Abstract

Toxicology is undergoing a digital revolution, with mobile apps, sensors, artificial intelligence (AI), and machine learning enabling better record-keeping, data analysis, and risk assessment. Additionally, computational toxicology and digital risk assessment have led to more accurate predictions of chemical hazards, reducing the burden of laboratory studies. Blockchain technology is emerging as a promising approach to increase transparency, particularly in the management and processing of genomic data related with food safety. Robotics, smart agriculture, and smart food and feedstock offer new opportunities for collecting, analyzing, and evaluating data, while wearable devices can predict toxicity and monitor health-related issues. The review article focuses on the potential of digital technologies to improve risk assessment and public health in the field of toxicology. By examining key topics such as blockchain technology, smoking toxicology, wearable sensors, and food security, this article provides an overview of how digitalization is influencing toxicology. As well as highlighting future directions for research, this article demonstrates how emerging technologies can enhance risk assessment communication and efficiency. The integration of digital technologies has revolutionized toxicology and has great potential for improving risk assessment and promoting public health.
Introduction
Digitalization or Digital technology refers to the use of electronic devices and software to process, store, and transmit information.1 These technologies include computers, smartphones, the Internet, and other electronic devices that use digital data and signals to perform various functions. Digital technology is an important enabler of digitalization and is widely used in various industries and sectors, including business, healthcare, education, and entertainment.2
Digital technology has had a significant impact on healthcare in recent years. Some examples of where digitalization is in practice include telemedicine, which involves the use of video or phone calls to consult with a healthcare provider remotely.3 It can be particularly useful for people in rural or underserved areas who may not have easy access to a healthcare facility. Medical imaging benefits from digital technologies such as CT scans and MRI tools that can produce detailed images of the inside of the body, which can help healthcare providers diagnose and treat various conditions.4 Furthermore, examples like robotics can be used in healthcare settings to assist with tasks such as dispensing medication or performing surgery. Therefore, the use of digital technology in healthcare and risk assessment has the potential to improve patient care and outcomes as well as increase efficiency and reduce costs.5
Timeline of Digital Advancement or Digitalization
Digital advancements have not only changed the way we live but also opened up new possibilities for people to explore and connect. Digital technology has a long and complex history, with many important developments and milestones as shown in Figure 1. In the history of digital technology, the foundation was laid when in 1937, John Atanasoff and Clifford Berry develop the Atanasoff-Berry Computer (ABC), a prototype electronic computer that used binary digits (bits) to represent data.6 In 1950, Alan Turing proposes the Turing test, a measure of a machine’s ability to exhibit intelligent behavior equivalent to, or indistinguishable from, that of a human which was a step ahead. Since then, there was no stop in digitalization progress including the release of the first smartphone, the IBM Simon (1964), Amazon was founded in 1995, becoming one of the first online retailers, Google launched in 1998, which became a dominant search engine over the period of time. Furthermore, Artificial intelligence becomes a hot topic, with the release of advanced AI systems such as Google’s AlphaGo (1998). The COVID-19 pandemic led to an acceleration in the adoption of digital technologies including remote work and online learning (see timeline below in Figure 1).7
Figure 1.
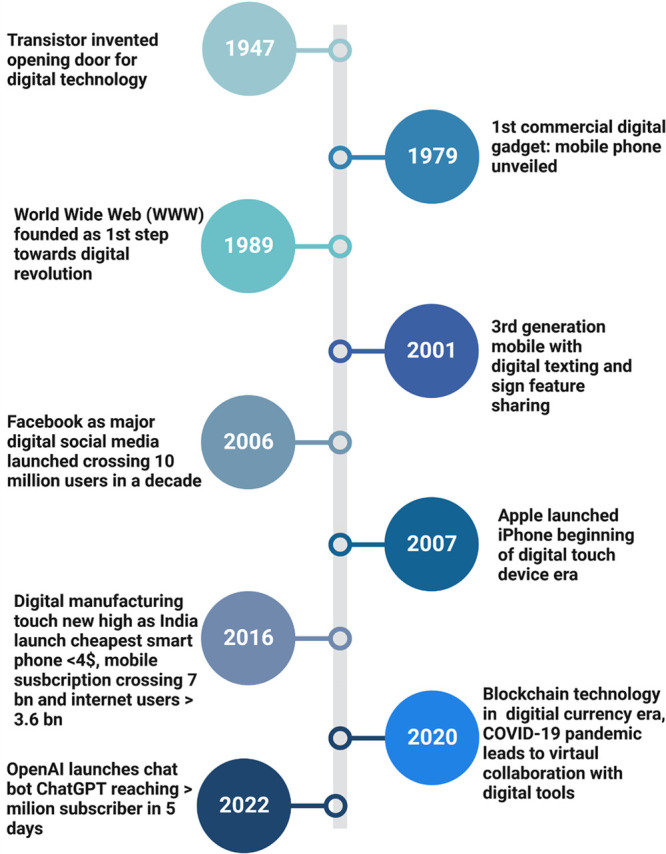
Timeline of digitalization showing invention of transistors in 1947, commercialization of mobile phones in 1979, founding worldwide web in 1989, the advent of 3rd generation mobiles with digital texting and sign feature in 2001, Facebook and Apple iPhone launch in 2006 and 2007, respectively, India launches the cheapest smartphone in 2016, and spread of digitalization in 2020 due to pandemic led virtual collaborative era. The latest revolution ChatGPT plays a crucial role in digitalization by providing human-like language capabilities to machines, enabling them to understand and communicate with users in natural language, thus facilitating the automation of various tasks and interactions.
Making Food Safety Decisions Using Digital Technologies
Digital technologies can play a significant role in supporting food safety decision-making by providing access to information and resources that can help identify and mitigate potential food safety risks. Some examples of digital technologies that can be used for food safety decision support include the use of food safety management systems.8 These are computer-based systems that can help organizations track and manage food safety processes, such as hazard analysis and critical control points (HACCP), and document and monitor food safety practices. Furthermore, food traceability systems are used to track food products from farm to fork, helping to identify the source of any potential food safety issues by using technology such as barcodes and radio frequency identification (RFID) tags.9
Food safety plan builder is a food safety database by the Food and Drug Administration (FDA)’s Food Safety Modernization Act (FSMA), which can provide access to information and resources to help organizations develop and implement food safety plans. Additionally, food safety apps can provide information on food safety best practices, safe food handling and storage, and food recalls.10
Digital technologies can play a significant role in supporting food safety decision-making. These technologies assists in monitoring, track, and trace food products throughout the supply chain, as well as to detect and predict food safety risks.11 For example, the utilization of sensors to monitor temperature, humidity, and other environmental factors that can impact food safety. These sensors can be installed in storage and transportation facilities, as well as in food processing plants, to provide real-time data on the conditions under which food is being handled. This data can identify potential food safety risks, such as the risk of bacterial growth due to high humidity levels, and to take corrective action to mitigate these risks.
Conclusively, the use of digital technologies can help to improve the accuracy and efficiency of food safety decision-making and can help in reducing the risk of foodborne illness.
Other digital technologies for food safety decision support include blockchain technology ed to trace the origin and movement of food products throughout the supply chain, helping to identify potential food safety risks and to track the effectiveness of food safety controls.12 Moving further, AI and machine learning (ML) technologies help to analyze large amounts of data, such as data from sensor networks or from food safety inspections, to identify patterns and trends that may indicate food safety risks. Additionally, mobile apps provide food safety information about food recalls or foodborne illnesses and to allow consumers to report food safety issues.
Digital FoodHub: Digitalization of Microbial Risk Assessment and Quality Parameters for Authenticating Food
Digitalization of microbial food safety risk assessment and quality parameters can help to improve the accuracy and efficiency of food authenticity certification.13 One way this can be achieved is through the use of FoodHub, which is a digital platform that connects food producers, food safety experts, and regulators to facilitate the exchange of information about food safety and quality. It assist to collect and analyze data about food products including data about microbial food safety risks and quality parameters such as pH, moisture content, and nutrient levels.14,15 This data can be used to assess the authenticity and safety of food products, and to identify potential food safety risks or quality issues. It can also be used to track the movement of food products throughout the supply chain, using technologies such as blockchain and RFID (radio frequency identification) to provide a secure and traceable record of food product movements.14 Furthermore, it ensures that food products are accurately labeled and that they meet relevant food safety and quality standards.
To sum it up, the digitalization of microbial food safety risk assessment and quality parameters through the use of platforms like FoodHub can help to improve the accuracy and efficiency of food authenticity certification, ultimately ensuring the safety and quality of the food supply (Figure 2).
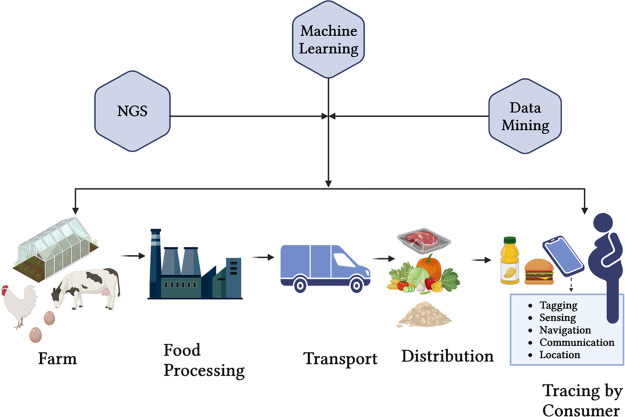
Figure 2.
Digitalization of food and feed classification for tracing purposes where integration of next-generation sequencing (NGS), machine learning, and data mining can provide traceability to consumers from farm to table. The food products have a tag describing their origin, contents, manufacturing details, etc. which could enhance transparency and tracing by consumer in food safety.
Traceability of Foods and Feeds through Digital Technology Ensures Transparency and Safety of Food Supply Chain
Digitalization of food and feed classification for tracing purposes, or FoodClass, is a digital platform that uses advanced analytical tools and techniques to classify food and feed products for tracing purposes. It is designed to provide food safety professionals with accurate and reliable data about the characteristics of food and feed products, including information about their composition, origin, quality, and processing history.
It is designed to help food safety professionals identify and classify food and feed products. This information can be used to trace the movement of food and feed products throughout the supply chain, helping to identify potential food safety risks and to track the effectiveness of food safety controls.16 It recruits a combination of advanced analytical techniques, such as next-generation sequencing, machine learning, and data mining, to analyze fruits, vegetables, and feed samples and identify their characteristics.17 It can also be integrated with other digital systems and technologies, such as blockchain, to provide a complete and transparent record of the movement and characteristics of food and feed products.18 Furthermore, a combination of advanced analytical techniques, such as machine learning, data mining, and chemical analysis, to analyze food and feed samples and classify them into specific categories is used.19 This can help to improve the transparency in food and feed tracing efforts as well as to identify potential food safety risks or quality issues.
One of the main benefits of FoodClass is its ability to provide rapid and accurate results, which can help to reduce the time and cost associated with traditional food and feed classification methods. It can also help to identify potential food safety risks and quality issues earlier in the supply chain, allowing for more timely corrective action to be taken. Therefore, FoodClass is an innovative digital platform that has the potential to improve the efficiency and effectiveness of food safety efforts, helping to protect consumers and ensure the safety of the food supply.
Digital Platform for Global Food Integrity and Traceability Promotes Safer Food in Mediterranean Supply Chains
The Digital Platform for Food Integrity and Traceability of relevant Mediterranean Supply Chains, or MEDIFIT, uses advanced analytical tools and techniques to support food traceability and integrity in Mediterranean supply chains.20 It is designed to provide food safety professionals with real-time data and analysis to help them trace the movement of food products throughout the supply chain and identify potential food safety risks or quality issues. It uses a combination of advanced analytical techniques, such as blockchain technology, machine learning, and data mining, to analyze food samples and trace their origin and movement through the supply chain. For example, newly developed unsupervised/semisupervised machine learning models can predict bioactivities of hazardous chemicals in human biological targets, which is a cost-effective and time-saving alternative to in vivo and in vitro biological experiments.21 The use of advanced data analysis approaches, using ML and AI, can revolutionize data analysis and modeling in the field of food technology, similar to their potential in the field of environmental science and engineering.22
The merit of MEDIFIT is its ability to provide rapid and precise traceability information, which can help to improve the proficiency and effectiveness of food safety efforts. It can also help to identify potential food safety risks and quality issues earlier in the supply chain, allowing for more timely corrective action to be taken. To conclude, MEDIFIT is an innovative digital platform that has the potential to revolutionize the way food traceability and integrity are managed in Mediterranean supply chains, helping to improve the safety and quality of the food supply.
Digital Tools to Enhance Safety in the Food Supply Chain
There are a number of digital to improve Food preservation in the supply chain. These tools help to monitor, track, and trace food products as well as to detect and predict food safety risks. Some examples of digital tools for more protection in the food chain include sensors to monitor temperature, humidity, and other environmental factors that can impact hygiene, blockchain technology supports to trace the origin and movement of food products throughout the supply chain, helping to identify potential food sanitation risks and to track the effectiveness of food security controls.23 Even artificial intelligence and machine learning technologies helps to analyze large amounts of data, such as data from sensor networks or from food security inspections, to identify patterns and trends that may indicate food safety risks.24 Additionally, mobile apps help to provide secure information to consumers, such as information about food recalls or foodborne illness outbreaks, and to allow consumers to report food safety issues.25
Therefore, the use of digital tools can help to improve the productivity and effectiveness of food safety efforts, ultimately helping to protect consumers and ensure the safety of the food supply.
Integration of Digital Technology into Smoking Related Health Hazard Assessment
Digital technologies can play a significant role in supporting toxicology research related to smoking. These technologies can be used to monitor the effects of smoking on human health as well as to identify the specific toxicants present in tobacco smoke and their potential health impacts. One example of digital technology used for smoking toxicology is the use of sensors to monitor the levels of various toxicants in the air, such as tobacco smoke.26,27 These sensors can be installed in homes, workplaces, and other public spaces to provide real-time data on the levels of toxicants present in the air.28,29 This data can identify potential health risks associated with smoking and to take corrective action to mitigate these related inhalation risks.27
Other digital technologies that are used for smoking toxicology include mobile apps which help to provide information about the health risks associated with smoking and to help individuals track their smoking habits. Artificial intelligence and machine learning technologies can be used to analyze large amounts of data, such as data from toxicology studies of published work or from sensor networks, to identify patterns and trends that may indicate health risks associated with smoking (Figure 3). Electronic cigarettes are the devices used in digital technology to deliver nicotine and other substances to the user without the need for combustion, potentially reducing the toxicants present in the smoke. There are a number of digital technologies that are to support smoking toxicology research and analysis. These technologies can be used to collect, analyze, and interpret data on the toxicological effects of smoking, including the potential risks to human health using mobile apps and self-intervention as shown in Figure 3.
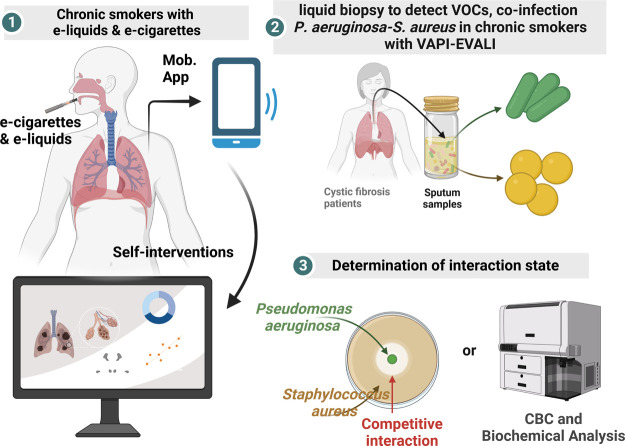
Figure 3.
Digitalization toward smoking toxicology. Electronic cigarettes (e-cigarettes) and e-liquids produce carcinogenic aerosols by heating a certain liquid that contains nicotine, are used as an alternative to regular cigarettes, which can be self-monitored via adds-on devices with mobile apps, which can provide deeper insight into ill-effect via communicating computational databases (1). The heated nicotine products cause Vaping-associated pulmonary injury (VAPI) or vaping product use-associated lung injury (EVALI) and may destroy beneficial microflora causing opportunistic infection (2). Digital toxicology further enables laboratory diagnosis and radiological interventions in clinics upon confirmation of toxicological symptoms (3). Created with BioRender.com.
Another example of digital technology used in smoking toxicology is electronic cigarettes (e-cigarettes). E-cigarettes are battery-powered devices that deliver nicotine, flavorings, and other chemicals in the form of an aerosol, which is inhaled by the user.30 They are often marketed as a safer alternative to traditional cigarettes, but there is ongoing debate and research into their potential toxicological effects. These devices are called electronic nicotine delivery systems (ENDS) and they deliver nicotine through an inhalable vapor, similar to e-cigarettes. They include products such as vape pens, e-hookahs, and e-cigars.31
For this reason, mobile apps can be used to track and monitor smoking behavior as well as to provide information and support to individuals attempting to quit smoking, whereas wearable devices can also be used to monitor smoking behavior and exposure to smoke as well as to track other behaviors and factors that may impact smoking-related health risks.32 Overall, the use of digital technologies for smoking toxicology can help to improve our understanding of the health risks associated with smoking and to identify effective strategies for reducing these risks.
Revolutionizing Chemical Toxicology: The Power of Digital Technology
There are a number of digital technologies to support chemical toxicology research and analysis. These technologies are used to collect, analyze, and interpret data on the toxicological effects of chemicals, including the potential risks to human health. One such example of a digital technology used in chemical toxicology is in vitro testing, which refers to the use of cell cultures or other lab-based models to study the toxicological effects of chemicals.33 In vitro testing is used to screen large numbers of chemicals for potential toxic effects, and can be more efficient and cost-effective than traditional animal testing methods.34 Furthermore, computational toxicology can be used to identify potential toxicants and to assess the potential risks to human health and can be described as the use of computer-based modeling and simulation techniques to predict the toxicological effects of chemicals.35 Mobile apps can also be used to track and monitor exposure to chemicals, as well as to provide information and support to individuals who may be at risk of chemical toxicity. Electronic databases and repositories aid to store, organize, and access data on the toxicological effects of chemicals, including data from toxicology studies, risk assessments, and regulatory decisions. Wearable tracking devices can also be used to monitor exposure to chemicals as well as to track other behaviors and factors that may impact chemical-related health risks.36
Therefore, the use of digital technologies in chemical toxicology can help to improve our understanding of the toxicological effects of chemicals and the potential risks to human health.
Exploring the Use of Mobile Applications in Advancements of Toxicology Research
There are a number of hand-held devices and mobile apps that support toxicology research. These apps can be used to collect, analyze, and interpret data on the toxicological effects of chemicals, including the potential risks to human health (Figure 4). One example of a mobile app used in toxicology research is an app that tracks and monitors exposure to chemicals. These apps aid in collecting data on the types and levels of chemicals an individual is exposed to, as well as to provide information on the potential health risks associated with these chemicals.37
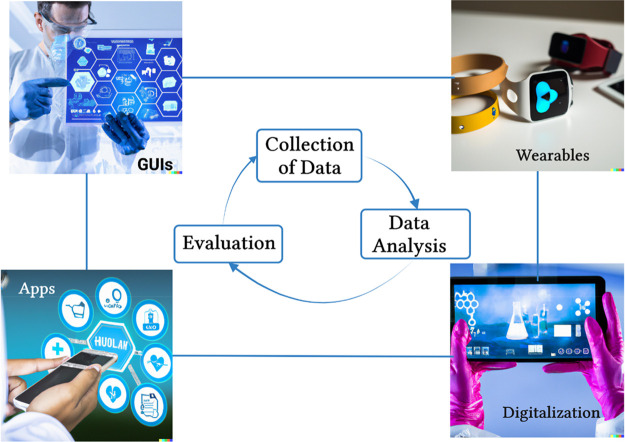
Figure 4.
Digitalization in toxicology using hand-held devices, mobile apps, and wearable devices. Wearable devices monitor and track fitness, blood pressure, and blood sugar levels. Wearable biosensors are wearable devices that allow patients to collect data on their movement, heart rate, respiratory rate, and temperature and can reduce and prevent chances of cardiac and respiratory arrest, while hand-held devices can keep records of patients and their prescriptions, demonstrate help to patients by a personal digital assistant (PDA), keep track of patients’ health, and eliminate delay or error on patients’ end. Certain mobile apps can also help in tracking fitness, setting reminders for medications, offering self-diagnosis solutions, checking in on the mental health of the patients, and much more. These digital tools collect, analyze, and evaluate the data acquired to monitor toxicological effects.
This data is used to better understand the toxicological effects of substances and to identify potential health risks.38 The mobile apps that provide information and resources on toxicology can also provide access to toxicology data, i.e., data on the toxicological effects of chemicals, as well as information on the safe use and handling of chemicals.39 Furthermore, apps that support in vitro testing can be used to collect and analyze data from in vitro toxicology studies, which prepares cell cultures or other biological systems to test the potentially toxic effects of chemicals.40 Risk assessment apps aid in evaluating the risks to human health from chemical exposure, covering both acute and chronic hazards.41
Therefore, mobile apps can be useful tools for toxicology research, helping to improve our understanding of the toxicological effects of chemicals and the potential risks to human health. A list of freely available mobile apps is given in Table 1.
Table 1. Mobile Apps for Medicine and Toxicology, Which Can Be Freely Downloaded from the App Store (App Store for iOS, Google Play Store for Android).
| Apps | Main Features | URL | OS |
|---|---|---|---|
| MedCalc | Aids to calculate Body mass index (BMI), glucose, creatinine clearance | http://apps.mdcalc.com/ | iOS, Android, Webbrowser |
| UpToDate | Clinical decision support tool for physicians for vidence-based medical information, drug information, and treatment guidelines | https://guides.upstate.edu/ | iOS, Android, Webbrowser |
| Drugs.com | Drug information, interaction checker, pill identifier; Personalized information, FDA alerts, drug news, and condition information | https://www.drugs.com/apps/ | iOS, Android |
| Clinical Tox | Drug and chemical information, antidotes, and treatment suggestions with omprehensive toxicology information | https://www.clintox.org/ | iOS, Android |
| Poison Control | Emergency poison exposure information and treatment guidance | https://www.poison.org/ | iOS, Android, Webbrowser |
| TOXBASE | Clinical cases and management suggestions on poisonous compounds | https://www.toxbase.org/TOXBASE-app-for-iOS-and-Android/ | iOS, Android |
| First Aid | Step-by-step guidance on handling emergency situations with first aid information and guidance | https://www.redcross.org/ | iOS, Android, Webbrowser |
| WebMD | Drug information, interaction checker, pill identifier, Disease and condition information, healthy living and wellness articles and tools, physician directory | https://www.webmd.com/ | iOS, Android |
| Epocrates | Medical calculators, drug–drug interaction, health insurance formulary information, disease and condition information | https://www.epocrates.com/ | iOS, Android, Webbrowser |
| Medscape | CME courses and resources, disease and condition information; Drug information, interaction checker, pill identifier | https://www.medscape.org/ | iOS, Android |
Design and Implementation of Digital Interface for Computational Toxicology
Computational toxicology is the use of computer models and simulations to predict the toxicological effects of chemicals. These models can be used to screen chemicals for potential toxic effects and to prioritize chemicals for further testing. A digital interface for computational toxicology is a software tool that allows users to access and interact with computational toxicology models and simulations.42 These interfaces are used as data input on the chemical of interest, such as its structure and properties, and to run simulations to predict its toxicological effects.43 It can be web-based, allowing users to access the models and simulations from any device with an Internet connection, or it can be installed locally on a computer or server. Some features that may be included in a digital interface for computational toxicology include:44
A user-friendly interface (GUI) that allows users to easily input data and run simulations.
A database of chemical structures and properties that assists as input for the models.
Visualization tools that allow users to view and interpret the results of the simulations.45
Integration with other toxicology databases and resources such as electronic databases of toxicology data and regulatory lists of chemicals.46
Therefore, digital interfaces for computational toxicology can provide a convenient and efficient way for users to access and utilize computational toxicology models and simulations.
Electronic ‘OMICS’ Databases and Repositories for Digital Toxicology
Electronic databases and repositories are digital tools that store, organize, and access data on the toxicological effects of chemicals. These databases and repositories can be used to support toxicology research and risk assessment, as well as to provide information and resources to the public on the toxicology of chemicals.47 Some examples of electronic databases and repositories that may be used in digital toxicology include toxicity data repositories which are databases containing data on the toxicological effects of chemicals, including data from toxicology studies, risk assessments, and regulatory decisions. These repositories may be organized by chemicals, by an end point (e.g., cancer, reproductive toxicity), or by other criteria.48
Chemical structure databases contain information on the chemical structures and properties of chemicals, including information on their physical and chemical characteristics, as well as their toxicological effects.49 Again, mobile apps help to provide information and resources on toxicology to the public including information on the safe use and handling of chemicals. The other examples of electronic databases and repositories that may be used in digital toxicology could be chemical hazard and toxicity OMICS databases containing information on the toxicological effects of chemicals. OMICS databases are a collection of databases that store information related to various omics fields such as genomics, transcriptomic, proteomics, and metabolomics in relation with actionable knowledge gathered from effect of chemical on individuals (Figure 5). Such repositories may further include data on the potential acute and chronic health effects of chemicals, as well as data on the safe use and handling of chemicals, environmental exposure databases containing information on the levels of chemicals present in the environment, including data on the levels of chemicals in the air, water, soil, and food.50 Even toxicology literature databases contain information on toxicology research and studies, including abstracts and full-text articles on toxicology topics.
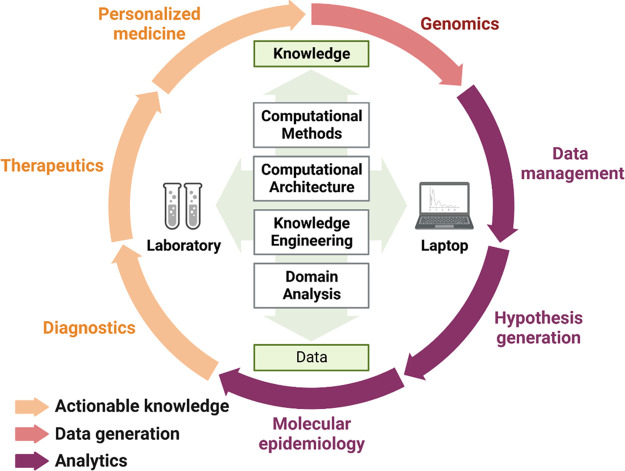
Figure 5.
Digital toxicology in handling patient ‘OMICS’ data and storage to monitor the health and detect toxińs response. Digital OMICS to provide actionable knowledge across molecular medicine, diagnostics, and epidemiology. Hypothesis-driven data generation knowledge engineering from personalized medicine will enable digitalization interfacing Environmental and industrial toxicology; clinical and regulatory toxicology; Forensic molecular toxicology (Created with BioRender.com).
Investigating the Potential of Wearable Devices in Digital Toxicology: Advancements in Real-Time Biomonitoring and Personalized Exposure Assessment
Wearable devices are electronic devices that can be worn on the body and used to monitor and track various health and environmental factors.51 These devices can be used to support digital toxicology by collecting data on chemical exposure and other toxicology-related factors.52 Wearable air monitoring devices can continuously measure airborne chemicals including pollutants and VOCs.53,54 Even wearable chemical exposure monitoring devices can be worn to measure the levels of chemicals present in the environment as well as the levels of chemicals present in the body.36 Wearable toxicology devices measure physiological responses to chemical exposure including heart rate, respiratory rate, and body temperature.
There is no doubt that potential exists for digital technologies to transform risk assessment in toxicology by enhancing communication and efficiency. However, it is essential to consider the ethical implications associated with the use of these technologies, particularly regarding wearable devices and the collection of personal data.55 Wearable sensors are increasingly being used to collect data on an individual’s exposure to various environmental toxins. This technology has the potential to improve the accuracy and timeliness of risk assessment by providing real-time data on an individual’s exposure. However, the use of wearable sensors raises concerns about privacy and consent.56 Individuals must be fully informed about the collection, use, and storage of their personal data and be given the option to opt-out. Additionally, the use of digital technologies in toxicology also raises concerns about data security. The sensitive nature of toxicological data means that it is crucial to ensure that the data is protected against unauthorized access or use. Appropriate safeguards must be put in place to protect the confidentiality and privacy of individuals’ personal data.57 While the potential benefits of using digital technologies in toxicology are significant, it is crucial to consider the ethical implications associated with their use. The collection and use of personal data must be done in a transparent and ethical manner, with appropriate safeguards in place to protect individuals’ privacy and confidentiality. Future research in this area should prioritize the development of ethical guidelines and best practices for the use of digital technologies in toxicology.
Blockchain in Digital Toxicology and Medicine: Improving Data Security, Traceability, and Transparency
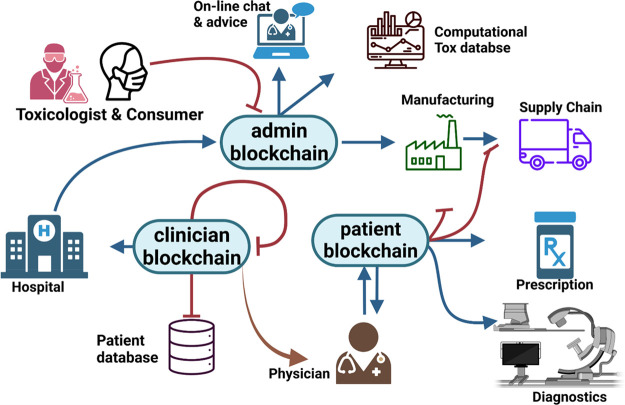
The use of blockchain technology has the potential to significantly enhance the safety, security, and transparency of digital medical practices, revolutionizing the field of healthcare for the better. As shown in Figure 6, Blockchain is a distributed database that allows for tamper-proof record-keeping of data collected from physicians for personalized medicine based on toxicity assessment.58 This makes it well-suited for use in the healthcare industry, where the security and privacy of patient data are of the utmost importance. Its potential applications in digital medicine are electronic health records (EHRs) used to securely store and manage electronic health records, allowing for easier and more secure access to patient data to clinicians and toxicologist.59 Moreover, blockchain could be used to track and verify the results of clinical trials, ensuring that the data is accurate and cannot be tampered.60 It could also be used in supply chain management to monitor the origin and movement of medical supplies and drugs, helping to ensure the safety and effectiveness of these products and to store and manage data related to personalized medicine, such as genetic information and treatment histories, allowing for more tailored and effective treatment.61 Therefore, the use of blockchain in digital medicine has the potential to improve the security and efficiency of healthcare systems, as well as to better protect patient privacy.
Figure 6.
Blockchain technology in toxicology can aid in decentralized data keeping and evaluate data that and potential risks to consumers with exposure risks through available chemical databases. MSDS (Material Safety Datasheet) contains information about potential hazard-causing agents and how to work with them. Blockchain also involves traceability in the distribution network and the use of product barcodes in manufacturing. Collection, analysis, and evaluation of the data in lab testing and pharmacies are possible due to blockchain (Created with BioRender.com).
Blockchain application in digital technologies have potential to revolutionize the field of toxicology by improving unbiased communication and efficiency in risk assessment.62 By using digital tools, toxicologists can streamline the process of collecting and analyzing data, collaborate more effectively with colleagues and stakeholders, and provide more accurate and timely assessments of the risks posed by chemicals and other substances. One example of the practical application of blockchain technologies in toxicology is the use of predictive modeling to assess the toxicity of chemicals.63 Predictive models can be developed using large data sets and machine learning algorithms to predict the toxicity of chemicals based on their chemical structure and other properties. This approach can significantly reduce the time and cost associated with traditional toxicity testing methods, while also providing more accurate predictions. Another example of the practical benefits of digital technologies in toxicology is the use of online databases to share and access information. Online databases can be used to store and share data on chemical properties, toxicity studies, and other relevant information in dynamic supply chain.64 This approach can improve communication and collaboration between toxicologists and other stakeholders, such as regulators and industry, and facilitate the development of more effective risk assessments. In addition, blockchain technologies can also improve the efficiency of risk assessments by automating certain tasks and reducing the need for manual data entry and analysis. For example, automated data processing tools can be used to extract relevant information from large data sets, reducing the time and effort required for data analysis.65
Blockchain in Genomic Medicine and Genomic Toxicology
Genomics is the study of the structure, function, and evolution of an organism’s genome, or its complete set of genetic material. The genomic sector deals with products and services in genomics, such as DNA sequencing, genetic testing, and personalized medicine.66,67 Blockchain technology has the potential to transform the genomic market by providing a secure and decentralized platform for storing and managing genomic data.68
Blockchain has potential applications in the genomic market, such as secure storage and management of genetic test data for improved accuracy and personalized genetic testing services; and storage and management of personalized medicine data in genomic medicine as shown in Figure 7. Such efforts with genomic data or medical device data, will enable more personalized and effective treatment for patients.70 Furthermore, blockchain supports to securely store and manage data from genomics research, enabling researchers to more easily share data and collaborate on projects. Overall, the use of blockchain in the genomic market has the potential to improve the accuracy, security, and efficiency of genomics-based products and services, ultimately leading to better patient outcomes.
Figure 7.
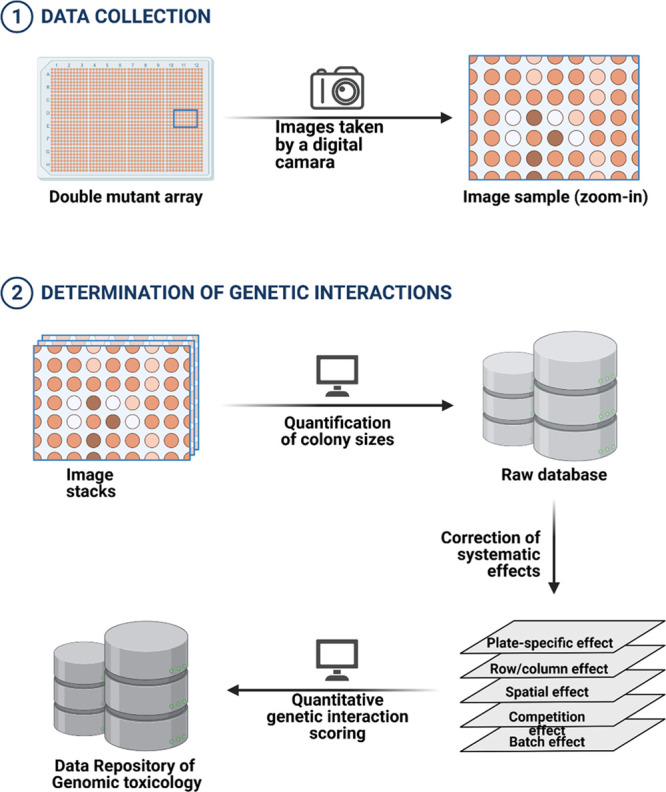
Blockchain in genomic toxicology. Digital gene interactions by Synthetic Gene array and storage of quantitative genetic interaction scores in a blockchain data repository. The query genes are extracted from toxin-led mutants to determine genetic interaction with the ones from the original genome. The images of an array are taken by a digital camera and these image stacks are quantified and a raw database is created.69 The correction in this raw data with respect to different effects leads to genetic interaction scores which are stored in blockchain data repositories of genomic toxicology (Created with BioRender.com).
Blockchain Technology in a GUI Format to Consumer
Blockchain allows for the creation of secure and decentralized networks using a distributed ledger to store data in a way that is transparent, immutable, and secure, making it well-suited for a variety of applications in the digital world, for example, for safe and encrypted data sharing between patients and clinicians.71 It enables the creation of decentralized and secure digital information which is useful for hospital transactions and store value.72 Blockchain can also be used to track the movement of goods through the supply chain, helping to ensure the authenticity and quality of products.73 For identity management, blockchain serves to securely store and manage digital identities, enabling individuals and organizations to prove their identity online.74 For smart contracts, blockchain can be used to facilitate the creation of smart contracts, which are self-executing contracts with the terms of the agreement between buyer and seller being directly written into lines of code.75
Utilizing Digital Technology for Risk Assessment and Hazard Prediction in Environmental and Occupational Health
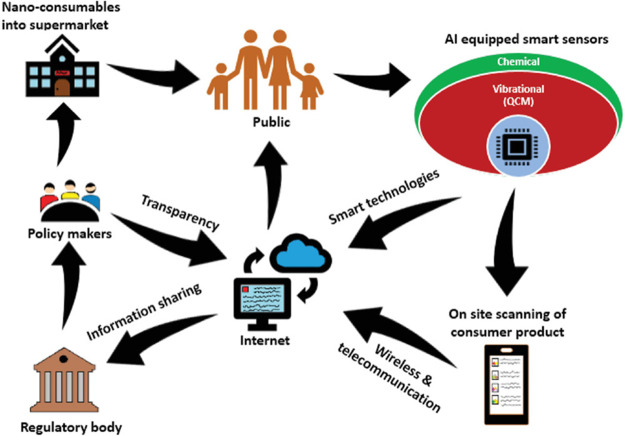
Digital technology can support risk assessment and hazard prediction by providing tools and resources for collecting, analyzing, and interpreting data on the potential risks to human health and the environment. Some examples of digital technologies for risk assessment and hazard prediction include computational toxicology, involving the use of computer models and simulations to predict the toxicological effects of chemicals and to evaluate the potential risks to human health from exposure to these chemicals.76 Even machine learning algorithms can be used to analyze large amounts of data, such as chemical structure, exposure levels, and toxicological effects, to identify patterns and trends that may indicate potential hazards or risks.77 Electronic databases and repositories are used to store, organize, and access data on the toxicological effects of chemicals, as well as data on exposure levels and risk factors, to support risk assessment and hazard prediction efforts.78 Conclusively, the use of digital technologies in risk assessment and hazard prediction can help to improve our understanding of the potential risks to human health and the environment to identify, and prioritize hazards for further evaluation or risk management (Figure 8).
Figure 8.
An envisioned future digital platform that would be designed to monitor and track the presence of nanomaterials in consumer products, and to take regulatory measures to ensure that these products are safe for human use. This type of platform would likely require the integration of several different submodules, including ones for pollutant generation and screening, data production and sharing, and communication with regulatory agencies and law enforcement. It would also likely involve the use of advanced computational technologies to analyze and interpret the data collected and to identify potential risks or hazards associated with the use of certain products. Reproduced with permission from ref (3). Copyright 2018, Wiley.
Challenges and Limitations Associated with Implementing Digital Technologies in Toxicology
One of the significant challenges of digital transformation in toxicology is data privacy and security.79 As the amount of data generated by toxicological studies increases, it becomes crucial to ensure that sensitive information is protected from unauthorized access or breaches. Moreover, data protection regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) mandate that organizations handling sensitive data must adhere to strict data privacy and security standards.79 In this regard, it is vital to develop robust cybersecurity measures that can mitigate risks associated with data breaches and ensure that confidential data is secure. Another challenge that arises from digital transformation is algorithmic bias. Algorithmic bias is the systematic error that occurs in machine learning algorithms due to biased data or flawed design. Biased algorithms can lead to unfair or discriminatory outcomes, especially in toxicology where risk assessments are made based on the results of machine learning models.80 Hence, it is crucial to recognize and address algorithmic bias in toxicology to ensure fair and unbiased risk assessments. In addition to these challenges, the implementation of digital technologies in toxicology also requires adequate infrastructure. The infrastructure needed to support digital transformation in toxicology includes hardware, software, and data management systems. Inadequate infrastructure can hinder the adoption and implementation of digital technologies, thereby affecting the efficiency and accuracy of toxicological risk assessments.
Toxicologists, regulators, and industry stakeholders play critical roles in the implementation and integration of digital technologies in toxicology.81 Each stakeholder brings a unique perspective and expertise that is necessary for the successful adoption of digital technologies. Toxicologists provide valuable scientific knowledge and expertise in the development and validation of digital tools, while regulators ensure that digital technologies meet regulatory standards and requirements. Industry stakeholders can provide financial and technical support for the development and implementation of digital technologies.3 Financial constraints can limit the implementation of digital technologies in toxicology. These technologies require significant investments in hardware, software, and personnel training. Therefore, it is essential to develop cost-effective solutions that balance the costs and benefits of digital technologies.
Collaboration among these stakeholders is essential for the successful implementation and integration of digital technologies in toxicology. For example, regulators can work with toxicologists to develop guidelines and standards for the development and validation of digital tools, while industry stakeholders can provide financial and technical support for the development and implementation of digital technologies.82 Collaborative efforts can also help identify and address potential challenges and limitations associated with the implementation of digital technologies, such as data privacy and security, algorithmic bias, and the need for adequate infrastructure. Moreover, collaboration can facilitate communication and knowledge sharing among stakeholders, leading to improved decision-making and more effective risk assessments. Collaboration can also enhance transparency and accountability in the development and implementation of digital technologies, ensuring that the interests of all stakeholders are taken into account.83
The implementation of digital technologies in toxicology requires essential collaboration among interdisciplinary researchers and stakeholders since there are technical barriers such as the complexity of integrating different systems and platforms. Digital technologies rely on a range of hardware and software solutions, and compatibility issues can arise when integrating these technologies with existing systems. Furthermore, the lack of standardization in data formats and quality assurance processes can also pose challenges to integrating digital technologies. The development and adoption of digital tools require the involvement of various stakeholders, including researchers, regulators, industry players, and the public. Collaboration can ensure that digital technologies are developed, implemented, and utilized in a manner that is transparent, ethical, and socially responsible. End-users, such as toxicologists, must be involved in the development and testing of digital tools to ensure that they are practical, user-friendly, and meet their needs. Moreover, there are concrete examples and case studies are crucial in illustrating the practical applications and benefits of collaborating toxicologists, engineers, and epidemiologists supporting digital technologies in toxicology. For instance, a recent report from our group aimed to explore the transmission of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) through speech droplets emitted by infected individuals.29 The study utilized a vibrating mesh nebulizer as a human patient simulator to mimic the release of speech aerosol droplets containing CorNPs. The study found that speech generates nanoaerosols with droplets of <5 μm in diameter that can travel distances longer than 1 m after release, and these aerosol droplets are a major element in the current COVID-19 pandemic. The study also explored the role of residual water in aerosol droplet stability and the drying dynamics of aerosol droplets. Additionally, a candle experiment was designed to determine whether air pollution might influence the transmission and air stability of respiratory virus-like nanoparticles. The study concludes that there is a need for systematic studies that take into account speech-generated aerosol/droplet experimental validation and their aerodynamics/particle kinetics analysis in the context of COVID-19 transmission. The findings of the study could inform the development of effective measures to mitigate the transmission of SARS-CoV-2 through speech droplets and nanoaerosols.
Future Outlook: Robotics, Super Artificial Intelligence, Smart Food/Feedstock, and Beyond
Robotics is an increasingly important field in science research, as robots are being used in a variety of applications including exploration, sample collection, and experimentation.84 One area where robotics is likely to have a significant impact in the future is enabling the 3D printed organ to enter the mass market, a major boost for healthcare. Recent developments in sensors that can measure gases and volatile compounds, as well as particulate matter, have made it possible to scan consumer products like textiles, toys, and food for nanomaterials.85 These sensors, which use AI and machine learning, can be complemented with advanced nanobiosensors to track toxicology nanomaterials from production to disposal.86 By using a unique universal material ID and potentially a blockchain-based ledger system, it will be possible to easily access information about the safety and handling requirements of each enhanced material through a simple scan with a hand-held device. If the data collected from the scan is complex, it can be wirelessly sent to regulatory experts for interpretation and feedback. This technology could enable “cradle-to-grave” monitoring of nano and other advanced material-enabled products.81
Super artificial intelligence (SAI) could access a patient’s medical history and other relevant data to predict potential health issues and provide recommendations for practitioners in vital sign tracking, and monitoring.87 Health sensors to establish major organ networks in the body will assist the SAI algorithm to predict the toxicological effect of consumed products ranging from therapeutics, and fast food to inhaled volatile organic chemicals.88 This will help clinicians and healthcare workers to make smart decisions in context with the toxicological effect of chemicals and new materials used in emerging technologies such as 3D printing and Inhalable electronics liquids/cigarettes.54 Another emerging application of SAI food and agriculture is in the area of crop monitoring and prediction.89 AI systems could analyze data from sensors and other sources to monitor the growth and health of crops, predict yield and quality, and identify potential issues that may need to be addressed.90
AI could also be used to optimize irrigation and fertilization practices, helping to reduce pesticides/biocide and improve the efficiency of these processes.91 In the livestock industry, AI could be used to monitor animal health and predict potential issues, as well as optimize feeding and breeding practices adding better meat/milk production as a major need to fight the food crisis in the future.92 With growing digitalization, many areas will be realized which are not yet thought of by the human mind but could become a reality when we will begin practicing those cutting-edge digital technologies.
Acknowledgments
A.V.S. thanks BfR for the internal grant (Award No. SFP-1322-735).
Author Contributions
Conceptualization, A.V.S., G.B., and M.M.; writing—original draft preparation, G.B. and A.V.S.; writing—review and editing, P.D., A.T., K.R., S.P.S., P.L., and D.R.; Graphic Design, A.M.B., S.K., K.R., A.G., and M.M.; funding acquisition: A.V.S., J.B., and S.K. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Bloomberg J. Digitization, digitalization, and digital transformation: confuse them at your peril. Forbes 2018, 28, 2019. [Google Scholar]
- Iyamu I.; Xu A. X.; Gómez-Ramírez O.; Ablona A.; Chang H.-J.; Mckee G.; Gilbert M. Defining digital public health and the role of digitization, digitalization, and digital transformation: scoping review. JMIR public health and surveillance 2021, 7 (11), e30399 10.2196/30399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. V.; Ansari M. H. D.; Rosenkranz D.; Maharjan R. S.; Kriegel F. L.; Gandhi K.; Kanase A.; Singh R.; Laux P.; Luch A. Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Adv. Healthcare Mater. 2020, 9 (17), 1901862. 10.1002/adhm.201901862. [DOI] [PubMed] [Google Scholar]
- Shen Y.-T.; Chen L.; Yue W.-W.; Xu H.-X. Digital technology-based telemedicine for the COVID-19 pandemic. Frontiers in Medicine 2021, 8, 933. 10.3389/fmed.2021.646506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. Y.; Yang Y.; Balakrishnan S.; Abinahed J.; Al-Ansari A.; Warfa M.; Almokdad O.; Barah A.; Omer A.; Singh A. V.; et al. A lightweight neural network with multiscale feature enhancement for liver CT segmentation. Sci. Rep. 2022, 12 (1), 1–12. 10.1038/s41598-022-20472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. V.; Rosenkranz D.; Ansari M. H. D.; Singh R.; Kanase A.; Singh S. P.; Johnston B.; Tentschert J.; Laux P.; Luch A. Artificial Intelligence and Machine Learning Empower Advanced Biomedical Material Design to Toxicity Prediction. Advanced Intelligent Systems 2020, 2 (12), 2000084. 10.1002/aisy.202000084. [DOI] [Google Scholar]
- El-Sherif D. M.; Abouzid M.; Elzarif M. T.; Ahmed A. A.; Albakri A.; Alshehri M. M.. Telehealth and Artificial Intelligence insights into healthcare during the COVID-19 pandemic. In Healthcare; MDPI, 2022; Vol. 10, p 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C.; Murphy S.; Orsi R.; Wiedmann M. How Can AI Help Improve Food Safety?. Annual Review of Food Science and Technology 2023, 14, 517. 10.1146/annurev-food-060721-013815. [DOI] [PubMed] [Google Scholar]
- Kleter G.; McFarland S.; Bach A.; Bernabucci U.; Bikker P.; Busani L.; Kok E.; Kostov K.; Nadal A.; Pla M.; et al. Surveying selected European feed and livestock production chains for features enabling the case-specific post-market monitoring of livestock for intake and potential health impacts of animal feeds derived from genetically modified crops. Food Chem. Toxicol. 2018, 117, 66–78. 10.1016/j.fct.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Jin C.; Bouzembrak Y.; Zhou J.; Liang Q.; Van Den Bulk L. M.; Gavai A.; Liu N.; Van Den Heuvel L. J.; Hoenderdaal W.; Marvin H. J. Big Data in food safety-A review. Current Opinion in Food Science 2020, 36, 24–32. 10.1016/j.cofs.2020.11.006. [DOI] [Google Scholar]
- Jouanjean M.-A.Digital technologies will profoundly impact the way we grow and distribute food: here’s how; Springer, 2019; Vol. 14, pp 103–104. [Google Scholar]
- Yu Z.; Jung D.; Park S.; Hu Y.; Huang K.; Rasco B. A.; Wang S.; Ronholm J.; Lu X.; Chen J. Smart traceability for food safety. Critical Reviews in Food Science and Nutrition 2022, 62 (4), 905–916. 10.1080/10408398.2020.1830262. [DOI] [PubMed] [Google Scholar]
- Fritsche J. Recent Developments and Digital Perspectives in Food Safety and Authenticity. J. Agric. Food Chem. 2018, 66 (29), 7562–7567. 10.1021/acs.jafc.8b00843. [DOI] [PubMed] [Google Scholar]
- Yu W.; Huang S. Traceability of Food Safety Based on Block Chain and RFID Technology. 2018 11th International Symposium on Computational Intelligence and Design (ISCID) 2018, 01, 339–342. 10.1109/ISCID.2018.00083. [DOI] [Google Scholar]
- Gaspar P. D.; Alves J.; Pinto P. Simplified Approach to Predict Food Safety through the Maximum Specific Bacterial Growth Rate as Function of Extrinsic and Intrinsic Parameters. ChemEngineering 2021, 5 (2), 22. 10.3390/chemengineering5020022. [DOI] [Google Scholar]
- Zhao Y.; Yang Q. E.; Zhou X.; Wang F.-H.; Muurinen J.; Virta M. P.; Brandt K. K.; Zhu Y.-G. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Critical Reviews in Environmental Science and Technology 2021, 51 (19), 2159–2196. 10.1080/10643389.2020.1777815. [DOI] [Google Scholar]
- Hernández-Rubio J.; Morillas-Guerrero J. J.; Galdeano Gómez E.; Pérez Mesa J. C.; Aznar-Sánchez J. Á.; Fernández-Olmos M.; Malorgio G.. Fruit and vegetables supply chain organization in Spain: effects on quality and food safety. Food safety regulations, market access and international competition, SAFEMED; Seventh Framework Programme, 2016.
- Kamilaris A.; Fonts A.; Prenafeta-Boldu F. X. The rise of blockchain technology in agriculture and food supply chains. Trends in Food Science & Technology 2019, 91, 640–652. 10.1016/j.tifs.2019.07.034. [DOI] [Google Scholar]
- Wang X.; Bouzembrak Y.; Lansink A. O.; van der Fels-Klerx H. J. Application of machine learning to the monitoring and prediction of food safety: A review. Comprehensive Reviews in Food Science and Food Safety 2022, 21 (1), 416–434. 10.1111/1541-4337.12868. [DOI] [PubMed] [Google Scholar]
- Chen K.; WANG X.-x.; SONG H.-y. Food safety regulatory systems in Europe and China: A study of how co-regulation can improve regulatory effectiveness. Journal of Integrative Agriculture 2015, 14 (11), 2203–2217. 10.1016/S2095-3119(15)61113-3. [DOI] [Google Scholar]
- Kwon H.; Ali Z. A.; Wong B. M. Harnessing Semi-Supervised Machine Learning to Automatically Predict Bioactivities of Per- and Polyfluoroalkyl Substances (PFASs). Environmental Science & Technology Letters 2022, 10.1021/acs.estlett.2c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S.; Zhang K.; Bagheri M.; Burken J. G.; Gu A.; Li B.; Ma X.; Marrone B. L.; Ren Z. J.; Schrier J.; et al. Machine Learning: New Ideas and Tools in Environmental Science and Engineering. Environ. Sci. Technol. 2021, 55 (19), 12741–12754. 10.1021/acs.est.1c01339. [DOI] [PubMed] [Google Scholar]
- Hirata E.; Lambrou M.; Watanabe D. Blockchain technology in supply chain management: insights from machine learning algorithms. Maritime Business Review 2021, 6, 114. 10.1108/MABR-07-2020-0043. [DOI] [Google Scholar]
- Kudashkina K.; Corradini M. G.; Thirunathan P.; Yada R. Y.; Fraser E. D. G. Artificial Intelligence technology in food safety: A behavioral approach. Trends in Food Science & Technology 2022, 123, 376–381. 10.1016/j.tifs.2022.03.021. [DOI] [Google Scholar]
- Nichols K. T.; McConnell D. T. Food Safety for the Consumer. Journal of Consumer Health on the Internet 2012, 16 (1), 85–92. 10.1080/15398285.2012.647563. [DOI] [Google Scholar]
- Singh A. V.; Maharjan R. S.; Kromer C.; Laux P.; Luch A.; Vats T.; Chandrasekar V.; Dakua S. P.; Park B.-W. Advances in Smoking Related In Vitro Inhalation Toxicology: A Perspective Case of Challenges and Opportunities from Progresses in Lung-on-Chip Technologies. Chem. Res. Toxicol. 2021, 34 (9), 1984–2002. 10.1021/acs.chemrestox.1c00219. [DOI] [PubMed] [Google Scholar]
- Singh A. V.; Romeo A.; Scott K.; Wagener S.; Leibrock L.; Laux P.; Luch A.; Kerkar P.; Balakrishnan S.; Dakua S. P.; et al. Emerging Technologies for In Vitro Inhalation Toxicology. Adv. Healthcare Mater. 2021, 10 (18), 2100633. 10.1002/adhm.202100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janarthanan A.; Paramarthalingam A.; Arivunambi A.; Vincent P. M. D. R. Real-time indoor air quality monitoring using the Internet of Things. 2022 Third International Conference on Intelligent Computing Instrumentation and Control Technologies (ICICICT) 2022, 99–104. 10.1109/ICICICT54557.2022.9917990. [DOI] [Google Scholar]
- Singh A. V.; Katz A.; Maharjan R. S.; Gadicherla A. K.; Richter M. H.; Heyda J.; Del Pino P.; Laux P.; Luch A. Coronavirus-mimicking nanoparticles (CorNPs) in artificial saliva droplets and nanoaerosols: Influence of shape and environmental factors on particokinetics/particle aerodynamics. Science of The Total Environment 2023, 860, 160503. 10.1016/j.scitotenv.2022.160503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R.; Benowitz N.; Glantz S. A. E-Cigarettes. Circulation 2014, 129 (19), 1972–1986. 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser A. M.; Collins L.; Pearson J. L.; Abudayyeh H.; Niaura R. S.; Abrams D. B.; Villanti A. C. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. American Journal of Preventive Medicine 2017, 52 (2), e33-e66 10.1016/j.amepre.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi I.; Tsow F.; Tanwar K.; Zhang L.; Iglesias R. A.; Chen C.; Rai A.; Forzani E. S.; Tao N. Novel monitor paradigm for real-time exposure assessment. Journal of Exposure Science & Environmental Epidemiology 2011, 21 (4), 419–426. 10.1038/jes.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A. K.; Singh D.; Dubey K.; Maurya R.; Mittal S.; Pandey A. K.. Chapter 3 - Models and Methods for In Vitro Toxicity. In In Vitro Toxicology, Dhawan A., Kwon S., Eds.; Academic Press, 2018; pp 45–65. [Google Scholar]
- Bakand S.; Winder C.; Khalil C.; Hayes A. Toxicity Assessment of Industrial Chemicals and Airborne Contaminants: Transition from In Vivo to In Vitro Test Methods: A Review. Inhalation Toxicology 2005, 17 (13), 775–787. 10.1080/08958370500225240. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J.; Ankley G.; Blancato J.; Breen M.; Conolly R.; Dix D.; Houck K.; Hubal E.; Judson R.; Rabinowitz J.; et al. Computational Toxicology—A State of the Science Mini Review. Toxicol. Sci. 2008, 103 (1), 14–27. 10.1093/toxsci/kfm297. [DOI] [PubMed] [Google Scholar]
- Sempionatto J. R.; Jeerapan I.; Krishnan S.; Wang J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2020, 92 (1), 378–396. 10.1021/acs.analchem.9b04668. [DOI] [PubMed] [Google Scholar]
- Alyani N.; Madya M. M. Training on the application of appropriate chemical-based technology using the mobile training unit model for behavior change in remote village communities. AIP Conf. Proc. 2022, 2659 (1), 070007. 10.1063/5.0113809. [DOI] [Google Scholar]
- Vashist S. K.; Schneider E. M.; Luong J. H. T. Commercial Smartphone-Based Devices and Smart Applications for Personalized Healthcare Monitoring and Management. Diagnostics 2014, 4 (3), 104–128. 10.3390/diagnostics4030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasap S.; AlRashedi M.; AlMutairi N.; AlDallal F.; AlShaddad S.; Alshaddad M. Developing a Mobile Application for Safety Engineers for Hazard Analysis. 2019 7th International Conference on Future Internet of Things and Cloud Workshops (FiCloudW) 2019, 36–43. 10.1109/FiCloudW.2019.00020. [DOI] [Google Scholar]
- Clark A. M.; Dole K.; Coulon-Spektor A.; McNutt A.; Grass G.; Freundlich J. S.; Reynolds R. C.; Ekins S. Open Source Bayesian Models. 1. Application to ADME/Tox and Drug Discovery Datasets. J. Chem. Inf. Model. 2015, 55 (6), 1231–1245. 10.1021/acs.jcim.5b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y.; Ahn G.-J.; Zhao Z.; Hu H.. Riskmon: Continuous and automated risk assessment of mobile applications. In Proceedings of the 4th ACM Conference on Data and Application Security and Privacy, 2014; pp 99–110.
- Abedini J.; Cook B.; Bell S.; Chang X.; Choksi N.; Daniel A. B.; Hines D.; Karmaus A. L.; Mansouri K.; McAfee E.; et al. Application of new approach methodologies: ICE tools to support chemical evaluations. Computational Toxicology 2021, 20, 100184. 10.1016/j.comtox.2021.100184. [DOI] [Google Scholar]
- Katritzky A. R.; Kuanar M.; Slavov S.; Hall C. D.; Karelson M.; Kahn I.; Dobchev D. A. Quantitative Correlation of Physical and Chemical Properties with Chemical Structure: Utility for Prediction. Chem. Rev. 2010, 110 (10), 5714–5789. 10.1021/cr900238d. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Chou W.-C. Machine Learning and Artificial Intelligence in Toxicological Sciences. Toxicol. Sci. 2022, 189 (1), 7–19. 10.1093/toxsci/kfac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif D. M.; Sypa M.; Lock E. F.; Wright F. A.; Wilson A.; Cathey T.; Judson R. R.; Rusyn I. ToxPi GUI: an interactive visualization tool for transparent integration of data from diverse sources of evidence. Bioinformatics 2013, 29 (3), 402–403. 10.1093/bioinformatics/bts686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Li F.; Chen J.; Ji C.; Wu H. Integration of Computational Toxicology, Toxicogenomics Data Mining, and Omics Techniques to Unveil Toxicity Pathways. ACS Sustainable Chem. Eng. 2021, 9 (11), 4130–4138. 10.1021/acssuschemeng.0c09196. [DOI] [Google Scholar]
- Davis A. P.; Wiegers J.; Wiegers T. C.; Mattingly C. J. Public data sources to support systems toxicology applications. Current Opinion in Toxicology 2019, 16, 17–24. 10.1016/j.cotox.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia F.; Pillo G.; Worth A.; Corvi R.; Prieto P. Integration of data across toxicity endpoints for improved safety assessment of chemicals: the example of carcinogenicity assessment. Arch. Toxicol. 2021, 95 (6), 1971–1993. 10.1007/s00204-021-03035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chandrasekar V.; Singh A. V.; Maharjan R. S.; Dakua S. P.; Balakrishnan S.; Dash S.; Laux P.; Luch A.; Singh S.; Pradhan M. Perspectives on the Technological Aspects and Biomedical Applications of Virus-Like Particles/Nanoparticles in Reproductive Biology: Insights on the Medicinal and Toxicological Outlook. Advanced NanoBiomed Research 2022, 2 (8), 2200010. 10.1002/anbr.202200010. [DOI] [Google Scholar]
- Sushko I.; Salmina E.; Potemkin V. A.; Poda G.; Tetko I. V. ToxAlerts: A Web Server of Structural Alerts for Toxic Chemicals and Compounds with Potential Adverse Reactions. J. Chem. Inf. Model. 2012, 52 (8), 2310–2316. 10.1021/ci300245q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R.; Dix D. Computational Toxicology as Implemented by the U.S. EPA: Providing High Throughput Decision Support Tools for Screening and Assessing Chemical Exposure, Hazard and Risk. Journal of Toxicology and Environmental Health, Part B 2010, 13 (2–4), 197–217. 10.1080/10937404.2010.483935. [DOI] [PubMed] [Google Scholar]; Dionisio K. L.; Frame A. M.; Goldsmith M.-R.; Wambaugh J. F.; Liddell A.; Cathey T.; Smith D.; Vail J.; Ernstoff A. S.; Fantke P.; et al. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicology Reports 2015, 2, 228–237. 10.1016/j.toxrep.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amjadi M.; Kyung K.-U.; Park I.; Sitti M. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016, 26 (11), 1678–1698. 10.1002/adfm.201504755. [DOI] [Google Scholar]
- Chai P. R. Wearable Devices and Biosensing: Future Frontiers. Journal of Medical Toxicology 2016, 12 (4), 332–334. 10.1007/s13181-016-0569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Zheng H.; Xiang H.; Wang W.; Wu H.; Li Z.; Zhuang J.; Zhou H. Paper-Based Wearable Sensors for Humidity and VOC Detection. ACS Sustainable Chem. Eng. 2021, 9 (50), 16937–16945. 10.1021/acssuschemeng.1c05156. [DOI] [Google Scholar]
- Singh A. V.; Maharjan R. S.; Jungnickel H.; Romanowski H.; Hachenberger Y. U.; Reichardt P.; Bierkandt F.; Siewert K.; Gadicherla A.; Laux P.; et al. Evaluating Particle Emissions and Toxicity of 3D Pen Printed Filaments with Metal Nanoparticles As Additives: In Vitro and in Silico Discriminant Function Analysis. ACS Sustainable Chem. Eng. 2021, 9 (35), 11724–11737. 10.1021/acssuschemeng.1c02589. [DOI] [Google Scholar]
- Chang V.; Xu Q. A.; Hall K.; Wang Y. A.; Kamal M. M. Digitalization in omnichannel healthcare supply chain businesses: The role of smart wearable devices. Journal of Business Research 2023, 156, 113369. 10.1016/j.jbusres.2022.113369. [DOI] [Google Scholar]
- London A. J.; Razin Y. S.; Borenstein J.; Eslami M.; Perkins R.; Robinette P. Ethical Issues in Near-Future Socially Supportive Smart Assistants for Older Adults. IEEE Transactions on Technology and Society 2023, 1. 10.1109/TTS.2023.3237124. [DOI] [Google Scholar]
- Dhirani L. L.; Mukhtiar N.; Chowdhry B. S.; Newe T. Ethical Dilemmas and Privacy Issues in Emerging Technologies: A Review. Sensors 2023, 23 (3), 1151. 10.3390/s23031151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux V. L. Blockchain and Recordkeeping: Editorial. Computers 2021, 10 (11), 135. 10.3390/computers10110135. [DOI] [Google Scholar]
- Shahnaz A.; Qamar U.; Khalid A. Using Blockchain for Electronic Health Records. IEEE Access 2019, 7, 147782–147795. 10.1109/ACCESS.2019.2946373. [DOI] [Google Scholar]
- Omar I. A.; Jayaraman R.; Salah K.; Yaqoob I.; Ellahham S. Applications of Blockchain Technology in Clinical Trials: Review and Open Challenges. Arabian Journal for Science and Engineering 2021, 46 (4), 3001–3015. 10.1007/s13369-020-04989-3. [DOI] [Google Scholar]
- Panda S. K.; Satapathy S. C. Drug traceability and transparency in medical supply chain using blockchain for easing the process and creating trust between stakeholders and consumers. Personal and Ubiquitous Computing 2021, 10.1007/s00779-021-01588-3. [DOI] [Google Scholar]
- Anaam E.; Ghazal T. M.; Haw S. C.; Alzoubi H. M.; Alshurideh M. T.; Mamun A. A. Utilization of Blockchain Technology In Human Resource Management. 2023 IEEE 2nd International Conference on AI in Cybersecurity (ICAIC) 2023, 1–5. 10.1109/ICAIC57335.2023.10044181. [DOI] [Google Scholar]
- Gruchmann T.; Elgazzar S.; Ali A. H.. Blockchain technology in pharmaceutical supply chains: a transaction cost perspective. Modern Supply Chain Research and Applications 2023. 10.1108/MSCRA-10-2022-0023. [DOI] [Google Scholar]
- Tashmanov G. D.; Toshmanov A. D.. Blockchain Technology – Innovation for Better Collaboration and Increased Efficiency. The U.S. Logistics and Trucking Industry Case. Cham, 2023; Springer Nature Switzerland: pp 618–627. [Google Scholar]
- Suryavanshi A.; A G.; M. B. T N.; R M.; A. H N. The integration of Blockchain and AI for Web 3.0: A security Perspective. 2023 4th International Conference on Innovative Trends in Information Technology (ICITIIT) 2023, 1–8. 10.1109/ICITIIT57246.2023.10068672. [DOI] [Google Scholar]
- Parisi C.; Rodríguez-Cerezo E. Current and future market applications of new genomic techniques. Publications Office of the European Union: Luxembourg 2021, JRC123830. 10.2760/02472. [DOI] [Google Scholar]
- Gemmati D.; Zeri G.; Orioli E.; De Gaetano F. E.; Salvi F.; Bartolomei I.; D’Alfonso S.; Dall’Osso C.; Leone M. A.; Singh A. V.; et al. Polymorphisms in the genes coding for iron binding and transporting proteins are associated with disability, severity, and early progression in multiple sclerosis. BMC Medical Genetics 2012, 13 (1), 70. 10.1186/1471-2350-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. V. Blockchain Applications for Healthcare Data Management. Healthc Inform Res. 2019, 25 (1), 51–56. 10.4258/hir.2019.25.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. V.; Jungnickel H.; Leibrock L.; Tentschert J.; Reichardt P.; Katz A.; Laux P.; Luch A. ToF-SIMS 3D imaging unveils important insights on the cellular microenvironment during biomineralization of gold nanostructures. Sci. Rep. 2020, 10 (1), 261. 10.1038/s41598-019-57136-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghazwi M.; Turkmen F.; Van Der Velde J.; Karastoyanova D. Blockchain for genomics: a systematic literature review. Distributed Ledger Technologies: Research and Practice 2022, 1 (2), 1–28. 10.1145/3563044. [DOI] [Google Scholar]
- Soomro N.; Soomro S. Applications of Blockchain Technology beyond Cryptocurrency. Annals of Emerging Technologies in Computing (AETiC) 2018, 2 (1), 7–16. 10.33166/AETiC.2018.01.002. [DOI] [Google Scholar]
- Pilkington M.; Olleros F. X.; Zhegu M.. Research Handbook on Digital Transformations. In Chapter 11: Blockchain technology: principles and applications; Edward Elgar Publishing, 2016. 10.4337/9781784717766 [DOI] [Google Scholar]
- Zhang H.; Sakurai K.. Blockchain for IoT-Based Digital Supply Chain: A Survey. Cham, 2020; Springer International Publishing: pp 564–573. [Google Scholar]
- Gao Z.; Xu L.; Turner G.; Patel B.; Diallo N.; Chen L.; Shi W. Blockchain-based identity management with mobile device. Proceedings of the 1st Workshop on Cryptocurrencies and Blockchains for Distributed Systems 2018, 66–70. 10.1145/3211933.3211945. [DOI] [Google Scholar]
- Wang S.; Ouyang L.; Yuan Y.; Ni X.; Han X.; Wang F. Y. Blockchain-Enabled Smart Contracts: Architecture, Applications, and Future Trends. IEEE Transactions on Systems, Man, and Cybernetics: Systems 2019, 49 (11), 2266–2277. 10.1109/TSMC.2019.2895123. [DOI] [Google Scholar]
- Ekins S. Progress in computational toxicology. Journal of Pharmacological and Toxicological Methods 2014, 69 (2), 115–140. 10.1016/j.vascn.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Hemmerich J.; Ecker G. F. In silico toxicology: From structure–activity relationships towards deep learning and adverse outcome pathways. WIREs Computational Molecular Science 2020, 10 (4), e1475 10.1002/wcms.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Thiessen P. A.; Bolton E. E.; Chen J.; Fu G.; Gindulyte A.; Han L.; He J.; He S.; Shoemaker B. A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44 (D1), D1202–D1213. 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauneck A.; Schmalhorst L.; Kazemi Majdabadi M. M.; Bakhtiari M.; Völker U.; Baumbach J.; Baumbach L.; Buchholtz G. Federated Machine Learning, Privacy-Enhancing Technologies, and Data Protection Laws in Medical Research: Scoping Review. Journal of Medical Internet Research 2023, 25, e41588 10.2196/41588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano T. P.; Loureiro R. B.; Lisboa F. V.; Cruz G. O.; Peixoto R. M.; Guimarães G. A. d. S.; Oliveira E. L.; Winkler I.; Nascimento E. G. S. Context-Based Patterns in Machine Learning Bias and Fairness Metrics: A Sensitive Attributes-Based Approach. Big data and cognitive computing 2023, 7 (1), 27. 10.3390/bdcc7010027. [DOI] [Google Scholar]
- Singh A. V.; Varma M.; Laux P.; Choudhary S.; Datusalia A. K.; Gupta N.; Luch A.; Gandhi A.; Kulkarni P.; Nath B. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: a comprehensive review. Arch. Toxicol. 2023, 97, 963. 10.1007/s00204-023-03471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.; Bilyanska A.; Bradley M.; Dinsdale J.; Hutt L.; Backhaus T.; Boons F.; Bott D.; Collins C.; Cornell S. E.. et al. A Horizon Scan to support Chemical Pollution related policymaking for sustainable and climate resilient economies. Environ. Toxicol. Chem. 2023. 10.1002/etc.5620 [DOI] [PubMed] [Google Scholar]
- Thakkar S.; Slikker Jr W.; Yiannas F.; Silva P.; Blais B.; Chng K. R.; Liu Z.; Adholeya A.; Pappalardo F.; Soares M. d. L. C.; et al. Artificial intelligence and real-world data for drug and food safety–A regulatory science perspective. Regul. Toxicol. Pharmacol. 2023, 140, 105388. 10.1016/j.yrtph.2023.105388. [DOI] [PubMed] [Google Scholar]
- Vikram Singh A.; Laux P.; Luch A.; Balkrishnan S.; Prasad Dakua S.. Bottom-UP assembly of nanorobots: extending synthetic biology to complex material design. Front. Nanosci. Nanotechnol 2019, 5 ( (1), ). 10.15761/FNN.1000S2005 [DOI] [Google Scholar]
- Singh A.; Liu C.; Wan K.; Wang H.. Optical properties of flame-synthesized carbon nanoparticles. In 10th US National Combustion Meeting of the Eastern States Section of the Combustion Institute, 2017. [Google Scholar]; Singh A. V.; Sigloch H.; Laux P.; Luch A.; Wagener S.; Tentschert J. Micro/nanoplastics: an emerging environmental concern for the future decade. Front. Nanosci. Nanotechnol 2020, 6, 1–2. 10.15761/FNN.1000191. [DOI] [Google Scholar]
- Singh A. V.; Chandrasekar V.; Laux P.; Luch A.; Dakua S. P.; Zamboni P.; Shelar A.; Yang Y.; Pandit V.; Tisato V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11 (18), 2801. 10.3390/cells11182801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep Ganesh G.; Kolusu A. S.; Prasad K.; Samudrala P. K.; Nemmani K. V. S. Advancing health care via artificial intelligence: From concept to clinic. Eur. J. Pharmacol. 2022, 934, 175320. 10.1016/j.ejphar.2022.175320. [DOI] [PubMed] [Google Scholar]
- Carreiro S.; Chai P. R.; Carey J.; Chapman B.; Boyer E. W. Integrating Personalized Technology in Toxicology: Sensors, Smart Glass, and Social Media Applications in Toxicology Research. Journal of Medical Toxicology 2017, 13 (2), 166–172. 10.1007/s13181-017-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelar A.; Nile S. H.; Singh A. V.; Rothenstein D.; Bill J.; Xiao J.; Chaskar M.; Kai G.; Patil R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Letters 2023, 15 (1), 54. 10.1007/s40820-023-01025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid M.; Haleem A.; Khan I. H.; Suman R. Understanding the potential applications of Artificial Intelligence in Agriculture Sector. Advanced Agrochem 2023, 2, 15. 10.1016/j.aac.2022.10.001. [DOI] [Google Scholar]
- Talaviya T.; Shah D.; Patel N.; Yagnik H.; Shah M. Implementation of artificial intelligence in agriculture for optimization of irrigation and application of pesticides and herbicides. Artificial Intelligence in Agriculture 2020, 4, 58–73. 10.1016/j.aiia.2020.04.002. [DOI] [Google Scholar]
- Shelar A.; Singh A. V.; Dietrich P.; Maharjan R. S.; Thissen A.; Didwal P. N.; Shinde M.; Laux P.; Luch A.; Mathe V.; et al. Emerging cold plasma treatment and machine learning prospects for seed priming: a step towards sustainable food production. RSC Adv. 2022, 12 (17), 10467–10488. 10.1039/D2RA00809B. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shelar A.; Singh A. V.; Maharjan R. S.; Laux P.; Luch A.; Gemmati D.; Tisato V.; Singh S. P.; Santilli M. F.; Shelar A.; et al. Sustainable Agriculture through Multidisciplinary Seed Nanopriming: Prospects of Opportunities and Challenges. Cells 2021, 10 (9), 2428. 10.3390/cells10092428. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chaudhry A. A.; Mumtaz R.; Zaidi S. M. H.; Tahir M. A.; School S. H. M. Internet of Things (IoT) and Machine Learning (ML) enabled Livestock Monitoring. 2020 IEEE 17th International Conference on Smart Communities: Improving Quality of Life Using ICT, IoT and AI (HONET) 2020, 151–155. 10.1109/HONET50430.2020.9322666. [DOI] [Google Scholar]; Horrigan L.; Lawrence R. S.; Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002, 110 (5), 445–456. 10.1289/ehp.02110445. [DOI] [PMC free article] [PubMed] [Google Scholar]