Abstract
Background
Breast cancer brain metastases (BCBM) are the most fatal, with limited survival in all breast cancer distant metastases. These patients are deemed to be incurable. Thus, survival time is their foremost concern. However, there is a lack of accurate prediction models in the clinic. What’s more, primary surgery for BCBM patients is still controversial.
Methods
The data used for analysis in this study was obtained from the SEER database (2010–2019). We made a COX regression analysis to identify prognostic factors of BCBM patients. Through cross-validation, we constructed XGBoost models to predict survival in patients with BCBM. Meanwhile, a BCBM cohort from our hospital was used to validate our models. We also investigated the prognosis of patients treated with surgery or not, using propensity score matching and K–M survival analysis. Our results were further validated by subgroup COX analysis in patients with different molecular subtypes.
Results
The XGBoost models we created had high precision and correctness, and they were the most accurate models to predict the survival of BCBM patients (6-month AUC = 0.824, 1-year AUC = 0.813, 2-year AUC = 0.800 and 3-year survival AUC = 0.803). Moreover, the models still exhibited good performance in an externally independent dataset (6-month: AUC = 0.820; 1-year: AUC = 0.732; 2-year: AUC = 0.795; 3-year: AUC = 0.936). Then we used Shiny-Web tool to make our models be easily used from website. Interestingly, we found that the BCBM patients with an annual income of over USD$70,000 had better BCSS (HR = 0.523, 95%CI 0.273–0.999, P < 0.05) than those with less than USD$40,000. The results showed that in all distant metastasis sites, only lung metastasis was an independent poor prognostic factor for patients with BCBM (OS: HR = 1.606, 95%CI 1.157–2.230, P < 0.01; BCSS: HR = 1.698, 95%CI 1.219–2.365, P < 0.01), while bone, liver, distant lymph nodes and other metastases were not. We also found that surgical treatment significantly improved both OS and BCSS in BCBM patients with the HER2 + molecular subtypes and was beneficial to OS of the HR−/HER2− subtype. In contrast, surgery could not help BCBM patients with HR + /HER2− subtype improve their prognosis (OS: HR = 0.887, 95%CI 0.608–1.293, P = 0.510; BCSS: HR = 0.909, 95%CI 0.604–1.368, P = 0.630).
Conclusion
We analyzed the clinical features of BCBM patients and constructed 4 machine-learning prognostic models to predict their survival. Our validation results indicate that these models should be highly reproducible in patients with BCBM. We also identified potential prognostic factors for BCBM patients and suggested that primary surgery might improve the survival of BCBM patients with HER2 + and triple-negative subtypes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-023-04277-2.
Keywords: Breast cancer, Brain metastases, XGBoost algorithm, SEER, Surgery
Introduction
Breast cancer (BC) is emerging as the top diagnosed cancer worldwide and the leading cause of cancer-related deaths in women [1]. BC metastasis to the central nervous system (CNS) is a devastating disease involving either the brain parenchyma or the leptomeninges. Of newly diagnosed BC patients annually, 10–16% will experience symptomatic brain metastases, and more than 30% of patients with metastatic BC are found in autopsy reports [2–5].
Patients with breast cancer brain metastases (BCBM) suffer from a particularly poor prognosis, with their median survival time being only 10 months [6]. Moreover, brain metastases usually lead to progressive neurologic deficits, which further reduce the quality of life [7]. Sadly, patients with BCBM are refractory to almost all currently available treatments, experiencing a traumatic deterioration of quality of life and a devastating < 20% 1-year survival [8]. A major reason for such a dreadful prognosis is that current treatment options for brain metastasis (e.g. steroids, cranial radiotherapy, and surgical resection in selected patients) are limited and merely palliative, not curative. Additionally, diverse clinical characteristics greatly affect the prognosis of BCBM patients [9]. Therefore, there is an urgent need for prognostic prediction models to accurately answer BCBM patients' concerns about survival and to help optimize their management.
Previous studies have built a few nomograms for predicting the prognosis of BCBM patients. To predict the prognosis of BCBM patients, a few nomograms have been developed in earlier investigations. These models' accuracy, however, is unsatisfactory (AUC value or C-index less than 0.7) [10–12]. Therefore, a more precise and robust model is required. To this end, machine learning has emerged as an absolutely crucial topic, offering tools and methods for evaluating the tremendous, high-dimensional, and multi-modal data generated by the biological sciences [13, 14]. It can also help us create an artificial intelligence (AI) prognostic model, significantly increasing the accuracy rate [14]. Extreme Gradient Boosting (XGBoost), one of the numerous machine learning algorithms, is created iteratively to minimize the loss function, which makes it perform well in various domains [15–17]. However, it is rarely applied in the prognostic prediction of cancer patients. We used 6 kinds of machine learning algorithms to create prognostic models and found that XGBoost performed best.
The Surveillance Epidemiology and End Results (SEER) database was exploited in this study to examine the variables affecting BCBM patients' prognoses. High-precision AI models were developed to predict the 6-month, 1, 2 and 3-year survival of BCBM patients. This study contributes to the development of clinical AI models to optimize the long-term follow-up of BCBM patients and provides insight into the prognosis of BCBM patients.
Materials and methods
Data source and study design
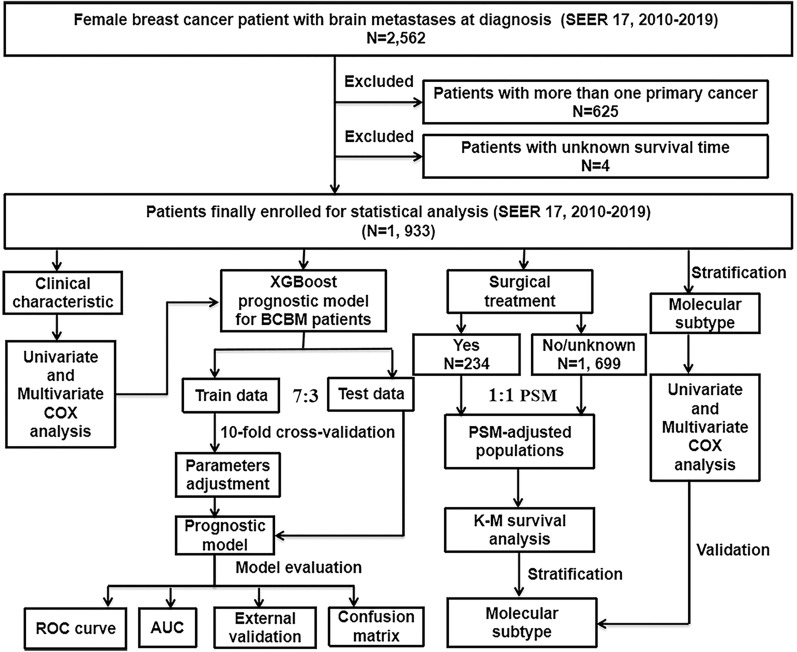
Figure 1 presents the workflow of our study design and its analyses. As the information on distant metastases was included from 2010, the data analyzed in this study were obtained from the SEER database [SEER 17 Regs study data, (changes 2010–2019); version 8.4.0] where the data is openly accessible. Data about women with BC were collected from this database. Inclusion criteria were as follows: (1) BC was the patients’ one and only cancer that had been identified; (2) all cancer patients showed histopathological and morphological evidence in accordance with the International Classification of Cancer Diseases Edition III (ICD-O-3); (3) all cancer patients developed brain metastases at the initial diagnosis. Exclusion criteria were as follows: (1) patients suffering from two or more primary cancers; (2) patients whose survival time was unknown. Follow up is sustained until patients died, loss to follow-up, or December 31, 2019.
Fig. 1.
The flowchart described the process of conducting the study and statistical analysis. SEER the surveillance, epidemiology, and end results database; BCBM breast cancer brain metastases, PSM propensity score matching, COX concordance index; ROC curve receiver operating characteristic curve, AUC area under the curve, K–M Kaplan–Meier, XGBoost extreme gradient boosting
XGBoost model
The XGBoost algorithm modifies the gradient boosting approach by utilizing Newton's method to solve for the extreme values of the loss function, conducting Taylor expansion of the loss function to the second order, and adding a regularization term to the loss function The gradient boosting algorithm loss and the regularization term make up the first and second parts of the objective function at training time, respectively. In addition, the XGBoost algorithm adopts a technique named "feature subsampling”, which can be understood as selecting a subset of all features to train each tree (similar to a random forest) so as to improve the generalization ability of the model, make it more diverse and prevent overfitting. The XGBoost algorithm operates under the following principle: feature vector with the corresponding (output) category yi:
Feature selection: univariate and multivariate COX analyses were performed on clinical characteristics obtained from the SEER database. Characteristics that were statistically significant in the multivariate COX, including age at diagnosis, marital status, histological type, molecular subtype, T stage, lung metastases and chemotherapy, median household income,, as well as grade, race, surgery, radiotherapy, liver metastases reported as independent prognostic factors in previous studies [10, 18–20], were incorporated into machine learning models to predict 6-month, 1-, 2- and 3-year overall BCBM patient survival. Prior to excluding the patients who survived but lived less than 6-month, 1-, 2- or 3-year at the follow-up cut-off date, these analyses were conducted. A response variable was collected for survival information before running the training program, in which 1 = survival and 0 = death. Patients were randomized into train data and test data in a 7:3 ratio. We also compared the area under the curve (AUC value) of logistic regression (LR), support vector machine (SVM), random forest (RF), K-Nearest Neighbor (KNN), decision tree (ID3), and XGBoost on test data. Receiver operating characteristic (ROC) analysis, area under the ROC curve (AUC) and confusion matrix were used to evaluate the model. Precision and accuracy are the primary assessment parameters in the confusion matrix.
External validation: to further validate the XGBoost prognostic model, we collected information on 67 patients diagnosed with BCBM from May 2015 to May 2022 in the Second Affiliated Hospital of Xi’an Jiaotong University. Exclusion criteria were as follows: (1) under the age of 20; (2) patients with second primary cancer of any kind; (3) male BC patients; (4) patients who were lost to follow-up. Follow proceeded until the patient's death or November 5th, 2022. Our retrospective cohort study was authorized by the Institutional Review Board of the Second Affiliated Hospital of Xi’an Jiaotong University, which consented to waive informed consent because the data used in this study have no personally identifiable information of patients.
Shiny app: we built a web-based application to make our new predictive models available online. The web-based application was built based on the R package “shiny”.
Statistical analysis
To explore the connection between various clinical and pathological features and the survival of patients, we sued univariate COX regression models. To assess patient mortality risk and identify independent prognostic markers, further multifactorial COX analysis was conducted. Patients undergoing surgical therapy and those who did not were matched on a 1:1 propensity score matching (PSM) based on the variables in the XGBoost model to examine the effect of surgical treatment on the prognosis of patients with BCBM. On the PSM-adjusted population, a Kaplan–Meier (K–M) survival analysis stratified by molecular subtype was also carried out. Finally, we performed subgroup univariate and multifactorial COX analyses in BCBM patients according to molecular subtype. We further investigated the role of treatment in patients with different molecular subtypes of BCBM. For all statistical calculations, the R programming language was utilized (version 4.0.2). Statistical significance was defined as a bilateral tail value of less than 0.05.
Results
Clinical characteristics of BCBM patients
Eventually, we obtained the information on 1933 eligible BCBM patients from the SEER database (2010 to 2019). The clinicopathological characteristics of BC patients with brain metastases are shown in Table 1 and summarized below. The median age of the patients was 60 years, of which 141 (7.29%) patients were younger than 40 years, and 129 (6.67%) patients were older than 80 years. While 739 patients (38.23%) received therapy more than a month following diagnosis, 877 patients (45.37%) received immediate medical attention. HR + /HER2− made up 37.09% of the molecular subtypes,, followed by HR−/HER2− (17.23%), HR + /HER2 + (15.93%) and HR−/HER2 + (12.36%). In terms of race, 74.86% of the patients were white. Invasive ductal carcinoma (IDC) was the predominant histopathological type (65.13%). Regarding marital status, 40.87% of the patients were married, and 25.61% were single. The proportions of staging T1 to T4 were 10.14%, 21.31%, 12.83% and 33.11%, respectively and N0 to N3 were 19.56%, 41.49%, 8.85% and 14.49%. Approximately 39.11% of the patients with tumors progressed to grade III or IV tumors, while only 3.47% had grade I. About 34.97% of the patients had a good annual family income of US$70,000. In the treatment field, only 12.11% of patients received surgery, 60.84% received radiotherapy, and 54.58% received chemotherapy. Bone, liver, and lung metastases, distant lymph nodes and other distant organ metastases accounted for 64.83%, 33.32%, 43.51%, 14.69% and 11.02% of patients, respectively.
Table 1.
Baseline characteristics of BC brain metastases (BCBM) patients included from SEER data cohort
| Characteristic | Cases | % | |
|---|---|---|---|
| Age at diagnosis | < 40 | 141 | 7.29 |
| 40–49 | 251 | 12.98 | |
| 50–59 | 529 | 27.37 | |
| 60–69 | 576 | 29.80 | |
| 70–79 | 307 | 15.88 | |
| 80 + | 129 | 6.67 | |
| Months from diagnosis to therapy | 0 month | 877 | 45.37 |
| ≥ 1 month | 739 | 38.23 | |
| Unknown | 317 | 16.40 | |
| Subtype | HR + /HER2− | 717 | 37.09 |
| HR + /HER2 + | 308 | 15.93 | |
| HR−/HER2 + | 239 | 12.36 | |
| HR−/HER2− | 333 | 17.23 | |
| Unknown | 336 | 17.38 | |
| Race | White | 1447 | 74.86 |
| Black | 307 | 15.88 | |
| Other | 179 | 9.26 | |
| Histological type | IDC | 1259 | 65.13 |
| ILC | 97 | 5.02 | |
| Mixed | 70 | 3.62 | |
| Other | 507 | 26.23 | |
| Marital status | Married | 790 | 40.87 |
| Singled | 495 | 25.61 | |
| Widow/divorced/other | 648 | 33.52 | |
| T Stage | T1 | 196 | 10.14 |
| T2 | 412 | 21.31 | |
| T3 | 248 | 12.83 | |
| T4 | 640 | 33.11 | |
| Unknown | 437 | 22.61 | |
| N Stage | N0 | 378 | 19.56 |
| N1 | 802 | 41.49 | |
| N2 | 171 | 8.85 | |
| N3 | 280 | 14.49 | |
| Unknown | 302 | 15.62 | |
| Grade | Well differentiated | 67 | 3.47 |
| Moderate differentiated | 448 | 23.18 | |
| Poorly differentiated | 756 | 39.11 | |
| Unknown | 662 | 34.25 | |
| Median household income(inflation adjusted) | < 40,000$ | 104 | 5.38 |
| 40,000–49,999$ | 219 | 11.33 | |
| 50,000–59,999$ | 292 | 15.11 | |
| 60,000–69,999$ | 642 | 33.21 | |
| 70,000$ + | 676 | 34.97 | |
| Chemotherapy | No/unknown | 878 | 45.42 |
| Yes | 1055 | 54.58 | |
| Radiotherapy | No/unknown | 757 | 39.16 |
| Yes | 1176 | 60.84 | |
| Surgery | No/unknown | 1699 | 87.89 |
| Yes | 234 | 12.11 | |
| Bone metastases | No/unknown | 680 | 35.18 |
| Yes | 1253 | 64.82 | |
| Liver metastases | No/unknown | 1289 | 66.68 |
| Yes | 644 | 33.32 | |
| Lung metastases | No/unknown | 1092 | 56.49 |
| Yes | 841 | 43.51 | |
| Distant lymph nodes metastases | No/unknown | 1649 | 85.31 |
| Yes | 284 | 14.69 | |
| Distant other metastases | No/unknown | 1720 | 88.98 |
| Yes | 213 | 11.02 |
Univariable and multivariable COX regression analysis
We practiced univariable COX regression analysis to spot variables that significantly influenced overall survival (OS) and breast cancer specific survival (BCSS) of BCBM patients, including age at diagnosis, race, marital status, histological type, months from diagnosis to therapy, median family income (inflation-adjusted), molecular subtype, T and N stage, grade, distant metastases and treatment information (Table 2).
Table 2.
Univariate and multivariate COX analysis of characteristics extracted from SEER database
| Univariate COX analysis | Multivariate COX analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | BCSS | OS | BCSS | |||||||||
| HR | 95%CI | P Value | HR | 95%CI | P Value | HR | 95%CI | P Value | HR | 95%CI | P Value | |
| Age at diagnosis | ||||||||||||
| < 40 | Reference | Reference | Reference | Reference | ||||||||
| 40–49 | 1.292 | 1.006–1.660 | * | 1.201 | 0.922–1.564 | 0.175 | 1.322 | 0.646–2.706 | 0.444 | 1.105 | 0.521–2.279 | 0.791 |
| 50–59 | 1.423 | 1.132–1.788 | ** | 1.371 | 1.080–1.740 | ** | 2.232 | 1.180–4.221 | * | 2.293 | 1.165–4.101 | * |
| 60–69 | 1.674 | 1.336–2.099 | *** | 1.608 | 1.270–2.037 | *** | 1.900 | 1.030–3.620 | * | 1.746 | 1.002–3.181 | * |
| 70–79 | 2.291 | 1.802–2.911 | *** | 2.175 | 1.692–2.796 | *** | 3.336 | 1.636–6.805 | *** | 3.737 | 1.845–7.569 | *** |
| 80 + | 2.710 | 2.056–3.574 | *** | 2.448 | 1.824–3.286 | *** | 1.908 | 0.764–4.767 | 0.166 | 2.282 | 1.061–5.617 | * |
| Months from diagnosis to therapy | ||||||||||||
| 0 month | Reference | Reference | Reference | Reference | ||||||||
| ≥ 1 month | 0.842 | 0.753–0.942 | ** | 0.846 | 0.751–0.953 | ** | 0.799 | 0.580–1.088 | 0.159 | 0.782 | 0.569–1.075 | 0.128 |
| Subtype | ||||||||||||
| HR + /HER2- | Reference | Reference | Reference | Reference | ||||||||
| HR + /HER2 + | 0.772 | 0.659–0.904 | ** | 0.790 | 0.668–0.934 | ** | 0.900 | 0.559–1.497 | 0.674 | 0.860 | 0.516–1.435 | 0.564 |
| HR-/HER2 + | 0.962 | 0.813–1.138 | 0.653 | 0.983 | 0.822–1.174 | 0.846 | 2.163 | 1.407–3.606 | *** | 2.029 | 1.251–3.290 | ** |
| HR-/HER2- | 1.744 | 1.511–2.015 | *** | 1.830 | 1.572–2.129 | *** | 3.575 | 2.380–5.771 | *** | 3.369 | 2.150–5.278 | *** |
| Race | ||||||||||||
| White | Reference | Reference | Reference | Reference | ||||||||
| Black | 1.266 | 1.107–1.448 | *** | 1.222 | 1.057–1.412 | ** | 1.002 | 0.601–1.496 | 0.994 | 0.936 | 0.581–1.510 | 0.787 |
| Other | 0.883 | 0.735–1.061 | 0.185 | 0.812 | 0.663–0.993 | * | 0.824 | 0.480–1.541 | 0.519 | 0.754 | 0.411–1.382 | 0.361 |
| Histological type | ||||||||||||
| IDC | Reference | Reference | Reference | Reference | ||||||||
| ILC | 0.992 | 0.779–1.262 | 0.947 | 0.955 | 0.738–1.236 | 0.728 | 2.794 | 1.266–6.197 | * | 2.838 | 1.297–6.214 | ** |
| Mixed | 0.887 | 0.679–1.158 | 0.449 | 0.857 | 0.644–1.139 | 0.287 | 1.040 | 0.536–2.027 | 0.908 | 0.934 | 0.468–1.862 | 0.846 |
| Other | 1.354 | 1.209–1.516 | *** | 1.225 | 1.083–1.386 | ** | 1.422 | 0.784–2.724 | 0.270 | 1.015 | 0.484–2.128 | 0.970 |
| Marital status | ||||||||||||
| Married | Reference | Reference | Reference | Reference | ||||||||
| Singled | 1.262 | 1.111–1.435 | *** | 1.251 | 1.091–1.435 | ** | 1.793 | 1.198–2.695 | ** | 1.879 | 1.253–2.819 | ** |
| Widow/divorced/other | 1.519 | 1.351–1.706 | *** | 1.531 | 1.353–1.734 | *** | 1.571 | 1.034–2.253 | * | 1.543 | 1.044–2.279 | * |
| T Stage | ||||||||||||
| T1 | Reference | Reference | Reference | Reference | ||||||||
| T2 | 1.064 | 0.878–1.291 | 0.526 | 1.146 | 0.931–1.412 | 0.199 | 1.839 | 0.986–3.360 | 0.051 | 1.915 | 0.998–3.650 | 0.056 |
| T3 | 1.137 | 0.921–1.405 | 0.233 | 1.231 | 0.981–1.545 | 0.073 | 1.652 | 0.784–3.289 | 0.168 | 1.543 | 0.756–3.146 | 0.233 |
| T4 | 1.248 | 1.042–1.495 | * | 1.337 | 1.099–1.626 | ** | 1.918 | 1.025–3.419 | * | 1.910 | 1.023–3.566 | * |
| N Stage | ||||||||||||
| N0 | Reference | Reference | refereNce | Reference | ||||||||
| N1 | 0.934 | 0.815–1.071 | 0.327 | 0.951 | 0.822–1.100 | 0.502 | 0.979 | 0.646–1.584 | 0.925 | / | / | / |
| N2 | 0.788 | 0.645–0.963 | * | 0.834 | 0.675–1.029 | 0.09 | 0.864 | 0.411–1.702 | 0.687 | / | / | / |
| N3 | 0.921 | 0.777–1.092 | 0.342 | 0.962 | 0.804–1.152 | 0.676 | 1.171 | 0.718–1.907 | 0.524 | / | / | / |
| Grade | ||||||||||||
| Well differentiated | Reference | Reference | Reference | Reference | ||||||||
| Moderately differentiated | 1.175 | 0.871–1.586 | 0.291 | 1.266 | 0.914–1.754 | 0.156 | 0.906 | 0.423–1.939 | 0.799 | 0.820 | 0.370–1.820 | 0.626 |
| Poorly differentiated | 1.435 | 1.072–1.921 | * | 1.561 | 1.137–2.145 | ** | 1.140 | 0.529–2.458 | 0.738 | 1.177 | 0.530–2.618 | 0.689 |
| Median household income(inflation adjusted) | ||||||||||||
| < 40,000$ | Reference | Reference | Reference | Reference | ||||||||
| 40,000–49,999$ | 1.055 | 0.823–1.352 | 0.676 | 1.006 | 0.774–1.308 | 0.965 | 0.559 | 0.254–1.230 | 0.148 | 0.604 | 0.277–1.320 | 0.207 |
| 50,000–59,999$ | 1.053 | 0.888–1.249 | 0.477 | 1.058 | 0.823–1.361 | 0.659 | 0.728 | 0.357–1.488 | 0.385 | 0.733 | 0.356–1.506 | 0.398 |
| 60,000–69,999$ | 0.931 | 0.805–1.076 | 0.748 | 0.936 | 0.742–1.181 | 0.579 | 0.724 | 0.381–1.377 | 0.325 | 0.651 | 0.341–1.243 | 0.193 |
| 70,000$ + | 0.760 | 0.656–0.879 | * | 0.751 | 0.595–0.948 | * | 0.522 | 0.271–1.006 | 0.052 | 0.523 | 0.273–0.999 | * |
| Chemotherapy | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.454 | 0.410–0.502 | *** | 0.477 | 0.428–0.531 | *** | 0.386 | 0.267–0.558 | *** | 0.436 | 0.301–0.637 | *** |
| Radiotherapy | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.629 | 0.568–0.697 | *** | 0.667 | 0.598–0.744 | *** | 0.721 | 0.499–1.042 | 0.082 | 0.753 | 0.520–1.092 | 0.135 |
| Surgery | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.619 | 0.529–0.724 | *** | 0.615 | 0.520–0.727 | *** | 0.693 | 0.430–1.117 | 0.132 | 0.742 | 0.464–1.186 | 0.212 |
| Bone metastases | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.956 | 0.859–1.063 | 0.407 | 1.002 | 0.894–1.123 | 0.973 | / | / | / | / | / | / |
| Liver metastases | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.290 | 1.159–1.436 | *** | 1.283 | 1.144–1.438 | *** | 1.286 | 0.892–1.860 | 0.179 | 1.264 | 0.877–1.823 | 0.209 |
| Lung metastases | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.434 | 1.294–1.589 | *** | 1.426 | 1.278–1.592 | *** | 1.606 | 1.157–2.230 | ** | 1.698 | 1.219–2.365 | ** |
| Distant Lymph nodes metastases | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.128 | 0.942–1.352 | 0.190 | 1.093 | 0.900–1.327 | 0.368 | / | / | / | / | / | / |
| Distant other metastases | ||||||||||||
| No/unknown | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.331 | 1.101–1.609 | ** | 1.322 | 1.079–1.620 | ** | 1.195 | 0.829–1.723 | 0.339 | 1.216 | 0.842–1.757 | 0.296 |
*P < 0.05,
**P < 0.01,
***P < 0.001
Then, we performed multivariable COX regression analysis to eliminate confounding factors and uncover the independent factors that influence OS and BCSS (Table 2). It showed that in patients aged > 50, ILC, T4 stage, lung metastases were greatly related to worse OS and BCSS. Patients with HR−/HER2 + and HR−/HER2-subtypes demonstrated poorer OS and BCSS than HR + /HER2-patients, whereas there was no difference between HR + /HER2- and HR + /HER2 + . In terms of treatment, it showed that only chemotherapy was able to prolong OS and BCSS in multivariable COX regression analysis rather than radiotherapy and primary tumor surgery. The prognosis was also influenced by a few social factors, including marital status and financial stability of the family. Married status and yearly household income of over USD$70,000 were tightly linked to higher survival.
Establishing and evaluating predictive models for estimating the prognosis of patients with BCBM
In light of the results obtained, we took steps to establish an XGBoost prediction model to predict the OS of BCBM patients at six months, one year, two years, and three years. We sorted the patients into train and test data group in a 7:3 ratio. And to ensure the stability of the model, we used ten-fold cross-validation in the training set for iterative testing and tuning so as to confirm the key hyperparameters and generate the optimal model (Table 3). For the train and validation sets, we formed the predicted ROC curves and computed the corresponding AUCs. Our XGBoost model performed exceptionally well in predicting survival of BCBM patients at 6-month (test set: AUC = 0.824; train set AUC = 0.828), 1-year (test set: AUC = 0.813; train set AUC = 0.831), 2-year (test set: AUC = 0.800; train set AUC = 0.819) and 3-year (test set: AUC = 0.803; train set AUC = 0.834) (Fig. 2). Compared to traditional machine learning algorithms, LR (6-month: AUC = 0.794; 1-year: AUC = 0.744; 2-year: AUC = 0.740; 3-year: AUC = 0.744), RF (6-month: AUC = 0.770; 1-year: AUC = 0.729; 2-year: AUC = 0.730; 3-year: AUC = 0.756), SVM (6-month: AUC = 0.730; 1-year: AUC = 0.647; 2-year: AUC = 0.525; 3-year: AUC = 0.509), KNN (6-month: AUC = 0.738; 1-year: AUC = 0.623; 2-year: AUC = 0.581; 3-year: AUC = 0.586) and ID3 (6-month: AUC = 0.692; 1-year: AUC = 0.628; 2-year: AUC = 0.685; 3-year: AUC = 0.639), XGBoost model performed best (Table 4).
Table 3.
Main parameters of the XGBoost model
| Parameter | Value |
|---|---|
| Gamma | 1 |
| Min_child_weight | 10 |
| Subsample | 0.8 |
| Max_delta_step | 6 |
| Alpha | 2 |
| Max_depth | 5 |
| Eta | 0.17 |
| nround | 25 |
Fig. 2.
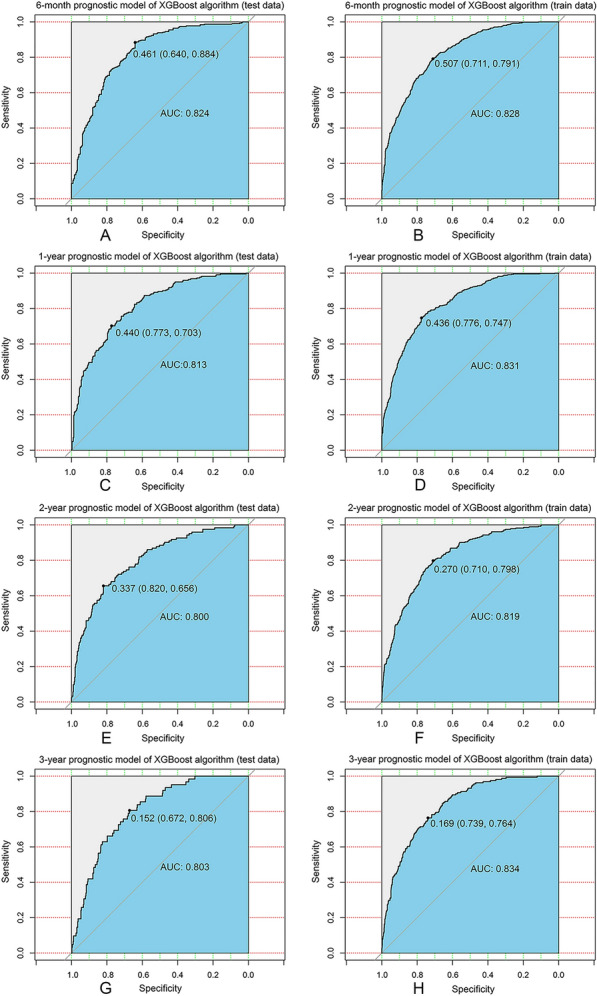
XGBoost model evaluation. A ROC curve for the 6-month prognostic model (test data); B ROC curve for the 6-month prognostic model (train data); C ROC curve for the 1-year prognostic model (test data); D ROC curve for the 1-year prognostic model (train data); E ROC curve for the 2-year prognostic model (test data); F ROC curve for the 2-year prognostic model (train data); G ROC curve for the 3-year prognostic model (test data); H ROC curve for the 3-year prognostic model (train data); ROC receiver operating characteristic curve, AUC area under the curve, XGBoost extreme Gradient Boosting
Table 4.
Performance of prognostic models built by machine learning algorithms on test data (area under the ROC curve)
| 6-month survival | 1-year survival | 2-year survival | 3-year survival | |
|---|---|---|---|---|
| XGBoost | 0.824 | 0.813 | 0.800 | 0.803 |
| LR | 0.794 | 0.744 | 0.740 | 0.744 |
| RF | 0.770 | 0.729 | 0.730 | 0.756 |
| SVM | 0.730 | 0.647 | 0.525 | 0.509 |
| KNN | 0.738 | 0.623 | 0.581 | 0.586 |
| ID3 | 0.692 | 0.628 | 0.685 | 0.639 |
XGBoost extreme gradient boosting, LR logistic regression, RF random forest, SVM support vector machine, ID3 decision tree, KNN K-Nearest Neighbor
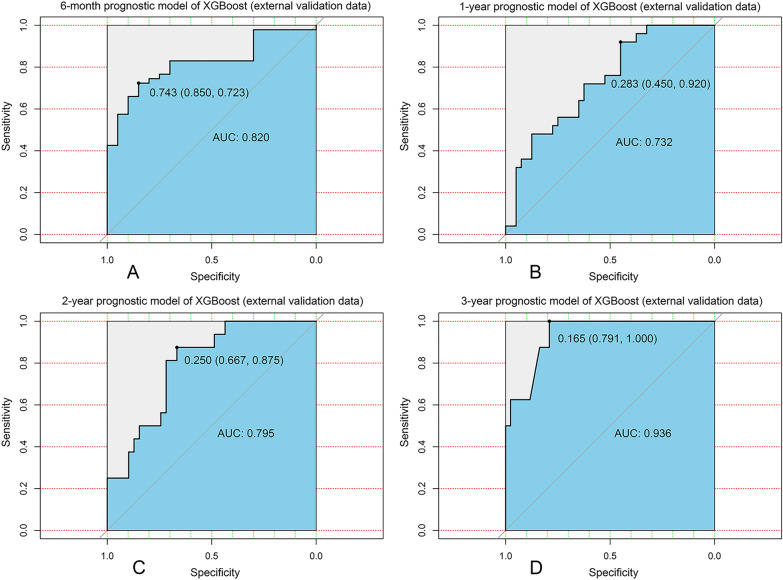
In order to further validate our models, we collected clinical and prognostic information from 67 patients with BCBM from our hospital (Additional file 1: Table S1). It showed that our XGBoost models still exhibited good robustness in an externally independent dataset [6-month: AUC = 0.820 (Fig. 3A); 1-year: AUC = 0.732 (Fig. 3B); 2-year: AUC = 0.795 (Fig. 3C); 3-year: AUC = 0.936 (Fig. 3D)].
Fig. 3.
Validation of XGBoost models from external database. A ROC curve for the 6-month prognostic model (external validation data); B ROC curve for the 1-year prognostic model (external validation data); C ROC curve for the 2-year prognostic model (external validation data); D ROC curve for the 3-year prognostic model (external validation data); ROC receiver operating characteristic curve; AUC area under the curve; XGBoost extreme gradient boosting
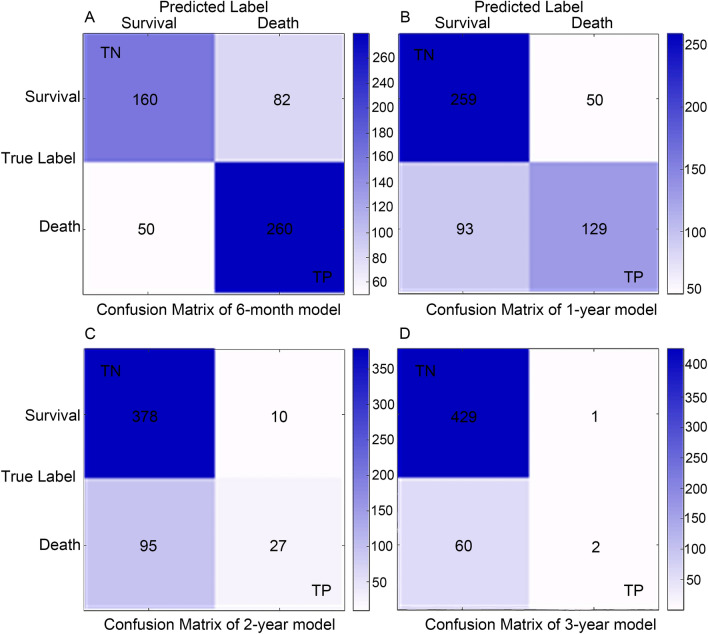
Then, the effectiveness and precision of our XGBoost model was then assessed using a confusion matrix. The 6-month survival prediction model was calculated to have a correctness of 0.76 and a precision of 0.76 (Fig. 4A); the 1-year survival model had a correctness of 0.73 and a precision of 0.72 (Fig. 4B); the 2-year survival model had a correctness of 0.79 and a precision of 0.73 (Fig. 4C). And the 3-year survival model had a correctness of 0.88 and a precision of 0.67 (Fig. 4D). In general, our models behaved efficiently and successfully.
Fig. 4.
Confusion matrix of the XGBoost model’s predicted results in the test data. A Confusion matrix in the 6-month prognostic model; B confusion matrix in the 1-year prognostic model; C confusion matrix in the 2-year prognostic model; D confusion matrix in the 3-year prognostic model. TP true positive, TN true negative
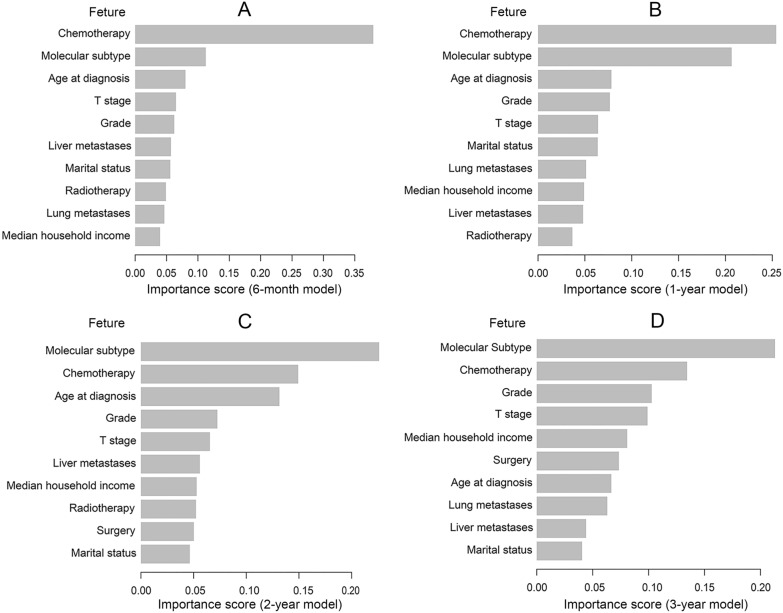
Additionally, we graded how prominent clinical traits were in the models. The findings revealed that the top 5 factors affecting prognosis were chemotherapy, molecular subtype, age at diagnosis, grade and T stage. Among them, chemotherapy was the most important factor for short-term prognostic models (6-month and 1-year) (Fig. 5A and B), while molecular subtype was more important for medium- to long-term prognostic models (2 and 3-year) (Fig. 5C and D).
Fig. 5.
The ranking of clinical characteristics in terms of importance in the XGBoost prognostic model. A The ranking of clinical characteristics in terms of importance in the 6-month prognostic model; B the ranking of clinical characteristics in terms of importance in the 1-year prognostic model; C the ranking of clinical characteristics in terms of importance in the 2-year prognostic model; D the ranking of clinical characteristics in terms of importance in the 2-year prognostic model. XGBoost: extreme Gradient Boosting
Web-based application development
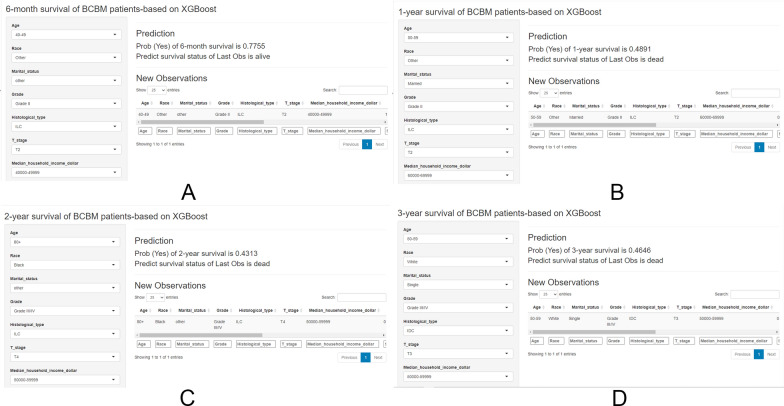
To help researchers and clinicians learn to use our prognostic models, we have developed user-friendly web applications based on the shiny platform. The web interfaces (Fig. 6A–D) allow users to input clinical characteristics of a new sample and then the web application can help predict survival probabilities and survival status according to BCBM patient’s information.
Fig. 6.
Screenshot of web app. A The screenshot of the 6-month prognostic model (https://lee2287171854.shinyapps.io/6-month_survival/); B the screenshot of the 1-year prognostic model (https://lee2287171854.shinyapps.io/1-year_survival/); C the screenshot of the 2-year prognostic model (https://lee2287171854.shinyapps.io/2-year_survival/); D the screenshot of the 3-year prognostic model (https://lee2287171854.shinyapps.io/3-year_survival/). XGBoost: extreme Gradient Boosting; BCBM: breast cancer brain metastases; NA: not applicable; 1 = yes, 0 = no; HR ± hormone receptor positive/negative, HER2 ± human epidermal growth factor receptor 2 positive/negative, IDC infiltrating ductal carcinoma, ILC infiltrating lobular carcinoma, Mixed: Infiltrating ductal and lobular carcinoma
Benefits of surgical treatment in BCBM patients subdivided by molecular subtypes
Previous studies proved that surgical treatment was an independent prognostic factor for BCBM patients [10–12]. However, our multivariable COX regression analysis gave us the opposite result (Table2). Furthermore, we explored how surgery affected the prognosis of BCBM patients. Patients undergoing surgical therapy and those not undergoing surgery were compared based on their baseline characteristics (Table 5). These two groups had different baselines. Therefore, PSM was employed to adjust for the observed imbalance. After PSM correction, there were ultimately no significant differences in baseline characteristics (Table 5).
Table 5.
Comparison of patient characteristics according to surgery treatment before and after propensity score matching (PSM)
| Characteristics | Unmatched Cohort | 1:1 propensity score matched (PSM) Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | Surgery not given | Unadjusted | Surgery | Surgery not given | PSM-adjusted | |||||
| N = 234 | % | N = 1699 | % | P value | N = 200 | % | N = 200 | % | P value | |
| Age at diagnosis | 0.004 | 0.951 | ||||||||
| < 40 | 24 | 10.26 | 117 | 6.89 | 19 | 9.50 | 18 | 9.00 | ||
| 40–49 | 41 | 17.52 | 210 | 12.36 | 34 | 17.00 | 32 | 16.00 | ||
| 50–59 | 72 | 30.77 | 457 | 26.90 | 60 | 30.00 | 59 | 29.50 | ||
| 60–69 | 57 | 24.36 | 519 | 30.55 | 49 | 24.50 | 50 | 25.00 | ||
| 70–79 | 23 | 9.83 | 284 | 16.72 | 23 | 11.50 | 29 | 14.50 | ||
| 80 + | 17 | 7.26 | 112 | 6.59 | 15 | 7.50 | 12 | 6.00 | ||
| Subtype | < 0.001 | 0.815 | ||||||||
| HR + /HER2- | 85 | 36.32 | 632 | 37.20 | 75 | 37.50 | 68 | 34.00 | ||
| HR + /HER2 + | 33 | 14.10 | 275 | 16.19 | 30 | 15.00 | 29 | 14.50 | ||
| HR-/HER2 + | 29 | 12.39 | 210 | 12.36 | 26 | 13.00 | 29 | 14.50 | ||
| HR-/HER2- | 64 | 27.35 | 269 | 15.83 | 48 | 24.00 | 46 | 23.00 | ||
| Unknown | 23 | 9.83 | 313 | 18.42 | 21 | 10.50 | 28 | 14.00 | ||
| Race | 0.830 | 0.786 | ||||||||
| White | 172 | 73.50 | 1275 | 75.04 | 143 | 71.50 | 148 | 74.00 | ||
| Black | 38 | 16.24 | 269 | 15.83 | 34 | 17.00 | 33 | 16.50 | ||
| Other | 24 | 10.26 | 155 | 9.12 | 23 | 11.50 | 19 | 9.50 | ||
| Histological type | < 0.001 | 0.700 | ||||||||
| IDC | 177 | 75.64 | 1082 | 63.68 | 149 | 74.50 | 149 | 74.50 | ||
| ILC | 11 | 4.70 | 86 | 5.06 | 10 | 5.00 | 6 | 3.00 | ||
| Mixed | 12 | 5.13 | 58 | 3.41 | 10 | 5.00 | 9 | 4.50 | ||
| Other | 34 | 14.53 | 473 | 27.84 | 31 | 15.50 | 36 | 18.00 | ||
| Marital status | 0.132 | 0.623 | ||||||||
| Married | 105 | 44.87 | 685 | 40.32 | 86 | 43.00 | 81 | 40.50 | ||
| Single | 64 | 27.35 | 430 | 25.31 | 56 | 28.00 | 52 | 26.00 | ||
| Others | 65 | 27.78 | 584 | 34.37 | 58 | 29.00 | 67 | 33.50 | ||
| T stage | < 0.001 | 0.802 | ||||||||
| T1 | 34 | 14.53 | 162 | 9.54 | 25 | 12.50 | 28 | 14.00 | ||
| T2 | 69 | 29.49 | 343 | 20.19 | 53 | 26.50 | 48 | 24.00 | ||
| T3 | 31 | 13.25 | 217 | 12.77 | 29 | 14.50 | 23 | 11.50 | ||
| T4 | 84 | 35.90 | 556 | 32.73 | 77 | 38.50 | 86 | 43.00 | ||
| Tx | 16 | 6.84 | 421 | 24.78 | 16 | 8.00 | 15 | 7.50 | ||
| Grade | < 0.001 | 0.923 | ||||||||
| Well | 9 | 3.85 | 58 | 3.41 | 8 | 4.00 | 7 | 3.50 | ||
| Moderately | 52 | 22.22 | 396 | 23.31 | 48 | 24.00 | 43 | 21.50 | ||
| Poorly | 146 | 62.39 | 610 | 35.90 | 117 | 58.50 | 121 | 60.50 | ||
| Unknown | 27 | 11.54 | 635 | 37.37 | 27 | 13.50 | 29 | 14.50 | ||
| Median household income (inflation adjusted) | 0.093 | 0.944 | ||||||||
| < 40,000$ | 19 | 8.12 | 85 | 5.00 | 15 | 7.50 | 14 | 7.00 | ||
| 40,000–49,999$ | 30 | 12.82 | 189 | 11.12 | 24 | 12.00 | 28 | 14.00 | ||
| 50,000–59,999$ | 41 | 17.52 | 251 | 14.77 | 35 | 17.50 | 30 | 15.00 | ||
| 60,000–69,999$ | 76 | 32.48 | 566 | 33.31 | 63 | 31.50 | 63 | 31.50 | ||
| 70,000$ + | 68 | 29.06 | 608 | 35.79 | 63 | 31.50 | 65 | 32.50 | ||
| Chemotherapy | < 0.001 | 0.586 | ||||||||
| No/unknown | 66 | 28.21 | 812 | 47.79 | 63 | 31.50 | 58 | 29.00 | ||
| Yes | 168 | 71.79 | 887 | 52.21 | 137 | 68.50 | 142 | 71.00 | ||
| Radiotherapy | < 0.001 | 0.517 | ||||||||
| No/unknown | 60 | 25.64 | 697 | 41.02 | 59 | 29.50 | 65 | 32.50 | ||
| Yes | 174 | 74.36 | 1002 | 58.98 | 141 | 70.50 | 135 | 67.50 | ||
| Liver metastases | < 0.001 | 0.370 | ||||||||
| No/unknown | 193 | 82.48 | 1096 | 64.51 | 159 | 79.50 | 166 | 83.00 | ||
| Yes | 41 | 17.52 | 603 | 35.49 | 41 | 20.50 | 34 | 17.00 | ||
| Lung metastases | 0.002 | 0.918 | ||||||||
| No/unknown | 154 | 65.81 | 938 | 55.21 | 124 | 62.00 | 123 | 61.50 | ||
| Yes | 80 | 34.19 | 761 | 44.79 | 76 | 38.00 | 77 | 38.50 | ||
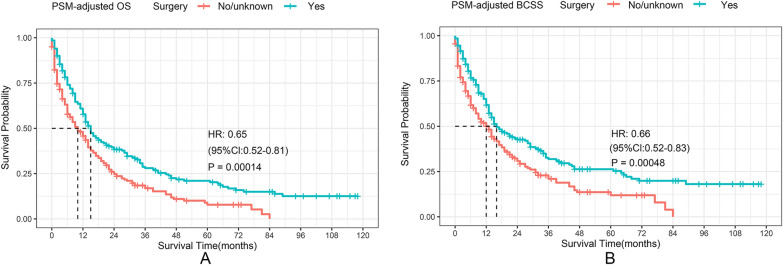
A 35% decrease in the overall risk of mortality in the surgery was observed in the PSM-adjusted data group (P = 0.00014, HR: 0.65; 95% CI 0.52–0.81) (Fig. 7A), with a similar reduction in the risk of BC-related death of approximately 34% (P = 0.00048, HR: 0.66; 95% CI 0.52–0.83) (Fig. 7B). The OS and BCSS of the BC patients with HR + /HER2 + and HR−/HER2 + subtypes enormously improved after surgery, according to the stratified K–M survival analysis. (Fig. 8B, C, F, G). However, no significant difference in HR + /HER2− subtype can be found (Fig. 8A, E). In addition, the effect of surgical treatment on OS and BCSS in patients with HR−/HER2− subtypes was different. To further validate these results, we divided all the 1933 eligible BCBM patients into four groups according to molecular subtype and performed univariate and multivariable COX analyses again (Additional file 2: Table S2). It showed that only HR + /HER2− subtype could not benefit from surgical treatment, which was consistent with our results of the PSM-adjusted K–M survival analysis.
Fig. 7.
PSM adjusted OS and BCSS of BCBM patients with surgical treatment. Kaplan–Meier (K–M) survival analysis: A unadjusted OS of BCBM patients with surgical treatment; B PSM adjusted OS of BCBM patients with surgical treatment. PSM propensity score matching, OS overall survival; BCBM BC brain metastases, HR hazard ratio, CI confidence interval
Fig. 8.
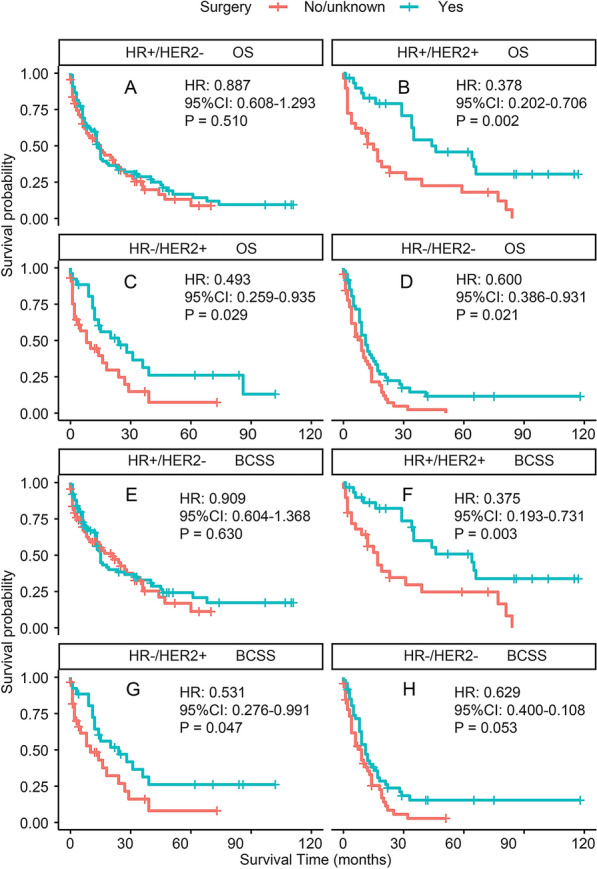
PSM adjusted OS and BCSS of BCBM patients with surgical treatment (Stratified by molecular subtype). Kaplan–Meier (K–M) survival analysis: A OS of BCBM patients with HR + /HER2− subtype; B OS of BCBM patients with HR + /HER2 + subtype; C OS of BCBM patients with HR−/HER2 + subtype; D OS of BCBM patients with HR−/HER2− subtype; E BCSS of BCBM patients with HR + /HER2− subtype; F BCSS of BCBM patients with HR + /HER2 + subtype; G BCSS of BCBM patients with HR−/HER2 + subtype; H BCSS of BCBM patients with HR−/HER2− subtype. OS overall survival, BCSS BC-specific survival, BCBM BC brain metastases, HR ± hormone receptor positive/negative, HER2 ± human epidermal growth factor receptor 2 positive/negative, PSM propensity score matching, HR hazard ratio, CI confidence interval
Discussion
The bone, lung, brain and liver etc. are the organs where BC might metastasis with a high probability of success. Different patient prognoses and reactions to therapy result from this organotropism [21]. Brain metastases are the most fatal. For these BCBM patients deemed, incurable, survival time is their foremost concern. The clinic practice, however, lacks reliable predictive models. In recent investigations, multiple nomogram prediction models for BCBM patients were constructed with the help of SEER datasets, but their accuracy rates are all less than 70% [10–12]. In consequence, more accurate and powerful models are needed. To our knowledge, the current study is the largest one to analyze the clinical characteristics and prognosis of BCBM patients. The 6-month, 1-, 2-, and 3-year OS of BCBM patients is 54.44% %, 40.51%, 23.78% and 13.61%, respectively. This study is the first one to create AI prognostic models for BCBM patients, and the models we made are the most accurate in predicting the survival of BCBM patients. In practice, our XGBoost models still exhibited good performance in an externally independent dataset. This demonstrates the high clinical utility of the models. Moreover, we have also created the first model for predicting the 3-year survival of BCBM patients with high accuracy.
This study identified several independent factors associated with better prognosis, including age < 50, HR + molecular subtype, IDC, married, low T stage, median household income over USD$70,000 and chemotherapy. Age > 40 years was a risk factor for BCBM patients to experience a worse OS, according to previous research [10, 18], whereas age 45–64 years was also a risk factor [12]. We analyzed more age groups and found that age > 50 was a feature for worse OS and BCSS. Compared to the HR + subtype, the patients with the HR− subtype showed poorer survival, similar to several previous studies [10, 11], and implied the importance of endocrine therapy for HR + BCBM patients. According to the research, the survival of BC patients could be impacted by household income [22]. Generally, patients with higher incomes have better prognoses. The OS and BCSS of BCBM patients with incomes over USD $70,000 were shown to be superior to those with incomes under USD $40,000 in our study. No income level boundary among BC patients was documented previously, while this may be a reflection of how well they cooperate with doctors throughout treatment. Several studies showed that extracranial organ metastases worsened the prognosis of patients with BCBM [10, 23]. Our study found that only lung metastasis is an independent poor prognostic factor for patients with BCBM, while bone, liver, distant lymph nodes and other metastases were not. In contrast, two previous studies indicated that liver metastasis was also an independent factor of BCBM patients [19, 20], but their studies only covered about 700 patients, which was much smaller than ours and incorporated fewer factors. For example, the study by Leone et al. did not even include chemotherapy as an important factor [20].
In terms of treatment, our analysis showed that only chemotherapy was an independent protective factor for all BCBM patients. Consistent with previous studies[10–12, 19], we also found radiotherapy was not an independent prognostic factor for BCBM patients, which further validated the effect of chemotherapy and radiotherapy on OS and BCSS of BCBM. One controversial topic is whether surgical therapy for the primary site improves the survival of BCBM patients. Previous studies showed that surgical treatment was an independent prognostic factor for BCBM patients [10–12]. However, our result was exactly the opposite of it, and another study indicated that surgical therapy, with the exception of brain metastases, positively affected the prognosis in primary metastatic BC patients with a single distant metastasis. [24]. Whether surgical therapy for the primary site prolongs survival time in patients with de novo metastatic BC has long been debatable, but current results imply that in well-selected patients, primary surgery might be a therapeutic option [25–33]. To more explicitly categorize the patients, we subsequently looked into the impact of surgery on the prognosis of BCBM patients with various molecular subtypes. In BCBM patients with HER2 + molecular subtypes, it was found that surgical intervention dramatically enhanced both OS and BCSS, suggesting that anti-HER2-targeted therapy combined with surgical treatment may prolong the survival of BCBM patients. We also found that for BCBM patients with HR-/HER2- subtype, the OS, but not BCSS, could benefit from surgery. In contrast, surgery could not help BCBM patients with HR + /HER2− subtype improve their prognosis, suggesting that chemotherapy and endocrine therapy are more important for these patients. Our findings suggested the necessity of surgery for HER2 + and triple-negative BCs (TNBC), which had the greatest incidence of brain metastases, compared with other BC subtypes [34, 35].
Our study may have some potential limitations despite its promising discoveries. First, although the SEER database includes about 30% of the USA population, this study’s sample size was constrained because the SEER database only incorporates the clinical data on tumor subtypes and distant metastatic sites following 2010. Second, the SEER database can greatly represent the general situation, but due to ethnic differences, it may not always apply to Asian and especially the Chinese. Third, the SEER database does not incorporate data on disease recurrence or subsequent sites of metastases. Therefore, we could not go further and look into the patients who developed brain metastases later in their remaining years, which may potentially result in some bias. Fourth, elaborate information on treatments of patients with brain metastases is not collected in the SEER database. We were unable to go deeper on this consequently. Furthermore, despite the extraordinary accuracy the machine learning prognostic model has achieved, external validation could be strengthened so that the study results can be more reliable.
Conclusion
In conclusion, we analyzed the clinical features of BCBM patients and constructed 4 machine-learning prognostic models to predict their survival. According to the findings of our validation, these models are considered to be highly reproducible in BCBM patients. We further revealed potential prognostic variables for BCBM patients, and the survival of BCBM patients with the HER2 + and triple-negative subtypes may be greatly improved by primary surgery.
Supplementary Information
Additional file 1: Table S1. Clinical and prognostic information from patients with BCBM from our hospital.
Additional file 2: Table S2. Univariate and multivariate COX analysis of characteristics (stratified by molecular subtype).
Acknowledgements
We thank all staff of the SEER database for their contribution in data collection, maintenance, distribution and so on. Also we would like to thank all the developers of the R programming package for selflessly sharing their code.
Abbreviations
- BCBM
Breast cancer brain metastases
- SEER
Surveillance, epidemiology, and end results
- AUC
Area under the curve
- COX
Concordance index
- PSM
Propensity score matching
- HR
Hazard ratio
- CI
Confidence interval
- OS
Overall survival
- BCSS
Breast cancer-specific survival
- HR ±
Hormone receptor positive/negative
- HER2
Human epidermal growth factor receptor 2
- BC
Breast cancer
- LR
Logistic regression
- SVM
Support vector machine
- RF
Random forest
- KNN
K-nearest neighbor
- ID3
Decision tree
- AI
Artificial intelligence
- ICD-O-3:
International classification of cancer diseases edition III
Author contributions
Conceptualization, CL, JQ and SZ; methodology, CL, JQ, ML and Y.Z.; formal analysis, CL and JL; data curation, SS and XL; writing—original draft preparation, YW, HW and CF; writing—review and editing, PY, YJ, YZ and XW; supervision, FW, CD and XZ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by the following: National Science Foundation of China (81903856, to X. Zhao; 82174164, to S.Q. Zhang, 82103569, to J.K. Qu); Key Science and Technology Program of Shaanxi Province (2021KW-57, to X Zhao; 2021KW-60, to J.K. Qu). Scientific research fund of the Second Affiliated Hospital of Xi’an Jiaotong University (RC (XM) 202004, to X Zhao). Free exploring fund of Xi’an Jiaotong University (xzy012022096, to X Zhao; xzy012022097 to J.K. Qu). Medical “basic—clinical” integration and innovation project of Xi’an Jiaotong University (YXJLRH2022088 to JQ).
Availability of data and materials
All data here are publicly available in the SEER database [https://seer.cancer.gov/ (accessed on April 15, 2022)].
Declarations
Ethics approval and consent to participate
Ethical review and approval were waived for this study due to the fact that the data are fully de-identified and no intervention on patients was performed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuqun Zhang, Email: shuqun_zhang1971@163.com.
Jingkun Qu, Email: qujingkun@xjtu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Eckel R, Aydemir U, Aydemir S, Kerr J, Schlesinger-Raab A, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1186–1195. doi: 10.1016/S0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 4.Shaffrey ME, Mut M, Asher AL, Burri SH, Chahlavi A, Chang SM, et al. Brain metastases. Curr Probl Surg. 2004;41(8):665–741. doi: 10.1067/j.cpsurg.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4:33. doi: 10.1186/s40164-015-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witzel I, Oliveira-Ferrer L, Pantel K, Müller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):8. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71(1):64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 8.Mayer M. A patient perspective on brain metastases in breast cancer. Clin Cancer Res. 2007;13(6):1623–1624. doi: 10.1158/1078-0432.CCR-06-2842. [DOI] [PubMed] [Google Scholar]
- 9.Tyuryumina EY, Neznanov AA. Consolidated mathematical growth model of the primary tumor and secondary distant metastases of breast cancer (CoMPaS) PLoS ONE. 2018;13(7):e0200148. doi: 10.1371/journal.pone.0200148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Kong X, Wang Z, Wang X, Zhang W, Ai B, et al. NCCBM, a nomogram prognostic model in breast cancer patients with brain metastasis. Front Oncol. 2021;11:642677. doi: 10.3389/fonc.2021.642677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun MS, Liu YH, Ye JM, Liu Q, Cheng YJ, Xin L, et al. A nomogram for predicting brain metastasis in patients with de novo stage IV breast cancer. Ann Transl Med. 2021;9(10):853. doi: 10.21037/atm-21-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong Y, Cao H, Zhang Y, Pan Z, Dong S, Wang G, et al. Nomogram-predicted survival of breast cancer brain metastasis: a SEER-based population study. World Neurosurg. 2019;128:e823–e834. doi: 10.1016/j.wneu.2019.04.262. [DOI] [PubMed] [Google Scholar]
- 13.Sajda P. Machine learning for detection and diagnosis of disease. Annu Rev Biomed Eng. 2006;8(1):537–565. doi: 10.1146/annurev.bioeng.8.061505.095802. [DOI] [PubMed] [Google Scholar]
- 14.Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13(1):152. doi: 10.1186/s13073-021-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan KC, Tsai LW, Lee KH, Cheng YW, Hsu SC, Lo YS, et al. The development an artificial intelligence algorithm for early sepsis diagnosis in the intensive care unit. Int J Med Informatics. 2020;141:104176. doi: 10.1016/j.ijmedinf.2020.104176. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Tran H. An XGBoost-based fitted Q iteration for finding the optimal STI strategies for HIV patients. IEEE Trans Neural Netw Learning Syst. 2022 doi: 10.1109/TNNLS.2022.3176204. [DOI] [PubMed] [Google Scholar]
- 17.Ye Q, Chai X, Jiang D, Yang L, Shen C, Zhang X, et al. Identification of active molecules against Mycobacterium tuberculosis through machine learning. Briefings Bioinform. 2021;22(5):bbab068. doi: 10.1093/bib/bbab068. [DOI] [PubMed] [Google Scholar]
- 18.Sun MS, Yun YY, Liu HJ, Yu ZH, Yang F, Xu L. Brain metastases in de novo breast cancer: an updated population-level study from SEER database. Asian J Surg. 2022 doi: 10.1016/j.asjsur.2021.12.037. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–1816. doi: 10.1007/s00432-018-2697-2. [DOI] [PubMed] [Google Scholar]
- 20.Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer brain metastases at initial diagnosis. Eur J Cancer. 2017;74:17–25. doi: 10.1016/j.ejca.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–548. doi: 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 23.Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. doi: 10.1001/jamaoncol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Huang R, Ma L, Liu S, Zong X. Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast. 2019;45:104–112. doi: 10.1016/j.breast.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247(5):732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 26.Fields RC, Jeffe DB, Trinkaus K, Zhang Q, Arthur C, Aft R, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14(12):3345–3351. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 27.Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol. 2007;14(8):2187–2194. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 28.Lang JE, Tereffe W, Mitchell MP, Rao R, Feng L, Meric-Bernstam F, et al. Primary tumor extirpation in breast cancer patients who present with stage IV disease is associated with improved survival. Ann Surg Oncol. 2013;20(6):1893–1899. doi: 10.1245/s10434-012-2844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pons-Tostivint E, Kirova Y, Lusque A, Campone M, Geffrelot J, Mazouni C, et al. Survival impact of locoregional treatment of the primary tumor in de novo metastatic breast cancers in a large multicentric cohort study: a propensity score-matched analysis. Ann Surg Oncol. 2019;26(2):356–365. doi: 10.1245/s10434-018-6831-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang X, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: a real-world observational study. Eur J Surg Oncol. 2019;45(8):1364–1372. doi: 10.1016/j.ejso.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16(13):1380–1388. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 32.Bjelic-Radisic V, Fitzal F, Knauer M, Steger G, Egle D, Greil R, et al. Primary surgery versus no surgery in synchronous metastatic breast cancer: patient-reported quality-of-life outcomes of the prospective randomized multicenter ABCSG-28 Posytive Trial. BMC Cancer. 2020;20(1):392. doi: 10.1186/s12885-020-06894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan SA, Zhao F, Goldstein LJ, Cella D, Basik M, Golshan M, et al. Early local therapy for the primary site in de novo stage IV breast cancer: results of a randomized clinical trial (EA2108) J Clin Oncol. 2022;40(9):978–987. doi: 10.1200/JCO.21.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam BH, Kim SY, Han HS, Kwon Y, Lee KS, Kim TH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Clinical and prognostic information from patients with BCBM from our hospital.
Additional file 2: Table S2. Univariate and multivariate COX analysis of characteristics (stratified by molecular subtype).
Data Availability Statement
All data here are publicly available in the SEER database [https://seer.cancer.gov/ (accessed on April 15, 2022)].