Abstract
Tofacitinib was the first Janus kinase inhibitor to be approved for the treatment of rheumatoid arthritis (RA), and there is a large body of data to inform the efficacy and safety of this drug for patients at different places in their treatment journeys and with diverse demographics and characteristics. Here, we summarize tofacitinib clinical efficacy and safety data from some clinical trials, post hoc analyses, and real-world studies, which provide evidence of the efficacy of tofacitinib in treating patients with RA at various stages of their treatment journeys, and with differentiating baseline characteristics, such as age, gender, race, and body mass index. In addition, we review the safety data available from different patient subpopulations in the tofacitinib clinical development program, real-world data, and findings from the ORAL Surveillance post-marketing safety study that included patients aged ⩾50 years with pre-existing cardiovascular risk factors. The available efficacy and safety data in these subpopulations can enable better discussions between clinicians and patients to guide informed decision-making and individualized patient care.
Keywords: Janus kinase inhibitors, rheumatoid arthritis, tofacitinib
Introduction
Rheumatoid arthritis (RA) is a systemic, chronic inflammatory disease that causes synovitis and is associated with increased risk of comorbidities and higher mortality, compared to the general population.1,2 Treatment options for RA have expanded beyond conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and aiming for remission, or at least low disease activity (LDA), per the ‘treat-to-target’ guidelines has become more achievable.3,4 Among the treatment options available to patients with RA are oral small molecule Janus kinase (JAK) inhibitors. Tofacitinib was the first JAK inhibitor to be approved for the treatment of RA, followed by baricitinib, upadacitinib, and filgotinib. As tofacitinib was first approved for RA in 2012, a large body of data has amassed from clinical trials, post hoc analyses, and real-world studies, which provides information to guide the appropriate use of this medication across different clinical scenarios.5–13 However, the volume of these data may also make it difficult for a clinician to quickly access the specific information they need for a particular clinical scenario. The goal of this article is to summarize the most pertinent tofacitinib efficacy and safety information based on commonly occurring clinical scenarios.
How to use this article
Table 1 provides a guide for our readers; each tofacitinib developmental study has a study number, alongside a study name used to refer to the studies more easily. The article is then divided into summaries of efficacy and safety information by patient characteristics and specific clinical scenarios.
Table 1.
Phase III, IIIb/IV, and LTE studies of tofacitinib in RA.
| Study name | ORAL Start 5 |
ORAL Solo 9 |
ORAL Sync 6 |
ORAL Standard 8 |
ORAL Scan 7 |
ORAL Step 10 |
ORAL Strategy 14 |
ORAL Shift 15 |
ORAL Surveillance 16 |
ORAL Sequel 17 |
A392104118 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Phase III RDB | Phase III RDB, PC | Phase III RDB, PC | Phase III RDB, PC | Phase III RDB, PC | Phase III RDB, PC | Phase IIIb/IV RDB, NI | Phase IIIb/IV, OL run-in phase, RDB MTX withdrawal phase, NI | Phase IIIb/IV randomized OL, NI | Phase III OL LTE | Phase III OL LTE |
| Population | MTX-naïve | DMARD-IR | DMARD-IR | MTX-IR | MTX-IR | TNFi-IR | MTX-IR | MTX-IR | MTX-IR | Patients had completed index studies | Japanese patients had completed index studies |
| Treatment arms | Tofacitinib 5 mg BID/tofacitinib 10 mg BID/MTX | Tofacitinib 5 mg BID/tofacitinib 10 mg BID/PBO a | Tofacitinib 5 mg BID + csDMARD/tofacitinib 10 mg BID + csDMARD/PBO + csDMARD b | Tofacitinib 5 mg BID + MTX/tofacitinib 10 mg BID + MTX/adalimumab SC 40 mg Q2W + MTX/PBO +MTX b | Tofacitinib 5 mg BID + MTX/tofacitinib 10 mg BID + MTX/PBO + MTX b | Tofacitinib5 mg BID + MTX/tofacitinib 10 mg BID + MTX/PBO + MTX a | Tofacitinib 5 mg BID/tofacitinib 5 mg BID + MTX/adalimumab SC 40 mg Q2W + MTX | Tofacitinib modified-release 11 mg QD ± MTX | Tofacitinib 5 mg BID + MTX/tofacitinib 10 mg BID + MTX/TNFi (adalimumab SC 40 mg Q2W or etanercept SC 50 mg QW) + MTX | Tofacitinib 5 mg BID c /tofacitinib 10 mg BID c | Tofacitinib 5 mg BID c /tofacitinib 10 mg BID c |
| N | 956 | 610 | 792 | 717 | 797 | 399 | 1146 | 694 | 4362 | 4481 | 486 |
| Duration | 2 years | 6 months | 1 year | 1 year | 2 years | 6 months | 1 year | 48 weeks | 5.5 years | 9.5 years | 5.5 years |
BID, twice daily; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; IR, inadequate response; LTE, long-term extension; MTX, methotrexate; N, total number of patients randomized and treated in the study; NI, non-inferiority; OL, open-label; PBO, placebo; PC, placebo-controlled; Q2W, every 2 weeks; QD, once daily; QW, once weekly; RA, rheumatoid arthritis; RDB, randomized, double-blind; SC, subcutaneous; TNFi, tumor necrosis factor inhibitor.
ClinicalTrials.gov identifiers: NCT01039688 (ORAL Start); NCT00814307 (ORAL Solo); NCT00856544 (ORAL Sync); NCT00853385 (ORAL Standard); NCT00847613 (ORAL Scan); NCT00960440 (ORAL Step); NCT02187055 (ORAL Strategy); NCT02831855 (ORAL Shift); NCT02092467 (ORAL Surveillance); NCT00413699 (ORAL Sequel); NCT00661661 (A3921041).
Participants receiving PBO advanced to tofacitinib 5 or 10 mg BID in a blinded manner at 3 months.
Participants receiving PBO advanced to tofacitinib 5 or 10 mg BID in a blinded manner at 3 months (non-responders) or 6 months (all remaining participants receiving PBO).
Concomitant csDMARD use was permitted. Patients could increase or decrease tofacitinib dose at the discretion of the investigator.
Use of tofacitinib based on a patient’s baseline characteristics
Tofacitinib use in seropositive versus seronegative RA
Elevated levels of rheumatoid factor (RF) and/or anti-cyclic citrullinated peptide (CCP) antibodies are seen in many, but not all, patients with a diagnosis of RA.19,20 Seropositivity for these antibodies may indicate greater disease severity and a higher risk of disease progression21,22 and has been shown to influence response to treatment for some medications. 23 A post hoc analysis of data pooled from ORAL Solo, Sync, Standard, Scan, and Step illustrated that tofacitinib significantly improved American College of Rheumatology (ACR) 20/50/70 responses, Disease Activity Score in 28 joints, erythrocyte sedimentation rate (ESR) LDA rates, and patient-reported outcomes (PROs), regardless of the patient’s serologic status. 24 However, anti-CCP-positive patients were more likely to achieve remission and LDA, and also had numerically greater improvement in physical functioning, regardless of RF status. 24 Another post hoc analysis of ORAL Scan and Start showed that tofacitinib generally reduced radiographic progression in patients with RA versus placebo (ORAL Scan) and methotrexate (MTX; ORAL Start), regardless of serologic status, but the impact on radiographic progression was larger in seropositive patients (either anti-CCP-positive or RF-positive). 25 Rates of adverse events (AEs) and discontinuations due to AEs were similar across serotype subgroups. 24
Tofacitinib use in RA based on sex, race, and geography
As with other studies in RA,26,27 the majority of patients with RA in the tofacitinib clinical development program were female; 28 however, tofacitinib has been shown to be efficacious regardless of biological sex. A post hoc analysis of ORAL Scan, Standard, and Sync specifically compared the outcomes of patients based on documented biologic sex, and indicated that male patients were generally more likely to reach remission, had slightly greater reduction in disease activity from baseline, and had greater improvement in disability index and functional assessments. 29 No consistent differences in safety findings were reported between males and females. 29 The observation that female patients have more residual symptoms has been reported with other advanced therapies and is poorly understood. 30
In another post hoc analysis of 15 phase II/III/IIIb/IV studies that evaluated the impact of race on safety and efficacy, patients in the tofacitinib clinical development program self-identified as White (n = 4145), Black (n = 213), Asian (n = 1348), or ‘Other’ (n = 649). 31 In general, patients responded to tofacitinib regardless of self-identified race, although numerically higher placebo responses were observed in Black patients compared with patients of other races, a finding that warrants further exploration. 31 Safety findings for tofacitinib were generally consistent across racial/treatment groups, except for higher rates of infections, herpes zoster (HZ), and hepatic events in Asian patients, who were mainly from Japan and Korea. 31 In a post hoc analysis of the upadacitinib RA phase III clinical program, upadacitinib was efficacious regardless of race; however, efficacy was generally lower in Black or African American patients versus patients who identified as White, Asian, or ‘Other’. Although safety was comparable across these groups, a higher overall AE rate was observed among non-White patients, while higher rates of specific AEs of special interest were noted in Asian (serious infections and HZ) and Black or African American patients [creatine phosphokinase elevations and adjudicated venous thromboembolisms (VTEs)]. 32
The consistency of tofacitinib efficacy and safety across racial groups in clinical studies are generally supported by real-world studies in patients with RA across geographic regions. For instance, in the Taiwan XTRA registry, the long-term (5 years) efficacy and safety of tofacitinib were shown to be comparable with tumor necrosis factor inhibitors (TNFi). 33 In addition, tofacitinib was effective and well-tolerated in patients enrolled in the Turkish HURBIO database, and in patients from the St. Gallen and Aarau hospitals in Switzerland.34,35 Tofacitinib was also shown to have comparable effectiveness and safety profiles to baricitinib in a real-world study in Japan, where HZ was the most common AE with both treatments. 36 Safety profiles of tofacitinib in real-world Latin American settings appeared to be generally consistent with those observed in a post hoc analysis of Latin American patients who received tofacitinib in the RA clinical program.37,38
Tofacitinib use in patients with pain and fatigue
Tofacitinib has been associated with rapid analgesic effects across multiple pain measures. 39 In ORAL Solo, where an interactive voice response system (IVRS) was used, patients receiving tofacitinib reported a reduction in their pain level in their IVRS daily diary 3 days from the start of therapy, and the difference between patients receiving tofacitinib versus placebo was statistically significant by the first post-baseline assessment at 2 weeks. 40 Supplementing this clinical trial data with recent real-world evidence, the majority (60%) of patients with RA receiving tofacitinib in clinical practice felt that their pain was reduced by half within 1 month of starting the therapy. 41 This decrease in pain level correlated with a reduction in disease activity measures. 41 Rapid pain relief has also been reported with other JAK inhibitors.42,43 In addition to targeting inflammation, JAK inhibitors may have a direct impact on the neurological pathways mediating pain; however, the exact mechanism through which this could happen has not been clearly described.
Patients with RA view fatigue as one of the most pervasive symptoms of their disease. 44 Fatigue was a pre-specified PRO in all the tofacitinib phase III studies, with the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scoring system used as an objective measure to compare baseline with Month 1 (first assessment of fatigue after therapy initiation), and time points through the remainder of each study. 45 Patients treated with tofacitinib were shown to have significant improvement in fatigue at Month 1 compared with MTX monotherapy (ORAL Start) 46 or placebo (ORAL Sync, Scan, and Standard).47–49 In ORAL Strategy, patients treated with tofacitinib either as monotherapy or in combination with MTX had similar improvements in fatigue from Month 3; improvements for patients receiving tofacitinib in combination with MTX were significantly greater at Month 6 than for those receiving adalimumab in combination with MTX. 50 Similarly, results from the SELECT-COMPARE phase III trial of upadacitinib in patients with RA with an inadequate response to MTX showed that patients treated with upadacitinib and background csDMARDs had significantly greater least squares mean changes from baseline in pain and fatigue at Week 12 versus those receiving adalimumab and background csDMARDs. 43
Tofacitinib use in a patient with depression and anxiety
A post hoc analysis of five phase III studies and one phase IIIb/IV study suggested that patients with signs of anxiety and depression had efficacy outcomes comparable with those of patients who did not have a past medical history of these diagnoses. Interestingly, the proportion of tofacitinib-treated patients who identified as having probable co-morbid major depressive disorder/anxiety (based on 36-item Short Form Health Survey [SF-36] mental component summary [MCS] score ⩽38) decreased from baseline with tofacitinib therapy (60% reduction after 6 months). 51 This analysis was limited by the use of the SF-36 MCS score to identify probable rather than confirmed major depressive disorder/anxiety. Further research using a gold-standard psychiatric interview is required to validate the use of SF-36 MCS score ⩽38.
Tofacitinib use in RA by weight and body mass index
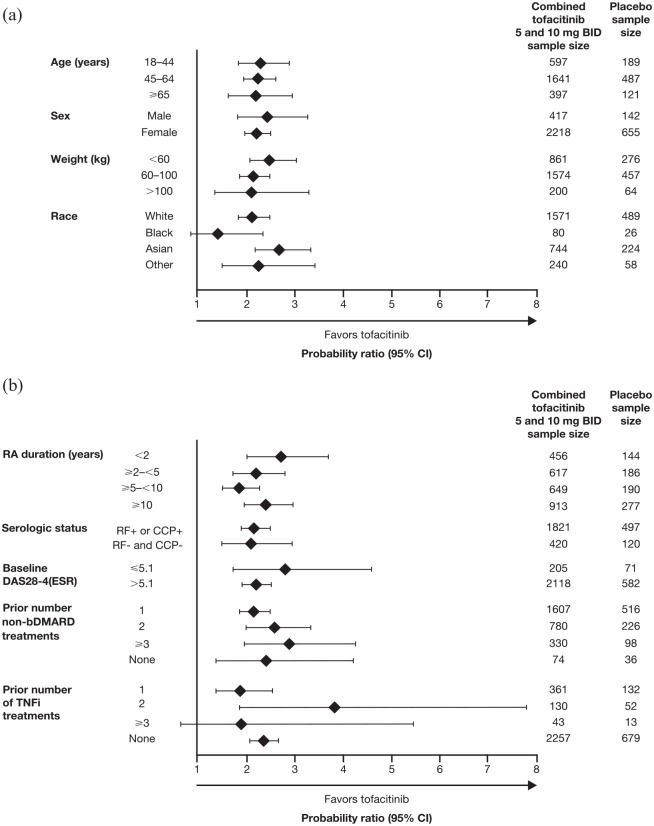
Pooled analyses of data from the tofacitinib clinical development studies have demonstrated consistent efficacy of tofacitinib in patients with RA, regardless of their weight (Figure 1) or body mass index (BMI) at baseline.52–54 Although the improvement in disease activity score from baseline is dampened as the patient’s BMI increases, 53 this is a trend also seen with other advanced therapies such as TNFi.55,56
Figure 1.
Probability ratios for achieving ACR20 response with tofacitinib plus MTX versus MTX alone in subpopulations of patients with RA stratified by (a) demographics and (b) disease characteristics. 52
ACR20, American College of Rheumatology >20% response criteria; bDMARD, biologic disease-modifying antirheumatic drug; BID, twice daily; CCP, cyclic citrullinated peptide; CI, confidence interval; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor; TNFi, tumor necrosis factor inhibitor.
Pooled analysis of data for tofacitinib 5 and 10 mg BID or placebo in phase II/III trials of ⩾3 months’ duration (NCT00550446, NCT00687193, NCT00814307, NCT00413660, NCT00603512, NCT00960440, NCT00847613, NCT00856544, and NCT00853385).
Tofacitinib use in older patients and those with comorbidities
Note 1: The recommended dosage for RA is tofacitinib 5 mg twice daily (BID) or modified-release 11 mg once daily (QD).57,58
The impact of age on the efficacy of tofacitinib has been investigated in two major data sets. The first is the pooled data from the phase III (ORAL Step, Sync, Scan, Solo and Standard) and long-term extension (LTE; ORAL Sequel and A3921041) trials of tofacitinib in patients with RA, which showed consistent effects of tofacitinib in improving disease activity and disability measures regardless of age (⩾65 years versus <65 years). 59 However, patients aged ⩾65 years in the LTEs had numerically higher incidence rates (IRs) of serious AEs and discontinuations, and higher rates of serious infections, than those aged <65 years. 59 Subsequent analysis of tofacitinib data from clinical trials (pooled phase II/III/IIIb/IV studies) has also confirmed a higher risk of serious infections in older versus younger patients. 60 Similarly, in an integrated safety analysis of upadacitinib clinical trials across RA, psoriatic arthritis, ankylosing spondylitis, and atopic dermatitis, higher rates of major adverse cardiovascular events (MACE), VTEs, malignancies, and serious infections were observed in patients aged ⩾65 years receiving upadacitinib, adalimumab, and MTX. 61 A higher IR for serious infections in older patients was also observed with tofacitinib and biologic disease-modifying antirheumatic drugs (bDMARDs) in US RA registry data, highlighting the need for careful monitoring of older patients. 60 The increased risk for adverse outcomes of therapy in older patients with RA is an established phenomenon that has been observed regardless of treatment.59,62
A key data set where the impact of age may be observed is from ORAL Surveillance (NCT02092467). The ORAL Surveillance post-authorization study was conducted per the directive of the US Food and Drug Administration owing to observed increases in serum lipids and some malignancies in the tofacitinib RA clinical development studies. The study enrolled >4300 patients with active RA despite MTX treatment and, to enrich for cardiovascular (CV) risk, patients had to be aged ⩾50 years and have one or more additional CV risk factors. 16 The patients had a median age of 60 years (range 50–88 years), and approximately one-third were aged ⩾65 years. For reference, patients in the tofacitinib RA clinical program had a median age of 53 years (range 18–86 years), and 16% were aged ⩾ 65 years. 63 Other than their age, key points to note about the patients in ORAL Surveillance are that half of the patients were current or ex-smokers (compared with approximately one-third across the RA program) 63 ; the majority (66%) had hypertension; 17% had diabetes; and 11% had history of coronary artery disease. 16
Although this was primarily a safety trial with an open-label design, it also provided data on the efficacy of tofacitinib 5 and 10 mg BID, compared with an active control (TNFi) in this relatively older population with comorbidities. 16 Across multiple efficacy endpoints, the three treatment arms (tofacitinib 5 mg BID, tofacitinib 10 mg BID, and TNFi) showed comparable efficacy. The first post-enrollment visit was at Month 2, then from Month 3 onwards patients were seen every 3 months while in the trial, and the data show improvement in composite disease activity measures and PROs at Month 2 through to study completion at around 5 years (Figure 2). 16 During the trial, 3.7% of patients who received tofacitinib 5 mg BID, and 1.8% of patients who received tofacitinib 10 mg BID, withdrew from treatment due to insufficient clinical response, compared with 5.4% of patients who received a TNFi. 16 By the end of the study, 8.0% of patients who received tofacitinib 5 mg BID, and 7.5% who received tofacitinib 10 mg BID, had permanently discontinued background MTX, compared with 4.9% for TNFi.
Figure 2.
Proportion of patients achieving (a) CDAI LDAa and (b) CDAI remissionb, and change from baseline in (c) DAS28-4(CRP)c and (d) HAQ-DId over time in ORAL Surveillance.
BID, twice daily; BL, baseline; CDAI, Clinical Disease Activity Index; DAS28-4(CRP), Disease Activity Score in 28 joints, C-reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; LSM, least squares mean; MMRM, mixed model for repeated measures; N, number of patients with observations at visit; SE, standard error; TNFi, tumor necrosis factor inhibitor.
Binary outcomes were analyzed using normal approximation of the difference in binomial proportions. Last Observation Carried Forward mixed components were applied to the missing data. On-treatment time included data on or prior to the study treatment end date. Only visits with >50 patients for each treatment group were included. For continuous outcomes, data were derived from an MMRM with fixed effects for treatment, visit, treatment group by visit interaction, baseline value, and baseline value by visit interaction, without imputation for missing values. A common heterogeneous compound symmetry covariance matrix was used. On-treatment time includes data on or prior to the study treatment end date. Only visits with >50 patients for each treatment group were included. 16
aDefined as CDAI ⩽ 10.
bDefined as CDAI ⩽ 2.8.
cScores range from 0 to 9.4, with higher scores indicative of worse disease activity.
dScores range from 0 to 3, with increasing scores indicative of worse functioning (0 indicating no functional impairment and 3 indicating complete impairment).
eFor patients randomized to the tofacitinib 10 mg BID group who had their dose of tofacitinib reduced to 5 mg BID, the data collected after patients were switched to tofacitinib 5 mg BID were counted in the tofacitinib 10 mg BID group.
ORAL Surveillance also provided important information regarding the safety of tofacitinib versus TNFi in this CV risk-enriched older patient population. The hypothesis of ORAL Surveillance was based on the non-inferiority of tofacitinib versus TNFi for two co-primary endpoints: MACE, and malignancies excluding non-melanoma skin cancers (NMSC); at study conclusion, non-inferiority criteria were not met. The hazard ratio (confidence interval [CI]) for MACE on tofacitinib (5 and 10 mg BID combined) compared with TNFi was 1.33 (0.91–1.94), and for malignancies excluding NMSC it was 1.48 (1.04–2.09). 16 Post hoc analyses of the data revealed that age ⩾65 years was a significant risk factor in this study for MACE and malignancies excluding NMSC across treatment groups.64,65 In addition to the findings on the co-primary endpoints, patients on tofacitinib were noted to have a higher risk than those on TNFi for other AEs of special interest. Details are presented in the ORAL Surveillance primary manuscript. 16 Table 2 provides a summary of numbers needed to harm (NNH) for AEs of special interest in ORAL Surveillance. The NNH is a derived statistic that tells us the number of patient-years of exposure to tofacitinib required to have one additional event relative to TNFi. ORAL Surveillance was a safety study of tofacitinib versus an active control but without a placebo arm. It can therefore not be determined whether the difference seen in the risk of these AEs in ORAL Surveillance is due to TNFi reducing risk, tofacitinib increasing risk, or a combination thereof, in patients with RA.
Table 2.
NNH for adjudicated MACE, malignancies (excluding NMSC), thrombosis, and serious infections in ORAL Surveillance.16,66,67
| Event | Tofacitinib 5 mg BID versus TNFi | Tofacitinib 10 mg BID versus TNFi |
|---|---|---|
| Patient years a | Patient years a | |
| MACE | 567 | 319 |
| Malignancies | 276 | 275 |
| VTE | 763 | 198 |
| DVT | 1347 | 589 |
| PE | 870 | 229 |
| Serious infections | 238 | 83 |
BID, twice daily; DVT, deep-vein thrombosis; MACE, major adverse cardiovascular event; NMSC, non-melanoma skin cancer; NNH, number needed to harm; PE, pulmonary embolism; TNFi, tumor necrosis factor inhibitor; VTE, venous thromboembolism.
Calculations were performed post hoc.
The NNH was the number of patient-years of exposure to tofacitinib required to have one additional event, relative to TNFi.
Supplementing the data from ORAL Surveillance are several post-marketing real-world studies that use data from registries and claims data; real-world data include patient populations that reflect those seen in clinical practice and are valuable in supplementing data from clinical trials. A study using the US CorEvitas RA Registry (formerly Corrona), which evaluated 5-year AE IRs between patients initiated on tofacitinib and those initiated on bDMARDs, showed that IRs for serious infections, MACE, VTEs, malignancies, NMSC, and deaths were comparable for tofacitinib and bDMARDs at 5 years after treatment initiation. 13 The CorEvitas study looked at all patients in the registry with a diagnosis of RA, and did not specifically separate out older patients or those with CV risk. In another study from US claims-based data, called STAR-RA, in which 102,263 patients were identified and of whom 12,852 initiated tofacitinib, there was no increased risk of CV outcomes (myocardial infarction or stroke) or malignancies (excluding NMSC) with tofacitinib versus TNFi when assessing the overall RA cohort; however, risk of MACE and malignancies (excluding NMSC) was numerically increased with tofacitinib relative to TNFi when they evaluated a CV risk-enriched population by mimicking the ORAL Surveillance inclusion criteria (aged ⩾ 50 years and with ⩾1 baseline CV risk factor).68,69 Conversely, analysis from the German RABBIT register did not show an increased risk of MACE with JAK inhibitors compared with TNFi, in both the overall RA population and a high CV risk cohort. 70
Tofacitinib use in RA based on smoking status
Smoking status (current smoker or ex-smoker compared with those who had never smoked) had not been found to have an effect on efficacy outcomes, 71 even when looking at the impact of smoking on efficacy in older (aged ⩾ 65 years) and younger (aged < 65 years) patients [post hoc analysis of phase III (ORAL Step, Sync, Scan, Solo, and Standard) and LTE studies (ORAL Sequel and A3921041)]. 59 However, as may be expected, smoking has been found to have a negative impact on the risk of AEs. In ORAL Surveillance, patients who were current or past smokers had a significantly higher risk for MACE and malignancies excluding NMSC compared with those who had never smoked across treatment groups.65,72 Similarly, in an analysis of the upadacitinib clinical program, smoking was shown to be a risk factor for MACE in patients with RA receiving upadacitinib. 73
Appropriate use of tofacitinib at different stages in a patient’s treatment journey
Note 2: Treatment guidelines regarding the appropriate use of tofacitinib in different lines of therapy are evolving; here, we only present the data for clinical consideration.
Impact of RA disease duration and inflammatory markers on tofacitinib efficacy
In three post hoc analyses investigating the impact of disease duration on tofacitinib efficacy, patients who were bDMARD-naïve, or early in their RA diagnosis (<1 year), had greater clinical responses to tofacitinib, compared with biologic-experienced patients or those with longer duration of disease, respectively.11,12,74 Those with higher levels of inflammation at baseline, as measured by C-reactive protein (CRP), have been shown to have a numerically higher response to tofacitinib (particularly those who had failed a TNFi previously); the effect of baseline ESR on efficacy outcomes was less clear. 75 No meaningful differences in the safety profile of tofacitinib were reported in these post hoc analyses of patients with early versus established RA (ORAL Start) or based on baseline CRP alone (nine phase II/III studies).11,75 While serious infections occurred more frequently with tofacitinib in patients with established versus early RA, the differences were not considered to be clinically meaningful. 11
Use of tofacitinib as first-line therapy for RA
The use of tofacitinib is not approved in MTX-naïve patients. In ORAL Start (MTX-naïve patients), tofacitinib monotherapy was superior to MTX monotherapy across disease activity measures and the pain and fatigue PROs (Figure 3), compared with MTX monotherapy.5,46,76 Furthermore, patients experienced more rapid improvement in physical function while receiving tofacitinib than MTX,5,46 and tofacitinib monotherapy inhibited the progression of structural damage more effectively than MTX monotherapy (Figure 3).5,76 The rates of AEs, serious AEs, and discontinuations due to AEs were similar between the two groups. 5
Figure 3.
Effects of tofacitinib monotherapy on RA (a) disease activity, (b) patient-reported outcomes, and (c) radiographic progression at Month 6 in ORAL Start, a phase III study of MTX-naïve patients.5,46
ACR70, American College of Rheumatology >70% response criteria; BID, twice daily; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, low disease activity; MCID, minimal clinically important difference; mTSS, modified Total Sharp Score; MTX, methotrexate; N, number of patients randomized and treated; RA, rheumatoid arthritis; SE, standard error; VAS, visual analog scale.
aLDA defined as DAS28-4(ESR) score ⩽ 3.2.
bRemission defined as DAS28-4(ESR) score < 2.6.
c⩾0.22-point decrease from baseline in HAQ-DI.
d⩾10 mm decrease from baseline in pain VAS score.
e⩾4-point increase from baseline in FACIT-F total score.
fNo radiographic progression defined as change from baseline in the mTSS ⩽ 0.5.
*p ⩽ 0.05;**p < 0.001 versus MTX.
Tofacitinib in patients with RA who are already on MTX or chronic steroids
Two clinical questions can be considered here. The first is whether being treated with MTX or corticosteroids (and the dose) impacts tofacitinib efficacy. To address this, two post hoc analyses were conducted to determine the impact of background MTX dose or concomitant corticosteroids on efficacy outcomes in tofacitinib phase III RA studies. These analyses demonstrated no impact of background concomitant MTX dose level (⩽12.5, >12.5–<17.5, or ⩾17.5 mg/week) or use of corticosteroids (⩽10 mg/day) on the efficacy of tofacitinib versus placebo.77,78 Details of studies on the efficacy of tofacitinib as monotherapy versus in combination with MTX are presented in the section ‘Tofacitinib as monotherapy’. Generally, the safety profile of patients on tofacitinib versus tofacitinib plus csDMARD was comparable. 79 However, the proportion of patients with elevated liver enzymes, as well as rates of serious infections and HZ, was numerically higher with combination therapy 79 ; the incidence of HZ increased in patients on concomitant corticosteroids. 80
The second question is whether patients on a combination of tofacitinib and MTX/chronic oral corticosteroids can be tapered off these add-on therapies while maintaining response on tofacitinib monotherapy. A post hoc analysis of pooled tofacitinib LTE data (combined doses) showed that approximately 70% of patients who had achieved Clinical Disease Activity Index (CDAI) remission at Month 3, and subsequently discontinued MTX or corticosteroids, remained in remission after 3 years. 81 This finding suggested that response to tofacitinib can be maintained regardless of MTX or corticosteroid withdrawal. ORAL Shift subsequently confirmed that MTX can be withdrawn when patients have achieved LDA. In that study, patients received modified-release tofacitinib (11 mg QD) plus MTX, and then MTX was withdrawn. If patients had already achieved CDAI LDA after 24 weeks of receiving the combination, their disease activity remained controlled without MTX. 15
Tofacitinib as an add-on therapy when RA activity is refractory to csDMARDs
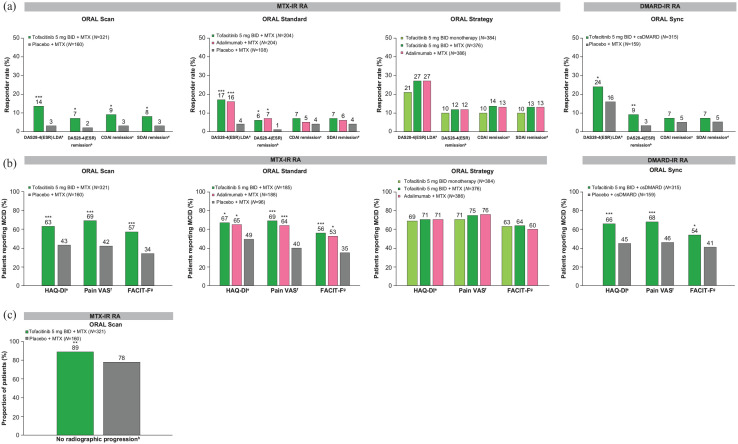
A post hoc analysis of phase II/III studies has suggested that the addition of tofacitinib can improve the symptoms of RA in patients who fail MTX or csDMARD alone. 52 When tofacitinib 5 mg BID was added to MTX in ORAL Scan, Standard, and Strategy (all of which included patients with an inadequate response to MTX), patients experienced a significant reduction in RA disease activity, pain, and fatigue, as well as improved functioning and sleep (Figure 4), and had a higher likelihood of achieving remission than on csDMARD alone after 6 months of treatment.7,8,14,47,49,50,82 Similar results were observed in ORAL Sync, wherein patients with an inadequate response to previous DMARD therapy received tofacitinib in addition to csDMARDs including MTX, leflunomide, sulfasalazine, or hydroxychloroquine (Figure 4).6,48 In ORAL Scan, adding tofacitinib, compared with placebo, to MTX, also inhibited the progression of structural damage (significantly with tofacitinib 10 mg BID), and both doses of tofacitinib were associated with significantly higher rates of non-progression at Month 6 (Figure 4), regardless of the background MTX dose used.7,78,83 It is known from a post hoc analysis that patients who achieved remission early with tofacitinib had significant long-term inhibition of radiographic progression. 76 A small open-label study in which patients with RA received tofacitinib 5 mg BID added to csDMARDs captured early improvements in musculoskeletal ultrasound and demonstrated an association between early ultrasound response and 12-week clinical outcomes. 84
Figure 4.
Effects of tofacitinib as monotherapy or in combination with csDMARD on (a) disease activity at Month 6, (b) patient-reported outcomes at Month 3, and (c) radiographic progression at Month 6 in phase III and IIIb/IV studies of patients with DMARD-IR.6–8,14,47–50,82,85
BID, twice daily; CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; LDA, low disease activity; MCID, minimal clinically important difference; mTSS, modified Total Sharp Score; MTX, methotrexate; N, number of patients randomized and treated (N may vary by outcome); RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; VAS, visual analog scale.
aDAS28-4(ESR) LDA defined as ⩽3.2 or <3.2 (ORAL Strategy).
bDAS28-4(ESR) remission defined as <2.6.
cCDAI remission defined as ⩽2.8.
dSDAI remission defined as ⩽3.3.
e⩾0.22-point decrease from baseline in HAQ-DI.
f⩾10 mm decrease from baseline in pain VAS score.
g⩾4-point increase from baseline in FACIT-F total score.
hNo radiographic progression defined as change from baseline in the mTSS ⩽ 0.5.
*p ⩽ 0.05; **p < 0.01; ***p < 0.001 versus MTX or csDMARD plus placebo.
Another important point to note from these trials in patients with RA refractory to csDMARDs is that two of the trials (ORAL Standard and Strategy) also included a group of patients who received a TNFi (subcutaneous adalimumab every other week added to MTX).8,14 In both studies, tofacitinib 5 mg BID added to MTX yielded similar clinical improvements as adalimumab added to MTX, although it should be noted that ORAL Standard was not designed for a formal comparison between the tofacitinib and adalimumab treatment groups (Figure 4).8,14
Safety findings for tofacitinib added to MTX or other csDMARDs were generally consistent with the monotherapy studies. 79 As mentioned previously, the proportion of patients with elevated liver enzymes, as well as rates of serious infections and HZ, were numerically higher with combination therapy compared with tofacitinib alone. 79 Increased risk of HZ is a repeated safety finding across tofacitinib studies, as well as other JAK inhibitor studies in RA.26,80,86,87 This highlights the importance of vaccination to prevent HZ in patients with RA, as discussed in the section ‘Use of tofacitinib in a patient requiring vaccination’.
Tofacitinib as monotherapy
In a phase IIb dose-ranging study in DMARD-IR patients, tofacitinib monotherapy (5 or 10 mg BID) achieved rapid and significant improvement versus placebo, as indicated by a higher proportion of patients achieving ACR20 responses at Month 3, while improvement with adalimumab monotherapy was not statistically significant in comparison with placebo. 88 In ORAL Strategy, tofacitinib plus MTX was non-inferior to adalimumab plus MTX. Tofacitinib monotherapy was not shown to be non-inferior to either combination. 14 These results suggest that in patients with an inadequate response to MTX, the addition of tofacitinib or adalimumab is equally efficacious, and the addition of tofacitinib to MTX is preferable to switching to tofacitinib monotherapy, although there was no difference in the remission rate (secondary endpoint) between groups.
Tofacitinib for biologic-experienced or JAK inhibitor-experienced (failed efficacy or intolerance) patients with RA
One challenging clinical scenario is the treatment of patients with RA who have already failed one or several bDMARDs. Approximately one-third of the patients in ORAL Step had tried and failed ⩾2 prior TNFi; 10 despite this, the treatment of TNFi-IR patients in this study with tofacitinib plus MTX led to improved disease activity and PROs versus placebo by Month 3 (Figure 5). The safety profile of tofacitinib in this population was generally consistent with that reported in other studies.10,28
Figure 5.
Effects of tofacitinib in combination with csDMARD on (a) disease activity and (b) patient-reported outcomes at Month 3 in a phase III study of patients with TNFi-IR RA,10,82 and change from baseline in (c) DAS28-4(ESR) and (d) HAQ-DI at Month 3 in phase II/III trials of bDMARD-naïve and bDMARD-IR patients (adapted by permission from BMJ publishing Group Limited [Charles-Schoeman C, et al. Ann Rheum Dis 2016]). 74
bDMARD, biologic disease-modifying antirheumatic drug; BID, twice daily; CDAI, Clinical Disease Activity Index; CI, confidence interval; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate response; LDA, low disease activity; LSM, least squares mean; MCID, minimal clinically important difference; MTX, methotrexate; N, number of patients randomized and treated; RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; TNFi, tumor necrosis factor inhibitor.
aDAS28-4(ESR) LDA defined as ⩽3.2.
bDAS28-4(ESR) remission defined as <2.6.
cCDAI remission defined as ⩽2.8.
dSDAI remission defined as ⩽3.3.
e⩾0.22-point decrease from baseline in HAQ-DI.
*p < 0.05 versus MTX plus placebo or placebo, ***p < 0.0001 versus MTX plus placebo or placebo.
Tofacitinib has shown efficacy (reduced signs and symptoms, and improved PROs) in patients who had previously failed bDMARDs. This was demonstrated in a post hoc analysis of phase II/III studies, including patients with prior inadequate response to ⩾2 TNFi and other bDMARDs (abatacept, rituximab, or tocilizumab). 74
Registry data in patients who failed a first JAK inhibitor suggest that switching to a second JAK inhibitor seems to lend similar efficacy as switching to a bDMARD, 89 and higher rates of drug retention than switching to a TNFi. 90 At this time, there are limited data regarding patients who have failed another JAK inhibitor and then started treatment with tofacitinib.
In general, we know that patients who have failed one or more advanced therapies tend to have more treatment-refractory RA. 91 While patients with RA have shown improvement with tofacitinib regardless of whether or not they have been on a previous biologic, the data suggest that the biologic-naïve patients had a greater response to tofacitinib than bDMARD-experienced patients (Figure 5). 74 Suboptimal treatment compliance among patients who have already failed multiple therapies has been shown to also negatively impact outcomes.92,93
Tofacitinib in RA when treatment is interrupted and resumed
Temporary discontinuation of tofacitinib treatment for surgery, vaccination, or other reasons may become necessary. In a substudy of ORAL Sequel, patients discontinued tofacitinib to receive pneumococcal and influenza vaccines, and then re-initiated treatment at the same dose 2 weeks later. 94 Clinically meaningful worsening of disease activity measures was observed during the two-week interruption period. 94 One month after re-initiation of treatment, patients had returned to their baseline disease control status, both in terms of disease activity measures and PROs. 94 These data suggest that efficacy could be re-established with tofacitinib after temporary treatment interruption. 94
Use of tofacitinib in a patient requiring vaccination
The impact of tofacitinib on vaccine efficacy has been studied for T-cell-dependent (tetanus, influenza, and 13-valent pneumococcal vaccine) and T-cell-independent (23-valent pneumococcal vaccine) vaccines in phase II/III studies. Patients newly starting tofacitinib, particularly those receiving concomitant MTX, had diminished responsiveness to the 23 valent pneumococcal vaccine, but not to the influenza vaccine. Temporarily discontinuing tofacitinib for 2 weeks following vaccination had minimal effect on the responses to either vaccine. 95
Similarly, a phase II study on the use of live attenuated zoster vaccination (Zostavax®), given 2–3 weeks prior to initiating tofacitinib treatment, found that tofacitinib did not impact vaccine response. 96 However, in this study, one patient without prior exposure to varicella zoster virus (VZV) developed vaccine disseminated virus, and clinical judgment should be used to preferably allow a longer interruption of tofacitinib when administering live vaccines. 96 Recombinant zoster vaccine (Shingrix®) is an important alternative to the live vaccine for immunocompromised patients, and data from a real-world study support its use in patients with RA; disease flares and AEs reported after vaccination were mild and self-limiting and did not require a change in RA therapy. 97 Clinical guidance on long-term strategies for the vaccination of patients with RA receiving JAK inhibitors is still evolving.
Recent guidance on the COVID-19 vaccination recommends interruption of therapy with JAK inhibitors, owing to concerns about the inhibition of interferons as a result of JAK inhibition. 98 Data from clinical studies of tofacitinib demonstrate small and variable changes in immune cell subsets during therapy. 99 As previously described above, from an analysis investigating changes in disease activity after only 2 weeks of treatment interruption, patients had steadily increasing disease activity levels, consistent with a clinically meaningful change, as well as increases in CRP. 94 This finding was not surprising given the short half-life of tofacitinib. Importantly, however, both patients in the group who continued tofacitinib therapy and those who interrupted therapy for influenza vaccination reached satisfactory immune responses (66.3% versus 63.7%, respectively). 95 Therefore, in the shared decision-making process, prescribers should consider both the benefits and potential risks of therapy interruption in the context of vaccination. 100 Evidence with regard to the management of immunosuppressants in the context of COVID-19 vaccination is still evolving. Available data from a population-based study of 315,101 US adults with COVID-19 demonstrate an increased risk of severe COVID-19 for patients with RA relative to a comparator cohort with COVID-19, but do not show a further increase in risk with use of JAK inhibitors, including tofacitinib. 101
Long-term use of tofacitinib
Data on the long-term effectiveness of tofacitinib are available from ORAL Surveillance (see section ‘Tofacitinib use in older patients and those with comorbidities’), LTE, and real-world studies.13,17,18 More than 4000 patients (>16,000 patient-years of exposure) who received tofacitinib as monotherapy or combination therapy in phase I to III clinical development trials participated in a global, open-label LTE study (ORAL Sequel), which showed sustained efficacy of tofacitinib for up to 8 years. 17 Median drug effectiveness was 5 years in the pooled analysis of data from the tofacitinib LTE studies. 102 The evidence for sustained efficacy in the clinical trials is supported by global post-marketing data 103 and real-world data from registries.104–107 One real-world study suggested higher drug retention for tofacitinib than for a TNFi, particularly when the TNFi was administered as monotherapy. 107 Patients with seronegative disease, diabetes, or hypertension were more likely to discontinue tofacitinib in the LTE studies. 102 A possible explanation is that patients with comorbidities might have experienced a higher frequency of AEs. Patient preferences regarding the frequency of therapy administration may also influence compliance and treatment outcomes; a real-world study from a US claims–based analysis demonstrated an adherence benefit for the modified release formulation of tofacitinib (11 mg QD), with comparable clinical outcomes as tofacitinib 5 mg BID. 105
Long-term safety data for tofacitinib can be obtained from the open-label LTE studies that had data for up to 9.5 years of treatment,17,18 as well as integrated safety summary (ISS) analyses, which combined the phase I/II/III/IIIb/IV and LTE trials with >7000 patients followed for up to 10.5 years.28,63 Long-term safety data were consistent with the safety profile established in the individual studies in the tofacitinib RA program. Long-term safety data in a CV risk-enriched older patient population can be gleaned from ORAL Surveillance (see ‘Tofacitinib use in older patients and those with comorbidities’). 16
Other safety data for tofacitinib in patients with RA
The most common side effects experienced by patients with RA receiving tofacitinib as monotherapy or in combination with MTX include upper respiratory tract infection, nasopharyngitis, urinary tract infection, bronchitis, HZ, diarrhea, headache, and hypertension.16,28,57 The most frequent serious AEs observed in patients with RA receiving tofacitinib are infections [8.2% of patients in tofacitinib RA ISS; IR (95% CI) 2.5 (2.3–2.7) patients with events/100 patient-years], most commonly pneumonia and HZ. 28 Similar results were observed in ORAL Surveillance, with serious infections reported in 9.7% [IR (95% CI) 2.9 (2.4–3.4) patients with events/100 patient-years] and 11.6% [IR (95% CI) 3.6 (3.1–4.2) patients with events/100 patient-years] of patients receiving tofacitinib 5 and 10 mg BID, respectively. 16 While the safety profile of tofacitinib is generally similar when given as monotherapy or in combination with csDMARDs, liver enzyme elevations are more frequent with combination therapy and serious infections and HZ appear to be less frequent with tofacitinib monotherapy (without csDMARDs or corticosteroids).67,79,80 For HZ in particular, post hoc analysis shows that the concomitant use of csDMARD and/or corticosteroids is associated with substantially higher IRs compared with tofacitinib monotherapy, and corticosteroid use has been identified as a significant risk factor for developing HZ while receiving tofacitinib. 80 For information on laboratory monitoring, local prescribing information should be consulted.
AEs of special interest: HZ
The elevated risk of HZ observed with tofacitinib108,109 is also seen with other JAK inhibitors 109 and is believed to be target related.110,111 Most cases of HZ reported in the tofacitinib RA ISS were monodermatomal and non-serious, although cases of multidermatomal (5.5% of patients with HZ) and disseminated (1.0%), including ocular HZ (0.1%), were reported. 28 In patients who received live attenuated zoster vaccination (Zostavax®) prior to tofacitinib treatment in a phase IIIb/IV study (N = 144), the VZV reactivation rate was 2.1%, compared with 1.5% for non-vaccinated patients (N = 616), with overall milder manifestations. 112 The efficacy of the live attenuated vaccine is 70% in patients aged 50–59 years and only 51% in those aged >60 years, 113 while the recombinant vaccine (Shingrix®) provides efficacy of >90% in older patients.114,115 A study on the use of recombinant zoster vaccine in patients with ulcerative colitis receiving tofacitinib is planned.
Conclusion
Tofacitinib was the first JAK inhibitor to be approved for the treatment of RA and has been in clinical use in different countries for a decade. An extensive evidence base on the efficacy, effectiveness, and safety of tofacitinib across different clinical scenarios and patient populations has accumulated from the clinical development program, post-marketing studies, and real-world use. Although not all the literature could be discussed in this narrative review, these data demonstrate the benefits of tofacitinib for patients with RA across populations. While the efficacy of tofacitinib appears generally consistent across patients with different demographic and disease characteristics, there is evidence that some patient groups might derive particular benefit (e.g. seropositive disease) and that some patients (e.g. older patients and those with CV risk factors, particularly smoking) are at greater risk of serious AEs while receiving tofacitinib, compared to TNFi. While we appreciate that everyday clinical scenarios can be complex, the summary of the available safety and efficacy data provided here is intended to help rheumatologists advise appropriate patients regarding treatment with tofacitinib, and, in conjunction, assess the risk/benefit profile of this medication.
Acknowledgments
This review was sponsored by Pfizer. New data presented are from studies sponsored by Pfizer. Medical writing support, under the direction of the authors, was provided by Kirsteen Munn, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med 2022; 175: 1298–1304). We thank Jose Rivas and David Gold, of Pfizer Inc, for their review and input.
Footnotes
ORCID iD: Mahta Mortezavi  https://orcid.org/0000-0003-4666-071X
https://orcid.org/0000-0003-4666-071X
Contributor Information
Mahta Mortezavi, Pfizer Inc, 66 Hudson Boulevard, New York, NY 10001, USA.
Eduardo F. Mysler, Organización Médica de Investigación, Buenos Aires, Argentina
Declarations
Ethics approval and consent to participate: The tofacitinib studies included in this review were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonization and were approved by the relevant Institutional Review Board and/or Independent Ethics Committee of the investigational centers. All patients provided written, informed consent to participate in the trials. No further approval was required for this review, in accordance with the policy of our institutions.
Consent for publication: Not applicable.
Author contributions: Mahta Mortezavi: Conceptualization; Writing – review & editing.
Eduardo F. Mysler: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was sponsored by Pfizer. New data presented are from studies sponsored by Pfizer. Medical writing support was funded by Pfizer, New York, NY, USA.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MM is an employee and shareholder of Pfizer Inc. EFM is a speaker or advisor to AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Glaxo SmithKline, Janssen, Lilly, Novartis, Pfizer Inc, Roche, and Sanofi.
Availability of data and materials: Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 2.Taylor PC, Atzeni F, Balsa A, et al. The key comorbidities in patients with rheumatoid arthritis: a narrative review. J Clin Med 2021; 10: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 4.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021; 73: 1108–1123. [DOI] [PubMed] [Google Scholar]
- 5.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014; 370: 2377–2386. [DOI] [PubMed] [Google Scholar]
- 6.Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013; 159: 253–261. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013; 65: 559–570. [DOI] [PubMed] [Google Scholar]
- 8.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012; 367: 508–519. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012; 367: 495–507. [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013; 381: 451–460. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmann RM, Huizinga TWJ, Kavanaugh AF, et al. Efficacy of tofacitinib monotherapy in methotrexate-naive patients with early or established rheumatoid arthritis. RMD Open 2016; 2: e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall S, Nash P, Rischmueller M, et al. Efficacy of tofacitinib in patients who are inadequate responders to disease-modifying antirheumatic drugs according to early versus late duration of rheumatoid arthritis: post-hoc analysis of data from phase 3 trials [Abstract]. Arthritis Rheum 2016; 68(suppl. 10): 1609. [Google Scholar]
- 13.Kremer JM, Bingham CO, 3rd, Cappelli LC, et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol 2021; 3: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017; 390: 457–468. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SB, Pope J, Haraoui B, et al. Methotrexate withdrawal in patients with rheumatoid arthritis who achieve low disease activity with tofacitinib modified-release 11 mg once daily plus methotrexate (ORAL Shift): a randomised, phase 3b/4, non-inferiority trial. Lancet Rheumatol 2019; 1: E23–E34. [DOI] [PubMed] [Google Scholar]
- 16.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022; 386: 316–326. [DOI] [PubMed] [Google Scholar]
- 17.Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther 2019; 21: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther 2016; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res 2000; 2: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bas S, Perneger TV, Seitz M, et al. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology (Oxford) 2002; 41: 809–814. [DOI] [PubMed] [Google Scholar]
- 21.Kastbom A, Strandberg G, Lindroos A, et al. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2003; 63: 1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niewold TB, Harrison MJ, Paget SA. Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM 2007; 100: 193–201. [DOI] [PubMed] [Google Scholar]
- 23.Courvoisier DS, Chatzidionysiou K, Mongin D, et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: results from a collaboration of 16 registries. Rheumatology (Oxford) 2021; 60: 820–828. [DOI] [PubMed] [Google Scholar]
- 24.Bird P, Hall S, Nash P, et al. Treatment outcomes in patients with seropositive versus seronegative rheumatoid arthritis in phase III randomised clinical trials of tofacitinib. RMD Open 2019; 5: e000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landewé RB, Connell CA, Bradley JD, et al. Is radiographic progression in modern rheumatoid arthritis trials still a robust outcome? Experience from tofacitinib clinical trials. Arthritis Res Ther 2016; 18: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 2022; 81: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burmester GR, Gordon KB, Rosenbaum JT, et al. Long-term safety of adalimumab in 29,967 adult patients from global clinical trials across multiple indications: an updated analysis. Adv Ther 2020; 37: 364–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 2020; 6: e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones N, Strand V, Schulze-Koops H, et al. Sex differences in the efficacy and safety of tofacitinib in rheumatoid arthritis patients: a post hoc analysis of phase 3 and long-term extension trials [Abstract]. Arthritis Rheumatol 2020; 72(Suppl. 10): 0825. [Google Scholar]
- 30.Bergstra SA, Allaart CF, Ramiro S, et al. Sex-associated treatment differences and their outcomes in rheumatoid arthritis: results from the METEOR register. J Rheumatol 2018; 45: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 31.Wright G, Mysler E, Chen Y-H, et al. Impact of race on the efficacy and safety of tofacitinib in patients with RA: a post hoc analysis of phase 2, 3, and 3b/4 clinical trials [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 1680. [Google Scholar]
- 32.Wright G, Mysler E, Navarro-Millan I, et al. Efficacy and safety of upadacitinib in patients across races with rheumatoid arthritis: a post hoc analysis of six phase 3 clinical trials [Abstract]. Arthritis Rheumatol 2022; 74(Suppl. 9): 0286. [Google Scholar]
- 33.Hsieh SC, Chen WS, Hu JC, et al. Real-world data of tofacitinib versus tumor necrosis factor inhibitors in Taiwanese patients with rheumatoid arthritis from a drug-based registry [Abstract]. Arthritis Rheumatol 2022; 74(Suppl. 9): 0289. [Google Scholar]
- 34.Bilgin E, Ceylan F, Duran E, et al. Efficacy, retention, and safety of tofacitinib in real-life: Hur-bio monocentric experience. Turk J Med Sci 2021; 51: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller RB, Hasler C, Popp F, et al. Effectiveness, tolerability, and safety of tofacitinib in rheumatoid arthritis: a retrospective analysis of real-world data from the St. Gallen and Aarau cohorts. J Clin Med 2019; 8: 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwamoto N, Sato S, Kurushima S, et al. Real-world comparative effectiveness and safety of tofacitinib and baricitinib in patients with rheumatoid arthritis. Arthritis Res Ther 2021; 23: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneeberger EE, Salas A, Medina LF, et al. Real-world use of tofacitinib in rheumatoid arthritis: data from Latin America [Abstract]. J Clin Rheumatol 2018; 24: 195. [Google Scholar]
- 38.Radominski SC, Cardiel MH, Citera G, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of Latin American patients with rheumatoid arthritis: pooled efficacy and safety analyses of Phase 3 and long-term extension studies. Reumatol Clin 2017; 13: 201–209. [DOI] [PubMed] [Google Scholar]
- 39.Ogdie A, de Vlam K, McInnes IB, et al. Efficacy of tofacitinib in reducing pain in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. RMD Open 2020; 6: e001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strand V, Kremer J, Wallenstein G, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther 2015; 17: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pogozheva E, Karateev A, Nasonov E, et al. Tofacitinib in rheumatoid arthritis: is there a correlation between a rapid analgesic effect and a decrease in activity after 3 and 6 months? [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 1704. [Google Scholar]
- 42.Taylor PC, Alten R, Álvaro Gracia JM, et al. Achieving pain control in early rheumatoid arthritis with baricitinib monotherapy or in combination with methotrexate versus methotrexate monotherapy. RMD Open 2022; 8: e001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strand V, Tundia N, Bergman M, et al. Upadacitinib improves patient-reported outcomes vs placebo or adalimumab in patients with rheumatoid arthritis: results from SELECT-COMPARE. Rheumatology (Oxford) 2021; 60: 5583–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowell WB, Gavigan K, Kannowski CL, et al. Which patient-reported outcomes do rheumatology patients find important to track digitally? A real-world longitudinal study in ArthritisPower. Arthritis Res Ther 2021; 23: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartlett SJ, Bingham CO, van Vollenhoven R, et al. The impact of tofacitinib on fatigue, sleep, and health-related quality of life in patients with rheumatoid arthritis: a post hoc analysis of data from Phase 3 trials. Arthritis Res Ther 2022; 24: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strand V, Lee EB, Fleischmann R, et al. Tofacitinib versus methotrexate in rheumatoid arthritis: patient-reported outcomes from the randomised phase III ORAL Start trial. RMD Open 2016; 2: e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strand V, van Vollenhoven RF, Lee EB, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford) 2016; 55: 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strand V, Kremer JM, Gruben D, et al. Tofacitinib in combination with conventional disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: patient-reported outcomes from a phase III randomized controlled trial. Arthritis Care Res (Hoboken) 2017; 69: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strand V, van der Heijde D, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: patient-reported outcomes from the 24-month phase 3 ORAL Scan study. Clin Exp Rheumatol 2020; 38: 848–857. [PubMed] [Google Scholar]
- 50.Strand V, Mysler E, Moots RJ, et al. Patient-reported outcomes for tofacitinib with and without methotrexate, or adalimumab with methotrexate, in rheumatoid arthritis: a phase IIIB/IV trial. RMD Open 2019; 5: e001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Citera G, Jain R, Irazoque F, et al. Tofacitinib in patients with rheumatoid arthritis and indicative of depression and/or anxiety: a post hoc analysis of phase 3 and phase 3b/4 clinical trials [Abstract]. Arthritis Rheumatol 2019; 71(Suppl. 10): 1444. [Google Scholar]
- 52.Kremer J, Zerbini C, Lee EB, et al. THU0143 Tofacitinib (CP-690,550), an oral Janus kinase inhibitor: analysis of efficacy endpoints by subgroups in a pooled Phase 2 and 3 rheumatoid arthritis study population [Abstract]. Ann Rheum Dis 2012; 71(Suppl. 3): THU0143. [Google Scholar]
- 53.Wollenhaupt J, Morel J, Daien C, et al. Analysis of the impact of tofacitinib treatment on weight in patients with rheumatoid arthritis [Abstract]. Arthritis Rheumatol 2020; 72(Suppl. 10): 1203.32017421 [Google Scholar]
- 54.Dikranian AH, Gonzalez-Gay MA, Wellborne F, et al. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by baseline body mass index: an analysis of pooled data from phase 3 studies. RMD Open 2022; 8: e002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gremese E, Carletto A, Padovan M, et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res (Hoboken) 2013; 65: 94–100. [DOI] [PubMed] [Google Scholar]
- 56.Ottaviani S, Gardette A, Tubach F, et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin Exp Rheumatol 2015; 33: 478–483. [PubMed] [Google Scholar]
- 57.Pfizer Inc. XELJANZ: highlights of prescribing information, http://labeling.pfizer.com/ShowLabeling.aspx?id=959 (2018, accessed 29 March 2023).
- 58.European Medicines Agency. Xeljanz® (tofacitinib): summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf (2022, accessed 29 March 2023).
- 59.Curtis JR, Schulze-Koops H, Takiya L, et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin Exp Rheumatol 2017; 35: 390–400. [PubMed] [Google Scholar]
- 60.Winthrop KL, Citera G, Gold D, et al. Age-based (<65 vs ⩾65 years) incidence of infections and serious infections with tofacitinib versus biological DMARDs in rheumatoid arthritis clinical trials and the US Corrona RA registry. Ann Rheum Dis 2021; 80: 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023; 9: e002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widdifield J, Bernatsky S, Paterson JM, et al. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013; 65: 353–361. [DOI] [PubMed] [Google Scholar]
- 63.Burmester GR, Nash P, Sands BE, et al. Adverse events of special interest in clinical trials of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and psoriasis with 37 066 patient-years of tofacitinib exposure. RMD Open 2021; 7: e001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curtis J, Yamaoka K, Chen Y-H, et al. Malignancies in patients aged ⩾ 50 years with RA and ⩾ 1 additional cardiovascular risk factor: results from a phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 1940. [Google Scholar]
- 65.Charles-Schoeman C, Buch M, Dougados M, et al. Risk factors for major adverse cardiovascular events in patients aged ⩾ 50 years with RA and ⩾ 1 additional cardiovascular risk factor: results from a phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 0958. [Google Scholar]
- 66.Charles-Schoeman C, Fleischmann R, Mysler E, et al. The risk of venous thromboembolic events in patients with RA aged ⩾50 years with ⩾1 cardiovascular risk factor: results from a phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 1941. [Google Scholar]
- 67.Balanescu AR, Citera G, Pascual-Ramos V, et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis 2022; 81: 1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khosrow-Khavar F, Kim SC, Lee H, et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis 2022; 81: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khosrow-Khavar F, Desai R, Lee H, et al. Risk of malignancy in patients treated with tofacitinib: results from the Safety of TofAcitinib in Routine Care Patients with Rheumatoid Arthritis (STAR-RA) study [Abstract]. Arthritis Rheumatol 2021; 73(Suppl. 10): 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meissner Y, Albrecht K, Kekow J, et al. OP0135 risk of cardiovascular events under Janus kinase inhibitors in patients with rheumatoid arthritis: observational data from the German RABBIT register [Abstract]. Ann Rheum Dis 2022; 81(Suppl. 1): OP0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kremer JM, Greenberg JD, Turesson C, et al. Effects of smoking status on response to treatment with tofacitinib in patients with rheumatoid arthritis [Abstract]. Arthritis Rheumatol 2013; 65(Suppl. 10): 1418. [Google Scholar]
- 72.Curtis JR, Yamaoka K, Chen YH, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis 2023; 82: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charles-Schoeman C, Choy E, McInnes IB, et al. MACE and VTE across upadacitinib clinical trial programs in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis [Abstract]. Arthritis Rheumatol 2022; 74(Suppl. 9): 0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charles-Schoeman C, Burmester G, Nash P, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2016; 75: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartzman S, van Vollenhoven RF, Matsumoto AK, et al. Efficacy of tofacitinib in patients with moderate to severe rheumatoid arthritis by baseline C-reactive protein levels and erythrocyte sedimentation rates [Abstract]. Arthritis Rheumatol 2017; 69(Suppl. 10): 495. [Google Scholar]
- 76.Strand V, Kavanaugh A, Kivitz AJ, et al. Long-term radiographic and patient-reported outcomes in patients with rheumatoid arthritis treated with tofacitinib: ORAL Start and ORAL Scan post-hoc analyses. Rheumatol Ther 2018; 5: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charles-Schoeman C, van der Heijde D, Burmester GR, et al. Effect of glucocorticoids on the clinical and radiographic efficacy of tofacitinib in patients with rheumatoid arthritis: a posthoc analysis of data from 6 phase III studies. J Rheumatol 2018; 45: 177–187. [DOI] [PubMed] [Google Scholar]
- 78.Fleischmann R, Mease PJ, Schwartzman S, et al. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by background methotrexate dose group. Clin Rheumatol 2017; 36: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kivitz AJ, Cohen S, Keystone E, et al. A pooled analysis of the safety of tofacitinib as monotherapy or in combination with background conventional synthetic disease-modifying antirheumatic drugs in a phase 3 rheumatoid arthritis population. Semin Arthritis Rheum 2018; 48: 406–415. [DOI] [PubMed] [Google Scholar]
- 80.Winthrop KL, Curtis JR, Lindsey S, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017; 69: 1960–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleischmann R, Wollenhaupt J, Cohen S, et al. Effect of discontinuation or initiation of methotrexate or glucocorticoids on tofacitinib efficacy in patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther 2018; 5: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smolen JS, Aletaha D, Gruben D, et al. Remission rates with tofacitinib treatment in rheumatoid arthritis: a comparison of various remission criteria. Arthritis Rheumatol 2017; 69: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Heijde D, Strand V, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol 2019; 71: 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Razmjou AA, Brook J, Elashoff D, et al. Ultrasound and multi-biomarker disease activity score for assessing and predicting clinical response to tofacitinib treatment in patients with rheumatoid arthritis. BMC Rheumatol 2020; 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Vollenhoven RF, Krishnaswami S, Benda B, et al. Tofacitinib and adalimumab achieve similar rates of low disease activity in rheumatoid arthritis – lack of improvement in disease activity score by 3 months predicts low likelihood of low disease activity at 1 year [Abstract]. Arthritis Rheum 2012; 64(Suppl. 10): 1297.22231914 [Google Scholar]
- 86.Bird P, Bensen W, El-Zorkany B, et al. Tofacitinib 5 mg twice daily in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs: a comprehensive review of phase 3 efficacy and safety. J Clin Rheumatol 2019; 25: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis 2021; 80: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012; 64: 617–629. [DOI] [PubMed] [Google Scholar]
- 89.Pombo-Suarez M, Sanchez-Piedra C, Gómez-Reino J, et al. After JAK inhibitor failure: to cycle or to switch, that is the question – data from the JAK-pot collaboration of registries. Ann Rheum Dis 2023; 82: 175–181. [DOI] [PubMed] [Google Scholar]
- 90.Amstad A, Papagiannoulis E, Scherer A, et al. Comparison of drug retention of TNF inhibitors, other biologics and JAK inhibitors in RA patients who discontinued JAK inhibitor therapy. Rheumatology (Oxford) 2022; 62: 89–97. [DOI] [PubMed] [Google Scholar]
- 91.Karlsson JA, Kristensen LE, Kapetanovic MC, et al. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 2008; 47: 507–513. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Cui Y, Yin R, et al. Medication adherence has an impact on disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Patient Prefer Adherence 2017; 11: 1343–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Salaffi F, Carotti M, Di Carlo M, et al. Adherence to anti-tumor necrosis factor therapy administered subcutaneously and associated factors in patients with rheumatoid arthritis. J Clin Rheumatol 2015; 21: 419–425. [DOI] [PubMed] [Google Scholar]
- 94.Kaine J, Tesser J, Takiya L, et al. Re-establishment of efficacy of tofacitinib, an oral JAK inhibitor, after temporary discontinuation in patients with rheumatoid arthritis. Clin Rheumatol 2020; 39: 2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016; 75: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol 2017; 69: 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stevens E, Weinblatt ME, Massarotti E, et al. Safety of the zoster vaccine recombinant adjuvanted in rheumatoid arthritis and other systemic rheumatic disease patients: a single center’s experience with 400 patients. ACR Open Rheumatol 2020; 2: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases – version 1. Arthritis Rheumatol 2021; 73: 1093–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hodge JA, Kawabata TT, Krishnaswami S, et al. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016; 34: 318–328. [PubMed] [Google Scholar]
- 100.Mortezavi M, Menon S, Lee K, et al. Use of tofacitinib in the context of COVID-19 vaccination: comment on the American College of Rheumatology clinical guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2021; 73: 1768–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Curtis JR, Zhou X, Rubin DT, et al. Characteristics, comorbidities, and outcomes of SARS-CoV-2 infection in patients with autoimmune conditions treated with systemic therapies: a population-based study. J Rheumatol 2022; 49: 320–329. [DOI] [PubMed] [Google Scholar]
- 102.Pope JE, Keystone E, Jamal S, et al. Persistence of tofacitinib in the treatment of rheumatoid arthritis in open-label, long-term extension studies up to 9.5 years. ACR Open Rheumatol 2019; 1: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen S, Curtis JR, DeMasi R, et al. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Ther 2018; 5: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bird P, Littlejohn G, Butcher B, et al. Real-world evaluation of effectiveness, persistence, and usage patterns of tofacitinib in treatment of rheumatoid arthritis in Australia. Clin Rheumatol 2020; 39: 2545–2551. [DOI] [PubMed] [Google Scholar]
- 105.Cohen SB, Greenberg JD, Harnett J, et al. Real-world evidence to contextualize clinical trial results and inform regulatory decisions: tofacitinib modified-release once-daily vs immediate-release twice-daily for rheumatoid arthritis. Adv Ther 2021; 38: 226–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reed GW, Gerber RA, Shan Y, et al. Real-world comparative effectiveness of tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol Ther 2019; 6: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finckh A, Tellenbach C, Herzog L, et al. Comparative effectiveness of antitumour necrosis factor agents, biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland. RMD Open 2020; 6: e001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winthrop KL, Curtis JR, Yamaoka K, et al. Clinical management of herpes zoster in patients with rheumatoid arthritis or psoriatic arthritis receiving tofacitinib treatment. Rheumatol Ther 2022; 9: 243–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bechman K, Subesinghe S, Norton S, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 2019; 58: 1755–1766. [DOI] [PubMed] [Google Scholar]
- 110.Almanzar G, Kienle F, Schmalzing M, et al. Tofacitinib modulates the VZV-specific CD4+ T cell immune response in vitro in lymphocytes of patients with rheumatoid arthritis. Rheumatology (Oxford) 2019; 58: 2051–2060. [DOI] [PubMed] [Google Scholar]
- 111.Kato M. New insights into IFN-γ in rheumatoid arthritis: role in the era of JAK inhibitors. Immunol Med 2020; 43: 72–78. [DOI] [PubMed] [Google Scholar]
- 112.Calabrese LH, Abud-Mendoza C, Lindsey SM, et al. Live zoster vaccine in patients with rheumatoid arthritis treated with tofacitinib with or without methotrexate, or adalimumab with methotrexate: a post hoc analysis of data from a phase IIIb/IV randomized study. Arthritis Care Res (Hoboken) 2020; 72: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.European Medicines Agency. ZOSTAVAX: summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/zostavax-epar-product-information_en.pdf (2019, accessed 29 March 2023).
- 114.US Food and Drug Administration. SHINGRIX: highlights of prescribing information, https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF (2019, accessed 29 March 2023).
- 115.European Medicines Agency. Shingrix: summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/shingrix-epar-product-information_en.pdf (2018, accessed 29 March 2023).