ABSTRACT
A balanced vaginal microbiome dominated by Lactobacillus can help promote women’s reproductive health, with Lactobacillus crispatus showing the most beneficial effect. However, the potential role of vaginal microbiomes in hypertensive disorders of pregnancy (HDP) development is not thoroughly explored. In this nested case-control study based on an assisted reproductive technology follow-up cohort, we prospectively assessed the association between pregestational vaginal microbiomes with HDP by collecting vaginal swabs from 75 HDP cases (HDP group) and 150 controls (NP group) and using 16S amplicon sequencing for bacterial identification. The vaginal microbial composition of the HDP group significantly differed from that of the NP group. The abundance of L. crispatus was significantly lower, and the abundances of Gardnerella vaginalis was significantly higher, in the HDP group than in the NP group. Of note, L. crispatus-dominated vaginal community state type was associated with a decreased risk for HDP (odds ratio = 0.436; 95% confidence interval, 0.229 to 0.831) compared with others. Additionally, network analysis revealed different bacterial interactions with 61 and 57 exclusive edges in the NP and HDP groups, respectively. Compared with the HDP group, the NP group showed a higher weighted degree and closeness centrality. Several taxa, including G. vaginalis, L. iners, and bacterial vaginosis-associated bacteria (Prevotella, Megasphaera, Finegoldia, and Porphyromonas), were identified as “drivers” for network rewiring. Notable alterations of predicted pathways involved in amino acid, cofactor, and vitamin metabolism; membrane transport; and bacterial toxins were observed in the HDP group.
IMPORTANCE The etiology of HDP remains unclear to date. Effective methods for the individualized prediction and prevention are lacking. Pregestational vaginal dysbiosis precedes the diagnosis of HDP, providing a novel perspective on the etiology of HDP. Early pregnancy is the critical period of placental development, and abnormal placentation initiates HDP development. Thus, disease prevention should be considered before pregnancy. Vaginal microbiome characterization and probiotic interventions before pregnancy are preferred because of their safety and potential for early prevention. This study is the first to prospectively assess associations between pregestational vaginal microbiome and HDP. L. crispatus-dominated vaginal community state type is linked to a reduced risk for HDP. These findings suggest that vaginal microbiome characterization may help identify individuals at high risk for HDP and offer potential targets for the development of novel pregestational intervention methods.
KEYWORDS: hypertensive disorders of pregnancy, vagina, microbiome, 16S ribosomal RNA, Lactobacillus crispatus
INTRODUCTION
Hypertensive disorders of pregnancy (HDP), a leading cause of maternal and neonatal morbidity and mortality, complicate 3% to 8% of pregnancies worldwide (1, 2). HDP is characterized by a new-onset elevated blood pressure with or without proteinuria and multisystem injury after 20 weeks of gestation in previously normotensive women. Risk factors for developing HDP include obesity, dysglycemia, lipid metabolic dysfunction, advanced maternal age, nulliparity, use of assisted reproductive technologies, genetic predisposition, and chronic medical conditions, including pregestational diabetes mellitus, chronic hypertension, and systemic lupus erythematosus (3 to 9). Some factors are amendable to pregestational modification, which might be beneficial in reducing HDP risk. However, few studies have explored the link between the vaginal microbiome, a novel modifiable target, and HDP risk.
Emerging evidence has linked maternal microbiome dysbiosis of the gut, oral cavity, uterus, placenta, and amniotic fluid to HDP development, in support of the multifactorial cause of HDP (10 to 14). Gut dysbiosis may induce immune imbalance, barrier dysfunction, and further bacterial translocation, which lead to intrauterine infections or placental inflammation that potentially contributes to preeclampsia (11). Prevotella, Porphyromonas, Dialister, Streptococcus, Ureaplasma, Sneathia, Salmonella, Bacillus, Escherichia, and Fusobacterium were detected in the amniotic fluid and placentas of women with preeclampsia (15 to 17). These bacteria, common in the oral cavity, lower reproductive tract, and gastrointestinal tract, gain access to the uterus through hematogenous spread (18, 19). Additionally, the anatomical proximity of the vagina to the uterus, the area where the placenta will be implanted for future fetal growth, facilitates potential cross talk between the two sites through vertical translocation. Vaginal microbes could ascend to the uterus, induce inflammation, and affect uterine microecology and endometrial health (20).
Until now, only two studies have explored the links between the vaginal microbiome and HDP; however, both studies were conducted in late pregnancy with a small sample size (13, 21). Nested case-control studies are needed to examine pregestational vaginal microbial profiles, which may be a relevant etiological window given that defective placentation occurs in early pregnancy. Derived from a population-based prospective cohort, the present nested case-control study aimed to characterize the pregestational vaginal microbiomes of women with and without HDP and investigate their association with HDP development. The results of this study may help identify individuals at high risk for HDP and offer probiotic supplementation as a novel pregestational intervention to prevent this disease.
RESULTS
Participant characteristics.
This study included 225 individuals with normal baseline blood pressure, including 75 women who developed HDP and 150 matched controls who delivered at term without any complications after embryo transfer (Fig. 1). The characteristics of the participants are detailed in Table 1. Risk factors for HDP, including maternal age, body mass index (BMI), baseline blood pressure, nullipara, indicators for glucose and lipid metabolism, number of fetuses, and incidence of polycystic ovary syndrome (PCOS), revealed no difference between the HDP and NP groups. Of the 75 women in the HDP group, one died of sudden elevated blood pressure at 35 weeks of gestation, and another had a late miscarriage at 26 weeks (Table S1 in the supplemental material).
FIG 1.
Overview of the study design. HDP, hypertensive disorders of pregnancy; PSM, propensity score matching; IVF/ICSI, in vitro fertilization or intracytoplasmic sperm injection; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
TABLE 1.
General characteristics of matched participantsa
| NP | HDP | |||
|---|---|---|---|---|
| Baseline | n = 150 | n = 75 | P value | |
| Age (yrs) | 32.08 (4.41) | 32.00 (4.72) | 0.895 | |
| BMI (kg/m2) | 23.93 (4.00) | 24.27 (3.40) | 0.532 | |
| SBP (mm Hg) | 115.51 (11.55) | 116.37 (11.19) | 0.546 | |
| DBP (mm Hg) | 72.28 (8.40) | 72.47 (9.35) | 0.856 | |
| TG (mmol/L) | 1.31 (0.90) | 1.38 (1.21) | 0.616 | |
| TC (mmol/L) | 4.27 (0.75) | 4.26 (0.82) | 0.924 | |
| HDL (mmol/L) | 1.31 (0.31) | 1.29 (0.31) | 0.684 | |
| LDL (mmol/L) | 2.67 (0.66) | 2.65 (0.67) | 0.828 | |
| FPG (mmol/L) | 5.35 (0.47) | 5.34 (0.43) | 0.847 | |
| Gravidity | 1 (0, 2) | 1 (0, 2.5) | 0.686 | |
| Parity | 0 (0, 1) | 0 (0, 1) | 0.850 | |
| Abortion | 0 (0, 1) | 0 (0, 2) | 0.645 | |
| Nullipara (no. (%)) | 0.827 | |||
| Yes | 102 (68.00) | 50 (66.67) | ||
| No | 48 (32.00) | 25 (33.33) | ||
| Education (no. (%)) | 0.446 | |||
| ≤ High school | 99 (66.00) | 53 (70.67) | ||
| College graduate | 51 (34.00) | 22 (29.33) | ||
| No. of embryos transferred | 0.850 | |||
| Mean (SD) | 1.45 (0.50) | 1.47 (0.50) | ||
| One embryo (no. (%)) | 82 (54.67) | 40 (53.33) | ||
| Two embryos (no. (%)) | 68 (45.33) | 35 (46.67) | ||
| Embryo transferred (no. (%)) | 0.631 | |||
| Fresh | 79 (52.67) | 37 (49.33) | ||
| Frozen | 71 (47.33) | 38 (50.67) | ||
| Embryo stage (no. (%)) | 0.849 | |||
| Blastocyst | 80 (53.33) | 39 (52.00) | ||
| Cleavage-stage embryo | 70 (46.67) | 36 (48.00) | ||
| Fetal no. | 0.906 | |||
| Mean (SD) | 1.33 (0.47) | 1.33 (0.47) | ||
| Singleton (no. (%)) | 101 (67.33) | 50 (66.67) | ||
| Twins (no. (%)) | 49 (32.67) | 25 (33.33) | ||
| PCOS (no. (%)) | 1.000 | |||
| Yes | 24 (16.00) | 12 (16.00) | ||
| No | 126 (84.00) | 63 (84.00) |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; TC, total cholesterol; FPG, fasting plasma glucose; PCOS, polycystic ovary syndrome.
Pregnancy outcomes, gestational weeks, and matched group of all participants. Download Table S1, DOCX file, 0.04 MB (40.2KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pregestational vaginal microbiome changes in women who developed HDP.
A total of 986 amplicon sequence variants (ASVs) were obtained in the two groups. The rarefaction curve of the ASV number revealed that the sequencing depth was sufficient to capture most of the microbial characteristics (Fig. S1). Moreover, alpha diversities indicated by Shannon, Richness, Chao1, and Simpson diversity indexes were comparable between the two groups (Fig. 2A, Fig. S2, conditional logistic regression, P > 0.05). The PCoA based on the weighted Unifrac distance indicated that the vaginal microbial composition of the HDP group significantly differed from that of the NP group (Fig. 2B, PERMANOVA, R2 = 0.014, P = 0.037). This result was further validated by the Bray-Curtis and Jaccard distances (Fig. S3A and B). Furthermore, the dissimilarities among individuals within the HDP group were significantly larger than those among individuals within the NP group (Fig. 2C, Fig. S3C and D, Wilcox’s rank-sum test, P < 0.001) and were comparable to the intergroup distances, indicating that the vaginal microbiome was highly divergent among individuals in the HDP group.
FIG 2.

Comparison of pregestational vaginal microbiome diversity and differential microbial compositions in women with and without hypertensive disorders of pregnancy. Comparison of Shannon diversity (A) between the NP and HDP groups. Significance in comparison of alpha diversity was determined using conditional logistic regression. (B) Principal coordinate analysis (PCoA) of the vaginal microbiome of the NP and HDP groups based on Unifrac distance. The vaginal bacterial community composition is significantly different between the HDP and NP groups. Each dot represents a sample and is colored by group. The difference in beta diversity between the NP and HDP groups was determined using PERMANOVA. (C) Unifrac distance of the vaginal microbial community among individuals within each group and between different groups. Significance in comparison of Unifrac distance was determined using a two-tailed Wilcox rank-sum test. (D) Differentially abundant bacterial taxa between the two groups. Significance was determined using conditional logistic regression corrected for multiple testing with significance identified at q < 0.2. (E) Community state type assignment of vaginal bacterial profiles based on the dominant bacteria in the NP and HDP groups. Violin plots in (A) show the density distribution of the alpha diversity within each group. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001. NP group, healthy controls with normal blood pressure during pregnancy; HDP group, women with the development of hypertensive disorders of pregnancy.
Rarefaction curves of the observed number of amplicon sequence variants (Richness) in the NP and HDP groups. Download FIG S1, EPS file, 1.2 MB (1.2MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of Chao1 (A), Richness (B), and Simpson indexes (C) between the NP and HDP groups. Significance was determined using conditional logistic regression. The violin plots show the density distribution of the alpha diversity within group. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. Download FIG S2, EPS file, 4.4 MB (4.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Principal coordinate analysis and dissimilarities of the vaginal microbiome of the NP and HDP groups based on Bray-Curtis distance and Jaccard distance. The vaginal community composition is significantly different between the HDP and NP groups based on Bray-Curtis distance (A) and Jaccard distance (B). Each dot represents a sample and is colored by group. Differences in beta diversity between the NP and HDP groups were determined using PERMANOVA. Bray-Curtis distance (C) and Jaccard distance (D) of the vaginal microbial community among individuals within the same group and between the NP and HDP groups. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. P values were determined using a two-tailed Wilcox’s rank-sum test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Download FIG S3, EPS file, 5.6 MB (5.6MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To further illuminate the difference in microbial community compositions between the two groups, we compared the relative abundance of the dominant bacterial taxa with mean relative abundances over 0.1%. At the phylum level, the two groups showed similar compositional patterns, both dominated by Firmicutes, Actinobacteria, Bacteroidetes, Proteobacteria, Tenericutes, and Fusobacteria (Fig. S4A). The top five abundant genus in the two groups were Lactobacillus, Gardnerella, Atopobium, Prevotella, and Streptococcus (Fig. S4B). The abundance of Lactobacillus crispatus was significantly lower (q = 0.046), while the abundance of Gardnerella vaginalis was higher (q = 0.046) in the HDP group than in the NP group (Fig. 2D).
Vaginal bacterial composition of the NP and HDP groups at the phylum level (A) and genus level (B). Download FIG S4, EPS file, 1.4 MB (1.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Associations of identified differential bacterial taxa with a gestational weeks at delivery and neonatal birth weight were examined using Pearson’s correlation analysis (Fig. S5A). L. crispatus was positively associated with gestational duration, whereas women with a high abundance of G. vaginalis were vulnerable to an early delivery (P = 0.038, P = 0.005, respectively). Noticeably, G. vaginalis was significantly linked with a low neonatal birth weight (P = 0.012), and a similar correlation of G. vaginalis can be found in twin and singleton pregnancies (P = 0.031).
Correlations between the relative abundance of differential bacteria taxa and gestational weeks at delivery and neonatal birth weight (A) and distributions of vaginal community state types in the NP and HDP groups (B). BW_singleton denotes birth weight in singleton pregnancy. BW_both denotes birth weight in both singleton and twin pregnancies (average neonatal birth weight). Red denotes positive correlation, while blue denotes negative correlation. *, P < 0.05; **, P < 0.01. Download FIG S5, EPS file, 1.4 MB (1.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Vaginal CSTs associated with HDP development.
The vaginal microbial communities were heterogeneous and diverse in the population. To further explore whether the vaginal community structure diverged between the two groups and to stratify populations and simplify the analysis of the vaginal microbiome, we assigned vaginal microbial profiles to community state types (CSTs) according to the most abundant taxon (Fig. 2E, Fig. S5B, Table S2). The L. crispatus- and L. iners-dominated CSTs accounted for 46.2% and 41.3% of the total vaginal samples, respectively (Table S2). The L. crispatus-dominated CSTs constituted more than half (52.0%) of the samples in the NP group but only 34.7% of the samples in the HDP group, which coincided with the higher L. crispatus abundance in the NP group than in the HDP group (Fig. 2E, Fig. S5B, Table S2). Conversely, the L. iners-dominated CST accounted for nearly half (49.3%) of the HDP group but only 37.3% of the NP group.
Summary of vaginal community state types (CSTs) assignment. Download Table S2, DOCX file, 0.01 MB (12.7KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The women presenting a vaginal microbiome dominated by L. crispatus tended to deliver at term without HDP (odds ratio [OR] = 0.436; 95% confidence interval [CI], 0.229 to 0.831, P = 0.012; Table S3). Nevertheless, the women with L. iners predominance were prone to HDP (OR = 1.732; 95% CI, 0.947 to 3.165, P = 0.074; Table S3).
Odds ratio and 95% confidence intervals of the association between the vaginal microbiome and risk of HDP. Download Table S3, DOCX file, 0.01 MB (11.9KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alterations in bacterial interactions of vaginal microbiota in the HDP group.
We investigated whether bacterial interactions, a critical part of the ecosystem, were altered in the women who developed HDP. Cooccurrence networks were constructed to analyze the relationships between bacterial taxa in the two groups (Fig. 3A). To assess the concordance of microbial cooccurrence between the HDP and NP groups, we first counted the number of exclusive and shared nodes and edges. Although more than half the nodes (33/57) were shared, 61 and 57 unique edges were found in the NP and HDP groups, respectively, with only 42 shared edges (Fig. 3B and C). Differences in the topological structure of networks were also found between the two groups. Compared with those in the HDP group, the bacterial interactions in the NP group were denser, with a higher weighted degree and closeness centrality (Fig. S6, Wilcox’s rank-sum test, P < 0.001, P = 0.008, respectively). A similar pattern was also observed in the shared nodes (Fig. 3A, Fig. S6, Wilcox’s rank-sum test, P < 0.001, P = 0.044). The comparable degree between the two networks indicated that the total connections of nodes in the two networks were similar (Fig. S6).
FIG 3.
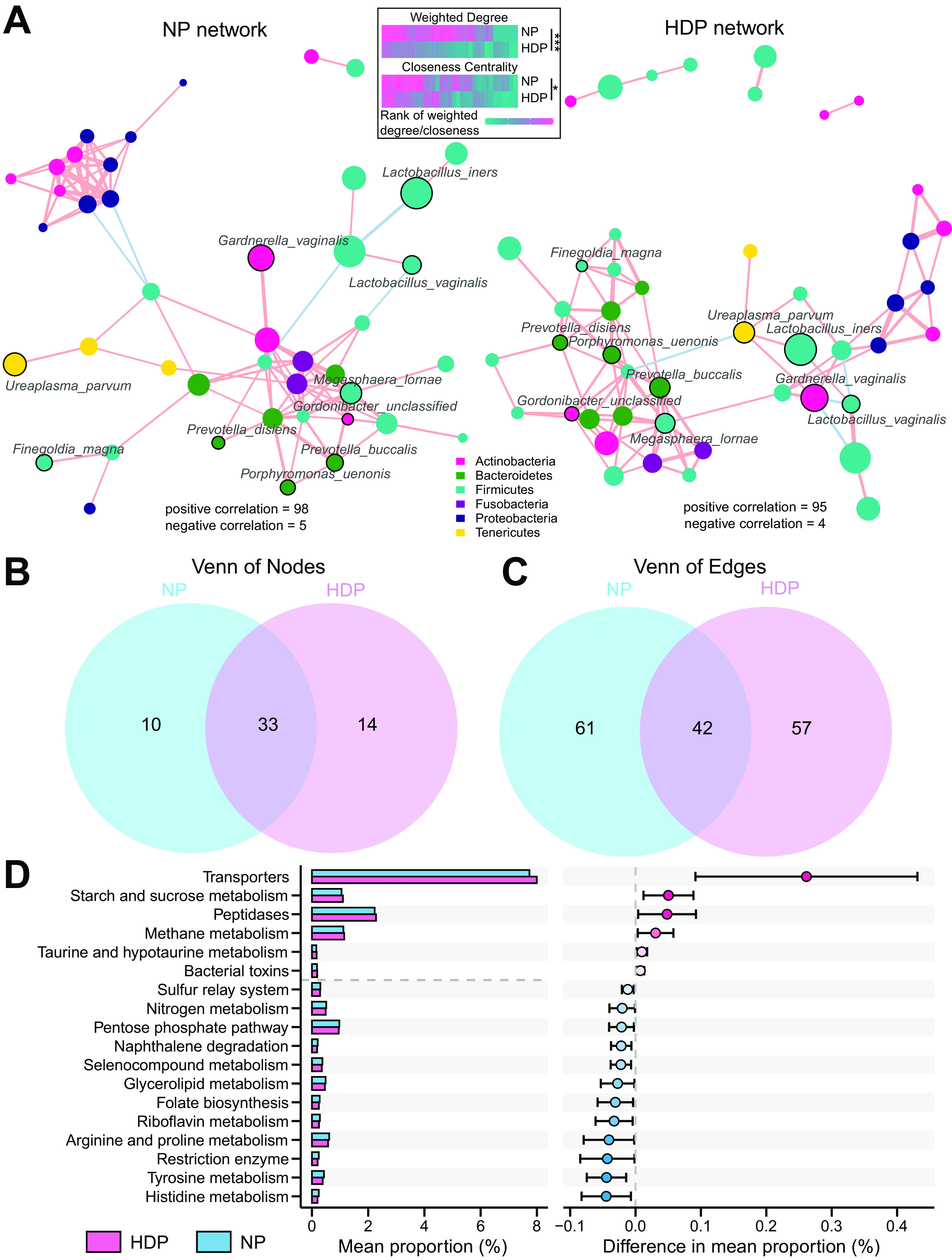
Discrepancies in bacterial cooccurrence networks and predicted functions in the NP and HDP groups. (A) The cooccurrence network was inferred for each group. Nodes are colored by the phylum level. Nodes identified as “drivers” of the network rewiring from NP to HDP by Netshift are labeled. The size of nodes represents the mean relative abundance of the taxa. The edge width reflects the strength of correlations. The edge color denotes the direction of correlations, with red and blue representing positive and negative correlations, respectively. Only significant bacterial connections beyond the cutoff mean ± three standard deviations are retained. The heatmaps shown at the top depict comparisons of weighted degree and closeness centrality of shared nodes in the two networks using a two-tailed Wilcox rank-sum test. The heatmaps are colored by rank of weighted degree or closeness centrality, with red denoting a high rank. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Exclusive and shared nodes (B) as well as edges (C) in the two networks. (D) Predicted KEGG pathways significantly different between the two groups. The left part depicts the mean relative abundance of differential predicted pathways (q < 0.2) between the NP and HDP groups. The right part depicts differences in abundance between the two groups with 95% CI. Red denotes pathways enriched in the HDP group, while blue denotes pathways enriched in the NP group.
Comparisons of weighted degree, closeness centrality, and degree of all nodes (A) as well as shared nodes (B) in the two networks. NP, healthy controls with normal blood pressure during pregnancy; HDP, women with hypertensive disorders of pregnancy. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. Significance was determined using a two-tailed Wilcox rank-sum test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Download FIG S6, EPS file, 2.7 MB (2.7MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Finally, we inferred “driver” taxa for network rewiring from NP to HDP by analyzing the most common subnetwork. The following 10 taxa essentially contributing to the remodeling of the entire microbial community structure were identified as potential “driver” taxa (22): G. vaginalis, L. iners, Ureaplasma parvum, Porphyromonas uenonis, Finegoldia magna, Lactobacillus vaginalis, Prevotella disiens, Prevotella buccalis, Gordonibacter_unclassified, and Megasphaera lornae (Fig. 3A). Overall, the distinct components and topology of the networks indicated that the discrepancy in vaginal ecology between the two groups was driven not only by differential abundant taxa but also by the different interactions of shared microbes.
Functional characteristics of vaginal microbiota in normotensive women who developed HDP.
The function profiles were inferred to determine the functional capacity of the vaginal microbiota (Fig. 3D, conditional logistic regression, all P < 0.05 with q < 0.2). A comparative analysis of the predicted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways showed that the HDP group was enriched in pathways associated with environmental information processing and membrane transport, such as bacterial toxins, transporters, starch and sucrose metabolism, peptidases, methane metabolism, and taurine and hypotaurine metabolism. In contrast, the vaginal microbiota of the NP group was mainly enriched in pathways involved in the metabolism of amino acids, cofactors, and vitamins, including histidine, tyrosine, arginine, proline, and riboflavin, and biosynthesis of folate.
DISCUSSION
The etiology of HDP remains unclear to date. Consequently, effective methods for the individualized prediction, prevention, and treatment of this disease are lacking. The role of the microbiota in HDP development has attracted increasing interest (10 to 14). To the best of our knowledge, this study is the first to prospectively assess associations between pregestational vaginal microbiota and HDP. Study participants were from a large cohort with high follow-up rates and detailed medical information before and during pregnancy, which enabled us to exclude women with pregestational diabetes mellitus (PGDM) or gestational diabetes mellitus (GDM), considering they have increased risks of HDP and vaginal dysbiosis (3, 23). The good match between groups on confounders, including age, BMI, baseline blood pressure, and nulliparity, made our observed associations reliable. This study showed that pregestational vaginal dysbiosis precedes the diagnosis of HDP in normotensive women receiving in vitro fertilization or intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET) treatment, who were considered to have a relatively high risk of HDP (3, 4, 6). Of note, L. crispatus-dominated vaginal microbiome was associated with a decreased likelihood of developing HDP by 0.436.
As demonstrated in the present study, the abundance of L. crispatus was lower, while the abundance of G. vaginalis was higher, in the women who developed HDP than in the healthy controls. L. crispatus enhances the trophoblastic invasion by upregulating the expression of matrix metalloproteinases (MMPs) (24), which might explain its beneficial effects on placental implantation and pregnancy outcome (25 to 28). MMPs are important regulators in vasodilation, placentation, and uterine expansion during normal pregnancy (29). The protein expression of MMP-1 in extravillous trophoblast is decreased in preeclampsia (30). Reduced MMP-2 expression, decreased uterine vascularization, and arterial expansive remodeling have been observed in a previous model of hypertensive pregnancy, possibly contributing to uteroplacental ischemia (31). Depletion of L. crispatus may be related to the impaired invasion of the trophoblast, which may result in shallow placentation and inadequate remodeling of the spiral artery, leading to placental ischemia and preeclampsia (4). Furthermore, high L. crispatus abundance was positively associated with gestational age at delivery, whereas the opposite association was found for G. vaginalis. This result is reasonable because HDP was closely related to medically indicated preterm birth and fetal growth restriction, which also indirectly reflect the severity of the disease.
A decreased abundance of L. crispatus and a higher level of G. vaginalis in the female reproductive tract were also associated with spontaneous preterm birth (32 to 36), early missed abortion (37), recurrent pregnancy loss (38), and lower fecundability (39). The overlap of bacterial associations between adverse pregnancy outcomes and HDP suggests that maternal diseases might have a common vaginal dysbiosis that resembles the common gut dysbiosis in different diseases (40, 41). Further studies are needed to depict detailed mechanisms underlying the overlap. The overlapping mechanisms could be partially mediated by the inflammation triggered by these pathogens because proinflammatory cytokines and complement proteins reportedly induce abnormal placentation (42 to 44).
Despite its lack of differential abundance, L. iners was identified as the “driver,” essentially contributing to the remodeling of the entire microbial community from NP to HDP together with G. vaginalis. Vaginal microbiomes dominated by L. iners transform more often into a diverse community than L. crispatus-dominated CSTs (45, 46). L. crispatus and G. vaginalis tend to be exclusive in the vagina, whereas L. iners often coexists with G. vaginalis (35). L. iners enhances the adhesion of Gardnerella to epithelial cells, and Gardnerella displaces L. crispatus but not L. iners from the epithelia (47). The decreased proportion of the L. crispatus-dominated CST and the increased proportion of the L. iners-dominated CST, and the latter’s propensity to shift toward diverse communities, could partly explain the larger disparity among individuals in the HDP group than among those in the NP group.
Additionally, notable alterations in bacterial interactions, which are important in the microecosystem, were found in the women susceptible to HDP. Despite shared nodes, a large number of edges were exclusive to the women in the HDP or NP group, indicating the remodeling of the entire microbial structure. Hence, the discrepancy between the two groups was not only driven by differentially abundant bacteria but also by the distinct bacterial interactions of shared microbes. Several taxa were identified as potential “drivers” during the remodeling, of which the majority belong to bacterial vaginosis-associated bacteria, namely, G. vaginalis, Porphyromonas, Finegoldia, Prevotella, and Megasphaera (48, 49). Gardnerella, also identified as a differential taxon, can initiate biofilm formation, which then serves as a scaffold for other species to adhere to during vaginal dysbiosis (50, 51). Prevotella, Porphyromonas, Streptococcus, Ureaplasma, and L. iners were detected in preeclampsia amniotic fluid and placenta by cultivation and sequence-based methods, supporting the involvement of dysbiosis in HDP development (15, 16).
In the present study, the NP group was enriched with pathways related to the metabolism of histidine, tyrosine, arginine, riboflavin, and folate, whereas the HDP group was enriched with pathways related to transporters and bacterial toxins as signs of bacterial virulence and predisposing factors of inflammation. High dietary histidine and tyrosine intake are associated with low blood pressure (52, 53). Decreased serum l-arginine, which plays a key role in nitric oxide metabolism, is a risk factor for HDP (54). Riboflavin, a methylenetetrahydrofolate reductase (MTHFR) cofactor for folate metabolism, could lower blood pressure by restoring MTHFR activity in vascular cells, improving nitric oxide availability and endothelial function (55, 56). Dietary deficiencies of folate and riboflavin are related to increased plasma homocysteine, reduced DNA methylation patterns, and genome instability events, which increase cardiovascular risk (57). These studies suggest that the microbiome participates in HDP development by modulating metabolism and inducing inflammation. A recent study has similarly demonstrated that reductions in short-chain fatty acid-producing bacteria accompanied by decreased propionate and butyrate contribute to HDP development by causing inflammation and impaired trophoblast invasion (14).
Several limitations of the present study need to be acknowledged. Although this study was based on a large-scale population, only 75 cases were included in the final analysis because of the low incidence of HDP (4.9%, 108/2224) and the exclusion of cases with other complications. Moreover, the study participants were infertile women of the Chinese Han population who received IVF/ICSI treatment. Caution should be taken when generalizing the results of this study to other populations in other geographical regions. Large-scale and longitudinal multicenter studies and animal experiments are therefore needed to verify these findings.
Conclusions.
In conclusion, pregestational vaginal dysbiosis in normotensive women precedes the diagnosis of HDP. L. crispatus-dominated vaginal microbiomes are associated with a reduced risk for HDP. Our study extends the understanding of potential links between vaginal perturbations and pregnancy complications, provides novel insights into the etiology of HDP, and sheds light on a novel path for the prevention of this disease.
MATERIALS AND METHODS
Study participants.
This study was derived from a comprehensive microbiome cohort established in 2019 with vaginal swabs collected from Chinese females aged 19 to 64 years at routine clinic visits at the Center for Reproductive Medicine, Shandong University.
The exclusion criteria were as follows: antibiotic treatment within 30 days, vaginal medications or vaginal douching within 7 days, engagement in sexual activity in the current menstrual cycle, vaginal bleeding, vaginal subjective symptoms (e.g., vaginal itching and abnormal discharge), any acute inflammation, and severe systemic disease. HDP is defined as new-onset hypertension during pregnancy (gestational hypertension or preeclampsia) after 20 weeks of gestation.
The workflow of the study is as follows (Fig. 1). A total of 5,849 normotensive women scheduled for IVF/ICSI-ET were recruited. Among them, 4,345 individuals received fresh or frozen embryo transfer. Then, 2,224 achieved persistent pregnancy after 12 weeks of gestation, and one was lost to follow-up after 16 weeks of gestation. Finally, 108 women were diagnosed with HDP, of which 75 women who were not complicated with GDM or PGDM were included as cases (here denoted as “HDP group”). Meanwhile, 150 uncomplicated controls (here denoted as “NP group”) with term delivery were matched by 1:2 nearest-neighbor propensity score matching (PSM) without replacement with a propensity score estimated using logistic regression models. For the model, the absence or presence of HDP was the outcome variable. The predictor variables included age, BMI, systolic blood pressure, diastolic blood pressure, fasting blood glucose, triglyceride, total cholesterol, high-density lipoprotein, low-density lipoprotein, transfer of fresh or frozen embryos, number of transferred embryos, and number of fetuses. Advanced maternal age, obesity, chronic hypertension, higher fasting blood glucose, multiple gestation, the use of assisted reproductive technologies, frozen embryo transfer, and certain cardiovascular risk factors such as elevated levels of triglyceride, total cholesterol, low-density lipoprotein cholesterol, and low levels of high-density lipoprotein cholesterol were in relation to a higher risk for HDP (3 to 9). These clinical risk factors were selected to estimate the risk for HDP, and further PSM would balance the baseline characteristics between the two groups by reducing selection bias. The PSM method has been widely used in observational studies to reduce selection bias (58 to 60), and the procedure was conducted by the MatchIt package with default parameters (61). Each case, in descending order of the propensity score, was successively matched to the control with the closest propensity score. Two rounds of one-to-one matches were conducted, resulting in a match ratio of 1:2. This process yielded an adequate balance, as evidenced by the standardized mean differences of all variables being within a threshold of 0.15.
Ethical approval and consent to participate.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Reproductive Medicine, Shandong University (no. 2019LSZ14). All participants provided written informed consent.
DNA extraction from vaginal swabs.
Vaginal swabs were collected from the posterior vaginal fornix of the women in the follicular phase of menstrual cycle at routine clinic visits. DNA extraction was performed using Magnetic Soil and Stool DNA kits (Tiangen Biotech, Beijing) following the manufacturer’s protocol. The V1–V2 hypervariable regions were amplified. Further details about sample collection, 16S rRNA gene sequencing, and preprocessing of raw data are provided in the supplemental material.
Bioinformatics analysis and statistical analyses.
An average of 59,635 reads per sample were obtained after quality filtering. The representative sequences were annotated against the Ribosomal Database Project (RDP) database (rdp_16s_v16_sp.fa accessible at http://www.drive5.com/sintax) (62) using the SINTAX algorithm with a confidence threshold of 0.6. Species allocation was performed by using the RDP and STIRRUPS databases (63).
Sequencing reads were subsampled to the lowest read count of 25,441, followed by rarefaction evaluation. Alpha diversity was quantified using Shannon, Richness, Chao1, and Simpson diversity indices. The weighted Unifrac distance, Bray-Curtis distance, and Jaccard distance were calculated to assess the dissimilarity of microbial composition among intragroup or intergroup samples. The dissimilarity was visualized using principal coordinate analysis (PCoA). Permutational multivariate analysis of variance (PERMANOVA) was conducted using the function adonis from the vegan package to evaluate community structure differences. Discriminatory bacteria were identified by conducting conditional logistic regression on high-abundance taxa with a mean relative abundance over 0.1%. Vaginal profiles were assigned to CSTs based on the most abundant taxon (32). Samples with the largest proportion of less than 30% were not assigned to any CST. Differences in the number of Lactobacillus crispatus-dominated and Lactobacillus iners-dominated CSTs between the two groups were evaluated using conditional logistic regression.
Microbial community networks were constructed as follows. Correlation coefficients were computed at the species level by using Fastspar v2.0 with 1,000 bootstrap replicates to estimate the P values. Bacterial taxa prevalent in at least 5% of the samples were used to filter rare taxa. Correlations with P values of <0.05 and coefficients beyond the threshold of mean ± three standard deviations were retained for further network analysis. Networks were visualized using the igraph package. Local property measures, such as degree, weighted degree, and closeness centrality, were calculated. Common and exclusive nodes and edges and the most common subnetwork between two networks were analyzed to decipher the network similarity and the extent of network rewiring. The taxon with an altered set of associations (identified by a high NESH score >1) and increasing importance in the case network (identified with a positive delta betweenness from control to case) was identified as a “driver” by using Netshift (22). Finally, the functions of vaginal microbiota were predicted using PICRUST, and the predicted KEGG pathways with mean relative abundances over 0.15% were compared between the two groups by using conditional logistic regression.
Conditional logistic regression was used to compare the difference between the case and control pairs unless otherwise specified. False discovery rate-corrected P values (q values) were used to define statistical significance at 0.2 for multiple testing.
Data availability.
The sequencing data reported in this article have been deposited in the Genome Sequence Archive at the BIG Data Center, Chinese Academy of Science, under accession number CRA009956 (BioProject: PRJCA015148). The data are publicly accessible at http://bigd.big.ac.cn/gsa.
Additional experimental details. Download Text S1, DOCX file, 0.02 MB (17.1KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
This research was supported by grants from the National Key Research and Development Program of China (2021YFC2700701 and 2021YFC2700400) to S.Z. and H.Z.; Basic Science Center Program of Natural Science Foundation of China (31988101), Research Unit of Gametogenesis and Health of ART-Offspring, Chinese Academy of Medical Sciences (2020RU001), Shandong Provincial Key Research and Development Program (2020ZLYS02), innovative research team of high-level local universities in Shanghai (SHSMU-ZLCX20210200), and CAMS Innovation Fund for Medical Sciences (2021-I2M-5-001) to Z.-J.C.; National Natural Science Foundation of China (82192874, 31871509, and 82071606) to H.Z. and S.Z.; Taishan Scholars Program of Shandong Province (ts20190988) to H.Z.; and Fundamental Research Funds of Shandong University to S.Z.
We thank all the participants who took part in the study. We appreciate the editor and anonymous reviews for providing invaluable feedback to improve our work. We thank Q.Z. and W.Y. from the Department of Epidemiology, School of Public Health, Shandong University, for their invaluable advice on statistical analysis.
The authors have no conflicts of interest to declare.
S.Z. conceived and supervised the study, and critically reviewed the manuscript. X.L. designed the study, recruited patients, collected samples, analyzed the data, and prepared and revised the manuscript. Z.T., J.L., and X.Y. performed the experiments and collected data. R.C. revised the manuscript. L.Q. recruited patients, collected samples, and critically reviewed the manuscript. Z.L. recruited patients and collected data. C.J. and Y.X. recruited patients and collected samples. C.Z. recruited patients. Z.-J.C. and H.Z. critically reviewed the manuscript. All the authors listed have read and approved the manuscript.
Contributor Information
Shigang Zhao, Email: zsg0108@126.com.
Krishna Rao, University of Michigan—Ann Arbor.
REFERENCES
- 1.Cameron NA, Everitt I, Seegmiller LE, Yee LM, Grobman WA, Khan SS. 2022. Trends in the incidence of new-onset hypertensive disorders of pregnancy among rural and urban areas in the United States, 2007 to 2019. J Am Heart Assoc 11:e023791. doi: 10.1161/JAHA.121.023791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong T, Mu Y, Liang J, Zhu J, Li X, Li J, Liu Z, Qu Y, Wang Y, Mu D. 2018. Hypertensive disorders in pregnancy and stillbirth rates: a facility-based study in China. Bull World Health Organ 96:531–539. doi: 10.2471/BLT.18.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappell LC, Cluver CA, Kingdom J, Tong S. 2021. Pre-eclampsia. Lancet 398:341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 4.Rana S, Lemoine E, Granger JP, Karumanchi SA. 2019. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 124:1094–1112. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 5.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. 2007. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ 335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chih HJ, Elias FTS, Gaudet L, Velez MP. 2021. Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses. BMC Pregnancy Childbirth 21:449. doi: 10.1186/s12884-021-03938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbib N, Pfeffer-Gik T, Sneh-Arbib O, Krispin E, Rosenblat O, Hadar E. 2020. The pre-gestational triglycerides and high-density lipoprotein cholesterol ratio is associated with adverse perinatal outcomes: a retrospective cohort analysis. Int J Gynaecol Obstet 148:375–380. doi: 10.1002/ijgo.13078. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Lan X, Cai C, Li R, Gao Y, Yang L, Wu C, Dong H, Pang X, Bai D, Zeng G. 2021. Associations between maternal lipid profiles and pregnancy complications: a prospective population-based study. Am J Perinatol 38:834–840. [DOI] [PubMed] [Google Scholar]
- 9.Wu D, Zhang J, Xiong Y, Wang H, Lu D, Guo M, Zhang J, Chen L, Fan J, Huang H, Lin X. 2022. Effect of maternal glucose and triglyceride levels during early pregnancy on pregnancy outcomes: a retrospective cohort study. Nutrients 14:3295. doi: 10.3390/nu14163295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadian E, Rahbar Saadat Y, Hosseiniyan Khatibi SM, Nariman-Saleh-Fam Z, Bastami M, Zununi Vahed F, Ardalan M, Zununi Vahed S. 2020. Pre-eclampsia: microbiota possibly playing a role. Pharmacol Res 155:104692. doi: 10.1016/j.phrs.2020.104692. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Li P, Liu M, Zheng H, He Y, Chen MX, Tang W, Yue X, Huang Y, Zhuang L, Wang Z, Zhong M, Ke G, Hu H, Feng Y, Chen Y, Yu Y, Zhou H, Huang L. 2020. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 69:513–522. doi: 10.1136/gutjnl-2019-319101. [DOI] [PubMed] [Google Scholar]
- 12.Contreras A, Herrera JA, Soto JE, Arce RM, Jaramillo A, Botero JE. 2006. Periodontitis is associated with preeclampsia in pregnant women. J Periodontol 77:182–188. doi: 10.1902/jop.2006.050020. [DOI] [PubMed] [Google Scholar]
- 13.Ivankiv VY, Malanchyn IM, Tkachuk NI. 2019. Microbiota of vagina and mammary glands skin in the pregnant women with preeclampsia. Int J Medicine and Medical Res 4:44–49. doi: 10.11603/ijmmr.2413-6077.2018.2.9347. [DOI] [Google Scholar]
- 14.Jin J, Gao L, Zou X, Zhang Y, Zheng Z, Zhang X, Li J, Tian Z, Wang X, Gu J, Zhang C, Wu T, Wang Z, Zhang Q. 2022. Gut dysbiosis promotes preeclampsia by regulating macrophages and trophoblasts. Circ Res 131:492–506. doi: 10.1161/CIRCRESAHA.122.320771. [DOI] [PubMed] [Google Scholar]
- 15.DiGiulio DB, Gervasi M, Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Seok KS, Gomez R, Mittal P, Gotsch F, Chaiworapongsa T, Oyarzun E, Kim CJ, Relman DA. 2010. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 38:503–513. doi: 10.1515/jpm.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH. 2015. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res 41:662–669. doi: 10.1111/jog.12619. [DOI] [PubMed] [Google Scholar]
- 17.Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. 2007. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol 78:670–676. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- 18.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. 2004. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaemsaithong P, Lertrut W, Kamlungkuea T, Santanirand P, Singsaneh A, Jaovisidha A, Pakdeeto S, Mongkolsuk P, Pongchaikul P. 2022. Maternal septicemia caused by Streptococcus mitis: a possible link between intra-amniotic infection and periodontitis. Case report and literature review. BMC Infect Dis 22:562. doi: 10.1186/s12879-022-07530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Li Z, Ma X, Du L, Jia Z, Cui X, Yu L, Yang J, Xiao L, Zhang B, Fan H, Zhao F. 2021. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat Commun 12:4191. doi: 10.1038/s41467-021-24516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CY, Lin CY, Yeh YM, Yang LY, Lee YS, Chao A, Chin CY, Chao AS, Yang CY. 2020. Severe preeclampsia is associated with a higher relative abundance of Prevotella bivia in the vaginal microbiota. Sci Rep 10:18249. doi: 10.1038/s41598-020-75534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuntal BK, Chandrakar P, Sadhu S, Mande SS. 2019. “NetShift”: a methodology for understanding “driver microbes” from healthy and disease microbiome datasets. ISME J 13:442–454. doi: 10.1038/s41396-018-0291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, Ji P, Zhang F, Jia Z, Wang Y, Zheng Z, Zhang H, Zhao F. 2018. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67:1614–1625. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Takada K, Komine-Aizawa S, Kamei Y, Ishihara O, Hayakawa S. 2021. Lactobacillus crispatus promotes invasion of the HTR-8/SVneo trophoblast cell line. Placenta 111:76–81. doi: 10.1016/j.placenta.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, Alonso R, Alama P, Remohi J, Pellicer A, Ramon D, Simon C. 2016. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 26.Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahceci M, Barrionuevo MJ, Taguchi S, Puente E, Dimattina M, Lim MW, Meneghini G, Aubuchon M, Leondires M, Izquierdo A, Perez-Olgiati M, Chavez A, Seethram K, Bau D, Gomez C, Valbuena D, Vilella F, Simon C. 2022. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 10:1. doi: 10.1186/s40168-021-01184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Serrano MG, Fettweis JM, Basta P, Rosen E, Ludwig K, Sorgen AA, Blakley IC, Wu MC, Dole N, Thorp JM, Siega-Riz AM, Buck GA, Fodor AA, Engel SM. 2022. Race, the vaginal microbiome, and spontaneous preterm birth. mSystems 7:e0001722. doi: 10.1128/msystems.00017-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaviani F, Hezelgrave NL, Kanno T, Prosdocimi EM, Chin-Smith E, Ridout AE, von Maydell DK, Mistry V, Wade WG, Shennan AH, Dimitrakopoulou K, Seed PT, Mason AJ, Tribe RM. 2021. Cervicovaginal microbiota and metabolome predict preterm birth risk in an ethnically diverse cohort. JCI Insight 6:e149257. doi: 10.1172/jci.insight.149257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Khalil RA. 2017. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci 148:87–165. doi: 10.1016/bs.pmbts.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lian IA, Toft JH, Olsen GD, Langaas M, Bjorge L, Eide IP, Bordahl PE, Austgulen R. 2010. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta 31:615–620. doi: 10.1016/j.placenta.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, He H, Cui N, Ren Z, Zhu M, Khalil RA. 2020. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy. Am J Physiol Heart Circ Physiol 318:H165–H180. doi: 10.1152/ajpheart.00602.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, Mistry SD, Duckworth RA, 3rd, Bradley SP, Koparde VN, Orenda XV, Milton SH, Rozycki SK, Matveyev AV, Wright ML, Huzurbazar SV, Jackson EM, Smirnova E, Korlach J, Tsai YC, Dickinson MR, Brooks JL, Drake JI, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Wijesooriya NR, Hendricks-Munoz KD, Jefferson KK, Strauss JF, III, Buck GA. 2019. The vaginal microbiome and preterm birth. Nat Med 25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, Fraser WD. 2019. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case-control study. Bjog 126:349–358. doi: 10.1111/1471-0528.15299. [DOI] [PubMed] [Google Scholar]
- 34.Payne MS, Newnham JP, Doherty DA, Furfaro LL, Pendal NL, Loh DE, Keelan JA. 2021. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am J Obstet Gynecol 224:206.e1–206.e23. doi: 10.1016/j.ajog.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, Biggio JR, Wong RJ, Druzin ML, Shaw GM, Stevenson DK, Holmes SP, Relman DA. 2017. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci USA 114:9966–9971. doi: 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bretelle F, Rozenberg P, Pascal A, Favre R, Bohec C, Loundou A, Senat MV, Aissi G, Lesavre N, Brunet J, Heckenroth H, Luton D, Raoult D, Fenollar F, Groupe de Recherche en Obstetrique Gynecologie . 2015. High Atopobium vaginae and Gardnerella vaginalis vaginal loads are associated with preterm birth. Clin Infect Dis 60:860–867. doi: 10.1093/cid/ciu966. [DOI] [PubMed] [Google Scholar]
- 37.Sun D, Zhao X, Pan Q, Li F, Gao B, Zhang A, Huang H, Xu D, Cheng C. 2022. The association between vaginal microbiota disorders and early missed abortion: a prospective study. Acta Obstet Gynecol Scand 101:960–971. doi: 10.1111/aogs.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peuranpaa P, Holster T, Saqib S, Kalliala I, Tiitinen A, Salonen A, Hautamaki H. 2022. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod Biomed Online 45:1021–1031. doi: 10.1016/j.rbmo.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Hong X, Zhao J, Yin J, Zhao F, Wang W, Ding X, Yu H, Ma X, Wang B. 2022. The association between the pre-pregnancy vaginal microbiome and time-to-pregnancy: a Chinese pregnancy-planning cohort study. BMC Med 20:246. doi: 10.1186/s12916-022-02437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta VK, Kim M, Bakshi U, Cunningham KY, Davis JM, III, Lazaridis KN, Nelson H, Chia N, Sung J. 2020. A predictive index for health status using species-level gut microbiome profiling. Nat Commun 11:4635. doi: 10.1038/s41467-020-18476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aasmets O, Krigul KL, Lull K, Metspalu A, Org E. 2022. Gut metagenome associations with extensive digital health data in a volunteer-based Estonian microbiome cohort. Nat Commun 13:869. doi: 10.1038/s41467-022-28464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. 2008. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol 198:385e1–9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tincani A, Cavazzana I, Ziglioli T, Lojacono A, De Angelis V, Meroni P. 2010. Complement activation and pregnancy failure. Clin Rev Allergy Immunol 39:153–159. doi: 10.1007/s12016-009-8183-5. [DOI] [PubMed] [Google Scholar]
- 44.Girardi G. 2018. Complement activation, a threat to pregnancy. Semin Immunopathol 40:103–111. doi: 10.1007/s00281-017-0645-x. [DOI] [PubMed] [Google Scholar]
- 45.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. 2009. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol 9:116. doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. 2017. Lactobacillus iners: friend or foe? Trends Microbiol 25:182–191. doi: 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Castro J, Henriques A, Machado A, Henriques M, Jefferson KK, Cerca N. 2013. Reciprocal interference between Lactobacillus spp. and Gardnerella vaginalis on initial adherence to epithelial cells. Int J Med Sci 10:1193–1198. doi: 10.7150/ijms.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 49.Muzny CA, Blanchard E, Taylor CM, Aaron KJ, Talluri R, Griswold ME, Redden DT, Luo M, Welsh DA, Van Der Pol WJ, Lefkowitz EJ, Martin DH, Schwebke JR. 2018. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect Dis 218:966–978. doi: 10.1093/infdis/jiy243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson JL, Stull-Lane A, Girerd PH, Jefferson KK. 2010. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology (Reading) 156:392–399. doi: 10.1099/mic.0.034280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verstraelen H, Swidsinski A. 2013. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr Opin Infect Dis 26:86–89. doi: 10.1097/QCO.0b013e32835c20cd. [DOI] [PubMed] [Google Scholar]
- 52.Tuttle KR, Milton JE, Packard DP, Shuler LA, Short RA. 2012. Dietary amino acids and blood pressure: a cohort study of patients with cardiovascular disease. Am J Kidney Dis 59:803–809. doi: 10.1053/j.ajkd.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Altorf-van der Kuil W, Engberink MF, De Neve M, van Rooij FJ, Hofman A, Van't Veer P, Witteman JC, Franco OH, Geleijnse JM. 2013. Dietary amino acids and the risk of hypertension in a Dutch older population: the Rotterdam Study. Am J Clin Nutr 97:403–410. doi: 10.3945/ajcn.112.038737. [DOI] [PubMed] [Google Scholar]
- 54.Maruta E, Wang J, Kotani T, Tsuda H, Nakano T, Imai K, Sumigama S, Niwa Y, Mitsui T, Yoshida S, Yamashita M, Nawa A, Tamakoshi K, Kajiyama H, Kikkawa F. 2017. Association of serum asymmetric dimethylarginine, homocysteine, and l-arginine concentrations during early pregnancy with hypertensive disorders of pregnancy. Clin Chim Acta 475:70–77. doi: 10.1016/j.cca.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Thakur K, Tomar SK, Singh AK, Mandal S, Arora S. 2017. Riboflavin and health: a review of recent human research. Crit Rev Food Sci Nutr 57:3650–3660. doi: 10.1080/10408398.2016.1145104. [DOI] [PubMed] [Google Scholar]
- 56.Liu M, Zhou C, Zhang Z, Li Q, He P, Zhang Y, Li H, Liu C, Qin X. 2020. Inverse association between riboflavin intake and new-onset hypertension: a nationwide cohort study in China. Hypertension 76:1709–1716. doi: 10.1161/HYPERTENSIONAHA.120.16211. [DOI] [PubMed] [Google Scholar]
- 57.Singh MD, Thomas P, Owens J, Hague W, Fenech M. 2015. Potential role of folate in pre-eclampsia. Nutr Rev 73:694–722. doi: 10.1093/nutrit/nuv028. [DOI] [PubMed] [Google Scholar]
- 58.Ressler AM, Patel A, Rao K. 2021. Non-steroidal anti-inflammatory drugs are not associated with increased risk of Clostridioides difficile infection: a propensity-score-matched case-control study. Anaerobe 72:102444. doi: 10.1016/j.anaerobe.2021.102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Yu X, Xu X, Ming J, Wang Z, Gao B, Xing Y, Zhou J, Fu J, Liu T, Liu X, Garstka MA, Wang X, Ji Q. 2021. The fecal microbiota is already altered in normoglycemic individuals who go on to have type 2 diabetes. Front Cell Infect Microbiol 11:598672. doi: 10.3389/fcimb.2021.598672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Chen X, Cheng H, Zhang L. 2022. Dietary copper intake and risk of stroke in adults: a case-control study based on National Health and Nutrition Examination Survey 2013–2018. Nutrients 14:409. doi: 10.3390/nu14030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Randolph J, Falbe K, Manuel AK, Balloun JL. 2014. A step-by-step guide to propensity score matching in R. Pract Assess Res Eval 19:1–6. [Google Scholar]
- 62.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fettweis JM, Serrano MG, Sheth NU, Mayer CM, Glascock AL, Brooks JP, Jefferson KK, Vaginal Microbiome C, Buck GA, Vaginal Microbiome Consortium (additional members) . 2012. Species-level classification of the vaginal microbiome. BMC Genomics 13(Suppl 8):S17. doi: 10.1186/1471-2164-13-S8-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pregnancy outcomes, gestational weeks, and matched group of all participants. Download Table S1, DOCX file, 0.04 MB (40.2KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Rarefaction curves of the observed number of amplicon sequence variants (Richness) in the NP and HDP groups. Download FIG S1, EPS file, 1.2 MB (1.2MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of Chao1 (A), Richness (B), and Simpson indexes (C) between the NP and HDP groups. Significance was determined using conditional logistic regression. The violin plots show the density distribution of the alpha diversity within group. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. Download FIG S2, EPS file, 4.4 MB (4.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Principal coordinate analysis and dissimilarities of the vaginal microbiome of the NP and HDP groups based on Bray-Curtis distance and Jaccard distance. The vaginal community composition is significantly different between the HDP and NP groups based on Bray-Curtis distance (A) and Jaccard distance (B). Each dot represents a sample and is colored by group. Differences in beta diversity between the NP and HDP groups were determined using PERMANOVA. Bray-Curtis distance (C) and Jaccard distance (D) of the vaginal microbial community among individuals within the same group and between the NP and HDP groups. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. P values were determined using a two-tailed Wilcox’s rank-sum test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Download FIG S3, EPS file, 5.6 MB (5.6MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Vaginal bacterial composition of the NP and HDP groups at the phylum level (A) and genus level (B). Download FIG S4, EPS file, 1.4 MB (1.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Correlations between the relative abundance of differential bacteria taxa and gestational weeks at delivery and neonatal birth weight (A) and distributions of vaginal community state types in the NP and HDP groups (B). BW_singleton denotes birth weight in singleton pregnancy. BW_both denotes birth weight in both singleton and twin pregnancies (average neonatal birth weight). Red denotes positive correlation, while blue denotes negative correlation. *, P < 0.05; **, P < 0.01. Download FIG S5, EPS file, 1.4 MB (1.4MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Summary of vaginal community state types (CSTs) assignment. Download Table S2, DOCX file, 0.01 MB (12.7KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Odds ratio and 95% confidence intervals of the association between the vaginal microbiome and risk of HDP. Download Table S3, DOCX file, 0.01 MB (11.9KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparisons of weighted degree, closeness centrality, and degree of all nodes (A) as well as shared nodes (B) in the two networks. NP, healthy controls with normal blood pressure during pregnancy; HDP, women with hypertensive disorders of pregnancy. For the boxplot, the central line, box, and whisker represent the median, interquartile range (IQR), and 1.5 times the IQR, respectively. Significance was determined using a two-tailed Wilcox rank-sum test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Download FIG S6, EPS file, 2.7 MB (2.7MB, eps) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Additional experimental details. Download Text S1, DOCX file, 0.02 MB (17.1KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The sequencing data reported in this article have been deposited in the Genome Sequence Archive at the BIG Data Center, Chinese Academy of Science, under accession number CRA009956 (BioProject: PRJCA015148). The data are publicly accessible at http://bigd.big.ac.cn/gsa.
Additional experimental details. Download Text S1, DOCX file, 0.02 MB (17.1KB, docx) .
Copyright © 2023 Li et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.



