone, Pfirrmann grades, responsible segments, and type of herniated disc. (Table 1).
FIGURE 2.
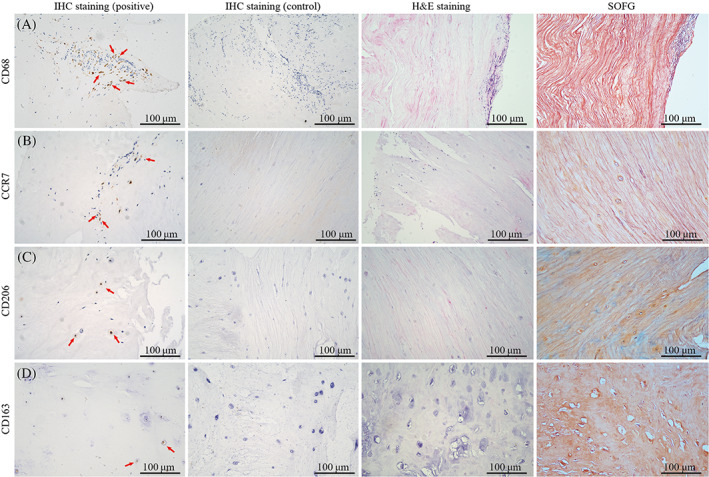
The histological staining and immunohistochemistry of herniated nucleus pulposus (NP) derived from patients with lumbar disc hernia (LDH). The hematoxylin and eosin (H&E) and Safranin O/Fast Green (SOFG) staining showed that NP tissues developed structural abnormalities, such as extracellular matrix disorder, with cracks and tears, reduced cell counts, and cell cluster formation. The accumulation of macrophage markers CD68, CCR7, CD206, and CD163 was detected in NP samples of LDH patients (red arrow).
TABLE 1.
Preoperative clinical findings
| Parameter | No inflammation (N = 41) | Inflammation (N = 76) | Total (N = 117) | Significance (p‐value) |
|---|---|---|---|---|
| Mean age (years) | 51 ± 14 | 53 ± 10 | 52 ± 11 | 0.373 |
| Women (%) | 13 (31.7%) | 27 (35.5%) | 34% | 0.678 |
| Radicular pain (%) | 36 (87.8%) | 72 (94.7%) | 92% | 0.328 |
| Mean duration of symptoms (days) | 165 ± 42 | 153 ± 40 | 157 ± 58 | 0.131 |
| Smokers (%) | 16 (39%) | 28 (36.8%) | 38% | 0.816 |
| Using of analgesics (%) | 15 (36.6%) | 35 (46.1%) | 43% | 0.323 |
| MCs | 14 (34.1%) | 30 (39.5%) | 38% | 0.570 |
| HIZ | 4 (9.8%) | 9 (11.8%) | 11% | 0.973 |
| Pfirrmann grades (%) | 0.780 | |||
| II | 2 (4.9%) | 5 (6.6%) | 7 (6%) | |
| III | 18 (43.9%) | 37 (48.7%) | 45 (47%) | |
| IV | 21 (51.2%) | 34 (44.7%) | 55 (47%) | |
| Responsible segments (%) | ||||
| L3–L4 | 2 (4.9%) | 4 (5.3%) | 6 (5.1%) | 0.285 |
| L4–L5 | 24 (58.4%) | 50 (65.8%) | 74 (63.2%) | |
| L5–S1 | 15 (36.6%) | 22 (28.9%) | 37 (31.7%) | |
| The type of herniated disc (%) | 0.651 | |||
| Protrusion | 6 (14.6%) | 10 (13.2%) | 16 (13.7%) | |
| Extrusion | 23 (56.1%) | 49 (64.5%) | 72 (61.5%) | |
| Sequestration | 12 (29.3%) | 17 (22.4%) | 29 (24.8%) | |
| VAS score (1D PreOP) | 7.2 ± 1.5 | 7.3 ± 1.4 | 7.2 ± 1.2 | 0.720 |
| ODI (1D PreOP) (%) | (56 ± 20)% | (58 ± 18)% | (57 ± 17)% | 0.583 |
Note: Pain assessment was evaluated using a visual analog scale (VAS) preoperatively 1 day before decompressive discectomy (1D PreOP). The low back functional assessment was evaluated using the Oswestry disability index (ODI) preoperatively 1D PreOP.The Mann–Whitney U test was used to compare nonparametric data, and the chi‐squared test was used to evaluate frequency data.
Abbreviations: HIZ, high‐intensity zone; MCs, modic changes.
3.2. Comparison of the clinical efficacy between macrophage (+) and macrophage (−) groups
When comparing the clinical efficacy of the VAS score and ODI, both macrophage (+) and macrophage (−) groups showed significant improvement at 1 week and 12 months after surgery compared with baseline measurements (all p < 0.0001, Table 2). When comparing the difference between macrophage (+) and macrophage (−) groups, no difference was detected at the time points of 1 week or 12 months after surgery (Table 2). When analyzing the macrophage marker, the CD68 (+) group showed a significantly lower VAS score 1 week after surgery than the CD 68(−) group (p < 0.05), while at 12 months after surgery, no difference in VAS score was detected between the two groups (Table 3). Regarding the VAS score in CCR7‐positive cells, the CCR7(+) group showed a lower VAS score 1 week after surgery than the CCR7(−) group (p < 0.05), while after 12 months of surgery, no difference in VAS score was detected between the two groups (Table 3). For CD206‐ and CD163‐positive cells, no difference was found in either group 1 week or 12 months after surgery (Table 3). In the ODI analysis, no difference was found in any of the four markers when the macrophage‐positive expression group was compared with the negative expression group (Table 4).
TABLE 2.
Clinical efficacy in the macrophage (+) and macrophage (−) groups
| Parameter | Macrophage (−) (n = 41) | p | Macrophage (+) (n = 76) | p |
|---|---|---|---|---|
| VAS score (1D PreOP) | 7.2 ± 1.5 | 7.3 ± 1.4 | ||
| VAS score (7D PostOP) | 3.4 ± 1.8 a | <0.001 | 3.3 ± 1.8 a | <0.001 |
| VAS score (12M PostOP) | 1.8 ± 1.6 a | <0.001 | 1.9 ± 1.6 a | <0.001 |
| ODI % (1D PreOP) | (56 ± 20)% | (58 ± 20)% | ||
| ODI % (7D PostOP) | (24 ± 17)% a | <0.001 | (21 ± 17)% a | <0.001 |
| ODI % (12M PostOP) | (12 ± 12)% a | <0.001 | (13 ± 12)% a | <0.001 |
Abbreviations: 1D PreOP, 1 day before discectomy; 7D PostOP, 7 days after discectomy; 12M PostOP, 12 months after discectomy; ODI, Oswestry disability index; VAS, visual analog scale.
Mann–Whitney U test was used as compared to the data at 1D PreOP.
TABLE 3.
Comparison the VAS score after surgery between macrophage (+) and macrophage (−)
| Markers | Expression | N | VAS score (7D PostOP) | p | VAS score (12M PostOP) | p |
|---|---|---|---|---|---|---|
| CD68 | (−) | 41 | 3.7 ± 1.7 a | 0.021 | 1.9 ± 1.6 | 0.757 |
| CD68 | (+) | 76 | 2.9 ± 1.8 | 1.8 ± 1.7 | ||
| CCR7 | (−) | 41 | 3.9 ± 1.6 a | <0.001 | 1.7 ± 1.5 | 0.751 |
| CCR7 | (+) | 76 | 2.6 ± 1.5 | 1.8 ± 1.6 | ||
| CD206 | (−) | 66 | 3.3 ± 1.7 | 0.759 | 1.8 ± 1.6 | 0.745 |
| CD206 | (+) | 51 | 3.4 ± 1.8 | 1.9 ± 1.7 | ||
| CD163 | (−) | 102 | 3.4 ± 1.4 | 0.624 | 1.9 ± 1.5 | 1.000 |
| CD163 | (+) | 15 | 3.2 ± 1.9 | 1.9 ± 1.8 |
Abbreviations: 7D PostOP, 7 days after discectomy; 12M PostOP, 12 months after discectomy; VAS, visual analog scale.
Mann–Whitney U test was used between the positive macrophage markers group and the control group.
TABLE 4.
Comparison the ODI after surgery between macrophage (+) and macrophage (−) groups
| Markers | Expression | N | ODI (7DPostOP) (%) | p | ODI (12M PostOP) (%) | p |
|---|---|---|---|---|---|---|
| CD68 | (−) | 41 | 23 ± 15 | 0.454 | 12 ± 10 | 0.529 |
| CD68 | (+) | 76 | 25 ± 13 | 11 ± 7 | ||
| CCR7 | (−) | 41 | 24 ± 13 | 0.709 | 13 ± 11 | 0.643 |
| CCR7 | (+) | 76 | 25 ± 13 | 12 ± 9 | ||
| CD206 | (−) | 66 | 22 ± 10 | 0.074 | 9 ± 8 | 0.059 |
| CD206 | (+) | 51 | 26 ± 14 | 12 ± 9 | ||
| CD163 | (−) | 102 | 25 ± 11 | 0.517 | 12 ± 8 | 0.392 |
| CD163 | (+) | 15 | 27 ± 12 | 14 ± 11 |
Note: Mann–Whitney U test was used between the positive macrophage markers group and the control group.
Abbreviations: 7D PostOP, 7 days after discectomy; 12M PostOP, 12 months after discectomy; ODI, Oswestry disability index.
3.3. Analysis of the correlation between clinical efficacy and CD68 (+) cells in patients with LDH
No correlation was detected between the VAS scores and CD68 (+) percentages at 7 days after discectomy (7D PostOP) and at 12 months after surgery (12M PostOP) (r = 0.03, p = 0.77 and r = 0.01, p = 0.98, respectively; Table 5). To assess clinical efficacy after surgery, the 7D PostOP VAS score was subtracted from the baseline 1D PreOP VAS score to define the improvement in VAS score at 1 week, and the 12M PostOP VAS score was subtracted from the baseline 1D PreOP VAS score to define the improvement in VAS score at 12 months. The CD68 (+) rate showed a positive association with the improvement in VAS score at 1 week (r = 0.35, p = 0.03; Figure 3A), and the VAS score at 12 months (r = 0.29, p = 0.04; Figure 3B). Regarding ODI, no significant association was detected between macrophage phenotypic marker positive rates and ODI at the two time points of either 7D PostOP (r = 0.12, p = 0.29) or 12M PostOP (r = 0.12, p = 0.3) (Table 5). Similarly, when subtracting the 7D PostOP ODI from the baseline ODI (1D PreOP) to define the improvement in ODI at 1 week and subtracting the 12M PostOP ODI from the baseline ODI (1D PreOP) as the improvement of 12 months ODI, no significant association was observed between the improvements of ODI at 1 week and 12 months (r = 0.22, p = 0.05 and r = 0.12, p = 0.03, respectively; Figure 3C,D).
TABLE 5.
Association between clinical efficacy after surgery and different macrophage markers
| Markers | Time | Correlation coefficient (r) | p‐value |
|---|---|---|---|
| CD68 | VAS score (7D PostOP) | 0.03 | 0.77 |
| CD68 | VAS score (12M PostOP) | 0.01 | 0.98 |
| CCR7 | VAS score (7D PostOP) | −0.09 | 0.42 |
| CCR7 | VAS score (12M PostOP) | −0.17 | 0.14 |
| CD206 | VAS score (7D PostOP) | 0.11 | 0.31 |
| CD206 | VAS score (12M PostOP) | 0.14 | 0.2 |
| CD163 | VAS score (7D PostOP) | −0.02 | 0.88 |
| CD163 | VAS score (12M PostOP) | 0.15 | 0.2 |
| CD68 | ODI (7D PostOP) | 0.12 | 0.29 |
| CD68 | ODI (12M PostOP) | 0.12 | 0.3 |
| CCR7 | ODI (7D PostOP) | −0.19 | 0.09 |
| CCR7 | ODI (12M PostOP) | −0.24 | 0.06 |
| CD206 | ODI (7D PostOP) | 0.11 | 0.31 |
| CD206 | ODI (12M PostOP) | −0.05 | 0.66 |
| CD163 | ODI (7D PostOP) | −0.02 | 0.76 |
| CD163 | ODI (12M PostOP) | −0.09 | 0.42 |
Abbreviations: 7D PostOP, 7 days after discectomy; 12M PostOP, 12 months after discectomy; ODI, Oswestry disability index; VAS, visual analog scale.
FIGURE 3.
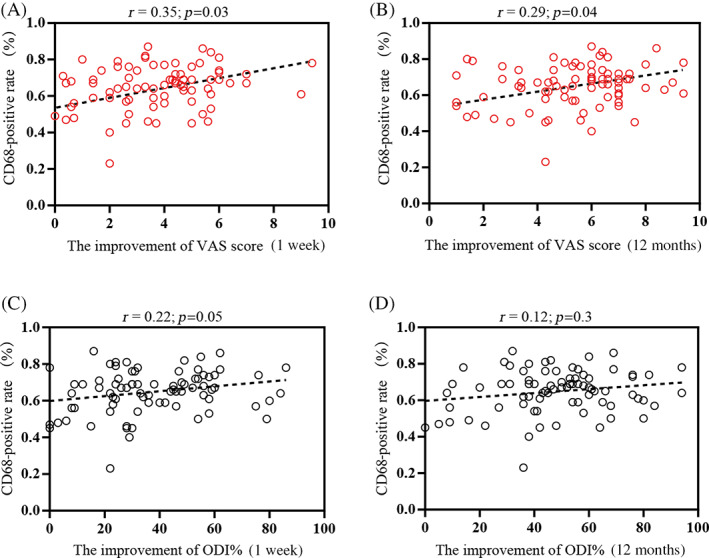
Correlation between clinical efficacy and CD68 positivity rates in patients with lumbar disc herniation (LDH). The correlation between the proportion of CD68 (+) cell populations and the improvement of visual analog scale (VAS) scores at 1 week (A) and 12 months (B) was determined. The association between the improvement of Oswestry disability index (ODI) at 1 week (C) and 12 months (D) with CD68 (+) cell percentages was assessed. r = Spearman's correlation coefficient (95% confidence interval).
3.4. Analysis of the correlation between clinical efficacy and CCR7 (+) cells in patients with LDH
Of the 76 patients evaluated, the VAS scores at 7D PostOP and 12M PostOP did not show a significant association with the frequency of CCR7 (+) cells (r = −0.09, p = 0.42 and r = −0.17, p = 0.14, respectively; Table 5). In particular, the improvements of the 1 week and 12 months VAS scores exhibited a medium‐positive association with the frequency of CCR7 (+) cells (r = 0.47, p = 0.002 and r = 0.43, p < 0.001; Figure 4A,B). ODI analysis did not reveal any association between the ODI% at 7D PostOP (r = −0.19, p = 0.09; Table 5) and 12M PostOP (r = −0.24, p = 0.03; Table 5). In addition, no association was observed with respect to changes in ODI (1 week and 12 months) (r = 0.18, p = 0.1 and r = 0.14, p = 0.22, respectively; Figure 4C,D).
FIGURE 4.
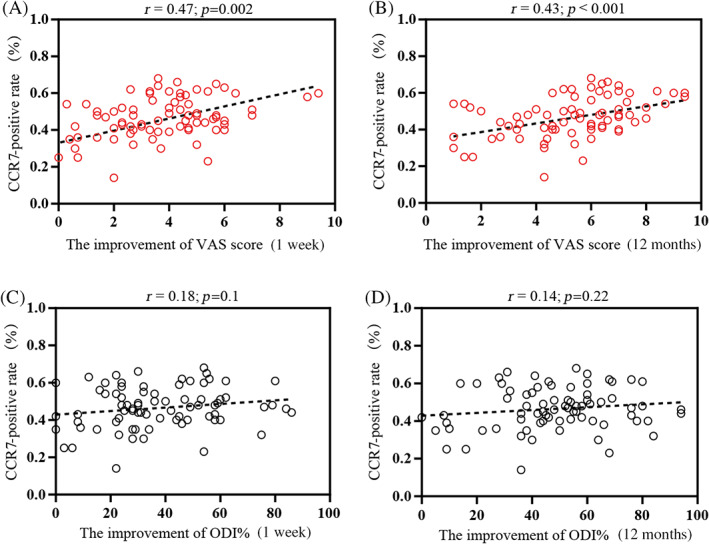
Correlation between clinical efficacy and CCR7 positivity rates in patients with lumbar disc herniation (LDH). The correlation between CCR7 (+) cell percentages and the improvement of visual analog scale (VAS) scores at 1 week (A) and 12 months (B) was analyzed. The association between the improvements of the ODI at 1 week (C) and 12 months (D) with the percentages of CCR7 (+) cells was analyzed. r = Spearman's correlation coefficient (95% confidence interval).
3.5. Analysis of the correlation between clinical symptoms and CD206 (+) cells in patients with LDH
For the M2a phenotypic marker CD206, no significant association was detected at 7D (r = 0.11, p = 0.31; Table 5) and at 12M PostOP (r = 0.14, p = 0.2; Table 5). The improvement in VAS scores at 1 week and at 12 months did not show a significant association with the frequency of CD206 (+) (r = 0.07, p = 0.56 and r = −0.07, p = 0.52, respectively; Figure 5A,B). Furthermore, ODI percentages at the two time points 7D PostOP (r = 0.11, p = 0.31; Table 5) and 12M PostOP (r = −0.05, p = 0.66; Table 5), and the improvements in ODI% at 1 week (r = 0.21, p = 0.06; Figure 5C) and 12 months (r = 0.19, p = 0.09; Figure 5D) did not show correlation with CD206 positivity.
FIGURE 5.
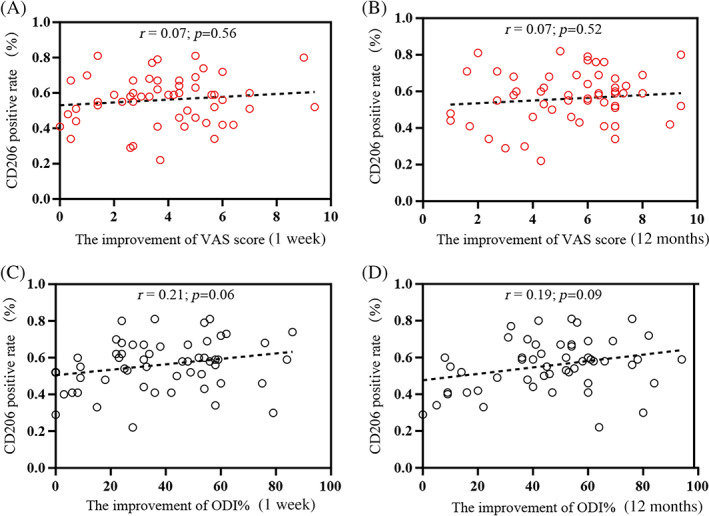
Correlation between clinical efficacy and CD206 positivity rates in patients with lumbar disc herniation (LDH). The correlation between CD206 (+) cell percentages and the improvement of visual analog scale (VAS) scores at 1 week (A) and 12 months (B) were determined. The association between ODI at 1 week (C) and 12 months (D) and the CD206 (+) proportion was analyzed. r = Spearman's correlation coefficient (95% confidence interval).
3.6. Analysis of the correlation between clinical symptoms and CD163 (+) cells in patients with LDH
The percentage of CD163(+) cells was not significantly associated with VAS scores at 7D PostOP (r = −0.02, p = 0.88) and at 12M PostOP (r = 0.15, p = 0.2; Table 5). Similarly, no correlation was detected with the improvement in VAS score at the same time points (1 week: r = 0.09, p = 0.43; 12 months: r = −0.04, p = 0.73; Figure 6A,B). The ODI% at 7D PostOP (r = −0.02, p = 0.76; Table 5) and 12M PostOP (r = −0.09, p = 0.42; Table 5) was not associated with CD163 (+) cells. Last, no association was observed with respect to ODI changes at the same time points (1 week: r = 0.12, p = 0.27; 12 months: r = 0.16, p = 0.15; Figure 6C,D).
FIGURE 6.
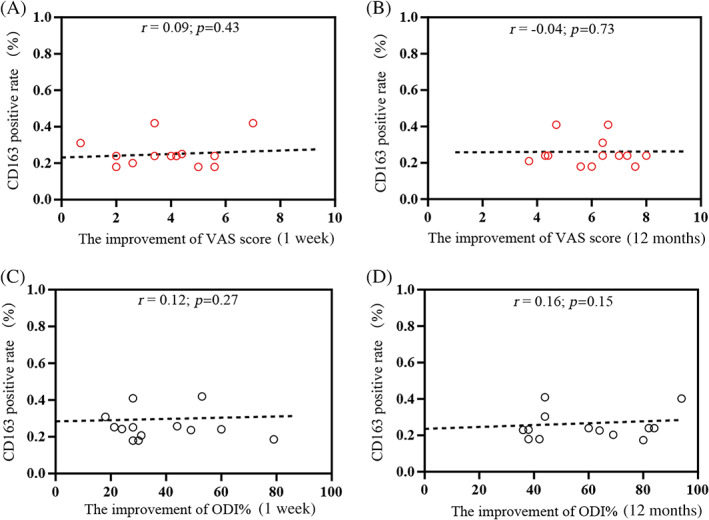
Correlation between clinical efficacy and CD163 positivity rates in patients with lumbar disc herniation (LDH). The correlation between CD163 (+) cell percentages and the improvement of visual analog scale (VAS) scores at 1 week (A) and 12 months (B) was determined. The association between ODI at 1 week (C) and 12 months (D) with CD163 (+) cell percentages was analyzed. r = Spearman's correlation coefficient (95% confidence interval).
4. DISCUSSION
A recent human NP samples study based on single‐cell RNA‐Seq analysis demonstrated only M1 and M2 macrophage polarization in degenerate IVD specimens. 26 Nakazawa et al (2017) 17 identified the localization of M1, M2a, and M2c cells within human cadaver IVDs, indicating that macrophage polarization is an important marker with immense potential for improving knowledge about pathophysiology and the development of future therapeutic targets of IVD. CD68, as a macrophage marker, has been reported only in a fraction of patients with LDH, 8 , 19 , 27 and two studies found a negative association with pain scores. 8 , 19 However, to date, little is known about the association between clinical efficacy after surgery and macrophage polarization in patients with LDH. The results of our study indicated that patients whose NP samples positively expressed CD68 and CCR7 showed significantly lower VAS scores 1 week after surgery compared with those that did not express CD68 and CCR7. Moreover, the improvement in VAS score showed a convincing positive correlation with CD68‐ or CCR7‐positive cell rate. Our results indicate that diminishing pro‐inflammatory M1 macrophages may be related to the mechanism of chronic pain reduction after surgery. Therefore, patients with LDH require personalized treatment targeting heterogeneous pain.
Previous studies have reported the association between sciatic pain and the release of pro‐inflammatory cytokines, such as TNF‐α, IL‐6, IL‐8, and IFN‐γ; anti‐inflammatory cytokines, such as IL‐10, IL‐4, and TGF‐β. 28 , 29 Of these, the most convincing positive association between pain‐related outcome measures and M1 excretion factors was provided by studies on TNF‐α. 5 , 30 , 31 However, there is still a lack of widely accepted evidence that TNF‐α contributes to sciatic symptoms after surgery. In contrast, M2‐related factors showed moderate quality evidence for an association between low pain scores and high levels of IL‐4, limited evidence for an association between low pain scores and high levels of IL‐10, and no association with TGF beta. 5 However, conclusions regarding these inflammatory cytokine analyses are controversial because cytokines were released from either NP tissue or inflammatory cells. 5 This limited the comparability of all the studies and confounded the results. Furthermore, chronic pain after surgery could also prove to increase serum levels of inflammatory cytokines during the follow‐up period. 8 Thus, the correction between the release of chronic inflammation‐related cytokines and postoperative clinical efficacy was ambiguous. In this study, four phenotypic markers (CD68, CCR7, CD206, and CD163) were used to directly identify macrophage polarization, which is in accordance with several previous studies that have applied similar markers. 17 , 18 , 21 Of these, CD68 was widely accepted as a macrophage marker, while CCR7, CD206, and CD163 were taken as M1, M2a, and M2c polarized macrophage markers respectively. 17 , 18 In addition, only those cells surrounding cracks, tears, or granulation tissue were selected for analysis, rather than a specified area in the IVD. This approach may truly and effectively reflect macrophage infiltration in the IVD, as infiltrated cells have been shown to migrate along the neovascularization in a linear form during the LDH process. 32 , 33
No significant differences in clinical symptoms, measured by the VAS score and ODI, after surgery were found between macrophage‐positive expression and negative expression. This may be because the macrophage population in this study contained both pro‐ and anti‐inflammatory macrophage phenotypes, finally confounding the results to some extent. When the different macrophage markers were analyzed, the CD68(+) and CCR7(+) marker rates showed significantly lower VAS scores 1 week after surgery, suggesting that more extensive polarization of M1 macrophages was associated with better clinical efficacy, at least for a short period of time. These findings are in accordance with previous reports that a higher CD68 (+) proportion in patients was associated with lower pain scores after 6 months of follow‐up. 8 Furthermore, our results also showed that the CCR7(+) percentage showed the same trend as CD68 positivity. Owing to CD68 (+) being used as a macrophage marker and CCR7 (+) being taken as a pro‐inflammatory M1 marker, 17 the current data suggest that, in LDH, sciatic pain was partially attributed to M1 macrophages in the herniated NP tissue. Therefore, our results demonstrated that macrophage polarization occurs only in a fraction of patients, indicating that for many cases, pain symptoms may be defined by personalized signs. Finally, macrophages may cause lumbar radicular pain through transition to M1 phenotype, and their activity on the dorsal nerve roots, at least for a short period of time.1
Furthermore, when the association between clinical efficacy and macrophage markers was analyzed respectively, no significant correlation was detected between any of the four markers and the VAS score or ODI, suggesting that higher macrophage polarization does not mean better clinical efficacy after surgery. This observation may be due to the following reasons: (i) During the perioperative period, the VAS score and ODI after surgery are usually reduced by drug administration. (ii) As an inflammatory response occurs in the discs, nerve outgrowth from the dorsal root ganglion infiltration into the disc begins and, subsequently, the sciatic symptoms appear. 34 , 35 Discectomy surgery could decrease mechanical compression and reduce inflammatory stimuli to some extent, but inflammatory cytokines whose levels are difficult to reduce promote sensitization of the disc, causing residual pain symptoms after surgery. Taken together, the clinical efficacy of LDH is regulated by a combination of comprehensive factors, which makes a specific analysis challenging. More studies with specific subpopulations are required to confirm this hypothesis.
When the improvement of VAS score was analyzed, a convincing positive correlation was detected in both CD68 (+) and CCR7 (+) cells after 1 week and 12 months of follow‐up. This is the most important finding because the improvement in VAS could truly reflect clinical efficacy; the improvement in VAS score (1 week or 12 months) in this study was calculated by subtracting the follow‐up VAS score (7D PostOP or 12M PostOP) from the baseline VAS score (1D PreOP). Our findings indicated that sciatic pain was partially caused by the presence of pro‐inflammatory M1 cells, and excision of the herniation likely not only relieved the compression but also a part of M1 macrophages. On the contrary, no significant association was detected with the improvements in the ODI, an index of lumbar spine function, and this may be ascribed to the ODI commonly being taken as comprehensive accumulated results. Therefore, a specific analysis according to the proportion of macrophage polarization at specific time points may be difficult to reflect the truly accumulating function of the lumbar spine. Further study is still needed to clarify this.
This study had some limitations that should be considered. First, this study lacked non‐injured disc tissue samples for comparison purposes, and the imaging field selection criteria were not enough to exclude bias to some extent. Second, a wide variety of accepted macrophage phenotype markers were not used in the study; only CD68, CCR7, CD206, and CD163 were used to identify macrophage polarization. Therefore, more studies are warranted using more markers and dual‐staining‐based immunofluorescence analysis to validate our findings. Finally, our study focused on the clinical efficacy of macrophage polarization after surgery, and only a few studies have investigated the potential mechanism underlying this phenomenon to date; thus, further investigation is needed.
In conclusion, the findings of the present study supported the involvement of macrophage polarization in herniated lumbar disc specimens. The NP tissue samples of patients with LDH exhibiting positivity for the phenotypic markers CD68 and CCR7 achieved better clinical efficacy, and there was a significantly positive correlation with the improvement in VAS score after surgery. This indicates that the presence of pro‐inflammatory M1 cells probably holds better clinical efficacy in many cases. However, it remains difficult to define to what extent macrophage polarization contributes to the pain experienced in these patients. Therefore, our findings emphasize the importance of treatment to deliver a more personalized approach considering the heterogeneity of sciatica symptoms.
AUTHOR CONTRIBUTIONS
Xiao‐Chuan Li, Mao‐Sheng Wang, and Xiao‐Chun Bai conceived and designed the experiments. Xiao‐Chuan Li, Shao‐Jian Luo, Zhen‐Hua Qiu, Jiong‐Hui Chen, Dan‐Qin Tan, Yong‐Long Chen, Cheng Jiang, Wei Wang, Zhen‐Wu Zhang, and Wu Fan performed, analyzed, and interpreted the data; and Xiao‐Chuan Li wrote the manuscript. Xiao‐Chuan Li, Chun‐Ming Huang, Mao‐Sheng Wang, and Xiao‐Chun Bai provided reagents and reviewed the manuscript for intellectual content. All authors have read and approved the final submitted manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 81802130), China Postdoctoral Science Foundation (Grant No. 2018M630968), and Natural Science Foundation of Guangdong Province (Grant No. 2018A030310462).
Li, X.‐C. , Luo, S.‐J. , Fan, W. , Jiang, C. , Wang, W. , Chen, J.‐H. , Chen, Y.‐L. , Zhang, Z.‐W. , Qiu, Z.‐H. , Tan, D.‐Q. , Huang, C.‐M. , Wang, M.‐S. , & Bai, X.‐C. (2023). Influence of macrophage polarization in herniated nucleus pulposus tissue on clinical efficacy after lumbar discectomy. JOR Spine, 6(2), e1249. 10.1002/jsp2.1249
Footnotes
[Correction added on 20 February 2023, after first online publication: Incorrect versions of Tables 3 and 4 and Figures 5 and 6 have been replaced]
Contributor Information
Mao‐Sheng Wang, Email: lixcgzph@163.com.
Xiao‐Chun Bai, Email: baixiaochunnfy@163.com.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in the published article.
REFERENCES
- 1. Deyo RA, Mirza SK. CLINICAL PRACTICE. Herniated lumbar intervertebral disk. N Engl J Med. 2016;374:1763‐1772. [DOI] [PubMed] [Google Scholar]
- 2. Cunha C, Silva AJ, Pereira P, Vaz R, Gonçalves RM, Barbosa MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther. 2018;20:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu ZH, Sun Z, Wang HQ, et al. FasL expression on human nucleus pulposus cells contributes to the immune privilege of intervertebral disc by interacting with immunocytes. Int J Med Sci. 2013;10:1053‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vizcaino Reves N, Mogel HM, Stoffel M, et al. Polarization of macrophages in epidural inflammation induced by canine intervertebral disc herniation. Front Vet Sci. 2020;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Djuric N, Lafeber GCM, Vleggeert‐Lankamp CLA. The contradictory effect of macrophage‐related cytokine expression in lumbar disc herniations: a systematic review. Eur Spine J. 2020;29:1649‐1659. [DOI] [PubMed] [Google Scholar]
- 6. Kawaguchi K, Harimaya K, Matsumoto Y, et al. Effect of cartilaginous endplates on extruded disc resorption in lumbar disc herniation. PLoS One. 2018;13:e0195946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun Z, Liu B, Luo ZJ. The immune privilege of the intervertebral disc: implications for intervertebral disc degeneration treatment. Int J Med Sci. 2020;17:685‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Woertgen C, Rothoerl RD, Brawanski A. Influence of macrophage infiltration of herniated lumbar disc tissue on outcome after lumbar disc surgery. Spine. 2000;25:871‐875. [DOI] [PubMed] [Google Scholar]
- 9. Shamji MF, La S, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandes TL, Gomoll AH, Lattermann C, Hernandez AJ, Bueno DF, Amano MT. Macrophage: a potential target on cartilage regeneration. Front Immunol. 2020;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothoerl R, Woertgen C, Holzschuh M, Brehme K, Rüschoff J, Brawanski A. Macrophage tissue infiltration, clinical symptoms, and signs in patients with lumbar disc herniation. A clinicopathological study on 179 patients. Acta Neurochir. 1998;140:1245‐1248. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. 2020;28:555‐561. [DOI] [PubMed] [Google Scholar]
- 13. Silva AJ, Ferreira JR, Cunha C, et al. Macrophages down‐regulate gene expression of intervertebral disc degenerative markers under a pro‐inflammatory microenvironment. Front Immunol. 2019;10:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao F, Guo Z, Hou F, Fan W, Wu B, Qian Z. Magnoflorine alleviates “M1” polarized macrophage‐induced intervertebral disc degeneration through repressing the HMGB1/Myd88/NF‐kappaB pathway and NLRP3 inflammasome. Front Pharmacol. 2021;12:701087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwiecien I, Polubiec‐Kownacka M, Dziedzic D, et al. CD163 and CCR7 as markers for macrophage polarization in lung cancer microenvironment. Cent Eur J Immunol. 2019;44:395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen MA‐O, Hvidbjerg Gantzel RA‐O, Clària JA‐O, et al. Macrophage activation markers, CD163 and CD206, in acute‐on‐chronic liver failure. Cells. 2020;9:1175. doi: 10.3390/cells9051175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakazawa KR, Walter BA, Laudier DM, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018;18:343‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang C, Cao P, Gao Y, et al. Differential expression of p38 MAPK alpha, beta, gamma, delta isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Sci Rep. 2016;6:22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothoerl RD, Woertgen C, Brawanski A. Pain resolution after lumbar disc surgery is influenced by macrophage tissue infiltration. A prospective consecutive study on 177 patients. J Clin Neurosci. 2002;9:633‐636. [DOI] [PubMed] [Google Scholar]
- 20. Kawakubo A, Uchida K, Miyagi M, et al. Investigation of resident and recruited macrophages following disc injury in mice. J Orthop Res. 2020;38:1703‐1709. [DOI] [PubMed] [Google Scholar]
- 21. Li L, Wei K, Ding Y, et al. M2a macrophage‐secreted CHI3L1 promotes extracellular matrix metabolic imbalances via activation of IL‐13Ralpha2/MAPK pathway in rat intervertebral disc degeneration. Front Immunol. 2021;12:666361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakawaki M, Uchida K, Miyagi M, et al. Changes in nerve growth factor expression and macrophage phenotype following intervertebral disc injury in mice. J Orthop Res. 2019;37:1798‐1804. [DOI] [PubMed] [Google Scholar]
- 23. Yokozeki Y, Kawakubo A, Miyagi M, et al. Reduced TGF‐beta expression and CD206‐positive resident macrophages in the intervertebral discs of aged mice. Biomed Res Int. 2021;2021:7988320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X‐C, Luo S‐J, Fan W, et al. Macrophage polarization regulates intervertebral disc degeneration by modulating cell proliferation, inflammation mediator secretion, and extracellular matrix metabolism. Front Immunol. 2022;13:922173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940‐2952. [DOI] [PubMed] [Google Scholar]
- 26. Ling Z, Liu Y, Wang Z, et al. Single‐cell RNA‐seq analysis reveals macrophage involved in the progression of human intervertebral disc degeneration. Front Cell Dev Biol. 2022;9:833420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dongfeng R, Hou S, Wu W, et al. The expression of tumor necrosis factor‐alpha and CD68 in high‐intensity zone of lumbar intervertebral disc on magnetic resonance image in the patients with low back pain. Spine. 2011;36:E429‐E433. [DOI] [PubMed] [Google Scholar]
- 28. Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911‐917. [DOI] [PubMed] [Google Scholar]
- 29. Zu B, Pan H, Zhang XJ, Yin ZS. Serum levels of the inflammatory cytokines in patients with lumbar radicular pain due to disc herniation. Asian Spine J. 2016;10:843‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrade P, Visser‐Vandewalle V, Philippens M, et al. Tumor necrosis factor‐alpha levels correlate with postoperative pain severity in lumbar disc hernia patients: opposite clinical effects between tumor necrosis factor receptor 1 and 2. Pain. 2011;152:2645‐2652. [DOI] [PubMed] [Google Scholar]
- 31. Andrade P, Hoogland G, Teernstra OP, et al. Elevated levels of tumor necrosis factor‐alpha and TNFR1 in recurrent herniated lumbar discs correlate with chronicity of postoperative sciatic pain. Spine J. 2016;16:243‐251. [DOI] [PubMed] [Google Scholar]
- 32. Sun Z, Zhang M, Zhao XH, et al. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6:1009‐1014. [PMC free article] [PubMed] [Google Scholar]
- 33. Ni S, Ling Z, Wang XA‐OX, et al. Sensory innervation in porous endplates by Netrin‐1 from osteoclasts mediates PGE2‐induced spinal hypersensitivity in mice. [DOI] [PMC free article] [PubMed]
- 34. Djuric N, Yang X, Ostelo R, et al. Disc inflammation and Modic changes show an interaction effect on recovery after surgery for lumbar disc herniation. Eur Spine J. 2019;28:2579‐2587. [DOI] [PubMed] [Google Scholar]
- 35. Aoki Y, Ohtori S, Ino H, et al. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine. 2004;29:2621‐2626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.


