Abstract
Disseminated coccidioidomycosis is associated with significant morbidity and mortality. Involvement of the meninges is often fatal if untreated, typically requiring lifelong antifungal therapy and neurosurgical intervention. We present the case of a young male without any known immunocompromising conditions who opted exclusively for medical management of newly diagnosed coccidioidomycosis meningitis with communicating hydrocephalus and discuss the controversy associated with this approach. This case highlights the importance of shared decision-making between patient and clinician, even if the plan diverges from available guidelines. Furthermore, we discuss clinical considerations in approaching the close outpatient monitoring of patients with central nervous system coccidioidomycosis with hydrocephalus.
Keywords: coccidioidal meningitis, communicating hydrocephalus, ventriculoperitoneal shunt, IDSA, Coccidioidomycosis Study Group
Introduction
Over the last 25 years, there has been an increased incidence of disseminated coccidioidomycosis of varying manifestations in hosts without known immunocompromise, from tenosynovitis to meningitis with resultant hydrocephalus.1-4 Presenting symptoms of coccidioidomycosis meningitis include progressively worsening headaches, nausea, vomiting, visual deficits, and mental status changes with fewer traditional meningeal signs like nuchal rigidity.5,6 In addition to antifungal therapy, standard of care for coccidioidomycosis meningitis with symptomatic hydrocephalus includes neurosurgical intervention for definitive management of increased intracranial pressure (ICP). Current guidelines do not support the exclusive use of medical management. 7 In this report, we describe a case of coccidioidomycosis meningitis-related hydrocephalus in an immunocompetent male, who declined recommended neurosurgical intervention and opted solely for medical management. Extending from this case, we discuss an approach for clinicians to closely monitor similar patients electing for non-shunt management of coccidioidomycosis meningitis-related hydrocephalus. We present this case to highlight the importance of shared decision-making between patient and clinician, even if the therapeutic plan diverges from clinical guideline recommendations.
Case Report
A 29-year-old Canadian male of Indian descent without a significant past medical history presented to the emergency department (ED) in Tucson, Arizona directly from a local optometry office after significant bilateral papilledema was noted on a routine exam required for an updated prescription for his corrective lenses. He denied nausea, vomiting, eye pain, and acute vision changes. However, he endorsed ongoing headaches for 6 months, initially starting in late spring following a job interview for a materials engineer position at a nearby mine and exploring other Sonoran Desert attractions in Arizona earlier in the year. He reported briefly living in India 4 years prior to presentation but was otherwise without notable travel history or high-risk social behaviors. He repeatedly presented to local EDs in Canada and underwent neuroimaging for his new-onset, severe headache postjob interview. Head computerized tomography (CT) and magnetic resonance imaging (MRI) during those visits revealed no acute abnormalities. At these ED visits, he was treated for migraines before discharging home. The patient also reported a 20-pound weight loss in the following months, but his weight stabilized and headache improved gradually after relocating to Arizona for his new job.
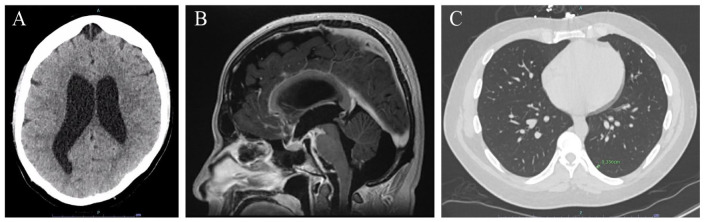
In our ED, a CT of the head showed ventriculomegaly with hydrocephalus (Figure 1A). Subsequently, an MRI of the brain confirmed extensive basilar meningeal enhancement with communicating hydrocephalus (Figure 1B). Neurosurgery was immediately consulted based on neuroimaging results. Following a successful lumbar puncture (LP), the patient was initially treated empirically for viral, fungal, bacterial, and mycobacterial meningitis, given his degree of hydrocephalus and hyperdense basilar meningeal enhancement on neuroimaging.
Figure 1.
CT and MRI findings showing disseminated coccidioidomycosis basilar meningitis with communicating hydrocephalus and left lower lobe primary pulmonary nodule. (A) CT brain without contrast showing diffuse ventriculomegaly without trans-ependymal flow, consistent with hydrocephalus. (B) MRI brain sagittal view on T1 postcontrast sequence showing extensive basilar meningitis and communicating hydrocephalus. (C) CT thorax without contrast showing 3 mm left lower lobe pulmonary nodule.
Abbreviations: CT, computerized tomography; MRI, magnetic resonance imaging.
Initial cerebrospinal fluid (CSF) studies showed low glucose (19 mg/dL), elevated protein (279.8 mg/dL) with lymphocytic predominant leukocytosis (87% of 335 per µL), and an opening pressure of 33 cmH2O. Empiric coverage was narrowed to cover Mycobacterium tuberculosis and Coccidiosis spp. based on his international travel history and endemic exposure. Cerebro spinal fluid Gram stain did not visualize any organisms, spherules, molds, or yeasts. Bacterial and fungal cultures of CSF fluid did not yield any growth. Diagnosis of disseminated coccidioidomycosis meningitis was confirmed with positive Coccidioides enzyme immunoassay (EIA) IgG antibody, positive Coccidioides immunodiffusion (IMDF) IgM tube precipitin antibody, positive Coccidioides IMDF IgG complement fixation (CF) antibody, serum Coccidioides CF titer of 1:128, and CSF Coccidioides CF titer of 1:32. Computed tomography thorax imaging also identified a 3-mm left lower lobe pulmonary nodule without parenchymal consolidation, cavitary lesion, or pleural effusion (Figure 1C).
Our patient was treated with intravenous amphotericin B for 5 days with concurrent intravenous fluconazole until therapeutic azole levels reached blood-brain barrier penetrance concentrations. Consistent with Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines, our patient was advised to undergo ventriculoperitoneal (VP) shunt surgery with neurosurgery for definitive management of symptomatic hydrocephalus. 7 However, he declined any invasive procedure due to his perception that his symptoms were not hindering his ability to perform his highly demanding occupational and daily activities. He was transitioned to oral fluconazole before discharging home with close outpatient follow-up in subspecialty clinics while waiting to establish care with a primary-care physician. Prior to discharge, he was educated on warning signs and symptoms (including but not limited to visual changes, new neurological symptoms, urinary or bowel dysfunction, worsening headaches, refractory nausea, and vomiting) and given strict ED return precautions. He was also scheduled for a repeat outpatient brain MRI 3 months postdischarge.
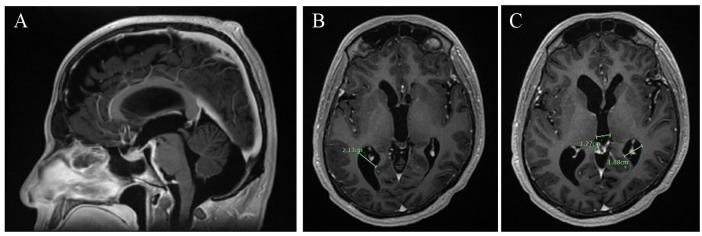
Since discharge, he has been closely monitored for any progression of his intermittent headaches or further clinical signs of neurologic impairment and completed repeat brain MRI imaging (Figure 2A-C). Thus far, our patient endorses a positive response to and tolerance of oral fluconazole treatment with symptomatic and radiographic improvement.
Figure 2.
Repeat MRI brain after 4 months of exclusive medical management showing interval improvement of basilar meningitis with decreased communicating hydrocephalus. (A) MRI brain sagittal view in T1 postcontrast showing interval improvement in basilar meningitis. (B-C) MRI brain coronal view in T1 MP-RAGE postcontrast showing interval reduction in hydrocephalus.
Abbreviations: MRI, magnetic resonance imaging; MP-RAGE, Magnetization Prepared - RApid Gradient Echo.
Discussion
Coccidioidomycosis meningitis results in proteinaceous debris and arachnoid fibrosis that contribute to the development of hydrocephalus and increased ICP. 8 In a recent Invited Commentary in Clinical Infectious Diseases authored by members of the IDSA Coccidioidomycosis Study Group, management of increased ICP and hydrocephalus in central nervous system (CNS) coccidioidomycosis was discussed. 9 The experts recommended placement of an MRI-compatible VP shunt as the only permanent management approach, with other approaches like repeated CSF removal via LP or external ventricular drain (EVD) as temporary treatments.9,10 The experts also agreed that shunt placement should not be delayed with concern that delays in shunting lead to worse patient outcomes.8,11
However, published guidelines do not specify optimal timing for a shunt in the management of hydrocephalus with increased ICP for minimally symptomatic patients. Some studies question the necessity of shunt placement early in the disease process due to risks and complications associated with indwelling shunts. 11 VP shunt placement is a lifelong process associated with a high likelihood of shunt failure requiring revisions due to catheter obstruction, infection with catheter biofilm formation and abscess development, hemorrhage, and other postprocedural complications, such as seizures.8,12,13 Continued ventriculomegaly despite a properly functioning shunt and 9% mortality rate in patients who receive VP shunting are additional considerations. 8
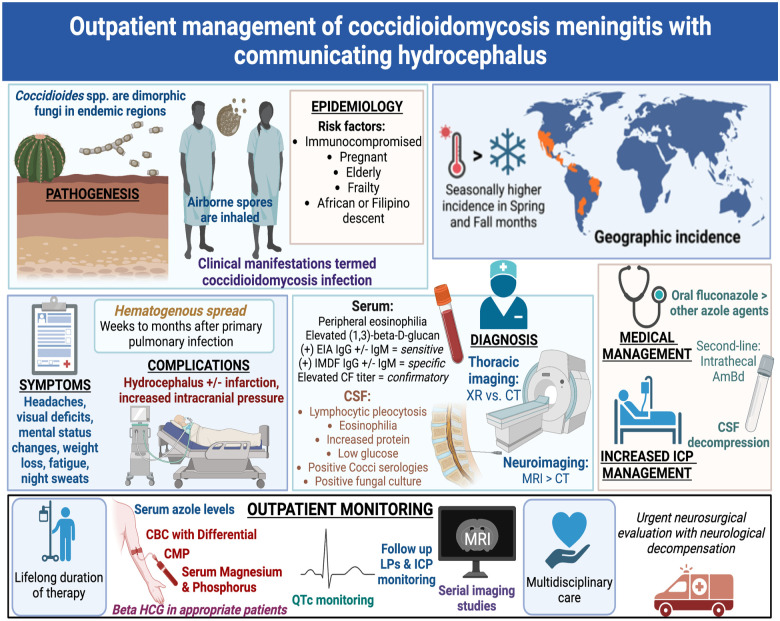
Our case emphasizes the importance of the general practitioner’s basic understanding of outpatient management of disseminated coccidioidomycosis (Figure 3). In addition to outpatient management by a primary-care physician, patients with CNS coccidioidomycosis complicated by hydrocephalus should follow-up in the clinic with infectious disease experts in coccidioidomycosis and with neurologists. Medication management requires lifelong antifungal therapy (Table 1).7,14-17 Oral fluconazole remains the standard of care for coccidioidomycosis meningitis therapy, but intrathecal amphotericin B (AmBd) is recommended for pregnant patients in the first trimester due to teratogenicity of azoles.7,10,15Table 1 provides a summary of adverse effects of anti-Coccidioidal agents and specific laboratory tests and studies to order for outpatient management of disseminated coccidioidomycosis.
Figure 3.
Central illustration: Outpatient management of coccidioidomycosis meningitis with communicating hydrocephalus.
Abbreviations: EIA, enzyme immunoassay; IMDF, immunodiffusion; CSF, cerebrospinal fluid; ICP, intracranial pressure; LP, lumbar puncture; CBC, complete blood count; CMP, comprehensive metabolic panel; Beta HCG, Human Chorionic Gonadotropin, QTc, corrected QT interval.
Table 1.
Summary of Coccidioidomycosis Meningitis Antifungal Management.
| Antifungal Agent | Oral Bioavailability | Absorption affected by food consumption | CSF Penetration | Clearance | Recommended oral daily dose | Adverse effects | Routine Monitoring |
|---|---|---|---|---|---|---|---|
| Fluconazole | >90% | Unaffected | Good-CSF concentration 80% that of serum concentration | Renal (80%); requires renal dose adjustment | 400-1200 mg daily | Hepatotoxicity | Liver function tests |
| Regular monitoring of vitals and electrolytes | |||||||
| Teratogenicity | Serum drug monitoring (can guide dose adjustments) | ||||||
| Hepatic (10%) | Higher dosing is frequently used due to high rates of failure with lower dose | Rarely, glucocorticoid suppression (i.e. Addison’s disease) | Drug interaction monitoring (CYP450 inhibitor) | ||||
| Itraconazole | 55% | Enhanced by consumption of high fat meal and increased gastric acidity (e.g. consuming an acidic beverage; Coca Cola was specifically studied) | Poor-CSF concentration: 0.2-12% that of serum concentration | Hepatic | 400-800 mg every 8 to 12 hours | Aldosterone-like effects: hypokalemia, hypertension, peripheral edema | Regular monitoring of vitals and electrolytes |
| With fatty food and acidic beverages | *BLACK BOX WARNING* for heart failure patients due to negative inotropic effects | Baseline and routine echocardiograms with serum brain natriuretic peptide levels prior to initiation and whenever there is a clinical change, respectively. | |||||
| Decreases by 40-50% when taken with proton pump inhibitors or H2 blockers that reduce gastric acidity | Teratogenicity | Serum drug monitoring | |||||
| Requires closer serum and clinical monitoring due to risk for toxicity and drug interactions | |||||||
| Voriconazole | >90% | Decreased by >30% when taken with food | Less than fluconazole | Hepatic | 4 mg/kg every 12 hours | Reversible visual deficits | Counseling on sun safety: protective clothing, sunscreen, and avoiding direct sunlight |
| Severe photodermatitis | |||||||
| Increased risk of skin cancer | |||||||
| Extensive tissue distribution | CSF concentration 46% that of serum concentration | Potassium, calcium, and magnesium electrolyte abnormalities | Electrolyte monitoring before and during therapy to correct abnormalities as needed | ||||
| QTc prolongation | Regular ECG monitoring | ||||||
| Teratogenicity | |||||||
| Increased risk for toxicity above 5.5 micrograms/mL | Serum drug monitoring | ||||||
| Posaconazole | >50% | Enhanced by consumption of a high fat meal and acidic beverage | Poor-CSF concentration: 0.9% that of serum concentration | Hepatic (15%) | 400 mg of oral suspension every 12 hours for cocci meningitis refractory to fluconazole | Reported in immunocompromised patients: Diarrhea, fever, nausea | Electrolyte monitoring before and during therapy to correct abnormalities as needed |
| Electrolyte abnormalities | Regular ECG monitoring | ||||||
| Oral absorption improved by dividing doses into 4 throughout the day | Minimally metabolized (77% excreted unchanged in feces) | However, delayed-release oral tablets have better oral absorption than liquid suspension | QTc prolongation | Serum drug monitoring | |||
| Teratogenicity | |||||||
| Intrathecal Amphotericin B deoxycholate | N/A | N/A | Excellent- bypasses blood-brain barrier | Unknown | Initial dose of 0.1 mg 3 times per week, gradually increasing the dose by 0.1 mg as tolerated | Headache | Electrolyte monitoring |
| Nausea | |||||||
| Vomiting | |||||||
| (Urinary and biliary excretion account for <5% of dose) | Dose titrated to patient tolerability | Neurotoxicity: hearing loss, ophthalmoplegia, ataxia, paraplegia, neurogenic bladder, erectile dysfunction | Serum drug monitoring |
Abbreviation: CSF, cerebrospinal fluid; QTc, corrected QT interval.
For this case, we followed a patient-centered approach for non-shunt management of coccidioidomycosis meningitis-related hydrocephalus. As the patient presented with papilledema and hydrocephalus, immediate lowering of ICP early into his hospitalization was necessary to prevent long-term disability and death in the short term. After stabilization from immediate harm, we used shared decision-making with the patient, only proceeding with therapies for which he provided informed consent, which did not include repeat LP for further CSF removal or long-term shunt placement. Through very close outpatient follow-up and repeat imaging, our patient’s symptoms improved and ventriculomegaly diminished on oral fluconazole. Subsequent outpatient follow-up for the patient should include formal ophthalmological evaluation to assess for resolution of papilledema and repeat LP to assess for objective decrease in ICP. Even if the treatment plan decided upon differs from clinical practice guidelines, patient safety should remain the paramount concern in the management of coccidioidomycosis meningitis-related hydrocephalus during patient counseling and shared decision-making.
Acknowledgments
This case was presented as a poster at the 67th Annual Coccidioidomycosis Study Group Meeting in Tucson, Arizona on March 31, 2023. Figure 3 was produced using the BioRender online platform available at BioRender.com.
Footnotes
Author Contributions: All authors contributed to the conception and design of the work; acquisition, analysis, or interpretation of the data for the work; drafting the work or revising it critically for important intellectual content; approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was supported by The University of Arizona Research, Innovation & Impact and BIO5 funding from the Improving Health Technology and Research Initiative Fund (TRIF) # 5844228.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Sanjay V. Menghani  https://orcid.org/0000-0003-2056-1879
https://orcid.org/0000-0003-2056-1879
References
- 1.Romeo JH, Rice LB, McQuarrie IG. Hydrocephalus in coccidioidal meningitis: case report and review of the literature. Neurosurgery. 2000;47(3):773-777. doi: 10.1097/00006123-200009000-00051 [DOI] [PubMed] [Google Scholar]
- 2.Campbell M, Kusne S, Renfree KJ, et al. Coccidioidal tenosynovitis of the hand and wrist: report of 9 cases and review of the literature. Clin Infect Dis. 2015;61:1514-1520. doi: 10.1093/cid/civ642 [DOI] [PubMed] [Google Scholar]
- 3.Sharifi S, Sharma R, Heidari A, Johnson RH. Disseminated coccidioidomycosis: cutaneous, soft tissue, osseous, and “shotgun intraparenchymal” brain disease. J Investig Med High Impact Case Rep. 2022;10:23247096221075906. doi: 10.1177/23247096221075906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Ruan Q, Jin J, et al. Disseminated coccidioidomycosis in immunocompetent patients in non-endemic areas: a case series and literature review. Eur J Clin Microbiol Infect Dis. 2022;41(6):925-939. doi: 10.1007/s10096-022-04447-y [DOI] [PubMed] [Google Scholar]
- 5.Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a descriptive survey of a reemerging disease. Medicine. 2004;83(3):149-175. doi: 10.1097/01.md.0000126762.91040.fd [DOI] [PubMed] [Google Scholar]
- 6.Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA—armed forces cooperative studies, 1955–1958. Clin Infect Dis. 1993;16(2):247-254. doi: 10.1093/clind/16.2.247 [DOI] [PubMed] [Google Scholar]
- 7.Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63:e112-146. doi: 10.1093/cid/ciw360 [DOI] [PubMed] [Google Scholar]
- 8.Hardesty DA, Ramey W, Afrasiabi M, et al. Patient outcomes and surgical complications in coccidioidomycosis-related hydrocephalus: an institutional review. J Neurosurg. 2014; 121(4):785-789. doi: 10.3171/2014.6.jns14111 [DOI] [PubMed] [Google Scholar]
- 9.Thompson GR, Ampel NM, Blair JE, et al. Controversies in the management of central nervous system coccidioidomycosis. Clin Infect Dis. 2022;75:555-559. doi: 10.1093/cid/ciac478 [DOI] [PubMed] [Google Scholar]
- 10.Bercovitch RS, Catanzaro A, Schwartz BS, Pappagianis D, Watts DH, Ampel NM. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53(4):363-368. doi: 10.1093/cid/cir410 [DOI] [PubMed] [Google Scholar]
- 11.Morshed RA, Lee AT, Egladyous A, et al. Shunt treatment for coccidioidomycosis-related hydrocephalus: a single-center series. World Neurosurg. 2020;138:e883-e891. doi: 10.1016/j.wneu.2020.03.135 [DOI] [PubMed] [Google Scholar]
- 12.Davis LE, Cook G, Costerton JW. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis. 2002;8(4):376-379. doi: 10.3201/eid0804.010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Johnson RH, David GR, Rahimifar M, Heidari A. A case of coccidioidal meningitis with biofilm obstructing VP shunt due to cutibacterium acnes. J Investig Med High Impact Case Rep. 2023;11:23247096231159810. doi: 10.1177/23247096231159810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange D, Pavao JH, Wu J, Klausner M. Effect of a cola beverage on the bioavailability of itraconazole in the presence of h2 blockers. J Clin Pharmacol. 1997;37(6):535-540. doi: 10.1002/j.1552-4604.1997.tb04332.x [DOI] [PubMed] [Google Scholar]
- 15.Johnson R, Ho J, Fowler P, et al. Coccidioidal meningitis: a review on diagnosis, treatment, and management of complications. Curr Neurol Neurosci Rep. 2018;18:19. doi: 10.1007/s11910-018-0824-8 [DOI] [PubMed] [Google Scholar]
- 16.Ho J, Fowler P, Heidari A, et al. Intrathecal amphotericin B: a 60-year experience in treating coccidioidal meningitis. Clin Infect Dis. 2017;64:519-524. doi: 10.1093/cid/ciw794 [DOI] [PubMed] [Google Scholar]
- 17.Gintjee TJ, Donnelley MA, Thompson GR. Aspiring antifungals: review of current antifungal pipeline developments. J Fungi. 2020;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]