Abstract
Cardiac transplantation remains the gold standard therapy for end stage heart failure. However, it remains limited by the number of available donor hearts and by complications such as primary graft dysfunction and graft rejection. The recent clinical use of an ex vivo perfusion device in cardiac transplantation introduces a unique opportunity for treating cardiac allografts with therapeutic interventions to improve function and avoid deleterious recipient responses. Establishing a translational, large-animal model for therapeutic delivery to the entire allograft is essential for testing novel therapeutic approaches in cardiac transplantation. The porcine, heterotopic heart transplantation model in the intra-abdominal position serves as an excellent model for assessing the effects of novel interventions as well as the immunopathology of graft rejection. This model additionally offers long-term survival for the pig given that the graft is not required to maintain the recipient’s circulation. The aim of this protocol is to provide a reproducible and robust approach for achieving ex vivo delivery of a therapeutic to the entire cardiac allograft prior to transplantation and provide technical details to perform a survival heterotopic transplant of the ex vivo perfused heart.
SUMMARY:
We present a protocol for utilizing a normothermic ex vivo sanguinous perfusion system for the delivery of therapeutics to an entire cardiac allograft in a porcine heterotopic heart transplant model.
INTRODUCTION:
Heart failure is a condition that affects an estimated 6 million adults in the United States and is projected to increase to 8 million adults by the year 20301. Cardiac transplantation remains the gold standard therapy for end stage heart failure. However, it is not without its limitations and complications. It remains limited by the number of available donor hearts, primary graft dysfunction, rejection of the heart, and the side effects of long-term immunosuppression2. These limitations are particularly important in young recipients who may experience allograft failure and require subsequent re-transplantation to achieve normal life expectancy.
An ideal intervention to overcome these limitations would treat entire cardiac allografts with therapeutics prior to implantation into the recipient that can improve the viability of the allograft and confer “cardioprotection.” Such interventions would be given prophylactically to minimize the incidence of ischemic insults, allograft rejection, cardiac allograft vasculopathy, and even repair marginal allografts. Translational studies for developing these types of interventions require a large-animal model of cardiac transplantation to allow for the long-term surveillance of the cardiac graft. The porcine, heterotopic heart transplantation model in the intra-abdominal position has proven to be ideal for this purpose. Heart transplantation in this position allows for testing the effects of novel therapies and assessing the immunopathology of graft rejection. Additionally, the heterotopic model is advantageous over the orthotopic model due to better overall survival of the recipient, no requirement for cardiopulmonary bypass, and no requirement of the graft to maintain the recipient’s circulation3.
Effective delivery of therapeutic interventions, such as gene, cell, or immuno- therapy, to the heart is a significant barrier to clinical application4,5. The technology introduced by ex vivo perfusion devices allows for grafts to be continually perfused, maintaining them in a nonworking but metabolically active state6–9. This offers a unique opportunity to treat a whole heart with advanced therapeutics while minimizing potential side effects of systemic delivery10–13. Another advantage of utilizing ex vivo perfusion devices for therapeutic delivery is that they allow for the administration of medications to the coronary circulation over extended periods that are not feasible using traditional cold static storage methods. This allows for more global delivery of the therapeutics to the graft14. Using the protocol presented here, we achieved successful gene delivery of the firefly luciferase gene to a whole porcine cardiac graft using adenoviral vectors15. The aim of this protocol is to provide a reproducible and robust approach for achieving delivery of a therapeutic to the entire cardiac allograft prior to transplantation.
PROTOCOL:
NOTE: Two female Yucatan pigs are selected with one designated to be the cardiac graft donor and the other the recipient. Pigs that are between 6–8 months of age, weigh approximately 30 kg, and have compatible blood types are recommended. Housing and the treatment procedures for the pigs are performed in accordance with the guidelines of the Animal Care and Use Committee of Duke University Medical Center.
1. Preparation of the ex vivo perfusion device
1.1. Prepare the ex vivo perfusion device and a cell saver device for use per the manufacturer’s guidelines.
1.2. Have a pacing box and defibrillator available and set them up.
1.3. Have a point-of-care (POC) testing device available to check a complete blood count (CBC), basic metabolic panel (BMP), and arterial blood gas (ABG).
1.4. Add the following medications to the perfusion priming solution provided by the manufacturer, if not already present in the manufacturer’s perfusion solution: 100 mL of 25% albumin, 10 mL of 200 mg/100 mL ciprofloxacin, 1 g of cefazolin sodium, two 5 mL vials of multivitamin injection, 250 mg of methylprednisolone, 10,000 IU of heparin, and 50 IU of insulin.
1.4.1. Titrate calcium gluconate, sodium bicarbonate, and dextrose based on the POC testing results.
1.5. To add the priming solution with the added medications, spike the solution and de-air the line delivering the solution to the ex vivo perfusion device.
NOTE: Skip to section 6 for instructions on priming the ex vivo perfusion device.
2. Initiation of anesthesia and IV access in the donor pig
2.1. After fasting the pig for 8–12 h, pre-medicate it with ketamine (5–33 mg/kg) and midazolam (0.2–0.5 mg/kg) and administer isoflurane (1–4%) using a face mask.
2.2. Place the pig in supine position and intubate with an endotracheal tube (ETT) (5.5–6.5 mm internal diameter) to protect the airway. Secure the ETT by tying it to the pig’s snout. Position the extremities using heavy ties attached to the table.
2.3. Apply vet ointment on the eyes to prevent dryness while under anesthesia.
2.4. Place an intravenous (IV) catheter (20–22 G) in an ear vein.
2.5. Initiate maintenance IV fluids (Lactated Ringer’s solution at 10 mL·(kg·h)−1).
2.6. Administer intramuscular (IM) Buprenorphine 0.005–0.01 mg/kg for analgesia.
3. Vital signs and central line settings
3.1. Start mechanical ventilation at a tidal volume of 10 mL·(kg·min)−1 and a rate of 10–15 breaths per minute with isoflurane (1–3%) maintained throughout the procedure such that reflexes are absent and the heart rate (>60 bpm, <100 bpm) and blood pressure (systolic blood pressure >90 mmHg, <130 mmHg) remain within physiologic range.
NOTE: Addition of a paralytic is optional.
3.2. Continuously monitor oxygen saturation and heart rates throughout the surgery.
Apply a sterile surgical drape around the immediate surgical site.
4.2 Make an incision using a no. 10 blade from the manubrium down to the xiphoid, measuring 20–30 cm, depending on the size of the pig.
4.3. Use electrocautery to divide the pectoralis major down from the sternum to the xiphoid, being careful to do this along the midline of the sternum. Once down to the sternum, score the midline and begin the sternotomy from the xiphoid by dividing it with heavy scissors.
4.4. Extend the sternotomy cephalad with heavy scissors. After each cut, bluntly separate the heart off the sternum using finger sweeps. In this manner, complete the sternotomy through the manubrium.
4.6. Place a sternal retractor and open to optimize exposure of the surgical field. Identify and remove the thymus with electrocautery. Enter the pericardium in a longitudinal fashion from the diaphragm to the aorta. Create a pericardial cradle using five to six, size: 2–0, silk sutures.
5.1. Fully divide the tissue between the aorta and pulmonary artery (PA) and visualize the location of the aortic arch and the brachiocephalic trunk to facilitate proper placement of the aortic cross clamp.
5.2. Circumferentially free the superior vena cava (SVC) using scissors and blunt dissection. Pass two, size: 0, silk ties around the SVC.
5.3. Circumferentially free the inferior vena cava (IVC) using scissors and blunt dissection. Similarly, pass two 0 silk ties around the IVC.
5.4. Apply a U-stitch, size: 4–0, polypropylene suture to the ascending aorta.
5.5. Apply a purse-string, size: 4–0, polypropylene suture to the right atrium (RA).
5.7. Insert a pediatric 4-Fr aortic root cannula, secured by the previously placed U-stitch. De-air the cannula and secure it in place with a Rummel tourniquet.
5.8. Connect the aortic root cannula to the cardioplegia tubing after the tubing has been flushed with Del Nido cardioplegia. Flush with the necessary amount to remove any air bubbles
5.9. Create a right atriotomy within the previously placed purse-string, insert a 24 Fr venous cannula into the RA, and secure with a Rummel tourniquet.
5.10. Connect the venous cannula to a sterile suction line that is connected to the cell saver cardiotomy and collect approximately 1–1.3 L of blood. Then, apply the aortic cross clamp, carefully ensuring that the clamp completely occludes the ascending aorta. Administer 500 mL of Del Nido cardioplegia into the root at a pressure of 100–150 mmHg using a pressure bag.
5.11. Place sterile ice slush on the heart.
5.13. Divide the following: the IVC, the SVC just proximal to the azygos vein, the aorta at the level of the arch just distal to the Innominate artery, the main PA at the bifurcation.
5.18. Identify the pulmonary veins and ligate them with size: 2–0, silk ties or large-sized clips.
5.19. Remove the heart from the chest and place it in a container with sterile ice slush.
6.1.2. Prime the cell saver device by spiking Plasmalyte A and selecting the prime function on the device. Add as much Plasmalyte A as the volume of blood that was collected from the donor pig in a 1:1 fashion.
6.3. Add the washed blood to the ex vivo perfusion device per the manufacturer’s guidelines.
7.1. Oversew the SVC. Place four pledgeted, size: 4–0, polypropylene sutures in a simple horizontal mattress fashion around the inside of the distal aorta, 5 mm below the cut edge and tie them down.
7.3. While holding up the 4, size: 4–0, pledgeted aortic sutures, insert the aortic connector into the aorta and tie an umbilical tape around the aorta to secure the connector.
7.4. Place a size: 4–0, polypropylene purse-string around the distal cut edge of the main PA. Insert the PA cannula and tie down the ends of the purse-string to secure the cannula.
7.6. Take the prepared graft from the backtable to the ex vivo perfusion device and connect the aortic connector to the device. Be sure to de-air the aorta/aortic connector before securing the heart to the device.
7.9. Connect the PA cannula to the PA connector on the device and secure it with a tie.
7.10. Place the left ventricle (LV) vent drain through the untied pulmonary vein into the left atrium and across the mitral valve into the LV. Secure the vent in place with a single stitch to
8.2. De-air the cardioplegia port by using a sterile 3 mL syringe to draw blood through the port. Administer the therapeutic into the cardioplegia port (or equivalent) such that the therapeutic is introduced directly into the aortic root.
Apply a sterile surgical drape around the immediate surgical site.
9.6. Use a 10 blade to incise the skin (20–30 cm incision) and switch to electrocautery to dissect down to the fascia.
9.7. Use two Kocher clamps to lift the fascia and peritoneum and carefully make a small incision (1 cm) into the peritoneal cavity using Metzenbaum scissors.
9.8. Extend the peritoneal opening for the full length of the incision using electrocautery, placing a finger underneath to protect the underlying viscera. Place a Balfour retractor to optimize exposure. Retract the small bowel cranially and with wet towels.
9.12. Carry the dissection down to the abdominal aorta and IVC. Ligate the lymphatics with medium and large clips.
10.7. Remove the heart from the ex vivo perfusion device by disconnecting the PA cannula and the aortic connector and cutting the pacing wires.
10.9. On the backtable, oversew the pulmonary vein/left atriotomy where the LV vent had been inserted. Trim (1 or 2 mm) of the distal aspect of the aorta and PA where attachment to the cannulas may have crushed the tissue.
11.2. Place a Satinsky clamp on the IVC and create a longitudinal venotomy measuring ~1.5 cm using an 11-blade and Pott’s scissors.
11.3. Anastomose the graft PA to the recipient’s infra-renal IVC in an end-to-side fashion using a running, size: 4–0, polypropylene suture. Perform the inner part of the anastomosis first and
3.4. Place a Satinsky clamp on the aorta and create a longitudinal aortotomy measuring ~1.5 cm using an 11-blade and Pott’s scissors.
11.5. Anastomose the graft aorta to the recipient’s infra-renal aorta in an end-to-side fashion using a running, size: 4–0, polypropylene suture. Perform the inner part of the anastomosis first
11.6. Remove the Satinsky clamps to reperfuse the heart; first, remove the IVC clamp followed by the aortic clamp.
11.9. Carefully place the heart into the right retroperitoneal space, such that there is no tension on the anastomoses and no kinking of the vessels. Replace the small bowel.
12.1. Close the fascia with looped, size: 0, Maxon suture in a running fashion starting from both ends of the incision and tying in the middle. Take care to avoid any injury to the bowel.
12.2. Close the deep dermal layer with size: 2–0, Vicryl in a running fashion. And the skin with size: 4–0, Monocryl in a running fashion.
12.4. Clean the skin incision and apply skin glue.
4. Post-surgical treatment
13.1. After completion of the surgery, turn off the isoflurane flow and monitor the pig for return of muscular tone and neuromuscular reflexes (corneal reflex, withdrawal to painful stimuli, swallowing).
13.2. After confirming the restoration of these functions, turn off mechanical ventilation and observe for spontaneous breathing. If there is spontaneous breathing, remove the endotracheal tube; if there is not, reconnect the endotracheal tube to mechanical ventilation.
13.3. Transfer the pig off the operating table to an isolated enclosure where its vital signs (rectal temperature, blood pressure, heart rate) can be closely monitored. Use a heating lamp to warm the pig as necessary. Provide an IV fluid bolus of 250 mL of Lactated Ringer’s solution in the setting of hypotension (systolic blood pressure < 100mmHg). Continue to monitor the pig until it is able to maintain sternal recumbency and vital signs are fully normalized.
NOTE: The animal is not left unattended until it has regained sufficient consciousness. Additionally, the animal is not returned to the company of other animals until fully recovered.
13.4. For pain management, administer a one-time dose of Buprenorphine (sustained release) subcutaneous injection 0.12 mg/kg for 72 h of analgesia.
REPRESENTATIVE RESULTS:
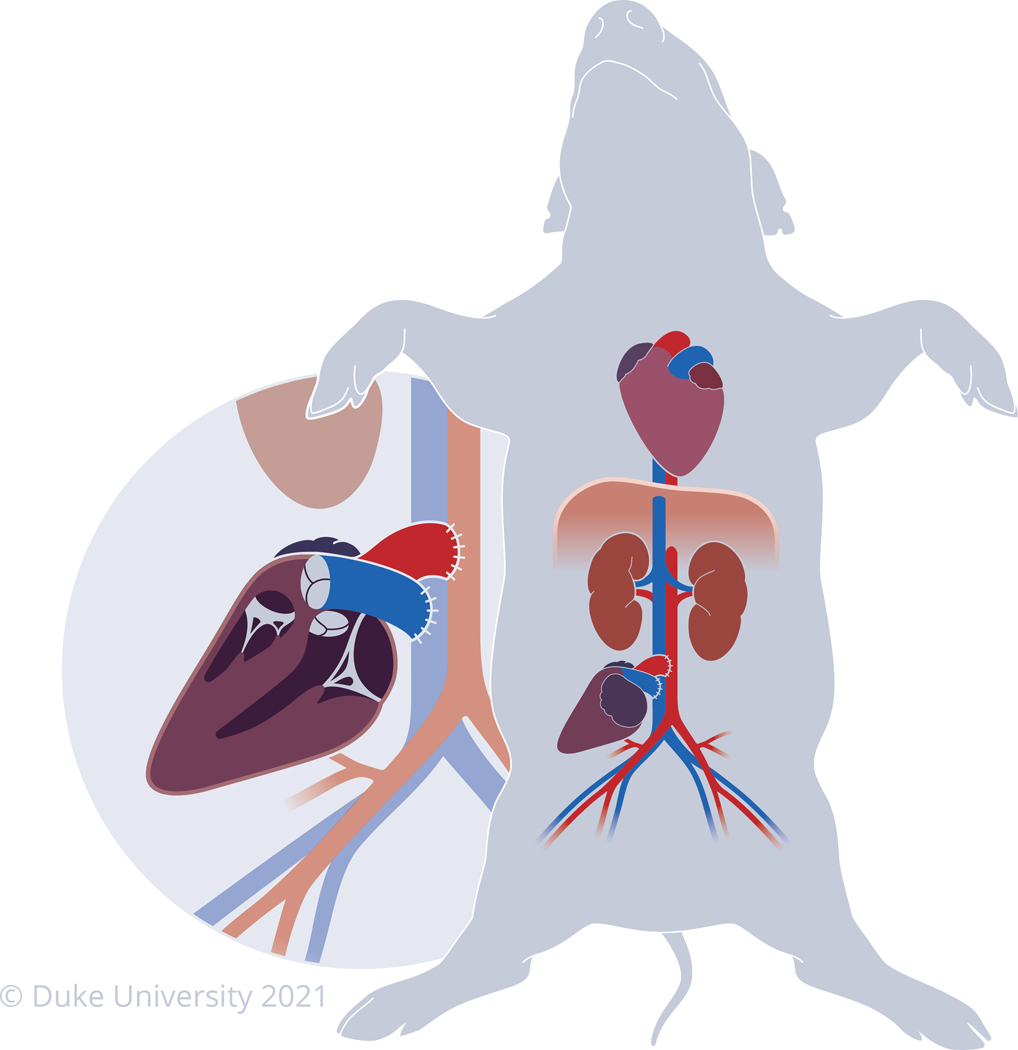
Following the protocol as presented here, this group has successfully survived 9 pigs between 5 and 35 days, depending on the study design. Out of 10 pigs that have undergone this protocol, only 1 died prematurely from surgical complications, yielding a 90% survival rate. Demonstrated in Figure 1 is a diagram of the configuration of a heterotopic heart transplanted in the intra-abdominal position in a pig. When determining the site for anastomosis of the allograft, select a site that minimizes any tension or kinking on the anastomosis. This is to assure that the anastomoses heal properly and that the allograft receives optimal perfusion and drainage of blood. Figure 5 demonstrates an image of an intra-abdominal heterotopic heart in situ 35 days after successful transplantation.
Figure 1: Porcine heterotopic heart model in the intra-abdominal position.
Diagram of the heterotopic heart model where the allograft is transplanted in the intra-abdominal position while the recipient’s native heart remains in its natural location. The pulmonary artery of the allograft is anastomosed to the infra-renal inferior vena cava while the aorta of the allograft is anastomosed to the infra-renal aorta of the recipient.
Figure 5: Cardiac allograft transplanted in the recipient.

A cardiac allograft on post-operative day 35 that was treated with therapeutic at the time of implantation. The donor was selected to be a perfect SLA match with the recipient. Abbreviation: SLA = Swine Leukocyte Antigen.
A representative image of a cardiac allograft being perfused on a normothermic ex vivo perfusion device is shown in Figure 3. Figure 4 outlines representative perfusion parameters acquired during a successful experiment (circulatory flow rate, aortic pressure, heart rate, temperature, mixed venous oxygen saturation, and hematocrit). Inability to achieve the parameter values demonstrated here may lead to compromised allograft function after transplantation. Representative results of the effectiveness of using the protocol presented here for therapeutic delivery was previously demonstrated by this group15. The cardiac allografts were perfused with perfusate treated with Adenoviral vector, and gene expression proved to be global and robust within the allografts 5 days after the treatment and transplantation.
Figure 3: Cardiac allograft on ex vivo perfusion device.
The cardiac allograft mounted on a normothermic, ex vivo perfusion device where it is perfused with therapeutic-infused perfusate for 2 h prior to implantation into the recipient.
Figure 4: Representative ex vivo perfusion parameters.
(A) Circulatory flow rates measured from the pulmonary artery (blue), the aorta (green), and the coronary arteries (red). (B) Representative aortic pressure measurements: mean pressure (blue), systolic pressure (red), diastolic pressure (green). (C) Heart rate of a cardiac allograft during ex vivo perfusion. (D) Recorded temperature of the cardiac allograft during ex vivo perfusion. (E) demonstrates the values of mixed venous oxygen saturation (SvO2) measured from the perfusate during the perfusion period. (F) Hematocrit (hct) values measured from the perfusate during the perfusion period.
DISCUSSION:
Delivery of therapeutics during ex vivo perfusion in cardiac transplantation offers a strategy to modify the allograft and potentially improve transplant outcomes. The protocol presented here incorporates the state-of-the-art normothermic ex vivo sanguinous perfusion storage and offers promising potential to test isolated delivery of cell, gene, or immunotherapies to the allograft11–13. To date, cardiac delivery techniques for these putative therapies for cardiovascular disease and end-stage heart failure have relied on systemic administration, intra-coronary perfusion via catheterization, and direct intra-myocardial injections; all of which have achieved poor results in terms of myocardial delivery5,18. We have previously demonstrated robust and global expression of a reporter gene to entire cardiac allografts when a viral vector was administered into the perfusate during ex vivo perfusion prior to transplantation15.
There are several critical steps presented in this protocol to highlight. (1) Every precaution must be taken to minimize blood loss during the procurement of the heart from the donor. At least 1 L of blood needs to be attained from the donor for the perfusion device to achieve adequate flow rates. (2) For therapeutic delivery using normothermic ex vivo sanguinous perfusion, it is necessary to wash the donor blood before adding it to the perfusate to remove any neutralizing components in the donor serum that may negatively affect the delivery of the therapeutic to the heart. (3) Minimize dissection of the heart in the donor, until after cardioplegic arrest, to avoid fatal arrhythmias. (4) When introducing the therapeutic to the perfusion device, it is important to introduce it through the port closest to the aortic root and always flush the port to ensure complete delivery of the suspension. This is to minimize any potential loss of the therapeutic to the oxygenator or tubing within the circuit and ensure that the graft is receiving as high of a therapeutic concentration as possible. (5) Finally, when selecting the site for graft implantation, it is critical that the location minimizes the potential for tension on the anastomosis and that there be no kinking of the blood vessels/anastomoses.
It is also recommended that the pigs be Swine Leukocyte Antigen (SLA)-typed (i.e., porcine major histocompatibility complex, MHC) beforehand to select for the appropriate degree of matching/mis-matching across SLA haplotypes comprising the cell-surface class I (SLA-1, SLA-2, and SLA-3) and/or class II (DR and DQ) antigens based on the investigator’s needs (SLA-typing performed by SH as previously described with slight modifications made to the typing primer panels)16,17. For example, ensuring that pigs match across all SLA antigens minimizes the risk of allograft rejection, whereas using pigs with mismatch across all SLA antigens maximizes the incidence of allograft rejection.
A limitation of this model is that while it allows for the study of the immunologic effects on the cardiac graft, it does not allow for a full assessment on the graft’s ability to support the cardiovascular system following an intervention. To achieve that, the graft would need to be implanted orthotopically. However, orthotopic transplantation in large-animal models has a higher associated mortality and requires cardiopulmonary bypass3. Another limitation of this model is limited access to an ex vivo perfusion device to conduct effective gene delivery to the graft. As these devices become more available in the field of organ transplantation, access is expected to improve. Furthermore, a non-commercial device may be an option for experimental purposes.
Cardiac transplantation offers a unique setting where therapeutics can be introduced to the allograft via ex vivo perfusion prior to implantation into the recipient. The use of an ex vivo perfusion device allows for grafts to be in transit from the donor to the recipient for periods of time that are much longer than what is safe using traditional cold static storage6. This extended perfusion period enables effective isolated delivery of therapeutics. This model serves as a translational step between pre-clinical animal testing of therapeutics and transformative clinical therapies.
Figure 2: Protocol schematic for therapeutic delivery to an entire cardiac allograft using normothermic ex vivo sanguinous perfusion.
(A) The heart and blood are procured from the donor pig. (B) The blood is washed using a cell saver device to remove any therapeutic neutralizing components from the donor serum. (C) The cardiac allograft is mounted onto the normothermic ex vivo perfusion device and perfused for 2 h. (D) Soon after the allograft is mounted, the therapeutic of interest is added to the perfusate. (E) After the allotted ex vivo perfusion period, the allograft is transplanted into the recipient pig in the intra-abdominal, heterotopic position. This figure has been modified from 15.
Figure 6: Luciferase activity after transduction of cardiac allografts.
Presented are the results of three cardiac allografts that were transduced with adenoviral vectors carrying a luciferase transgene. Demonstrated is the average fold-change in luciferase protein activity in each area of the cardiac allograft. This figure has been modified from Bishawi et al15.
ACKNOWLEDGMENTS:
We would like to thank Duke Large Animal Surgical Core and Duke Perfusion Services for their assistance during these procedures. We would also like to thank Paul Lezberg and TransMedics, Inc. for support.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/63114.
DISCLOSURES:
Paul Lezberg is employed by TransMedics, Inc. The other authors have no conflicts of interest to declare.
REFERENCES:
- 1.Virani SS et al. Heart Disease and Stroke Statistics-2021 Update: A report from the American Heart Association. Circulation. 143 (8), e254–e743 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK Honoring 50 Years of Clinical Heart Transplantation in Circulation: In-Depth State-of-the-Art Review. Circulation. 137 (1), 71–87, doi: 10.1161/CIRCULATIONAHA.117.029753, (2018). [DOI] [PubMed] [Google Scholar]
- 3.Kadner A, Chen RH & Adams DH Heterotopic heart transplantation: experimental development and clinical experience. Eur J Cardiothorac Surg. 17 (4), 474–481, doi: 10.1016/s1010-7940(00)00362-6, (2000). [DOI] [PubMed] [Google Scholar]
- 4.Hastings CL et al. Drug and cell delivery for cardiac regeneration. Adv Drug Deliv Rev. 84 85–106, doi: 10.1016/j.addr.2014.08.006, (2015). [DOI] [PubMed] [Google Scholar]
- 5.Sahoo S, Kariya T. & Ishikawa K. Targeted delivery of therapeutic agents to the heart. Nat Rev Cardiol. 18 (6), 389–399, doi: 10.1038/s41569-020-00499-9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamp NL et al. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart, Lung and Circulation. 24 (6), 611–613, doi: 10.1016/j.hlc.2015.01.005, (2015). [DOI] [PubMed] [Google Scholar]
- 7.Ragalie WS & Ardehali A. Current status of normothermic ex-vivo perfusion of cardiac allografts. Curr Opin Organ Transplant. 25 (3), 237–240, doi: 10.1097/MOT.0000000000000759, (2020). [DOI] [PubMed] [Google Scholar]
- 8.Koerner MM et al. Normothermic ex vivo allograft blood perfusion in clinical heart transplantation. Heart Surg Forum. 17 (3), E141–145, doi: 10.1532/HSF98.2014332, (2014). [DOI] [PubMed] [Google Scholar]
- 9.Rosenbaum DH et al. Perfusion preservation versus static preservation for cardiac transplantation: effects on myocardial function and metabolism. J Heart Lung Transplant. 27 (1), 93–99, doi: 10.1016/j.healun.2007.10.006, (2008). [DOI] [PubMed] [Google Scholar]
- 10.Cullen PP, Tsui SS, Caplice NM & Hinchion JA A state-of-the-art review of the current role of cardioprotective techniques in cardiac transplantation. Interact Cardiovasc Thorac Surg. 32 (5), 683–694, doi: 10.1093/icvts/ivaa333, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rurik JG, Aghajanian H. & Epstein JA Immune Cells and Immunotherapy for Cardiac Injury and Repair. Circ Res. 128 (11), 1766–1779, doi: 10.1161/CIRCRESAHA.121.318005, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rincon MY, VandenDriessche T. & Chuah MK Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 108 (1), 4–20, doi: 10.1093/cvr/cvv205, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieserman JM, Myers VD, Dubey P, Cheung JY & Feldman AM Current Landscape of Heart Failure Gene Therapy. J Am Heart Assoc. 8 (10), e012239, doi: 10.1161/JAHA.119.012239, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perin EC in Stem Cell and Gene Therapy for Cardiovascular Disease eds Perin Emerson C., Miller Leslie W., Taylor Doris A., & Willerson James T.) 279–287 (Academic Press, 2016). [Google Scholar]
- 15.Bishawi M. et al. A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci Rep. 9 (1), 8029, doi: 10.1038/s41598-019-43737-y, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho CS et al. Molecular characterization of swine leucocyte antigen class I genes in outbred pig populations. Animal Genetics. 40 (4), 468–478, doi: 10.1111/j.1365-2052.2009.01860.x, (2009). [DOI] [PubMed] [Google Scholar]
- 17.Ho CS et al. Molecular characterization of swine leucocyte antigen class II genes in outbred pig populations. Anim Genet. 41 (4), 428–432, doi: 10.1111/j.1365-2052.2010.02019.x, (2010). [DOI] [PubMed] [Google Scholar]
- 18.Hulot JS, Ishikawa K. & Hajjar RJ Gene therapy for the treatment of heart failure: promise postponed. Eur Heart J. 37 (21), 1651–1658, doi: 10.1093/eurheartj/ehw019, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]