Abstract

Catalytic ozonation is an effective and promising advanced oxidation technology for organic pollutant removal. Herein, CexMn1–xO2 metal oxides loaded on Al2O3 catalysts (Mn–Ce/Al2O3) were synthesized for catalytic ozonation of the wastewater containing ciprofloxacin. The morphology, crystal structure, and specific surface area of the prepared catalyst were characterized. The characteristics of the Mn–Ce/Al2O3 catalyst revealed that the loaded MnO2 could interfere with the formed CeO2 crystals and then produced complex CexMn1–xO2 oxides. Compared with an ozone-alone system (47.4%), the ciprofloxacin degradation efficiency in the Mn–Ce/Al2O3 catalytic ozonation system elevated to 85.1% within 60 min. The ciprofloxacin degradation kinetic rate over the Mn–Ce/Al2O3 catalyst is 3.0 times that of the ozone-alone system. The synergetic corporation of redox pairs between Mn(III)/Mn(IV) and Ce(III)/Ce(IV) in the Mn–Ce/Al2O3 catalyst could accelerate ozone decomposition to generate active oxygen species and further significantly improve the mineralization efficiency of ciprofloxacin. The work demonstrates the great potential of developing dual-site ozone catalysts for advanced treatment of wastewater.
1. Introduction
Ciprofloxacin is widely used as an antibiotic, which can inhibit the growth of both Gram-positive and Gram-negative bacteria.1−3 After being metabolized by humans or animals, more than 50% of ciprofloxacin will be excreted with feces and urine in the form of antibiotics itself and its metabolites into the wastewater system.4 Unfortunately, the detection concentration of antibiotics in the tail-water of wastewater treatment plants indicates that the conventional wastewater treatment process is not effective for ciprofloxacin removal.5 As a result, ciprofloxacin is widespread in surface waters. Considering the potential adverse effects of ciprofloxacin on the ecological environment, there is an urgent need to develop an efficient wastewater treatment technology for ciprofloxacin removal.
Recently, the catalytic ozonation technology has been considered as an effective technology for refractory organic pollutant treatment. Especially, heterogeneous catalytic ozonation could improve the mass-transfer efficiency of ozone from the gas phase to the liquid phase and accelerate O3 decomposition into hydroxyl radicals (•OH).6−8 The advantages of catalytic ozonation, such as high oxidation efficiency and reusability of the catalyst, make it to be a potential and environmentally friendly ozone treatment method for wastewater treatment. Due to the important role of catalyst materials in affecting catalytic oxidation efficiency,9 intensive research has been conducted to design efficient catalysts for catalytic ozonation.
Due to the large specific surface area, mesoporous structure, high hardness, and high mechanical strength, the activated alumina-supported metal catalyst is one of the most used components in the field of catalytic ozonation process.10−13 Especially, transitional-metal oxides, such as Fe, Cu, Mn, and Ce oxides, displayed great potential for ozone decomposition and reactive oxygen species (ROS) generation, subsequently improving the degradation efficiency of the organic pollutants.14−16 Compared with single-component metal-loaded catalysts, the construction of multiple metal-loaded catalysts may achieve the synergic effect among the active components for the catalytic ozonation process. The development of the multiple metal-loaded catalysts is of great significance for the catalytic ozonation technology in treating antibiotic-containing wastewater.
In this work, a highly efficient and stable multiple metal-loaded alumina catalyst Mn–Ce/Al2O3 was prepared and ciprofloxacin was selected as a typical quinolone antibiotic pollutant. The catalytic ozonation performance of Mn–Ce/Al2O3 for ciprofloxacin degradation was tested. The physicochemical properties of the catalysts were investigated to explore the relationship between the structure properties and catalytic activity. The catalytic mechanism of treating wastewater containing ciprofloxacin by catalytic ozonation with Mn–Ce/Al2O3 was revealed.
2. Experimental Section
2.1. Experimental Materials
Ciprofloxacin and ciprofloxacin-D8 were purchased from Tianjin Alta Scientific Co., Ltd. Activated alumina was purchased from Shanghai Macklin Biochemical Co., Ltd. Both cerium nitrate and manganese nitrate were produced by Sinopharm Group Chemical Reagent Co., Ltd. All chemical reagents were used without any further purification. The water used in the experimental process was deionized water. All pieces of glassware were washed with deionized water several times before use and dried in a 105 °C oven.
2.2. Preparation of Catalysts
2.2.1. Activation Treatment of Al2O3 Microspheres
The activated alumina microspheres (Φ 3–5 mm) with uniform shape were selected and washed three times with deionized water to remove the residual impurities and dust on the surfaces of particles and then dried in an oven at 110 °C for 10 h. Dried activated alumina microspheres were transferred to a muffle furnace, calcined at 400 °C for 3 h, and then cooled to room temperature and stored for future use.
2.2.2. Preparation of the Loaded Activated Al2O3 Catalyst
A certain amount of activated Al2O3 microspheres were put into 0.5 mol L–1 manganese nitrate, cerium nitrate, and manganese nitrate–cerium nitrate mixed solution (the molar concentration ratio of manganese nitrate to cerium nitrate was set to 1:1). The suspension was placed in a conical flask and then vibrated in a shaker for 6 h to achieve the adsorption equilibrium. After filtration, activated Al2O3 microspheres were placed in an oven at 60 °C for drying and then placed in a muffle furnace and calcined at 500 °C for 3 h to obtain metal oxide-loaded Al2O3 catalysts. Three catalysts with different components were prepared under the same conditions and labeled as Mn/Al2O3, Ce/Al2O3, and Mn–Ce/Al2O3.
2.3. Catalyst Characterization
The microscopic morphology of the catalysts was analyzed by scanning electron microscopy (SEM). The crystal structure of the metal-loaded-activated Al2O3 catalysts was analyzed by X-ray diffraction (XRD). The elemental composition and its chemical state on the catalyst surface were analyzed by X-ray photoelectron spectrometry (XPS). The specific surface area and pore size distribution were determined with the Brunauer–Emmett–Teller method and Barrett–Joyner–Halenda method using a specific surface area analyzer. The surface charge of the catalysts and the zero charge pH point (pHzpc) were determined using a Zeta potential meter. The hydroxyl radical (•OH) generation was identified by a Bruker ER073 spectrometer (Karlsruhe, Germany) using 5,5-dimethylpyridine-N-oxide (DMPO) as the spin-trapping reagent. tert-Butanol was employed as an •OH scavenger to verify the contribution of •OH to the degradation of ciprofloxacin.
2.4. Catalytic Ozonation of Ciprofloxacin
A 15 L of simulated wastewater containing ciprofloxacin (5.0 mg L–1) and a certain mass of solid catalyst were added into a reactor and evenly mixed by the magnetic stirrer. The ozone generator was used to provide O3 (Figure S1 in the Supporting Information). After a certain reaction time, water samples were taken out and 0.5 mL of Na2S2O3 solution (0.01 mol L–1) was added dropwise to terminate the ozonation reaction. After the catalyst in the water sample was precipitated, the supernatant was taken for ciprofloxacin detection. In the ozone-alone experiment, no solid catalyst was added into the reactor. In the catalyst adsorption experiment, oxygen was directly introduced into the reactor without opening the ozone generator, while other experimental conditions were kept the same as the catalytic ozonation experiment.
3. Results and Discussion
3.1. Morphology and Structure Characterization
The surface structure of the catalyst would affect not only the diffusion process of pollutants on the surface but also the exposure of the surface active sites.17Figure 1 shows SEM images of the prepared catalysts. The surface of the Al2O3 microsphere displays irregular morphology, which is mainly due to the inhomogeneous packing of Al2O3 particles on the surface. The pores in the Al2O3 microsphere would facilitate the diffusion of ozone molecules or organic molecules into the interior of Al2O3 microspheres. Compared to the bare Al2O3 catalyst, some dispersed nanoparticles were displayed on the surface of Mn/Al2O3, Ce/Al2O3, and Mn–Ce/Al2O3 catalysts, which should be the formed MnO2 or CeO2 nanoparticles. When Al2O3 microspheres were immerged into a metal nitrate solution, metal ions would get adsorbed on the surface of Al2O3 particles. After the high-temperature calcination, the metal nitrates were further converted into metal oxide particles and tightly fixed on the surface of the activated Al2O3 particles.
Figure 1.
SEM images of the prepared catalysts. (a) Al2O3 microspheres, (b) Mn/Al2O3, (c) Ce/Al2O3, and (d) Mn–Ce/Al2O3.
The XRD spectra of the prepared Al2O3 catalysts are shown in Figure 2. The active Al2O3 exhibits characteristic diffraction peaks at 38.7 and 67.3° (Standard Card PDF#80-0956). After loading metal oxides, the Mn/Al2O3 catalyst exhibits the characteristic diffraction peak of MnO2 at 37.5° (Standard Card PDF#44-0141). The curve of the Ce/Al2O3 catalyst shows the characteristic peaks of CeO2 at 28.5 and 47.5° (standard card PDF#43-1002), indicating that manganese nitrate and cerium nitrate have been converted into MnO2 or CeO2 nanoparticles after the impregnation-calcination process.18,19
Figure 2.
XRD patterns of the metal-loaded Al2O3 catalyst.
The XPS spectra of metal-supported active Al2O3 catalysts are shown in Figure 3. It can be seen that the Mn–Ce/Al2O3 catalyst shows characteristic peaks of Al 2p, O 1s, Mn 2p, and Ce 3d, demonstrating that Mn and Ce species have been successfully loaded in the activated Al2O3 catalyst. For Mn/Al2O3 and Mn–Ce/Al2O3 catalysts, the Mn 2p spectrum presents two sets of characteristic peaks at 642.1 and 653.2 eV, corresponding to Mn 2p1/2 and Mn 2p3/2, respectively. Using the Gaussian fitting, the Mn 2p3/2 peaks could be fitted to the characteristic energies of 642.2 and 643.5 eV, corresponding to Mn (III) and Mn (IV), respectively.20 These results indicate that Mn oxides mainly exist in the form of MnO2, with a small amount present in the form as Mn (III).
Figure 3.
High-resolution XPS spectra of (a) Mn 2p and (b) Ce 3d for the metal-loaded Al2O3 catalyst.
Due to the high localization of 4f electrons in CeO2 nanocrystals, it is easy to form oxygen vacancies on CeO2 surfaces in Ce/Al2O3 and Mn–Ce/Al2O3 catalysts, which further induces the part reduction of Ce(IV) to Ce(III). The high-resolution XPS curve to the Ce 3d XPS spectrum was further fitted with Gaussian curves; 10 spin–orbit peaks appeared, in which v represented the split peak generated by the Ce 3d3/2 spin–orbit and u represented the split peak generated by the Ce 3d5/2 spin–orbit.21,22Table 1 lists the binding energy positions where different splitting peaks appeared, among which Ce (III) exhibited a quadruple split peak structure, corresponding to v0, v′, v‴, and u′, respectively. Ce(IV) exhibited a sixfold split peak structure, corresponding to v, v″, u0, u, u″, and u‴, respectively. The ratio of Ce(III)/Ce(IV) can be estimated in terms of the peak areas.23 The results show that the atomic ratios of Ce(III)/Ce(IV) in Ce/Al2O3 and Mn–Ce/Al2O3 catalysts are 0.79 and 0.83, respectively. Indeed, the Ce (III) content in Ce oxides plays an important role in determining the catalytic performance.
Table 1. Energy Positions of Ce 3d3/2 and Ce 3d5/2 Spin–Orbit Components.
| sample name | v0 | v | v′ | v″ | v‴ | u0 | u | u′ | u″ | u‴ |
|---|---|---|---|---|---|---|---|---|---|---|
| Ce/Al2O3 | 882.1 | 882.8 | 885.4 | 888.6 | 898.0 | 899.1 | 900.8 | 903.6 | 907.4 | 916.5 |
| Mn–Ce/Al2O3 | 882.2 | 882.9 | 885.3 | 888.9 | 898.3 | 899.4 | 901.0 | 903.4 | 907.3 | 916.4 |
The specific surface area of the catalyst is closely related to the exposed active sites, which in turn affects the catalyst activity. Figure 4 shows the N2 adsorption–desorption isotherms of the Al2O3, Mn/Al2O3, Ce/Al2O3, and Mn–Ce/Al2O3 catalysts. The activated Al2O3 microspheres have a relatively large specific surface area (289.1 m2 g–1). The loading of active metal components does not significantly affect the specific surface area of Al2O3 microspheres. There are large numbers of mesoporous structures in the catalyst with pore size mainly varying from 3 to 10 nm (Table 2). These porous structures in the microspheres would facilitate the diffusion and adsorption of ozone and organic pollutants into the catalyst and subsequently enhance the interaction between ozone molecules and the catalytic active sites.
Figure 4.
N2 adsorption–desorption isotherms of different Al2O3 catalysts.
Table 2. Surface Area, Pore Diameter, and Pore Volume of Different Catalysts.
| sample name | specific surface area (m2 g–1) | pore size (nm) | pore volume (cm3 g–1) | pHzpc |
|---|---|---|---|---|
| Al2O3 | 289.1 | 5.63 | 0.46 | 7.3 |
| Mn/Al2O3 | 281.3 | 6.50 | 0.48 | 5.3 |
| Ce/Al2O3 | 273.4 | 5.92 | 0.51 | 6.9 |
| Mn–Ce/Al2O3 | 280.9 | 6.20 | 0.49 | 6.6 |
3.2. Catalytic Ozonation Performance toward Ciprofloxacin
Ciprofloxacin was selected as the model pollutant to investigate the catalytic ozonation degradation performance of the metal-supported active Al2O3 catalysts. Before the catalytic degradation experiment, the adsorption abilities of active Al2O3, Mn/Al2O3, Ce/Al2O3, and Mn–Ce/Al2O3 catalysts to ciprofloxacin were investigated. It indicated that the adsorbed amount of ciprofloxacin by all catalysts is <3% within 30 min, indicating that the adsorption capacities of the prepared Al2O3 catalyst are weak. Figure 5 shows the ciprofloxacin degradation curves in different catalytic ozonation systems. In the ozone-alone system, about 47% ciprofloxacin was degraded in 60 min. The introduction of Al2O3 catalysts improved the ciprofloxacin degradation efficiency (56%), indicating that the Al2O3 catalyst could enhance the catalytic ozonation reaction. Al2O3 microspheres are beneficial to the transfer of ozone molecules at the solid–liquid interface, and then the Lewis acidic sites on Al2O3 surface could serve as active sites for catalyzing ozone decomposition to generate ROS.
Figure 5.
(a) Degradation performance of ciprofloxacin during the catalytic ozonation over different Al2O3-based catalysts and (b) the corresponding pseudo-first-order reaction kinetic analysis. ([ciprofloxacin] = 5 mg L–1, [catalyst] = 10.0 g L–1, pH = 6.40).
However, the catalytic ozonation efficiency of the Al2O3 catalyst is low. After loading the active metal components, the degradation efficiency of ciprofloxacin on Mn/Al2O3, Ce/Al2O3, and Mn–Ce/Al2O3 catalysts reached 65, 69, and 85%, respectively, within 60 min. The degradation curves of ciprofloxacin were fitted by the first-order kinetic equation, which fitted well with the pseudo-first-order kinetic process. The degradation rate constant of ciprofloxacin was 0.0102 min–1 in the ozone-alone system. The presence of Al2O3 catalysts accelerates the ciprofloxacin degradation rate (0.0135 min–1). Compared with the single-metal-loaded Mn/Al2O3 or Ce/Al2O3 catalysts, the Mn–Ce/Al2O3 catalyst further improved the ciprofloxacin degradation rate, which was 3.0 times (0.0309 min–1) that of the ozone-alone system, indicating that loading of bimetallic active components remarkably improves the catalytic ozonation performance of activated Al2O3.
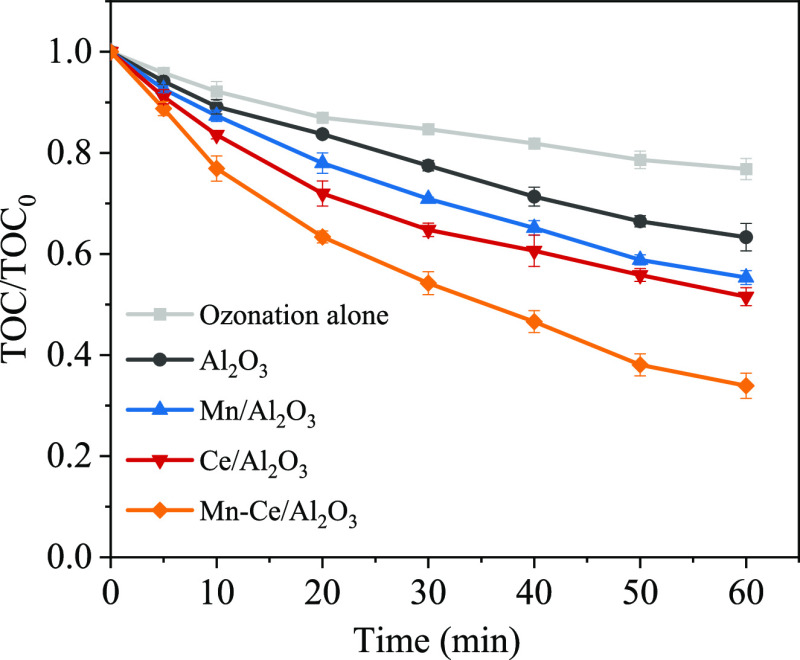
Figure 6 shows the mineralization efficiency curves of ciprofloxacin in different catalyst systems. In the ozone-alone system, the removal efficiency of total organic carbon (TOC) was 23% at 60 min, indicating that the single ozonation system has limited ability to mineralize ciprofloxacin due to the generation of intermediates. The introduction of Al2O3, Mn/Al2O3, and Ce/Al2O3 catalysts significantly improved the mineralization efficiency of ciprofloxacin. Especially, TOC removal efficiency of ciprofloxacin over the Mn–Ce/Al2O3 catalyst reached 67% at 60 min, which should be attributed to the fact that the Mn–Ce/Al2O3 catalyst could greatly catalyze and decompose ozone molecules to generate reactive oxygen radicals.
Figure 6.
Mineralization performance of ciprofloxacin during the catalytic ozonation over different Al2O3-based catalysts ([ciprofloxacin] = 5 mg L–1, [catalyst] = 10.0 g L–1, pH = 6.40).
3.3. Optimization of Catalyst Preparation Conditions
The composition of the prepared catalyst determines the content of active components in Al2O3 support, which in turn affects its catalytic ozonation performance. Figure 7 displays the effect of precursor concentration on the performance of the Mn–Ce/Al2O3 catalyst for ozonation degradation of ciprofloxacin. With the increase of active component concentration in the impregnation solution, the ciprofloxacin degradation on Mn–Ce/Al2O3 shows a trend of increasing first and then decreasing, which can be attributed to the lack of sufficient sites at low loadings and the pore-clogging on Al2O3 microsphere surface at high loadings. The highest degradation efficiency of ciprofloxacin on the Mn–Ce/Al2O3 catalyst occurred at an active component concentration of 0.5 mol L–1. Thus, proper catalyst composition in the Mn–Ce/Al2O3 catalyst is important to promote the catalytic ozonation performance.
Figure 7.

Ciprofloxacin degradation efficiency over the Mn–Ce/Al2O3 catalyst prepared at different impregnation concentrations ([ciprofloxacin] = 5 mg L–1, [catalyst] = 10.0 g L–1, pH = 6.40).
The effect of the initial pH value of the reaction solution on the catalytic ozonation performance of the Mn–Ce/Al2O3 catalyst was further investigated. As shown in Figure 8, the removal efficiencies of ciprofloxacin were 54.3, 73.7, 83.5, 86.3, and 85.1% within 60 min at the initial pH of the reaction solution of 3.5, 5.3, 6.4, 7.8, and 8.6, respectively. It can be observed that the degradation efficiency of ciprofloxacin significantly increases as the initial pH value increases. The catalytic ozonation degradation efficiency of ciprofloxacin under neutral and alkaline conditions is much higher than that under acidic conditions. This can be attributed to the increased −OH groups on Mn–Ce/Al2O3 catalyst surface at high solution pH, which can act as the active sites for catalyzing ozone decomposition.
Figure 8.
Effect of solution pH on ciprofloxacin removal efficiency in the Mn–Ce/Al2O3 catalytic ozonation process.
3.4. Stability of Mn–Ce/Al2O3 Catalyst
The stability of the catalyst during the catalytic process is critical in a heterogeneous catalysis reaction. To investigate the stability of the Mn–Ce/Al2O3 catalyst during catalytic ozonation, the cyclic catalytic ozonation degradation experiments were conducted (Figure 9). The performance of the Mn–Ce/Al2O3 catalyst for catalytic ozonation degradation of ciprofloxacin was not significantly reduced after five repeated cycles. In the fifth repeated experiment, the degradation efficiency of ciprofloxacin still reached >80%, indicating that the prepared Mn–Ce/Al2O3 catalyst has good stability during the catalytic ozonation process. In addition, the dissolution of Mn and Ce ions on the catalyst surface during the catalytic ozonation process was further measured (Table 3). After the reaction, the dissolved concentration of Mn and Ce ions in the solution was lower than 0.01 mg L–1. These results indicate that the synthesized Mn–Ce/Al2O3 catalyst possesses good stability and reusability, which is of great importance for the engineering applications of the catalytic ozonation technology.
Figure 9.
Cycling runs of the Mn–Ce/Al2O3 catalyst for the catalytic ozonation degradation of ciprofloxacin.
Table 3. Dissolution of Metal Ions during the Catalytic Ozonation Degradation of Ciprofloxacin by the Mn–Ce/Al2O3 Catalyst.
| the reused times | Mn ion concentration (mg L–1) | Ce ion concentration (mg L–1) |
|---|---|---|
| 1 | 0.0042 | 0.0024 |
| 2 | 0.0031 | 0.0017 |
| 3 | 0.0035 | 0.0019 |
| 4 | 0.0016 | 0.0016 |
| 5 | 0.0021 | 0.0012 |
3.5. Reaction Mechanism Analysis of Catalytic Ozonation
Since the adsorption of ciprofloxacin over different metal-supported active Al2O3 catalysts was less than 5%, it is concluded that most of the degradation of ciprofloxacin was completed by catalytic ozonation pathways. Electron spin resonance (ESR) spectroscopy was used to verify the produced ROS during the catalytic ozonation process.24 DMPO was used as the spin capture reagent to detect the generated •OH radicals. As shown in Figure 10a, a typical four-line characteristic signal of DMPO–•OH (1:2:2:1) was clearly observed, which demonstrates the generation of •OH in the catalytic ozonation system. Compared to the Al2O3 catalyst system, the significantly enhanced DMPO–•OH signal in the Mn–Ce/Al2O3 catalyst system suggests the promoted ozone decomposition and ROS generation, which would enhance the degradation of ciprofloxacin in wastewater.
Figure 10.
(a) ESR spectra of the DMPO–•OH adduct recorded at 5 min over different catalytic ozonation systems. (b) Effect of tert-butyl alcohol on ciprofloxacin removal efficiency over different catalytic ozonation systems.
To determine the critical active species during ciprofloxacin degradation, radical quenching experiments were further performed. As shown in Figure 10b, the degradation efficiency of ciprofloxacin was greatly inhibited in the presence of tert-butyl alcohol (•OH scavenger) in the Mn–Ce/Al2O3 catalyst system. This result suggests that •OH plays an important role in promoting ciprofloxacin degradation. According to the analysis of the free-radical capture experiment and free-radical quenching experiment, the enhanced generation of OH in the Mn–Ce/Al2O3 system mainly contributes to ciprofloxacin degradation.
Based on the above results, the mechanism of the catalytic ozonation reaction over the Mn–Ce/Al2O3 catalyst is proposed as follows (Figure 11). The hydroxyl group on the catalyst surface plays an important role in the ozone decomposition.25,26 In the aqueous environment, the water molecules coordinate with Lewis acid on the Mn–Ce/Al2O3 catalyst surface, and then water molecules dissociate and form hydroxyl groups and H+ on the catalyst surface. The highly active surface resonance structure of ozone molecules makes it coordinate with the hydroxyl group on Mn–Ce/Al2O3 catalyst surface to form a five-membered ring structure, which subsequently induces the coordination structure to release O2 molecules and generate •O2H or •O2- on catalyst surface. Meanwhile, ozone molecules and •O2H- would generate •OH through a series of reactions and then achieve oxidative degradation of ciprofloxacin molecules to achieve wastewater purification (eqs 1–6)
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
Figure 11.

Schematic representation of the zone oxidation reaction on Mn–Ce/Al2O3 catalyst surface.
4. Conclusions
The multiple activated metal component catalyst Mn–Ce/Al2O3 was successfully prepared by an impregnation-calcination method for catalytic ozonation degradation. The Mn–Ce/Al2O3 catalyst exhibited superior catalytic performance, resulting in 85% degradation of ciprofloxacin within 60 min. The degradation kinetic constant with Mn–Ce/Al2O3 is 3.0, 1.8, and 1.6 times than that with the ozone-alone system, Mn/Al2O3 system, and Ce/Al2O3 system, respectively. Although there is a direct oxidation process by ozone molecules in both the ozone-alone system and Mn–Ce/Al2O3 catalytic system, Mn–Ce/Al2O3 catalyst could more efficiently catalyze the decomposition of ozone to generate reactive oxygen radicals, which significantly improves the mineralization efficiency of ciprofloxacin. The synergetic corporation of bimetallic active components Mn(III)/Mn(IV) and Ce(III)/Ce(IV) in the Mn–Ce/Al2O3 catalyst could promoted the catalytic ozone decomposition and subsequently enhance ROS generation in the catalytic ozonation process for ciprofloxacin degradation. The highly catalytic ozonation performance Mn–Ce/Al2O3 will help the development of a high-efficiency ozone catalytic oxidation system to achieve the effective degradation of antibiotics in water and high purification of reclaimed water.
Acknowledgments
This work was supported by the Open Foundation of Beijing Key Laboratory of Water Environmental and Ecological Technology for River Basins (BKL-KF-2021-001-SZY).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c01302.
Schematic diagram of the experimental apparatus for catalytic ozonation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- De Witte B.; Dewulf J.; Demeestere K.; Van Langenhove H. Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water. J. Hazard. Mater. 2009, 161, 701–708. 10.1016/j.jhazmat.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Rocha D. C.; da Silva Rocha C.; Tavares D. S.; de Morais Calado S. L.; Gomes M. P. Veterinary antibiotics and plant physiology: an overview. Sci. Total Environ. 2021, 767, 144902. 10.1016/j.scitotenv.2020.144902. [DOI] [PubMed] [Google Scholar]
- Zhang G. F.; Liu X.; Zhang S.; Pan B.; Liu M. L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. 10.1016/j.ejmech.2018.01.078. [DOI] [PubMed] [Google Scholar]
- Hayes A.; May Murray L.; Catherine Stanton I.; Zhang L.; Snape J.; Hugo Gaze W.; Kaye Murray A. Predicting selection for antimicrobial resistance in UK wastewater and aquatic environments: Ciprofloxacin poses a significant risk. Environ. Int. 2022, 169, 107488. 10.1016/j.envint.2022.107488. [DOI] [PubMed] [Google Scholar]
- Qalyoubi L.; Al-Othman A.; Al-Asheh S. Removal of ciprofloxacin antibiotic pollutants from wastewater using nano-composite adsorptive membranes. Environ. Res. 2022, 215, 114182. 10.1016/j.envres.2022.114182. [DOI] [PubMed] [Google Scholar]
- Vittenet J.; Aboussaoud W.; Mendret J.; Pic J.-S.; Debellefontaine H.; Lesage N.; Faucher K.; Manero M. H.; Thibault-Starzyk F.; Leclerc H.; et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal., A 2015, 504, 519–532. 10.1016/j.apcata.2014.10.037. [DOI] [Google Scholar]
- Ikhlaq A.; Brown D. R.; Kasprzyk-Hordern B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal., B 2012, 123–124, 94–106. 10.1016/j.apcatb.2012.04.015. [DOI] [Google Scholar]
- Yu D.; Wu M.; Hu Q.; Wang L.; Lv C.; Zhang L. Iron-based metal-organic frameworks as novel platforms for catalytic ozonation of organic pollutant: Efficiency and mechanism. J. Hazard. Mater. 2019, 367, 456–464. 10.1016/j.jhazmat.2018.12.108. [DOI] [PubMed] [Google Scholar]
- Nawrocki J.; Kasprzyk-Hordern B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal., B 2010, 99, 27–42. 10.1016/j.apcatb.2010.06.033. [DOI] [Google Scholar]
- Bing J.; Hu C.; Zhang L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal., B 2017, 202, 118–126. 10.1016/j.apcatb.2016.09.019. [DOI] [Google Scholar]
- Nawaz F.; Cao H.; Xie Y.; Xiao J.; Chen Y.; Ghazi Z. Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 2017, 168, 1457–1466. 10.1016/j.chemosphere.2016.11.138. [DOI] [PubMed] [Google Scholar]
- Wu M.; Wang X.; Dai Q.; Li D. Catalytic combustion of chlorobenzene over Mn–Ce/Al2O3 catalyst promoted by Mg. Catal. Commun. 2010, 11, 1022–1025. 10.1016/j.catcom.2010.04.011. [DOI] [Google Scholar]
- Wang Z.; Li C.; Guo Y.; Cheng J.; Song Z.; Sun D.; Qi F.; Ikhlaq A. Model prediction and mechanism analysis of PPCPs abatement in secondary effluent by heterogeneous catalytic ozonation: A case study with MnO2-Co3O4 depends on DOM concentration. Chem. Eng. J. 2023, 455, 140792. 10.1016/j.cej.2022.140792. [DOI] [Google Scholar]
- Ma N.; Ru Y.; Weng M.; Chen L.; Chen W.; Dai Q. Synergistic mechanism of supported Mn–Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere 2022, 308, 136192. 10.1016/j.chemosphere.2022.136192. [DOI] [PubMed] [Google Scholar]
- Ma N.; Ru Y.; Weng M.; Chen L.; Chen W.; Dai Q. Synergistic mechanism of supported Mn–Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere 2022, 308, 136192. 10.1016/j.chemosphere.2022.136192. [DOI] [PubMed] [Google Scholar]
- Liu H.; Gao Y.; Wang J.; Pan J.; Gao B.; Yue Q. Catalytic ozonation performance and mechanism of Mn-CeOx@γ-Al2O3/O3 in the treatment of sulfate-containing hypersaline antibiotic wastewater. Sci. Total Environ. 2022, 807, 150867. 10.1016/j.scitotenv.2021.150867. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yu G. Challenges and pitfalls in the investigation of the catalytic ozonation mechanism: A critical review. J. Hazard. Mater. 2022, 436, 129157. 10.1016/j.jhazmat.2022.129157. [DOI] [PubMed] [Google Scholar]
- Li P.; Zhan S.; Yao L.; Xiong Y.; Tian S. Highly porous alpha-MnO2 nanorods with enhanced defect accessibility for efficient catalytic ozonation of refractory pollutants. J. Hazard. Mater. 2022, 437, 129235. 10.1016/j.jhazmat.2022.129235. [DOI] [PubMed] [Google Scholar]
- Wang D.; He Y.; Chen Y.; Yang F.; He Z.; Zeng T.; Lu X.; Wang L.; Song S.; Ma J. Electron transfer enhancing the Mn(II)/Mn(III) cycle in MnO/CN towards catalytic ozonation of atrazine via a synergistic effect between MnO and CN. Water Res. 2023, 230, 119574. 10.1016/j.watres.2023.119574. [DOI] [PubMed] [Google Scholar]
- Gao E.; Meng R.; Jin Q.; Yao S.; Wu Z.; Li J.; Du E. Highly effective mineralization of acetic acid wastewater via catalytic ozonation over the promising MnO2/γ-Al2O3 catalyst. Chem. Phys. Impact 2023, 6, 100149. 10.1016/j.chphi.2022.100149. [DOI] [Google Scholar]
- Wang J.; Quan X.; Chen S.; Yu H.; Liu G. Enhanced catalytic ozonation by highly dispersed CeO2 on carbon nanotubes for mineralization of organic pollutants. J. Hazard. Mater. 2019, 368, 621–629. 10.1016/j.jhazmat.2019.01.095. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Xu Z.; Jiang M.; Chen S.; Han Z.; Liu Y.; Liu Y. Enhanced activity of CuOy/TNTs doped by CeOx for catalytic ozonation of 1,2-dichloroethane at normal temperatures: Performance and catalytic mechanism. Sep. Purif. Technol. 2023, 311, 123255. 10.1016/j.seppur.2023.123255. [DOI] [Google Scholar]
- Ma N.; Ru Y.; Weng M.; Chen L.; Chen W.; Dai Q. Synergistic mechanism of supported Mn–Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere 2022, 308, 136192. 10.1016/j.chemosphere.2022.136192. [DOI] [PubMed] [Google Scholar]
- Qi F.; Chen Z.; Xu B.; Shen J.; Ma J.; Joll C.; Heitz A. Influence of surface texture and acid–base properties on ozone decomposition catalyzed by aluminum (hydroxyl) oxides. Appl. Catal., B 2008, 84, 684–690. 10.1016/j.apcatb.2008.05.027. [DOI] [Google Scholar]
- Joseph C. G.; Farm Y. Y.; Taufiq-Yap Y. H.; Pang C. K.; Nga J. L. H.; Li Puma G. Ozonation treatment processes for the remediation of detergent wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 106099. 10.1016/j.jece.2021.106099. [DOI] [Google Scholar]
- Rekhate C. V.; Srivastava J. K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020, 3, 100031. 10.1016/j.ceja.2020.100031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.