Abstract
Background
Although several studies have confirmed the prognostic value of the consolidation to tumor ratio (CTR) in non-small cell lung cancer (NSCLC), there still remains controversial about it.
Methods
We systematically searched the PubMed, Embase, and Web of Science databases from inception to April, 2022 for eligible studies that reported the correlation between CTR and prognosis in NSCLC. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were extracted and pooled to assess the overall effects. Heterogeneity was estimated by I2 statistics. Subgroup analysis based on the cut-off value of CTR, country, source of HR and histology type was conducted to detect the sources of heterogeneity. Statistical analyses were performed using STATA version 12.0.
Results
A total of 29 studies published between 2001 and 2022 with 10,347 patients were enrolled. The pooled results demonstrated that elevated CTR was associated with poorer overall survival (HR = 1.88, 95% CI 1.42–2.50, P < 0.01) and disease-free survival (DFS)/recurrence-free survival (RFS)/progression-free survival (PFS) (HR = 1.42, 95% CI 1.27–1.59, P < 0.01) in NSCLC. According to subgroup analysis by the cut-off value of CTR and histology type, both lung adenocarcinoma and NSCLC patients who had a higher CTR showed worse survival. Subgroup analysis stratified by country revealed that CTR was a prognostic factor for OS and DFS/RFS/PFS in Chinese, Japanese, and Turkish patients.
Conclusions
In NSCLC patients with high CTR, the prognosis was worse than that with low CTR, indicating that CTR may be a prognostic factor.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03081-y.
Keywords: Non-small cell lung cancer, Consolidation to tumor ratio, Prognostic, Meta-analysis
Introduction
An estimated 1.8 million cancer-related deaths were recorded in 2020 due to lung cancer, according to the latest global statistics on cancer [1]. Most of the pathologic subtypes of lung cancer are NSCLC, in which adenocarcinoma accounts for a large proportion [2]. Although many cases can be detected early and the treatment has been significantly improved, patients with lung cancer continue to face unsatisfactory prognoses, due to metastasis or recurrence [3].
Recently, with the extensive use of chest computed tomography (CT), many lung cancers have been detected to contain ground-glass opacity (GGO), which was defined as an area of a slight, homogenous increase in density without obscuring the underlying vascular markings on CT in previous studies [4]. Several studies have demonstrated that GGO components on CT indicated improved survival in NSCLC, especially in lung adenocarcinoma [5, 6]. The relationship between preoperative radiological findings, such as the maximal standardized uptake value (SUVmax), and tumor disappearance ratio, and the prognosis of NSCLC has attracted close attention, due to improvements in imaging technology [7–9].
The consolidation to tumor ratio, which was calculated as the ratio of the maximum consolidation size to the maximum tumor size measured using the lung window setting on CT in several studies, has been used to select patients for sublobar resection or to predict the prognosis of NSCLC patients [10–12]. There are, however, controversies over the prognostic value of CTR in NSCLC. Kim and his colleagues found that CTR was not an independent prognostic indicator for lung adenocarcinoma patients treated with surgery [13]. Xi et al. confirmed the prognostic value of CTR in lung adenocarcinomas [12]. To assess its prognostic value in NSCLC, we performed this meta-analysis.
Materials and methods
Registration
Our study has been registered in the International Prospective Registry of Systematic Reviews (PROSPERO) (registration number: CRD42022360462). Details of the protocol can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022360462.
Literature retrieval
Relevant studies were collected through systematic searches of the PubMed, Embase and Web of Science databases up to April, 2022. The following MeSH terms were used: “cancer”, “tumor”, “neoplasm”, “carcinoma”, “lung”, “pulmonary”, and “consolidation to tumor ratio”. Additionally, references of all included studies and relevant review articles were searched for available articles.
Inclusion and exclusion criteria
Inclusion criteria: (1) patients were clearly grouped according to the CTR value. (2) Retrospective or prospective studies evaluating the prognostic relationship between CTR and NSCLC. (3) The hazard ratios of OS and/or DFS/RFS/PFS with 95% CIs were reported or sufficient data were obtained to calculate them. (4) NSCLC was confirmed by postoperative pathology. (5) Full-texts were available.
Exclusion criteria: (1) overlapping studies; (2) reviews, case reports, editorials, conference abstracts, or animal trials; (3) the HR or 95% CIs were not available.
Data extraction and quality assessment
Two researchers (Yongming Wu and Wenpeng Song) independently screened the literature. Any disagreement that arose during the study was resolved through team discussion. The following information was extracted: first author, year of publication, country, study time, sample size, median follow-up time, histology type, TNM stage, clinical outcome, cut-off value of CTR, HR, and 95% CIs. The HR information was recorded directly or gathered from Kaplan‒Meier curves using Engauge Digitizer Version 4.1.
The quality of all eligible studies was evaluated by two researchers (Yongming Wu and Wenpeng Song) using the Newcastle‒Ottawa quality assessment scale (NOS). A study was considered high quality if it had an NOS score of 6 or greater.
Statistical analysis
All statistical analyses were conducted using Stata 12.0 software. The pooled HRs of OS or DFS/PFS/RFS and 95% CIs were used to evaluate the relationship between CTR and prognosis in NSCLC. I2 statistics were used to evaluate the heterogeneity. When I2 > 50% and/or P < 0.1, there was obvious heterogeneity, and the random effect model was used, otherwise, the fixed effect model was used [14]. Subgroup analysis based on the cut-off value of CTR, country, source of HR and histology type was performed to explore the source of heterogeneity or further demonstrate the results of the meta-analysis. Sensitivity analysis was used to assess the stability of the results in the enrolled studies. Begg's funnel plot and Egger's test were used to detect publication bias [15, 16]. The trim-and-fill method was used if obvious publication bias was detected. P values less than 0.05 were considered statistically significant.
Results
Literature search
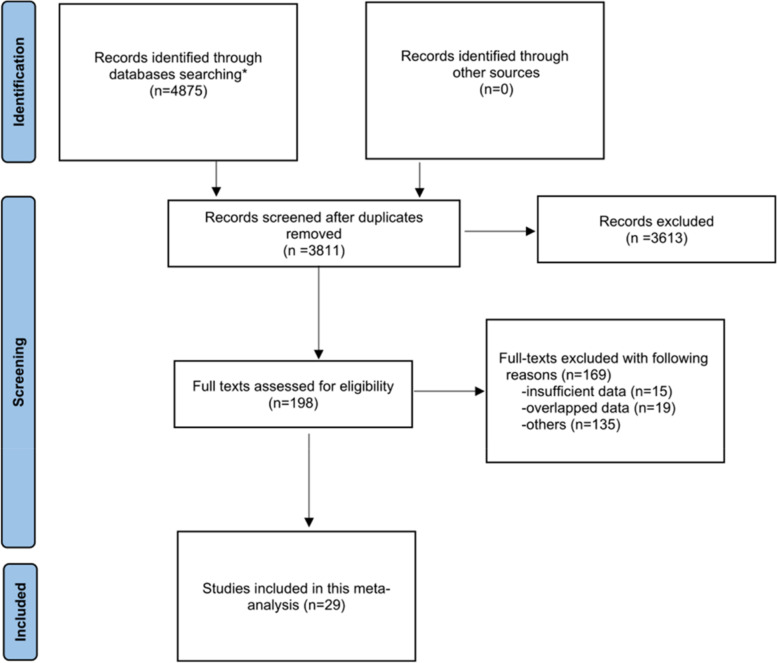
According to the search strategies, 4875 articles were retrieved. After duplicates were removed, we carefully read the titles and abstracts of the 3811 studies, and 3613 studies were excluded. Subsequently, 198 potential articles were further evaluated by reading the full text, of which 169 were excluded due to inclusion and exclusion criteria. Finally, 29 qualified studies including 10,347 patients were eligible for pooled analysis [6, 12, 13, 17–42]. The selection process is shown in Fig. 1.
Fig. 1.
Flowchart of the study search and selection. *PubMed (n = 1428), Embase (n = 648), Web of Science (n = 2799)
Characteristics of the included studies
In total, 29 studies published between 2001 and 2022 with 10,347 patients were included. All included studies were retrospective in our study. Most of the studies included were conducted in China and Japan; only two studies originated from Korea (n = 2) [13, 21] and one from Turkey (n = 1) [30]. All included studies had an NOS score of at least 7 (with a mean value of 7.45), which meant they were all high-quality studies (Supplementary file Table S2). The characteristics of all qualified literature sources are recorded in Table 1.
Table 1.
Basic characteristics of included studies
| Authors | Year | Study period | Country | Sample size |
MFP (months) |
Study type | Histology type | Cut-off | Endpoint | Source of HR | Nos |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aoki et al. [17] | 2001 | 1990–1999 | Japan | 127 | 37.1 | Retro | ADA | 0.5 | OS | E | 7 |
| Higashi et al. [18] | 2009 | 1997–2005 | Japan | 87 | 18 | Retro | ADA | 0.5 | DFS | E | 7 |
| Koike et al. [19] | 2012 | 1992–2009 | Japan | 223 | 70 | Retro | NSCLC | 0.75 | OS | R | 8 |
| Kishimoto et al. [20] | 2014 | 2006–2010 | Japan | 169 | 42 | Retro | NSCLC | C | DFS | R | 8 |
| Nakamura et al. [22] | 2015 | 2005–2011 | Japan | 113 | 46 | Retro | ADA | 0.5 | OS/DFS | R | 8 |
| Shimada et al. [23] | 2015 | 2004–2010 | Japan | 67 | 58.9 | Retro | NSSLC | 0.5 | OS/RFS | R | 8 |
| Cho et al. [21] | 2015 | 2001–2010 | Korea | 97 | 44.7 | Retro | ADA | 0.25 | OS/RFS | E | 8 |
| Tsurugai et al. [24] | 2016 | 2005–2014 | Japan | 155 | 34.7 | Retro | NSCLC | 0.5 | OS/DFS | R | 8 |
| Suzuki et al. [25] | 2017 | 2003–2007 | Japan | 392 | 84 | Retro | ADA | 0.5 | OS/RFS | E | 7 |
| Tsunezuka et al. [26] | 2017 | 2008–2012 | Japan | 62 | 55.1 | Retro | NSCLC | 0.5 | OS/RFS | R | 7 |
| Huang et al. [27] | 2018 | 2004–2013 | China | 789 | 87 | Retro | ADA | 0.75 | OS/DFS | E | 7 |
| Ye et al. [28] | 2018 | 2008–2014 | China | 736 | 38 | Retro | ADA | C | RFS | R | 7 |
| Kamigaichi et al. [29] | 2019 | 2007–2016 | Japan | 166 | 49.3 | Retro | NSCLC | 0.85 | OS/RFS | E | 7 |
| Kim et al. [13] | 2019 | 2009–2015 | Korea | 691 | 39 | Retro | ADA | 0.5 | DFS | R | 7 |
| Ye et al. [6] | 2019 | 2008–2014 | China | 329 | 42.2 | Retro | ADA | 0.5 | OS | R | 8 |
| Kuroda et al. [31] | 2020 | 2006–2010 | Japan | 260 | 83.8 | Retro | NSCLC | 0.5 | OS/DFS | R | 7 |
| Kabalak et al. [30] | 2020 | 2013–2016 | Turkey | 156 | 40 | Retro | ADA | 0.5 | OS/PFS | E | 8 |
| RYOJI IWAMOTO et al. [33] | 2021 | 2000–2009 | Japan | 73 | 77 | Retro | ADA | 0.8 | OS | R | 7 |
| Takamori et al. [37] | 2021 | 2006–2014 | Japan | 85 | 87.6 | Retro | NSCLC | 0.5 | OS | E | 7 |
| Sun et al. [36] | 2021 | 2014.01–2014.12 | China | 257 | 76 | Retro | NSCLC | C | OS/RFS | R | 7 |
| Zhong et al. [39] | 2021 | 2011–2012 | China | 620 | 72.4 | Retro | ADA | C | OS/RFS | R | 7 |
| Ji et al. [34] | 2021 | 2014–2015 | China | 190 | 51 | Retro | ADA | 0.5 | PFS | R | 7 |
| Lin et al. [35] | 2021 | 2013–2015 | China | 372 | 55 | Retro | ADA | 0.5 | RFS | R | 7 |
| Xi et al. [12] | 2021 | 2011–2016 | China | 862 | 47 | Retro | ADA | C | RFS | R | 7 |
| Chiang et al. [32] | 2021 | 2011–2017 | China | 1002 | 43.2 | Retro | ADA | 0.5 | DFS | R | 8 |
| Tsai et al. [38] | 2021 | 2003–2015 | China | 149 | 74 | Retro | ADA | 0.5 | OS/DFS | R | 8 |
| Hattori et al. [40] | 2022 | 2008–2017 | Japan | 603 | 54 | Retro | ADA | C | OS | R | 8 |
| Nakao et al. [41] | 2022 | 2010–2017 | Japan | 1014 | 61 | Retro | ADA | C | OS | R | 8 |
| Zhai et al. [42] | 2022 | 2008–2018 | China | 501 | 64.8 | Retro | ADA | 0.75 | OS/DFS | R | 8 |
MFP median follow-up time, HR hazard ratio, NOS Newcastle–Ottawa scale, NSCLC non-small cell lung cancer, Retro retrospective, ADA adenocarcinoma, C continuous, OS overall survival, DFS disease-free survival; RFS, recurrence-free survival, PFS progression-free survival, E estimated, R reported, NA not available
Association between CTR and prognosis in NSCLC patients
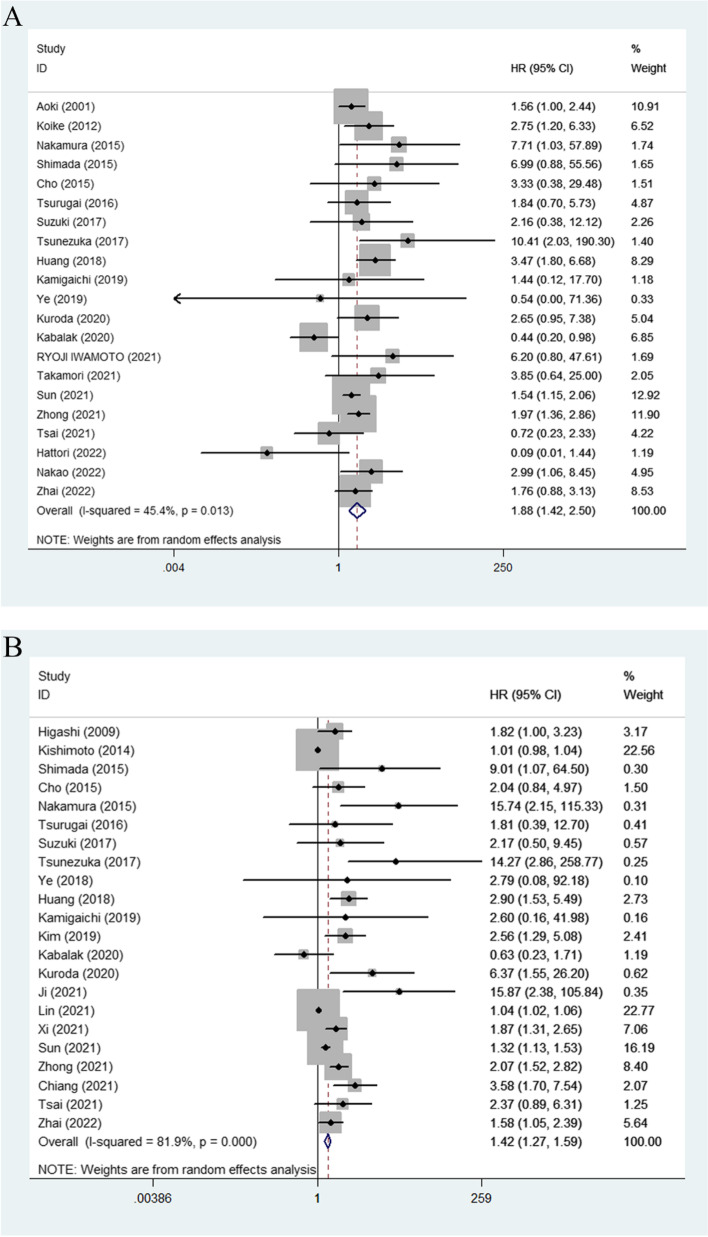
The relationship between CTR and OS was reported in 21 studies with 6238 participants [6, 17, 19, 21–27, 29–31, 33, 36–42], and the pooled HR demonstrated that a higher CTR was associated with a worse prognosis (HR = 1.88, 95% CI 1.42–2.50, P < 0.01) (Fig. 2A).
Fig. 2.
A Forest plot for the relationship between CTR and overall survival. B Forest plot for the relationship between CTR and DFS/RFS/PFS. CTR, consolidation to tumor ratio; DFS, disease-free survival; RFS, recurrence-free survival; PFS, progression-free survival
There were 22 articles with 7893 participants reporting the impact of CTR on DFS/RFS/PFS [12, 13, 18, 20–32, 34–36, 38, 39, 42], and the results showed that a higher CTR was significantly correlated with poorer prognosis (HR = 1.42, 95% CI 1.27–1.59, P < 0.01) (Fig. 2B).
Subgroup analysis
Subgroup analysis by the cut-off value of CTR indicated that CTR was not a prognostic factor for OS when the cut-off values of CTR were 0.25, 0.8 and 0.85. However, when the cut-off value of CTR was 0.5 or 0.75 or when CTR was a continuous variable, CTR was a prognostic factor for OS. For DFS/RFS/PFS, when the cut-off value of CTR was 0.50 or 0.75 or CTR was a continuous variable, CTR could predict the prognosis of NSCLC patients. Subgroup analysis by country showed that among patients from China, Japan and Turkey, a higher CTR was associated with worse OS and DFS/RFS/PFS. Subgroup analysis stratified by histology type demonstrated that CTR was a predictor for OS and DFS/RFS/PFS in both lung adenocarcinoma and NSCLC patients (Tables 2 and 3). Nine studies had investigated the correlation between CTR and OS in stage I NSCLC patients, and the pooled HR for OS was 1.63 (95% CI 1.05–2.54) (Supplementary file Figure S2). Ten studies were conducted to explore the correlation between CTR and DFS/RFS/PFS in stage I NSCLC patients, the pooled HR for DFS/RFS/PFS was 1.89 (95% CI 1.26–2.85) (Supplementary file Figure S3).
Table 2.
Subgroup analysis for overall survival
| Number of studies | HR | 95% CI | P value | Heterogeneity (P, I2 (%)) | |
|---|---|---|---|---|---|
| Overall survival | 21 | 1.88 | 1.42–2.50 | < 0.01 | 0.013, 45.4 |
| Country | |||||
| China | 6 | 1.81 | 1.35–2.44 | < 0.01 | 0.172, 35.3 |
| Japan | 13 | 2.37 | 1.59–3.53 | < 0.01 | 0.222, 21.9 |
| Korea | 1 | 3.33 | 0.38–29.33 | 0.278 | –, – |
| Turkey | 1 | 0.44 | 0.19–0.97 | 0.043 | –, – |
| Cut-off value | |||||
| 0.25 | 1 | 3.33 | 0.38–29.33 | 0.278 | –, – |
| 0.5 | 11 | 1.79 | 1.03–3.11 | 0.039 | 0.022, 52.0 |
| 0.75 | 3 | 2.52 | 1.65–3.83 | < 0.01 | 0.335,8.5 |
| 0.8 | 1 | 6.20 | 0.80–47.83 | 0.080 | –, – |
| 0.85 | 1 | 1.44 | 0.12–17.49 | 0.775 | –, – |
| Continuous variable | 4 | 1.69 | 1.06–2.72 | 0.027 | 0.056,60.3 |
| Source of HR | |||||
| Reported | 14 | 1.98 | 1.46–2.68 | < 0.01 | 0.114, 32.6 |
| Estimated | 7 | 1.70 | 0.86–3.37 | 0.126 | 0.010, 64.6 |
| Histology type | |||||
| Adenocarcinoma | 13 | 1.68 | 1.10–2.56 | 0.016 | 0.004,59.0 |
| NSCLC | 8 | 1.88 | 1.41–2.51 | < 0.01 | 0.400,3.9 |
HR hazard ratio, CI confidence interval, NSCLC non-small cell lung cancer
Table 3.
Subgroup analysis for DFS/RFS/PFS
| Number of studies | HR | 95% CI | P value | Heterogeneity (P, I2 (%)) | |
|---|---|---|---|---|---|
| Progress-free survival | 22 | 1.42 | 1.27–1.59 | < 0.01 | < 0.01, 81.9 |
| Country | |||||
| China | 10 | 1.83 | 1.39–2.42 | < 0.01 | < 0.01, 87.7 |
| Japan | 9 | 2.92 | 1.47–5.81 | 0.002 | < 0.01, 72.6 |
| Korea | 2 | 2.35 | 1.37–4.05 | 0.278 | 0.692, 0.0 |
| Turkey | 1 | 0.63 | 0.23–1.72 | 0.043 | –, – |
| Cut-off value | |||||
| 0.25 | 1 | 2.04 | 0.84–4.96 | 0.116 | –, – |
| 0.50 | 13 | 2.73 | 1.63–4.58 | < 0.01 | < 0.01, 78.7 |
| 0.75 | 2 | 2.03 | 1.13–3.66 | 0.018 | 0.117,59.3 |
| 0.85 | 1 | 2.60 | 0.16–42.12 | 0.501 | –, – |
| Continuous variable | 5 | 1.46 | 1.08–1.99 | 0.015 | < 0.01,90.7 |
| Source of HR | |||||
| Reported | 16 | 1.36 | 1.22–1.52 | < 0.01 | < 0.01, 84.8 |
| Estimated | 6 | 1.85 | 1.21–2.83 | 0.004 | 0.264, 22.6 |
| Histology type | |||||
| Adenocarcinoma | 15 | 2.06 | 1.48–2.87 | < 0.01 | < 0.01,83.6 |
| NSCLC | 7 | 1.44 | 1.02–2.02 | 0.037 | < 0.01,78.9 |
HR hazard ratio, CI confidence interval, NSCLC non-small cell lung cancer
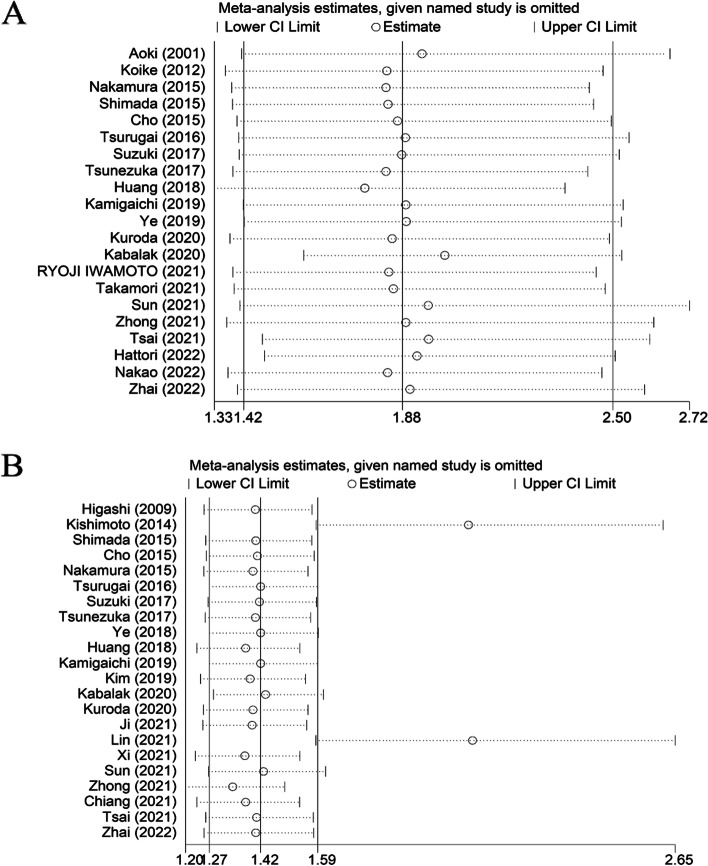
Sensitivity analysis
Sensitivity analysis revealed that the study for OS was stable and reliable (Fig. 3A). However, sensitivity analysis for the study on the relationship between CTR and DFS/RFS/PFS suggested that the studies of Kishimoto et al. [20] Lin et al. [35] and Zhong et al. [39] had a certain impact on our results (Fig. 3B). There was no significant change in the pooled HR (HR = 2.23, 95% CI = 1.69–2.94, p < 0.01) or heterogeneity (I2 = 57.2%, p < 0.01) after we discarded these three studies.
Fig. 3.
A Sensitivity analysis of the relationship between CTR and overall survival. B Sensitivity analysis of the relationship between CTR and DFS/RFS/PFS
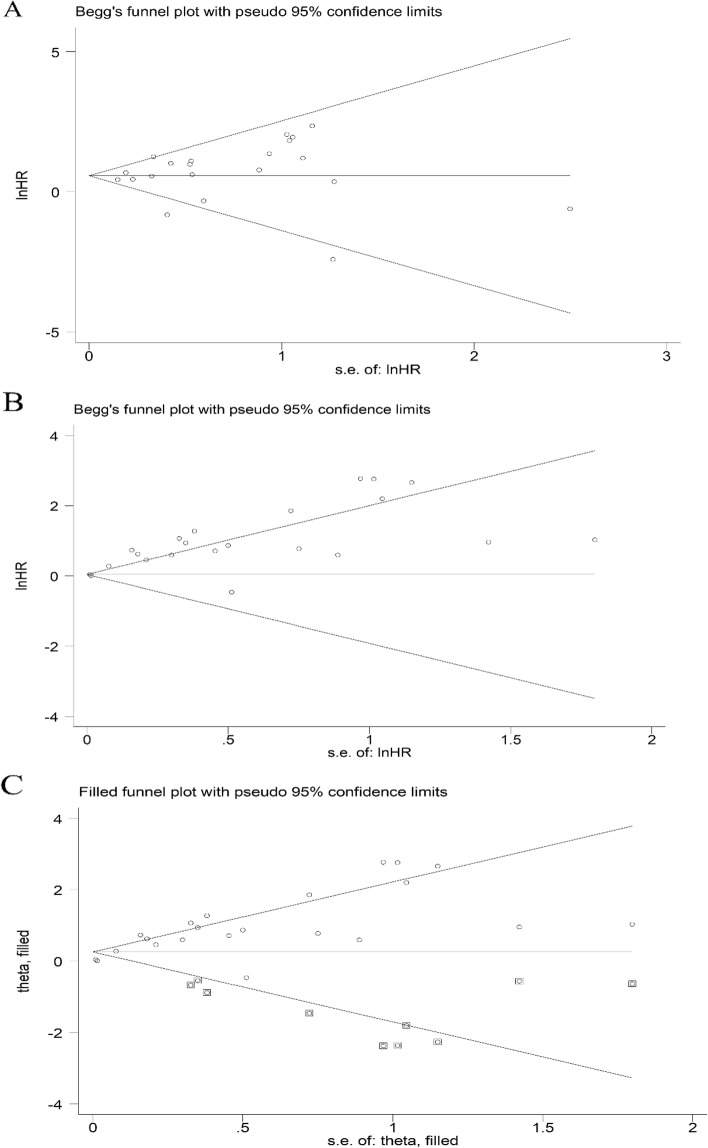
Publication bias
A symmetrical funnel plot revealed no significant publication bias (P > 0.1) in the study for the correlation between CTR and OS (Fig. 4A).
Fig. 4.
A Begg’s funnel plot for the relationship between CTR and overall survival. B Begg’s funnel plot for the relationship between CTR and DFS/RFS/PFS. C Begg’s funnel plot for the relationship between CTR and DFS/RFS/PFS after using the trim-and-fill method
The asymmetrical funnel plot implied significant publication bias for the study on DFS/RFS/PFS. Ten potentially unpublished studies were found using the trim-and-fill method. After adding the 10 potentially unpublished studies, the pooled HR was 1.29 (95% CI 1.15–1.45, p < 0.01) using the random effects model, which indicated that the 10 unpublished studies had no significant effect on the result, indicating that the result was reliable and stable (Fig. 4B, C).
Discussion
In this study, 29 studies with 10,347 patients were included to analyze the prognostic value of CTR. The results suggested that higher CTR was associated with worse prognosis in NSCLC patients. Subgroup analysis by cut-off value demonstrated that this result was valid when the cut-off value was 0.5 or 0.75 or when CTR was a continuous variable. Simultaneously, subgroup analysis stratified by country implied that CTR could be a prognostic factor for patients with NSCLC from Japan and China. Significant heterogeneity was observed among studies focusing on DFS/RFS/PFS as the outcome, warranting cautious interpretation of the findings. The findings from subgroup analysis indicated that the pathology type and source of HR did not exert a significant impact on the statistical significance of the study results. However, within the Korean population and when employing the CTR cut-off values of 0.25 and 0.85, the results were not significant, which may be related to the small number of studies. Given the observed heterogeneity, future studies are recommended to investigate the prognostic significance of CTR in different national populations and the optimal cut-off value of CTR.
An increasing number of studies have found that some CT-based radiomics signatures can be used to predict tumor aggressiveness and prognosis [43, 44]. Our study found that CTR can be used to predict the prognosis of NSCLC patients, and this factor can be included in future research on the prognostic model construction of NSCLC. Nguyen et al. found that the use of imaging features combined with clinical information can improve the accuracy of predicting epidermal growth factor receptor (EGFR) mutation status in patient with NSCLC [45]. Due to our study's limitation in lacking information on EGFR mutations within different CTR groups, we failed to elucidate the relationship between CTR and EGFR mutations, future investigations should be undertaken to address this issue. Lin et al. [35] demonstrated that a higher CTR subgroup had more invasive adenocarcinomas, lymphovascular invasion, and visceral pleural invasion than the lower CTR subgroup. Ono et al. [46] conducted a study to evaluate the association between CTR and immune-related factors, and found that small-sized lung adenocarcinoma with high CTR might be correlated with immunosuppressive conditions in the antitumor immune response compared with low CTR. The results of the above studies may be the reason why NSCLC patients with high CTR have a worse prognosis than those with low CTR, but more high-quality research is needed. Based on the above findings, we believe that CTR can be used to guide the preoperative decision-making of patients with NSCLC, and more aggressive surgical methods and more aggressive adjuvant therapy after surgery may be required for patients with higher CTR.
Although the prognostic value of CTR in NSCLC has been proven by many studies, consensus on the cut-off value of CTR has not yet been reached. Based on our results, when the cut-off value was 0.5 or 0.75 or when CTR was a continuous variable, CTR could predict prognosis. Huang et al. [27] showed that a GGO ratio greater than 75%, conversely, means that a CTR less than 25%, has value in predicting a favorable prognosis in resected lung adenocarcinoma patients. RYOJI IWAMOTO et al. [33] performed an ROC analysis to find the appropriate cut-off value of the CT solid score, which was equal to the CTR. They found that when the cut-off value was 80%, the area under the curve (AUC) for predicting recurrence had the highest sensitivity and specificity. Multivariate analysis indicated that a CT solid score > 80% was associated with an elevated likelihood of recurrence. However, in our studies, no obvious survival differences were observed between the low CTR and high CTR groups when the cut-off value was 0.8 or 0.85. Most of the studies we included used 0.5/0.75 as a cut-off, which limited our further analysis of the prognostic value of CTR with different cut-off values. Therefore, further studies are needed to confirm the appropriate cut-off to predict the prognosis of NSCLC patients.
According to previous studies, the survival and clinicopathological characteristics of part-solid nodules (PSNs) differ from those of pure solids [47]. Therefore, some experts suggested that NSCLC manifesting as PSNs should be treated as a special subtype. Most of our included studies included pure GGOs or pure solids, which each represent a different prognosis than PSNs. We could not perform subgroup analysis to analyze the prognosis of CTR in PSNs due to the lack of studies on PSNs. Kim et al. [13] demonstrated that CTR was not an independent prognostic factor for part-solid lung adenocarcinomas from cT1mi to cT1c. Fu et al. [48] found that a higher CTR indicated worse survival in NSCLC patients with part-solid nodules excluding lepidic pattern–predominant adenocarcinoma. The different conclusions of these two studies remind us that the predictive value of CTR in PSNs is worthy of further study.
It was reported by Pan et al.’s meta-analysis that no significant difference in DFS was found between patients with higher and lower GGO ratios in pathological stage I pulmonary adenocarcinoma [49], which was not consistent with our results. However, only four studies were included in their study, and some studies used the tumor shadow disappearance rate (TDR) [50], which was calculated as the ratio between the tumor area in the mediastinal window setting and that in the lung window setting, to calculate the GGO ratio. Our study not only unified the definition of CTR, but also included more references, and conducted subgroup analysis on different cut-off values. Therefore, our results may be more convincing.
There were still several limitations in our study. First, all included studies were retrospective observational studies, which might cause potential selection and publication bias. Second, the potential impact of our results may be influenced by the unequal distribution of disease stages and treatments among groups, warranting further clarification. Third, HR information for some studies was extracted from Kaplan‒Meier curves, which may generate bias. Fourth, all of the patients included in this study were from China, Japan, Korea, and Turkey, which may limit the generalizability of our findings to other populations and ethnics. Fifth, as a result of a lack of detailed baseline data, such as age, TNM stage, and sex, we could not perform subgroup analyses by these factors. Sixth, significant heterogeneity was observed in our study, and we failed to find the source of the heterogeneity. Seventh, the lack of molecular marker information in our study may have affected our ability to comprehensively analyze the predictive value of CTR.
According to our study, CTR is a good prognostic factor for NSCLC patients, but further studies need to be conducted to verify this.
Supplementary Information
Additional file 2. Supplementary Table S1. Search strategy. Supplementary Table S2. NOS score. Figure S1. Measurement of CTR, CTR was defined as the maximum size of consolidation to the maximum tumor size in the lung window on computed tomography of the chest with or without IV contrast. CTR, consolidation to tumor ratio. Figure S2. Forest plot for the relationship between CTR and overall survival in stage I patients. CTR, consolidation to tumor ratio. Figure S3. Forest plot for the relationship between CTR and DFS/RFS/PFS in stage I patients. CTR, consolidation to tumor ratio; DFS, disease-free survival; RFS, recurrence-free survival; PFS, progression-free survival.
Abbreviations
- CT
Computed tomography
- CTR
Consolidation to tumor ratio
- CI
Confidence interval
- DFS
Disease-free survival
- EGFR
Epidermal growth factor receptor
- HR
Hazard ratio
- NSCLC
Non-small cell lung cancer
- NOS
Newcastle‒Ottawa quality assessment scale
- OS
Overall survival
- PFS
Progression-free survival
- PROSPERO
The International Prospective Registry of Systematic Reviews
- PSN
Part-solid nodule
- RFS
Recurrence-free survival
- SUV max
Maximal standardized uptake value
- TDR
Tumor shadow disappearance rate
Authors’ contributions
Yongming Wu: Conceptualization, Literature selection, Data extraction, Data curation, Writing-review and editing. Wenpeng Song: Literature retrieval, Selection, Data extraction, Data curation, Writing-review and editing. Denian Wang: Visualization, Writing—Review and Editing, Formal Analysis, Supervision. Junke Chang: Data curation, Statistical analysis,Writing-review and editing. Yan Wang: Methodology; Data curation; Software. Jie Tian: Conceptualization; Data curation; Writing-review and editing. Sicheng Zhou: Data curation; Conceptualization; Writing original draft. Jing Zhou: Data curation; Conceptualization; Writing original draft. Yingxian Dong: Data curation, Statistical analysis,Writing-review and editing. Jue Li: Visualization; Investigation. Guowei Che: Conceptualization, Supervision, Writing-review and editing.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All procedures performed in studies which involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For our study, formal consent is not required.
Consent for publication
All the authors consent to publish the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yongming Wu, Wenpeng Song, and Denian Wang contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in china: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134(7):783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical ia lung cancer (japan clinical oncology group 0201) J Thorac Oncol. 2011;6(4):751–756. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 5.Shigefuku S, Shimada Y, Hagiwara M, et al. Prognostic significance of ground-glass opacity components in 5-year survivors with resected lung adenocarcinoma. Ann Surg Oncol. 2021;28(1):148–156. doi: 10.1245/s10434-020-09125-x. [DOI] [PubMed] [Google Scholar]
- 6.Ye T, Deng L, Wang S, et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol. 2019;14(4):617–627. doi: 10.1016/j.jtho.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez Londoño GA, García Vicente AM, Bosque JJ, et al. Suvmax to tumor perimeter distance: a robust radiomics prognostic biomarker in resectable non-small cell lung cancer patients. Eur Radiol. 2022;32(6):3889–3902. doi: 10.1007/s00330-021-08523-3. [DOI] [PubMed] [Google Scholar]
- 8.Kwak YK, Park HH, Choi KH, et al. Suvmax predicts disease progression after stereotactic ablative radiotherapy in stage i non-small cell lung cancer. Cancer Res Treat. 2020;52(1):85–97. doi: 10.4143/crt.2019.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Kim HK, Kim SH, et al. Prognostic significance of histologic classification and tumor disappearance rate by computed tomography in lung cancer. J Thorac Dis. 2018;10(1):388–397. doi: 10.21037/jtd.2017.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of japan clinical oncology group 0201. J Thorac Cardiovasc Surg. 2013;146(1):24–30. doi: 10.1016/j.jtcvs.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (jcog0802/wjog4607l): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 12.Xi J, Yin J, Liang J, et al. Prognostic impact of radiological consolidation tumor ratio in clinical stage ia pulmonary ground glass opacities. Front Oncol. 2021;11:616149. doi: 10.3389/fonc.2021.616149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Goo JM, Kim YT, Park CM. Consolidation-to-tumor ratio and tumor disappearance ratio are not independent prognostic factors for the patients with resected lung adenocarcinomas. Lung Cancer. 2019;137:123–128. doi: 10.1016/j.lungcan.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: Heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27(3):317–321. doi: 10.1093/icvts/ivy163. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 16.Biljana M, Jelena M, Branislav J, Milorad R. Bias in meta-analysis and funnel plot asymmetry. Stud Health Technol Inform. 1999;68:323–328. [PubMed] [Google Scholar]
- 17.Aoki T. Peripheral lung adenocarcinoma correlation of thin-section ct findings with histologic prognostic factors and survival. 2001. [DOI] [PubMed] [Google Scholar]
- 18.Higashi K, Sakuma T, Ito K, et al. Combined evaluation of preoperative fdg uptake on pet, ground-glass opacity area on ct, and serum cea level: Identification of both low and high risk of recurrence in patients with resected t1 lung adenocarcinoma. Eur J Nucl Med Mol Imaging. 2009;36(3):373–381. doi: 10.1007/s00259-008-0961-4. [DOI] [PubMed] [Google Scholar]
- 19.Koike T, Koike T, Yamato Y, Yoshiya K, Toyabe S. Prognostic predictors in non-small cell lung cancer patients undergoing intentional segmentectomy. Ann Thorac Surg. 2012;93(6):1788–1794. doi: 10.1016/j.athoracsur.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto M, Iwano S, Ito S, Kato K, Ito R, Naganawa S. Prognostic evaluations of small size lung cancers by 18f-fdg pet/ct and thin-section ct. Lung Cancer. 2014;86(2):180–184. doi: 10.1016/j.lungcan.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015;99(1):218–222. doi: 10.1016/j.athoracsur.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura S, Fukui T, Kawaguchi K, Fukumoto K, Hirakawa A, Yokoi K. Does ground glass opacity-dominant feature have a prognostic significance even in clinical t2an0m0 lung adenocarcinoma? Lung Cancer. 2015;89(1):38–42. doi: 10.1016/j.lungcan.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer. 2015;88(2):174–180. doi: 10.1016/j.lungcan.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Tsurugai Y, Kozuka T, Ishizuka N, Oguchi M. Relationship between the consolidation to maximum tumor diameter ratio and outcomes following stereotactic body radiotherapy for stage i non-small-cell lung cancer. Lung Cancer. 2016;92:47–52. doi: 10.1016/j.lungcan.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki S, Aokage K, Yoshida J, et al. Thin-section computed tomography findings of lung adenocarcinoma with inherent metastatic potential. Surg Today. 2017;47(5):619–626. doi: 10.1007/s00595-016-1416-3. [DOI] [PubMed] [Google Scholar]
- 26.Tsunezuka H, Kato D, Okada S, Furuya T, Shimada J, Inoue M. Surgical outcome of wide wedge resection in poor-risk patients with clinical-n0 non-small cell lung cancer. Gen Thorac Cardiovasc Surg. 2017;65(10):581–586. doi: 10.1007/s11748-017-0803-z. [DOI] [PubMed] [Google Scholar]
- 27.Huang TW, Lin KH, Huang HK, et al. The role of the ground-glass opacity ratio in resected lung adenocarcinoma. Eur J Cardiothorac Surg. 2018;54(2):229–234. doi: 10.1093/ejcts/ezy040. [DOI] [PubMed] [Google Scholar]
- 28.Ye T, Deng L, Xiang J, et al. Predictors of pathologic tumor invasion and prognosis for ground glass opacity featured lung adenocarcinoma. Ann Thorac Surg. 2018;106(6):1682–1690. doi: 10.1016/j.athoracsur.2018.06.058. [DOI] [PubMed] [Google Scholar]
- 29.Kamigaichi A, Tsutani Y, Fujiwara M, Mimae T, Miyata Y, Okada M. Postoperative recurrence and survival after segmentectomy for clinical stage 0 or ia lung cancer. Clin Lung Cancer. 2019;20(5):397–403.e391. doi: 10.1016/j.cllc.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Akın Kabalak P, Yılmaz Ü, Ertürk H, et al. Prognostic significance of preoperative consolidation to maximum tumour diameter ratio and suvmax in pathological stage i lung adenocarcinoma. Clin Respir J. 2020;14(2):71–77. doi: 10.1111/crj.13102. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda H, Nakada T, Oya Y, Takahashi Y, Matsusita H, Sakakura N. Clinical adjustability of radiological tools in patients with surgically resected ct1n0-staged non-small-cell lung cancer from the long-term survival evaluation. J Thorac Dis. 2020;12(11):6655–6662. doi: 10.21037/jtd-20-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang X-H, Lu T-P, Hsieh M-S, et al. Thoracoscopic wedge resection versus segmentectomy for ct1n0 lung adenocarcinoma. Ann Surg Oncol. 2021;28(13):8398–8411. doi: 10.1245/s10434-021-10213-9. [DOI] [PubMed] [Google Scholar]
- 33.Iwamoto R, Tanoue S, Nagata S, et al. T1 invasive lung adenocarcinoma: Thin-section ct solid score and histological periostin expression predict tumor recurrence. Mol Clin Oncol. 2021;15(5):228. doi: 10.3892/mco.2021.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Bai G, Qiu B, et al. The surgical management of early-stage lung adenocarcinoma: Is wedge resection effective? J Thorac Dis. 2021;13(4):2137–2147. doi: 10.21037/jtd-20-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin B, Wang R, Chen L, Gu Z, Ji C, Fang W. Should resection extent be decided by total lesion size or solid component size in ground glass opacity-containing lung adenocarcinomas? Transl Lung Cancer Res. 2021;10(6):2487–2499. doi: 10.21037/tlcr-21-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun K, You A, Wang B, et al. Clinical t1an0m0 lung cancer: Differences in clinicopathological patterns and oncological outcomes based on the findings on high-resolution computed tomography. Eur Radiol. 2021;31(10):7353–7362. doi: 10.1007/s00330-021-07865-2. [DOI] [PubMed] [Google Scholar]
- 37.Takamori S, Oizumi H, Suzuki J, Suzuki K, Kabasawa T. Video-assisted thoracoscopic segmentectomy for deep and peripheral small lung cancer. Thorac Cardiovasc Surg. 2022;70(3):233–238. doi: 10.1055/s-0040-1722172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai PC, Liu C, Yeh YC, et al. Prognostic histologic subtyping of dominant tumor in resected synchronous multiple adenocarcinomas of lung. Sci Rep. 2021;11(1):9539. doi: 10.1038/s41598-021-88193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Y, Xu Y, Deng J, et al. Prognostic impact of tumour spread through air space in radiological subsolid and pure solid lung adenocarcinoma. Eur J Cardiothorac Surg. 2021;59(3):624–632. doi: 10.1093/ejcts/ezaa361. [DOI] [PubMed] [Google Scholar]
- 40.Hattori A, Matsunaga T, Fukui M, Takamochi K, Suzuki K. Prognostic influence of a ground-glass opacity component in hypermetabolic lung adenocarcinoma. Eur J Cardiothorac Surg. 2022;61(2):249–256. doi: 10.1093/ejcts/ezab436. [DOI] [PubMed] [Google Scholar]
- 41.Nakao M, Oikado K, Sato Y, et al. Prognostic stratification according to size and dominance of radiologic solid component in clinical stage ia lung adenocarcinoma. JTO Clin Res Rep. 2022;3(2):100279. doi: 10.1016/j.jtocrr.2022.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhai W, Gong L, Zheng Y, et al. Ground glass opacity and adjuvant chemotherapy in pathological stage ib-iia lung adenocarcinoma. Front Oncol. 2022;12:851276. doi: 10.3389/fonc.2022.851276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Huang S, Xu Y, Wu J. Diagnostic accuracy of artificial intelligence based on imaging data for preoperative prediction of microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:763842. doi: 10.3389/fonc.2022.763842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le VH, Kha QH, Minh TNT, Nguyen VH, Le VL, Le NQK. Development and validation of ct-based radiomics signature for overall survival prediction in multi-organ cancer. J Digit Imaging. 2023. Published online ahead of print. [DOI] [PMC free article] [PubMed]
- 45.Nguyen HS, Ho DKN, Nguyen NN, Tran HM, Tam KW, Le NQK. Predicting egfr mutation status in non-small cell lung cancer using artificial intelligence: a systematic review and meta-analysis. Acad Radiol. 2023. Published online ahead of print. [DOI] [PubMed]
- 46.Ono Y, Tagawa T, Kinoshita F, et al. Relationship between consolidation tumor ratio and tumor-infiltrating lymphocytes in small-sized lung adenocarcinoma. Thorac Cancer. 2022;13(15):2134–2141. doi: 10.1111/1759-7714.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T, Li M, Lin M, Zhao M, Zhan C, Feng M. Meta-analysis of comparing part-solid and pure-solid tumors in patients with clinical stage ia non-small-cell lung cancer in the eighth edition tnm classification. Cancer Manag Res. 2019;11:2951–2961. doi: 10.2147/CMAR.S196613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu F, Zhang Y, Wen Z, et al. Distinct prognostic factors in patients with stage i non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol. 2019;14(12):2133–2142. doi: 10.1016/j.jtho.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Pan XL, Liao ZL, Yao H, et al. Prognostic value of ground glass opacity on computed tomography in pathological stage i pulmonary adenocarcinoma: a meta-analysis. World J Clin Cases. 2021;9(33):10222–10232. doi: 10.12998/wjcc.v9.i33.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takamochi K, Yoshida J, Nishimura M, et al. Prognosis and histologic features of small pulmonary adenocarcinoma based on serum carcinoembryonic antigen level and computed tomographic findings. Eur J Cardiothorac Surg. 2004;25(5):877–883. doi: 10.1016/j.ejcts.2004.01.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Supplementary Table S1. Search strategy. Supplementary Table S2. NOS score. Figure S1. Measurement of CTR, CTR was defined as the maximum size of consolidation to the maximum tumor size in the lung window on computed tomography of the chest with or without IV contrast. CTR, consolidation to tumor ratio. Figure S2. Forest plot for the relationship between CTR and overall survival in stage I patients. CTR, consolidation to tumor ratio. Figure S3. Forest plot for the relationship between CTR and DFS/RFS/PFS in stage I patients. CTR, consolidation to tumor ratio; DFS, disease-free survival; RFS, recurrence-free survival; PFS, progression-free survival.
Data Availability Statement
Not applicable.