Abstract

A divergent two-step process has provided access to optically pure enantiomers of MDMA and MDA, clinically relevant phenylisopropylamine entactogens. Target compounds were synthesized from commercially available alanine-derived aziridines. Critical process parameters were identified, and the reactions were optimized to avoid chromatographic purifications toward gram-scale isolations, providing (R)-(−)-MDMA, (S)-(+)-MDMA, (R)-(−)-MDA, and (S)-(+)-MDA each in greater than 98% purity by UPLC, >99% enantiomeric excess, and net yields between 50 and 60% for the complete process.
3,4-Methylenedioxymethamphetamine (MDMA, 1, Figure 1) was first synthesized in 1912 by Merck, but it was not until the 1970s that reports emerged on the compound’s unique pharmacology and psychoactive properties.1−3 In 1985, the US government classified it as a Schedule I drug, asserting that it had high potential for abuse and no accepted medical use. Despite the imposed legal restrictions, limited research continued to explore potential therapeutic uses of MDMA in the following decades. In the 2000s, a small number of controlled studies guided in part by underground therapy practices helped provide evidence that MDMA may have therapeutic potential in the treatment of post-traumatic stress disorder (PTSD).4 As a result, the Multidisciplinary Association for Psychedelic Studies (MAPS) funded several Phase 2 studies that showed promising results in the treatment of PTSD with MDMA. In 2021, the US Food and Drug Administration (FDA) granted Breakthrough Therapy designation for MDMA-assisted psychotherapy for PTSD, which expedited the regulatory development and review process. The same organization has recently completed advanced-stage clinical trials supporting a New Drug Application (NDA) toward possible FDA approval and commercial medical use.5 Research into the therapeutic potential of MDMA is ongoing and continues to evolve. Similarly, MDA (2) is a structurally and pharmacologically related compound and, though less rigorously studied than MDMA, has been evaluated in small preliminary clinical trials with consideration for potential clinical use.6
Figure 1.
Structures of MDMA (1), MDA (2), and corresponding stereoisomers.
MDMA and MDA are characterized by a chiral carbon alpha to the amine, and racemic material has been most widely studied in humans. However, numerous studies have consistently indicated that the pharmacological and toxicological effects of enantiomers of both (R/S)-MDMA and (R/S)-MDA are distinct and divergent.7−15 Systematic research into enantiodivergent pharmacology of MDMA and MDA stereoisomers has been hindered by a lack of availability of enantiopure materials due to both regulatory challenges and the inherent difficulties in the production of single enantiomers. Improved access to optically pure stereoisomers of both MDMA and MDA could therefore enable better understanding of their pharmacology, toxicology, and therapeutic potential and help enable the development of new treatments for challenging psychiatric conditions. To facilitate more systematic study into the effects of single enantiomers of these potential therapeutic entactogens and to expand the public domain of available manufacturing methods, an operationally simple synthetic method leveraging chiral pool synthesis was developed.
Chiral pool synthesis is a strategy used to prepare chiral compounds from readily available, naturally occurring chiral starting materials and is generally considered to be preferable to chiral resolution by diastereomeric crystallization or by chromatographic separation, which have been used previously for producing enantiopure MDMA.13,16 A key advantage of chiral pool synthesis is that the approach provides a direct strategy for obtaining a single enantiomer with a predefined known stereocenter. Provided racemization is avoided in subsequent synthetic steps, the enantiopurity of the feedstock material is preserved providing an efficient and cost-effective method of enantiomer synthesis.
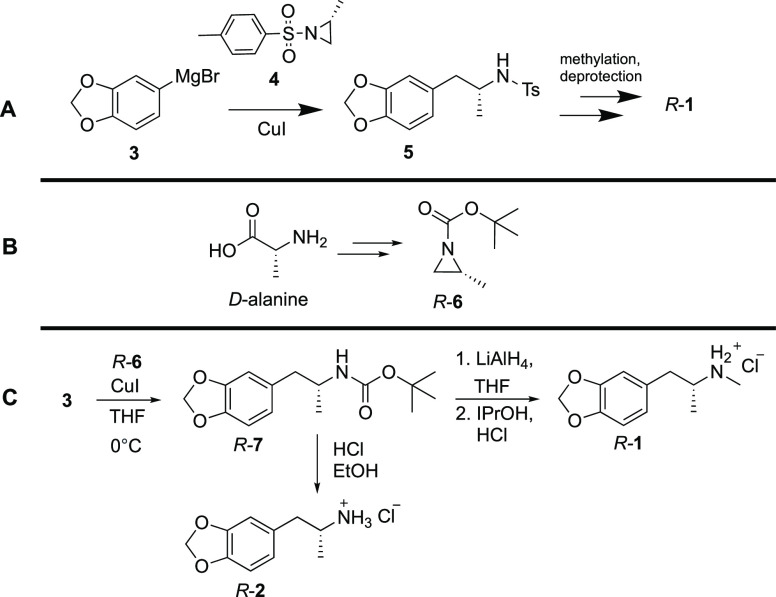
Dunlap et al. recently reviewed reported methods for producing both racemic and enantiopure MDMA.17 In particular, two analogous methods were highlighted by Huot et al. and Nenajdenko et al. leveraging ring-opening reactions of alanine-derived aziridines to produce MDMA and related amphetamines.14,18 Nucleophilic ring-opening reactions of aziridines are well described,19 and in these two examples, the reactions proceeded through the corresponding tosyl-protected aziridines (4, Scheme 1A). From intermediate 5, subsequent N-methylation and tosyl deprotection provided access to enantiopure N-methylated target compounds such as MDMA. A notable drawback of this multistep approach is that each unit operation required silica gel chromatography to purify intermediates, limiting practicality and scalability.
Scheme 1. (A) Regiospecific Ring Opening of Tosyl-Protected Aziridine 4 As Described by Huot et al.14 and Nenajdenko et al.;18 (B) Chiral Pool Synthesis of Requisite boc-Aziridine R-6 from d-Alanine; and (C) Optimized Two-Step Divergent Route to 1 and 2 Employing boc-Aziridine 6 (This Work).
An alternative optimized approach was developed (Scheme 1C), instead employing enantiopure tert-butoxycarbonyl (boc)-protected aziridines (6), which are commercially available (Ambeed Inc.) and efficiently prepared from the corresponding natural or unnatural enantiomers of the amino acid alanine by a three-step process (Scheme 1B).20 Access to intermediate 7 via copper-catalyzed regioselective nucleophilic ring opening of boc-protected aziridine 6 with Grignard 3 enabled convenient divergent access to enantiomers of both MDA and MDMA by either acid-mediated carbamate hydrolysis deprotection or by lithium aluminum hydride (LiAlH4) reduction of the carbamate in the final transformation, respectively, as depicted in Scheme 1C. The reactions were further optimized to enable multigram-scale preparation of intermediate 7 with sufficient quality to telescope into subsequent hydrolysis or LiAlH4 reduction reactions, avoiding silica gel chromatography for the complete process to access enantiopure MDMA or MDA hydrochloride salts in two steps overall.
Results and Discussion
Regioselective ring opening of aziridine 6 to provide intermediate 7 was accomplished by fast addition of 1.35 equivalents of freshly prepared Grignard solution 3 to an azirine solution in THF at −5 °C with a 20 mol % copper iodide catalyst. Although solutions of 3 in THF/toluene were commercially available, in our hands, this material performed inconsistently, possibly due to reduced solubility of the copper catalyst in the presence of toluene cosolvent, and freshly prepared 3 was found to react more cleanly. With reaction monitoring by LCMS, short addition times of the Grignard solution were found to provide cleaner reaction profiles, an observation that was consistent with reports on optimizations of analogous epoxide ring-opening reactions.21 UPLC-MS analysis of isolated 7 revealed several minor and two prominent impurities with strong absorbance at 269 nm but no ionization response by ESI-MS (Figure 2 red trace). Analysis of quenched Grignard solution 3 in the absence of aziridine 6 provided an identical impurity profile (Figure 2, blue trace), indicating that these byproducts were derived exclusively from this starting material and not from degradation of 7 or reactions with aziridine 6. Given the slight excess of 3 used in the reaction, the putative structures 1,3-benzodioxole (8) and the homocoupled biphenyl product 9 were assigned to these chromatographic impurity peaks, as depicted in Figure 2. Aromatic structures 8 and 9 derived from a solution of 3 are sufficiently nonpolar, lack ionizable functional groups, and could be conveniently purged by acid–base workups, leveraging selective partitioning of ionized MDMA and MDA hydrochloride salts. Though appearing as significant chromatographic impurities in samples of isolated 7, the presence of products derived from 3 in the blue trace shown in Figure 2 proved to be entirely noncritical to the overall process.
Figure 2.

Overlaid chromatograms for isolated 7 (red), quenched Grignard solution 3 (blue), and labeled impurity peaks with putative structures 8 and 9 derived from 3.
The reaction was typically complete within minutes with consumption of the aziridine limiting reagent; in experiments where extended reaction times occurred, additional byproduct formation became evident. Notably, increased levels of the putative byproduct amide 10 (Scheme 2) were identified by its corresponding high-resolution mass spectrum, formed by attack of the Grignard reactant at the carbamate. To avoid necessitating chromatographic purification of intermediate 7 in the production of high-purity MDMA, the reaction time was found to be a critical process parameter and quenching the Grignard reaction within 30 min of addition of solution 3 was found to correlate with minimizing the formation of amide 10. When elevated levels of 10 were telescoped into the LiAlH4 reduction in the production of MDMA, the amide was reduced to the corresponding benzylamine 11, which subsequently formed an intractable hydrochloride salt that cocrystallized alongside MDMA-HCl. In contrast, with the alternate transformation to MDA by acid hydrolysis of 7, unreacted impurity 10 was found to be inconsequential as the neutral amide was readily washed from the precipitated hydrochloride salt of MDA by trituration in acetone.
Scheme 2. Byproduct Formation and Fate with Extended Reaction Times in the Formation of 7.
With the described optimizations, intermediate 7 was isolated without further purification and subjected to reduction with lithium aluminum hydride to provide the (R)-MDMA freebase. The reaction was monitored for disappearance of the formamide intermediate 12 (m/z: 208.0968, Figure 3), which was the rate-limiting transformation. The workup and hydrochloride salt formation were carried out using approximately the same process recently described in the large-scale GMP (good manufacturing practice) synthesis of MDMA.22 The isolated hydrochloride salt was subsequently recrystallized from isopropanol to provide gram-scale quantities of (R)-MDMA-HCl in 52% overall yield from aziridine 6 with enantiomeric excess >99% determined by chiral chromatography.
Figure 3.

Monitored intermediate in lithium aluminum hydride reduction of 7.
When the crude carbamate intermediate 7 was instead treated with excess hydrochloric acid in refluxing ethanol, MDA was conveniently precipitated as the corresponding hydrochloride salt. Subsequent trituration in acetone to remove nonpolar unionized byproducts provided a material with >98% purity by UPLC in 62% yield over two steps.
The same reaction sequences depicted in Scheme 1C with optimizations outlined above were repeated employing the S-enantiomer of aziridine 6 to provide S-MDMA and S-MDA hydrochloride salts in 51 and 61% overall yields, respectively. Enantiomeric excesses for both were >99% by chiral chromatography.
Conclusions
A synthetic process leveraging the chiral pool has been developed to access enantiomers of both MDA and MDMA. Critical aspects of the process were identified and include utilization of freshly prepared Grignard solution (3) with rapid addition of this solution to aziridine 6 and subsequent shortened reaction times. Characterization data have also been provided to enable in-process monitoring for key reaction intermediates as well as critical and noncritical byproducts with consideration of their impact on final product quality. Given the recent resurgence in interest in the clinical exploration of psychedelics and entactogens and the fact that many are built upon the phenylisopropylamine core bearing a chiral alpha methyl group, the reported process provides operationally simple access to enantiopure MDMA and MDA and may also be sufficiently general to provide access to numerous analogous compounds for further biological characterization and pharmacological study.
Experimental Section
General Experimental Methods
Reactions were performed using commercially obtained solvents. Unless otherwise stated, all commercially obtained reagents were used as received. Reactions were monitored by UPLC-UV-HRMS (described below). Flash column chromatography was performed using prepackaged RediSepRf columns on a CombiFlash Rf system (Teledyne Isco Inc.). 1H and 13C NMR spectra were recorded on a Bruker 400 (at 400 and 101 MHz, respectively) and are reported relative to internal CHCl3 (1H, δ = 7.26) and CDCl3 (13C, δ = 77.0). High-resolution mass spectra were obtained on Waters Xevo G2-XS QToF in ESI-positive mode. Purity analysis was conducted on a Thermo Vanquish UHPLC utilizing a YMC ODS-AQ (150 mm × 2.0 mm, 3 μm) column. Separation was achieved with a gradient mobile phase consisting of 20 mM ammonium formate adjusted to pH = 3 with formic acid and [1:1] ACN/methanol at 0.4 mL/min. Samples were diluted in [70:30] water/acetonitrile to approximately 1 mg/mL and 2 μL was injected. Chromatographic peaks were detected by a diode array detector at 269 nm. HRMS data were acquired (inline with UV) on Waters Xevo G2-XS QTof in ESI-positive mode. Low and high collision energy mass spectra were acquired using the Waters MSe experiment. Enantiomeric excesses were determined with a Thermo Vanquish UHPLC utilizing a Phenomenex Lux AMP (150 mm × 3.0 mm, 3 μm) column. For the separation of MDMA stereoisomers (R/S-1), an isocratic mobile phase of [60:40] 5 mM ammonium bicarbonate adjusted to pH 11 with ammonium hydroxide/ACN was used at 0.4 mL/min, while the separation of MDA (R/S-2) required an isocratic mobile phase of [55:45] 5 mM ammonium bicarbonate adjusted to pH 11 with ammonium hydroxide: [1:1] ACN/methanol at 0.5 mL/min. Samples were diluted in [70:30] water/acetonitrile to approximately 1 mg/mL and 2 μL was injected. Optical rotations were measured on a Reichert Polar3 polarimeter using a 100 mm glass sample tube.
Benzo[d][1,3]dioxol-5-ylmagnesium Bromide (1 M in THF, 3)
To a dry 50 mL round-bottom flask (RBF) equipped with a Claisen adapter and a reflux condenser, Mg granules (608 mg, 1 equiv) were added. The apparatus was sealed with a rubber septum, and an argon atmosphere was established. Anhydrous tetrahydrofuran (THF, 22 mL) and iodine (approximately 10 mg) were added to a flask, and the slurry was stirred for 5 min. 1-Bromo-3,4-methylenedioxybenzene (5.03 g, 3.0 mL, 25.0 mmol) was drawn into a syringe. About 20% of the total volume was added to the slurry, and the mixture was stirred for 30 min with gentle warming until the pink color disappeared. The remaining bromide was added dropwise to the stirring suspension, and gentle heat was maintained until all magnesium had dissolved, which took about 2 h. This process yielded a 1 M solution of benzo[d][1,3]dioxol-5-ylmagnesium bromide (3).
tert-Butyl (R)-(1-(Benzo[d][1,3]dioxol-5-yl)propan-2-yl)carbamate (R-7)
To a flame-dried 50 mL RBF was added CuI (0.42 g, 2.2 mmol, 20 mol %), THF (10 mL), and aziridine R-6 (1.75 g, 11.1 mmol) under argon. The slurry was cooled in an ice-salt bath (−5 °C) and stirred for 15 min. Grignard solution 3 (1 M, 15 mL, 15 mmol, 1.35 equiv) was added to the aziridine suspension steadily over 60 s via a syringe. The suspension was stirred for 30 min then quenched by dropwise addition of saturated aqueous NH4Cl (15 mL) and water (5 mL). The resulting suspension was extracted with EtOAc (2 × 60 mL). Combined organic extracts were washed with water (2 × 60 mL) and brine (80 mL), dried over Na2SO4, filtered, and concentrated to a yellow residue that solidified under high vacuum at 30 °C over 24 h to provide an off-white crude solid (3.48 g, 112%, superstoichiometric yield as a result of inconsequential byproducts from excess 3). An analytical sample was prepared by purification with flash column chromatography on silica (0–10% EtOAc/Hex) to give the title compound as a white crystalline solid. Melting point: 82–83 °C (lit.23 60–62 °C). 1H NMR (400 MHz, CDCl3): δ = 6.74 (d, J = 7.8 Hz, 1H), 6.68 (s, 1H), 6.63 (d, J = 7.8 Hz, 1H), 5.93 (s, 2H), 4.36 (br s, 1H), 3.84 (br s, 1H), 2.76 (br dd, J = 5.5, 13.3 Hz, 1H), 2.58 (dd, J = 7.3, 13.4 Hz, 1H), 1.44 (s, 9H), 1.08 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ = 155.15, 147.50, 146.01, 131.99, 122.37, 109.81, 108.09, 100.79, 28.41, 20.10. HRMS (ESI+): calcd for [C15H21NO4Na+] [M + Na]+: 302.1363; found: 302.1355. [α]D20 +5.88 (c 0.5, CHCl3) {lit.23 [α]D +5.6 (c 1.0, CHCl3)}.
tert-Butyl (S)-(1-(Benzo[d][1,3]dioxol-5-yl)propan-2-yl)carbamate (S-7)
The procedure for the synthesis of S-7 was essentially the same as that for R-7, with the following differences in reagent amounts: CuI (0.24 g, 1.3 mmol, 20 mol %), THF (6 mL), aziridine S-6 (1.04 g, 6.55 mmol), and Grignard solution 3 (1 M, 8.4 mL, 8.4 mmol, 1.3 equiv). The resulting off-white crude solid was obtained in a yield of 103% (1.8 g, superstoichiometric yield as a result of inconsequential byproducts from excess 3). An analytical sample was prepared by purification of 100 mg of the crude residue by flash column chromatography on silica (0–10% EtOAc/Hex) to give the title compound as a white crystalline solid. Melting point: 83–84 °C. 1H NMR (400 MHz, CDCl3): δ = 6.74 (d, J = 7.8 Hz, 1H), 6.71–6.68 (m, 1H), 6.63 (d, J = 7.8 Hz, 1H), 5.93 (s, 2H), 4.55–4.19 (m, 1H), 3.98–3.68 (m, 1H), 2.76 (br dd, J = 5.6, 13.4 Hz, 1H), 2.58 (dd, J = 7.3, 13.4 Hz, 1H), 1.44 (s, 9H), 1.08 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ = 155.15, 147.50, 146.01, 131.99, 122.37, 109.81, 108.09, 100.79, 77.20, 28.41, 20.09. HRMS (ESI+): calcd for [C15H21NO4Na+] [M + Na]+: 302.1363; found: 302.1355. [α]D20 −5.76 (c 2.0, CHCl3).
(R)-1-(Benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-aminium Chloride (R-1)
A 50 mL RBF fitted with a reflux condenser was charged with a solution of crude R-7 (3.48 g, assuming a quantitative yield from the previous step, 3.10 g, 11.1 mmol) in anhydrous THF (15 mL) under an argon atmosphere. LiAlH4 solution in THF (2.4 M, 13.9 mL, 33.3 mmol, 3 equiv) was added. The reaction mixture was heated at reflux and monitored by LCMS for the disappearance of the formamide intermediate (12, m/z 208.0968). After 4 h, the reaction was deemed complete. The mixture was diluted with THF (20 mL), cooled using an external ice bath, and quenched with 100:27 THF/H2O (13 mL) dropwise, maintaining a reaction temperature between 0 and 10 °C. The suspension was stirred at room temperature for 30 min and then silica (3 g) and anhydrous sodium sulfate (3 g) were added, stirring for an additional hour. The mixture was filtered over a pad of Celite and washed with MeOH (3 × 30 mL). The filtrate was concentrated to afford a semisolid residue (ca. 3 g). The residue was partitioned between methyl tert-butyl ether (MtBE,50 mL) and water (50 mL). The organic layer was collected, and the aqueous layer was extracted with MtBE (2 × 50 mL). The combined organic layers were washed with water (50 mL) and then extracted with 2 N HCl (2 × 50 mL). The aqueous extract was washed with MtBE (50 mL) and adjusted to pH 10–11 by the dropwise addition of 4 N NaOH. The crude free base was extracted with MtBE (2 × 50 mL). The organic extract was washed with brine (50 mL), dried over Na2SO4, and concentrated to yield a yellow oil (1.47 g, 68%, two steps) with a 90.1% peak area by UPLC. The crude free base was dissolved in isopropanol (15 mL), cooled using an external ice bath, and HCl in cyclopentyl methyl ether (3 M, 2.6 mL, 1.1 eq) was added dropwise to precipitate the hydrochloride salt. The suspension was distilled to remove volatile components, and the resulting crude solid was recrystallized with isopropanol (15 mL) and held at 0 °C for 12 h. The suspension was filtered, and the collected solid was washed with cold isopropanol (10 mL) and acetone (10 mL). The solid was dried under high vacuum to afford a shiny white solid (1.3 g, 51%) with a 98.30% peak area by UPLC. Melting point: 184–185 °C (lit. 192–193 °C). HRMS (ESI+): calcd for [C11H16NO2+] [M + H]+: 194.1176; found: 194.1165. [α]D20 −17.5 (c 2.0, H2O) {lit. [α]D −17.54 (c 1.0, H2O)}. Enantiomeric excess by chiral UPLC: 99.96%. Physical and spectroscopic characterizations were consistent with previously reported literature values.16
(S)-1-(Benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-aminium Chloride (S-1)
A 50 mL RBF fitted with a reflux condenser was charged with a solution of crude S-7 (750 mg, 2.7 mmol, assuming a quantitative yield from the previous step) in anhydrous THF (5 mL) under an argon atmosphere. LiAlH4 solution in THF (2.4 M, 3.4 mL, 8.05 mmol, 3 equiv) was added. The reaction was conducted and worked up following the same procedure as described for the R-enantiomer. The crude-free base was dissolved in isopropanol (10 mL), cooled using an external ice bath, and HCl in cyclopentyl methyl ether (3 M, 1.0 mL, 1.1 equiv) was added dropwise to precipitate the hydrochloride salt. The recrystallization and isolation steps were performed similarly as for the R-enantiomer. The solid was dried under high vacuum to afford a shiny white solid (320 mg, 52%) with a 98.43% peak area by UPLC. Melting point: 183–184 °C (lit. 192–193 °C). HRMS (ESI+): calcd for [C11H16NO2+] [M + H]+: 194.1176; found: 194.1174. [α]D20 +17.2 (c 2.0, H2O) {lit. [α]D +17.43 (c 1.0, H2O)}. Enantiomeric excess by chiral UPLC: 99.55%. Physical and spectroscopic characterizations were consistent with previously reported literature values.16
(R)-1-(Benzo[d][1,3]dioxol-5-yl)propan-2-aminium Chloride (R-2)
Carbamate R-7 (crude mass 1.65 g isolated from reaction with 6.2 mmol aziridine 6) was dissolved in EtOH (12 mL) and then HCl in cyclopentyl methyl ether (3 M, 5.6 mL, 16.7 mmol) was added. The solution was brought to a reflux and maintained for 20 min when the reaction was determined to be complete by LCMS analysis. The volatile components were removed by rotary evaporation and the residue was triturated in acetone (25 mL) to provide a fine white suspension that was collected by vacuum filtration, washed with acetone, and dried to afford R-2-HCl as a pearly white solid (0.83 g, 3.9 mmol, 62%, two steps) with a 98.42% peak area by UPLC. Melting point: 193–194 °C (lit. 200–202 °C). HRMS (ESI+): calcd for [C10H14NO2+] [M + H]+: 180.1019; found: 180.1016. [α]D20 −25.0 (c 2.0, H2O) {lit. [α]D −25.70 (c 2.0, H2O)}. Enantiomeric excess by chiral UPLC: 99.89%. Physical and spectroscopic characterizations were consistent with previously reported literature values.16
(S)-1-(Benzo[d][1,3]dioxol-5-yl)propan-2-aminium Chloride (S-2)
Carbamate S-7 (crude mass 750 mg) was dissolved in EtOH (5 mL) and then HCl in cyclopentyl methyl ether (3 M, 3.6 mL, 10 mmol) was added. The reaction was carried out and worked up following the same procedure as described for the R-enantiomer. The solid obtained was S-2-HCl, and it was afforded as a pearly white solid (304 mg, 63%) with a 99.23% peak area by UPLC. Melting point: 195–197 °C (lit. 200–202 °C). HRMS (ESI+): calcd for [C10H14NO2+] [M + H]+: 180.1019; found: 180.1008. [α]D20 +25.9 (c 2.0, H2O) {lit. [α]D +26.52 (c 2.0, H2O)}. Enantiomeric excess by chiral UPLC: 99.83%. Physical and spectroscopic characterizations were consistent with previously reported literature values.16
Acknowledgments
The authors thank Joshua Kimball for supporting analytical characterization efforts. ChatGPT-4 was utilized for proofreading and formatting assistance in portions of the Abstract, Introduction, Experimental Section, and References.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02358.
NMR spectroscopy and chiral UPLC chromatograms for final compounds and intermediates (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was funded by Usona Institute.
The authors declare no competing financial interest.
Supplementary Material
References
- Shulgin A. T.History of MDMA. In Ecstasy: The Clinical, Pharmacological and Neurotoxicological Effects of the Drug MDMA. Topics in the Neurosciences; Peroutka S. J., Ed.; Kluwer Academic Publishers: Boston, MA, 1990; vol 9, pp 1–20, 10.1007/978-1-4613-1485-1_1. [DOI] [Google Scholar]
- Freudenmann R. W.; Öxler F.; Bernschneider-Reif S. The Origin of MDMA (Ecstasy) Revisited: The True Story Reconstructed from the Original Documents. Addiction 2006, 101, 1241–1245. 10.1111/j.1360-0443.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A. M.; Prisinzano T. E. Novel Psychotherapeutics–a Cautiously Optimistic Focus on Hallucinogens. Expert Rev. Clin. Pharmacol. 2018, 11, 1–3. 10.1080/17512433.2018.1415755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa B.; Higbed L.; Nutt D. A Review of 3,4-Methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy. Front. Psychiatry 2019, 10, 138. 10.3389/fpsyt.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. M.; Bogenschutz M.; Lilienstein A.; Harrison C.; Kleiman S.; Parker-Guilbert K.; Otalora G. M.; Garas W.; Paleos C.; Gorman I.; Nicholas C.; Mithoefer M.; Carlin S.; Poulter B.; Mithoefer A.; Quevedo S.; Wells G.; Klaire S. S.; van der Kolk B.; Tzarfaty K.; Amiaz R.; Worthy R.; Shannon S.; Woolley J. D.; Marta C.; Gelfand Y.; Hapke E.; Amar S.; Wallach Y.; Brown R.; Hamilton S.; Wang J. B.; Coker A.; Matthews R.; De Boer A.; Yazar-Klosinski B.; Emerson A.; Doblin R. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. 10.1038/s41591-021-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggott; Garrison K. J.; Coyle J. R.; Galloway G. P.; Barnes A. J.; Huestis M. A.; Mendelson J. E. Effects of the Psychedelic Amphetamine MDA (3,4-Methylenedioxyamphetamine) in Healthy Volunteers. J. Psychoact. Drugs 2019, 51, 108–117. 10.1080/02791072.2019.1593560. [DOI] [PubMed] [Google Scholar]
- Murnane K. S.; Murai N.; Howell L. L.; Fantegrossi W. E. Discriminative Stimulus Effects of Psychostimulants and Hallucinogens in S(+)-3,4-Methylenedioxymethamphetamine (MDMA) and R(−)-MDMA Trained Mice. J. Pharmacol. Exp. Ther. 2009, 331, 717–723. 10.1124/JPET.109.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna D. J.; Guan X. M.; Shulgin A. T. 3,4-Methylenedioxyamphetamine (MDA) Analogues Exhibit Differential Effects on Synaptosomal Release of 3H-Dopamine and 3H-5-Hydroxytryptamine. Pharmacol. Biochem. Behav. 1991, 38, 505–512. 10.1016/0091-3057(91)90005-M. [DOI] [PubMed] [Google Scholar]
- Curry D. W.; Young M. B.; Tran A. N.; Daoud G. E.; Howell L. L. Separating the Agony from Ecstasy: R(−)-3,4-Methylenedioxymethamphetamine Has Prosocial and Therapeutic-like Effects without Signs of Neurotoxicity in Mice. Neuropharmacology 2018, 128, 196–206. 10.1016/j.neuropharm.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquas E.; Pisanu A.; Spiga S.; Plumitallo A.; Zernig G.; Chiara G. D. Differential Effects of Intravenous R,S-(±)-3,4- Methylenedioxymethamphetamine (MDMA, Ecstasy) and Its S(+)- and R(−)-Enantiomers on Dopamine Transmission and Extracellular Signal Regulated Kinase Phosphorylation (PERK) in the Rat Nucleus Accumbens Shell. J. Neurochem. 2007, 102, 121–132. 10.1111/j.1471-4159.2007.04451.x. [DOI] [PubMed] [Google Scholar]
- Baker L. E.; Taylor M. M. Assessment of the MDA and MDMA Optical Isomers in a Stimulant-Hallucinogen Discrimination. Pharmacol. Biochem. Behav. 1997, 57, 737–748. 10.1016/S0091-3057(96)00334-6. [DOI] [PubMed] [Google Scholar]
- Pitts E. G.; Curry D. W.; Hampshire K. N.; Young M. B.; Howell L. L. (±)-MDMA and Its Enantiomers: Potential Therapeutic Advantages of R(−)-MDMA. Psychopharmacology 2018, 235, 377–392. 10.1007/s00213-017-4812-5. [DOI] [PubMed] [Google Scholar]
- Lourenço T. C.; Bósio G. C.; Cassiano N. M.; Cass Q. B.; Moreau R. L. M. Chiral Separation of 3,4-Methylenedioxymethamphetamine (MDMA) Enantiomers Using Batch Chromatography with Peak Shaving Recycling and Its Effects on Oxidative Stress Status in Rat Liver. J. Pharm. Biomed. Anal. 2013, 73, 13–17. 10.1016/j.jpba.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Huot P.; Johnston T. H.; Lewis K. D.; Koprich J. B.; Reyes M. G.; Fox S. H.; Piggott M. J.; Brotchie J. M. Characterization of 3,4-Methylenedioxymethamphetamine (MDMA) Enantiomers in Vitro and in the MPTP-Lesioned Primate: R-MDMA Reduces Severity of Dyskinesia, Whereas S-MDMA Extends Duration of ON-Time. J. Neurosci. 2011, 31, 7190–7198. 10.1523/JNEUROSCI.1171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro N.; Farré M.; Pujadas M.; Peiró A. M.; Roset P. N.; Joglar J.; de la Torre R. Stereochemical Analysis of 3,4-Methylenedioxymethamphetamine and Its Main Metabolites in Human Samples Including the Catechol-Type Metabolite (3,4-Dihydroxymethamphetamine). Drug Metab. Dispos. 2004, 32, 1001–1007. [PubMed] [Google Scholar]
- Nichols D. E.; Hoffman A. J.; Oberlender R. A.; Jacob P.; Shulgin A. T. Derivatives of l-(l,3-Benzodioxol-5-yl)-2-butanamine: Representatives of a Novel Therapeutic Class. J. Med. Chem. 1986, 29, 2009–2015. 10.1021/jm00160a035. [DOI] [PubMed] [Google Scholar]
- Dunlap L. E.; Andrews A. M.; Olson D. E. Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 2018, 9, 2408–2427. 10.1021/acschemneuro.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenajdenko V. G.; Karpov A. S.; Balenkova E. S. A New Convenient Approach to Chiral β-Aryl(Heteroaryl)Alkylamines. Tetrahedron: Asymmetry 2001, 12, 2517–2527. 10.1016/S0957-4166(01)00442-6. [DOI] [Google Scholar]
- Hu X. E. Nucleophilic Ring Opening of Aziridines. Tetrahedron 2004, 60, 2701–2743. 10.1016/j.tet.2004.01.042. [DOI] [Google Scholar]
- Dellaria J. F.; Sallin K. J. Wittig Olefination in the Absence of an Exogenous Base: A New Synthesis of α-Substituted Primary Allylic Amines. Tetrahedron Lett. 1990, 31, 2661–2664. 10.1016/S0040-4039(00)94666-5. [DOI] [Google Scholar]
- Alam M.; Wise C.; Baxter C. A.; Cleator E.; Walkinshaw A. Development of a Robust Procedure for the Copper-Catalyzed Ring-Opening of Epoxides with Grignard Reagents. Org. Process Res. Dev. 2012, 16, 435–441. 10.1021/op200329x. [DOI] [Google Scholar]
- Nair J. B.; Hakes L.; Yazar-Klosinski B.; Paisner K. Fully Validated, Multi-Kilogram CGMP Synthesis of MDMA. ACS Omega 2022, 7, 900–907. 10.1021/acsomega.1c05520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaka T.; Nagayama S. I.; Desadee W.; Yajima N.; Kumamoto T.; Watanabe T.; Ishikawa T.; Kawahata M.; Yamaguchi K. Ring-Opening Reactions of 3-Aryl-1-Benzylaziridine-2-Carboxylates and Application to the Asymmetric Synthesis of an Amphetamine-Type Compound. Helv. Chim. Acta 2007, 90, 128–142. 10.1002/hlca.200790006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.