Abstract
Objective
Digital devices have demonstrated benefits to patients with chronic and neurodegenerative diseases. But when patients use medical devices in their homes, the technologies have to fit into their lives. We investigated the technology acceptance of seven digital devices for home use.
Methods
We conducted 60 semi-structured interviews with participants of a larger device study on their views on the acceptability of seven devices. Transcriptions were analysed using qualitative content analysis.
Results
Based on the unified theory of acceptance and use of technology, we evaluated effort, facilitating conditions, performance expectancy and social influence of each device.
In the effort category, five themes emerged: (a) the hassle to use the device; (b) its usability; (c) comfort; (d) disturbance to daily life; and (e) problems during usage. Facilitating conditions consisted of five themes: (a) expectations regarding a device; (b) quality of the instructions; (c) insecurities with usage; (d) possibilities of optimization; and (e) possibilities to use the device longer. Regarding performance expectancy, we identified three themes: (a) insecurities with the performance of a device; (b) feedback; and (c) motivation for using a device. In the social influence category, three themes emerged: (a) reactions of peers; (b) concerns with the visibility of a device; and (c) concerns regarding data privacy.
Conclusions
We identify key factors that determine the acceptability of medical devices for home use from the participants’ perspective. These include low effort of use, minor disruptions to their daily lives and good support from the study team.
Keywords: Chronic disease; digital health, general; connected devices, personalised medicine; remote patient monitoring, personalised medicine; wearables, personalised medicine; qualitative studies
Introduction
The use of digital technology and devices has increased in frequency and importance within clinical research in recent years.1–3 Digital devices have demonstrated benefit in research of patients with a range of chronic conditions,3–5 for example, neurodegenerative diseases such as Parkinson's disease (PD). 6 Patients can be monitored continuously for a longer period of time and in their home environment.3,5 Thus, digital devices can provide information for determining health and functional status, monitoring disease course or aiding diagnosis. 6 They can also lessen the burden on patients and work load on clinicians, as conventional disease monitoring is time-consuming and costly. 1 They can help to improve the health literacy of patients, address the health management needs of the home and consecutively help in maintaining health.7,8
When digital devices or technologies are applied in a patient's home, patients need to be able to use the technology and integrate it into their daily lives. To yield quality data, a technology must fulfil its measurement function while minimally impacting activities of daily living (ADLs).9,10 As the use of digital technology in healthcare grows, understanding factors that influence patient acceptance of technology is increasingly important.11,12 Considering patients opinions and experiences of technologies can increase the likelihood of successful use. 10
In this study, we investigated patient acceptance of seven different digital and wearable devices for home use. By examining different devices that require varying input on behalf of the users, we wanted to further explore usability in real-time monitoring of patients. Our aim was to identify properties of a device that facilitate its acceptance in home use.
Technology acceptance
The acceptance and intention to use digital technologies are of relevance for developers, businesses and the medical sector. The unified theory of acceptance and use of technology (UTAUT) is designed to combine different models of user acceptance of new technology. 13 The model has been used in various contexts including the intention to use medical technologies.14,15 UTAUT postulated the prediction of the use of a technology by the behavioural intention to use. Behavioural intention is in turn influenced by four aspects: performance expectancy, effort expectancy, social influence and facilitating conditions. Performance expectancy describes the degree to which the user of a technology expects this technology to be helpful to them, while effort expectancy focuses on ease of use. Social influence describes the perceived expectations of others regarding the use of a technology. Facilitating conditions are described as the perceived infrastructure that supports the use of the technology, such as instructions or assistance. 13
IDEA-FAST
The study ‘Identifying Digital Endpoints to Assess Fatigue, Sleep and Activities of daily living in Neurodegenerative disorders and Immune-mediated inflammatory diseases’ (IDEA-FAST) aims to identify ‘digital biomarkers’ of fatigue and sleep disturbances in patients with PD and Huntington's disease (HD), rheumatoid arthritis, systemic lupus erythematosus, primary Sjögren's syndrome (PSS) and inflammatory bowel disease. To achieve this, selected digital devices will be used to measure different aspects of the daily life of patients, their symptoms and physiology. This will then be correlated with patient reported outcomes (PROs) measured via remote technology.
In order to choose suitable devices, a feasibility study (FS) was conducted. Focus of this study was the usability of different medical devices in the larger observational study.
Methods
As part of a mixed methods study, qualitative semi-structured interviews were conducted with patients and healthy controls. All were participants of the IDEA-FAST FS and were interviewed after they had finished the device wearing period.
Design of the FS
Study procedures. The goal of the FS was to identify candidate digital measurements of fatigue and sleep disturbances, which can be further explored in a larger-scale validation study. To achieve this, digital technologies were implemented along with standardised questionnaires, qualitative interviews and PRO measures such as an e-diary. These different data sets were combined to select the most accurate digital measures for further exploration.
The FS was approved by ethics committees of all involved research institutions. All participants gave informed consent in written form. The FS was conducted from August 2020 until June 2021. Participants in Kiel (Germany), Münster (Germany), Newcastle (England) and Rotterdam (Netherlands) received the technologies and used them over a course of 4 weeks. The sample was comprised of six different patient populations as described, and healthy participants (HP). Each site was responsible for at least one patient population and one group of HP.
During the study period, participants used seven different devices. At the start of the study period, participants visited their local research centre, where they were introduced to the technologies and trained in their use. After this baseline visit, participants used these technologies for a 36-day period including technology-free periods. Each device package was used over two 5-day periods, followed by a rest period of at least 2 days. To ensure that participants understood and were able to use the devices, additional support and information were provided via written support material, tutorial videos and telephone and/or in-person support by specially trained clinical staff.
In some centres, such as Kiel, participants received weekly home visits, while in others, support was given mostly using telephone and videoconference tools.
To minimise the burden put on the participants, simultaneous use of wearables was limited for each device package. While technologies that only needed to be installed once were used over the whole study period, most devices were only used for 2 weeks and then exchanged for others. The order in which the participants used the devices differed between participants.
Devices. Participants were asked to use seven different devices, each for at least 10 days. These devices were selected to cover a range of concepts of interest (COI) related to sleep quality and fatigue. In some cases, the functions of the devices overlap. All devices are CE-certified. Additional information on the devices can be found in the appendix.
Axivity AX6. The AX6 is a movement sensor containing an accelerometer and gyroscope. The device was fitted into a wristband to be worn similarly to a watch and used continuously aside from contact with water. It does not rely on Wi-Fi and requires no charging by the participant.
MoveMonitor. The MoveMonitor is a sensing unit for activity and sleep tracking. The device is placed in the lower back and is attached to a belt that can be fixated around the waist using a hook and loop fastener. The participant can continuously wear the device, only taking it off for water contact. The device does not rely on Wi-Fi and does not need to be charged during study duration.
Byteflies Sensor Dots. The Sensor Dot is a wearable device that continuously records physiological signals in different locations. Participants were asked to wear three Sensor Dots located at the ankle, chest and neck to measure activity, electrocardiography (ECG) and electroencephalography (EEG). The Sensor Dots were attached using adhesive patches. The ECG and EEG Sensor Dots were connected with two biopotential electrodes each on the chest and behind the ears. In this study, commercially available electrodes were purchased separately. This system was worn continuously but had to be removed for showering, requiring new adhesives and electrodes every time. The Sensor Dots were charged every day and were therefore switched every morning and evening to be put into a docking station, which needs a Wi-Fi connection.
Vital Patch. The Vital Patch is an adhesive device attached to the left chest. It can measure ECG, heart rate, breathing rate and body temperature. The device has to be paired with a smartphone app using Bluetooth prior to use. After attaching, the participant can continuously wear the device for up to 7 days as it is waterproof, and dispose of it after use.
Dreem Headband. The Dreem Headband is a wireless headband worn during sleep. It records physiological data. The device needs to be paired with a smartphone app every evening. In the morning, data is transferred to the app, and the participant gets a visual representation of the collected data such as sleep stages and duration. The Dreem Headband has to be charged every day.
Bed Sensor. The Bed Sensor is a force-sensitive film that is placed under a mattress topper or protector during sleep. It detects heart rate, breathing rate and movements. After being set up on the bed, participants do not need to manipulate the device in any way. The Bed Sensor uses Wi-Fi.
ZKOne. The ZKOne is a stationary sensing device for respiration and heart rate. It senses breathing patterns when a person is stationary, and it uses radio frequency. The ZKOne has a box-like shape and is placed near the bed to monitor sleep. The device needs a Wi-Fi connection.
Interview guideline and implementation
Interviews were structured according to a protocol that was based on literature regarding Technology Acceptance. After an introduction and explanation including verbal consent by participants, they were asked questions about their background, followed by questions about health and well-being, experiences with technical devices and packaging. In the next section of the interview, participants were asked about each device and technology separately. First, they were asked to describe their experiences with the device, then more detailed questions. This was repeated for each device.
Interviews were conducted during the whole duration of the FS. Most interviews were conducted by JG in German or English, who had no prior experience with qualitative interviews (female research assistant with a diploma in psychology). The interviews with Rotterdam participants were conducted by EW in Dutch (female research assistant with an M.Sc. in Human Movement Sciences, no prior interview experience). Participants of the FS were approached by their contact person, informed about the study and its goals and asked about their willingness to be interviewed. They did not know the interviewers before. The first six interviews were conducted face-to-face in Kiel. The other interviews with participants from Kiel, Newcastle and Rotterdam were conducted using a videoconference tool. Interviews with participants from Münster were conducted by telephone. In most cases, there were no other people present, with the exception of interviews with HD patients, where a local contact person was present. After an introduction and the consent of the participant, the interviews were recorded using either a recording device or a recording function. Interviews lasted between 0:15 and 1:31 hours.
Participants
For the qualitative evaluation of the FS, interviews were conducted for each group separately until saturation was reached. This led to different sample sizes for each group. Because interviews with HP and PD patients in Kiel as well as PSS patients in Newcastle were conducted first, these groups have the highest participation rate. Taken together, interviews with 60 participants were conducted. Table 1 shows the number of interviews per group and location.
Table 1.
Number of interviews per group and location.
| Location | HP | PD | HD | PSS | RA | IBD |
|---|---|---|---|---|---|---|
| Kiel | 11 | 12 | 5 | |||
| Münster | 6 | 4 | ||||
| Newcastle | 2 | 11 | 1 | |||
| Rotterdam | 2 | 6 |
HP: healthy participants; PD: Parkinson's disease; HD: Huntington's disease; PSS: primary Sjögren's syndrome; RA: rheumatoid arthritis; IBD: irritable bowel disease.
Table 2 gives an overview of the demographic characteristics of the interviewed participants in each group. Participants were between 21 and 82 years old. A total of 26 participants were male. A total of 12 participants reported living alone or in a dorm, while 49 lived with their spouses or families in one household.
Table 2.
Demographic data of the interview participants.
| Gender | Living Situation | |||||
|---|---|---|---|---|---|---|
| Group | Number of Participants | Age (Range) | Male | Female | Alone | Not Alone |
| HP | 21 | 21–77 | 12 | 9 | 7 | 14 |
| PD | 12 | 37–80 | 8 | 4 | 1 | 11 |
| HD | 4 | 29–59 | 2 | 2 | 1 | 3 |
| PSS | 11 | 52–82 | 2 | 9 | 2 | 9 |
| RA | 6 | 40–68 | 0 | 6 | 1 | 5 |
| IBD | 6 | 22–53 | 2 | 4 | 1 | 5 |
| total | 60 | 21–82 | 26 | 34 | 12 | 48 |
HP: healthy participants; PD: Parkinson's disease; HD: Huntington's disease; PSS: primary Sjögren's syndrome; RA: rheumatoid arthritis; IBD: irritable bowel disease.
A total of 19 participants wore every device, while 41 did not wear at least one device for the whole period. This includes participants who were not offered every device. HD patients and participants from Rotterdam were not asked to wear the Byteflies Sensor Dots. The Bed Sensor was not used in Rotterdam. Details on how many participants used each device are given in Table 3. The device worn by most participants for the full duration was the Axivity AX6, with two drop-outs. In contrast, the Sensor Dots were worn by 30 participants for the full duration, 10 participants discontinued use earlier than planned, 9 declined using the device and 11 were not offered to use it.
Table 3.
Number of participants using each device.
| Duration of Use Period | |||
|---|---|---|---|
| Device | Full Time | Partly | Refusal (Thereof Not Offered) |
| AX6 | 58 | 2 | 0 |
| MoveMonitor | 56 | 4 | 0 |
| Sensor Dots | 30 | 10 | 20 (11) |
| Vitalpatch | 50 | 7 | 3 |
| Dreem Headband | 48 | 9 | 3 |
| Bed Sensor | 50 | 1 | 9 (8) |
| ZKOne | 43 | 3 | 14 |
Transcription and analysis
Verbatim transcriptions were done partly by JG using the software MAXQDA 16 and partly by a transcription service. Afterwards, all transcripts were checked for errors and pseudonymised. Interviews in Dutch were translated into English after transcription using an online translation tool. All translations were double-checked by EW. Interviews in German and English were left in their original form for analysis. For two interviews, a summary of the interview had to be used for analysis, because recording was flawed. These interviews did not offer new insights, but supported insights from other interviews. One interview could not be transcribed due to bad audio quality. This interview is not included in the following analysis. Overall, 58 complete interviews and two summaries were included in the analysis.
The analysis was done by JG using the software MAXQDA. 16 To analyse the content of the interviews, qualitative content analysis following Mayring 17 was used. Each device was analysed separately. The code system for each device was derived in a hybrid method. For the top-level codes, knowledge from literature research on technology acceptance was used. The UTAUT model 13 was used for both the interview guideline and the top-level codes for each technology. Second level codes were defined based on the interview guideline and derived inductively from the interview data. Additionally, a separate analysis of the general experiences in the study was conducted using the same method. This process led to separate analyses for each device, while still maintaining a similar structure. In this paper, we arranged the following results by codes to facilitate comparability between devices.
While the UTAUT has proven useful in the context of medical technology use,5,14,15 it does not fit the purpose of this study perfectly. In this case, the adoption of a medical technology was in the context of a medical study. Participants of the study used different devices without a clear benefit for themselves and without the possibility to evaluate their usefulness themselves. Therefore, we expected performance expectancy to play a lesser role in the acceptance of the technology used in this study. Additionally, users were interviewed after their use period, thus the evaluation of ‘ease of use’ includes only past use. For this reason, we named this aspect ‘effort’ for the sake of this study.
Results
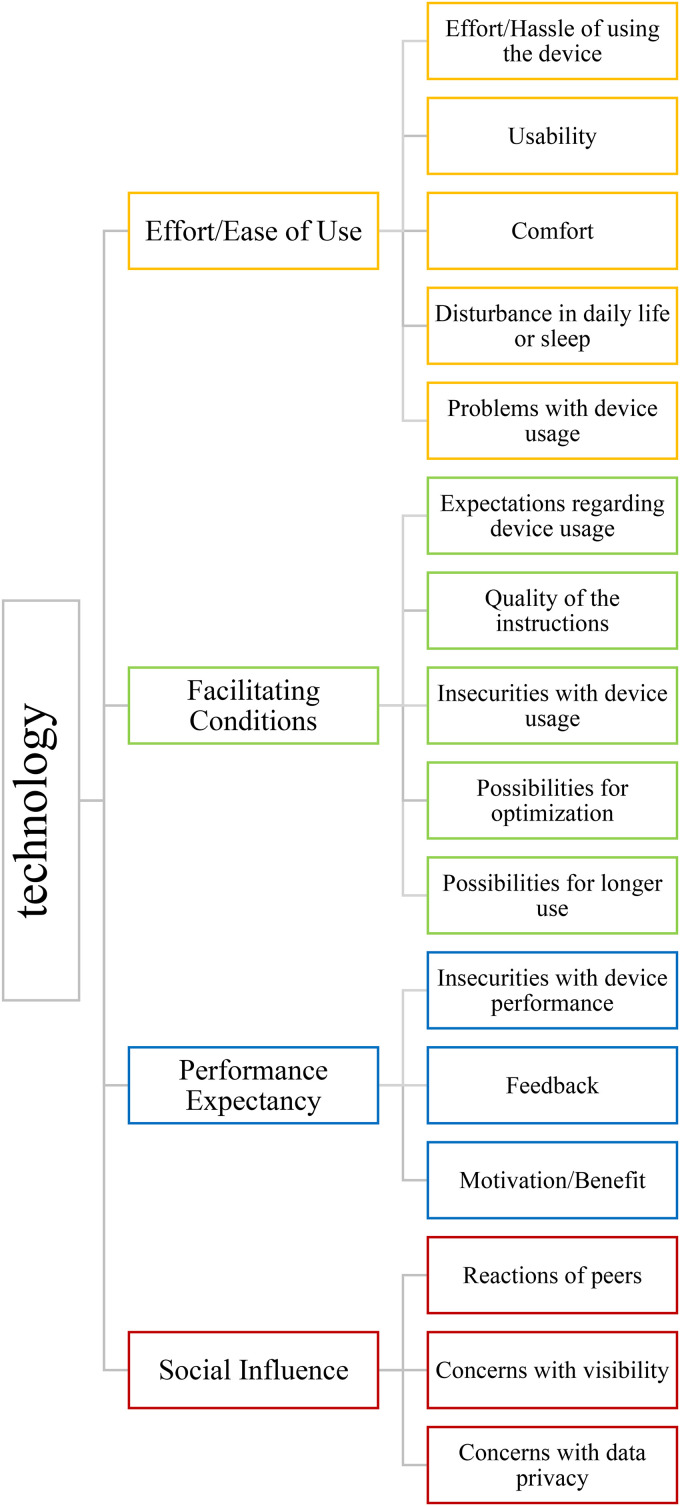
Results are sorted along the first level codes effort/ease of use, performance expectancy, facilitating conditions and social influence. Each code is comprised of different second level codes. For each code, all devices containing this code are described. The general code system is presented in Figure 1. Each quotation can be precisely assigned to the interview participant quoted. An attribution number has been omitted from the publication, as this information is not further-reaching for the readers.
Figure 1.
General code system.
Effort/ease of use
Effort/hassle of using the device. Generally, participants labelled the effort of the study as high due to the number of different devices: ‘It was all different things. Of course, if you only have one such device, then it is a lot less complicated’, reported one participant. The majority of participants felt that devices that only had to be worn with no other action needed required only small effort. This was the case for the Axivity, MoveMonitor and Vital Patch. For the latter, participants appreciated that they were able to shower with it and could leave it on for 5 days. Both Bed Sensor and ZKOne were also mentioned as not a lot of effort, as they only had to be installed. For the Dreem Headband, some participants found charging the headband every day disrupting. Also, connecting the phone before sleeping was seen as a specific effort, because at that time, users were tired. A few participants reported needing help with connecting the Dreem. While many participants perceived the effort for using the Byteflies Sensor Dots as acceptable, others perceived it as very time-consuming. ‘Yes, it was time-consuming and you had to be careful that you got the right ones in the right place, that you’ve got them charged up for the next time’, one participant reported. Many reported needing help from family members for the sensor on the neck: ‘My son had to do it from the back for us […]. I think if you were elderly, and there was nobody there, I think you might have problems connecting them once the device was on’*.
Usability. Participants rated most devices as easy to use, though reported instructions were a necessity: ‘It wouldn’t have worked at all without the manual. But it’s just that every device has a different way of handling’*. It was also mentioned that the weather can have a big impact on the usability of the devices. Two participants reported that the material of the strap of the AX6 tended to stick together, making it hard to take the watch off. For the McRoberts MoveMonitor, participants reported having to be careful with the hook and loop fastener and having to readjust the belt regularly during the day. Participants reported that the Vital Patch did not stick or that it was hard to take off. Most participants found the Bed Sensor easy to use, though many participants mentioned that it often slid out of position. The ZKOne was described by most participants as easy to use and to set up, though some people had problems with the set-up due to connectivity issues: ‘It was really hard to get it to connect […]. I think somebody who isn’t very technically minded might get frustrated with it’. In some cases, set-up was done by the clinical staff. A few participants said that the button was very small and hard to use. For two devices, participants disagreed about its usability. While some participants described installing the Sensor Dots as ‘fiddly’ or had problems connecting the device to their Wi-Fi, others reported ease of use. The EEG sensor was described as complicated to put on due to the position. Regarding the Dreem Headband, most participants found it easy to put on, but connecting it to the app proved difficult for some. ‘In the first week I was so frustrated. I always sat at least half an hour […] on the bed, because I couldn’t get it to work’*, reported one user. For many participants, the Headband fell off during the night because it did not fit well enough or they moved around during sleep: ‘Sometimes that fell off. […] When I woke up, I would put the thing back on my head. It wasn’t like that every night, but when I woke up in the morning, it was sometimes lying next to me’*, reported one participant.
Comfort. Participants rated the comfort of each device differently. Most concerns were mentioned regarding the Sensor Dots and Dreem Headband. The AX6, MoveMonitor, Vital Patch and Bed Sensor were overall perceived as comfortable by most users. For some participants, the sizing of the AX6 did not fit well. ‘One, it was too tight, and then the next one down it was too loose and it moved […] And there [were] times during the day where I just took it off for an hour’ reported one participant. Three users reported that the material of the strap caused sweating, and seven reported that it caused irritations on their skin. In two cases, skin irritations lead participants to drop the device. Some participants had problems with clothes getting caught on the long strap or perceiving the watch as too bulky. Regarding the MoveMonitor, some participants reported the belt sliding into uncomfortable positions or being uncomfortable when sitting or lying down. It was mentioned that the material chafed into the skin, especially when the hook and loop fastener was not perfectly aligned: ‘You just have to be accurate with how you fasten the Velcro[hook and loop fastener], so it doesn’t scratch, but once you get the hang of it, […] it’s fine’. Some users reported having to wear clothes underneath the belt. While most participants described the Vital Patch as unobtrusive and comfortable to wear, it caused minor skin irritations for eleven people: ‘It was red when I took it off, but it wasn’t irritable like the other ones’. Six people had bad skin reactions to the patch, some of them taking it off due to that. A few participants reported feeling pressure or bruising underneath the patch. Some people reported feeling uncomfortable when lying down or that their brassiere was putting pressure on the patch. Many participants mentioned it being painful to take off, especially if the area was hairy: ‘When you take it off, it is a wax hair removal’*, said one participant. The Bed Sensor was mostly described as comfortable, though some users mentioned noticing it when lying on it: ‘But still, you notice that you are lying on something […]. I can’t say it bothered me. But […] I was glad when I could remove it after five days’*. While some participants found the Dreem Headband very comfortable, others felt differently: ‘That was awful. […] Because it is somehow too thick. […] That started to hurt my ear’*. The Sensor Dots were perceived as uncomfortable by most users. A lot of participants reported the adhesive patches and electrodes being irritable on their skin, some described them as uncomfortable or even painful: ‘The sticky pads were itchy. And then the pads I got to link the wires, that was unpleasant’*. Six participants mentioned big problems with allergies or bad skin reactions to the electrodes. ‘It looked like a burn and it caused bleeding and scabbing, and it’s left a mark’, reported one participant. Some users also mentioned that taking the patches off was painful. Other people felt irritated by the wires of the EEG electrodes. For a lot of participants, the long wiring of the electrodes for the ECG sensor proved uncomfortable: ‘I could feel this wire hanging down me all the time, because I had to actually curl it up and tie it with some sticky tape. Anything sticky kind of close to a hairy body is just terrible’. Some participants described being constantly aware of the device or having a general feeling of discomfort.
Disturbance in daily life or sleep. Even though people reported the study disturbed their daily routines, most were satisfied with the experience. One user said: ‘Overall, I wouldn’t say it was a negative experience in any way. There were minor irritations as we’ve discussed but, no, fine, fine’. The majority of participants did not feel disturbed in their daily lives by using the AX6, MoveMonitor or Vital Patch. ‘This is one of the devices I was least bothered about having’, described one user her experience with the AX6. Some people reported refraining from wearing their own watch or wearing two watches. For others, the device was problematic at work or they had to take it off. Regarding the MoveMonitor, participants noted that it might be more uncomfortable or visible in summer. A few participants reported a general feeling of disturbance in their life or feeling constantly aware of the belt. Many participants said that they liked that the Vital Patch was waterproof. It was mentioned that participants wore different clothing or were careful while showering. In some cases, male participants had to shave their body hair. Some participants reported almost forgetting about the patch. While some participants said the Sensor Dots did not disturb them in their daily life or reported enjoying it, some participants limited showering to avoid taking it off or changed their habits like getting up earlier, limiting the use of skin lotion or wearing socks in bed to avoid losing the sensor. Some male participants reported shaving their body hair to avoid pain from the electrodes: ‘I was continuously shaving bigger and bigger areas of my chest, so it wouldn’t pull’. Other participants reported hindrances in their job, feeling limited in their movement or constantly worrying about the sensors or electrodes. ‘That was probably the most invasive […], I would swap it twice a day […]. These pads, […] you’re conscious when you’re pulling those off, you have skin irritation and discomfort’ said one user.
Most participants did not feel disturbed when sleeping while using the Bed Sensor or ZKOne. Some participants mentioned changing their routines while using the Bed Sensor, such as using a mattress protector or not being able to change positions during the night, while others reported feeling disturbed by the light: ‘It is very bright. […] At some point I put a sock over it or a pillow or something’. Regarding the ZKOne, some people reported feeling bothered by the light: ‘This lamp, I covered it after a while, because it was too bright for me. […] I can’t stand it when something is bright like that’*. Other people had to change their bedroom in order to find a fitting place for the device, for example, putting it on books or removing things that were usually on the bedside table. While many people reported that wearing the Dreem did not disturb their sleep, others found it ‘strange’ or changed their sleep routines such as sleeping on their back. Some users felt bothered by the infrared light. Others got nervous about the device or generally slept worse while wearing it: ‘But I couldn’t sleep properly because I knew it was there and I thought if I turn over it might come off. And then I was looking at the red light all the time. Because I could see it when I looked up’†.
Problems with device usage. There were a few problems with the implementation of the study. Two participants did not have Wi-Fi in their homes. For most participants, the AX6, MoveMonitor, Vital Patch and Bed Sensor caused no problems. In case of the AX6, one participant reported that the loop on the strap broke. In many cases, the casing of the MoveMonitor broke or almost broke during use. Some participants mentioned the Vital Patch becoming unstuck prematurely and that it got wet underneath the patch. One person had problems with the notification on their phone not going away. Other participants reported problems with the app, such as problems installing it or losing the connection to the device. One participant mentioned that the app picked up their partner's Vital Patch. Regarding the Bed Sensor, some participants mentioned problems with the touchscreen or with reconnecting the device. In two cases, the device got warm: ‘It wasn’t so warm that I couldn’t touch it. […] It wasn’t going to burn anything. […] You are conscious that there is an electrical device that's warm in your bed’. Participants reported more problems with the Sensor Dots, Dreem and ZKOne. The most frequently mentioned problem with the Sensor Dots was the devices falling off, especially the one placed at the ankle. In a few cases, participants lost a sensor dot. Also, the electrodes behind the ears did not stick very well and had to be reattached frequently. In other cases, the glue left residue which was hard to remove. Some participants had problems with the docking station not charging the Sensor Dots, and in other cases. it did not connect to the Wi-Fi. There were some technical problems with the Dreem Headband as well. A few users reported that their headband had to be swapped because it did not work. In some cases, data was not visible in the morning or there were problems connecting the headband to the app: ‘The headband could not connect, would not connect to the mobile telephone. […] And just no matter what I did, […] it just would not connect’†, said one user. Most reported problems with the ZKOne were concerning the Wi-Fi connection. As the device needs a specific Wi-Fi frequency, not all participants could connect the device. In some cases, the connection could be established after some time, in other cases it was not successful at all: ‘It wouldn’t reconnect. No. I have a healthy sort of Wi-Fi setup in my house so I don’t believe there’s any sort of home technical reason why it shouldn’t. So, I don’t know what that was’ reported one user. Some participants also had no Wi-Fi in their bedroom. Some other problems included data not being available or a defective contact.
Facilitating conditions
Expectations regarding device usage. Participants had different expectations before the start of the study. Some participants had not expected the study to be so demanding: ‘I think it was probably more demanding than I realized it was going to be. I was involved with so many different devices and things to do, and apps on the phone, and paperwork to write on at the end of each section of the research’, reported one user. Others said that the study met their expectations or turned out to be less demanding than previously thought: ‘It wasn’t as invasive as I thought it was going to be. And was very manageable, really. So no, no, there was no problem at all’. For most devices, participants did not have negative expectations before their use. A few participants reported expecting the MoveMonitor to be uncomfortable or impractical in daily life: ‘I thought it would be uncomfortable. I was expecting to […] be more conscious of it and more uncomfortable but that didn’t turn out to be the case’. Some users were already familiar with the Vital Patch from previous studies. Some participants were surprised that it was waterproof, others had suspected it would irritate their skin: ‘I was a bit afraid that it would irritate the skin too much. That was not the case, it was very good’*. Most participants did not have bad expectations regarding the use of the Bed Sensor. One participant mentioned that she expected it would be uncomfortable. While some participants expected the Dreem to be uncomfortable, disturb their sleep or fall off during the night, this was not always the case. Regarding the Sensor Dots, some participants expected it to be a lot of effort, while others found it an interesting new technology.
Quality of the instructions. Most participants were satisfied with the way the devices were packaged and labelled. Some users mentioned feeling overwhelmed at first, but most reported finding their way easily due to the labels and instructions. In one case, a participant overlooked a device. The instructions for most devices were described as helpful and sufficient by many users. Regarding the Sensor Dots, some participants had to call for assistance. ‘If I had any queries I could contact for some assistance. So being talked through was a lot, was a lot easier and being shown what to do’, added one participant. One participant suggested adding information about the connection between the Vital Patch and the study phone to the instructions: ‘Maybe there should also be a rough timeframe in the description […]. When do I have to have the phone with me again for data to be transferred?’*. Some other suggestions were adding that men should shave before use, and women should check for a placing that does not hurt when wearing a brassiere. Some users mentioned needing more help for the set-up of the Bed Sensor: ‘I had to ring […]. Just the setting up of the little box. I couldn’t get the time and date right’. A few participants felt that the information in the set-up of the ZKOne was not helpful. Many participants were not satisfied with the instructions for the Dreem Headband. In some cases, participants reported that the instructions for connecting the device did not fit the instructions given by the app: ‘You only needed to do three things to set it up. Not go through these eight or nine screens. And the screens were wrong, anyway. […] I think two of the screens didn’t have the same information that you actually had on the phone’. Other participants suggested having more thorough instructions, better pictures or including information on charging the device.
Insecurities with device usage. One user was concerned whether the AX6 had to be charged, and some were unsure about which way the device had to be put in the strap, as ‘The symbol was hard to see’*. Three participants reported feeling insecure about the location of the MoveMonitor. ‘I don’t know whether it got all the results […], you know, if it does move, it might matter. But I did feel that it was moving.’, one participant said. Regarding the Sensor Dots, two participants felt unsure whether they had put it on correctly. Others were worried about the skin irritations it caused or that they might lose the sensors. Some participants felt insecure about the water resistance of the Vital Patch. ‘I did make a little kind of waterproof patch and I stuck the patch over the patch, just to make sure I didn’t get it any wet’, reported one person. There was some insecurity about the correct placing of the patch. Other insecurities concerned the connection to the app, for example, how far away the smartphone could be for how long. The usability problems with the Dreem Headband led to some insecurity for users. Some participants reported feeling insecure about using the app and whether they used it correctly. On the other hand, some people reported feeling reassured by the possibility to directly view their data in the morning. Other participants were unsure when to put on the headband: ‘What time do you go to bed. Well, I go to bed earlier than I go to sleep, so I was thinking, what do I put? Do I put my sleep timing now or when I’m actually in bed?’ asked one participant. A few users reported feeling insecure whether the Bed Sensor could be flipped or whether it had to be turned on before sleeping. Regarding the ZKOne, some participants reported feeling insecure about the correct positioning of the device. ‘You ask yourself whether it is in the right place. […] You can’t check whether it is the correct angle’* said one participant. Other insecurities concerned whether other people in bed might set the device off or whether the device was turned on.
Possibilities for optimization. Participants had suggestions for optimization for all devices. For the AX6, participants suggested changes to the strap to improve comfort. Participants also found the AX6 bulky and felt the device would benefit from a clockface when worn on the wrist. Users found the wires of the electrodes used for the Sensor Dots too long, and to prevent the sensors falling off, different clasps were suggested. Participants suggested an easier way to connect Dreem Headband to the phone, offering more practice or improving the instructions, as well as having smaller sizes available. Regarding the Bed Sensor, participants suggested improving the touchscreen, highlighting parts that are important for set-up and preventing the sensor from sliding. Suggestions to improve the ZKOne covered the light, making the button more visible or bigger or having better explanations what the device measures.
Possibilities for longer use. Many participants reported they would not have liked to continue with the study for a longer time. ‘I don’t know whether four weeks was a bit long. Whether it could be shortened or you could have a gap between the two sets of two weeks, rather than continuing straight through’, said one participant. Looking at the individual devices, most participants agreed that the AX6, MoveMonitor, Vital Patch, Bed Sensor and ZKOne could be used for a longer time. One participant said about the AX6: ‘It’s just there. It's like an item of jewellery, really, at the end of the day. Made no difference at all’. Regarding the Bed Sensor, some participants mentioned that they would only use it if it was necessary or with a topper. Participants were split on a recommendation for longer use for the Dreem. While many participants said this was possible, others disagreed or said that this would depend on the person's sleep habits. Many participants would not recommend the Sensor Dots for longer use, especially for older people, those with physical disabilities or sensitive skin. Still, many users agreed that it was possible to use for a longer time if it was a necessity.
Performance expectancy
Insecurities with device performance. Participants mentioned feeling insecure about the performance of the devices, and that they would have liked to get feedback on whether the devices worked. One participant said: ‘I could say that with all the devices, that in the end you couldn’t see anything. You feel a bit like: “Is this right now?” or: “Did I wear it right, put it on right?”’*. The AX6, the MoveMonitor, the Bed Sensor and the ZKOne have no indication of whether they are working properly, mentioned participants. One user commented on the AX6: ‘I mean, the thing that surprised me about it really was that you couldn’t get any information out of it. It was just a black screen all the time. So, you just wore it and sort of hoped for the best’. Regarding the MoveMonitor, one user asked: ‘I was wearing it but I didn’t know whether it was working. Do you know whether all of these units did actually work for me?’. In some cases, the casing of the device broke, which lead to further insecurities about the functionality. With the Bed Sensor, another insecurity was due to it slipping out of position: ‘It slides back and forth a bit, but I wasn’t sure whether it was still lying correctly at the end of the night’*. There were also some insecurities regarding the performance of the Sensor Dots. Users felt insecure whether the device was working, whether it was charging correctly or what it was measuring. ‘If I’m doing this, I need to be sure that something is coming out of it. Because if I’m wearing this for five days and there's no data coming out, I’m thinking, “Well, this is not very good”’, said one participant. Regarding the Vital Patch, insecurities concerned mostly the transfer of data and whether the device was working: ‘One time, I was not sure whether I had turned it on, you couldn’t see that afterwards’*, reported one participant. In other cases, users reported feeling insecure whether the Dreem Headband was working correctly, especially in cases that it did not fit well: ‘That’s what I was worried about. I mean, sometimes it would be down my eyes, it would be over my eyes rather than on my forehead. And then other times it was up in my hair. So, I was a bit concerned that it didn’t get the right data’. Other users reported feeling reassured because they could see that data had been recorded in the morning: ‘It was nice to see how it was going, because with that I could see if it was working […]. So, I knew I was doing it right’.
Feedback. For most devices, participants did not receive direct feedback on their data. Only the Dreem Headband gives users the possibility to retrieve their own data. In some cases, participants were shown their data from the MoveMonitor and ZKOne after their study period. Getting feedback on the data gathered by the devices was mentioned as a motivating factor by many participants: ‘[I would have liked] some indication of what kind of data was being gathered. Not from a data protection kind of aspect, but more from interest’. Most participants reported being very interested in the data they could see on the Dreem app and viewed this as motivating. ‘I enjoyed that information because I could see it was working’, reported one participant. On the other hand, a few participants felt that the data seemed inaccurate when they compared it to their own experiences or the data from other devices. ‘The results did not reflect what I experience at night when I sleep. […] I checked in the morning and thought: “It just doesn’t fit”’*, reported one user. Some participants were not interested in the data or felt it was too complicated. Regarding the MoveMonitor, many participants liked or would have liked to receive this information, and some argued it would motivate them to wear the device. Of those participants that received feedback from the ZKOne, many found it interesting. ‘That is quite interesting. What does [the device] show and what were my experiences… […] It's a confirmation [of my own experiences]’*, said one user. The data of the AX6, Sensor Dots, Vital Patch and Bed Sensor was not provided to participants. Many users reported interest in data from these devices: ‘But if there had been some kind of information […], it would have been a bit more motivation’*.
Motivation/benefit. Many participants mentioned that their motivation for taking part in the study was helping future patients with problems regarding fatigue and sleep: ‘I’m just happy that the information that you get might help people in the future to deal with fatigue. […] I know it will probably not benefit me personally’. Another participant said: ‘I have the feeling, because of my disability, that I am a bit less useful to society […]. This was a kind of replacement of work for me, so I really liked that’. Aside from receiving feedback, participants reported a few motivating aspects of wearing the MoveMonitor and Dreem Headband. A few participants mentioned, a benefit of using the MoveMonitor could be that it can be motivating to exercise more.
Social influence
Reaction of peers. For the AX6, MoveMonitor and Sensor Dots, there were no reports of negative reactions from peers. Most comments about reactions by participant's peers concerned the sleep devices. A few participants reported their peers were interested in the Dreem Headband, especially in the provided data. In two cases, spouses felt bothered by the device: ‘All [my wife] could see was a red light on the top of my brow from the unit itself to see it was working. […] She kept away from the flashing lights’. Regarding the Bed Sensor, one participant reported a negative reaction to the device: ‘Sometimes [the lid] slid out of position and my husband said, “it's so bright [here]”’*.
Concerns with visibility. Regarding the MoveMonitor, participants mentioned they would not want to be seen wearing the device, for example in warmer weather or during work: ‘If you [are wearing] a thin summer dress, and you have such a [bulky device] around here, that might not be so nice’*. Some users mentioned the Vital Patch was visible when wearing low-cut clothing, in warmer weather or when swimming. The device causing most concerns regarding its visibility was the Sensor Dots. A total of 21 participants mentioned it being very visible. Many participants mentioned feeling self-conscious while wearing them, or that they would not be suitable at their workplace. Two participants declined wearing the device, citing its visibility: ‘Walking around with something like this, this was recently when I was more active again, no, I didn’t feel like doing that’*.
Concerns with data privacy. Some participants talked about concerns with data privacy. One user was bothered by the use of Wi-Fi for many devices. Another user said: ‘What really frustrated me the most was the data that went away, over which I had no control whatsoever. I would actually like to know what I am sending out into the world’*. For most individual devices, no concerns with data privacy were mentioned by participants. For the ZKOne, some participants reported concerns regarding data privacy. In one case, this concern led to not using the device.
Discussion
For the success of a medical study or a therapy involving devices for home use, the acceptance of the devices by users is vital.
This paper examined the technology acceptance of seven devices with a qualitative approach, with the goal to gather insights into how digital devices for home use should be designed in order to ensure their use in studies. With the use of the UTAUT model, an interview guideline was developed and used in 60 interviews with participants of a medical study.
Practical implications
Summarizing the experiences with the different devices, we conclude key take-aways on properties of medical devices that lead to good acceptance for home use. These results can be a guideline for the selection of suitable devices in medical studies, focussing on their acceptance by users. They can also be useful in the development of health technology, as they indicate aspects to look out for during development in order to assure a device will be used by patients. Generally, participants emphasised the importance of low effort, good support from the study team and minimal disruption to their daily lives.
Effort. In the design of medical devices, good usability, such as easy installation and use, is an important factor. As other studies have shown, aspects such as the time and mental effort and the disturbance to the daily routines should be considered. 18 Users preferred devices requiring low effort, such as requiring not a lot of time or a specific time to use them. Installing a device should be easy for laypeople. Regarding comfort, one device does not necessarily fit all. 19 Devices should be adjustable and fit different sizes. Devices that use adhesives can be prone to problems. Support should be available.
Facilitating conditions. Kohnke et al. 11 have suggested that the confidence of users in their ability to use a device has an influence on their intention to use it. With good instructions and in-person explanations, insecurities with device use can be reduced. Contact with the research team seems to be an important factor in providing security and motivation for participants.
Performance expectancy. As pointed out by Rainey et al., 20 participants want to know that the devices actually work and that using them has a benefit for the study. Many devices do not have indications of whether they work. Participants need to feel confident that they are contributing data. Often, feedback on their data is the only direct benefit a user gets from using a device in a study. Our results support Elwyn et al., 21 who noted that providing feedback from sensors can promote effective self-management strategies. Still, most participants cited altruistic motivations for their participation.
Social influence. Devices should be as invisible as possible to be usable in home and workplace settings, especially for participants who are active. The visibility of a device can be a critical point for participants, while there were few negative reactions from family and peers. Regarding data privacy, many patients trust their doctors and medical science. If there are good explanations how their data is handled, most of the participants have no problems to contribute private data.
Limitations and strengths
The design of our parent study imposed several limitations on this sub-study. It is uncertain whether the fact that participants used multiple devices at once influenced their experiences and opinions regarding each device separately. Also, due to the progress of the FS, the number of interviews for each disease group varied, and there might be aspects of device acceptance influenced by single diseases that could not be captured due to this. Another possible limitation of the study concerns the COVID-19 pandemic. Many participants were living in lockdown, working from home and not participating in as many social activities as they normally would. There might be possible influences of the devices on their lives that participants did not experience, for example, social experiences of participation were limited. Another factor might be the weather. Most participants used the devices during winter with colder weather. Some participants mentioned that they would have felt different about aspects such as visibility or water resistance of devices during warmer weather. While this study did not focus on differences between the groups of participants, the interviews suggest a difference between participants from Kiel and Newcastle. Participants from Newcastle voiced insecurities with the performance of a device more often than participants from Kiel. This difference might be connected with differences in participant support. While new participants were visited and helped with device set-up in Kiel, this was not possible in Newcastle, where participants received the devices in a package and had to set up themselves. This might have led to more insecurities with the performance of the devices.
We had the opportunity to conduct 60 interviews with participants of different disease groups, ages, genders and living situation. This large pool of opinions and voices using the same medical devices is an opportunity to see the similarities and differences in requirements patients have for medical devices during home use. During the progression of the FS, the interviews proved helpful in the selection of suitable devices for the planned Clinical Observation Study. Due to information from the interviews, one device was dropped from consideration before the end of the study and not offered to some patient groups. The results from the interviews were included in the device selection process.
In addition to being useful for our process in selecting suitable devices, interviewing participants might also be a good way to involve patients’ opinions in medical research. While the interview participants did not have the opportunity to be directly involved in selecting the devices, talking about their experiences might have contributed to their feelings of appreciation from the researchers. As one patient put it: ‘I especially like the fact that […] there is someone who takes the time to seriously deal with what you experienced’.
Conclusions
This study discusses the technology acceptance of seven medical devices for home use using a qualitative approach. Participants stressed the importance of low effort and minimal disruption to their lives, as well as good support from a study team in case of insecurities or problems with a device. Regarding their motivation for participation, users mainly cited altruistic reasons. Still, many expressed the wish to receive feedback on their data. For some participants, the visibility of a device can be a critical factor, while we did not find a lot of concerns regarding data privacy. These findings can be useful not only in our selection process but also for other medical research projects planning to administer devices for home use.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076231181239 for Technology acceptance of digital devices for home use: Qualitative results of a mixed methods study by Johanna Graeber, Elke Warmerdam, Svenja Aufenberg, Christopher Bull, Kristen Davies, Jan Dixon, Kirsten Emmert, Claire Judd, Corina Maetzler, Ralf Reilmann, Wan-Fai Ng, Victoria Macrae, Walter Maetzler, Hanna Kaduszkiewicz and in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076231181239 for Technology acceptance of digital devices for home use: Qualitative results of a mixed methods study by Johanna Graeber, Elke Warmerdam, Svenja Aufenberg, Christopher Bull, Kristen Davies, Jan Dixon, Kirsten Emmert, Claire Judd, Corina Maetzler, Ralf Reilmann, Wan-Fai Ng, Victoria Macrae, Walter Maetzler, Hanna Kaduszkiewicz and in DIGITAL HEALTH
Appendix
Supplementary information on devices
Axivity AX6
– Manufacturer: Axivity Ltd
– Model: AX6
– Six-axis movement sensor
– Accelerometer and gyroscope
– Weight: 11g
– Can be fitted in a wristband
MoveMonitor
– Manufacturer: McRoberts
– Model: MoveMonitor
– Sensing unit for activity and sleep tracking
– Weight: 55g
– Can be integrated in a belt to be worn at the lower back
Sensor Dots
– Manufacturer: Byteflies
- – Model: 1.X.X
- Consists of six sensor dots (two for each sensing unit), one docking station, three sensor patches and electrodes for EEG and EKG
– Sensing unit for EEG (one dot), EKG (one dot) and motion data (accelerometer and gyroscope, one dot)
– Weight: 6.3 g per sensor dot
Vital Patch
– Manufacturer: MediBioSense Ltd
– Model: VitalPatch
– Sensing unit for ECG, accelerometer and thermistor
– Weight: 11g
– Water resistant
– Skin friendly adhesive
– Comes with an app
Dreem Headband
– Manufacturer: Dreem
– Model: Dreem 2
– Sensors: EEG, accelerometer, sonometer
– Weight: 130g
– Three size adjusters (three sizes available), head perimeter: 530 mm–610mm
– Comes with an app compatible with Android and iOS
Bed Sensor
– Manufacturer: eLive ecosystem
– Model: eLive.wellbeing
– Sensing unit for heart rate, respiratory rate and motion activity
– 580 mm × 380–620 mm × 1–14mm
ZKOne
– Manufacturer: Zhongke Intelihealth Technology (Shenzhen) Co., Ltd
– Model: Yoli Sleep Monitor 2.0
– Non-contact sensing unit for vital sign data such as heart rate, analysis of sleep phases and body movement
Notes: *Translated from German.
†Translated from Dutch.
Footnotes
Acknowledgements: The authors would like to thank the participants for their time and contribution to the feasibility study and the interviews. Also, the authors would like to thank the IDEA-FAST consortium, consisting of Newcastle University, University Medical Center Schleswig-Holstein. University of Brescia, Erasmus University Medical Center Rotterdam, University of Glasgow, University of Limerick, ECRIN European Clinical Research Infrastructure Network, Queen Mary University of London, Imperial College London, Byteflies NV, DREEM, University of Cambridge, Lixoft SAS, Asociación Parkinson Madrid, Stichting MLC Foundation, TMF e.V., Medibiosense Ltd, empirica GmbH, FCiências.ID, Pluribus One S.r.l., Instituto de Medicina Molecular Joao Lobo Antunes, Teknologian tutkimuskeskus VTT Oy, Cambridge Cognition Ltd, Universidad Autonoma de Madrid, Institut Mines-Télécom, McRoberts BV, George-Huntington-Institut GmbH, Instytut Psychiatrii Neurologii, Medical University Innsbruck, Helse Stanvanger Hf, iXscient Ltd, European Federation of Crohn's and Ulcerative Colitis Association, Leids University Medical Center, University of Manchester, Janssen Pharmaceutica NV, Takeda Development Centre Europe Ltd, AbbVie Inc, AstraZeneca AB, Eli Lilly & Co. Ltd, CHDI Foundation, Parkinson's Disease Society of the United Kingdom, Pfizer Ltd, F. Hoffmann-La Roche AG, Sanofi Aventis Recherche et Développement, UCB Biopharma SPRL, Biogen IDEC Ltd, Orion OYJ and Let it Care.
Contributorship: WN, WM, JG and HK conceived the study and were involved in protocol development with KE. WM, WN, JG and KE were involved in gaining ethical approval. JG, EW, DK, VM, SA, RR and JD were responsible for patient recruitment. Interviews were conducted by JG, EW, HK and VM. Data analysis and the first draft of the manuscript were completed by JG. All authors reviewed and edited the manuscript and approved the submission version of the manuscript.
Dr. Reilmann is founding director and owner of the George-Huntington-Institute, a private research institute focused on clinical and preclinical research in Huntington's disease, and QuantiMedis, a clinical research organization providing Q-Motor (quantitative motor) services in clinical trials and research. Dr. Reilmann served as elected member of the Steering Committees of the European Huntington Disease Network (EHDN) and the Huntington Study Group (HSG), cochair of the Task Force on Huntington's disease and member of the Task Force on Technology of the International Parkinson and Movement Disorder Society (IPMDS). He has provided consulting services, advisory board functions, clinical trial services, quantitative motor analyses, and/or lectures for Actelion, Alnylam, Amarin, Annexon, AOP Orphan Pharmaceuticals, AskBio, Cure Huntington Disease Initiative Foundation (CHDI), Desitin, Hoffmann-La Roche, IONIS, Ipsen, Lundbeck, MEDA Pharma, Medivation, Mitoconix, Neurosearch, Novartis, Omeros, Pfizer, Prana Biotechnology, Prilenia, PTC Therapeutics, Raptor, Siena Biotech, Temmler Pharma, Teva, uniQure, Vaccinex, Voyager, Wave Life Sciences, and Zevra. He has received grant support from the Bundesministerium für Bildung und Forschung (BMBF), the Cure Huntington Disease Initiative Foundation (CHDI), the Deutsche Forschungsgemeinschaft (DFG), the Deutsches Zentrum für Neurodegeneration und Entzündung (DZNE), the European Union's 7th Framework Program (EU-FP7) and Horizon2020 Innovative Medicine Initiative 2 program (IMI2), the European Huntington Disease Network (EHDN), the High-QFoundation, the National Institute of Health (NIH), and the National Science Foundation (NSF). The other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was granted by the ethics committee of Kiel University, Germany (Ethikkommission der Medizinischen Fakultät der CAU zu Kiel, approval number D491/20) and the HRA and Health and Care Research Wales (HCRW) (IRAS project ID 282329, REC reference 20/PR/0185)
Funding: This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) [grant number 853981]. The JU receives support from the European Union's Horizon 2020 research and innovation programme, EFPIA and PARKINSON'S DISEASE SOCIETY OF THE UNITED KINGDOM LBG. The authors acknowledge the financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
Guarantor: JG.
ORCID iDs: Johanna Graeber https://orcid.org/0000-0002-5126-2612
Christopher Bull https://orcid.org/0000-0002-9811-4190
Supplemental material: Supplemental material for this article is available online.
References
- 1.Guk K, Han G, Lim J, et al. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019; 9: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan M, Estève D, Fourniols J-Y, et al. Smart wearable systems: current status and future challenges. Artif Intell Med 2012; 56: 137–156. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Li H, Luo Y. An empirical study of wearable technology acceptance in healthcare. Indus Manag Data Syst 2015; 115: 1704–1723. [Google Scholar]
- 4.Iqbal SMA, Mahgoub I, Du E, et al. Advances in healthcare wearable devices. NPJ Flex Electron 2021; 5: 1–14. [Google Scholar]
- 5.Kitsiou S, Paré G, Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. J Med Internet Res 2013; 15: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovini E, Maremmani C, Cavallo F. How wearable sensors can support Parkinson’s disease diagnosis and treatment: a systematic review. Front Neurosci 2017; 11: 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condry M, Quan XI. Digital health innovation, informatics opportunity, and challenges. IEEE Eng Manag Rev 2021; 49: 81–88. [Google Scholar]
- 8.Awad A, Trenfield SJ, Pollard TD, et al. Connected healthcare: improving patient care using digital health technologies. Adv Drug Delivery Rev 2021; 178: 113958. [DOI] [PubMed] [Google Scholar]
- 9.Hardisty AR, Peirce SC, Preece A, et al. Bridging two translation gaps: a new informatics research agenda for telemonitoring of chronic disease. Int J Med Inform 2011; 80: 734–744. [DOI] [PubMed] [Google Scholar]
- 10.Middlemass JB, Vos J, Siriwardena AN. Perceptions on use of home telemonitoring in patients with long term conditions—concordance with the Health Information Technology Acceptance Model: a qualitative collective case study. BMC Med Inform Decis Mak 2017; 17: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohnke A, Cole ML, Bush R. Incorporating UTAUT predictors for understanding home care patient’s and clinician’s acceptance of healthcare telemedicine equipment. J Technol Manag Innov 2014; 9: 29–41. [Google Scholar]
- 12.Wills MJ, El-Gayar OF, Bennett D. Examining healthcare professionals’ acceptance of electronic medical records using UTAUT. Issues Infor Syst 2008; 2: 396–401. [Google Scholar]
- 13.Venkatesh V, Morris MG, Davis GB, et al. User acceptance of information technology: toward a unified view. Manage Inf Syst Q 2003; 27: 425–478. [Google Scholar]
- 14.Chang C-C. Exploring the usage intentions of wearable medical devices: a demonstration study. Interact J Med Res 2020; 9: e19776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Lee K-H, Hwang Het al. et al. Analysis of the factors influencing healthcare professionals’ adoption of mobile electronic medical record (EMR) using the unified theory of acceptance and use of technology (UTAUT) in a tertiary hospital. BMC Med Inform Decis Mak 2016; 16: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MAXQDA. Berlin, Deutschland: Sozialforschung GmbH; 2022.
- 17.Mayring P. Qualitative Inhaltsanalyse: Grundlagen und Techniken. 12th ed. Weinheim, Basel: Beltz, 2015, ISBN: 978-3-407-25730-7. [Google Scholar]
- 18.Hensel BK, Demiris G, Courtney KL. Defining obtrusiveness in home telehealth technologies: a conceptual framework. J Am Med Inform Assoc 2006; 13: 428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherton J, Sugarhood P, Procter R, et al. Co-production in practice: how people with assisted living needs can help design and evolve technologies and services. Implementation Sci 2015; 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainey J, Verweij D, Dodds C, et al. Data contribution summaries for patient engagement in multi-device health monitoring research. In: Adjunct Proceedings of the 2021 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2021 ACM International Symposium on Wearable Computers (UbiComp-ISWC ‘21 Adjunct), Virtual, USA, September 21–26, 2021, pp.536-541. New York: ACM. DOI: 10.1145/3460418.3479371 [DOI]
- 21.Elwyn G, Hardisty AR, Peirce SC, et al. Detecting deterioration in patients with chronic disease using telemonitoring: navigating the ‘trough of disillusionment’. J Eval Clin Pract. 2012; 18:896–903. DOI: 10.1111/j.1365-2753.2011.01701.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076231181239 for Technology acceptance of digital devices for home use: Qualitative results of a mixed methods study by Johanna Graeber, Elke Warmerdam, Svenja Aufenberg, Christopher Bull, Kristen Davies, Jan Dixon, Kirsten Emmert, Claire Judd, Corina Maetzler, Ralf Reilmann, Wan-Fai Ng, Victoria Macrae, Walter Maetzler, Hanna Kaduszkiewicz and in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076231181239 for Technology acceptance of digital devices for home use: Qualitative results of a mixed methods study by Johanna Graeber, Elke Warmerdam, Svenja Aufenberg, Christopher Bull, Kristen Davies, Jan Dixon, Kirsten Emmert, Claire Judd, Corina Maetzler, Ralf Reilmann, Wan-Fai Ng, Victoria Macrae, Walter Maetzler, Hanna Kaduszkiewicz and in DIGITAL HEALTH