Abstract
Objective
Cystic fibrosis (CF) is a genetic condition that causes abnormal mucus secretions in affected organs. MUC5AC and MUC5B are gel-forming mucins and frequent targets for investigations in CF tissues. Our objective was to qualify MUC5AC and MUC5B immunohistochemical techniques to provide a useful tool to identify, localize and interpret mucin expression in ferret tissues.
Results
MUC5AC and MUC5B mucins were detected most commonly in large airways and least in small airways, consistent with reported goblet cell density in airway surface epithelia. We evaluated whether staining method affected the detection of goblet cell mucins in serial sections of bronchial surface epithelia. Significant differences between stains were not observed suggesting common co-expression MUC5AC and MUC5B proteins in goblet cells of airway surface epithelia. Gallbladder and stomach tissues are reported to have differential mucin enrichment, so we tested these tissues in wildtype ferrets. Stomach tissues were enriched in MUC5AC and gallbladder tissues enriched in MUC5B, mucin enrichment similar to human tissues. Mucin immunostaining techniques were further qualified for specificity using lung tissue from recently generated MUC5AC−/− and MUC5B−/− ferrets. Qualified techniques for MUC5AC and MUC5B immunohistochemistry will be useful tools for mucin tissue studies in CF and other ferret models.
Keywords: Cystic fibrosis, Ferret, Immunohistochemistry, Lung, Mucin, Mucus, MUC5AC, MUC5B, Tissue, Pathology
Introduction
Cystic fibrosis (CF) is a life-limiting condition caused by mutations in the CF transmembrane conductance regulator (CFTR) [1, 2]. Clinical disease can begin before birth and produces lesions in several organ systems including: respiratory tract, gastrointestinal tract, skin, and reproductive tract [3–5]. Mouse models were developed by 1992, but these lacked significant phenotypes in key organs, thus accelerating the search for other novel animal models. With the advent of somatic cell nuclear transfer technology, the CF pig [6]and CF ferret [7] models were some of the first new animal models developed. Phenotypic analysis of the CF ferret model was reported in 2010 and has since been useful for study of lung, gastrointestinal, and pancreatic disease as well as novel treatment strategies [7–11].
Mucins are high molecular weight glycoproteins that provide the characteristic viscoelastic features of mucus. In the respiratory tract, MUC5AC (goblet cells) and MUC5B (goblet in surface epithelia and mucous cells of submucosal glands) are the major gel-forming mucins [12]. As part of mucociliary clearance, thin strands of secreted mucus sweep airways of inhaled debris and pathogens [13]. CF mucus is abnormal and described as thick, sticky and tenacious, features that contribute to its pathological role in disease development [12, 14]. Qualified immunohistochemical staining for MUC5AC and MUC5B can augment tissue studies for CF mucus [15].
The aims of the current study were to qualify the MUC5AC and MUC5B immunostaining techniques through use of control ferret tissues including the lung.
Main text
Methods
Archival paraffin-embedded tissue blocks from wildtype (WT) ferrets or those with ongoing research projects at the University of Iowa. All studies were performed under the approval and guidance of the University of Iowa Animal Care and Use Committee and followed all pertinent federal/national/international standards of care. Adult WT ferrets (1–2 years of age, n = 2–3 per sex) were used to evaluate mucin expression in select tissues (lung, sinonasal cavity, pancreas, gallbladder, stomach) that are relevant to CF research. Additionally, lung tissue of recently developed MUC5AC−/− (male, n = 1, > 1 yr age) and MUC5B−/− (female, n = 1, > 1 yr age) ferrets were obtained from an ongoing separate phenotypic study of these novel ferret models. Disruption of the two mucin genes was achieved using Cas9/gRNA ribonuclear protein complexes into ferret zygotes followed by adaptive transfer into pseudo-pregnant jills [16]. These exclusive, few lung tissues from novel mucin models were used to help qualify the specificity of mucin immunohistochemistry in the current study and any additional data about the generation or characterization of these models will be published in a separate study.
In tissue sections (~ 4 µm), diastase-pretreated periodic acid Schiff (dPAS) histochemical stain was applied to detect and localize mucus in tissues [17]. Baseline protocol parameters for evaluation of immunohistochemical staining for MUC5AC and MUC5B were guided from previous reports in CF models [15, 18–20]. The primary antibody concentration was preliminarily tested via a panel of concentrations (1:250, 1:500, 1:1000, 1:2000) to evaluate for staining of known positive cells (e.g. goblet cells) and absence of staining in off target cells to yield the initial baseline techniques used in this study. For both mucins, tissues were exposed to heat-induced epitope retrieval (citrate buffer pH 6.0, 110 °C × 15 min), followed by primary antibody for MUC5AC (mouse monoclonal 1:500 × 1 h, clone 45M1, #ab3649, Abcam, Waltham, MA, USA) or MUC5B (rabbit polyclonal 1:1000 × 20 min, #HPA008246, Sigma Aldrich, St. Louis, Mo, USA). Secondary kits of Mouse EnVision + and Rabbit Envison (Dako North America, Inc., Carpentaria, CA, USA) were respectively applied followed by 3,3′-Diaminobenzidine as chromogen and Harris hematoxylin as counterstain. Qualification of the immunostaining was primarily evaluated in gallbladder, stomach and lung tissues of wildtype ferrets (see results section). Anatomic definitions for airway size in ferret lungs were as follows: large bronchi (50–100% circumferential cartilage), small bronchi (< 1–50% circumferential cartilage), bronchioles (no cartilage in airway wall).
Representative high-resolution digital images were collected and analyzed (BX53 microscope, DP73 digital camera and CellSens Dimension Software, Olympus). Area of immunostaining relative to total area of airway surface epithelium produced the area fraction of immunostaining. These results were statistically analyzed with either two-way ANOVA or Kruskal–Wallis test as warranted using Prism software (Graphpad, Sand Diego, CA, USA). Airway epithelia height (as a metric of airway caliber) [21] and mucin expression were analyzed using Spearman correlation to define r and P values (significance defined as P < 0.05).
Results
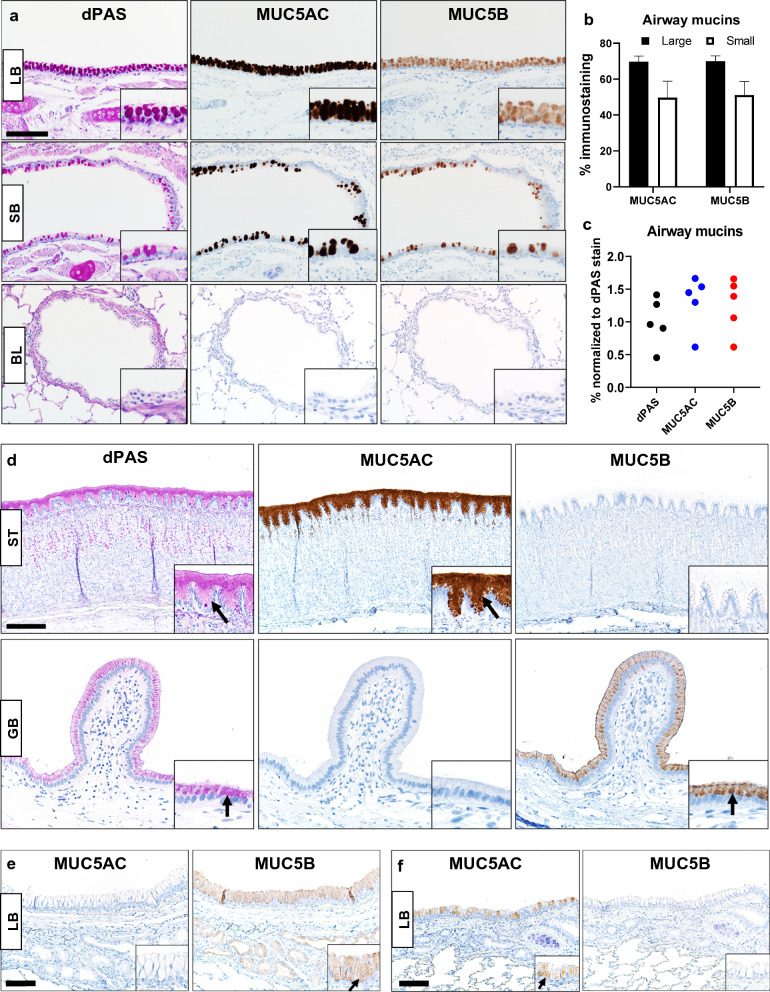
Positive and negative cellular or tissue expression of protein targets are useful to qualify the specificity and utility to immunohistochemical techniques [22, 23]. We evaluated mucin detection in bronchi (large and small) and bronchioles of WT ferret lungs. Mucins were detected more abundantly in large bronchi than small bronchi, but detection in bronchioles was rare to absent (Fig. 1a). Digital image analysis of airway mucins showed that the size of ferret bronchi significantly influenced mucin detection (P = 0.0072, two-way ANOVA, Fig. 1b), indicating that MUC5AC and MUC5B were more prevalent in larger than smaller bronchi. Correlation analysis of the airway mucin expression and airway epithelia height (a surrogate marker of airway caliber) demonstrated a significant relationship for MUC5AC (r = 0.6606, P = 0.044, Spearman correlation) and MUC5B (r = 0.6848, P = 0.035, Spearman correlation). In serial sections of bronchi surface epithelium, dPAS, MUC5AC and MUC5B techniques were digitally analyzed. We saw no significant differences between these mucin stains (Fig. 1c, P = 0.2938, Kruskal–Wallis test), suggesting MUC5AC and MUC5B have common co-expression in goblet cells.
Fig. 1.
Mucin detection in ferret tissues. a Representative mucin detection (insets) by dPAS, MUC5AC and MUC5B in surface epithelia of large bronchi (LB) or small bronchi (SB) and bronchioles (BL) from WT ferrets (bar = 130 µm). b Evaluation of MUC5AC and MUC5B immunostaining in surface epithelia of large and small bronchi (N = 5 WT ferrets) using digital image analysis. Airway size was a significant factor for the variance in mucin detection (P = 0.0072, Two-way ANOVA). c Comparison of dPAS, MUC5AC and MUC5B in serial sections of WT bronchus (N = 5 WT ferrets) showed no significant differences in mucin detection between stain method (P = 0.2938) Kruskal–Wallis test). d Representative images of differential mucin expression (arrows and insets) in WT ferret stomach (ST, bar = 170 µm) and gallbladder (GB, bar = 85 µm). dPAS, MUC5AC and MUC5B. e Mucin detection (arrow and insets) in a large bronchus (LB) from a MUC5AC−/− ferret (N = 1). Surface epithelia and submucosal glands were MUC5B + confirming the tissue was viable for immunostaining. MUC5AC immunostaining was absent from the surface epithelia, and this was consistent with the ferret’s genotype, bar = 85 µm. f Mucin detection (inset and arrows) in a large bronchus (LB) from a MUC5B−/− ferret (N = 1). The surface epithelia of the bronchus was MUC5AC+ confirming the tissue was viable for immunostaining, but it was MUC5B- consistent with the tissue genotype, bar = 85 µm
Healthy gallbladder and stomach tissues have been reported to have distinct tissue enrichment of MUC5AC in stomach and MUC5B in gallbladder [24, 25]. We evaluated stomach and gallbladder tissues from WT ferrets to see if similar mucin-specific enrichment was observed. MUC5AC immunostaining was detected in ferret stomach but was absent in ferret gallbladder. In contrast, MUC5B immunostaining was detected in ferret gallbladder, but absent in ferret stomach (Fig. 1d). These data parallel mucin enrichment observed in humans and several animal models (Table 1) [24–36].
Table 1.
Species comparison of mucin enrichment in healthy gallbladder and stomach tissues
| Species | Gallbladder | Stomach |
|---|---|---|
| Human |
MUC5AC high [25] MUC5B low (absent except in fetal development or disease) [26] |
|
| Ferret | MUC5B high, MUC5AC low (see results section) | MUC5AC high, MUC5B low (see results section) |
| Pigs |
MUC5B high [36] MUC5AC low [36] |
MUC5AC high [31, 33] |
| Sheep | NA | MUC5AC high [30] |
| Rabbits | NA | MUC5AC high [31] |
| Rat | Rats do not have gallbladders | MUC5AC high [31, 32] |
| Mice | MUC5B high [34] | MUC5AC high [29, 35] |
"High" = Moderate to strong expression, "Low" = Minor to lack of expression
Immunohistochemical techniques can also be qualified by testing tissues that lack antigen/epitope expression due to genomic editing. We acquired access to rare bronchial tissues from two novel models (a MUC5AC−/− ferret and a MUC5B−/− ferret) that are being phenotypically characterized for an ongoing separate study. We evaluated serial sections of a bronchus from a MUC5AC−/− ferret and both the surface epithelium and submucosal glands had MUC5B + immunostaining, but MUC5AC immunostaining was negative consistent with the tissue genotype (Fig. 1e, see Fig. 1a for reference of WT staining). We then evaluated a bronchus from a MUC5B−/− ferret. The surface epithelium was positive for MUC5AC immunostaining but it was negative for MUC5B consistent with expected expression patterns the model (Fig. 1f, see Fig. 1a for reference of WT staining).
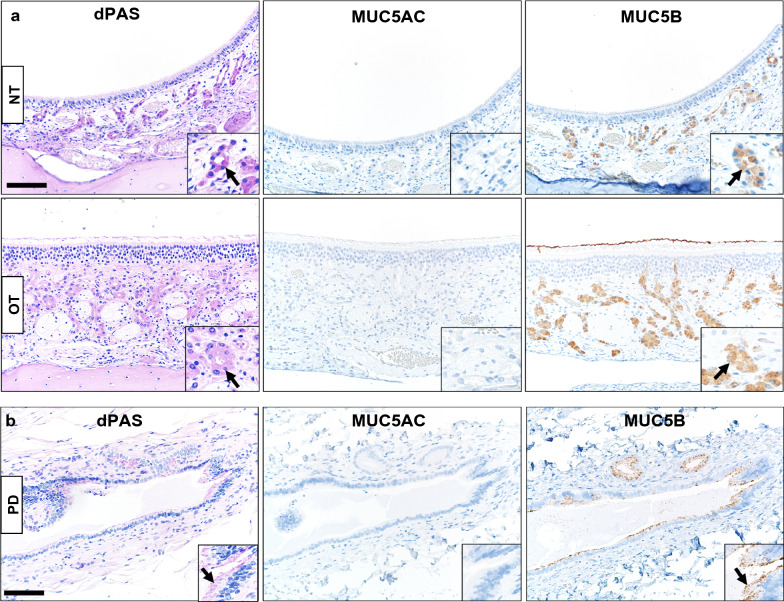
We then applied the MUC5AC and MUC5B immunostaining on two other WT ferret tissues that might be of interest for study in the CF, specifically sinonasal cavity and pancreas. In the sinonasal cavity, the glands of the respiratory epithelia and Bowman glands of olfactory epithelia were dPAS + and MUC5B + while MUC5AC immunostaining was lacking (Fig. 2a). These findings parallel a report of healthy human nasal glands and Bowman glands with MUC5B + expression and minimal/lack of MUC5AC immunostaining [37, 38]. In the pancreas, large secretory ducts had evidence of dPAS + mucins in mucous cells and these sites paralleled with MUC5B + immunostaining but was negative for MUC5AC (Fig. 2b). Healthy human pancreas ducts are reported to have MUC5B expression and lack MUC5AC expression, but both may be present in diseased pancreas (e.g. cancer) [39].
Fig. 2.
Representative mucin detection in WT tissues. a dPAS + and MUC5B + mucin detection (arrows and insets) in glands of nasal (NT) and olfactory (OT) tissues from WT ferret sinonasal cavity, but the glands were MUC5AC−, bar = 85 µm. b dPAS + and MUC5B + (arrows and insets) mucin detection in a large pancreas duct (PD) from a WT ferret, but duct epithelia were MUC5AC−, bar = 85 µm
In all of the studies, we did not see evidence for overt sex-specific differences in localization or distribution of mucin expression.
Discussion
Immunohistochemical techniques can be qualified by multiple approaches through use of appropriate controls [23, 40, 41]. In this current study, our control cell/tissue were defined from previous reports of airway mucin expression patterns in lungs, known anatomic / tissue enrichment differences, and use of gene-edited tissues. We were able apply these mucin immunohistochemical techniques in ferret tissues to define mucin expression for CF-relevant tissues such as lung, gallbladder, stomach, pancreas and sinonasal cavity and show its assessment through digital image analysis [9, 20, 42]. Mucin expression patterns in healthy tissues are useful controls to clarify expression changes that can appear in diseased tissues, such as prospective studies on ferret tissues to evaluate CF mucins [7, 28, 39, 43, 44]. Even so, mucins may be a pertinent parameter for study in ferret tissues modelling other diseases such as transplantation rejection [45], chronic obstructive pulmonary disease [46], COVID19 [21, 47], influenza [48], filoviruses [49] and cancer [50] to name a few.
For quality control, immunohistochemical techniques should be initially qualified before use in investigative studies [22, 51]. Multiple layers of immunohistochemical qualification (as seen in this study) provide added confidence in the use of these mucin detection techniques for tissue studies of CF and other ferret models. Additionally, requalification of any immunohistochemistry protocol is also recommended after changes in pre-analytic tissue factors or protocol reagents (lots, reagents, etc.) as these can affect the qualities of the final immunostaining [52, 53]. Our qualification of MUC5AC and MUC5B techniques in ferret tissues, use of control tissues and application of digital image analysis confirm that these mucin detection techniques will be vital tools to identify, localize and interpret mucin expression. Additionally, these tools will be useful in future studies to analyze the spatial and cellular expression of mucins in ferret lungs and compare to human lungs [54].
Limitations
This study is not without potential limitations. First, we studied select archival tissues from adult ferrets, but we cannot fully assume that our results will be directly applicable to other ferret tissues/organs or other ferret breeds/strains. Second, we focused our evaluation of healthy tissues, so we cannot rule out that diseased tissues with inflammation or remodeling changes might display differences in cellular localization or intensity of mucins. Lastly, it is well-recognized that pre-analytic factors (tissue handling, fixation quality, etc.) can greatly influence immunostaining and digital image analysis [22, 41, 55]. Thus, differences in pre-analytical could feasibly produce minor lab-to-lab variations in immunostaining results.
Acknowledgements
Not applicable.
Abbreviations
- BL
Bronchiole
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- COVID19
Coronavirus disease of 2019
- GB
Gallbladder
- LB
Large bronchi
- NT
Nasal tissue
- OT
Olfactory tissue
- SB
Small bronchi
- ST
Stomach
- dPAS
Diastase-pretreated periodic acid Schiff
- WT
Wildtype
Author contributions
DM and JE developed the conceptual ideas and all authors contributed to planning the experimental design. ML, JG, TB, AA, SV, IE, and YZ, identified/secured appropriate studies and tissues for inclusion. ML, AA, and SV optimized, and performed the histotechnology and immunohistochemistry procedures. DM, ML, and JAG optimized and performed analysis of digital images and immunostaining evaluation. All authors contributed to the drafting/revision of the manuscript and all authors approved the final manuscript.
Funding
JE (NIH Grants: P30 DK054759; P01 HL152960; R01 HL165404), DKM (HL163556, HL152960, HL091842, HL147366).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All tissues were collected from a repository of archival paraffin-embedded tissue blocks that originated in separate studies approved by the University of Iowa Animal Care and Use Committee and that followed appropriate federal guidelines on animal studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David K. Meyerholz, Email: david-meyerholz@uiowa.edu
Mariah R. Leidinger, Email: mariah-leidinger@uiowa.edu
J. Adam Goeken, Email: adam-goeken@uiowa.edu
Thomas R. Businga, Email: thomas-businga@uiowa.edu
Sebastian Vizuett, Email: sebastian-vizuett@uiowa.edu.
Allison Akers, Email: allison-akers@uiowa.edu.
Idil Evans, Email: idil-apak@uiowa.edu.
Yan Zhang, Email: yan-zhang-3@uiowa.edu.
John F. Engelhardt, Email: john-engelhardt@uiowa.edu
References
- 1.Garcia LCE, Petry LM, Germani P, Xavier LF, de Barros PB, Meneses ADS, Prestes LM, Bittencourt LB, Pieta MP, Friedrich F, et al. Translational research in cystic fibrosis: from bench to beside. Front Pediatr. 2022;10:881470. doi: 10.3389/fped.2022.881470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56(2):344–399. doi: 10.1001/archpedi.1938.01980140114013. [DOI] [Google Scholar]
- 4.Andersen DH. Pathology of cystic fibrosis. Ann Ny Acad Sci. 1962;93(12):500–517. doi: 10.1111/j.1749-6632.1962.tb30490.x. [DOI] [Google Scholar]
- 5.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176(3):1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321(5897):1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122(10):3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Olivier AK, Yi Y, Pope CE, Hayden HS, Liang B, Sui H, Zhou W, Hager KR, Zhang Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol. 2014;184(5):1309–1322. doi: 10.1016/j.ajpath.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, Olivier AK, Liang B, Yi Y, Sui H, Evans TI, Zhang Y, Zhou W, Tyler SR, Fisher JT, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol. 2014;50(3):502–512. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Yi Y, Yan Z, Rosen BH, Liang B, Winter MC, Evans TIA, Rotti PG, Yang Y, Gray JS et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med. 2019; 11(485). [DOI] [PMC free article] [PubMed]
- 12.Morrison CB, Markovetz MR, Ehre C. Mucus, mucins, and cystic fibrosis. Pediatr Pulmonol. 2019;54(Suppl 3):S84–S96. doi: 10.1002/ppul.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, Powers LS, Gansemer ND, Bouzek DC, Cook DP, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci USA. 2017;114(26):6842–6847. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372(4):351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerholz DK, Lambertz AM, Reznikov LR, Ofori-Amanfo GK, Karp PH, McCray PB, Jr, Welsh MJ, Stoltz DA. Immunohistochemical detection of markers for translational studies of lung disease in pigs and humans. Toxicol Pathol. 2016;44(3):434–441. doi: 10.1177/0192623315609691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He N, Liu X, Vegter AR, Evans TIA, Gray JS, Guo J, Moll SR, Guo LJ, Luo M, Ma N et al. Ferret models of alpha-1 antitrypsin deficiency develop lung and liver disease. JCI Insight. 2022; 7(5). [DOI] [PMC free article] [PubMed]
- 17.Meyerholz DK, Beck AP, Goeken JA, Leidinger MR, Ofori-Amanfo GK, Brown HC, Businga TR, Stoltz DA, Reznikov LR, Flaherty HA. Glycogen depletion can increase the specificity of mucin detection in airway tissues. BMC Res Notes. 2018;11(1):763. doi: 10.1186/s13104-018-3855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarei K, Stroik MR, Gansemer ND, Thurman AL, Ostedgaard LS, Ernst SE, Thornell IM, Powers LS, Pezzulo AA, Meyerholz DK, et al. Early pathogenesis of cystic fibrosis gallbladder disease in a porcine model. Lab Invest. 2020;100(11):1388–1399. doi: 10.1038/s41374-020-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostedgaard LS, Price MP, Whitworth KM, Abou Alaiwa MH, Fischer AJ, Warrier A, Samuel M, Spate LD, Allen PD, Hilkin BM et al. Lack of airway submucosal glands impairs respiratory host defenses. Elife. 2020; 9. [DOI] [PMC free article] [PubMed]
- 20.Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, Rector MV, Suter MJ, Kao S, McLennan G, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Resp Crit Care. 2010;182(10):1251–1261. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerholz DK, Reznikov LR. Influence of SARS-CoV-2 on airway mucus production: a review and proposed model. Vet Pathol. 2022;59(4):578–585. doi: 10.1177/03009858211058837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyerholz DK, Beck AP. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest. 2018;98(7):844–855. doi: 10.1038/s41374-018-0057-0. [DOI] [PubMed] [Google Scholar]
- 23.Meyerholz DK, Leidinger MR, Goeken JA, Businga TR, Akers A, Vizuett S, Kaemmer CA, Kohlmeyer JL, Dodd RD, Quelle DE. Utility of CD138/syndecan-1 immunohistochemistry for localization of plasmacytes is tissue-dependent in B6 mice. BMC Res Notes. 2022;15(1):219. doi: 10.1186/s13104-022-06100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Klinken BJ, Dekker J, van Gool SA, van Marle J, Buller HA, Einerhand AW. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998;274(5):G871–878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- 25.Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL, Jenkins MA, et al. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol. 2013;26(12):1642–1656. doi: 10.1038/modpathol.2013.101. [DOI] [PubMed] [Google Scholar]
- 26.Buisine MP, Devisme L, Maunoury V, Deschodt E, Gosselin B, Copin MC, Aubert JP, Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. I. Stomach. A relationship to gastric carcinoma. J Histochem Cytochem. 2000;48(12):1657–1666. doi: 10.1177/002215540004801209. [DOI] [PubMed] [Google Scholar]
- 27.Espinoza JA, Riquelme I, Sagredo EA, Rosa L, Garcia P, Bizama C, Apud-Bell M, Leal P, Weber H, Benavente F, et al. Mucin 5B, carbonic anhydrase 9 and claudin 18 are potential theranostic markers of gallbladder carcinoma. Histopathology. 2019;74(4):597–607. doi: 10.1111/his.13797. [DOI] [PubMed] [Google Scholar]
- 28.Finzi L, Barbu V, Burgel PR, Mergey M, Kirkwood KS, Wick EC, Scoazec JY, Peschaud F, Paye F, Nadel JA, et al. MUC5AC, a gel-forming mucin accumulating in gallstone disease, is overproduced via an epidermal growth factor receptor pathway in the human gallbladder. Am J Pathol. 2006;169(6):2031–2041. doi: 10.2353/ajpath.2006.060146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonckheere N, Van Der Sluis M, Velghe A, Buisine MP, Sutmuller M, Ducourouble MP, Pigny P, Buller HA, Aubert JP, Einerhand AW, et al. Transcriptional activation of the murine Muc5ac mucin gene in epithelial cancer cells by TGF-beta/Smad4 signalling pathway is potentiated by Sp1. Biochem J. 2004;377(Pt 3):797–808. doi: 10.1042/bj20030948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karakoc Z, Sagsoz H, Ketani MA. Mucin profiles of the abomasum in bulls and rams: a comparative study. Microsc Res Tech. 2016;79(9):856–868. doi: 10.1002/jemt.22713. [DOI] [PubMed] [Google Scholar]
- 31.Lacunza E, Bara J, Segal-Eiras A, Croce MV. Expression of conserved mucin domains by epithelial tissues in various mammalian species. Res Vet Sci. 2009;86(1):68–77. doi: 10.1016/j.rvsc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Nam SY, Kim N, Lee CS, Choi KD, Lee HS, Jung HC, Song IS. Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005;50(11):2110–2120. doi: 10.1007/s10620-005-3016-8. [DOI] [PubMed] [Google Scholar]
- 33.Padra M, Adamczyk B, Benktander J, Flahou B, Skoog EC, Padra JT, Smet A, Jin C, Ducatelle R, Samuelsson T, et al. Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes. Virulence. 2018;9(1):898–918. doi: 10.1080/21505594.2018.1460979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portal C, Gouyer V, Magnien M, Plet S, Gottrand F, Desseyn JL. In vivo imaging of the Muc5b gel-forming mucin. Sci Rep. 2017;7:44591. doi: 10.1038/srep44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Pineiro AM, Bergstrom JH, Ermund A, Gustafsson JK, Schutte A, Johansson ME, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G348–G356. doi: 10.1152/ajpgi.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarei K, Meyerholz DK, Stoltz DA. Early intrahepatic duct defects in a cystic fibrosis porcine model. Physiol Rep. 2021;9(14):e14978. doi: 10.14814/phy2.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Yan C, Tao S. Association of MUC2, MUC5AC and MUC5B genes with the recurrence of nasal polyps. Exp Ther Med. 2020;20(2):1808–1814. doi: 10.3892/etm.2020.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amini SE, Gouyer V, Portal C, Gottrand F, Desseyn JL. Muc5b is mainly expressed and sialylated in the nasal olfactory epithelium whereas Muc5ac is exclusively expressed and fucosylated in the nasal respiratory epithelium. Histochem Cell Biol. 2019;152(2):167–174. doi: 10.1007/s00418-019-01785-5. [DOI] [PubMed] [Google Scholar]
- 39.Balague C, Audie JP, Porchet N, Real FX. In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology. 1995;109(3):953–964. doi: 10.1016/0016-5085(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz ME, Thurman A, Pezzulo AA, Leidinger MR, Klesney-Tait JA, Karp PH, Tan P, Wohlford-Lenane C, McCray PB, Jr, Meyerholz DK. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60:102976. doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janardhan KS, Jensen H, Clayton NP, Herbert RA. Immunohistochemistry in investigative and toxicologic pathology. Toxicol Pathol. 2018;46(5):488–510. doi: 10.1177/0192623318776907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang EH, Pezzulo AA, Meyerholz DK, Potash AE, Wallen TJ, Reznikov LR, Sieren JC, Karp PH, Ernst S, Moninger TO, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope. 2012;122(9):1898–1905. doi: 10.1002/lary.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helke KL, Meyerholz DK, Beck AP, Burrough ER, Derscheid RJ, Lohr C, McInnes EF, Scudamore CL, Brayton CF. Research relevant background lesions and conditions: ferrets, dogs, swine, sheep, and goats. ILAR J. 2021;62(1–2):133–168. doi: 10.1093/ilar/ilab005. [DOI] [PubMed] [Google Scholar]
- 44.Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, Zhang Y, Jensen-Cody CW, Swatek AM, Zhou W, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med. 2018;197(10):1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch TJ, Ahlers BA, Swatek AM, Ievlev V, Pai AC, Brooks L, Tang Y, Evans IA, Meyerholz DK, Engelhardt JF, et al. Ferret lung transplantation models differential lymphoid aggregate morphology between restrictive and obstructive forms of chronic lung allograft dysfunction. Transplantation. 2022;106(10):1974–1989. doi: 10.1097/TP.0000000000004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussain SS, Edwards YJK, Libby EF, Stanford D, Byzek SA, Sin DD, McDonald ML, Raju SV, Rowe SM. Comparative transcriptomics in human COPD reveals dysregulated genes uniquely expressed in ferrets. Respir Res. 2022;23(1):277. doi: 10.1186/s12931-022-02198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An D, Li K, Rowe DK, Diaz MCH, Griffin EF, Beavis AC, Johnson SK, Padykula I, Jones CA, Briggs K et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci Adv. 2021; 7(27). [DOI] [PMC free article] [PubMed]
- 48.Zeng H, Goldsmith CS, Kumar A, Belser JA, Sun X, Pappas C, Brock N, Bai Y, Levine M, Tumpey TM et al. Tropism and infectivity of a seasonal A(H1N1) and a highly pathogenic avian A(H5N1) influenza virus in primary differentiated ferret nasal epithelial cell cultures. J Virol. 2019; 93(10). [DOI] [PMC free article] [PubMed]
- 49.Schiffman Z, Liu G, Cao W, Zhu W, Emeterio K, Qiu X, Banadyga L. The ferret as a model for filovirus pathogenesis and countermeasure evaluation. ILAR J. 2022;61(1):62–71. doi: 10.1093/ilar/ilab011. [DOI] [PubMed] [Google Scholar]
- 50.Yui T, Ohmachi T, Matsuda K, Okamoto M, Taniyama H. Histochemical and immunohistochemical characterization of chordoma in ferrets. J Vet Med Sci. 2015;77(4):467–473. doi: 10.1292/jvms.14-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyerholz DK, Ofori-Amanfo GK, Leidinger MR, Goeken JA, Khanna R, Sieren JC, Darbro BW, Quelle DE, Weimer JM. Immunohistochemical markers for prospective studies in neurofibromatosis-1 porcine models. J Histochem Cytochem. 2017;65(10):607–618. doi: 10.1369/0022155417729357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward JM, Rehg JE. Rodent immunohistochemistry: pitfalls and troubleshooting. Vet Pathol. 2014;51(1):88–101. doi: 10.1177/0300985813503571. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgibbons PL, Bradley LA, Fatheree LA, Alsabeh R, Fulton RS, Goldsmith JD, Haas TS, Karabakhtsian RG, Loykasek PA, Marolt MJ, et al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138(11):1432–1443. doi: 10.5858/arpa.2013-0610-CP. [DOI] [PubMed] [Google Scholar]
- 54.Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Dang H, et al. Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med. 2019;199(6):715–727. doi: 10.1164/rccm.201804-0734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chlipala EA, Butters M, Brous M, Fortin JS, Archuletta R, Copeland K, Bolon B. Impact of preanalytical factors during histology processing on section suitability for digital image analysis. Toxicol Pathol. 2021;49(4):755–772. doi: 10.1177/0192623320970534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.