Abstract
Background
Benign prostatic hyperplasia (BPH) is a non‐malignant enlargement of the prostate, which can lead to obstructive and irritative lower urinary tract symptoms (LUTS). The pharmacologic use of plants and herbs (phytotherapy) for the treatment of LUTS associated with BPH is common. The extract of the berry of the American saw palmetto or dwarf palm plant, Serenoa repens (SR), which is also known by its botanical name of Sabal serrulatum, is one of several phytotherapeutic agents available for the treatment of BPH.
Objectives
To assess the effects of Serenoa repens in the treatment of men with LUTS consistent with BPH.
Search methods
We performed a comprehensive search of multiple databases (the Cochrane Library, MEDLINE, Embase, Scopus, Web of Science, and LILACS), trials registries, other sources of grey literature, and conference proceedings published up to 16 September 2022, with no restrictions on language or publication status.
Selection criteria
We included randomized controlled trials of participants with BPH who were treated with Serenoa repens or placebo/no treatment.
Data collection and analysis
Two review authors independently assessed studies for inclusion at each stage and undertook data extraction and risk of bias assessment and GRADE assessment of the certainty of the evidence. We considered review outcomes measured up to 12 months after randomization as short term, and beyond 12 months as long term. Our main outcomes included urologic symptom scores, quality of life, and adverse events.
Main results
For this update, we narrowed the review question to only comparisons with placebo. We included 27 studies (of which 9 were new) involving a total of 4656 participants, 19 studies comparing Serenoa repens with placebo, and 8 studies comparing Serenoa repens in combination with other phytotherapeutic agents versus placebo. Most studies included men aged > 50 (mean age range 52 to 68) with moderate urologic symptoms (International Prostate Symptom Score [IPSS] range 8 to 19). Ten studies were funded by the pharmaceutical industry; two studies were funded by government agencies; and the remaining studies did not specify funding sources.
Serenoa repens versus placebo or no intervention
Results for this comparison are based on predefined sensitivity analyses limited to studies at low risk of bias. Serenoa repens results in little to no difference in urologic symptoms at short‐term follow‐up (3 to 6 months; IPSS score range 0 to 35, higher scores indicate worse symptoms; mean difference (MD) −0.90, 95% confidence interval (CI) −1.74 to −0.07; I2 = 68%; 9 studies, 1681 participants; high‐certainty evidence). Serenoa repens results in little to no difference in the quality of life at short‐term follow‐up (3 to 6 months; IPSS quality of life domain range 0 to 6, higher scores indicate worse quality of life; MD −0.20, 95% CI −0.40 to −0.00; I2 = 39%; 5 studies, 1001 participants; high‐certainty evidence). Serenoa repens probably results in little to no difference in adverse events (1 to 17 months; risk ratio (RR) 1.01, 95% CI 0.77 to 1.31; I2 = 18%; 12 studies, 2399 participants; moderate‐certainty evidence). Based on 164 cases per 1000 men in the placebo group, this corresponds to 2 more (38 fewer to 51 more) per 1000 men in the Serenoa repens group.
Serenoa repens results in little to no difference in urologic symptoms at long‐term follow‐up (12 to 17 months, IPSS score, MD 0.07, 95% CI −0.75 to 0.88; I2 = 34%; 3 studies, 898 participants; high‐certainty evidence). Serenoa repens results in little to no difference in quality of life at long‐term follow‐up (12 to 17 months, IPSS quality of life, MD −0.11, 95% CI −0.41 to 0.19; I2 = 65%; 3 studies, 882 participants; high‐certainty evidence). There were no data on long‐term adverse events for this comparison.
Serenoa repens in combination with other phytotherapy versus placebo or no intervention
Different phytotherapeutic agents that include Serenoa repens may result in little to no difference in urologic symptoms compared to placebo at short‐term follow‐up (12 to 24 weeks, IPSS score, MD −2.41, 95% CI −4.54 to −0.29; I2 = 67%; 4 studies, 460 participants; low‐certainty evidence). We are very uncertain about the effects of these agents on quality of life (very low‐certainty evidence). These agents may result in little to no difference in the occurrence of adverse events; however, the CIs included substantial benefits and harms (12 to 48 weeks, RR 0.91, 95% CI 0.58 to 1.41; I2 = 0%; 4 studies, 481 participants; low‐certainty evidence). Based on 132 cases per 1000 men in the placebo group, this corresponds to 12 fewer (55 fewer to 54 more) per 1000 men in the combined phytotherapeutic agents with Serenoa repens group.
Authors' conclusions
Serenoa repens alone provides little to no benefits for men with lower urinary tract symptoms due to benign prostatic enlargement. There is more uncertainty about the role of Serenoa repens in combination with other phytotherapeutic agents.
Keywords: Aged, Humans, Male, Middle Aged, Plant Extracts, Plant Extracts/adverse effects, Prostatic Hyperplasia, Prostatic Hyperplasia/complications, Prostatic Hyperplasia/drug therapy, Quality of Life, Serenoa
Plain language summary
Serenoa repens for benign prostatic hyperplasia
Review question
Does Serenoa repens alone or in combination with other phytotherapeutic agents improve symptoms in men with benign prostatic enlargement?
Background
An enlarged prostate may cause bothersome urinary tract symptoms, such as having to urinate often during the day or night, having a weak stream, and the feeling of not completely emptying the bladder. Besides other common drug interventions, using plants and herbs (phytotherapy) is common and has been growing steadily in most Western countries. The extract of the berry of the American saw palmetto, or dwarf palm plant, Serenoa repens, which is also known by its botanical name of Sabal serrulatum, is one of several phytotherapeutic agents available for the treatment of this condition.
Study characteristics
We found 27 studies with 4656 men comparing Serenoa repens alone or in combination with other herbal products to a placebo (participants are made to believe they received treatment when in fact they did not). Most studies included men over 50 with moderate symptoms. Ten studies were funded by pharmaceutical organizations; two studies received government funding; and the remaining studies did not specify funding sources.
Key results
Based on the most trustworthy studies, Serenoa repens alone results in little to no difference in urinary tract symptoms or quality of life compared to placebo at three to six months. This treatment is also likely not associated with adverse events. Results were similar at 12 to 17 months.
Serenoa repens in combination with other herbal products may result in little to no difference in urinary tract symptoms, but there is more uncertainty about effects on quality of life and adverse events.
The findings of this review are current to 16 September 2022.
Certainty of the evidence
The certainty of the evidence is primarily high or moderate for Serenoa repens alone, but low for Serenoa repens in combination with other agents, meaning our confidence in the results is high, moderate, or low.
Summary of findings
Summary of findings 1. Serenoa repens compared to placebo or no intervention.
| Serenoa repens compared to placebo or no intervention | |||||
|
Patient or population: lower urinary tract symptoms due to benign prostatic hyperplasia Setting: outpatient (Australia, Asia, Europe, and the USA) Intervention: Serenoa repens Comparison: placebo/no treatment | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo/no treatment | Risk difference with Serenoa repens | ||||
|
Urologic symptom score
Measured by IPSS scores (range 0 to 35)
Higher scores indicate worse symptoms.
Follow‐up: 3 to 6 months MCID: 3 points |
1681 (9 RCTs) |
⊕⊕⊕⊕ Higha | MD −0.90 (−1.74 to −0.07) |
The mean score was 14.33. | MD 0.90 lower (1.74 lower to 0.07 lower) |
|
Quality of life
Measured by IPSS‐QoL score (range 0 to 6)
Follow‐up: 3 to 6 months MCID: 0.5 points |
1001 (5 RCTs) |
⊕⊕⊕⊕ Higha | MD −0.20 (−0.40 to 0.00) |
The mean score was 3.11. | MD 0.20 lower (0.40 lower to 0.00 lower) |
|
Adverse events
Cumulative incidence
Follow‐up: 1 to 17 months MCID: relative risk reduction/increase of 0.25 |
2399 (12 RCTs) |
⊕⊕⊕⊝ Moderateb |
RR 1.01 (0.77 to 1.31) |
164 per 1000 | 2 more per 1000 (38 fewer to 51 more) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MCID: minimal clinically important difference; MD: mean difference; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe did not downgrade the certainty of the evidence for risk of bias as these results were robust following sensitivity analysis excluding studies at high risk of bias. bWe did not downgrade the certainty of the evidence for risk of bias as these results were robust following sensitivity analysis excluding studies at high risk of bias. We downgraded one level due to imprecision as the CI included little to no benefit and also harms (based on a 25% relative risk reduction).
Summary of findings 2. Serenoa repens in combination with other phytotherapy versus placebo or no intervention.
| Serenoa repens in combination with other phytotherapy versus placebo or no intervention | |||||
|
Patient or population: lower urinary tract symptoms due to benign prostatic hyperplasia Setting: outpatient (Europe/USA) Intervention: Serenoa repens with other phytotherapy Comparison: placebo/no intervention | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo/no treatment | Risk difference with Serenoa repens | ||||
|
Urologic symptom score
Measured by IPSS scores (range 0 to 35)
Higher scores indicate worse symptoms.
Follow‐up: 12 to 24 weeks MCID: 3 points |
460 (4 RCTs) |
⊕⊕⊝⊝ Lowa,b | MD −2.41 (−4.54 to −0.29) |
The mean score was 12. | MD 2.41 lower (4.54 lower to 0.29 lower) |
|
Quality of life
Measured by IPSS‐QoL score (range 0 to 6)
Follow‐up: 2 to 6 months MCID: 0.5 points |
265 (2 RCTs) |
⊕⊝⊝⊝ Very lowc,d,e | 1 study reported improvements (P < 0.05), while the other did not. | ||
|
Adverse events
Cumulative incidence
Follow‐up: 12 to 48 weeks MCID: relative risk reduction/increase of 0.25 |
481 (4 RCTs) |
⊕⊕⊝⊝ Lowf |
RR 0.91 (0.58 to 1.41) |
132 per 1000 | 12 fewer per 1000 (55 fewer to 54 more) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IPSS: International Prostate Symptom Score; MCID: minimal clinically important difference; MD: mean difference; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to concerns about inconsistency: high statistical inconsistency (I2 = 67%). bDowngraded one level due to imprecision: wide CI including substantial benefit and little to no effect. cDowngraded one level due to risk of bias: high risk of bias in included studies. dDowngraded one level due to inconsistency: the included studies reported different effects. eDowngraded one level due to imprecision: the included studies reported P values, and we are uncertain about effect sizes. fDowngraded two levels due to imprecision: CI includes substantial benefits and harms.
Background
Description of the condition
Description of the condition
The prostate gland is an organ approximately the size of a walnut located below the urinary bladder encircling the urethra (Leissner 1979). Benign prostatic hyperplasia (BPH) is a histological diagnosis defined as an increased number of epithelial and stromal cells in the prostate; this may cause prostatic enlargement and, subsequently, compression of the urethra and obstruction (Roehrborn 2008). BPH may therefore develop with or without lower urinary tract symptoms (LUTS) in men aged over 40 years (Dunphy 2015). BPH acquires clinical significance when associated with bothersome LUTS (Roehrborn 2008). 'Symptom bother' typically correlates with the increased number and severity of symptoms, which relate to both quality of life impairment and treatment seeking (Agarwal 2014). Self‐administered questionnaires (e.g. the International Prostate Symptom Score [IPSS]) include the quality of life domain to evaluate the relative degree of bother across all LUTS (Barry 1995). Increased LUTS severity is associated with worsening men's overall distress using the man's perception of bladder condition, which is a single‐item global question (ranging from 1 [causes no problems at all] to 6 [causes severe problems]) (Chapple 2017). In this Cochrane Review, we consider the term BPH as prostatic enlargement with LUTS to define the disease condition and potential need for intervention.
BPH can progress and cause serious consequences such as acute urinary retention, infection, and upper urinary tract deterioration. BPH also negatively impacts public health and a reduction in a person's quality of life (Kozminski 2015; Martin 2014). In Europe, 30% of men over 50 years of age, equivalent to 26 million men, are affected by bothersome LUTS, including storage symptoms (such as urinary frequency, urgency, and nocturia) or voiding symptoms (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding), or both. The yearly reported associated number of medical prescriptions is estimated to be around 11.6 million for 74 million people at risk from 2004 to 2008 (Cornu 2010). According to an international study involving 7588 men, the prevalence of LUTS was 18% in 40‐year‐olds, 29% of men in their 50s, 40% of men in their 60s, and 56% of men in their 70s (Homma 1997). In the USA, an estimated eight million men over 50 years of age have BPH (Roehrborn 2008). More recent data show that the lifetime prevalence of BPH was 26.2% (95% confidence interval 22.8 to 29.6%) (Lee 2017).
Diagnosis
Initial evaluation of LUTS suggestive of BPH includes patient history and physical examination, which may include a digital rectal examination, urinalysis, prostate‐specific antigen (PSA), and IPSS (Gravas 2022; Lerner 2021). A digital rectal examination may be performed to assess the prostate for size and any lesions suspicious of cancer. PSA is secreted by the prostate gland and is found to be abnormally elevated in conditions such as prostate cancer, BPH, infection, or inflammation of the prostate (Gravas 2022; Lerner 2021). The IPSS is used to assess urinary symptom severity and quality of life. It is also used to document subjective responses to treatment. Measurements of maximum flow rate (Qmax) and postvoid residual (PVR) are also used in diagnosis and treatment decisions (Gravas 2022; Lerner 2021). A low Qmax and a large PVR predict an increased risk of symptom progression (Crawford 2006). Further evaluations may be needed for differential diagnosis or pre‐surgical assessments (Gravas 2022; Lerner 2021).
Treatment
Treatment decisions are based on symptoms and the degree of bother noted by the patient. Initial treatment options for BPH include conservative management (watchful waiting and lifestyle modification) and medication (alpha‐blockers and 5‐alpha reductase inhibitors) (Gravas 2022; Lerner 2021). If patients have been refractory to conservative and medical treatment, and BPH causes subsequent complications, such as acute urinary retention, recurrent urinary tract infection, bladder stones or diverticula, hematuria, or renal insufficiency, surgical options are considered (Gravas 2022; Lerner 2021). Currently, guidelines do not recommend the routine use of Serenoa repens, but they state that in instances where patients want to avoid adverse side effects of other treatments, these patients should be informed of its modest benefits (Gravas 2022).
Description of the intervention
There are about 30 phytotherapeutic compounds available for the treatment of BPH, and one of the most widely used is an extract from the berry of the American saw palmetto or dwarf palm plant, Serenoa repens, which is also known by its botanical name of Sabal serrulatum. The extracts can be classified as hexane, ethanolic, and supercritical carbon dioxide. The hexane extract (commercially known as Permixon) is proposed to have a higher biological activity and the lowest variability from batch to batch in free fatty acid content, possibly suggesting a higher efficacy and fewer adverse events (Habib 2004; Scaglione 2008).
Serenoa repens is usually taken in a daily dose of 320 mg, although some studies have investigated higher doses (Barry 2011). The most frequently reported adverse events are minor gastrointestinal symptoms, genitourinary problems, musculoskeletal complaints, and upper respiratory tract infections.
How the intervention might work
The causes of LUTS related to BPH are not entirely known; however, it is theorized that a combination of prostatic cellular proliferation (BPH) and smooth muscle dysfunction are likely reasons (Roehrborn 2020). The purported mechanisms of action for Serenoa repens include:
alteration in cholesterol metabolism (Christensen 1990);
antiestrogenic and antiandrogenic effects (Dreikorn 1990; Marwick 1995), with Serenoa repens (Permixon) acting as a weak surrogate 5‐ARI inhibiting the conversion of testosterone to dihydrotestosterone (DHT), Dedhia 2008, and the dependent inhibition of 5‐ARI in the stroma and epithelium of the prostate (Weisser 1996);
anti‐inflammatory effects by a decrease in available sex hormone‐binding globulin (Di Silverio 1993);
pro‐apoptotic properties and inhibition of cellular proliferation (Vacherot 2000; Vela‐Navarrete 2005);
the relaxation of smooth muscles of the detrusor and the prostate via alpha‐1 adrenergic receptors (Roehrborn 2020);
placebo effect (Roehrborn 2020).
Why it is important to do this review
Phytotherapy is widely used for the relief of lower urinary symptoms attributed to BPH. Since the last update (Tacklind 2012), several new trials have been published. Whereas some newer non‐Cochrane reviews have been published, none has included GRADE methods (Novara 2016; Russo 2021; Vela‐Navarrete 2018).
Objectives
To assess the effects of Serenoa repens in the treatment of men with LUTS consistent with BPH.
Methods
Criteria for considering studies for this review
Types of studies
The methods for this update have been extensively modified since its last publication to meet current methodological expectations; please refer to the Differences between protocol and review section. We included parallel‐group randomized controlled trials (RCTs). We excluded cluster‐RCTs, as these study designs are not relevant in this setting. We included the first phase of cross‐over studies. We did not include single‐armed studies. We included studies regardless of their publication status or language.
Types of participants
We defined the eligible participant population as men over the age of 40 years with a prostate volume of 20 mL or greater (as assessed by ultrasound or cross‐sectional imaging), with lower urinary tract symptoms (LUTS) as determined by International Prostate Symptom Scores (IPSS) of eight or over, and a maximum flow rate (Qmax) of less than 15 mL/second, as measured by non‐invasive uroflowmetry, invasive pressure flow studies, or both (Dunphy 2015; EAU 2022; McNicholas 2016; McVary 2011). We based the age limit on the fact that the prevalence of BPH increases in middle‐aged and older men and is infrequent in younger men (Barry 1997; EAU 2022; Egan 2016). We included studies in which only a subset of participants was relevant to this review (i.e. studies in which more than 75% of participants were relevant to this review) if data were available separately for the relevant subset.
We excluded studies of men with active urinary tract infection, bacterial prostatitis, chronic renal failure, untreated bladder calculi or large diverticula, prostate cancer, and urethral stricture disease, as well as those who had undergone prior prostate, bladder neck, or urethral surgery. We also excluded studies of people with other conditions that affect urologic symptoms, such as neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease.
Types of interventions
Experimental intervention
Serenoa repens alone (hexanic and non‐hexanic extract)
Serenoa repens in combination with other phytotherapy
Comparator intervention
Placebo or no intervention
Comparisons
Serenoa repens versus placebo or no intervention
Serenoa repens in combination with other phytotherapy versus placebo or no intervention
To establish fair comparisons, we required that concomitant interventions be the same in the experimental and comparator groups.
Types of outcome measures
We did not use measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Urologic symptom scores (continuous outcome)
Quality of life (continuous outcome)
Adverse events
Secondary outcomes
We did not include secondary outcomes.
Method and timing of outcome measurement
We considered the clinically important differences for the review outcome measures to rate the overall certainty of evidence in the summary of findings tables following a minimally contextualized approach (Jaeschke 1989; Johnston 2013). We considered outcomes measured up to and including 12 months after randomization as short term, and later than 12 months as long term. For adverse events, the timing of outcome assessment was not well‐defined across studies, and outcome data were not disaggregated by follow‐up, so we did not divide them into short and long term.
Urologic symptom scores
Mean change from baseline or final mean value, measured using a validated scale (such as IPSS). We considered the improvement of an IPSS score of three points as the minimal clinically important difference (MCID) to assess the efficacy and comparative effectiveness (Barry 1995). If possible, we used different thresholds of MCID based on the severity of IPSS, with a threshold of three points for men with mild LUTS, five for moderate LUTS, and eight for severe LUTS (Barry 1995).
Quality of life
Mean change from baseline or final mean value measured as a validated scale (such as IPSS‐quality of life or BPH Impact Index). No threshold has been established for IPSS quality of life in the literature. However, we used an MCID of 0.5 to assess the efficacy and comparative effectiveness. A BPH Impact Index score of one as an MCID was used to indicate improvement (Barry 2013; Franco 2021; Rees 2015).
Adverse events
The number of participants experiencing at least one adverse event (e.g. gastrointestinal discomfort). There were no reported thresholds in adverse events, thus we considered a clinically important difference a risk ratio reduction or increase of at least 25% (Guyatt 2011).
Search methods for identification of studies
Electronic searches
We searched the following sources from the inception of each database to the date of search with no restrictions on the language of publication:
the Cochrane Central Register of Controlled Trials (CENTRAL; www.cochranelibrary.com/) (2022, Issue 9) searched 16 September 2022;
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to 16 September 2022);
Embase (www.embase.com/) from 1974 to 16 September 2022;
Scopus (www.scopus.com/home.uri) from 1966 to 16 September 2022;
Science Citation Index Expanded (SCI‐E) Web of Science Clarivate (www.webofscience.com; from 1970 to 16 September 2022);
Latin American and Caribbean Literature in Health Sciences (LILACS; lilacs.bvsalud.org/es/; from 1982 to 16 September 2022);
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 16 September 2022);
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) (searched 16 September 2022).
Details of the search strategies are provided in Appendix 1.
Searching other resources
We attempted to identify other potentially eligible trials and ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We contacted the study authors of included trials to identify further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished trials. We searched abstract proceedings of relevant meetings of the American Urological Association, the European Association of Urology, and the International Continence Society from 2020 to 2022 for unpublished studies (see Appendix 2).
Data collection and analysis
Selection of studies
We used Covidence to identify and remove potential duplicate records (Covidence). Two review authors (out of LT, NJS, GAA, and CF) scanned abstracts, titles, or both to determine which studies should be assessed further using the same software. Two review authors (out of LT, NJS, GAA, and CF) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies following the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). Any discrepancies were resolved through consensus or recourse to a third review author (JVAF or JHJ). We documented the reasons for exclusion of excluded studies. We presented a PRISMA flow diagram showing the process of study selection (Page 2021).
Data extraction and management
We developed a dedicated data abstraction form that we pilot‐tested ahead of time. For studies that fulfilled our inclusion criteria, two review authors (out of LT, NJS, GAA, and CF) independently abstracted the following information.
Study design
Study dates
Study settings and country
Participant inclusion and exclusion criteria (e.g. age, baseline IPSS)
Participant details, baseline demographics (e.g. age, prostate size, IPSS)
Numbers of participants by study and by study arm
Details of relevant experimental intervention (e.g. dose, posology, type of Serenoa repens extract)
Definitions of relevant outcomes and methods (e.g. type of instrument, such as IPSS) and timing of outcome measurement (e.g. in months), as well as relevant subgroups (e.g. based on age, prostate volume, and the severity of LUTS)
Study funding sources
Declarations of interest by primary investigators
For dichotomous outcomes, we presented numbers of events and totals for populations in a 2 × 2 table and summary statistics with corresponding measures of variance. We obtained the means and standard deviations or data necessary for continuous outcomes to calculate this information. Any disagreements were resolved by discussion or in consultation with a third review author (JVAF or JHJ) if required.
We provided information about potentially relevant studies, including the trial identifiers, in tables. In addition, we contacted the authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we prioritized the publication reporting the most extended follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (out of LT, NJS, GAA, CF) independently assessed the risk of bias for the results of the main outcomes (those included in the summary of findings table, see below) in each included study using the recently developed revision of the Cochrane risk of bias tool, RoB 2 (Flemyng 2023; Higgins 2022b). Any disagreements were resolved by discussion or by involving another review author (JVAF). We assessed the risk of bias according to the following domains, focusing on the effect of assignment to the intervention at baseline:
the randomization process;
deviations from intended interventions;
missing outcome data;
measurement of the outcome;
selection of the reported results.
Answers to signaling questions and supporting information collectively lead to a domain‐level judgment of 'low risk,' 'some concerns,' or 'high risk' of bias. These domain‐level judgments informed an overall risk of bias judgment for the outcome based on the algorithm in the guidance document of RoB 2.
We provided a quote from the study report together with a justification for our judgment in the risk of bias table. We aimed to source published protocols in order to assess selective reporting. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. We made summary assessments of the risk of bias for each important outcome across domains and an overall narrative across studies.
We used a Microsoft Excel spreadsheet tool to manage data supporting the answers to the signaling questions and risk of bias judgments (Microsoft Excel 2023). All of these data are publicly available as supplementary material in the Open Science Framework platform.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs, unless different studies used different measures to assess the same outcome, in which case we re‐expressed the data as standardized mean differences (SMDs) with 95% CIs.
Unit of analysis issues
Where multiple trial arms were reported in a single trial, we would include only the treatment arms relevant to the review topic. Had two comparisons from the same trial (e.g. drug A versus placebo and drug B versus placebo) been combined in the same meta‐analysis, we would have followed the guidance in Section 6.2 of the Cochrane Handbook (Higgins 2022a). Our preferred approach was to combine groups to create a single pair‐wise comparison.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was available as an abstract only).
Where numerical outcome data were missing, such as standard deviations or correlation coefficients, and they could not be obtained from the authors, we calculated them from other available statistics such as 95% CI or P values, according to the methods described in the Cochrane Handbook (Higgins 2022a). If this was not possible, and the missing data could have introduced serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and by using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis. We interpreted the I2 statistic as follows (Deeks 2022):
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
In the case of heterogeneity, we attempted to identify possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting. When we included 10 or more studies in a meta‐analysis, we used funnel plots to assess small‐study effects (Page 2022). Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design (and hence bias of small studies), and publication bias. We therefore used caution in our interpretation of results.
Data synthesis
Unless there was good evidence for homogeneous effects across studies, we summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. We also performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook (Higgins 2022a). For dichotomous outcomes, we used the Mantel‐Haenszel method. For continuous outcomes, we used the inverse variance method. We used Review Manager Web software to perform the analyses (RevMan Web 2022).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to potentially introduce clinical heterogeneity, and carried out subgroup analyses to investigate interactions.
Type of Serenoa repens preparation (hexanic versus non‐hexanic extract)
Participant age (less than 65 years versus 65 years or more)
Severity of LUTS based on IPSS (score less than or equal to 19 [moderately symptomatic] versus greater than 19 [severely symptomatic])
These subgroup analyses are based on the following observations.
Other reviews highlight that there might be different effects of different Serenoa repens extracts (Russo 2021).
Age is a well‐known risk factor for BPH surgery. Older men have a higher rate of postoperative complications compared with younger men (Bhojani 2014; Pariser 2015). The age cut‐off is based on the World Health Organization's (WHO) definition of old age (WHO 2002).
The relationship between changes in IPSS scores and patient global ratings of improvement is influenced by the baseline scores (Barry 1995).
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors (when applicable) on effect size:
restricting the analysis to RCTs by considering risk of bias, restricting to studies with an overall low risk of bias.
If the sensitivity analyses provided moderate‐ to high‐certainty evidence, we would draw our main conclusions (summary of findings tables) based on this estimate. This is a similar approach to the previous version of this review (Tacklind 2012).
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach (Guyatt 2008). For each comparison, two review authors (JVAF and LT) independently rated the certainty of the evidence for each outcome as 'high,' 'moderate,' 'low,' or 'very low,' using GRADEpro GDT software (GRADEpro GDT). Any discrepancies were resolved by consensus or if needed by arbitration from a third review author (JHJ). For each comparison, we presented a summary of the evidence for the main outcomes in the summary of findings table, which provides key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of our overall confidence in the effect estimates for each outcome (Schünemann 2022).
We considered five criteria, not only related to internal validity (overall risk of bias, inconsistency, imprecision, and publication bias) but also external validity (directness of results), for downgrading the certainty of the evidence for a specific outcome (Schünemann 2022). We included the following comparisons.
Serenoa repens versus placebo or no intervention
Serenoa repens in combination with other phytotherapy versus placebo or no intervention
Each summary of findings table includes the following outcomes.
Urologic symptom scores
Quality of life
Adverse events
We followed GRADE guidance for detailed footnotes and language to describe the certainty of the evidence (Santesso 2016; Santesso 2020).
Results
Description of studies
Results of the search
For this update, we narrowed the focus of the review questions, focusing on the effects of Serenoa repens versus placebo or in combination with other psychotherapeutic agents versus placebo. As a result, we excluded 7 of 32 studies included in the previous update because they were not included in this focused review question (Braeckman 1997; Carraro 1996; Debruyne 2002; Engelmann 2006; Pannunzio 1986; Roveda 1994; Sökeland 1997), and moved another 7 older studies to awaiting classification because of missing full text (see Characteristics of studies awaiting classification) (Cukier 1985; Emili 1983; Gabric 1987; Löbelenz 1992; Mattei 1990; Mohanty 1999; Tasca 1985). A total of 18 studies from the previous version of this review were relevant to our review question.
We conducted a de novo search and identified 14,362 records from electronic databases. We found no relevant records in additional sources. After removing duplicates, we screened the titles and abstracts of the remaining 6957 records, of which 6870 were excluded. We assessed 87 full‐text articles and excluded 49 records for various reasons (see Excluded studies). We identified nine new studies through this search (Argirović 2013; BASTA 2010; Carbin 1990; Coulson 2013; Hong 2009; Iacono 2015; Ryu 2015; Sudeep 2020; Ye 2019). Considering the 18 relevant studies from the previous version of the review, we included 27 studies with 4656 participants in this update. A PRISMA flow diagram illustrating the flow of literature through the assessment process is presented in Figure 1.
1.
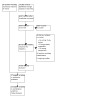
Study flow diagram.
Included studies
See Characteristics of included studies and Table 3.
1. Summary of the characteristics of included studies.
| Study | Trial period | Country | n | Follow‐up | Brand and daily dosage (when available) | Co‐intervention | Age mean (SD) | IPSS mean (SD) | Prostate volume mean (SD) | |||
| I | C | I | C | I | C | |||||||
| Studies comparing Serenoa repens with placebo | ||||||||||||
| Argirović 2013 | 2008 to 2010 | Serbia | 199 | 6 months | Prostamol Uno 320 mg | Tamsulosin | 65.9 (7.4) | 56.8 (7.7) | 15.6 (3.2) | 16.2 (4.9) | 31.2 (4.2) | 38.6 (11.6) |
| BASTA 2010 | 2006 to 2008 | International | 1011 | 12 months | Permixon* 320 mg daily Prostamol Uno 320 mg |
None | 64.61 (7.69) 65.14 (7.67) |
64.14 (7.69) | N/A | N/A | N/A | N/A |
| Barry 2011 | 2008 to 2010 | USA | 369 | 72 weeks | Prosta Urgenin Uno 320 mg | None | 61.25 (8.72) | 60.7 (8.08) | 14.42 (4.29) | 14.69 (4.75) | N/A | N/A |
| Bauer 1999 | N/A | Germany/Italy | 101 | 6 months | Talso Uno 320 mg daily | None | 66.1 | 9.6 | 8.9 | 34.5 | 31.7 | |
| Bent 2006 | 2001 to 2004 | USA | 225 | 14 months | Carbon dioxide extract 320 mg | None | 62.9 (8.0) | 63.0 (7.4) | 15.7 (5.7) | 15.0 (5.3) | 34.7 (13.9) | 33.9 (15.2) |
| Boccafoschi 1983 | N/A | Italy | 22 | 60 days | Permixon* 320 mg | None | 68 (55 to 80) | 68 (54 to 78) | N/A | N/A | N/A | N/A |
| Champault 1984 | N/A | France | 110 | 30 days | Permixon* 320 mg | None | N/A | N/A | N/A | N/A | N/A | N/A |
| Descotes 1995 | 1995 | France | 176 | 30 days | Permixon* 320 mg | None | 65.6 (8.4) | 67 (7.6) | N/A | N/A | N/A | N/A |
| Gerber 2001 | 1999 to 2000 | USA | 85 | 6 months | SR 320 mg | None | 64.6 (± 9.9) | 65.3 (± 9.7) | 16.7 (± 4.9) | 15.8 (± 4.8) | N/A | N/A |
| Glémain 2002 | N/A | France | 329 | 52 weeks | Permixon* 320 mg | Tamsulosin | 65.2 (7.9) | 64.4 (7.7) | 16.2 (5.2) | 16.3 (5.6) | 40.8 (16.5) | 38.6 (15) |
| Hizli 2007 | 2005 | Turkey | 60 | 6 months | Permixon* 320 mg | Tamsulosin | 60.2 (6.3) | 58.9 (5.7) | 15.6 (3.2) | 16.2 (4.7) | 31.2 (4.2) | 38.6 (11.6) |
| Hong 2009 | N/A | Korea | 62 | 12 months | SR 320 mg | None | 52.0 | 53.1 | 18.3 | 15.4 | 26.1 | 23.2 |
| Mandressi 1983 | N/A | Italy | 60 | 1 month | Permixon* 320 mg | None | N/A | N/A | N/A | N/A | N/A | N/A |
| Reece Smith 1986 | N/A | UK | 70 | 12 weeks | Permixon* 320 mg | None | 66.15 (5.86) | 67.03 (6.03) | N/A | N/A | N/A | N/A |
| Ryu 2015 | 2012 to 2013 | Korea | 120 | 12 months | Permixon* 320 mg | Tamsulosin | 62.5 (1.21) | 63.4 (1.44) | 19.6 (0.73) | 20 (0.85) | 30.1 (0.93) | 30.2 (0.67) |
| Shi 2008 | N/A | China | 94 | 3 months | Prostataplex (dosing not reported) | None | 65.91 | 64.04 | 16.85 | 14.46 | 47.72 | 48.38 |
| Sudeep 2020 | N/A | India | 99 | 12 weeks | VISPO/SPO 400 mg | None | 57.76 (7.25) | 55.18 (8.56) | 20.00 (4.41) | 20.00 (3.74) | N/A | N/A |
| Willetts 2003 | 1999 to 2000 | Australia | 100 | 12 weeks | Carbon dioxide extract 320 mg | None | 62.1 (1.2) | 63.9 (1.3) | N/A | N/A | N/A | N/A |
| Ye 2019 | 2014 to 2016 | China | 354 | 24 weeks | SR 320 mg | None | 61.47 (5.20) | 60.32 (5.96) | 14.42 (3.88) | 14.34 (4.08) | 37.0 (19.7) | 37.3 (25.4) |
| Studies comparing phytotherapy containing Serenoa repens with placebo | ||||||||||||
| Carbin 1990 | 1990 | Sweden/Denmark | 55 | 3 months | Curbicin (PSO 480 mg + SR 480 mg) |
None | 62.0 (6.7) | 61.2 (5.8) | N/A | N/A | N/A | N/A |
| Coulson 2013 | N/A | Australia | 60 | 3 months | ProstateEZE Max (PSO 160 mg, epilobium 500 mg, lycopene 2.1 mg, pygeum 15 g + SR 660 mg) |
None | 63 (10.1) | 64.9 (9.6) | 19.5 | 18 | N/A | N/A |
| Iacono 2015 | N/A | Italy | 185 | 6 months | Tradamixina (Eisenia 80 mg, Tribulus 100 mg, chitosan oligosaccharide (Biovis) 100 mg + SR 320 mg) |
None | 64.2 (8.6) | 20.6 (5.4) | N/A | N/A | N/A | |
| Lopatkin 2005 | 1997 to 2000 | Russia | 257 | 24 weeks | PRO 160/120 (Sabal‐Urtica 240 mg + SR 320 mg) |
None | 67 (7) | 68 (6) | 17.4 (3.3) | 17.8 (3.3) | 43.5 (17.6) | 44.8 (17.6) |
| Marks 2000 | 1997 to 1998 | USA | 44 | 6 months | Nettle root 240 mg, PSO 480 mg, lemon 99 mg, vitamin A 570 IU + SR 318 mg | None | 65.1 (8.1) | 62.9 (9.3) | 18.1 (7.2) | 16.6 (5.3) | 58.5 (29.8) | 55.6 (26.7) |
| Metzker 1996 | N/A | Germany | 40 | 12 months | Prostagutt forte (Sabal‐Urtica 240 mg + SR 320 mg) |
None | 66.0 | 65.1 | 18.6 | 19.0 | N/A | N/A |
| Morgia 2014 | 2011 to 2012 | Italy | 225 | 12 months | Profluss (selenium and lycopene + SR 320 mg) |
Tamsulosin | 65 | 66 | 20 | 19 | 45 | 45 |
| Preuss 2001 | N/A | USA | 144 | 3 months | Cernitin AF (Cernitin 378 mg, vitamin E 100 IU + SR with beta‐sitosterol 286 mg) |
None | N/A | N/A | 18.9 | 17.1 | N/A | N/A |
(*) Hexanic extract of Serenoa repens; C: control; I: intervention; IPSS: International Prostate Symptom Score; IU: international units; N/A: not available (not described); PSO: pumpkin seed oil; SR: Serenoa repens;SD: standard deviation
Study design and settings
All studies were RCTs. The median sample size was 100 participants (interquartile range 61 to 212), and the median follow‐up was 24 weeks (interquartile range 12 to 52). Studies were conducted in Serbia (Argirović 2013), the USA (Barry 2011; Bent 2006; Gerber 2001; Marks 2000; Preuss 2001), Germany (Bauer 1999; Metzker 1996), Italy (Boccafoschi 1983; Iacono 2015; Mandressi 1983; Morgia 2014), France (Champault 1984; Descotes 1995; Glémain 2002), Turkey (Hizli 2007), Korea (Hong 2009; Ryu 2015), the UK (Reece Smith 1986), China (Shi 2008; Ye 2019), India (Sudeep 2020), Sweden (Carbin 1990), Australia (Coulson 2013; Willetts 2003), and Russia (Lopatkin 2005), and one study was conducted in more than one country (BASTA 2010).
Participants
Most studies included men aged > 50 (mean age range 52 to 68), and a few studies included men with a mean age > 65 (Boccafoschi 1983; Descotes 1995; Glémain 2002; Lopatkin 2005; Marks 2000; Reece Smith 1986). Most studies included men with moderate urologic symptoms (IPSS range 8 to 19), with only three studies including a mean score of 20 or more (Iacono 2015; Morgia 2014; Sudeep 2020). Less than half of the studies reported prostate size, which was mostly small to moderate size (mean size range 26.1 to 58.5 mL) (Argirović 2013; Bauer 1999; Bent 2006; Glémain 2002; Hizli 2007; Hong 2009; Lopatkin 2005; Marks 2000; Morgia 2014; Ryu 2015; Shi 2008; Ye 2019).
Interventions and comparisons
We included 27 studies for the following comparisons.
-
Serenoa repens versus placebo (19 studies)
Nine studies included the hexanic extract of Serenoa repens (BASTA 2010; Boccafoschi 1983; Champault 1984; Descotes 1995; Glémain 2002; Hizli 2007; Mandressi 1983; Reece Smith 1986; Ryu 2015).
The other studies included other formulations: Prostamol Uno (Argirović 2013, BASTA 2010), Prosta‐Urgenin Uno (Barry 2011), Talso Uno (Bauer 1999), carbon dioxide extract (Bent 2006; Willetts 2003), Prostablex (Shi 2008), VISPO/SPO (Sudeep 2020), or other unspecified compounds of Serenoa repens (Gerber 2001; Hong 2009; Ye 2019).
-
Phytotherapy containing Serenoa repens versus placebo (8 studies)
Curbicin: pumpkin seed oil and Serenoa repens (Carbin 1990)
ProstaEZE Max: pumpkin seed oil, Epilobium, lycopene, pygeum, and Serenoa repens (Coulson 2013)
Tradaximina: Eisenia, Tribulus, chitosan oligosaccharide (Biovis), and Serenoa repens (Iacono 2015)
PRO 160/120: sabal urtica and Serenoa repens (Lopatkin 2005)
Lipoidal extract of Serenoa repens with other phytotherapeutics (Marks 2000)
Prostagutt forte: sabal urtica and Serenoa repens (Metzker 1996)
Profluss: selenium, lycopene, and Serenoa repens (Morgia 2014)
Cernitin AF: Cernitin, B‐sitoesterol, vitamin E, and Serenoa repens (Preuss 2001)
The most commonly used daily dose was 320 mg daily, either as a single dose or 160 mg twice daily. For the comparisons to placebo, one study did not specify the dosing (Shi 2008), and one study used a 200 mg extract twice daily (400 mg daily total; Sudeep 2020). Whereas the doses of Serenoa repens in combined treatment with another phototherapy usually ranged from 286 to 320, one study included a higher dose of 480 mg daily (Carbin 1990).
Co‐interventions were described in five studies, and in all cases included tamsulosin (Argirović 2013; Glémain 2002; Hizli 2007; Morgia 2014; Ryu 2015).
Outcomes
Twenty‐one studies reported data on urologic symptoms (Argirović 2013; Barry 2011; BASTA 2010; Bauer 1999; Bent 2006; Coulson 2013; Gerber 2001; Glémain 2002; Hizli 2007; Hong 2009; Iacono 2015; Lopatkin 2005; Marks 2000; Metzker 1996; Morgia 2014; Preuss 2001; Ryu 2015; Shi 2008; Sudeep 2020; Willetts 2003; Ye 2019), but only a subset of 12 of these studies reported data on quality of life (Argirović 2013; Barry 2011; Bent 2006; Gerber 2001; Glémain 2002; Hizli 2007; Hong 2009; Metzker 1996; Morgia 2014; Ryu 2015; Willetts 2003; Ye 2019). Twenty‐four studies reported data on adverse events (Argirović 2013; Barry 2011; BASTA 2010; Bauer 1999; Bent 2006; Boccafoschi 1983; Carbin 1990; Champault 1984; Coulson 2013; Descotes 1995; Gerber 2001; Glémain 2002; Hizli 2007; Lopatkin 2005; Marks 2000; Metzker 1996; Morgia 2014; Preuss 2001; Reece Smith 1986; Ryu 2015; Shi 2008; Sudeep 2020; Willetts 2003; Ye 2019). One study did not report any outcomes relevant to this review (Mandressi 1983).
Funding sources
Ten studies were funded by the pharmaceutical industry (BASTA 2010; Coulson 2013; Gerber 2001; Lopatkin 2005; Marks 2000; Morgia 2014; Preuss 2001; Sudeep 2020; Willetts 2003; Ye 2019); two studies were funded by government agencies (Barry 2011; Bent 2006); and the remaining studies did not specify funding sources.
Excluded studies
See Characteristics of excluded studies.
We excluded 49 studies for the following reasons.
Sixteen studies were non‐randomized studies or had no control group (Al‐Shukri 2000; Alcaraz 2022; Authié 1987; Di Maida 2020; Gerber 1998; Giannakopoulos 2002; Giulianelli 2012; Gurzhenko 2020; Ju 2015: Pavone 2010; Popa 2005; Sinescu 2011; Stepanov 1999; Taieb 2010; Vinarov 2010; Zlotta 2005).
Eight studies included the wrong study population (i.e. men with prostatitis or focus on changes in prostatic tissue) (Aliaev 2009; Di Silverio 1992; Morgia 2013; Pecoraro 2004; Suardi 2014; Vela‐Navarrete 2005; Veltri 2002; Weisser 1997).
Twenty‐five studies compared Serenoa repens with active components or different doses (Adriazola Semino 1992; Ali 2020; Bartsch 1998; Braeckman 1997; Cai 2013; Carraro 1996; Comar 1986; CTRI/2012/10/003049; CTRI/2020/09/027521; Debruyne 2002; Duborija‐Kovacevic 2010; Engelmann 2006; EUCTR2011‐005307‐33‐FR; Grasso 1995; Guzman 2016; Hamdv 1997; Latil 2015; Morgia 2018; NCT00797394; Pannunzio 1986; Romaniuk 2013; Roveda 1994; Sökeland 1997; Strauch 1994; Yamanishi 2004).
Studies awaiting classification
See Characteristics of studies awaiting classification.
We identified 24 studies (7 from the previous version of the review) with no available full text (Aliaev 2007; Anonymous 2005; Bercovich 2010; Buck 2002; Carreras 1987; Cukier 1985; Dathe 1991; Diehl 2005; Emili 1983; Fabricius 1993; Gabric 1987; Green 2000; Löbelenz 1992; Martínez 1987; Mattei 1990; Mohanty 1999; Neumann 1993; Razumov 2001; Sekikawa 2020; Tasca 1985; Tkachuk 2002; Vahlensieck 1993; Vinarov 2009; Wehr 1995).
Ongoing studies
We also identified five ongoing studies (ISRCTN84633360; JPRN‐UMIN000023274; JPRN‐UMIN000027902; NCT00497939; NCT02121613). See Characteristics of ongoing studies.
Risk of bias in included studies
The risk of bias assessments for each result in Table 1 and Table 2, including all domain judgments and support for judgment, is located in the risk of bias section, at the side of all forest plots. The signaling questions' responses can be found on the Open Science Framework storage (osf.io/65m8e).
The risk of bias of outcomes across all results and domains was mostly 'some concerns' due to a lack of prespecification of outcomes and analysis plans. We assessed three studies as at overall low risk of bias (Barry 2011; Bent 2006; Sudeep 2020). We assessed three studies as at high risk of bias due to missing outcome data or bias in the measurement of the outcome (due to lack of blinding), in addition to some concerns regarding selective reporting (Hizli 2007; Hong 2009; Ryu 2015).
Effects of interventions
1. Serenoa repens versus placebo or no intervention (short term)
Results for this comparison are based on predefined sensitivity analyses limited to studies at low risk of bias. See Table 1.
1.1. Urologic symptoms
Serenoa repens results in little to no difference in urologic symptoms at short‐term follow‐up (3 to 6 months; mean difference (MD) −0.90, 95% confidence interval (CI) −1.74 to −0.07; I2 = 68%; 9 studies, 1681 participants; high‐certainty evidence). All heterogeneity was explained by a single study of 304 participants that compared Serenoa repens to placebo and showed a difference in IPSS scores of −2.77 (95% CI −3.71 to −1.83) (Ye 2019), which is statistically significant but clinically unimportant. We did not downgrade the certainty of the evidence for inconsistency, considering a minimally contextualized approach and our predefined MCID. We also did not downgrade for risk of bias, since our main analysis was based on a sensitivity analysis excluding studies at high risk of bias (Analysis 1.1). However, this analysis did not materially differ from the analysis including all studies (Analysis 1.2).
1.1. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 1: Urologic symptom score (short term—sensitivity analysis)
1.2. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 2: Urologic symptom score (short term—all studies)
One study with 101 participants found a reduction of urologic symptoms with Serenoa repens (P < 0.01) (Bauer 1999). Another study with 1011 participants found a decrease in urologic symptoms with Serenoa repens compared to placebo at 12 months follow‐up (P = 0.04) (BASTA 2010).
1.2. Quality of life
Serenoa repens results in little to no difference in quality of life at short‐term follow‐up (3 to 6 months, MD −0.20, 95% CI −0.40 to −0.00; I2 = 39%; 5 studies, 1001 participants; high‐certainty evidence). We did not downgrade the certainty of the evidence for risk of bias since, our main analysis was based on a sensitivity analysis excluding studies at high risk of bias (Analysis 1.3). However, this analysis did not materially differ from the analysis including all studies (Analysis 1.4).
1.3. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 3: Quality of life (short term—sensitivity analysis)
1.4. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 4: Quality of life (short term—all studies)
1.3. Adverse events
Serenoa repens probably results in little to no difference in adverse events (1 to 17 months, risk ratio (RR) 1.01, 95% CI 0.77 to 1.31; I2 = 18%; 12 studies, 2399 participants; moderate‐certainty evidence). Based on 164 cases per 1000 men in the placebo group, this corresponds to 2 more (38 fewer to 51 more) per 1000 men in the Serenoa repens group. We did not downgrade the certainty of the evidence for risk of bias, since our main analysis was based on a sensitivity analysis excluding studies at high risk of bias (Analysis 1.5). However, this analysis did not materially differ from the analysis including all studies (Analysis 1.6). Nonetheless, we downgraded one level due to imprecision.
1.5. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 5: Adverse events (sensitivity analysis)
1.6. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 6: Adverse events
We did not incorporate three studies into the meta‐analysis because they reported no adverse events in either the treatment or control group (Bauer 1999; Shi 2008; Sudeep 2020).
The most commonly reported adverse events were headache, gastrointestinal disorders (e.g. diarrhea, nausea and vomiting, stomach upset), upper respiratory symptoms (e.g. rhinitis), ejaculation disorders, musculoskeletal symptoms (e.g. arthralgia in the knees and muscular arm pain), and dizziness. Many of these symptoms may be attributable to co‐interventions (alpha‐blockers).
Few studies in each category precluded subgroup analyses according to age, symptom severity, prostate size, and type of extract (see Included studies).
1.4. Subgroup analysis
1.4.1. Type of Serenoa repens preparation
We were unable to detect differences in urologic symptoms when comparing the effects of hexanic versus non‐hexanic extract (P = 0.23, see Analysis 1.7).
1.7. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 7: Urologic symptom score (subgroup analysis—hexanic vs non‐hexanic extract)
1.4.2. Other subgroup analyses
Few studies in each category precluded subgroups based on participant age and severity of lower urinary tract symptoms (see Included studies).
1.5. Sensitivity analysis
We conducted a sensitivity analysis excluding studies at overall high risk of bias. Given that these analyses provided moderate‐ to high‐certainty evidence, we incorporated them into the main results and summary of findings table (see above outcomes 1.1, 1.2, and 1.3 and Analysis 1.1; Analysis 1.3; Analysis 1.5).
2. Serenoa repens versus placebo or no intervention (long term)
2.1. Urologic symptoms
Serenoa repens results in little to no difference in urologic symptoms at long‐term follow‐up (12 to 17 months, MD 0.07, 95% CI −0.75 to 0.88; I2 = 34%; 3 studies, 898 participants; high‐certainty evidence). We did not downgrade the certainty of the evidence for risk of bias, since our main analysis was based on a sensitivity analysis excluding studies at high risk of bias (Analysis 1.8). However, this analysis did not materially differ from the analysis including all studies (Analysis 1.9).
1.8. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 8: Urologic symptom score (long term—sensitivity analysis)
1.9. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 9: Urologic symptom score (long term—all studies)
2.2. Quality of life
Serenoa repens results in little to no difference in quality of life at long‐term follow‐up (12 to 17 months, MD −0.11, 95% CI −0.41 to 0.19; I2 = 65%; 3 studies, 882 participants; high‐certainty evidence). We did not downgrade the certainty of the evidence for risk of bias, since our main analysis was based on a sensitivity analysis excluding studies at high risk of bias (Analysis 1.10). However, this analysis did not materially differ from the analysis including all studies (Analysis 1.11).
1.10. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 10: Quality of life (long term—sensitivity analysis)
1.11. Analysis.

Comparison 1: Serenoa repens versus placebo or no intervention, Outcome 11: Quality of life (long term—all studies)
2.3. Adverse events
None of the included studies reported this outcome.
2.4. Subgroup analysis
Few studies in each category precluded these subgroup analyses (see Included studies).
2.5. Sensitivity analysis
We conducted a sensitivity analysis excluding studies at overall high risk of bias. Given that these analyses provided high‐certainty evidence, we incorporated them into the main results and summary of findings table (see above outcomes 2.1 and 2.2 and Analysis 1.8; Analysis 1.10).
3. Serenoa repens in combination with other phytotherapy versus placebo or no intervention
See Table 2.
3.1. Urologic symptoms
Different phytotherapeutic agents that include Serenoa repens may result in little to no difference in urologic symptoms compared to placebo at short‐term follow‐up (12 to 24 weeks, MD −2.41, 95% CI −4.54 to −0.29; I2 = 67%; 4 studies, 460 participants; low‐certainty evidence; Analysis 2.1). The certainty of the evidence is low due to imprecision and inconsistency.
2.1. Analysis.

Comparison 2: Serenoa repens in combination with other phytotherapy versus placebo or no intervention, Outcome 1: Urologic symptom score
3.1.1. Studies not included in meta‐analysis
One study with 60 participants found a 36% reduction in the total IPSS median score in the active group (Serenoa repens, lycopene, Prunus africana, Epilobium parviflorum, and Cucurbita pepo) compared to 8% in the placebo group at three months follow‐up (P < 0.05) (Coulson 2013). Another study with 225 participants found a greater decrease in IPSS scores for combination therapy (Serenoa repens, lycopene, and selenium) compared to control at 12‐month follow‐up (median change 2.0, range −3 to −1, P < 0.01) (Morgia 2014). One study reported as an abstract did not provide comparative data (only a decrease in IPSS in the intervention group) (Iacono 2015).
3.2. Quality of life
We are very uncertain about the effects of these agents on quality of life (very low‐certainty evidence). In one study with 40 participants, 84.2% of participants in the intervention group had improvements in their quality of life after six months of treatment compared to 11.1% of participants in the placebo group (P < 0.001) (Metzker 1996). Another study with 225 participants found little to no difference in quality of life scores (median change 0, range −0.1 to 1) (Morgia 2014). The certainty of the evidence is very low due to risk of bias, inconsistency, and imprecision.
3.3. Adverse events
Different phytotherapeutic agents that include Serenoa repens may result in little to no difference in occurrence of adverse events; however, the CIs included substantial benefits and harms (12 to 48 weeks, RR 0.91, 95% CI 0.58 to 1.41; I2 = 0%; 4 studies, 481 participants; low‐certainty evidence; Analysis 2.2). Based on 132 cases per 1000 men in the placebo group, this corresponds to 12 fewer (55 fewer to 54 more) per 1000 men in the combined phytotherapeutic agents with Serenoa repens group. We did not incorporate two studies into the meta‐analysis because they reported no adverse events in either the treatment or control group (Carbin 1990; Coulson 2013). The certainty of the evidence is low due to severe imprecision.
2.2. Analysis.

Comparison 2: Serenoa repens in combination with other phytotherapy versus placebo or no intervention, Outcome 2: Adverse events
Another study with 225 participants reported no significant differences in treatment‐related adverse events (P = 0.67) (Morgia 2014).
The most commonly reported adverse events were headache, gastrointestinal disorders (e.g. diarrhea, nausea and vomiting, dyspepsia), upper respiratory symptoms (e.g. rhinitis), ejaculation disorders, musculoskeletal symptoms (e.g. arthralgia in the knees and pain), and dizziness. Many of these symptoms may be attributable to co‐interventions (alpha‐blockers).
3.4. Subgroup analysis
Few studies in each category precluded these subgroup analyses (see Included studies).
3.5. Sensitivity analysis
We were unable to conduct a sensitivity analysis because the meta‐analyses did not include studies at overall high risk of bias.
Discussion
Summary of main results
For this update, we narrowed the review question and included 27 studies (of which 9 were new studies) with 4656 participants, 19 studies comparing Serenoa repens with placebo and 8 studies comparing Serenoa repens in combination with other phytotherapeutic agents versus placebo.
Serenoa repens versus placebo or no intervention
Based on predefined sensitivity analyses limited to studies at low risk of bias, Serenoa repens results in little to no difference in urologic symptoms and quality of life at short‐term follow‐up. Serenoa repens probably results in little to no difference in adverse events.
Serenoa repens results in little to no difference in urologic symptoms and quality of life at long‐term follow‐up. There were no data on long‐term adverse events for this comparison.
Serenoa repens in combination with other phytotherapy versus placebo or no intervention
Phytotherapeutic agents with various agents, including Serenoa repens, may result in little to no difference in urologic symptoms compared to placebo at short‐term follow‐up. We are very uncertain about the effects of these agents on quality of life. These agents may result in little to no difference in the occurrence of adverse events; however, the confidence intervals included substantial benefits and harms.
Overall completeness and applicability of evidence
While there has been a growing body of research since the last update of this review, our conclusions remain unchanged. Clinical practice guidelines have since deprioritized Serenoa repens in their treatment pathways.
The 2021 Guideline of the American Urological Association focuses on the treatment of LUTS attributed to BPH using common surgical techniques and minimally invasive surgical therapies, thus the information on the different types of medical interventions is not deepened, much less the use of Serenoa repens (Lerner 2021).
A previous version of this guideline from 2010 mentioned that the available data do not suggest that Serenoa repens has a clinically significant effect on LUTS secondary to BPH (McVary 2011). Furthermore, it adds that no dietary supplement, combined herbal medicine, or other unconventional therapy is recommended to manage LUTS secondary to BPH due to the paucity of high‐quality published trials (McVary 2011).
The European Association of Urology guidelines on the management of non‐neurogenic male LUTS recommends several therapeutic and surgical interventions in men with BPH (EAU 2022). This guideline recommends offering the hexane extract of Serenoa repens to men with LUTS who want to avoid possible adverse events, especially those related to sexual function (weak recommendation), informing the patient that the magnitude of efficacy may be modest (strong recommendation) (EAU 2022). Our review offers a further cautionary note about the use of Serenoa repens.
The Korean Urological Association guidelines for the evidence‐based diagnosis and treatment of BPH provide basic information on diagnostic testing, drug therapy, and surgical treatment, but do not mention Serenoa repens as a management option (Yeo 2016).
Considering cut‐off points of 40 mL and 80 mL for small, medium, and large prostates, all studies included men with small‐ to average‐size prostates and moderate urologic symptoms. We found no studies in men with large prostates, and only a few studies of men with more severe urologic symptoms (see Table 3). This evidence is therefore only be applicable to this population (Franco 2023).
Few studies included co‐interventions such as tamsulosin (Argirović 2013; Glémain 2002; Hizli 2007; Morgia 2014; Ryu 2015). However, this did not contribute to statistical heterogeneity when analyzing the outcomes of adverse events. Nonetheless, many of those adverse events described narratively (see footnotes in Analysis 1.5 and Analysis 2.2) include dizziness and ejaculatory disorders, which are typically associated with alpha‐blockers (Mansbart 2022).
Quality of the evidence
The overall certainty of the evidence was high for the main comparison, except for adverse events, for which we identified imprecision. We followed a similar approach to previous versions of this review, excluding studies at high risk of bias from our primary analysis. For the second comparison, however, we had additional concerns about precision and inconsistency across outcomes.
Not all studies provided full details of critical outcomes such as urologic symptoms, quality of life, and adverse events, which would be desirable considering men’s values and preferences (Dahm 2021).
Potential biases in the review process
We could not locate the full text of seven of the original studies in the review, which could not be re‐analyzed using the updated methods (Cukier 1985; Emili 1983; Gabric 1987; Löbelenz 1992; Mattei 1990; Mohanty 1999; Tasca 1985). We contacted the original authors of the review and updates, and they did not hold copies of those studies. In addition to our existing library resources, we also posted a task in Cochrane TaskExchange (currently known as Cochrane Engage) to ask for help on this issue, without success. Based on the characteristics described in the previous version of the review (and available in the Characteristics of studies awaiting classification section), these studies primarily focused on non‐validated outcome measures and Qmax, which would not have contributed to the main analyses of this review. Moreover, we identified 17 additional references that were also assessed as awaiting classification because we could not retrieve a full text to determine their eligibility. These references were mostly from the 1980s and 1990s, so it is likely that their outcomes would not be able to be incorporated into our main analyses.
Although reporting of the timing of adverse events has improved in recent years, we were unable to identify the timing of their occurrence in the included reports, as required by the CONSORT‐Harms statement (Junqueira 2023; Phillips 2019). We therefore did not disaggregate data according to the length of follow‐up of the studies, since most of them described adverse events that were related to treatment initiation (gastrointestinal intolerance) or the effect of co‐interventions (e.g. dizziness and hypotension due to tamsulosin), which resulted in our assumption that they were all short term.
We could not incorporate the results of five studies in our meta‐analyses due to missing data (missing standard deviation or standard error), but we reported these results separately (BASTA 2010; Bauer 1999; Coulson 2013; Iacono 2015; Morgia 2014). Finally, we could not perform many predefined funnel plots and subgroup and sensitivity analyses due to the scarcity of data, low heterogeneity across comparisons, and few trials included in each comparison.
Agreements and disagreements with other studies or reviews
A recent systematic review and network meta‐analysis on the same topic included 22 randomized clinical trials with multiple comparisons of hexanic and non‐hexanic extracts of Serenoa repens with alpha‐adrenergic agonists and placebo (Russo 2021). While the authors concluded that there were clinically insignificant improvements in IPSS at 12 weeks, their confidence intervals included little to no difference compared to placebo (MD −0.47, 95% CI −2.69 to 1.74 for hexanic extract; MD −1.69, 95% CI −4.36 to 0.98 for non‐hexanic extract). Moreover, the authors reported greater improvements in hexanic extracts than in non‐hexanic extracts. Still, the quantitative estimate included little to no difference between subgroups, similar to the findings of our review (MD −2.16, 95% CI −5.64 to 1.30). Finally, this review was limited due to fewer studies comparing Serenoa repens with placebo (7 in that review compared to 15 in ours), with a substantial imprecision in their results.
Another systematic review included seven randomized clinical trials comparing hexanic extract (restricted to the Permixon formulation) with placebo for the outcomes of nocturia, Qmax,and adverse events, but did not assess IPSS (Novara 2016). The authors found a decrease in the episodes of nocturia that may be clinically insignificant (MD −0.31, range −0.59 to −0.03); however, the findings on adverse events were similar to ours.
Finally, a systematic review including 15 randomized clinical trials and 12 observational studies comparing Permixon with placebo assessed nocturia, Qmax,and adverse events, but did not assess IPSS (Vela‐Navarrete 2018). This review also found a small reduction in nocturia that may be clinically insignificant (MD −0.64, range −0.98 to −0.31), and similar results regarding adverse events.
Whereas the dose for almost all studies was 320 mg daily, higher concentrations may result in small but positive improvements in LUTS symptoms, as described in a single study that included doses of 400 mg (Sudeep 2020). The data were insufficient to conduct a subgroup analysis.
Authors' conclusions
Implications for practice.
Serenoa repens alone provides little to no benefits for men with lower urinary tract symptoms due to benign prostatic enlargement. There is more uncertainty about the role of Serenoa repens in combination with other phytotherapeutic agents.
Implications for research.
Considering the uncertainties about the effects of Serenoa repens in higher doses or combined with other herbal treatments, future high‐quality, placebo‐controlled randomized controlled trials are needed in this area that focus on patient‐important outcomes, including urologic symptoms, quality of life, and adverse events.
Feedback
Anna Rita Bilia, et al, 31 August 2009
Summary
Feedback: Quality of a herbal medicinal product is essential. Both the safety profile and the efficacy of a multi‐component herbal medicinal product are irrevocably linked to quality. Quality should be assessed according to the monographs reported in the European Pharmacopoeia or in other Pharmacopoeias or pharmaceutical reference books [1, 2]. These record the methods to define the quality of multi‐component herbal drugs and also of defined selected extracts, according to classification of active constituents, pharmacologically active markers and quality markers [3, 4]. Additionally, pharmacopeial methods are fully validated to perform correctly under the given analytical proceedings irrespectively of the environment where they are performed (ICH guideline Q2(R1); www.ich.org).
Quality of a defined multi‐component herbal extract is strictly related to the quality of the botanical source (herbal drug) defined by the botanical name of the plant according to the binomial system (genus, species, variety and author) and the part used (e.g. leaf, root or fruit). In addition other factors should be considered such as the method of preparation (extraction process, solvents used; solubility and stability of the plant constituents), the drug extract ratio (DER), time and temperature operations, which could be crucial not only for safety but also for the efficacy of the product [5‐8]. Ideally, in analogy with the analytical procedures for testing, also the production‐process should be fully validated, in order to guarantee consistency of the final product, as far as possible.
For these reasons the final mix of constituents in a multi‐component extract may exert different activities and in some circumstances, may even have a different safety profile from another type of extract, that is derived from the identical herb. These facts are taken into consideration and documented for well‐defined herbal extracts in a new series of published European Community Monographs, authorised by the EMEA [9]. It is noteworthy that, among the various types of plant products, e.g. food and botanical products, on the world market, only Herbal Medicinal Products are produced under rigid quality systems, such as Good Sourcing Practices (GSP), Good Agricultural Practices (GAP), Good Field Collection Practices (GFCP), Good Processing Practices (GPP), as well as Good Manufacturing Practices (GMP). As a consequence the quality can be assessed and the final product can be considered reproducible.
According to the above arguments, it is crucial to realise, that the identical botanical source cannot guarantee the bioequivalence of its various multi‐component extracts and of the resulting different Herbal Medicinal Products. The situation in the Cochrane review on Serenoa repens [10] leaves no doubt, that various different Serenoa extracts (not always defined) and their subsequently varying final medicinal products, have been summarised, then analysed, in order to obtain the final conclusions of the review. This left the reader to assume, that both comparable (bioequivalent) and non‐comparable products were included and compared in this study, in spite of the fact, that they might have exerted different, e.g. non‐comparable safety and/or efficacy profiles.
Considering statements and definitions mentioned above, the Cochrane Review on Serenoa repens [10] has been evaluated by the contributors to the present 'Letter to the editor.' Four in part‐related problems were encountered. In the following four comments, these problems have been addressed.
Feedback 1. Problem, missing conclusion regarding studies with a positive control Serenoa alone, was compared in 4 of the 30 investigated clinical trials with known BPH drugs, such as Finasteride, Tamsulosin and Gestonorone caproate as positive controls. Reported in the review were a few minor differences and many comparable results for the various evaluated symptoms and no difference for the overall urinary symptom scores, between treatments with either Serenoa extract or these BPH drugs in different studies with up to 1098 patients. This apparently demonstrated, that the efficacy of these drugs was not different from that of the Serenoa products. Selected results are shown here, to exemplify the commentary: 1 study compared Serenoa to finasteride (MD, mean difference, ‐0,40 Points, 95% CI ‐0.57 to 1.37, P > 0.05); 2 studies compared Serenoa to tamsulosin (WMD, weighted mean difference, ‐0.52 points, 95% CI ‐1.91 to 0.88, P > 0.05).
The reader of the review, even without being in the position to repeat the full statistical analysis, could conclude, that efficacy of Serenoa should be similar or comparable to these BPH drugs. The final statement of the authors, that "Sereoa is not different from placebo", is in clear contradiction to these reports. This contradiction has not been addressed, nor discussed, by the authors of the review.
Conclusion to comment 1
Contradictions described here, unless resolved, prohibit a final conclusion about the efficacy of Serenoa repens products.
Comment 2. Problem, chemical complexity of a multi‐component plant‐extract; 'non‐equivalence' of analysed products The authors of the review appear to have treated the various Serenoa fruit preparations, derived from different extracts, used in the 30 clinical trials, which they analysed, as if these extracts were identical single chemical entities. i.e. the authors appear not to have considered in their analysis, that components of different multi‐component preparations vary, according to their extraction procedure, the solvent used, the drug‐extract‐ratio, the total constituents probably vary, the co‐active constituents probably vary and the standardization can vary. Thus the doses can vary.
The authors have stated in the review, that "of the 15 trials (in true only 14 appeared to have been actually analysed in the review), that were placebo‐controlled and compared to Serenoa repens monotherapy, 7 utilized the commercialized Permixon®, which assured that our comparators were equivalent" [page 13]. Thus the reader may conclude, that 7 out of 14 placebo‐controlled trials were included in this analysis, that were 'non‐equivalent' (i.e. 50% of the comparators). This causes concern about the validity of the authors' statement as well as the authors' conclusions.
Comment 3. Problem, variation of dose
The dosage relates directly to the composition of a multi‐component extract (see comment 2 as well). Thus, the dosage between studies can vary, even if identical amounts are given. Naturally, the dosage must also vary, if the administered amount differs. The 'daily dose' of an extract administered, varied from study to study, in the 30 studies analysed: from 20 drops, 100 mg, 160 mg, 212 mg, 286 mg, 320 mg (a number of studies), 480 mg up to 640 mg, mostly applied in two portions. There was no statistical evaluation in the review taking these different dosages that were used in the 30 clinical trials, into account.
The BPH treatments using non‐identical Serenoa preparations at strongly varying dosages, were summarized and investigated in the review, as if identical treatments with defined dosages, had been used. This is, as if one would assume, that apples, pears and even lemons will taste the same, merely because they are round.
Conclusion to comments 2 and 3
The statistical comparative analysis by the authors of the review, focuses on clinical symptom‐scores of BPH in 30 trials, but they have omitted to fully address the consequences of analysing heterogenous Serenoa preparations administered in heterogenous dosage schemes, in those trials. For example the 7 'non‐equivalent' placebo‐controlled trials should not have been considered as a valid part of a comparative clinical analysis of the placebo‐controlled studies.
Comment 4. Problem, studies conducted with Serenoa‐containing combination products.
Nine (9) of 30 analysed studies, were conducted with combination products containing Serenoa repens extracts, besides one or more other potentially active phytotherapeutic agent (there was no consideration of the dosage, the various extracts were not defined, in the Cochrane review).
Conclusion to comment 4
These studies do not give evidence concerning the efficacy of Serenoa. Any efficacy or lack of efficacy cannot be attributed to Serenoa, such as would be the case in mono‐therapy, but could be influenced by the other plant components in each product. The reader may conclude, that these studies do not qualify for a comparative analysis and cannot support a conclusive statement concerning the activity of Serenoa repens.
Summarising conclusions from comments 1‐4
‐Of 30 analysed studies, 7 placebo‐controlled studies with "non‐comparable" Serenoa products and 9 studies with combination products, could be deleted for good reasons, possibly leaving 14 studies for a revision of the comparative analysis.
‐The authors final conclusion in this review "Sereoa is not different from placebo," does not appear to have been corroborated by rigorous scientific reasoning. Even without repeating the full statistical evaluation (which appears to be necessary as well), the authors final conclusion regarding the efficacy of Serenoa repens, needs to be reconsidered.
References
Saw Palmetto Fruit. Sabalis serrulatae fructus. European Pharmacopoeia. Council of Europe.
Saw palmetto. USP, United States Pharmacopeia.
Guideline on declaration of herbal substances and herbal preparations in herbal medicinal products/traditional herbal medicinal products in the SPC (Summary of product characteristics). EMEA adopted guideline. London, 26 July 2007 Doc. Ref: EMEA/HMPC/CHMP/CVMP/287539/2005
Reflection papers on markers used for quantitative and qualitative analyses of herbal medicinal products and traditional herbal medicinal products. EMEA adopted guideline. London, 15 July 2008. Doc. Ref. EMEA/HMPC/253629/2007
Gaedcke F, Steinhoff B. In: Herbal medicinal products. Scientific and regulatory basis for development, quality assurance and marketing authorisation. CRC Press, Boca Raton, 2003.
Guideline on specifications: test procedures and acceptance criteria for herbal substances, herbal preparations and herbal medicinal products/traditional herbal medicinal products. EMEA adopted guideline. London 30 March 2006. CPMP/QWP/2819/00 Rev 1. EMEA/CVMP/814/00 Rev 1
Guideline on quality of herbal medicinal products/traditional herbal medicinal products. London, 30 March 2006. EMEA adopted guideline. CPMP/QWP/2819/00 Rev 1. EMEA/CVMP/814/00 Rev 1.
Vlietinck A, Pieters L, Apers S. Legal requirements for the quality of herbal substances and herbal preparations for the manufacturing of herbal medicinal products in the European Union. Planta Med 2009;75:683‐8.
a) about European Community Monographs http://www.emea.europa.eu/htms/human/hmpc/hmpcmonographs.htm ; b) homepage of HMPC (Herbal Medicinal Product Committee of EMEA) http://www.emea.europa.eu/htms/human/hmpc/index.htm; c) about HMPC documents (Herbal Medicinal Product Committee of EMEA) http://www.emea.europa.eu/htms/human/hmpwp/working.htm.
Tacklind J, MacDonald R, Rutks I, Wilts TJ. Serenoa repens for benign prostatic hyperplasia (Review). The Cochrane Collaboration, The Cochrane Library 2009, Issue 2, pp.1‐56. Publishers John Wiley & Sons, Ltd.
Submitter agrees with default conflict of interest statement:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you for your comments.
The reviewer remarks that we are acting non‐scientifically by lumping, for example, Permixon® and generic Serenoa repens, is mistaken. Others have made claims that Serenoa repens (whether generic or as Permixon®) alleviates symptoms associated with BPH. We have merely tested their hypothesis.
The reviewer makes two excellent points on bio equivalency and dosages, and in a forthcoming update we will address both. However, we disagree with the reviewers' suggestion that we should have utilized only the Permixon® trials. We conducted a systematic review of the evidence related to all of these products.
The Permixon® trials, which the reviewer urges us to use exclusively, are of almost uniformly poor quality. For example, the 7 RCTs that compared Permixon® to placebo had study populations of 22, 30, 60, 80, 110, 168, and 215. These trials were conspicuously underpowered, with the possible exception of the last two. Follow‐up for the 7 Permixon®‐versus‐placebo trials, measured in weeks, was 4, 4, 4, 4, 8.5, 10 and 12. Only one of the six Permixon® trials that were compared to an active control (or combination therapy with either Permixon® or the active control) utilized a placebo arm.
The reviewer states: "The reader of the review, even without being in the position to repeat the full statistical analysis, could conclude that efficacy of Serenoa should be similar or comparable to these BPH drugs. The final statement of the authors that 'Serenoa is not different from placebo,' is in clear contradiction to these reports." We are not contradictory, but the evidence is ambiguous, as we put it in the review. For example, Carraro [1] (Permixon® versus finasteride) reported a decrease in IPSS symptom scores for both arms (‐37% versus ‐39%, respectively); unfortunately, he did not include a placebo arm. Carraro's trial was certainly well powered (N = 1098), but follow‐up was only 26 weeks.
The consequence of the reviewers' recommendation to use only the Permixon® trials would eliminate the highest quality trial of the thirty, and Bent’s NEJM trial [2] (Serenoa repens versus placebo) is methodologically superior to all of the other twenty‐nine. Bent writes "these studies [previous RCTs] are limited by the small numbers of subjects enrolled, their short duration, their failure to use standard outcome measures, and the lack of information from participants concerning how effectively the placebo was blinded."
After 12‐month follow‐up Bent reported "[b]oth groups also had a small decrease in the AUASI score. . . : the score decreased by 0.68 in the saw palmetto group (95 percent confidence interval, ‐1.37 to 0.01) and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ( Table 3 ( Barry 2011 ; Bent 2006 ; Braeckman 1997a ; Cukier 1985 ; Mohanty 1999a). There was, however, no significant difference between groups in the mean change in AUASI scores over time (difference in mean change, 0.04 point; 95 percent confidence interval, ‐0.93 to 1.01)."
Can these results be extrapolated to European populations using Permixon®? We think so. Nevertheless, we welcome an equivalent European trial utilizing Permixon® when it becomes available.
References
Carraro J‐C, Raynaud J‐P, Koch G, Chisholm GD, Di Silverio F, Teillac P, Da Silva FC, Cauquil J, Chopin DK, Hamdy FC, et al. Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29:231‐40.
Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. The New England Journal of Medicine 2006;354(6):557‐66.
Contributors
The consumers
Corresponding author: Prof. Dr. Anna Rita Bilia, Phytolab, University of Florence, Department of Pharmaceutical Sciences, via Ugo Schiff 6, I‐50019 Sesto Fiorentino (Florence), Italy. E‐mail: ar.bilia@unifi.it.
Co‐contributors names and addresses:
S. F.A.J. Horsten, PhD., Steenmarteakker 1, NL‐3994 GE Houten;
S. Sturm, PhD., University of Innsbruck, Inst. of Pharmacy/Pharmacognosy, A‐6020 Innsbruck;
M. Frater‐Schröder, PhD., Schwand, CH‐9642 Ebnat‐Kappel.
The authors
James Tacklind
Rod MacDonald
Indy Rutks
Tim Wilt
(2009)
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2023 | New search has been performed | Updated search (replacement of previous search) and updated methods (harmonizing with other reviews on this topic), with a narrower focus (comparisons and outcomes) |
| 22 June 2023 | New citation required but conclusions have not changed | Incorporation of nine new studies by a new author team |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 31 July 2016 | Amended | Under Abstract/Search methods, deleted Google Scholar |
| 24 June 2013 | Amended | Editorial changes requested by Prostatic Diseases and Urologic Cancers Group |
| 4 March 2013 | Amended | Under 'Serenoa repens alone or in combination versus placebo,' changed "There were 25 trials" to "24" trials. Added Carbin 1990 to the list. Under 'Serenoa repens alone or in combination versus active control,' we deleted Carbin 1990 from this listing. We changed "of 10 trials comparing SR" to "of 9 trials." We changed "Three trials reported nocturia" to "Two trials." Deleted Carbin 1990 from Analysis 1.5 because it was combination therapy not monotherapy |
| 31 October 2012 | New search has been performed | Search updated 27 January 2012; two new studies included. |
| 31 October 2012 | New citation required but conclusions have not changed | Two new studies included. Byline changed; conclusions not changed. |
| 6 May 2011 | Amended | For this update (2012) we added adverse events (harms) to 'Secondary outcomes.' |
| 4 March 2010 | Amended | Under 'Feedback/1 Anna Rita Bilia, et al, 31 August 2009/Summary/Reply,' a clause read: "and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ('Table 3')." It has been changed to "and by 0.72 in the placebo group (95 percent confidence interval, ‐1.40 to ‐0.04) ('Table 3')." |
| 29 September 2008 | New citation required and conclusions have changed | We have modified our findings of the efficacy of Serenoa repens. |
| 10 July 2008 | New search has been performed | This is a substantial update with nine new trials. |
| 25 March 2008 | Amended | Converted to new review format |
| 21 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
The text in the Background section and the Methods has been recycled from other Cochrane Reviews from the senior author (Franco 2021). Most of the evidence up to December 2020 was extracted and published as an open‐access non‐Cochrane review in which fewer databases were consulted (Trivisonno 2021), from which we also recycled text for the Discussion.
Acknowledgements
Acknowledgements from the authors
Juan Víctor Ariel Franco is a PhD candidate in the Programme of Methodology of Biomedical Research and Public Health, Universitat Autònoma de Barcelona (Spain).
We thank the authors of the previous version of this review: James Tacklind, Roderick MacDonald, Indy Rutks, Judith Stanke, and Timothy Wilt.
Acknowledgements from the previous version of this review
This work was partially funded by the National Center for Complementary and Alternative Medicine (NCCAM) Grant Number R24 AT001293 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant Number 1R01 DK063300‐01A2. The authors are employees of the US Department of Veterans Affairs. The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, the National Institutes of Health, or the Department of Veterans Affairs. We also wish to thank Maurizio Tiso, Margaret Haugh, Rich Crawford, Tatyana Shamilyan, Philipp Dahm, Yelena Slinin, and Joan Barnes for their work in translating and abstracting data from non‐English language studies.
Editorial and peer‐reviewer contributions
The Cochrane Urology Group supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Philipp Dahm, MD, Urology Section, Minneapolis VA Health Care System, Minneapolis, Minnesota, USA;
Handling Editor (selected peer reviewers, provided editorial guidance to authors, and made editorial decisions): Muhammad Imran Omar, MD, University of Aberdeen, Aberdeen, UK;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Jennifer Mariano, Cochrane Urology, USA;
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support;
Peer reviewers (provided comments and recommended an editorial decision): Brendan Brwne, Emory University Department of Urology (clinical/content review), Michael Lardas, 2nd Department of Urology, Sismanoglio General Hospital, Athens, Greece (clinical/content review). One additional peer reviewer provided clinical/content peer review, but chose not to be publicly acknowledged.
The authors JVAF and JHJ are contact editors for the Cochrane Urology Group but were excluded from the editorial processing of this article.
Appendices
Appendix 1. Search strategies
1. Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library .
#1 MeSH descriptor: [Prostatic Hyperplasia] explode all trees
#2 MeSH descriptor: [Prostatism] explode all trees
#3 (Prostat*near/3 hyperplasia*):ti,ab,kw
#4 ((Prostat* near/3 hypertroph*)):ti,ab,kw
#5 ((Prostat* near/3 adenoma*)):ti,ab,kw
#6 (BPH or BPO or BPE):ti,ab,kw
#7 (prostat* near/3 enlarg*):ti,ab,kw
#8 (Prostatism):ti,ab,kw
#9 (Bladder* near/3 obstruct*):ti,ab,kw
#10 (BOO):ti,ab,kw
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12 (phytoterap*):ti,ab,kw
#13 (Herb* near/3 Therap*):ti,ab,kw
#14 MeSH descriptor: [Plant Extracts] explode all trees
#15 (plant*):ti,ab,kw
#16 MeSH descriptor: [Serenoa] explode all trees
#17 (serenoa):ti,ab,kw
#18 (sabal*):ti,ab,kw
#19 (Phytomedicin*):ti,ab,kw
#20 (phytosterol*):ti,ab,kw
#21 (sitosterols*):ti,ab,kw
#22 (permixon):ti,ab,kw
#23 ("saw palmetto"):ti,ab,kw
#24 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
#25 #11 AND #24
2. Medline (Ovid)
1. exp Prostatic Hyperplasia/
2. exp Prostatism/
3.exp Urinary Bladder Neck Obstruction/
4. (Prostat* adj3 hyperplasia*).tw.
5. (Prostat* adj3 hypertroph*).tw.
6. (Prostat* adj3 adenoma*).tw.
7. (BPH or BPO or BPE).tw.
8. (prostat* adj3 enlarg*).tw.
9. Prostatism.tw.
10. (Bladder* adj3 obstruct*).tw.
11. BOO.tw.
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13 phytoterap*.tw.
14 (Herb* adj3 Therap*).tw.
15 exp Plant Extracts/
16 plant*.tw.
17 exp Serenoa/
18 serenoa.tw.
19 sabal*.tw.
20 Phytomedicin*.tw.
21 phytosterol*.tw.
22 sitosterols*.tw.
23 permixon.tw
24 "saw palmetto".tw.
25 13 or 14 or 15 or 16 0r 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
26 12 and 25
3. Embase (Elsevier.com)
#26. #12 AND #25
#25. #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 )
#24. 'saw palmetto':ti,ab,kw
#23. permixon:ti,ab,kw
#22. sitosterols*:ti,ab,kw
#21. phytosterol*:ti,ab,kw
#20. phytomedicin*:ti,ab,kw
#19. sabal*:ti,ab,kw
#18. serenoa:ti,ab,kw
#17. 'serenoa'/exp
#16. plant*:ti,kw
#15. 'plant extract'/exp
#14. (herb* NEAR/3 therap*):ti,ab,kw
#13. phytoterap*:ti,ab,kw
#12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
#11. boo:ti,ab,kw
#10. (bladder* NEAR/3 obstruct*):ti,ab,kw
#9. prostatism:ti,ab,kw
#8. (prostat* NEAR/3 enlarg*):ti,ab,kw
#7. bph:ti,ab,kw OR bpo:ti,ab,kw OR bpe:ti,ab,kw
#6. (prostat* NEAR/3 adenoma*):ti,ab,kw
#5. (prostat* NEAR/3 hypertroph*):ti,ab,kw
#4. (prostat* NEAR/3 hyperplasia*):ti,ab,kw
#3. 'bladder obstruction'/exp
#2. 'prostatism'/exp
#1. 'prostate hypertrophy'/exp
4. Scopus
TITLE‐ABS‐KEY ( ( "Prostatic Hyperplasia" OR prostatism OR "Urinary Bladder Neck Obstruction" OR bph OR bpo OR bpe OR boo OR "Bladder obstruction" ) ) AND TITLE‐ABS‐KEY ( ( "Plant Extracts" OR plant* OR serenoa OR sabal* OR phytomedicin* OR phytosterol* OR sitosterols* OR permixon OR "saw palmetto" )
5. Science Citation Index Expanded (SCI‐E) Web of Science Clarivate
1: Ti=("Prostatic Hyperplasia" OR Prostatism OR "Urinary Bladder Neck Obstruction" OR BPH OR BPO OR BPE OR BOO OR "Bladder obstruction")
2: AB=("Prostatic Hyperplasia" OR Prostatism OR "Urinary Bladder Neck Obstruction" OR BPH OR BPO OR BPE OR BOO OR "Bladder obstruction")
3: #2 OR #1
4: TI=("Plant Extracts" OR plant* OR serenoa OR sabal* OR Phytomedicin* OR phytosterol* OR sitosterols* OR permixon OR "saw palmetto")
5: AB=("Plant Extracts" OR plant* OR serenoa OR sabal* OR Phytomedicin* OR phytosterol* OR sitosterols* OR permixon OR "saw palmetto") 1304899
6: #4 OR #5
7: #3 AND #6
6. Latin American and Caribbean Literature in Health Sciences (LILACS)
título, resumen, asunto: ("Prostatic Hyperplasia" OR "Hiperplasia prostática" OR Prostatism OR prostatismo OR "Urinary Bladder Neck Obstruction" OR "Obstrucción del cuello de la vejiga urinaria" OR "Obstrução do colo da bexiga urinária" OR BPH OR BPO OR BPE OR BOO) AND (phytotherapy OR fitoterapia OR "Plant Extracts" OR "Extratos de plantas" OR "Extractos de plantas" OR plants OR plantas OR serenoa OR sabal OR Phytomedicine OR Fitomedicina OR phytosterol OR fitosterol OR sitosterols OR sitoesteroles OR sitoesteróis OR permixon OR permixon OR "saw palmetto" OR "saw palmetto" OR Palma)
7. ClinicalTrials.gov
#1 phytoterap* OR Serenoa OR permixon OR "saw palmetto" OR sitosterols* OR sabal* OR Plant Extracts
8. World Health Organization International Clinical Trials Registry Platform (ICTRP)
#1 phytoterap* OR Serenoa OR permixon OR "saw palmetto" OR sitosterols* OR sabal* OR Plant Extracts
Appendix 2. Searches in conference proceedings
| Conference | Website (last access 29 Dec 2022) |
| American Urology Association 2020 | https://www.aua2020.org/abstracts |
| American Urology Association 2021 | https://www.aua2021.org/abstracts |
| American Urology Association 2022 | https://www.auanet.org/AUA2022/publications |
| International Continence Society 2020 | https://www.ics.org/2020/programme#Abstracts |
| International Continence Society 2021 | https://www.ics.org/2021/programme |
| International Continence Society 2022 | https://www.ics.org/2022/programme |
| European Association of Urology 2020, 2021, 2022 | https://resource‐centre.uroweb.org/ |
Appendix 3. Methods from the previous version of the review
Criteria for considering studies for this review
Types of studies
Randomized, controlled clinical trials.
Types of participants
Men with lower urinary tract symptoms (LUTS) consistent with benign prostatic hyperplasia (BPH).
Types of interventions
Comparison of preparations of SR with placebo or medical therapies for BPH with a treatment duration of at least 30 days.
Types of outcome measures
Primary outcomes
Urologic symptom scores (Boyarsky, American Urologic Association Symptom Index (AUA), and the International Prostate Symptom Score (IPSS)), with validated scores taking precedence over non validated ones. Both the AUA and IPSS use an identical scale of zero to 35, with mild symptoms scored 1 to 8, medium 9 to 18, and severe ≥ 19.
Secondary outcomes
Change in peak urine flow (mL/s).
Change in prostate size (measured in cubic centimeters (cc)).
Nocturia (times/evening).
Overall physician or patient assessment of urinary symptoms.
Adverse events (harms).
Search methods for identification of studies
See Appendices.
Electronic searches
We electronically searched the Cochrane Central Register of Controlled trials (CENTRAL), (including the database of the Cochrane Prostatic Diseases and Urologic Cancers Group) MEDLINE® (from 2008 to 2011), EMBASE (from 2001 to 1 January 2012), Web of Science®, Scopus, BIOSIS Previews®, LILACS, http://clinicaltrial.gov/, http://www.controlled‐trials.com/, and http://www.who.int/ictrp/en/.
Searching other resources
We handsearched systematic reviews, references, clinical‐practice guidelines, and conference abstracts.
Data collection and analysis
We assessed mean urologic symptom scores (IPSS, AUA), nocturia (# times), peak urine flow (mL/s), and prostate size (cc). The number and per cent of men reporting specific harms were also evaluated.
For the primary analysis (of the stated primary and secondary outcomes), all trials including SR in mono preparations and in combination were analyzed separately (e.g., SR versus placebo or active controls, SR + Urtica dioica versus placebo or controls). We pooled studies that were deemed clinically similar and provided sufficient information.
Selection of studies
In this update, two review authors (JT, RM) decided on eligibility.
Data extraction and management
Two review authors (JT, RM) independently assessed study characteristics and extracted data. Missing or additional information was sought from authors/sponsors. Extracted data were reviewed by the principal review author and discrepancies were resolved by discussion.
Assessment of risk of bias in included studies
As a measure of overall methodologic study quality – and bias – we assessed scales and criteria developed by Schulz and The Cochrane Collaboration (Cochrane Handbook 2011; Schulz 1995). The following seven criteria were addressed.
Selection bias I (Was there an articulated rule for allocating interventions based on chance?).
Selection bias II (Was there any foreknowledge of the allocation of interventions by anyone?).
Blinding bias I (During the course of the trial were study participants and personnel blinded to the knowledge of who received which intervention?).
Blinding bias II (Were the outcome assessors blinded to who received the intervention and who did not?).
Attrition bias (Did the trial assess all patients, or account for those not assessed?).
Reporting bias (Were outcomes selectively reported?).
Other bias (Were arms assessed differently?).
Each criterion was answered by 'low risk', 'unclear risk', and 'high risk', and summarized here (Figure 1; Figure 1). For the main outcome, we also assessed the quality of evidence in the summary of findings Table for the main comparison using GRADEPro (GRADEPro 2008).
Measures of treatment effect
We performed our statistical analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook 2011). Dichotomous outcomes were expressed using risk ratios (RR) or absolute risk reductions (RD), using the Mantel‐Haenszel method. Continuous outcomes were expressed as mean differences (MD), or, for unequal scales, standardized mean differences (SMD). To minimize the uncertainty of the pooled‐effect estimate, we used an inverse variance method, which allowed larger trials with smaller SEM (standard error of the mean) more weight over smaller trials with larger SEM.
All outcome measures utilized 95% confidence intervals (CI), with a P value of ≤ 0.05 considered to be statistically significant.
Unit of analysis issues
Quasi‐randomized trials were not included.
Dealing with missing data
To assess the per cent change of patients' urologic symptoms, a modified intention‐to‐treat (ITT) was performed (i.e., men who dropped out or were lost to follow‐up were considered to have had worsening symptoms) (Lavori 1992). The denominator for the modified ITT analysis included the number randomized to treatment at baseline and the numerator included the number completing the trial and showing improvement.
Assessment of heterogeneity
We assessed for heterogeneity by using the I₂ statistic. If a meta‐analysis had an I₂ of > 50%, we conducted a descriptive sensitivity analysis.
Assessment of reporting biases
To minimize reporting bias, we cross‐referenced trials and their protocols, compared systematic reviews to their included studies, and contacted authors.
Data synthesis
Assuming some level of unexplained heterogeneity among trials, we used a random‐effects model to adjust for effect size inconsistency.
Subgroup analysis and investigation of heterogeneity
We conducted no a priori analyses by subgroup. For investigation of heterogeneity, see Assessment of heterogeneity.
Sensitivity analysis
See Assessment of heterogeneity.
Previous search methods (appendix)
A. We searched Google Scholar using all combinations of the following categories:
prostatic hyperplasia OR bph OR benign prostatic hyperplasia;
serenoa repens OR s. repens OR sabal serrulata OR saw palmetto;
rct OR randomized controlled trial OR randomised controlled trial.
Restrictions were by years (2008 to 2011) and category (Medicine, Pharmacology, and Veterinary Science).
B. We searched Ovid MEDLINE® from 2008 to 2011 by crossing an optimally sensitive search strategy for trials from The Cochrane Collaboration with the following MeSH search terms.
prostatic hyperplasia.mp.
phytosterols.mp.
plant extracts.mp.
sitosterols.mp.
serenoa repens.mp.
sabal serrulata.mp.
saw palmetto.mp.
or/2‐7
1 and 8
limit 9 to randomized controlled trial
limit 11 to yr="2008 ‐ 2011"
We included all subheadings (Dickersin 1994).
C. We also searched the following using the same key terms we used for the Ovid MEDLINE® search and limited by the dates 2008 to 2011:
The Cochrane Library, including the database of the Cochrane Prostatic Diseases and Urologic Cancers Group, the Cochrane Field for Complementary Medicine, and the Cochrane Central Register of Controlled Trials (CENTRAL);
Web of Science®;
CINAHL®;
BIOSIS Previews®;
LILACS;
D. EMBASE was searched from 2001 to 1 January 2012 using the following strategy.
| Set | Items | Description |
| S1 | 8684 | PROSTATIC (W) HYPERPLASIA |
| S2 | 7071 | BPH/TI,AB |
| S3 | 240 | SAW (W) PALMETTO |
| S4 | 179 | SERENOA (W) REPENS |
| S5 | 150 | PERMIXON |
| S6 | 31 | SABAL (W) SERRULATA |
| S7 | 252 | (S1 OR S2) AND (S3 OR S4 OR S5 OR S6) |
| S8 | 276370 | RANDOMIZED (W) CONTROLLED (W) TRIAL? OR RANDIMISED (W)CONTROLLE‐D (W) TRIAL? |
| S9 | 11351 | RCT? |
| S10 | 51 | S7 AND (S8 OR S9) |
| S11 | 37 | S10/2001:2011 |
There were no language restrictions.
Data and analyses
Comparison 1. Serenoa repens versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Urologic symptom score (short term—sensitivity analysis) | 9 | 1681 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.74, ‐0.07] |
| 1.2 Urologic symptom score (short term—all studies) | 12 | 1841 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.44, ‐0.11] |
| 1.3 Quality of life (short term—sensitivity analysis) | 5 | 1001 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.40, 0.00] |
| 1.4 Quality of life (short term—all studies) | 8 | 1161 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.32, 0.00] |
| 1.5 Adverse events (sensitivity analysis) | 12 | 2399 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.31] |
| 1.6 Adverse events | 14 | 2539 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.26] |
| 1.7 Urologic symptom score (subgroup analysis—hexanic vs non‐hexanic extract) | 12 | 1841 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.44, ‐0.11] |
| 1.7.1 Hexanic extract | 3 | 437 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.99, 0.44] |
| 1.7.2 Non‐Hexanic extract | 9 | 1404 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.91, ‐0.07] |
| 1.8 Urologic symptom score (long term—sensitivity analysis) | 3 | 898 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.75, 0.88] |
| 1.9 Urologic symptom score (long term—all studies) | 5 | 1018 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.58, 0.59] |
| 1.10 Quality of life (long term—sensitivity analysis) | 3 | 882 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.41, 0.19] |
| 1.11 Quality of life (long term—all studies) | 5 | 1002 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.37, 0.13] |
Comparison 2. Serenoa repens in combination with other phytotherapy versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Urologic symptom score | 4 | 460 | Mean Difference (IV, Random, 95% CI) | ‐2.41 [‐4.54, ‐0.29] |
| 2.2 Adverse events | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Argirović 2013.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: June 2008 to September 2010 Setting: outpatient, single center, national Country: Serbia |
| Participants |
Inclusion criteria: men > 50 years of age, a total IPSS of 7 to 18, QoL > 3, Qmax of 5 to 15 mL/s, with PVR < 150 mL, prostate volume < 50 mL, measured by transrectal ultrasound (TRUS) and serum PSA 1.5 to 4 ng/mL Exclusion criteria: significant bladder outlet obstruction (PVR > 200 mL, Qmax < 5 mL/s), history of bladder disease likely to affect micturition, urethral stenosis, prostate and/or bladder cancer, bladder stone, previous pelvic radiotherapy, recurrent urinary retention, neurogenic lower urinary tract dysfunction, repeated infection of the urinary tract, chronic bacterial prostatitis, or any other disease that can cause urinary problems Sample size: 199 participants Age (years): Group 1 (SD): 65.9 ± 7.4 Group 2 (SD): 56.8 ± 7.7 IPSS score (baseline) Group 1 (SD): 15.6 ± 3.2 Group 2 (SD): 16.2 ± 4.9 Prostate size: Group 1 (SD): 31.2 ± 4.2 Group 2 (SD): 38.6 ± 11.6 |
| Interventions |
Group 1 (n = 81): Serenoa repens (Prostamol Uno) 320 mg daily Group 2 (n = 87): only co‐intervention Co‐interventions: tamsulosin (Tamsol) 0.4 mg A third group of only Serenoa repens (without tamsulosin) was not included in the analysis as it was not relevant to this review. |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
|
| Notes |
Funding sources: not available Conflict of interest: not available |
Barry 2011.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: June 2008 to October 2010 Setting: multicenter, outpatient, national Country: USA |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 369 randomized participants Age (years): Group 1 mean (SD): 61.25 (8.72) Group 2 mean (SD): 60.7 (8.08) IPSS/AUASI score (baseline): Group 1 mean (SD): 14.42 (4.29) Group 2 mean (SD): 14.69 (4.75) Prostate size: Group 1: not available Group 2: not available |
| Interventions |
Group 1 (n = 176): one, two, and then three 320 mg chocolate‐colored gelcaps daily containing a standardized saw palmetto fruit extract with dose escalations at 24 and 48 weeks. Saw palmetto extract used was standardized to a reference chromatogram (with 85% to 95% fatty acids as marker substances). The phytotherapy used in this trial was a proprietary lipidic ethanolic extract of ripe, dried saw palmetto berries, Serenoa repens (W.Bartram) Small (Arecaceae), manufactured by Rottapharm/Madaus, Cologne, Germany and sold as PROSTAURGENIN UNO capsules. Group 2 (n = 181): an identical number of placebo gelcaps escalated similarly Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: Qmax, a global assessment of improvement, PVR, PSA, erectile and ejaculatory function, incontinence, sleep quality, NIH‐CPSI |
| Notes |
Funding sources: this study was funded by co‐operative agreements from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK63795, U01 DK63797, U01 DK63825, U01 DK63835, U01 DK63866, U01 DK63833, U01 DK63862, U01 DK63840, U01 DK63883, U01 DK63831, U01 DK63778, and U01 DK63788. Support was also provided by the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, NIH. Rottapharm/Madaus, Cologne, Germany donated the saw palmetto fruit extract and matching placebo. Declarations of interest: Rottapharm/Madaus had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation or approval of the manuscript. Rottapaharm/Madaus provided nonbinding comments to the authors on a draft of the manuscript. Individual authors:
|
BASTA 2010.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: December 2006 to November 2008 Setting: outpatient, multicenter Country: Germany, Lithuania, Romania, Slovak Republic, Poland, and Russia |
| Participants |
Inclusion criteria:
Exclusion criteria (men with a history of the following):
Sample size: 1011 participants Age (years): Group 1 mean (SD): 65.14 (7.67) Group 2 mean (SD): 64.61 (7.69) Group 4 mean (SD): 64.14 (7.69) IPSS score (baseline): not available Prostate size (cm3): not available |
| Interventions |
Group 1 (n = 334; per‐protocol set): ethanol extract of Sabal serrulata (Prostamol) once daily 320 mg orally for 12 months Group 1 (n = 330; per‐protocol set): hexane extract of Sabal serrulata (Permixon) once daily 2 x 160 mg orally for 12 months Group 2 (n = 126; per‐protocol set): placebo of similar characteristics for 12 months Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: PVR and Qmax |
| Notes |
Funding sources: Berlin‐Chemie AG (Menarini Group) Declarations of interest: not reported Unpublished data only from the clinical trial registry: www.clinicaltrialsregister.eu/ctr-search/trial/2006-003532-30/results |
Bauer 1999.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, multicenter, international Country: Germany/Italy |
| Participants |
Inclusion criteria: confirmed diagnosis of BPH with enlargement of the prostate, symptoms of obstruction, and a maximum flow of < 15 mL/s Exclusion criteria: men treated for BPH within 1 month of the trial start; prostate cancer; acute urinary tract infection; chronic prostatitis; neurogenic bladder Sample size: 101 participants Age (years): mean 66.1 IPSS score (baseline): Sabal extract 9.6; placebo 8.9 Prostate size: Sabal extract 34.5 cm3; placebo 31.7 cm3 |
| Interventions |
Group 1 (n = not available): Sabal extract (LG166/S ‐ Talso Uno) 160 mg twice daily Group 2 (n = not available): matching placebo |
| Outcomes |
Urinary symptoms
Adverse events
Other outcomes measured in the trial: Qmax, prostate volume, and sexual function |
| Notes |
Funding sources: Sanofi (industry) Conflicts of interest: not available |
Bent 2006.
| Study characteristics | |
| Methods |
Study design: parallel group randomized trial Study dates: July 2001 to May 2004 Setting: multicenter, outpatient, national Country: USA |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 225 participants Age (years): Group 1 mean (SD): 62.9 (8.0) Group 2 mean (SD): 63.0 (7.4) AUASI score (baseline): Group 1 mean (SD): 15.7 (5.7) Group 2 mean (SD): 15.0 (5.3) Prostate size (mL): Group 1 mean (SD): 34.7 (13.9) Group 2 mean (SD): 33.9 (15.2) |
| Interventions |
Group 1 (n = 112): saw palmetto carbon dioxide extract capsules, 160 mg twice daily orally for 14 months Group 2 (n = 113): placebo capsules twice a day orally for 14 months Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: Qmax, prostate size, PVR, PSA, creatinine, testosterone, and other laboratory values |
| Notes |
Funding sources: supported by a grant (1 RO1 DK56199‐01, to Dr Avins) from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant (1 K08 ATO1338‐01, to Dr Bent) from the National Center for Complementary and Alternative Medicine Declarations of interest: Dr Kane reports receiving consulting fees from American Medical Systems and Intuitive Surgical and lecture fees from Merck and TAP. Dr Shinohara reports having received lecture fees from GlaxoSmithKline and Pfizer. Dr Avins reports receiving grant support from Merck. |
Boccafoschi 1983.
| Study characteristics | |
| Methods |
Study design: double‐blind, controlled clinical trial Study dates: not available Setting: outpatient, single center and national Country: Italy |
| Participants |
Inclusion criteria: not available Exclusion criteria: not available Sample size: 22 randomized participants Age (years): Group 1 mean (range): 68 (55 to 80) Group 2 mean (range): 68 (54 to 78) IPSS score (baseline): not available Prostate size: not available |
| Interventions |
Group 1 (n = 11): Permixon 160 mg, 2 capsules orally (1 in the morning and the other in the evening) for 60 days (total dose per day: 320 mg) Group 2 (n = 11): placebo 2 capsules orally (1 in the morning and the other in the evening) for 60 days Co‐interventions: none |
| Outcomes |
Adverse events
Other outcomes measured in the trial: dysuria, nocturia, and urinary frequency |
| Notes |
Funding: not specified Conflicts of interest: not specified |
Carbin 1990.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, multicenter, international Country: Sweden/Denmark |
| Participants |
Inclusion criteria:
Exclusion criteria: need for surgery or residual urine > 300 mL Sample size: 55 Age (years): Group 1 mean (SD): 62.0 (± 6.7) Group 2 mean (SD): 61.2 (± 5.8) IPSS/AUASI score (baseline): Group 1 mean (SD): 6.8 (± 1.4) Group 2 mean (SD): 6.6 (± 1.3) Prostate size: not available |
| Interventions |
Group 1 (n = 26): Curbicin contains 160 mg of standardized extract PS6 from Cucurbita pepo L. seeds (80 mg) and Sabal serrulata fruits (80 mg). The tablets were swallowed whole in a dose of 2 tablets 3 times a day (possibly 3 months). Group 2 (n = 27): placebo tables were identical in smell, consistency, shape, color, and taste to the active compound (possibly 3 months) Co‐interventions: none |
| Outcomes |
Adverse events
Other outcomes measured in the trial: Qmax, micturition time, residual volume, diurnal frequency, nocturnal frequency, and duration of illness. Subjective symptoms such as dysuria and global assessment of the therapy were evaluated with a scale: much better, better, the same, worse. |
| Notes |
Funding sources: not available Declarations of interest: not available |
Champault 1984.
| Study characteristics | |
| Methods |
Study design: parallel‐group, double‐blind, placebo‐controlled randomized trial Study dates: study dates not available Setting: outpatient, single center, national Country: France |
| Participants |
Inclusion criteria: not available Exclusion criteria:
Sample size: 110 outpatients randomized Age (years): not available IPSS score (baseline): not available Prostate size: not available |
| Interventions |
Group 1 (n = 55): hexane extract of Serenoa repens (PA109/Permixon) 320 mg per day (2 x 80 mg x 2) orally for 30 days Group 2 (n = 55): placebo 320 mg per day (2 x 80 mg x 2) orally for 30 days Co‐interventions: none |
| Outcomes |
Adverse events
Other outcomes measured in the trial: dysuria, patient self‐rating and physician’s rating, Qmax |
| Notes |
Funding sources: not available Declarations of interest: not available |
Coulson 2013.
| Study characteristics | |
| Methods |
Study design: randomized, double‐blind, placebo‐controlled clinical trial Study dates: study dates not available Setting: outpatient, single center, national Country: Australia |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 60 randomized participants Age (years): Group 1 mean (SD): 63 (10.1) Group 2 mean (SD): 64.9 (9.6) IPSS score (baseline): Group 1 median: 19.5 Group 2 median: 18 Prostate size: not available |
| Interventions |
Group 1 (n = 32): ProstateEZE Max is a commercially available capsule‐form herbal formulation containing Cucurbita pepo seed oil (160 mg), Epilobium parviflorum extracts (equivalent to 500 mg dry herb), lycopene (2.1 mg), Prunus africana (equivalent to 15 g dry stem, standardized to ‐sitosterol), and Serenoa repens (equivalent to 660 mg of dry leaf per capsule) with the excipients lecithin, hydrogenated vegetable oil and beeswax and soya oil in a blue soft gel capsule. Administered as 1 capsule per day with food for 3 months Group 2 (n = 25): the placebo product contained the same amounts of lecithin, hydrogenated vegetable oil, and beeswax, but had higher levels of soya oil in the same soft gel capsule. Administered as 1 capsule per day with food for 3 months Co‐interventions: none |
| Outcomes |
Urinary symptoms
Adverse events
Other outcomes measured in the trial: daytime and nighttime urinary frequency |
| Notes |
Funding sources: funding and study medication for the project were received from the clinical trial sponsor, Totally Natural Products, Sydney, Australia Declarations of interest: funding and study medication for the project were received from the clinical trial sponsor, Totally Natural Products, Sydney, Australia. The sponsor had no involvement in the collection, analysis or interpretation of the data, writing of the report, or the decision to submit the paper for publication. Retrospectively registered: ACTRN12610000168055 |
Descotes 1995.
| Study characteristics | |
| Methods |
Study design: double‐blind, placebo‐controlled randomized trial Study dates: not available Setting: outpatient, multicenter and national Country: France |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 176 randomized participants Age (years): Group 1 mean (SD): 65.6 (8.4) Group 2 mean (SD): 67 (7.6) IPSS score (baseline): not available Prostate size: not available |
| Interventions |
Group 1 (n = 82): Permixon 160 mg orally twice daily (morning and evening) for 30 days Group 2 (n = 94): placebo orally twice daily (morning and evening) for 30 days Co‐interventions: none |
| Outcomes |
Adverse events
Other outcomes measured in the trial: patient‐based global efficacy and physician‐based global efficacy, improvement of dysuria, Qmax |
| Notes |
Funding sources: not available Declarations of interest: 1 author (P Deschaseaux) works in Laboratoires Pierre Fabre, which manufactures Permixon |
Gerber 2001.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: January 1999 to July 2000 Setting: outpatient, multicenter, national Country: USA |
| Participants |
Inclusion criteria:
Exclusion criteria: men were ineligible if they had:
Sample size: 85 Age (years): Group 1 mean (SD): 64.6 (± 9.9) Group 2 mean (SD): 65.3 (± 9.7) IPSS/AUASI score (baseline): Group 1 mean (SD): 16.7 (± 4.9) Group 2 mean (SD): 15.8 (± 4.8) Prostate size: not available |
| Interventions |
Group 1 (n = 41): men received saw palmetto 160 mg twice daily. The saw palmetto product was standardized to contain 85% to 95% fatty acids and sterols and was manufactured by Nutraceutical, Ogden, Utah. Group 2 (n = 44): the only other ingredients in the saw palmetto and placebo capsules were extra‐virgin olive oil, gelatin, and glycerin Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: sexual function, Qmax |
| Notes |
Funding sources: the study drug and placebo were provided by Nutraceutical Corp., Ogden, Utah Declarations of interest: not available |
Glémain 2002.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, multicenter and national Country: France |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 329 randomized participants Age (years): Group 1 mean (SD): 64.4 (7.7) Group 2 mean (SD): 65.2 (7.9) IPSS score (baseline): Group 1 mean (SD): 16.3 (5.6) Group 2 mean (SD): 16.2 (5.2) Prostate size: Group 1 mean (SD): 38.6 (15) Group 2 mean (SD): 40.8 (16.5) |
| Interventions |
Group 1 (n = 161): placebo orally after breakfast and dinner for 52 weeks Group 2 (n = 165): Serenoa repens 160 mg orally (Permixon) after breakfast and dinner for 52 weeks Co‐interventions: tamsulosin (Omix LP 0.4 mg) orally after breakfast |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: sexual function was assessed using the Brief Male Sexual Function Inventory (BMSFI), Qmax |
| Notes |
Funding sources: not available Declarations of interest: not available |
Hizli 2007.
| Study characteristics | |
| Methods |
Study design: open‐label prospective study Study dates: May 2005 to November 2005 Setting: outpatient, multicenter, national Country: Turkey |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 60 randomized participants Age (years): Group 1 mean (SD): 58.9 (5.7) Group 2 mean (SD): 56.8 (7.8) Group 3 mean (SD): 60.2 (6.3) IPSS score (baseline): Group 1 mean (SD): 16.2 (4.7) Group 2 mean (SD): 18.0 (4.9) Group 3 mean (SD): 15.6 (3.2) Prostate size: Group 1 mean (SD): 38.6 (11.6) Group 2 mean (SD): 35.2 (10.3) Group 3 mean (SD): 31.2 (4.2) |
| Interventions |
Group 1 (n = 20): tamsulosin 0.4 mg once a day for 6 months Group 2 (n = 20): Serenoa repens (Permixon) 320 mg once a day for 6 months is the lipidosterolic extract of Serenoa repens Group 3 (n = 20): Serenoa repens 320 mg plus tamsulosin 0.4 mg once a day for 6 months Co‐interventions: tamsulosin (for comparison of group 2 and 3) |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: PSA, Qmax, and acute urinary retention |
| Notes |
Funding sources: not available Declarations of interest: not available |
Hong 2009.
| Study characteristics | |
| Methods |
Study design: placebo‐controlled randomized trial Study dates: not available Setting: outpatient, single center, national Country: Korea |
| Participants |
Inclusion criteria: BPH patients with an IPSS of 8 or more Exclusion criteria: any major diseases. BPH‐related treatment (5‐a‐reductase inhibitor, a‐receptor blocker, or urogenital surgery) Sample size: 62 randomized participants Age (years): Group 1 mean (SD): 52 (1.8) Group 2 mean (SD): 53.1 (4.29) IPSS score (baseline): Group 1 mean (SD): 18.3 (2.0) Group 2 mean (SD): 15.4 (2.8) Prostate size: Group 1 mean (SD): 26.1 (1.7) Group 2 mean (SD): 23.2 (2.3) |
| Interventions |
Group 1 (n = 13): saw palmetto oil, 320 mg/day. Men took 2 capsules per day in the morning and in the evening after a meal over a 12‐month period. Group 2 (n = 7): placebo, control, sweet potato starch, 320 mg/day. Men took 2 capsules per day in the morning and in the evening after a meal over a 12‐month period. Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: Qmax and other uroflowmetry parameters; PSA. |
| Notes |
Funding sources: not available Declarations of interest: not available |
Iacono 2015.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, single center, national Country: Italy |
| Participants |
Inclusion criteria: men affected by lower urinary tract symptoms due to BPH Exclusion criteria: not specified Sample size: 185 participants Age (years): mean 64.5 (SD 8.6) IPSS score (baseline): Group 1 (SD): 20.6 (5.4) Group 2 (SD): not available Prostate size: not available |
| Interventions |
Group 1 (n = 95): Tramaxadina (Alga Ecklonia bicylis 80 mg, Tribulus terrestris 100 mg, chitosan oligosaccharide (Biovis) 100 mg) and Serenoa repens 320 mg daily for 6 months Group 2 (n = 90): placebo |
| Outcomes |
Urinary symptoms
|
| Notes |
Funding sources: not available Conflict of interest: not available Study available only as an abstract of the 17th Annual Congress of the European Society for Sexual Medicine, 2015 February 5‐7; Copenhagen. |
Lopatkin 2005.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, multicenter, national Country: possibly Germany or Russia |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 257 randomized participants Age (years): Group 1 mean (SD): 68 (7) Group 2 mean (SD): 67 (7) IPSS score (baseline): Group 1 mean (SD): 18 (4) Group 2 mean (SD): 18 (3) Prostate size: Group 1 mean (SD): 44.9 (18.1) cm3 Group 2 mean (SD): 46.4 (19.2) cm3 |
| Interventions |
Group 1 (n = 127): PRO 160/120 mg (Sabal fruit extract WS 1473 90% ethanol and Urtica root dry extract WS 1031, respectively) orally daily in the morning and in the evening for 24 weeks Group 2 (n = 126): placebo orally daily in the morning and in the evening for 24 weeks Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: prostate size, Qmax, and urinary retention |
| Notes |
Funding sources: Wilmar Schwabe GmbH & Co. KG, Karlsruhe (Germany) Conflicts of interest: not available At 24 weeks all men were unblinded and received the experimental treatment, with follow‐up to 48 and 96 months (no control group). |
Mandressi 1983.
| Study characteristics | |
| Methods |
Study design: double‐blind parallel‐group randomized trial Study dates: not available Setting: outpatient, single center, national Country: Italy |
| Participants |
Inclusion criteria:
Exclusion criteria: not available Sample size: 60 randomized participants Age (years): not available IPSS score (baseline): not available Prostate size: not available |
| Interventions |
Group 1 (n = 19): Serenoa repens (Permixon 160 mg twice daily) Group 2 (n = 15): placebo (capsule) Group 3 (n = 15): Pygeum africanum Co‐interventions: none |
| Outcomes |
This study did not report any outcomes relevant to this review. Other outcomes: individual symptom scales |
| Notes |
Funding sources: not available Declarations of interest: not available |
Marks 2000.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: September 1997 to January 1998 Setting: outpatient Country: USA |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 44 participants Age (years): Group 1 mean (SD): 65.1 (8.1) Group 2 mean (SD): 62.9 (9.3) IPSS score (baseline): Group 1 mean (SD): 18.1 (7.2) Group 2 mean (SD): 16.6 (5.3) Prostate size (cm3): Group 1 mean (SD): 58.5 (29.8) Group 2 mean (SD): 55.6 (26.7) |
| Interventions |
Group 1 (n = 21): saw palmetto berry lipoidal extract and other herbs (nettle root extract 80 mg; pumpkin seed oil extract 160 mg; lemon extract 33 mg; vitamin A 190 international units), 106 mg, 3 times daily with meals, orally for 6 months Group 2 (n = 23): placebo capsules (glycerin, yellow wax, lecithin, and soybean oil), 3 times daily with meals, orally for 6 months Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes: postvoid residual urine volume, total serum PSA, free serum PSA, % free PSA, testosterone, dihydrotestosterone, estradiol, whole prostate volume, transition zone prostate volume, Qmax, urinary retention |
| Notes |
Funding sources: supported by an unrestricted educational grant from the Nutrilite Division of Amway Corp., Buena Park, California. Financial interest and/or other relationship with Nutrilite Division of Amway Corp. Declarations of interest: saw palmetto extract capsules were provided and blinding was done by the Nutrilite Division of Amway Corp., Buena Park, California |
Metzker 1996.
| Study characteristics | |
| Methods |
Study design: double‐blind, parallel‐group randomized trial Study dates: not available Setting: inpatient, single center, national Country: Germany |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 40 randomized participants Age (years): Group 1 mean (SD): 66.0 (52.4 to 84.0) Group 2 mean (SD): 65.1 (52.8 to 80.6) IPSS score (baseline): Group 1 mean (SD): 18.6 (15.0 to 22.2) Group 2 mean (SD): 19.0 (15.1 to 22.9) Prostate size: not available |
| Interventions |
Group 1 (n = 20): Sabal‐Urtica (Prostagutt forte) capsule twice a day for 1 year. 1 capsule of Prostagutt forte contains: WS 1473 extract of saw palmetto fruits, 160 mg and WS 1031 dry extract of nettle root, 120 mg.
Group 2 (n = 20): placebo capsule twice a day for 1 year Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: blood pressure, Qmax, residual volume |
| Notes |
Funding sources: not available Conflicts of interest: not available After 24 weeks, all men were offered the interventional treatment (no control group). |
Morgia 2014.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: March 2011 to March 2012 Setting: outpatient, multicenter and national Country: Italy |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 225 randomized participants Age (years): Group 1 median (range): 65 (56 to 76) Group 2 median (range): 66 (56 to 79) IPSS score (baseline): Group 1 median (range): 20 (12 to 35) Group 2 median (range): 19 (12 to 33) Prostate size: Group 1 median (range): 45 (20 to 60) Group 2 median (range): 45 (20 to 60) |
| Interventions |
Group 1 (n = 75): Serenoa repens 320 mg, selenium, lycopene (Profluss) for 1 year Group 2 (n = 79): placebo for 1 year Co‐interventions: tamsulosin 0.4 mg |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: International Index of Erectile Function (IIEF‐5), PVR, prostate volume, PSA and Ejaculation Questionnaire (EjQ), Qmax |
| Notes |
Funding sources: Konpharma provided support for this study Declarations of interest: the authors declare no conflicts of interest. This study has been designed and conducted independently. |
Preuss 2001.
| Study characteristics | |
| Methods |
Study design: parallel‐group, double‐blind, placebo‐controlled randomized trial Study dates: not available Setting: outpatient, multicenter, national Country: USA |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 144 randomized participants Age (years): not available AUASI score (baseline): Group 1 mean (SD): 18.9 Group 2 mean (SD): 17.7 Prostate size: not available |
| Interventions |
Group 1 (n = 75): Cernitin 378 mg, saw palmetto complex and phytosterol (saw palmetto fruit standardized to 40% to 50% free fatty acids and B‐sitosterol standardized to 43%) 286 mg, and vitamin E 100 international units once a day for 3 months Group 2 (n = 69): placebo once a day for 3 months Co‐interventions: none |
| Outcomes |
Urinary symptoms
Adverse events
Other outcomes measured in the trial: PSA, Qmax, and nocturia |
| Notes |
Funding sources: Rexall/Sundown, Inc., Boca Raton, FL through the National Research Council for Health, Washington, DC and Meridian ID Declarations of interest: not available |
Reece Smith 1986.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: inpatient, multicenter, national Country: UK |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 70 randomized participants Age (years): Group 1 mean (SD): 66.15 (5.86) Group 2 mean (SD): 67.03 (6.03) IPSS score (baseline): not available Prostate size: not available |
| Interventions |
Group 1 (n = 33): Permixon (2 capsules of 160 mg) once a day for 12 weeks. Contains fruits of Serenoa repens, a kind of palm tree Group 2 (n = 37): placebo once a day for 12 weeks Co‐interventions: none |
| Outcomes |
Adverse events
Other outcomes measured in the trial: blood studies, urinary retention, and Qmax. |
| Notes |
Funding sources: not available Declarations of interest: not available |
Ryu 2015.
| Study characteristics | |
| Methods |
Study design: randomized controlled trial Study dates: March 2012 to March 2013 Setting: outpatient, single center, national Country: Korea |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 120 randomized participants Age (years): Group 1 mean (SD): 62.5 (1.21) Group 2 mean (SD): 63.4 (1.44) IPSS score (baseline): Group 1 mean (SD): 19.6 (0.73) Group 2 mean (SD): 20 (0.85) Prostate size: Group 1 mean (SD): 30.1 (0.93) Group 2 mean (SD): 30.2 (0.67) |
| Interventions |
Group 1 (n = 60): Serenoa repens (Permixon) 320 mg/day for 12 months Group 2 (n = 60): only the co‐intervention Co‐interventions: tamsulosin 0.2 mg/day |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: PSA and prostate volume, Qmax |
| Notes |
Funding sources: not available Declarations of interest: not available |
Shi 2008.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: not available Setting: outpatient, multicenter, national Country: China |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 94 randomized participants Age (years): Group 1 mean (SD): 65.91 (63.77 to 68.05) Group 2 mean (SD): 64.04 (61.57 to 66.52) IPSS score (baseline): Group 1 mean (SD): 16.85 (14.92 to 18.77) Group 2 mean (SD): 14.46 (13.13 to 15.78) Prostate size: Group 1 mean (SD): 47.72 (45.38 to 50.06) Group 2 mean (SD): 48.38 (46.25 to 50.52) |
| Interventions |
Group 1 (n = 46): 2 Prostataplex soft gels daily for 12 weeks. The main active ingredient of Prostataplex soft gel is saw palmetto. Other components include soybean oil, beeswax, soy lecithin, gelatin, glycerin, deionized water, titanium dioxide, US Federal Food, Drug and Cosmetic Act carmine red, and natural vanilla flavor. Group 2 (n = 48): 2 placebo soft gels for 12 weeks. The main ingredient of the placebo soft gel was corn oil. Other components include gelatin, glycerin, purified water and titanium dioxide, and the Federal Food, Drug and Cosmetic Act red, yellow, and blue. Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial: PSA, BUN and creatinine, Qmax and urinary retention |
| Notes |
Funding sources: not available Declarations of interest: not available |
Sudeep 2020.
| Study characteristics | |
| Methods |
Study design: double‐blind, parallel‐group randomized trial Study dates: not available Setting: outpatient, single center, national Country: India |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 99 randomized participants Age (years): Group 1 mean (SD): 57.76 (7.25) Group 2 mean (SD): 55.18 (8.56) Group 3 mean (SD): 57.48 (7.04) IPSS score (baseline): Group 1 mean (SD): 20.00 (4.41) Group 2 mean (SD): 20.00 (3.74) Group 3 mean (SD): 19.45 (3.82) Prostate size: not available |
| Interventions |
Group 1 (n = 33): saw palmetto extract VISPO (containing 3% beta‐sitosterol ‐ 200 mg of Serenoa repens extract) 500 mg doses twice daily for 12 weeks Group 2 (n = 33): placebo (maltodextrin) for 12 weeks Group 3 (n = 33): saw palmetto oil SPO (0.2% beta‐sitosterol ‐ 200 mg of Serenoa repens extract) for 12 weeks Co‐interventions: none |
| Outcomes |
Urinary symptoms
Adverse events
Other outcomes measured in the trial: urinary retention, Qmax, PSA, 5‐alpha‐reductase, testosterone, AMS, and ADAM |
| Notes |
Funding sources: Vidya Herbs Private Ltd Declarations of interest: none |
Willetts 2003.
| Study characteristics | |
| Methods |
Study design: parallel‐group, double‐blind randomized controlled trial Study dates: January 1999 to March 2000 Setting: outpatient, national Country: Australia |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 100 participants Age (years): Group 1 mean (SEM): 62.1 (1.2) Group 2 mean (SEM): 63.9 (1.3) IPSS score (baseline): not reported Prostate size: not reported |
| Interventions |
Group 1 (n = 50): Serenoa repens extract (2 x 160 mg of carbon dioxide extract; Blackmores Ltd, Sydney, Australia) 2 capsules per day for 12 weeks Group 2 (n = 50): placebo (paraffin oil), 2 capsules per day for 12 weeks Co‐interventions: none |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events
Other outcomes measured in the trial were: Qmax and sexual function |
| Notes |
Funding sources: Blackmores Ltd provided funding for this study Declarations of interest: not available |
Ye 2019.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized trial Study dates: March 2014 to June 2016 Setting: outpatient, multicenter, national Country: China |
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 354 Age (years): Group 1 mean (SD): 61.47 (± 5.20) Group 2 mean (SD): 60.32 (± 5.96) IPSS/AUASI score (baseline): Group 1 mean (SD): 14.42 (± 3.88) Group 2 mean (SD): 14.34 (± 4.08) Prostate size: Group 1: 37.01 (± 19.68) Group 2: 37.30 (± 25.40) |
| Interventions |
Group 1 (n = 159): Serenoa repens extract 320 mg (160 mg twice daily soft capsule) manufactured by TAD Pharma GmbH Group 2 (n = 166): placebo 160 mg twice daily soft capsule |
| Outcomes |
Urinary symptoms
Quality of life
Adverse events:
Other outcomes measured in the trial: voiding symptoms, prostate volume, urinary frequency, and total PSA level. Other parameters assessed were MSF‐4 score, IIEF, and Qmax. |
| Notes |
Funding sources: Serenoa Repens was provided by TAD Pharma GmbH Declarations of interest: the authors report no conflicts of interest |
AUASI: American Urological Association Symptom Index; BPH: benign prostatic hyperplasia; BUN: blood urea nitrogen; CFU: colony‐forming units; HCL: hydrochloride; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; MedDRA: Medical Dictionary for Regulatory Activities; MFR: maximum flow rate; MSF‐4: Male Sexual Function 4‐item; NIH‐CPSI: National Institutes of Health Chronic Prostatitis Symptom Index; PSA: prostate‐specific antigen; PVR: postvoid residual; Qmax: peak urinary flow; QoL: quality of life; SD: standard deviation; SEM: standard error of the mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adriazola Semino 1992 | Wrong study design/comparison: Serenoa repens vs active control (Prazosin). No indication of randomization |
| Al‐Shukri 2000 | Wrong study design: non‐randomized study |
| Alcaraz 2022 | Wrong study design: observational study |
| Ali 2020 | Wrong comparison: Serenoa repens vs alpha‐blockers |
| Aliaev 2009 | Wrong study population: men had chronic abacterial prostatitis |
| Authié 1987 | Wrong study design: non‐randomized study |
| Bartsch 1998 | Wrong comparison: Serenoa repens vs finasteride |
| Braeckman 1997 | Wrong comparison: different doses of Serenoa repens |
| Cai 2013 | Wrong comparison: IDIProst vs Serenoa repens |
| Carraro 1996 | Wrong comparison: Serenoa repens vs finasteride |
| Comar 1986 | Comparison with mepartricin |
| CTRI/2012/10/003049 | Wrong comparison: different doses of phytotherapy |
| CTRI/2020/09/027521 | Wrong comparison: Serenoa repens vs SAW‐1720 |
| Debruyne 2002 | Wrong comparison: with alpha‐blocker |
| Di Maida 2020 | Wrong study design: non‐randomized comparative study |
| Di Silverio 1992 | Tissue study investigating the antiestrogenic effect of Serenoa repens vs placebo |
| Duborija‐Kovacevic 2010 | Wrong comparison: Serenoa repens vs doxazosin and finasteride |
| Engelmann 2006 | Wrong comparison: Serenoa repens vs tamsulosin |
| EUCTR2011‐005307‐33‐FR | Wrong comparison: Serenoa repens vs tamsulosin |
| Gerber 1998 | Wrong study design: no control group |
| Giannakopoulos 2002 | Wrong study design: no control group |
| Giulianelli 2012 | Wrong study design: observational study |
| Grasso 1995 | Wrong comparison: Serenoa repens vs alpha‐blocker |
| Gurzhenko 2020 | Wrong study design: observational study |
| Guzman 2016 | Wrong comparison: D‐004 vs Serenoa repens |
| Hamdv 1997 | Wrong comparison: Serenoa repens vs finasteride |
| Ju 2015 | Wrong study design: no comparison |
| Latil 2015 | Wrong comparison: Serenoa repens vs alpha‐blockers |
| Morgia 2013 | Wrong study population: men subject to histologic sampling |
| Morgia 2018 | Wrong comparison: Serenoa repens vs tadalafil |
| NCT00797394 | Wrong comparison: Serenoa repens as a co‐intervention |
| Pannunzio 1986 | Wrong comparison: Serenoa repens vs gestonorone caproate |
| Pavone 2010 | Wrong study design: not a randomized controlled trial |
| Pecoraro 2004 | Wrong population: study focused on the use of the drug as preoperative adjuvant treatment |
| Popa 2005 | Wrong study design: independent re‐analysis of the included study Metzker 1996 |
| Romaniuk 2013 | Wrong comparison: Serenoa repens vs doxazosin and finasteride |
| Roveda 1994 | Wrong comparison: Serenoa repens oral vs rectal route |
| Sinescu 2011 | Wrong study design: non‐randomized study |
| Stepanov 1999 | Wrong study design: no control group |
| Strauch 1994 | Wrong comparison: Serenoa repens vs finasteride |
| Suardi 2014 | Wrong patient population: men with prostatitis |
| Sökeland 1997 | Wrong comparison: Serenoa repens vs finasteride |
| Taieb 2010 | Wrong study design: observational study |
| Vela‐Navarrete 2005 | Study focused on tissue samples of the prostate. |
| Veltri 2002 | Wrong study population: study focused on prostatic tissue |
| Vinarov 2010 | Wrong study design: no active or placebo control |
| Weisser 1997 | Wrong study population: prostatic tissue (enzyme study) |
| Yamanishi 2004 | Wrong comparison: Serenoa repens vs alpha‐blockers |
| Zlotta 2005 | Wrong study design: re‐analysis of previous studies |
Characteristics of studies awaiting classification [ordered by study ID]
Aliaev 2007.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Anonymous 2005.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Bercovich 2010.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Buck 2002.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Carreras 1987.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Cukier 1985.
| Methods | Multisite study Randomization: numbered or coded identical containers administered sequentially Blinding: participants, providers |
| Participants | Geographic region: France Study setting: community N = 168 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 69 (NR) years Race: white Diagnostic criteria: patients with "prostatism" or for whom surgery was not indicated (no mechanical or infectious complications) |
| Interventions | Control: matching placebo Treatment: Permixon 160 mg twice daily Study duration: 10 weeks Lost to follow‐up: 13% |
| Outcomes | Symptom score (# of daily mictions) Dysuria (4‐point scale) Bladder residual volume Nocturia Dropouts due to side effects: NR |
| Notes | Exclusions: symptoms for at least 6 months Full text not available for this update. |
Dathe 1991.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Diehl 2005.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Emili 1983.
| Methods | Single‐site study Randomization: noted, but method not stated Blinding: participants, providers |
| Participants | Geographic region: Italy Study setting: community N = 30 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): NR (44 to 78) years Race: white Diagnostic criteria: men with manageable BPH |
| Interventions | Control: matching placebo Treatment: Permixon 160 mg twice daily Study duration: 4 weeks Lost to follow‐up: n = 0 |
| Outcomes | Peak urine flow Mean urine flow Bladder residual volume Prostate size (qualitative scale used) Nocturia Dropouts due to side effects: none |
| Notes | Exclusions: prior treatment for BPH Full text not available for this update. |
Fabricius 1993.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Gabric 1987.
| Methods | Multisite study Randomization: unclear Blinding: participants, providers |
| Participants | Geographic region: Croatia Study setting: community N = 30 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): 65 (40 to 82) years Race: white Diagnostic criteria: BPH, stages I, II (Vahlensieck) |
| Interventions | Control: placebo Treatment: Prostagutt forte (SR + Urtica dioica) 20 drops 3 times daily Study duration: 6 weeks Lost to follow‐up: none |
| Outcomes | Physician rating of improvement Peak urine flow Bladder residual volume Dropouts due to side effects: none |
| Notes | Exclusions: stage IV prostate adenoma, bacterial prostatitis, cystitis, urethritis Full text not available for this update. |
Green 2000.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Löbelenz 1992.
| Methods | Multisite study Randomization: computer‐generated randomization code Blinding: participants, providers |
| Participants | Geographic region: Germany Study setting: community N = 60 Baseline IPSS: NR Baseline prostate volume: NR Mean age (range): NR (48 to 82) years Race: white Diagnostic criteria: BPH, stages I, II; peak urine flow < 20 mL/s |
| Interventions | Control: matching placebo Treatment: Sabal extract 100 mg daily Study duration: 6 weeks Lost to follow‐up: n = 0 |
| Outcomes | Peak urine flow Mean urine flow Dropouts due to side effects: n = 0 |
| Notes | Exclusions: NR Full text not available for this update. |
Martínez 1987.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Mattei 1990.
| Methods | Single‐site study Randomization: noted, but method not stated Blinding: participants, providers |
| Participants | Geographic region: Italy Study setting: community N = 40 Baseline symptom score: NR Baseline prostate volume: Talso (SR extract) 36 mm (diameter); placebo 37 mm Mean age (range): NR (45 to 72) years Race: white Diagnostic criteria: men with manageable BPH |
| Interventions | Control: matching placebo Treatment: Talso (SR extract) 160 mg twice daily Study duration: 13 weeks Lost to follow‐up: 5% |
| Outcomes | Dysuria (symptom score 0 to 4) Bladder residual volume (incomplete emptying ‐ symptom score 0 to 4) Discomfort (pollakiuria ‐ symptom score 0 to 4) Daytime frequency Nocturia Prostate size Dropouts due to side effects: 1 participant from each group due to "stomach pains"; relation to therapy unclear |
| Notes | Exclusions: urogenital disease, prostate cancer Full text not available for this update. |
Mohanty 1999.
| Methods | Site not described. Randomization not described. Blinding: double‐blind |
| Participants | Geographic region: NR Study setting: clinic N = 75 Baseline modified Boyarsky: NR Baseline prostate volume: SR 28.78 mL; placebo 29.89 mL Mean age (range): NR (40 to 90) years Race: NR Diagnostic criteria: BPH grades I or II; symptomatic; without surgical indication; took no BPH drug treatment for last 30 days |
| Interventions | Control: matching placebo, 1 capsule twice daily Treatment: SR, 1 capsule twice daily Study duration: 2 months Lost to follow‐up: SR n = 2; placebo n = 0 |
| Outcomes | Modified Boyarsky (range 0 to 27; higher score indicates worse symptoms) Frequency Nocturia Peak urine flow Residual volume Prostate size (ultrasound) Adverse events |
| Notes | Exclusions: men with prostate conditions other than BPH (carcinoma of the prostate, infective prostatitis); serious renal, hepatic, and cardiac conditions Full text not available for this update. |
Neumann 1993.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Razumov 2001.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full text not available. |
Sekikawa 2020.
| Methods | Double‐blind randomized controlled trial |
| Participants |
Key inclusion criteria:
Key exclusion criteria:
|
| Interventions | Intervention: capsule containing saw palmetto extract with 200 mL of water daily for 8 weeks Control: placebo, same posology |
| Outcomes |
Primary outcomes IPSS: change after 8 weeks Key secondary outcomes*
*1,2,3 Assess these at screening (before consumption) and 4 and 8 weeks after consuming. *4 Assess at screening (before consumption) and 8 weeks after consuming. |
| Notes |
Tasca 1985.
| Methods | Single‐site trial Randomization: noted, but method not stated Blinding: participants, providers |
| Participants | Geographic region: Italy Study setting: community N = 30 Baseline symptom score: NR Baseline prostate volume: NR Mean age (range): 61.5 (49 to 81) years Race: white Diagnostic criteria: stage I and II prostatic adenomas |
| Interventions | Control: matching placebo Treatment: PA109 (Permixon) 160 mg twice daily Study duration: 8 weeks Lost to follow‐up: 10% |
| Outcomes | Dysuria (% reporting) Peak urine flow Mean urine flow Total voided volume Pollakiuria ‐ daytime (% reporting) Pollakiuria ‐ nocturnal (% reporting) Urgency (% reporting) Dropouts due to side effects: 1 participant from the treatment group |
| Notes | Exclusions: details not provided Full text not available for this update. |
Tkachuk 2002.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full‐text not available |
Vahlensieck 1993.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full‐text not available for this update |
Vinarov 2009.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full‐text not available |
Wehr 1995.
| Methods | Not available |
| Participants | Not available |
| Interventions | Not available |
| Outcomes | Not available |
| Notes | Full‐text not available |
IPSS: International Prostate Symptom Score
Characteristics of ongoing studies [ordered by study ID]
ISRCTN84633360.
| Study name | Permixon (R) 320 mg once a day open label treatment for patients participating in the placebo controlled study |
| Methods | Randomized controlled trial |
| Participants | Participant inclusion criteria: not available. However, condition of interest was benign prostatic hyperplasia. |
| Interventions | Permixon 320 mg once a day vs placebo |
| Outcomes | Primary outcome measure: not provided at time of registration Secondary outcome measures: not provided at time of registration |
| Starting date | Recruitment start date: 20 February 2000 Recruitment end date: 19 February 2004 |
| Contact information | Mr T Terry University Hospitals of Leicester c/o Research and Development Office Leicester General Hospital NHS Trust Leicester LE1 4PW United Kingdom +44 (0)116 258 4109 research@lri.org.uk (contacted on 30 December 2022) |
| Notes |
JPRN‐UMIN000023274.
| Study name | A clinical trial to investigate the effect of the foods containing plant extract |
| Methods | Double‐blind randomized controlled trial |
| Participants |
Inclusion criteria: men aged 50 to 80 giving written informed consent Key exclusion criteria:
|
| Interventions | Intervention: food containing plant extract for 8 weeks Control: food without plant extract for 8 weeks |
| Outcomes |
Primary outcomes Change from baseline of IPSS total score at 4‐ and 8‐week consumption Key secondary outcomes
|
| Starting date | 23 July 2016 |
| Contact information | Masayuki Sugimoto
Email: info.food@kb‐clinic.com Takashi Sone Email: t.sone@kb‐clinic.com (contacted both on 30 December 2022) |
| Notes |
JPRN‐UMIN000027902.
| Study name | A verification study on improvement of problems with urination due to ingestion of the food containing saw palm extracts |
| Methods | Double‐blind randomized controlled trial |
| Participants |
Key inclusion criteria:
Key exclusion criteria:
|
| Interventions | Intervention: saw palmetto extracts containing capsule B, once a day for 12 weeks. Swallowing a capsule after breakfast with water or warm water (approximately 200 mL) without chewing. If the participant forgets to ingest the test food, they would take it as soon as they remember within the day. Control: placebo, same posology for 12 weeks |
| Outcomes |
Primary outcomes IPSS: assessed at 0, 4, 8, and 12 weeks after consuming Key secondary outcomes* 1. King's Health Questionnaire (KHQ) 2. Overactive Bladder Symptom Score (OABSS) 3. Subjective symptoms 3‐1. Visual analogue scale: pleasantness of waking, sleep quality 3‐2. Diary: frequency of urination per day, frequency of urination at night 3‐3. Interview sheet: time required for urination *1,2 Assessed at 0, 4, 8, and 12 weeks after consuming. *3‐2 Keep it from a week before consuming to 12 weeks after consuming. *3‐1,3‐3 Assessed at 0 and 12 weeks after consuming. |
| Starting date | 24 June 2017 |
| Contact information | Kazuo YAMAMOTO
CEO, ORTHOMEDICO Inc.
2F Sumitomo Fudosan Korakuen Bldg., 1‐4‐1 Koishikawa, Bunkyo‐ku, Tokyo
Telephone: 03‐3818‐0610
Email: kazu@orthomedico.jp (contacted on 30 December 2022) |
| Notes | The description of this study is identical to the registry of Sekikawa 2020. |
NCT00497939.
| Study name | The effectiveness of saw palmetto and sanmiaoshan on benign prostatic hyperplasia in Chinese patients |
| Methods | Cross‐over randomized controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Saw palmetto and sanmiaoshan capsule plus alpha‐blockers |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | January 2006 |
| Contact information | Annie YF Wong
Tel: (852) 2632 2501
anniewong@surgery.cuhk.edu.hk (contacted on 30 December 2022) |
| Notes |
NCT02121613.
| Study name | PERmixon® in LUTS Evaluation Study (PERLES) |
| Methods | Quadruple‐blinded randomized controlled trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental: Permixon 160 mg and placebo Drug: Permixon 160 mg. Oral administration, 160 mg twice daily Drug: placebo matching Permixon 160 mg. Oral administration, twice daily Drug: placebo matching Tamsulosine LP. Oral administration, daily Placebo comparator: placebo Drug: placebo matching Permixon 160 mg. Oral administration, twice daily Drug: placebo matching Tamsulosine LP. Oral administration, daily Tamsulosine LP and placebo: active control arm Drug: Tamsulosine LP. Oral administration, 0.4 mg daily Drug: placebo matching Permixon 160 mg. Oral administration, twice daily |
| Outcomes | IPSS score change from baseline to 180 days |
| Starting date | Completed study (unpublished) |
| Contact information | Study Director: Karim Keddad, MD (Pierre Fabre Medicament) No other contact information available. |
| Notes |
DRE: digital rectal examination; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; QoL: quality of life
Differences between protocol and review
2023 update: The Methods section has been extensively modified to fit current methodological standards, including the incorporation of RoB 2 for risk of bias assessment (we chose a 'replacement approach' as defined by Methodological Expectations of Cochrane Intervention Reviews (MECIR) criteria UR3). The previous version of the protocol can be found in Appendix 3. This is a similar approach to that used in other updates (Franco 2021).
We adjusted the wording of the Objectives to fit those of other reviews by the Cochrane Review Group, and we focused on the main critical outcomes for pharmacological treatments: urologic symptoms, quality of life, and adverse events. We provided greater specifications as to the study designs and population included in our update. We narrowed the scope of the comparisons to placebo or no treatment.
2012 update: The previous authors added adverse events (harms) to Secondary outcomes.
Contributions of authors
Research conception and design: Juan Víctor Ariel Franco and Jae Hung Jung.
Data acquisition: Camila Micaela Escobar Liquitay (search strategy), Leonel Fabrizio Trivisonno, Cecilia Fieiras, Nadia Sgarbossa, and Gustavo Ariel Alvez.
Statistical analysis: Juan Víctor Ariel Franco and Jae Hung Jung.
Data analysis and interpretation: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript: All authors.
Administrative, technical, or material support: Juan Víctor Ariel Franco.
Supervision: Juan Víctor Ariel Franco.
Approval of the final manuscript: All authors.
Sources of support
Internal sources
-
Instituto Universitario Hospital Italiano de Buenos Aires, Argentina
Provides a salary for her work in Cochrane to CMEL
-
Heinrich‐Heine‐University Düsseldorf, Germany
Provides a salary for his work in Cochrane to JVAF
External sources
No sources of support provided
Declarations of interest
JVAF: none known.
LT: none known.
NJS: none known.
GAA: none known.
CF: none known.
CMEL: none known.
JHJ: none known.
JVAF and JHJ are contact editors for the Cochrane Urology Group but were excluded from the editorial processing of this article.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Argirović 2013 {published data only}
- Argirovic A, Argirovic D. Does the addition of Serenoa repens to tamsulosin improve its therapeutical efficacy in benign prostatic hyperplasia? Vojnosanit Pregl 2013;70(12):1091-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Argirovic D. Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: The Comb TAMSR trial. European Urology Supplements 2009;8(8):574-574. [Google Scholar]
Barry 2011 {published data only}
- Andriole GL, McCullum-Hill C, Sandhu GS, Crawford ED, Barry MJ, Cantor A. The effect of increasing doses of saw palmetto fruit extract on serum prostate specific antigen: analysis of the CAMUS randomized trial. Journal of Urology 2013;189(2):486‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avins AL, Lee JY, Meyers CM, Barry MJ. Safety and toxicity of saw palmetto in the CAMUS trial. Journal of Urology 2013;189(4):1415-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MJ, Meleth S, Lee JY, Kreder KJ, Avins AL, Nickel JC, et al. Effect of increasing doses of Saw palmetto extract on lower urinary tract symptoms. JAMA 2011;306(12):1344-51. [clinicaltrials.gov ID: NCT00603304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor A, Barry M. Factors that affect men's feelings about their urination: findings from a multi-center clinical trial of phytotherapy to treat lower urinary tract symptoms. Clinical Trials (London, England) 2012;9(4):498. [Google Scholar]
- Crawford ED, Sullivan KF, Arangua P, Lee J, Lensing S, O'Donnell CI. Comparison of the american urological association symptom index with a short version, uwin, in a clinical phytotherapy trial. Journal of Urology 2012;187(4 SUPPL. 1):e700. [Google Scholar]
- Helfand BT, Lee JY, Sharp V, Foster H, Naslund M, Williams OD, et al. Associations between improvements in lower urinary tract symptoms and sleep disturbance over time in the CAMUS trial. Journal of Urology 2012;188(6):2288-2293. [DOI] [PubMed] [Google Scholar]
- Helfand BT, McVary KT, Meleth S, Sharp V, Foster H, Naslund M, et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. Journal of Urology 2011;185(6):2223-2228. [DOI] [PubMed] [Google Scholar]
- Helfand BT, Sharp V, Williams OD, Foster H, Naslund M, Lee J, et al. Improvements in lower urinary tract symptom severity are associated with improvements in sleep disturbance over time. Journal of Urology 2012;187(4):e703-e704. [Google Scholar]
- Lee J, Andriole G, Avins A, Crawford ED, Foster H, Kaplan S, et al. Redesigning a large-scale clinical trial in response to negative external trial results: the CAMUS study of phytotherapy for benign prostatic hyperplasia. Clinical Trials (London, England) 2009;6(6):628‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Barry M, Kusek J. Participant perception of treatment assignment is related to symptom severity in a clinical trial of phytotherapy. Clinical Trials 2012;9(4):521. [Google Scholar]
- Lee JY, Foster HE, McVary KT, Meleth S, Stavris K, Downey J, et al. Recruitment of participants to a clinical trial of botanical therapy for benign prostatic hyperplasia. Journal of Alternative and Complementary Medicine (New York, NY) 2011;17(5):469‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Moore P, Kusek J, Barry M. Treatment assignment guesses by study participants in a double-blind dose escalation clinical trial of saw palmetto. Journal of Alternative and Complementary Medicine (New York, NY) 2014;20(1):48‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00603304. Complementary and alternative medicine for urological symptoms (CAMUS). https://clinicaltrials.gov/ct2/show/NCT00603304 (first received 29 January 2008). [NCT00603304]
- Roehrborn C, McVary K, Nickel J, Crawford E, Kreder K, Naslund M, et al. The effect of increasing doses of a saw palmetto fruit extract on lower urinary tract symptoms attributed to benign prostatic hyperplasia: a randomized trial. Urology 2011;78(3 SUPPL. 1):S14. [Google Scholar]
BASTA 2010 {unpublished data only}
- Efficacy of two formulations of Sabal serrulata; a double-blind; randomized; placebo-controlled phase III study. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2006-003532-30 (Published 12 March 2010).
Bauer 1999 {published data only}
- Bauer HW, Casarosa C, Cosci M, Fratta M, Blessmann G. Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo-controlled double-blind study [Behandlung der benignen Prostatahyperplasie mit Sabalfrucht-Extrakt: Wirksamkeit und Verträglichkeit - Ergebnisse einer plazebokontrollierten Doppelblindstudie]. MMW Fortschritte der Medizin 1999;141(25):127-32. [PubMed] [Google Scholar]
Bent 2006 {published data only}
- Bent S, Kane C, Shinohara K, Neuhaus J, Hudes E, Goldberg H, et al. Saw palmetto for benign prostatic hyperplasia. The New England Journal of Medicine 2006;354(6):557-66. [CLINICALTRIALS.GOV: NCT00037154] [DOI: 10.1056/NEJMoa053085] [DOI] [PubMed] [Google Scholar]
- Bent S, Kane CJ, Shinohara K, Goldberg H, Neuhaus J, Avins A. A randomized controlled trial of saw palmetto for the treatment of benign prostatic hyperplasia. Journal of Urology 2005;173(4):443-443. [Google Scholar]
- NCT00037154. Saw Palmetto Extract in Benign Prostatic Hyperplasia. https://clinicaltrials.gov/ct2/show/NCT00037154 First Posted: May 17, 2002.
Boccafoschi 1983 {published data only}
- Boccafoschi C, Annoscia S. Serenoa repens extract and placebo in prostatic benign hyperplasia: clinical results [Confronto fra estratto di serenoa repens e placebo mediate prova clinica controllata in pazienti con adenomatosi prostatica]. Urologia 1983;50:1257-68. [DOI: 10.1177/039156038305000615] [DOI] [Google Scholar]
Carbin 1990 {published data only}
- Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. British Journal of Urology 1990;66(6):639-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Champault 1984 {published data only}
- Champault G, Patel JC, Bonnard AM. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. British Journal of Clinical Pharmacology 1984;18:461-2. [DOI: 10.1111/j.1365-2125.1984.tb02491.x] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulson 2013 {published data only}12610000168055
- ACTRN12610000168055. A study looking at the effectiveness of a oral herbal supplement for helping to manage the symptoms of enlarged prostate. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000168055 19/02/2010.
- Coulson S, Rao A, Beck SL, Steels E, Gramotnev H, Vitetta L. A phase II randomised double-blind placebo-controlled clinical trial investigating the efficacy and safety of ProstateEZE Max: a herbal medicine preparation for the management of symptoms of benign prostatic hypertrophy. Complementary Therapies in Medicine 2013;321(3):172-9. [ANZCTR: 12610000168055] [DOI: 10.1016/j.ctim.2013.01.007] [PMID: ] [DOI] [PubMed]
Descotes 1995 {published data only}
- Descotes JL, Rambeaud JJ, Deschaseaux P, Faure G. Placebo-controlled evaluation of the efficacy and tolerability of Permixon® in benign prostatic hyperplasia after the exclusion of placebo responders. Clinical Drug Investigation 1995;5:291-7. [Google Scholar]
Gerber 2001 {published data only}
- Gerber GS, Kuznetsov D, Johnson BC, Burstein JD. Randomized, double-blind, placebo-controlled trial of saw palmetto in men with lower urinary tract symptoms. Urology 2001;58(6):960-4. [DOI: 10.1016/s0090-4295(01)01442-x] [DOI] [PubMed] [Google Scholar]
Glémain 2002 {published data only}
- Glémain P, Coulange C, Billebaud T, Gattegno B, Muszynski R, Loeb G, et al. Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: the OCOS trial [Tamsulosine avec ou sans Serenoa repens dans l'hypertrophie bénigne de la prostate: l'essai OCOS]. Progrès en Urologie 2002;12:395-403. [PubMed] [Google Scholar]
Hizli 2007 {published data only}
- Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. International Urology and Nephrology 2007;39:879-86. [DOI] [PubMed] [Google Scholar]
Hong 2009 {published data only}
- Hong H, Kim CS, Lee JS, Maeng WJ. Effects of Saw Palmetto Extracted Oil Supplementation on Human Benign Prostatic Hyperplasia. FASEB Journal 2008;22:1108.6-1108.6. [Google Scholar]
- Hong H, Kim CS, Maeng S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutrition Research and Practice 2009;3(4):323-7. [DOI: 10.4162/nrp.2009.3.4.323] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iacono 2015 {published data only}
- Iacono F, Ruffo A, Prezioso D, Romeo G, Illiano E, Romis L, et al. Combined treatment with Tradamixina® and Serenoa repens decreases PSA levels and prostate inflammation, improving the lower urinary tract symptoms (LUTS). A randomized, double-blind, placebo-controlled study on 185 patients: PS-04-009. Journal of Sexual Medicine 2015;2(Suppl 3):201. [DOI: 10.1111/jsm.12872_2] [DOI]
Lopatkin 2005 {published data only}
- Lopatkin N, Sivkov A, Schläfke S, Funk P, Medvedev A, Engelmann U. Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms—long-term follow-up of a placebo-controlled, double-blind, multicenter trial. International Urology and Nephrology 2007;39(4):1137‐1146. [DOI] [PubMed] [Google Scholar]
- Lopatkin N, Sivkov A, Walther C, Schläfke S, Medvedev A, Avdeichuk J, et al. Combined extract of sabal palm and nettle in the treatment of patients with lower urinary tract symptoms in the double blind, placebo-controlled trial. World Journal of Urology 2005;23(2):139-46. [DOI] [PubMed] [Google Scholar]
- Lopatkin N, Sivkov A, Walther C, Schläfke S, Medvedev A, Avdeichuk J, et al. Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms—a placebo-controlled, double-blind, multicenter trial. World Journal of Urology 2005;23(2):139‐146. [DOI] [PubMed] [Google Scholar]
- Lopatkin NA, Sivkov AV, Medvedev AA, Walter K, Schlefke S, Avdeĭchuk IuI, et al. Combined extract of Sabal palm and nettle in the treatment of patients with lower urinary tract symptoms in double blind, placebo-controlled trial. Urologiia (Moscow, Russia : 1999) 2006;(2):12, 14‐9. [PubMed] [Google Scholar]
- Sivkov A, Köhler S, Engelmann U, Medvedev A, Lopatkin N. Long-term efficacy and safety of a combination of sabal and urtica extracts in LUTS—a placebo-controlled, double-blind, multicenter trial. Urologe A 2001;40 (Suppl 1):S19. [Google Scholar]
Mandressi 1983 {published data only}
- Mandressi S, Tarallo U, Maggioni A, Tombolini P, Rocco F, Quadraccia. Medical treatment of benign prostatic hyperplasia: efficacy of the extract of Serenoa repens (Permixon®) compared to that of the extract of Pygeum africanum and a placebo [Terapia medica dell'adenoma prostatico: confronto della efficacia dell'estratto di serenoa repens (Permixon®) versus l'estratto di pigeum africanum e placebo]. Urologia 1983;50(4):752-8. [Google Scholar]
Marks 2000 {published data only}
- Marks LS, Partin AW, Epstein JI, Tyler VE, Simon I, Macairan ML, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. The Journal of Urology 2000;163(5):1451-6. [PubMed] [Google Scholar]
- Sokoll LJ, Marks LS, Mikolajczyk SD, Bruzek DJ, Mangold LA, Rittenhouse HG, et al. Effects of finasteride and saw palmetto on BPSA, propsa, and CPSA in men with benign prostatic hyperplasia. Journal of Urology 2004;171(4):438-439. [Google Scholar]
Metzker 1996 {published data only}
- Metzker H, Kieser M, Holscher U. Efficacy of a Sabal-Urtica Combination Drug in the Treatment of Benign Prostatic Hyperplasia (BPH) [Wirksamkeit eines Sabal-Urtica-kombinationspraparates bei der behandlung der benignen prostatahyperplasie (BPH)]. Der Urologe B 1996;36(4):292-300. [Google Scholar]
Morgia 2014 {published data only}ISRCTN78639965
- ISRCTN78639965. Serenoa repens, lycopene and selenium vs. tamsulosin for the treatment of lower urinary tract symptoms (LUTS)/ Benign prostatic hyperplasia (BPH). https://www.isrctn.com/ISRCTN78639965 Registration date: 11/12/2013.
- Morgia G, Russo GI, Voce S, Palmieri F, Gentile M, Giannantoni A, et al. Serenoa repens, lycopene and selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicenter double-blinded randomized study between single or combination therapy (PROCOMB trial). Prostate 2014;74(15):1471-80. [DOI: 10.1002/pros.22866] [PMID: ] [DOI] [PubMed]
Preuss 2001 {published data only}
- Preuss HG, Marcusen C, Regan J, Klimberg IW, Welebir TA, Jones WA. Randomized trial of a combination of natural products (Cernitin, saw palmetto, beta-sitosterol, vitamin E) on symptoms of benign prostatic hyperplasia (BPH). International Urology and Nephrology 2001;33:217-25. [DOI] [PubMed] [Google Scholar]
Reece Smith 1986 {published data only}
- Reece Smith H, Memon A, Smart CJ, Dewbury K. The value of Permixon in benign prostatic hypertrophy. British Journal of Urology 1986;58:36-40. [DOI] [PubMed] [Google Scholar]
Ryu 2015 {published data only}
- Ryu YW, Lim SW, Kim JH, Ahn SH, Choi JD. Comparison of tamsulosin plus serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Urologia Internationalis 94;2:187-93. [DOI: 10.1159/000366521] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shi 2008 {published data only}
- Shi R, Xie Q, Gang X, Lun J, Life C, Pantuck A, et al. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. The Journal of Urology 2008;179:610-5. [DOI] [PubMed] [Google Scholar]
Sudeep 2020 {published data only}
- Sudeep HV, Thomas JV, Shyamprasad K. A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urology 2020;20(1):86. [DOI: 10.1186/s12894-020-00648-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Willetts 2003 {published data only}
- Willetts KE, Clements MS, Champion S, Ehsman S, Eden JA. Serenoa repens extract for benign prostate hyperplasia: a randomized controlled trial. British Journal of Urology International 2003;92:267-70. [DOI] [PubMed] [Google Scholar]
Ye 2019 {published data only}
- ChiCTR-TRC-13003575. Effects of Saw Palmtto extract in binign prostatic hyperplasia and sexual function: a multicenter, randomized, double-blind, placebo-controlled trial. http://www.chictr.org.cn/showproj.aspx?proj=5985 10 September 2013.
- Ye Z, Huang J, Zhou L, Chen S, Wang Z, Ma L, et al. Efficacy and Safety of Serenoa repens Extract Among Patients with Benign Prostatic Hyperplasia in China: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. Urology 2019;129:172-179. [DOI: 10.1016/j.urology.2019.02.030] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adriazola Semino 1992 {published data only}
- Adriazola Semino M, Lozano Ortega JL, Garcia Cobo E, Tejeda Banez E, Romero Rodriguez F. Symptomatic treatment of benign hypertrophy of the prostate. Comparative study of prazosin and Serenoa repens [Tratamiento sintomatico de la hipertrofia benigna de prostata. Estudio comparativo entre prazosin y Serenoa repens]. Archivos Espanoles de Urologia 1992;45(3):211-3. [MEDLINE: ] [PubMed] [Google Scholar]
Alcaraz 2022 {published data only}
- Alcaraz A, Gacci M, Ficarra V, Medina-Polo J, Salonia A, Fernández-Gómez JM et al. Efficacy and Safety of the Hexanic Extract of Serenoa repens vs. Watchful Waiting in Men with Moderate to Severe LUTS-BPH: Results of a Paired Matched Clinical Study. Journal of Clinical Medicine 2022;11(4):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz A, Rodríguez-Antolín A, Carballido-Rodríguez J, Castro-Díaz D, Esteban-Fuertes M, Cózar-Olmo JM, et al. Clinical benefit of tamsulosin and the hexanic extract of serenoa repens, in combination or as monotherapy, in patients with moderate/severe luts-bph: A subset analysis of the qualiprost study. Journal of Clinical Medicine 2020;9(9):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2020 {published data only}
- Ali L, Hayat F, Opal S, Kahn S, Kahn Q, Tariq K. Comparison of phytotherapy with alpha adrenocepters blockers in the treatment of symptomatic benign prostatic hyperplasia. Journal of Postgraduate Medical Institute 2020;34(2):129-133. [Google Scholar]
Aliaev 2009 {published data only}
- Aliaev IG, Apolikhin OI, Mazo EB, Vinarov AZ, Lokshin KL, Medvedev AA, et al. Efficacy and safety of prostamol-Uno in patients with chronic abacterial prostatitis [ЭФФЕКТИВНОСТЬ И БЕЗОПАСНОСТЬ ПРОСТАМОЛА-УНО У БОЛЬНЫХ ХРОНИЧЕСКИМ АБАКТЕРИАЛЬНЫМ ПРОСТАТИТОМ]. Urologiia 2006;1:47-50. [PubMed] [Google Scholar]
Al‐Shukri 2000 {published data only}
- Al-Shukri SH, Deschaseaux P, Kuzmin IV. Early urodynamic effects of the lipido-sterolic extract of Serenoa repens (Permixon®) in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 2000;3:195-9. [DOI] [PubMed] [Google Scholar]
Authié 1987 {published data only}
- Authié D, Cauquil J. Appreciation of the efficacy of Permixon in daily practice: multicenter study [Appréciation de l'efficacité de Permixon en pratique quotidienne: étude multicentrique]. Comptes Rendus de Thérapeutique et de Pharmacologie Clinique 1987;5(56):4-13. [Google Scholar]
Bartsch 1998 {published data only}
- Bartsch G, Dreikorn K, Schontoger PS. Combined Sabal extract and Urtica extract versus finasteride in benign prostatic hyperplasia (stages I-II). UROLOGE A 1998;37(1):83-84. [DOI] [PubMed] [Google Scholar]
Braeckman 1997 {published data only}
- Braeckman J, Bruhwyler J, Vanderkerckhove K, Géczy J. Efficacy and safety of the extract of Serenoa repens in the treatment of benign prostatic hyperplasia: therapeutic equivalence between twice and once daily dosage forms. Phytotherapy Research 1997;1:558-63. [Google Scholar]
- Braeckman J, Denis L, Leval J, Keuppens F, Cornet A, De Bruyne, et al. A double-blind, placebo-controlled study of the plant extract Serenoa repens in the treatment of benign hyperplasia of the prostate. European Journal of Clinical Research 1997;9:247-59. [Google Scholar]
- Braeckman J. The extract of Serenoa repens in the treatment of benign prostatic hyperplasia: a multicenter open study. Current Therapeutic Research 1994;55(7):776-85. [Google Scholar]
Cai 2013 {published data only}
- Cai T, Morgia G, Carrieri G, Terrone C, Imbimbo C, Verze P, et al. An improvement in sexual function is related to better quality of life, regardless of urinary function improvement: results from the IDIProst Gold Study. Archivio italiano di urologia e andrologia 2013;85(4):184‐189. [DOI] [PubMed] [Google Scholar]
Carraro 1996 {published data only}
- Carraro JC, Raynaud JP, Koch G, Chisholm GD, Di Silverio F, Teillac P, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29(4):231-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carraro JC, Raynaud JP, Koch G, Chisholm GD, Di Silverio F, Teiucic P, et al. Extract of saw palmetto for treatment of symptomatic benign prostatic hyperplasia. Integrative Medicine 1998;1(1):39-40. [Google Scholar]
Comar 1986 {published data only}
- Comar OB, Di Rienzo A. Mepartricin versus Serenoa repens: double-blind study in 20 cases of benign prostate hypertrophy. Rivista Italiana di Biologia e Medicina 1986;6(2):122-5. [Google Scholar]
CTRI/2012/10/003049 {published data only}
- CTRI/2012/10/003049. A Clinical Study to Evaluate the Safety and Efficacy of Capsule Outflo in Patients with Benign Prostatic Hyperplasia. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2012/10/003049 Date of registration: 08-10-2012.
CTRI/2020/09/027521 {published data only}
- CTRI/2020/09/027521. A clinical study to assess the Efficacy and Safety of SAW-1720 in Benign Prostate Hyperplasia patients. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/09/027521 Date of registration: 01-09-2020.
Debruyne 2002 {published data only}
- Debruyne F, Boyle P, Calais Da Silva F, Gillenwater JG, Hamdy FC, Perrin P, et al. Evaluation of the clinical benefit of Permixon and tamsulosin in severe BPH patients—PERMAL study subset analysis [Evaluation du benefice clinique de Permixon® et de la tamsulosine dans le traitement de l'hypertrophie bénigne de la prostate (HBP) sévére: Analyse d'une sous-groupe de l'étude Permal]. European Urology 2004;45:773-80. [DOI] [PubMed] [Google Scholar]
- Debruyne F, Boyle P, Da Silva FC, Gillenwater JG, Hamdy FC, Perrin P, et al. Evaluation of the clinical benefit of permixon((R)) and tamsulosin in severe BPH patients—PERMAL study subset analysis. PROGRES EN UROLOGIE 2004;14(3):326-331. [PubMed] [Google Scholar]
- Debruyne F, Koch G, Boyle P, Da Silva FC, Gillenwater JG, Hamdy FC, et al. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomized international study [Comparaison d'un produit de phytotherapie (Permixon) et d'un alpha-bloquant (tamsulosine) dans le traitement de l'hypertrophie benigne de la prostate: etude internationale randomisee d'une duree de 12 mois]. Progres en Urologie 2002;12(3):384-94. [PubMed] [Google Scholar]
- Debruyne F, Koch G, Da Silva FC, Gillenwater JG, Hamdy FC, Perrin P, et al. Comparison of a phytotherapeutic agent (Permixon®) with an a-blocker (tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomized international study [Comparaison d'un produit de phytothérapie (Permixon®) et d'un alpha-bloquant (tamsulosine) dans le traitement de l'hypertrophie bénigne de la prostate: étude internationale randomisée d'une durée de 12 mois]. Progrès en Urologie 2002;41:497-507. [PubMed] [Google Scholar]
Di Maida 2020 {published data only}
- Di Maida F, Mari A, Rubino R, Minervini A, Carini M, Siena G. A Prospective, Open-Label Comparison of Tamsulosin plus Serenoa repens and Bovine Colostrum versus Tamsulosin Alone in the Treatment of Benign Prostatic Hyperplasia. Urologia Internationalis 2020;104(5-6):351-355. [DOI] [PubMed] [Google Scholar]
Di Silverio 1992 {published data only}
- Di Siverio F, D'Eramo G, Lubrano C, Flammia GP, Sciarra A, Palma E, et al. Evidence that Serenoa repens extract displays an antiestrogenic activity in prostatic tissue of benign prostatic hypertrophy patients. European Urology 1992;21(4):309-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duborija‐Kovacevic 2010 {published data only}
- Duborija-Kovacevic N, Sabo A, Pajovic B. Pharmacoeconomic evaluation of three different medications for lower urinary tract symptoms associated with BPH: Herbal extract versus standard drugs. European Urology, Supplements 2010;9(6):597-598. [Google Scholar]
Engelmann 2006 {published data only}
- Engelmann U, Walther C, Bondarenko B, Funk P, Schläfke S. Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin. Arzneimittel-Forschung 2006;56(3):222-9. [DOI] [PubMed] [Google Scholar]
EUCTR2011‐005307‐33‐FR {published data only}
- EUCTR2011-005307-33-FR. Exploratory study of L.S.E.S.r. (PERMIXON® 160 mg hard capsule) versus Tamsulosine LP activity on inflammation biomarkers in the treatment of urinary symptoms related to BPH, A multinational, multicentric, randomised, double-blind, parallel-group prospect. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-005307-33/results Start Date: 24-09-2012.
Gerber 1998 {published data only}
- Gerber GS, Zagaja GP, Bales GT, Chodak GW, Contreras BA. Saw palmetto (Serenoa repens) in men with lower urinary tract symptoms: effects on urodynamic parameters and voiding symptoms. Urology 1998;51(6):1003-7. [DOI] [PubMed] [Google Scholar]
Giannakopoulos 2002 {published data only}
- Giannakopoulos X, Baltogiannis D, Giannakis D, Tasos A, Sofikitis N, Charalabopoulos K, et al. The lipidosterolic extract of Serenoa repens in the treatment of benign prostatic hyperplasia: a comparison of two dosage regimens. Advances in Natural Therapy 2002;19(2):285-96. [DOI] [PubMed] [Google Scholar]
Giulianelli 2012 {published data only}
- Giulianelli R, Pecoraro S, Sepe G, Leonardi R, Gentile BC, Albanesi L, et al. Multicentre study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation. Archivio Italiano di Urologia e Andrologia 2012;84(2):94-98. [PubMed] [Google Scholar]
Grasso 1995 {published data only}
- Grasso M, Montesano A, Buonaguidi A, Castelli M, Lania C, Rigatti P, et al. Comparative effects of alfuzosin versus Serenoa repens in the treatment of symptomatic benign prostatic hyperplasia. Archivos Españoles de Urología 1995;48(1):97-103. [MEDLINE: ] [PubMed] [Google Scholar]
Gurzhenko 2020 {published data only}
- Gurzhenko I, Spyrydonenko V. Improvement of sexual functions in men with benign prostatic hyperplasia with long-term use of the Serenoa repens extract. Journal of Sexual Medicine 2020;17(6):S160-S161. [Google Scholar]
Guzman 2016 {published data only}
- Guzman R, Illnait J, Mas R, Fernandez L, Pedroso M, Fernandez J, et al. Efficacy and tolerability of Roystonea regia (D-004) and saw palmetto lipid extracts in men with symptomatic benign prostatic hyperplasia: a 24 weeks study. Gazzetta medica italiana archivio per LE scienze mediche 2016;175(10):413‐420. [Google Scholar]
- RPCEC00000138. D-004 in patients with Bening Prostatic Hyperplasia. https://rpcec.sld.cu/en/trials/RPCEC00000138-En 04 January 2013.
Hamdv 1997 {published data only}
- Hamdv FC, Chopin DK, Authie D, Deschaseaux P, Périer A, Raynaud J-P. Prostatic volume does not correlate with efficacy of treatment for mild to moderate benign prostatic hyperplasia using either finasteride or phytotherapy (permixon®). British Journal of Urology 1997;80(SUPPL. 2):195. [Google Scholar]
- Raynaud JP, Authié D, Deschaseaux P, Périer A, Fiet J, Perrin P. Influence of age and endocrine treatments (permixon ® and finasteride) on sexual function in benign prostatic hyperplasia (BPH) patients. British Journal of Urology 1997;80(Suppl 2):206. [Google Scholar]
Ju 2015 {published data only}
- Ju X, Gu X, Zhang Z, Wei Z, Xu Z, Miao H, et al. Efficacy and safety of Saw Palmetto Extract Capsules in the treatment of benign prostatic hyperplasia. Zhonghua nan ke xue [National journal of andrology] 2015;21(12):1098-1101. [PubMed] [Google Scholar]
Latil 2015 {published data only}
- Latil A, Pétrissans MT, Rouquet J, Robert G, la Taille A. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 2015;75(16):1857‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgia 2013 {published data only}
- Morgia G, Cimino S, Favilla V, Russo GI, Squadrito F, Mucciardi G, et al. Effects of Serenoa repens, selenium and lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: results of "FLOG" (Flogosis and Profluss in Prostatic and Genital Disease), a multicentre Italian study. International braz j urol: official journal of the Brazilian Society of Urology 2013;39(2):214-221. [DOI] [PubMed] [Google Scholar]
Morgia 2018 {published data only}
- ISRCTN73316039. Sprite study. https://www.isrctn.com/ISRCTN73316039 Registration date: 22/05/2015.
- Morgia G, Vespasiani G, Pareo RM, Voce S, Madonia M, Carini M, et al. Serenoa repens + selenium + lycopene vs tadalafil 5 mg for the treatment of lower urinary tract symptoms secondary to benign prostatic obstruction: a Phase IV, non-inferiority, open-label, clinical study (SPRITE study). BJU International 2018;122(2):317‐325. [DOI] [PubMed] [Google Scholar]
NCT00797394 {unpublished data only}
- NCT00797394. Efficacy of natural extract 2007RD01 combined with Saw Palmetto in benign prostatic hyperplasia patients compared to Saw Palmetto. https://clinicaltrials.gov/ct2/show/NCT00797394 First Posted: November 25, 2008. [NCT00797394]
Pannunzio 1986 {published data only}
- Pannunzio E, Dascenzo R, Giardinetti F, Civili P, Persichelli E. Serenoa repens in the treatment of human benign prostatic hyperplasia (BPH). Journal of Urology 1987;137(4):A226-A226. [Google Scholar]
- Pannunzio E, D’Ascenzo R, Giardinetti F, Civili P, Persichelli E. Serenoa repens vs. Gestorone caproate in the treatment of benign prostatic hypertension: a randomized study [Serenoa repens vs. gestonorone caproato nel trattamento dell’ipertofia prostatica benigna: Studio randomizzato]. Urologia 1986;53(5):696-705. [Google Scholar]
Pavone 2010 {published data only}
- Pavone C, Abbadessa D, Taraintino ML, Oxenius I, Laganà A, Lupo A, et al. Association of Serenoa repens, Urtica dioica and Pinus pinaster. Safety and efficacy in the treatment of lower tract symptoms. Prospective study on 320 patients [Associazione di Serenoa repens , Urtica dioica e Pinus pinaster. Sicurezza ed efficacia nel trattamento dei sintomi del basso tratto ruinario. Studio prospettico su 320 pazienti]. Urologia 2010;77(1):43-51. [PubMed] [Google Scholar]
Pecoraro 2004 {published data only}
- Pecoraro S, Annecchiarico A, Gambardella M-C, Sepe G. Efficacy of pretreatment with Serenoa repens on bleeding associated with transurethral resection of prostate [Effetto del pretrattamento con serenoa repens sul sanguinamento associato a resezione transuretrale della prostata]. Minerva Urologica e Nefrologica 2004;56:73-8. [PubMed] [Google Scholar]
Popa 2005 {published data only}
- Popa G, Hägele-Kaddour H, Walther C. Symptomatic efficacy of a sabal-urtica combination preparation in the therapy of benign prostatic syndrome: results of a placebo-controlled double-blind study [Symptomatische wirksamkeit eines sabal-urtica-kombinationspräparats in der therapie des benignen prostatasyndroms: ergebnisse einer plazebokontrollierten doppelblindstudie]. MMW Fortschritte der Medizin 2005;147(Suppl 3):103-8. [PubMed] [Google Scholar]
Romaniuk 2013 {published data only}
- Romaniuk M, Gorpynchenko I, Gurzhenko J, Klimenko P, Shulyak A, Spiridonenko V. PROSPECT study (PROStamol: perspectives of combined therapy) in patients with BPH. European urology, supplements 2013;12(4):e1150. [Google Scholar]
Roveda 1994 {published data only}
- Roveda S, Colombo P. Clinical controlled trial on therapeutical bioequivalence and tolerability of Serenoa repens oral capsules 160 mg or rectal capsules 640 mg [Sperimentazione clinica controllata sulla bioequivalenza terapeutica e sulla tollerabilita dei prodotti a base di Serenoa repens in capsule da 160 mg o capsule rettali da 640 mg]. Archivio di Medicina Interna 1994;46(2):61-75. [Google Scholar]
Sinescu 2011 {published data only}
- Sinescu I, Geavlete P, Multescu R, Gangu C, Miclea F, Coman I, et al. Long-term efficacy of Serenoa repens treatment in patients with mild and moderate symptomatic benign prostatic hyperplasia. Urologia Internationalis 2011;86:284-9. [DOI: 10.1159/000322645] [DOI] [PubMed] [Google Scholar]
Sökeland 1997 {published data only}
- Sökeland J, Albrecht J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome [Kombination aus Sabal- und Urticaestrakt vs. finasterid bei BPH (Stad. I bis II nach Alken); Vergleich der therapeutischen wirksamkeit in einer einjahrigen doppelblindstudie]. Urologe A 1997;36(4):327-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sökeland J, Walther C. A combination preparation of sabal and urtica extract versus finasteride in benign prostatic hyperplasia (BPH)—an analysis of data regarding prostatic volume and treatment success. Zeitschrift fur Phytotherapie 2000;21(6):299-305. [Google Scholar]
- Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. British Journal of Urology International 2000;86:439-42. [DOI] [PubMed] [Google Scholar]
Stepanov 1999 {published data only}
- Stepanov VN, Siniakova LA, Sarrazin B, Raynaud JP. Efficacy and tolerability of the lipidosterolic extract of Serenoarepens (Permixon®) in benign prostatic hyperplasia: a double-blind comparison of two dosage regimens. Advances in Therapy 1999;16(5):231-41. [PubMed] [Google Scholar]
Strauch 1994 {published data only}
- Strauch G, Perles P, Vergult G, Gabriel M, Gibelin B, Cummings S, et al. Comparison of finasteride (Proscar®) and Serenoa repens (Permixon®) in the inhibition of 5-alpha reductase in healthy male volunteers. European Urology 1994;26(3):247-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suardi 2014 {published data only}
- Suardi N, Gandaglia G, Nini A, Montorsi F, Pellucchi F, Agostini A, et al. Effects of Difaprost R on voiding dysfunction, histology and inflammation markers in patients with benign prostatic hyperplasia who are candidates for surgical treatment. Minerva urologica e nefrologica [The Italian journal of urology and nephrology] 2014;66(2):119-25. [PubMed] [Google Scholar]
Taieb 2010 {published data only}
- Taieb C, Auges M, Cabanac F. BPH patients treated with phytotherapy: Results at 6 months. Urology 2012;80(3):S220-S221. [Google Scholar]
- Taieb C, Auges M, Perrin P. Patients with BPH: Results at 6 months following treatment with phytotherapy versus other treatments. Value in Health 2010;13(7):A480. [Google Scholar]
- Taieb C, Cabanac F. Patients with BPH: Results at 6 months with phytotherapy vs other treatments. Urology 2012;80(3):S220. [Google Scholar]
Vela‐Navarrete 2005 {published data only}
- Vela-Navarrete R, Escribano-Burgos M, Lopez Farre A, Garcia-Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies BAX/BCL-2 index expression and CASPASE-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. The Journal of Urology 2005;173:507-10. [DOI] [PubMed] [Google Scholar]
- Vela-Navarrete R, Garcia Cardoso JV, Barat A, Manzarbeitia F, Lopez Farre A. BPH and inflammation: pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. European Urology 2003;44:549-55. [DOI] [PubMed] [Google Scholar]
Veltri 2002 {published data only}
- Veltri RW, Marks LS, Miller CM, Bales WD, Fan J, MacAiran ML, et al. Saw palmetto alters nuclear measurements reflecting DNA content in men with symptomatic BPH: evidence for a possible molecular mechanism. Urology 2002;60:617-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vinarov 2010 {published data only}
- Vinarov AZ, Aliaev IuG, Apolikhin OI, Mazo EB, Darenkov SP, Demidko IuL, et al. Results of three-year clinical study of prostamol Uno efficacy and safety in patients with initial symptoms of prostatic adenoma and risk of its progression. Urologiia 2010;6:3-10. [21433319] [PubMed] [Google Scholar]
Weisser 1997 {published data only}
- Weisser H, Behnke B, Helpap B, Bach D, Krieg M. Enzyme activities in tissue of human benign prostatic hyperplasia after three months' treatment with the Sabal serrulata extract IDS 89 (Strogen) or placebo. European Urology 1997;31(1):97-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamanishi 2004 {published data only}
- Yamanishi T, Yasuda K, Kamai T, Tsujii T, Sakakibara R, Uchiyama T, et al. Single-blind, randomized controlled study of the clinical and urodynamic effects of an alpha-blocker (naftopidil) and phytotherapy (eviprostat) in the treatment of benign prostatic hyperplasia. International journal of urology 2004;11(7):501‐509. [DOI] [PubMed] [Google Scholar]
Zlotta 2005 {published data only}
- Zlotta AR, Teillac P, Raynaud JP, Schulman CC. Evaluation of male sexual function in patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon (R)), Tamsulosin or Finasteride. European Urology 2005;48(2):269-276. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aliaev 2007 {published data only}
- Aliaev IG, Vinarov AZ, Lokshin KL, Spivak LG. Extracts Serenoa repens in the treatment of prostatic adenoma and chronic abacterial prostatitis: results of short-term (3-month courses) therapy. Urologiia (Moscow, Russia : 1999) 2007;(2):80-82. [PubMed] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Benign prostatic syndrome. Urinary urgency and micturition frequency reduced with plant preparation [Benignes Prostatasyndrom. Harndrang und Miktionsfrequenz mit Pflanzenpraparat reduzieren]. MMW Fortschritte der Medizin 2005;147(40):52-3. [PubMed] [Google Scholar]
Bercovich 2010 {published data only}
- Bercovich E, Saccomanni M. Analysis of the results obtained with a new phytotherapeutic association for LUTS versus control. Urologia 2010;77(3):180‐186. [PubMed] [Google Scholar]
Buck 2002 {published data only}
- Buck AC, Mahendra V, Bayne CW, Ross M, Habib FK. An investigation into the "in vivo" effect of Permixon, an extract of the American dwarf palm, Serenoa repens, on human benign prostatic hyperplasia. Journal of Urology 2002;167(4):374-374. [Google Scholar]
Carreras 1987 {published data only}
- Carreras JO. Our experience with hexanic extract of Serenoa repens in the treatment of benign prostatic hypertrophy. Archivos Espanoles de Urologia 1987;40(5):310-313. [PubMed] [Google Scholar]
Cukier 1985 {published data only}
- Cukier J, Ducassou J, Le Guillou M, Leriche A, Lobel B, Toubol J. Permixon versus placebo; results of a multicenter study [Permixon versus placebo; resultats d'une étude multicentrique]. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1985;4:15-21. [Google Scholar]
Dathe 1991 {published data only}
- Dathe G, Schmid H. Phytotherapy of benign prostate hyperplasia with Serenoa repens extract (permixon®). Urologe - Ausgabe B 1991;31(5):220-223. [Google Scholar]
Diehl 2005 {published data only}
- Diehl KF, Kromer F, Horak T, Nahler G. Treatment of benign prostate hyperplasia with a combination preparation from sabal and urtica extract in urological practice. Journal fur Urologie und Urogynakologie 2005;12(2):21-24. [Google Scholar]
Emili 1983 {published data only}
- Emili E, Lo Cigno M, Petrone U. Clinical results on a new drug in the therapy of prostate hypertrophy (Permixon) [Risultati clinici su un nuovo farmaco nella terapia dell'ipertofia della prostata (Permixon)]. Urologia 1983;50:1042-8. [Google Scholar]
Fabricius 1993 {published data only}
- Fabricius PG, Vahlensieck W Jr. Sabal extract in a single daily dose in the treatment of benign prostate hyperplasia. Therapiewoche 1993;43(30-31):1616-1620. [Google Scholar]
Gabric 1987 {published data only}
- Gabric V, Miskic H. Treatment of benign prostate adenoma and chronic prostatitis [Behandlung des benignen prostata-adenoms und der chronischen prostatatitis]. Therapiewoche 1987;37:1775-88. [Google Scholar]
Green 2000 {published data only}
- Green KA, Campbell PS. Saw palmetto: An effective form of phytotherapy for the treatment of benign prostate hyperplasia (BPH)? Biology of Reproduction 2000;62:187-187. [Google Scholar]
Löbelenz 1992 {published data only}
- Löbelenz J. Extractum Sabal fructus in benign prostatic hyperplasia (BPH). Stage I and II clinical trial [Extractum Sabal fructus bei benigner prostatahyperplasie (BPH). Klinische prufung im stadium I und II]. Therapeutikon 1992;6(1-2):34-7. [Google Scholar]
Martínez 1987 {published data only}
- Martínez Agulló E, Gallego Gómez J, Jiménez Cruz FJ. Medical treatment of the prostatism syndrome. Comparative study. Archivos espanoles de urologia 1987;40(4):247‐253. [PubMed] [Google Scholar]
Mattei 1990 {published data only}
- Mattei FM, Capone M, Acconcia A. Drug therapy of benign prostatic hyperplasia with saw palmetto extract [Medikamentose therapie der benignen prostatahyperplasie mit einem extrakt der sagepalme]. TW Urologie Nephrologie 1990;2:346-50. [Google Scholar]
- Mattei FM, Capone M, Acconcia A. Liposterolic extract of Serenoa Repens in management of benign prostatic hypertrophy. Urologia 1988;55(5):547-552. [Google Scholar]
Mohanty 1999 {published data only}
- Mohanty NK, Jha RJ, Dutt C. Randomized double-blind controlled clinical trial of Serenoa repens versus placebo in the management of patients with symptomatic Grade I to Grade II benign prostatic hyperplasia (PBH). Indian Journal of Urology 1999;16(1):26-31. [Google Scholar]
- Mohanty NK. Serenoa repens versus placebo in management of benign prostatic hyperplasia (bph)—a randomised double blind control clinical trial. Nephrology, urology, transplantation society of SAARC, Sri Lanka 1999:137. [Google Scholar]
Neumann 1993 {published data only}
- Neumann HG. Combination treatment of benign prostate hyperplasia with phytotherapy and selective alpha 1 blocking agents. Urologe - Ausgabe B 1993;33(6):384-385. [Google Scholar]
Razumov 2001 {published data only}
- Razumov SV, Derevianko II, Sivkov AV. Prostamol-uno in the treatment of benign prostatic hyperplasia. Urologiia (Moscow, Russia : 1999) 2001;(2):35-37. [PubMed] [Google Scholar]
Sekikawa 2020 {published data only}
- JPRN-UMIN000035036. A verification study on improvement of urination issues with ingestion of saw palmetto fruit extract: a randomized double-blind, parallel-group, placebo-controlled study. https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000039948 Date of registration: 27/11/2019.
- Sekikawa T, et al. Verification Study on the Effects of Saw Palmetto Fruit Extract on Urination Issues―A Randomized Double-blind, Parallel-group, Placebo-controlled Study. Yakuritochiryō [Pharmacology and Therapy] 2020;48:429-440. [Google Scholar]
Tasca 1985 {published data only}
- Tasca A, Barulli M, Cavazzana A, Zattoni F, Artibani W, Pagano F. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens. Double-blind clinical study vs placebo [Trattamento della sintomatologia ostruttiva da adenoma prostatico con estratto di Serenoa repens. Studio clinico in doppio cieco vs. placebo]. Minerva Urologica e Nefrologica 1985;37:87-91. [PMID: ] [PubMed] [Google Scholar]
- Tasca A, Barulli M, Cavazzana A, Zattoni F, Artibani W, Pagano F. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens. Double-blind clinical study vs. placebo [Trattamento della sintomatologia ostruttiva da adenoma prostatico con estratto di Serenoa repens. Studio clinico in doppio cieco vs. placebo]. Minerva Urologica e Nefrologica 1985;37:87-91. [MEDLINE: ] [PubMed] [Google Scholar]
Tkachuk 2002 {published data only}
- Tkachuk VN, Al'-Shukri SK, Aleksandrov VP, Kniaz'kin IV. Treatment of benign prostatic hypertrophy with prostaplant. Urologiia (Moscow, Russia : 1999) 2002;(3):16-18. [PubMed] [Google Scholar]
Vahlensieck 1993 {published data only}
- Vahlensieck W Jr, Fabricius PG, Muschter R, Uysal A. Therapy of benign prostate hyperplasia with sabal fruit extract SG 291 is effective and causes few side-effects. Therapiewoche 1995;45(29):1728-1736. [Google Scholar]
- Vahlensieck W Jr, Volp A, Kuntze M, Lubos W. Change of miction in patients with benign prostate hyperplasia during treatment with sabal extract. Urologe - Ausgabe B 1993;33(6):380-383. [Google Scholar]
Vinarov 2009 {published data only}
- Vinarov AZ, Aliaev IG, Lokshin KL. Safety of continuous (more than 1 year) intake of Serenoa repens extract by patients with prostatic adenoma. Urologiia (Moscow, Russia : 1999) 2009;(1):84, 86-87. [PubMed] [Google Scholar]
Wehr 1995 {published data only}
- Wehr A. Therapy of benign prostate hyperplasia with sabal extract IDS 89: Long-term study proofs the efficiency. Therapiewoche 1995;45(5):313-314. [Google Scholar]
References to ongoing studies
ISRCTN84633360 {published data only}
- ISRCTN84633360. Permixon (R) 320 mg once a day open label treatment for patients participating in the placebo controlled study. https://www.isrctn.com/ISRCTN42774128 Registration date: 22/04/2009.
JPRN‐UMIN000023274 {unpublished data only}
- JPRN-UMIN000023274. A double-blind, randomized, placebo-controlled clinical trial to investigate the effect of the foods containing plant extract (No.SB2805). https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000026820 22 July 2016.
JPRN‐UMIN000027902 {published data only}
- JPRN-UMIN000027902. A verification study on improvement of problems with urination due to ingestion of the food containing saw palm extracts: a randomized double-blind, parallel-group, placebo-controlled study. https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000031969 23 June 2017.
NCT00497939 {published data only}
- NCT00497939. The Effectiveness of Saw Palmetto and Sanmiaoshan on Benign Prostatic Hyperplasia in Chinese Patients. https://clinicaltrials.gov/show/NCT00497939 First Posted: July 9, 2007.
NCT02121613 {unpublished data only}
- NCT02121613. PERmixon® in LUTS Evaluation Study (PERLES). https://ClinicalTrials.gov/show/NCT02121613 First Posted: April 23, 2014. [GERMAN CLINICAL TRIALS REGISTER: DRKS00008796]
Additional references
Agarwal 2014
- Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. European Urology 2014;65(6):1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995;154(5):1770-1774. [DOI] [PubMed] [Google Scholar]
Barry 1997
- Barry MJ, Fowler FJ, Bin L, Pitts JC, Harris CJ, Mulley AG. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10-4; discussion 14-5. [PubMed] [Google Scholar]
Barry 2013
- Barry MJ, Cantor A, Roehrborn CG, CAMUSSG. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. Journal of Urology 2013;189(3):987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhojani 2014
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831-840. [DOI] [PubMed] [Google Scholar]
Chapple 2017
- Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, et al. Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based study. Advances in Therapy 2017;34(8):1953-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 1990
- Christensen MM, Bruskewitz RC. Clinical manifestations of benign prostatic hyperplasia and the indications for therapeutic intervention. Urology Clinics of North America 1990;17:509-16. [MEDLINE: ] [PubMed] [Google Scholar]
Cornu 2010
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. European Urology 2010;58(3):450-456. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org, Accessed 7 June 2023.
Crawford 2006
- Crawford ED, Wilson SS, McConnell JD, Slawin KM, Lieber MC, Smith JA, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. Journal of Urology 2006;175(4):1422-6; discussion 1426-7. [DOI] [PubMed] [Google Scholar]
Dahm 2021
- Dahm P, MacDonald R, McKenzie L, Jung JH, Greer NL, Wilt T. Newer minimal-invasive treatment modalities to treat lower urinary tract symptoms attributed to benign prostatic hyperplasia. European Urology Open Science 2021;26:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dedhia 2008
- Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. The Journal of Urology 2008;179:2119-25. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Di Silverio 1993
- Di Silverio F, Flammia GP, Sciarra A, Caponera M, Mauro M, Buscarini M, et al. Plant extracts in benign prostatic hyperplasia. Minerva Urologica e Nefrologica 1993;45:143-9. [MEDLINE: ] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Indentifying relevant studies for systematic reviews. BMJ 1994;312:944-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreikorn 1990
- Dreikorn K, Richter R, Schönhöfer PS. Conservative nonhormonal treatment of patients with benign prostatic hyperplasia. Urologe A 1990;29(1):8-16. [PMID: ] [PubMed] [Google Scholar]
Dunphy 2015
- Dunphy C, Laor L, Te A, Kaplan S, Chughtai B. Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Reviews in Urology 2015;17(2):51-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2022
- European Association of Urology. Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). Amsterdam: European Association of Urology, 2022. [Google Scholar]
Egan 2016
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289-297. [DOI] [PubMed] [Google Scholar]
Flemyng 2023
Franco 2021
- Franco JV, Garegnani L, Escobar Liquitay CM, Borofsky M, Dahm P. Transurethral microwave thermotherapy for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD004135. [DOI: 10.1002/14651858.CD004135.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franco 2023
- Franco JVA, Tesolin P, Jung JH. Update on the management of benign prostatic hyperplasia and the role of minimally invasive procedures. Prostate International 2023;11(1):1-7. [DOI: 10.1016/j.prnil.2023.01.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University and Evidence Prime, Date accessed 7 June 2023. Available at gradepro.org.
Gravas 2022
- Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, et al. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). European Association of Urology. Available at: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts (Accessed 7 June 2023) 2022.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-394. [DOI] [PubMed] [Google Scholar]
Habib 2004
- Habib FK, Wyllie MG. Not all brands are created equal: a comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer and Prostatic Diseases 2004;7(3):195-200. [DOI: 10.1038/sj.pcan.4500746] [DOI] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022b
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Homma 1997
- Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. International Journal of Urology : Official Journal of the Japanese Urological Association 1997;4(1):40-46. [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407-415. [DOI] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses—Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Junqueira 2023
- Junqueira DR, Zorzela L, Golder S, Loke Y, Gagnier JJ, Julious SA, et al. CONSORT Harms 2022 statement, explanation, and elaboration: updated guideline for the reporting of harms in randomised trials. BMJ 2023;381:e073725. [DOI: 10.1136/bmj-2022-073725] [DOI] [PubMed] [Google Scholar]
Kozminski 2015
- Kozminski MA, Wei JT, Nelson J, Kent DM. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH). BJU International 2015;115(2):308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavori 1992
- Lavori PW. Clinical trials in psychiatry: should protocol deviation censor patient data? Neuropsychopharmacology 1992;6:39-63. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 2017
- Lee SW, Chan EM, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Scientific Reports 2017;7(1):7984-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leissner 1979
- Leissner KH, Tisell LE. The weight of the human prostate. Scandinavian Journal of Urology and Nephrology 1979;13(2):137-142. [DOI] [PubMed] [Google Scholar]
Lerner 2021
- Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART I-Initial Work-up and Medical Management. Journal of Urology 2021;206(4):806-817. [DOI: 10.1097/JU.0000000000002183] [DOI] [PubMed] [Google Scholar]
Mansbart 2022
- Mansbart F, Kienberger G, Sönnichsen A, Mann E. Efficacy and safety of adrenergic alpha-1 receptor antagonists in older adults: a systematic review and meta-analysis supporting the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatrics 2022;22(1):771. [DOI: 10.1186/s12877-022-03415-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2014
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130-137. [DOI] [PubMed] [Google Scholar]
Marwick 1995
- Marwick C. Growing use of medicinal botanicals forces assessment by drug regulators. JAMA 1995;273:607-9. [MEDLINE: ] [PubMed] [Google Scholar]
McNicholas 2016
- McNicholas TA. Benign prostatic hyperplasia and new treatment options—a critical appraisal of the UroLift system. Medical Devices 2016;9:115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
McVary 2011
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. Journal of Urology 2011;185(5):1793-1803. [DOI] [PubMed] [Google Scholar]
Microsoft Excel 2023 [Computer program]
- Microsoft Excel. Microsoft Corporation, 2023. Available from office.microsoft.com/excel.
Novara 2016
- Novara G, Giannarini G, Alcaraz A, Cózar-Olmo JM, Descazeaud A, Montorsi F, et al. Efficacy and Safety of Hexanic Lipidosterolic Extract of Serenoa repens (Permixon) in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: Systematic Review and Meta-analysis of Randomized Controlled Trials. Eur Urol Focus 2016;2(5):553-561. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1136/bmj.n71] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Pariser 2015
- Pariser JJ, Pearce SM, Patel SG, Bales GT. National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721-725. [DOI] [PubMed] [Google Scholar]
Phillips 2019
- Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open 2019;9(2):e024537. [DOI: 10.1136/bmjopen-2018-024537.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2015
- Rees J. Patients not P values. BJU International 2015;115(5):678-679. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.3. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Roehrborn 2008
- Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Reviews in Urology 2008;10(1):14-25. [PMC free article] [PubMed] [Google Scholar]
Roehrborn 2020
- Roehrborn CG. Chapter 103: Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology, and natural history. In: Partin AW, Peters CA, Kavoussi LR, Dmochowski RR, Wein AJ, editors(s). Campbell Walsh Wein Urology. 13th edition. Elsevier, 2020. [Google Scholar]
Russo 2021
- Russo GI, Scandura C, Di Mauro M, Cacciamani G, Albersen M, Hatzichristodoulou G, et al. Clinical Efficacy of Serenoa repens Versus Placebo Versus Alpha-blockers for the Treatment of Lower Urinary Tract Symptoms/Benign Prostatic Enlargement: A Systematic Review and Network Meta-analysis of Randomized Placebo-controlled Clinical Trials. Eur Urol Focus 2021;7(2):420-431. [DOI] [PubMed] [Google Scholar]
Santesso 2016
- Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol 2016;74:28-39. [DOI: 10.1016/j.jclinepi.2015.12.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126-135. [DOI: 10.1016/j.jclinepi.2019.10.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Scaglione 2008
- Scaglione F, Lucini V, Pannacci M, Caronno A, Leone C. Comparison of the potency of different brands of Serenoa repens extract on 5alpha-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology 2008;82(4):270-5. [DOI: 10.1159/000161128] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Vacherot 2000
- Vacherot F, Azzouz M, Gil-Diez-de-Medina S, Colombel M, la Taille A, Lefrere Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon®) in benign prostatic hyperplasia. The Prostate 2000;45:507. [DOI] [PubMed] [Google Scholar]
Vela‐Navarrete 2018
- Vela-Navarrete R, Alcaraz A, Rodríguez-Antolín A, Miñana López B, Fernández-Gómez JM, Angulo JC, et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon(®) ) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int 2018;122(6):1049-1065. [DOI] [PubMed] [Google Scholar]
Weisser 1996
- Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. The Prostate 1996;28:300. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 17 August 2017).
Yeo 2016
- Yeo JK, Choi H, Bae JH, Kim JH, Yang SO, Oh CY, et al. Korean clinical practice guideline for benign prostatic hyperplasia. Investigative and clinical urology 2016;57(1):30-44. [DOI: 10.4111/icu.2016.57.1.30] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Tacklind 2009
- Tacklind J, MacDonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD001423. [DOI: 10.1002/14651858.CD001423.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tacklind 2012
- Tacklind J, Macdonald R, Rutks I, Stanke JU, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD001423. [DOI: 10.1002/14651858.CD001423.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trivisonno 2021
- Trivisonno LF, Sgarbossa N, Alvez GA, Fieiras C, Escobar Liquitay CM, Jung JH, Franco JVA. Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement: A systematic review and meta-analysis. Investigative Clinical Urology 2021;62(5):520-534. [DOI: 10.4111/icu.20210254] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilt 2000
- Wilt TJ, Ishani A, Stark G, MacDonald R, Lau J, Mulrow C, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD001423. [DOI: 10.1002/14651858.CD001423] [DOI] [PubMed] [Google Scholar]
Wilt 2002
- Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD001423. [DOI: 10.1002/14651858.CD001423] [DOI] [PubMed] [Google Scholar]


