Abstract
Sex differences are prominent defining features of neurodevelopmental disorders. Understanding the sex biases in these disorders can shed light on mechanisms leading to relative risk and resilience for the disorders, as well as more broadly advance our understanding of how sex differences may relate to brain development. The prevalence of neurodevelopmental disorders is increasing, and the two most common neurodevelopmental disorders, Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) exhibit male-biases in prevalence rates and sex differences in symptomology. While the causes of neurodevelopmental disorders and their sex differences remain to be understood, increasing evidence suggests that the immune system plays a critical role in shaping development. In this chapter we discuss sex differences in prevalence and symptomology of ASD and ADHD, review sexual differentiation and immune regulation of neurodevelopment, and discuss findings from human and rodent studies of immune dysregulation and perinatal immune perturbation as they relate to potential mechanisms underlying neurodevelopmental disorders. Ultimately this chapter will give an overview of how understanding sex differences in neuroimmune function in the context of neurodevelopmental disorders could lend insight into their etiology and better treatment strategies.
Keywords: Hormones, Development, Inflammation, Cytokines, Microglia, Sex Differences
1. Introduction
Most neurological and psychiatric disorders exhibit sex biases in either prevalence rates, symptomology, or trajectory. Understanding the sex biases in these disorders can shed light on mechanisms of risk and resilience, as well as advance the understanding we have of the brain and human behavior. Neurodevelopmental disorders are a group of conditions usually diagnosed early in development and characterized by impairments in personal, social, and occupational functioning.1 Notably, prevalence of neurodevelopmental disorders is increasing.2 Potential reasons for the rising prevalence are manifold, and could be due to differences in research methodology or diagnostic criteria,3–5 earlier age at diagnosis,4 or an increase in risk factors for the disorders.6, 7 Additionally, reduced stigma/elevated awareness might be a factor: a recent study found that rates of mental health literacy for neurodevelopmental disorders were higher than reported previously.8 Nonetheless, a true increase in prevalence cannot be ruled out,4, 7 and a near 18% prevalence rate merits investigation into a better understanding of these conditions.2 The two most common neurodevelopmental disorders, Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) exhibit male-biases in prevalence rates (3:1 for ASD and 2:1 for ADHD).9, 10 The 2016 National Survey of Children’s Health found that of children in the United States aged 2–17 years of age, 8.4% were diagnosed with ADHD,10 and 3.8% were diagnosed with ASD.9 The causes of neurodevelopmental disorders are poorly understood, and the reasons underlying their sex-biases remain elusive. The immune system plays a critical role in shaping neurodevelopment, and there is increasing evidence that shifts in immune activation and thus potentially differential programming of neurodevelopment are associated with neurodevelopmental disorders. In the following chapter, we will describe symptoms of ASD and ADHD and their sex differences, review sexual differentiation and the role of immune cells in neurodevelopment, detail immune dysregulation found in ASD and ADHD, and discuss findings from rodent models of perinatal immune perturbation as they relate to potential mechanisms underlying neurodevelopmental disorders.* Ultimately this chapter will give an overview of how understanding sex differences in neuroimmune function in the context of neurodevelopmental disorders could lend insight into their etiology and better treatment strategies.
2. ASD and ADHD Symptomology
2.1. ASD:
Autism Spectrum Disorder is a newer diagnostic category established in the DSM-5 to account for what were previously known as four separate diagnoses: Autistic Disorder, Asperger’s Disorder, Childhood Disintegrative Disorder, or Pervasive Developmental Disorder Not Otherwise Specified. The impetus for the diagnostic reconceptualization was to provide a more accurate and scientifically useful diagnostic category,12 since these diagnoses were not reliably differentiated from one another in the clinic.13, 14 Using the DSM-5 criteria, a diagnosis of ASD requires persistent and pervasive deficits in social communication and interaction, as well as restricted repetitive patterns of behaviors, interests, or activities. These symptoms must be present in early childhood and impair everyday functioning. To account for heterogeneity of support needs and symptom severity, clinicians can categorize ASD severity ranging from “requiring support” to “requiring very substantial support.”1
In addition to the 3:1 male biased sex difference in ASD diagnosis rate, many studies have documented sex differences in ASD symptomology (see Table 1; for review, see 15), though some other studies do not report such sex differences.16–20 In the studies that have reported sex differences in symptoms, autistic males exhibit more externalizing behaviors, such as aggression, hyperactivity, and restricted or repetitive behavior compared to females.21–29 The evidence appears strongest for increased restricted and repetitive behavior in males, as multiple studies that do not find other sex differences in symptoms still observe this pattern.16, 19 Conversely, autistic females are more likely to exhibit internalizing symptoms, such as anxiety and depression.22, 23, 30 While one study in autistic children found better verbal, motor, and social skills in autistic males relative to females,28 other studies in children have found that females have better communication, social skills, and social cognition compared to males,31–33 but manifest more severe symptoms as they age.26, 32 This is possibly because females’ social skills appear to decline with age whilst males’ social skills improve.33 Moreover, the trajectory of ASD is influenced by sex. Collapsing across the ages eliminated sex differences in social and communication abilities,33 which may underlie null findings in some studies that do not find sex differences in social or communication domains.
Table 1:
Summary of sex differences that have been documented in ASD and ADHD
| Sex Differences in ASD | |
| ENDPOINT | SEX DIFFERENCE |
|
| |
| Social Skills | Males have more severe symptoms as children, but females have more severe symptoms as adolescents and adults.32, 33 |
| Communication Skills | Males have more severe symptoms as children, but females have more severe symptoms as adolescents and adults.32, 33 |
| Restricted/Repetitive Behavior | Males more severe.16, 19, 22, 26 |
|
Externalizing Behavior (Hyperactivity, Aggression) |
Males more severe.21–23 |
|
Internalizing Behavior (Anxiety, Depression) |
Females more severe.22, 23, 30 |
| Camouflaging Behavior | Adult autistic females camouflage more.34 |
| Peripheral Immune Markers | IL-1β, IL-8, MIP-1β, and VEGF negatively correlate with symptom severity in females but not males. PDGF negatively correlates with symptom severity in both sexes.48 |
| Males primarily have elevations in cytokines relative to controls while females primarily have elevations in growth factors relative to controls.49 | |
| Sex Differences in ADHD | |
| ENDPOINT | SEX DIFFERENCE |
|
| |
| Sub-types | Females more likely to have inattentive subtype, while males are more likely to have the combined subtype.37, 38 |
| Age at Symptom Onset | Females diagnosed later.50, 51 |
| Social Skills | Males have more severe symptoms as children, but females have more severe symptoms as adolescents and adults.33 |
| Communication Skills | Males have more severe symptoms as children, but females have more severe symptoms as adolescents and adults.33 |
|
Externalizing Behavior (Hyperactivity, Aggression) |
Males more severe.40, 43 |
|
Internalizing Behavior (Anxiety, Depression, Suicidality) |
Females more severe.40, 42, 44, 46, 47 |
Females may also be underdiagnosed with ASD due to higher rates of camouflaging behavior, whereby they hide or “mask” symptoms that are considered socially unacceptable or performatively offer socially acceptable behaviors.34 Adult autistic women camouflage more than men, 34 adolescent autistic females have been shown to be better at using subtle language skills compared to males and a separate study in children aged 5–10 found autistic females used more vivid gestures compared to males.27, 35 Teachers also report fewer concerns regarding the social skills of autistic girls relative to autistic boys.36 These phenomena are likely influenced by gendered expectations and perceptions regarding the behaviors of girls and boys. More work must be done to assess how common camouflaging is, the way that we measure ASD-like symptoms, and gender-related behavioral expectations as they may contribute to reduced prevalence rates in females.
In summary, the findings regarding sex differences in ASD symptomatology are heterogeneous, with the most conclusive evidence supporting higher levels of restricted and repetitive behaviors in males relative to females, and studies are beginning to show that symptoms may worsen with age in females.
2.2. ADHD:
For Attention-Deficit/Hyperactivity Disorder, the DSM-5 requires a persistent pattern of inattention, hyperactivity and/or impulsivity that interferes with functioning or development for diagnosis. These symptoms must be present prior to age 12, and they must manifest in multiple settings to produce significant impairment. There are three sub-classifications of ADHD: predominately inattentive, predominately hyperactive/impulsive, or a combined type.1 In the general population, males are more likely to be diagnosed with any of the ADHD subtypes.37 Of those who receive a diagnosis of ADHD, males are more likely to meet criteria for the combined subtype,37 while females are more likely to be diagnosed with the inattentive subtype.37–39 ADHD severity is generally worse in males,40, 41 and this may be explained by the fact that males in the general population display more ADHD symptoms and by the fact that males have greater overall variance in ADHD symptoms compared to females.18, 41
A recent cross-sectional study in children ages 7–13 years old found that younger females with ADHD generally outperformed their male counterparts in terms of social and communication skills, though the relationship for communication was reversed in older children, where older males outperformed females in communication skills.33 As with ASD, males are more likely to display externalizing behaviors like aggression,40and they have higher rates of comorbid conduct disorder and oppositional defiant disorder compared to females;38, 42, 43 however, individuals with ADHD have higher likelihood of being diagnosed with oppositional defiant disorder and this association is higher in females with ADHD compared to males.42 Males with ADHD also show higher rates of learning disability,38 and perform worse on tasks involving processing speed, inhibition, and working memory compared to females.41 These trends continue into adulthood, as adult males with ADHD display higher rates of criminality,44 and have greater impairment on complex cognitive tasks.45 Conversely, females with ADHD tend to display more internalizing problems such as anxiety, depression, and suicidality,40, 42, 44, 46, 47 though a recent study did not replicate this finding.18
The sex bias in diagnosis may also be driven by symptomology, such that the disruptiveness of males in classroom, social, or home settings may mean that they are more likely to be referred for diagnosis by teachers or caregivers.52 Studies in clinical samples of children with ADHD have found large male-biases (10:1) while studies in non-referred community-based samples have shown less of a male-bias in prevalence, 3:1.37, 41 Additionally, males receive their diagnoses and manifest symptoms earlier relative to females.50, 51 Individuals with the inattentive subtype, the subtype females are more prone to, also tend to manifest their symptoms at later ages,37 and females are also more likely to manifest hyperactivity symptoms at later ages.51 This discrepancy may contribute to the differing and reduced prevalence ratios (1:1 and 2:1) seen in studies of adults with ADHD.53 Moreover, as with ASD, not much is known regarding the developmental trajectory of symptoms of ADHD, but evidence suggests that both disorders are influenced by sex.
3. Sexual Differentiation: Y do sex differences matter?
To better understand how and why sex differences in ASD and ADHD come about, we must understand the basics of sexual differentiation. In early mammalian development, every embryo contains a bipotential structure called the gonadal ridge that can differentiate into ovaries or testes. The process by which the gonadal ridge becomes either ovaries or testes, known as sex determination, depends on the combination of chromosomes inherited from the parents. Mothers can pass on one of two X chromosomes, whereas fathers may pass down an X or a Y chromosome to the embryo. The presence of a Y chromosome, specifically the sex-determining region of the Y chromosome (SRY), will lead to synthesis of a secreted protein, called testis-determining factor (TDF). TDF causes the bipotential gonads to differentiate into testes which then initiate the process of male-specific sexual differentiation via secretion of androgens. The major mammalian androgens are testosterone and its metabolite, dihydrotestosterone (DHT), which induce development of male-specific accessory structures and external genitalia, respectively, in a process known as masculinization. Meanwhile, the testes also secrete anti-Mullerian hormone, which causes regression of female-specific accessory structures in a process known as defeminization. In the absence of a Y chromosome and thus an absence of SRY/TDF and androgenic hormones, the bipotential gonads will develop into ovaries via a process known as feminization, and the male accessory structures will regress (demasculinization) to initiate female-specific sexual differentiation.54 However, just because feminization does not depend on hormones, does not mean it is not an active process. Carefully orchestrated genetic programs direct female-specific brain organization, and perturbations in this programming can shift the trajectory of brain feminization. Additionally, feminization also requires active repression of male-specific genetic programming, such that higher DNA methylation levels in the developing female brain maintain feminization by silencing genes implicated in brain masculinization.55
Sexual differentiation of the brain is likewise driven by steroid hormones. In humans, sexual differentiation takes place prenatally during the second trimester of gestation.56 In rodents, this critical period begins when the testes start to secrete androgens on E18 and extends into neonatal life (<P10), even after androgen secretion stops around the day of birth.57 Given their nonpolar structure, sex steroid hormones can readily cross the blood brain barrier (BBB) to produce enduring cellular and molecular changes during this period of brain development. Once in the brain, testosterone can be converted into DHT via the enzyme, 5α reductase, or 17-β-estradiol via the enzyme, p450 aromatase.58 Androgens like testosterone and DHT are important for masculinization in primates, whereas estradiol is the main masculinizing hormone in rodents,57, 59 thus the aromatase enzyme is highly expressed in the rodent brain during the critical period for sexual differentiation to convert androgens into estrogens.60, 61 Later in life, steroid hormones, including androgens, estrogens, and progestins, act on the previously organized brain circuitry to permit expression of, or “activate” sex-specific aspects of brain and behavior.62 In this way, early life exposure to sex-typical hormones prepares the brain for later life hormonal surges that drive sex differences in a variety of behaviors, from cognition to social and sexual behavior.
Differences in typical sex steroid exposure during this early life organizational period have been linked to neurodevelopmental disorder risk. For example, females with polycystic ovary syndrome, a condition associated with elevated testosterone and other sex steroids, are more likely have children with ADHD or ASD.63, 64 Likewise, individuals with congenital adrenal hyperplasia (CAH), another disorder characterized by ectopically high levels of androgen exposure in utero, exhibit higher rates of autistic traits.65 Studies that have measured levels of sex steroid hormones in the amniotic fluid suggest that testosterone correlates positively with parent-reported autistic traits.66, 67 Another study showed that combined elevations in androgens, progesterone, and cortisol in amniotic fluid, dubbed a “latent steroidogenic factor”, was statistically associated with individuals going on to develop autism in childhood.68 Further studies implicate higher levels of prenatal estrogens as a risk factor for an ASD diagnosis and internalizing behaviors.69, 70 Sex steroids may impact brain development and later life behavior in a sex-dependent manner, as high levels of testosterone in the amniotic fluid were associated with reduced functional connectivity in the social brain default mode network during adolescence in males but not females.71 Nonetheless, not all studies support the idea that elevated levels of prenatal steroids program an increased likelihood of ASD. For example, conditions associated with reduced levels of sex steroids, like Klinefelter’s syndrome, are also associated with increased risk of both ASD and ADHD-like traits,72 and some studies have failed to find a relationship between perinatal sex steroids and autism.73–75 Overall, the take-home point from these steroid hormone studies is that deviations in either direction from ‘typical’ levels of prenatal steroids may be associated with higher risk of ASD. More work must be done to establish whether or not, and, if so, how prenatal sex steroids may contribute to the etiology of ASD at a mechanistic, rather than purely correlational level.
There is also evidence for persistent differences in steroid hormone-related function in the ASD brain outside of the prenatal period. Aromatase, the enzyme responsible for converting testosterone to estradiol, is reduced in the frontal cortex of adult autistic brains.76 Likewise, retinoic acid-related orphan receptor-alpha (RORA), a transcription factor that regulates expression of the gene for aromatase along with 438 other autism candidate genes, is downregulated in the postmortem frontal cortex of autistic adults.76 Testosterone itself downregulates RORA expression,76 suggestive of a positive feedback loop leading to further levels of elevated testosterone in the ASD brain. However, estrogens upregulate RORA, and may provide a protective buffering mechanism in females who have higher levels of these hormones.76 Indeed, control females express marginally higher levels of RORA in the frontal cortex, and this trend disappears in ASD brains.77 The percent reduction in aromatase protein is higher in ASD males, suggesting that females may offset aromatase deficiency and lead to the male-bias in ASD prevalence.77 Whether these trends are unique to adulthood or also exist prenatally must be parsed out in animal studies.
While steroid-mediated mechanisms of neurodevelopmental disorders is an area of active research interest, much remains to be established. Particularly of interest is how basic mechanisms of brain sexual differentiation, including hormone secretion and sensitivity, may be shifted in the context of ASD risk genes, early life perturbations or combined genetic and environmental risk exposure. However, steroid hormones do program the function of many cells throughout the brain during early life, including brain-resident immune cells called microglia. In the next section, we discuss how the immune system regulates neurodevelopment, sex differences in neuroimmune cell function, and evidence for neuroimmune cell dysregulation in ASD and ADHD.
3. Immune Cells Shape Neurodevelopment and Behavior
3.1. Microglia
Microglia are macrophages located in the central nervous system (CNS), though they are not derived from neural stem cells. Microglia are derived from myeloid progenitor cells from the yolk sac and infiltrate the brain during early embryogenesis, on embryonic day (E) 9.5 in rodents and gestational week 4.5 in humans.78, 79 Once microglia progenitors have entered the developing brain they differentiate, proliferate, and migrate widely.80 After microglia have seeded the brain, they form a long-lived and self-sustaining cellular population, where they provide local host defense and perform a variety of homeostatic and developmentally relevant functions.81 Microglia secrete factors such as cytokines and chemokines, which are small proteins that enable communication between and recruitment of immune cells, respectively, though both cytokines and chemokines also have neuromodulatory functions.82, 83 In the developing brain of both sexes, microglia populate proliferative and neurogenic zones,84, 85 and regulate cell genesis and survival through secretion of cytokines and growth factors.84, 86, 87 Microglia likely also support cell genesis throughout the forebrain, as neonatal depletion of microglia reduces cell genesis in the rat cortex, hippocampus, and amygdala of males and females.88 Microglia also participate in the cell death process, sensing cell death signals and releasing reactive oxygen species to advance apoptosis.89–91
Microglia also phagocytose, or “eat” viable precursor cells and dead and dying cells in the developing brain of both sexes.92, 93 Microglia phagocytosis is also crucial for developmental synaptic patterning. During normal brain development, neurons form an excess of excitatory synapses, and maturation of brain circuitry is marked by pruning of excess synapses.94, 95 Microglia facilitate the synaptic pruning process, though most of the work supporting this role for microglia has been done in males, so confirmational studies to show similar, or perhaps differential, effects occurring in females must still be performed. Microglia engulf pre- and postsynaptic elements in the juvenile hippocampus, and mice lacking the receptor for fractalkine, a “find me” signal released by neurons to attract microglia, had a transient increase in dendritic spines and immature synapses in the third postnatal week, indicative of reduced synaptic pruning.96 Microglia also sense “eat me” signals from the complement cascade, an immune pathway traditionally involved in marking pathogens and apoptotic cells for phagocytosis/elimination, to direct synaptic pruning activity. This happens in the neonatal thalamus, where microglia prune less active retinogeniculate axons via detection of the protein complement component (C)3.97 Conversely, microglia can also partially engulf, or nibble at (“trogocytose”) presynaptic elements to alter their shape, and microglia-dendrite contact can induce spine head and filopodia formation.98, 99 Microglia signaling may also affect synaptic activity, as microglial-derived brain-derived neurotrophic factor (BDNF) influences glutamate receptor subtype composition, and depletion decreases learning-dependent formation of dendritic spines in the cortex.100 Perturbation of embryonic microglia via knockout of either fractalkine receptor, complement receptors, or DAP12 altered the outgrowth of forebrain dopaminergic axons and disturbed laminar organization of neocortical interneurons.101
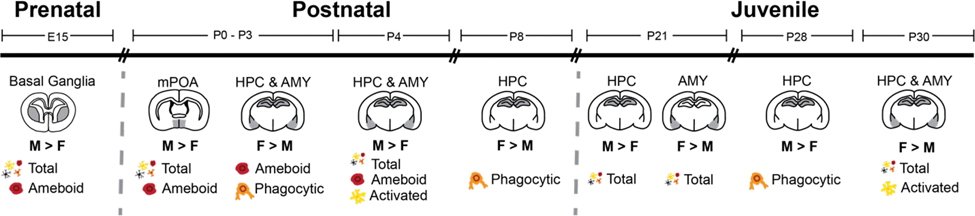
Studies in rodents indicate that microglia undergo several stages of development, reaching maturity during the juvenile period, around postnatal day (P)28.80, 102, 103 As microglia mature, they express higher levels of immune-response genes, at least in the hippocampus,104 and adult cortical and hippocampal microglia exhibit sex differences in their gene expression.105 Furthermore, various sex differences in microglia number and morphology in the developing brain have been documented (see Figure 1).106 Sex differences in microglia morphology or number in the developing brain are in some cases dramatic in magnitude but are highly dynamic across developmental stage and region-specific.
Figure 1:
Summary of known sex differences in microglia number and morphology during rodent neurodevelopment. On E15, males have more total and ameboid microglia in the basal ganglia compared to females.107 During the first three days of life, males have more total and ameboid microglia in the medial preoptic area (mPOA),108 while females have more ameboid and phagocytic microglia in the hippocampus (HPC)85, 88 and amygdala (AMY).109 Conversely, males have more total, ameboid and “reactive” microglia in the HPC and AMY on P4.110 On P8, females display more phagocytic microglia in the HPC.111 Subsequently, during the third week of life (P21), males have more microglia in the HPC, while females have more microglia in the AMY.105 By week 4 (P28), males have more phagocytic microglia in the HPC,111 while females have more total and “reactive” microglia in the HPC and AMY (P30).110 Collectively, these data suggest there are dynamic fluctuations in sex differences of microglia number and phenotype throughout development, particularly in the hippocampus and amygdala, though this may be reflective of greater experimental focus on these limbic regions across time relative to other brain regions.
Microglia sex differences are organized by early life steroid hormone exposure (e.g., estradiol in rodents) and may have long-term effects on development and behavior. For example, microglia phagocytose more viable neural progenitor cells in the neonatal hippocampus of female rats compared to male rats, and this sex difference is eliminated by administration of a masculinizing dose of estradiol to females.85 Likewise, neonatal ablation of microglia reduces sex differences in hippocampal cell genesis.88 Male rats also have more ameboid microglia in the neonatal preoptic area (POA), and microglia depletion during the neonatal period prevents the development of male-specific synaptic patterning and adult sexual behavior.108 Female synaptic patterning in the POA and sexual behavior can be masculinized via neonatal administration of estradiol or prostaglandin E2, an inflammatory mediator that can be released by immunocompetent cells as well as neurons and astrocytes,108, 112 and microglia inhibition prevents this masculinization.108 Microglia also regulate social play behavior in a sex-dependent manner. Juvenile social play is an adaptive and conserved behavior across mammalian species important for social, cognitive, and neural development.113, 114 Moreover, complement-dependent phagocytosis of dopamine (D)1 receptors in the adolescent nucleus accumbens programs social play in male rats, as inhibition of microglial phagocytosis increased play behavior in a D1r-dependent manner.115 Conversely, while inhibition of complement-dependent phagocytosis also increased play in female rats, it was not dependent on D1r.115 A separate study found that microglial phagocytosis of viable newborn astrocytes in the neonatal amygdala programs sex differences in juvenile play, and administration of testosterone to females during the neonatal period masculinizes play, along with phagocytosis and the number of newborn cells.109 Meanwhile, neonatal inhibition of phagocytosis in males feminizes juvenile play behavior.109 Because social behavior is a key domain impacted in neurodevelopmental disorders, these studies suggest that abnormal microglia activity may be involved in shaping abnormal social behavior found in ASD and ADHD.
Studies that inhibit, deplete, or knockout microglia during development recapitulate behavior associated with neurodevelopmental disorders. For example, embryonic depletion of microglia via maternal dietary treatment with a colony stimulating factor receptor 1 (CSF1R) inhibitor produced hyperactivity in adolescent female mice.116 Transient microglia depletion in the neonatal period leads to locomotor hyperactivity, increased risk assessment behavior, impaired cognition, reduced juvenile social play, and increased adult social avoidance in rats.117, 118 Likewise, a study employing fractalkine receptor (CX3CR1) knockout, which reduces neuron-microglia communication and causes a transient reduction of microglia during development, impairs juvenile social behavior, and increases repetitive grooming behavior in mice.119 Beyond the possible relevance to neurodevelopmental disorders, these rodent studies make clear that microglia are important mediators of brain development and resultant behavioral programming.
3.1.1. Microglia are dysregulated in ASD.
While human studies on microglia in ADHD are lacking, there is evidence for abnormal microglia activation in the brains of autistic individuals, at least in males. Positron emission tomography (PET) imaging of 18kDa translocator protein (TSPO), a protein found in activated microglia and astrocytes, revealed decreased expression in several cortical brain areas of ASD males.120 Conversely, a different PET study analyzing a radiocarbon that specifically binds to activated microglia ([11C])-labeled (R)-(1-[2-chrorophynyl]-N-methyl-N- [1-methylpropyl]-3 isoquinoline carboxamide) found elevated microglia activation in the cerebellum, midbrain, pons, and cingulate and orbitofrontal cortices of ASD males.121 This result complements other work in postmortem autistic tissue finding elevated microglia number, density, and/or activation in the cerebellum,122 dorsolateral prefrontal cortex,123 and fronto-insular and visual cortices of ASD males 124 compared to controls.
Studies investigating gene expression in the cortex of ASD versus control brains generally find downregulations in expression of genes associated with neuronal and synaptic function125–127 and upregulations in expression of genes associated with inflammatory and immune responses, including genes associated with microglia and activated microglia.125–128 The expression of genes associated with activated microglia was also negatively correlated with the expression of genes associated with neuronal activity, suggesting that dysregulation of microglia operates in tandem with altered neuronal activity.126 Likewise, a transcriptomic profiling and comparison of major psychiatric disorders discovered that microglial genes were uniquely upregulated in ASD cortex while multiple neuron-associated modules were downregulated.129 Alterations in gene expression may be region-specific, as another study found decreased microglial markers in the cerebellum.128 A more recent study using single nucleus RNA sequencing found that the most differentially expressed genes in ASD versus control brains were found in cortical layer 2/3 excitatory neurons, VIP interneurons, and microglia, and changes in these neurons and microglia were predictive of clinical severity.130 Autistic brains also display signs of exaggerated microglial maturity, suggesting inflammation.104
One study found that when a microglial maturity index was applied to the human brain transcriptome, the male microglial transcriptome was more developmentally mature compared to that of females,104 and autistic brains displayed markers of exaggerated microglial maturity, suggesting greater neuroinflammation.104 Exaggerated protein synthesis in microglia leads to autism-like behavior in male but not female mice,131 and a genetic mouse model of autism revealed differentially expressed genes in juvenile male brains were positively enriched for glia-specific gene sets, whereas those in the female brain were generally negatively enriched for glia-specific gene sets.132 Despite this, the above studies in humans are all biased towards male participants, and none analyzed sex differences. However, a study on samples from fetal and adult brains from non-autistic individuals of both sexes found that ASD-elevated glial and immune molecules were more likely to be expressed in the cortex of fetal and adult males compared to females, whereas ASD-reduced neuronal/synaptic genes were more likely to be expressed in the adult female cortex.133 This finding suggests that males and females may be differentially vulnerable to different mechanisms of ASD. Said another way, males may be more susceptible to neuroinflammatory-associated risk factors, whereas females may be more susceptible to neuronal dysfunction-associated risk factors. Seemingly conflicting with this, ASD candidate genes were more highly co-expressed in the female fetal brain compared to the male fetal brain, and that females displayed higher numbers of de novo mutations compared to autistic males, even after controlling for ASD severity.134 This finding supports the “female protective effect” theory of autism – the idea that mutation-induced gene deficiencies may be compensated for more readily in females, and that females may require higher etiological or genetic load to manifest autistic symptoms.25, 135 Overall, while there appear to be sex differences in expression of genes dysregulated in ASD, more work needs to ascertain whether specific ASD candidate genes are expressed or co-expressed in a sex dependent manner.
3.2. Astrocytes
Another immunocompetent cell located in the CNS is the astrocyte. Unlike microglia, astrocytes are neural progenitor cell-derived glial cells. Their primary functions in the brain include maintenance of the BBB, glutamate homeostasis, and metabolic and trophic support.136 They can also receive and relay immune signals, particularly through communication with microglia.137 Like microglia, astrocytes display sex differences in number, differentiation, and function. For example, in the POA, male rats have more complex astrocytes with increased process number and length relative to females, and this can be masculinized with estradiol treatment.138 Primary astrocytes cultured from cortical samples of male mice release more inflammatory cytokines compared to females following immune stimulation with bacterial endotoxin lipopolysaccharide (LPS), and this was masculinized in females with neonatal testosterone treatment.139
Post-mortem and genetic studies corroborate the idea that astrocytes may be involved in neurodevelopmental disorders, although little has been done to investigate sex differences as most of these studies’ subjects consist primarily of males and/or findings were not analyzed by sex. Some of the previously mentioned studies on post-mortem autistic tissue found increased astrocytic activation in the cerebellum, middle frontal gyrus, and anterior cingulate gyrus,122 and elevated astrocytic genes in prefrontal cortex and cerebellum.128 Likewise, both astrocyte and microglia markers are elevated in the autistic cortex.125 A separate study found increased number of protoplasmic astrocytes, a subtype of astrocyte that is highly branched and distributed throughout the gray matter,140 in autistic cortex.130 This same study observed dysregulation of developmental transcription factors in protoplasmic astrocytes.130 Astrocyte associated gene modules are also upregulated in ASD, schizophrenia, and bipolar disorder cortical samples of both sexes.129 As mentioned in section 3.1, ASD-elevated glial genes are expressed at higher levels in the male brain relative to the female brain, and this includes astrocytic markers,133 suggesting again that males may be more susceptible to immune-related pathways of ASD. Moreover, astrocytic cytokine signaling via IL-33 release directs microglial synaptic pruning during development,141 and astrocytes may also release ATP/ADP to regulate histamine-induced microglial phagocytosis in both sexes.142 Given that many potential avenues exist for communication between astrocytes and microglia and given that both cell types are dysregulated in ASD, astrocytes, too, may contribute to neurodevelopmental disorders, potentially through interaction with microglia and other immune cells. Future research is needed in this area, particularly within the domains of sex differences, neuroimmune crosstalk, and brain development in neurodevelopmental disorders.
3.3. Mast Cells
Mast cells are often-overlooked immune cells that play a role in sexual differentiation, neurodevelopment, and behavior. Mast cells are hematopoietic immune cells found in tissues throughout the body and are primarily known for their role in allergic and atopic disease.143 They can also be found in the brain and leptomeninges during embryonic development,144, 145 and they are highest in number in the brain during the perinatal period, where they are located preferentially at sites of blood vessel growth or on vessels sheathed by astrocytic processes.144 Mast cells house granules which contain neuroactive and inflammatory mediators, including cytokines, proteases, histamine, and serotonin.146, 147 When activated, mast cells undergo a process called degranulation, which involves bolus or piecemeal release of their granules into the extracellular space.148 Mast cells can also synthesize and release inflammatory mediators such as prostaglandins and cytokines independent of degranulation.149 Like microglia, mast cells are capable of sensing and responding to complement proteins, neuropeptides, steroid hormones, cytokines, and microbes,150 making them versatile cells. Interestingly, mast cell-mediated diseases are more common in females.151–153 This may stem from the fact that peripheral mast cells synthesize and store more immune mediators in females compared to males, resulting in more severe responses to immunological and psychological stress in females.154 This sex difference is established by perinatal androgen exposure.155
Sex differences have also been found in mast cells in the brain. For example, mast cells are sexually dimorphic in the rodent hippocampus and POA, where males have more mast cells compared to females.107, 156, 157 Such sex differences contribute to neurodevelopment and behavior, as mast cells are the first step in microglia-mediated sex-specific synaptic patterning of the POA. In the developing POA of males, estradiol binds to estrogen receptors on mast cells, inducing degranulation and release of histamine. This histamine binds to histamine receptors on microglia to facilitate microglia synthesis and release of prostaglandin, which binds to GPCRs on dendrites to facilitate dendritic spine formation – all of which is required for male-specific adult sexual behavior.156
Moreover, many studies in adult rodents have implicated mast cell in behaviors relevant to neurodevelopmental disorders, at least in males. Blockade of central, but not peripheral mast cell activity increases anxiety like behavior.158 Mast cell-deficient mice also display elevated anxiety-like behavior,158 and have deficits in hippocampal-dependent spatial learning and memory and reduced neurogenesis.159 Increasing the levels of mast cell mediator serotonin via chronic treatment with selective serotonin reuptake inhibitor fluoxetine reverses cognitive and neurogenic deficits in male mast cell-deficient mice.159 Application of histamine into the lateral septum of male rats decreases anxiety and novelty-suppressed feeding,160, 161 and spontaneous locomotor activity correlates with degranulation of mast cells in the meninges.162 Mast cell activity is also important for social behavior. Intracerebroventricular infusion of a mast cell activator increased sociability in male mice, whilst removal of mast cells in a diptheria toxin inducible knockout model reduced social preference levels without influencing anxiety- or depressive-like behavior.163 Given the sex differences that have been documented in mast cell number and function, future research determining whether these findings extend to females is merited.
Although few studies have specifically investigated mast cells in the context of neurodevelopmental disorders, evidence is mounting for altered peripheral mast cell activity in neurodevelopmental disorders, primarily due to the mast cell’s role in mediating allergy and atopy. The incidence of ASD is 10x higher in children with mastocytosis, a disease caused by mast cell hyperactivity, compared to children in the general population.164 Allergic diseases are associated with psychological and behavioral issues in preschoolers,165 and both ASD and ADHD have been linked to allergic disease.166 For example, allergic rhinitis, atopic dermatitis, asthma, eczema, food allergy, and urticaria have all been found to associate with either ASD167–170 and/or ADHD.171–178 The development of atopic dermatitis and asthma in early life increases risk for subsequent diagnosis of both ASD and ADHD.175, 179 Furthermore, allergic symptoms may contribute abnormal behavior, as overall allergy in autistic children was associated with higher measures of stereotyped behavior,169 and a study on children with ADHD found increased levels of allergic markers (IgE and eosinophils) in the blood and decreased levels of 5-HT and hemoglobin, and ADHD risk increased with the number of these biochemical factors present.177 Combined with evidence from rodent studies on mast cell regulation of behavior, this work suggests that peripheral and central mast cell activity may be involved in ASD and ADHD. Moreover, this work also demonstrates that we should consider peripheral inflammation when it comes to studying neurodevelopmental disorders and their rodent models. In the next section, we will discuss peripheral immune dysregulation documented in ASD and ADHD.
4. Peripheral Inflammation, Neurodevelopmental Disorders, and Brain Development
The brain was historically thought to be privileged from the immune system, as the BBB generally bars peripheral immune cells from freely entering the brain under normal conditions.180 However, the immune system communicates with the brain through three general mechanisms: diffusion at the sites of the brain’s circumventricular organs (CVOs), interactions at the endothelial interface of the BBB, and direct stimulation of the vagus nerve and sympathetic afferents.181 Cytokines are integral for immune to brain communication, and peripheral immune perturbation can readily affect this signaling.181 Moreover, given the ability of the immune system to communicate with the brain, and given the role that the immune system plays in neurodevelopment, it makes sense that peripheral immune dysregulation and perturbation are associated with neurodevelopmental disorders like ASD and ADHD. In this section, we will detail peripheral immune dysregulation documented in ASD and ADHD and discuss how rodent studies of early life inflammation model endophenotypes of neurodevelopmental disorders and provide insight into potential mechanisms of neurodevelopmental disorder-associated alterations in neurobehavioral development.
4.1. Peripheral immune dysregulation in ASD and ADHD
Evidence for immune dysregulation in ASD stems from multiple fields of study, though note that this work has been conducted primarily in male subjects with minimal attention paid to sex (see 4.1.1 for a discussion of sex differences in peripheral immune dysregulation). Certain haplotypes in the major histocompatibility complex (MHC), a major gene locus on chromosome 6 that codes for various immune-related proteins, have been associated with autism182 and many top gene sets associated with ASD involve immune pathways, including genes associated with recruitment of immune cells and the inflammatory response to viral infection.183 Likewise, autistic children exhibit cytokine dysregulation that is developmentally dynamic. Blood samples from neonates that subsequently received an ASD diagnosis display decreased levels both pro- and anti-inflammatory cytokines.184 Conversely, older autistic children have increased levels of proinflammatory cytokines 185–190 and reduced levels of the anti-inflammatory cytokine TGFβ.189–192 Altered cytokine levels may also contribute to symptomatology, as elevated levels of proinflammatory cytokines185, 188 and reduced levels of anti-inflammatory TGFβ191 are associated with increased symptom severity.
T cells, a term for a diverse group of lymphocytes involved in the adaptive immune response, are also dysregulated in ASD. Helper T cells, a subset of T cells, are some of the most prolific cytokine generators and can take on multiple phenotypes, three of which are Th1, Th2, and Th17.193 Th1 and Th17 cells are generally considered proinflammatory, whereas Th2 cells are generally considered anti-inflammatory. Peripheral immune cells isolated from autistic children show enhanced production of both Th1- and Th2-like cytokines,194 as well as Th1-, Th2-, and Th17-associated transcription factors.195 Autistic children also display reduced expression of transcription factors associated with a different class of T cell, the immunosuppressive regulatory T cell.195 Moreover, specific analysis of T cell numbers have found that autistic children have reduced numbers of regulatory T cells196 and Th1 cells,197 and increased numbers of Th2, Th17, and activated Th17 cells.196–198 A high ratio of Th17 cells to regulatory T cells is associated with more severe autistic symptoms.196 Increases in helper T cell activity in ASD may be due to decreased regulation by regulatory T cells, and the balance of T cell phenotypes in ASD may also be moderated by comorbidity with other syndromes. Rose et al. found that autistic children with comorbid gastrointestinal (GI) symptoms displayed elevated Th17 populations whereas autistic children without comorbid GI symptoms had increased frequency of Th2 populations, and both ASD groups had reduced regulatory T cell populations.199
Regarding ADHD, a study investigating gene expression in peripheral blood mononuclear cells found that genes differentially expressed in adults with ADHD (prior to statistical correction) were enriched for pathways relating to the immune and inflammatory response, with statistical correction still supporting differential expression of immune genes such as C1qA, TNFSF8, and IL7R.200 A recent meta-analysis on DNA methylation studies found six differentially methylated regions within the major histocompatibility complex, including complement-related genes C4A and C4B.201 Notably, overexpression of human C4A in mice increased microglia-mediated synaptic pruning during development in thalamus and cortex and reduced social preference behavior, increased anxiety, and impaired spatial working memory.202 Moreover, other studies have associated polymorphisms in various genes coding for proinflammatory cytokines with increased risk for ADHD and/or symptom severity,203, 204 and 2 polymorphisms in the IL-16 gene are associated with the inattentive ADHD phenotype.205 One study found a trend for elevated protein levels of pro and anti-inflammatory cytokines in the serum of patients with untreated ADHD, but not medicated ADHD patients.206 Another study found statistically elevated levels of serum IL-6 and IL-10 in children with ADHD,207 and serum IL-16 is positively associated with hyperactivity/impulsivity whereas serum IL-13 is positively associated with inattention.208
Some studies in peripheral blood suggest that the response to oxidative stress, a process associated with cellular damage, death, and immune activation, may be insufficient in individuals with ADHD.209 A separate study examining differential expression of genetic and epigenetic markers in peripheral blood cells found that expression of three microRNAs were predictive of ADHD diagnostic status.210 MicroRNAs interact with DNA to repress gene expression, and subsequent downstream analysis of genes normally targeted by these microRNAs that were differentially expressed in ADHD found enrichment of genes associated with “proliferation of neuroglia,” among other annotations.210 This finding suggests that epigenetic regulation of neuroglial proliferation may be altered in ADHD, though the ability to extrapolate findings from peripheral immune cells to the brain is limited.
Evidence also exists for a potential autoimmune component of ASD and ADHD. Antibodies that react to one’s own tissue, called autoantibodies, have been documented in both syndromes. In ASD, autoantibodies have been detected against proteins such as Hexokinase-1, brain derived neurotrophic factor (BDNF), glial fibrillary acid protein, myelin proteins, and heat shock protein 90, among others.211 Autoantibodies for unidentified proteins have also been detected in the hypothalamus, thalamus, and cerebellum tissue from the postmortem adult autistic brain.212, 213 A recent systematic review of studies examining autoantibodies in serum or CSF concluded that autistic children expressed higher levels of antibodies reactive to myelin proteins, endothelial cells proteins, nuclear antigens, and folate receptors, though more work needs to be done due to significant heterogeneity between studies.214
A small study in children has documented autoantibodies for GAD65, an enzyme expressed in GABAergic neurons in both ASD and ADHD,215 though another study in older patients could not replicate this finding.216 In line with evidence for cerebellar dysfunction in ADHD, two studies on children with ADHD found antibodies directed against Yo (PCA-1), a protein found in the cytoplasm of cerebellar Purkinje cells, in most ADHD cases (≥77.5%),217, 218 although again this finding was not replicated in a subsequent study.219 Another group found that a subset of individuals with ADHD had autoantibodies against the dopamine transporter (DAT),220, 221 and this correlated positively with ADHD symptoms in patients with a 10-repeat variant of the DAT1 gene.220 These autoantibodies may be related to neuroanatomical or functional abnormalities associated with neurodevelopmental disorders, as administration of myelin-specific antibodies isolated from autistic donors to rodent hippocampal slices reduced myelination and long-term potentiation in the hippocampus.222 Maternal autoimmune disease is associated with increased risk for ASD in offspring,223 and studies have also documented autoantibodies in the blood of some mothers of autistic children.211
As with individual autoantibodies, maternal autoantibodies may have consequences for offspring brain structure. For example, head and brain enlargement is found in about 16% and 9% of autistic patients (primarily examined males),224 and autistic male children from mothers with maternal autoantibodies displayed exacerbated ASD-associated brain enlargement (females not examined).225 This could be related to behavior, as brain enlargement was more severe in lower functioning patients.224 Likewise, a mouse model of maternal autoantibody exposure produced repetitive grooming, reduced play, and altered vocalization behavior in offspring of both sexes.226 However, there may be sex-specific effects regarding how these maternal autoantibodies causally relate to brain and behavioral deficits. A follow up study in the same mouse model found that maternal autoantibody exposure increased brain volume in female but not male offspring.227 Brain region volumes generally correlated positively with behavior in maternal antibody-exposed females whilst correlating negatively or not at all in exposed males.227 Thus begs the question: What other outcomes relating to neurodevelopmental disorder risk and etiology may vary according to sex that have not yet been systematically assessed?
4.1.1. What about sex differences?
As mentioned, a major problem persists in most studies mentioned in this section: no comparisons have been made by sex. The few studies that have examined sex as a biological variable in the context of neurodevelopmental disorder-associated immune dysregulation have found sex differences. For example, decreased levels of peripheral IL-1β, IL-8, MIP-1β, and VEGF were each associated with higher autism severity in females but not males.48 Conversely, platelet-derived growth factor (PDGF)-BB was negatively associated with ASD severity in both sexes.48 A separate study examining serum biomarkers in adults with and without Asperger’s syndrome (AS), an ASD characterized by less severe symptoms and the absence of language difficulty, found distinct molecular profiles in males and females. Males with AS primarily displayed increases in various cytokines whereas females with AS primarily displayed increases in levels of growth factors like BDNF.49 These data call into question the applicability and validity of previous research in neurodevelopmental disorder-related immune dysregulation and underscore the need for the inclusion of both males and females in clinical research.
4.2. Maternal/prenatal immune activation
In humans, maternal immune activation (MIA) during pregnancy, whether it be through infection, allergy, autoimmunity, or pollution, increases risk for neurodevelopmental disorders in the offspring, including ASD, ADHD, and schizophrenia (see Box 1 for a brief discussion of schizophrenia).175, 228–232 Rodent studies of maternal immune activation (MIA) during pregnancy have improved our understanding of the underlying physiological mechanisms through which immune perturbation during pregnancy may confer increased risk for neurobehavioral and immune abnormalities in the offspring, including in some cases sex differences in this risk or resilience.
Box 1: Maternal immune activation and sex differences: Relevance to Schizophrenia.
Schizophrenia is a long-term psychiatric disorder that typically shows onset in the late teenage years. Despite the latent nature of onset in late adolescence to early adulthood, there are early life risk factors for schizophrenia that overlap with the neurodevelopmental disorders covered in this chapter. In particular, maternal immune activation during pregnancy, such as viral infection, is associated with on average a 3-fold increased risk for schizophrenia in offspring,232, 233 as are obstetric complications, preeclampsia, and nutritional deficiencies during pregnancy (for review, see 234). There is also clinical evidence of inflammatory differences in male and female schizophrenic individuals, including elevated peripheral cytokines and altered TSPO PET labeling (sometimes increased, sometimes decreased) in schizophrenic individuals that correlate with symptom severity, and increased markers of inflammation in the postmortem brains of schizophrenic individuals.234–236 Interestingly, in a study that has directly compared gene expression profiles across psychiatric disorders, the same astrocyte gene modules are seen to be elevated in both schizophrenia and ASD but with noteworthy differences were seen in microglia-related gene module expression between the two disorders.129
Given this relationship between immune activation and schizophrenia, maternal immune activation rodent models are also relevant to the study of schizophrenia, and many research groups have assessed neural systems hypothesized to be implicated in schizophrenia (e.g., dopaminergic system, excitatory/inhibitory balance) as well as behavioral domains of particular relevance to schizophrenia (e.g., sensory gating of startle responses, attentional and cognitive flexibility deficits) (e.g., 237–239) to understand the mechanisms through which MIA could contribute to the etiology of schizophrenia. Thus, many of the studies which we report on in this chapter as relevant to the understanding of ASD may also be relevant to our understanding of schizophrenia, and in fact, were conceptualized that way by the researchers who performed them.
With regard to sex differences, there is a slight male sex bias in the overall rate of schizophrenia (1.4:1) that is far less dramatic than that seen with ASD and ADHD, but there are also sex differences in age of onset, with males typically showing significantly earlier age of symptom onset than females (in late teens-early twenties in males versus late twenties-early thirties in females).240, 241 Symptom-wise, female tend to show higher rates of affective symptoms, particularly later in the course of the disorder as they undergo menopause (which may show a modulatory role for female-typical hormones in this sex difference), whereas males show more severe symptoms overall and more positive symptoms, such as hallucinations and delusions (reviewed in 241). Regarding the genetics of schizophrenia, interesting recent work has now implicated the complement system, in particular the C4 gene locus in the major histocompatibility complex, as highly associated with sex-specific risk for schizophrenia in males.242 While mutations in the C4 allele are associated with elevated schizophrenia risk in both sexes, they are more pronounced in males, and the group found that C4 protein levels are higher in schizophrenic individuals, and higher in males in CSF during earlier ages (twenties versus fifties), which may explain male-biased risk for earlier onset schizophrenia.242 Interestingly this same group demonstrated collaboratively that C4 is necessary for developmental synaptic pruning by microglia to occur in the brain.243 Thus, overactive C4 function in schizophrenia, and particularly in males, may in part be developmentally responsible for the onset of the disorder, and maternal immune activation may in turn further perturb this developmental function of the neuroimmune system and be relevant to sex-specific schizophrenia risk or onset.
Two models of prenatal infection have been used in the majority of preclinical MIA studies in rodents: LPS, which models bacterial infection, and the synthetic double stranded RNA polyinosinic:polycytidylic acid (Poly I:C), which models viral infection.244 LPS binds to toll-like receptor (TLR) 4 while Poly I:C binds to TLR 3 on immune cells to mimic the acute-phase response to infection, and both cascades converge on the nuclear factor kappa B (NFkB) pathway to produce strong Th1-like cytokine responses.244 While LPS is a potent inducer of TNFα, Poly I:C is a potent inducer of interferons.244 However, these infection models are not the only rodent models of MIA. Given the increasing links between pollution, allergy, and neurodevelopmental disorders,166, 175, 231 other more recently developed MIA paradigms have focused on pollution-induced inflammation or allergy. These models generally involve inflammation beginning in the airway that result in the expansion of the Th2-like cytokine response, as opposed to the Th1-like cytokine response.245, 246 Generally, MIA studies across models reproduce behavioral endophenotypes associated with neurodevelopmental disorders.
Most MIA studies have been performed exclusively in male rodents. These studies have found that MIA impairs communication behavior, either reducing247, 248 or increasing249 neonatal ultrasonic vocalizations. MIA paradigms have found reduced social behavior, 248–251 impaired learning and memory,239, 247 anxiety-like behavior,239, 250, 252 and altered sensorimotor gating.250, 253 Additionally, some MIA studies have found increased repetitive behaviors, as assessed by self-grooming or marble burying.247–249, 254 At the neurochemical level, these male-focused studies have found evidence for reduced dopaminergic activity, which aligns well with hypotheses of reduced motivation and reward in human neurodevelopmental disorders. Specifically, levels of the dopamine-synthesis enzyme tyrosine hydroxylase, as well as dopamine and dopamine metabolite levels in are reduced in the striatum of adult offspring,247, 255 as well as dopamine hypofunction in the hypothalamus.254 Reduced levels of GABA synthesis enzyme, GAD67, in medial prefrontal cortex interneurons in adult male offspring have also been seen after MIA,239 suggestive of altered excitatory-inhibitory balance in regions critical for cognition.
In terms of immune mechanisms, two peripheral cytokines, IL-6 and IL-17 are necessary for the behavioral effects of Poly I:C induced-MIA, at least in males. Poly I:C injection on E12.5 reduces sensorimotor gating, exploration, and social behavior in adult offspring, and genetic knockout or blockade of IL-6 action prevents these behavioral alterations as well as MIA-induced changes in cortical gene expression.250 A different group found that the same paradigm increased levels of serum and placental IL-17a and IL-17 receptor subunit A (Ra) expression in the fetal brain in an IL-6-dependent manner, suggesting that IL-17a acts downstream of IL-6.249 These offspring displayed abnormal cortical lamination, increased ultrasonic vocalizations, repetitive behavior, and impaired sociability, and maternal treatment with an IL-17a blocking antibody protected offspring from these effects.249 Th17 cells may be the drivers of poly I:C induced behavioral deficits, as expression of RORγt, a transcriptional regulator of Th17 cell development, in maternal T cells was required for the abnormal behavioral phenotype in the offspring.249 Importantly, the evidence for elevated IL-17Ra levels in the fetal brain suggests increased IL-17a or Th17 activity in the fetus as well. IL-17 activity downregulates both Th1 and Th2 responses,256 thus this data goes well with research from humans showing that blood samples from neonates that would eventually receive an ASD diagnosis had decreased levels of both Th1-like and Th2-like cytokines.184 Meanwhile, a separate study found that Poly I:C offspring display decreased regulatory T cells and hyper responsive helper T cells in the periphery of adult offspring,257 which also goes along with work in older autistic patients that was discussed above.
Microglia changes have been examined in the context of MIA, but findings are heterogenous and thus challenging to make broad conclusions about. Most MIA studies that have investigated microglia examine effects on juvenile and adult male offspring, with some studies finding increased microglial activation or density and others finding the opposite or no change.258 Some studies on adult offspring have found no changes in microglia markers, number, or density in multiple brain regions.247, 253, 259 However, other studies have found elevated microglia number and activation in neonatal and adolescent amygdala,260 as well as the juvenile hippocampus and striatum,261 all regions important for social behavior, cognition, and locomotor activity. Additionally, lack of change in microglia number or staining density does not necessarily reflect a lack of change in function. Studies in a Poly I:C model have found impaired hippocampal neurogenesis, reduced expression of inflammatory response genes, and impaired microglial phagocytosis in adult male offspring (females not examined).253, 262 Notably, microglial inhibition with chronic minocycline treatment in adulthood after MIA rescued neurogenesis, gene expression, phagocytosis, and behavior in these males, suggesting a causal role for microglia in the neurogenic deficits and behavioral phenotype.253, 262 Evidence suggests that microglia may also be affected proximally to the immune insult, as Poly I:C MIA alters the transcriptome of early-stage microglia, indicating increased maturity and inflammatory capacity, at least in males (females not examined).102 This study goes along with other work suggesting accelerated microglial development in ASD.104 Thus, microglia may be affected proximally to the immune insult and during a critical period of development, programming long-term changes in brain and behavior. Furthermore, MIA-induced microglia alterations may vary according to paradigm, age at immune challenge, and age at assessment.
All the above-mentioned studies were conducted in males, so it is unknown the extent to which these particular findings apply in females, which will hopefully be addressed in future work. Studies that examine both sexes enable scientists to tease out potential sex differences in vulnerability to prenatal inflammation. Some studies across paradigms have documented inflammation-induced behavioral changes in both sexes, including decreased sociability,107, 245, 263, 264 altered sexual behavior,265 increased rearing and/or repetitive behavior,245, 263, 264, 266, 267 disrupted sensorimotor gating,237 increased baseline startle response,268 and impaired cognitive flexibility.107 Studies have also found increased anxiety-like behavior 269 and increased107, 263, 266, 267, 270 or decreased268 locomotor activity in both sexes. However, other MIA studies suggest that males may be preferentially affected by MIA, which would make sense given the sex bias in prevalence rates, though findings vary across paradigms (See Table 2 for a summary). For example, LPS-induced MIA reduces juvenile social play behavior in male but not female Wistar rats.247, 251, 271, 272 Additionally, these MIA males displayed altered vasopressin expression in the amygdala,271 blunted play-induced neuronal activation in PFC, NAc, and striatum, and potentiated play-induced neuronal activation in the amygdala.272 Likewise, one study found that Poly I:C preferentially impaired social play in male mice,267 while another found social deficits in both sexes.264 However, male mice were more susceptible to Poly I:C-induced anxiety-like behavior and sensorimotor gating impairments.264 Likewise, a combined model of diesel exhaust particle exposure and maternal stress (DEP+MS) increases anxiety-like behavior only in male offspring.273 This same paradigm preferentially impairs neonatal ultrasonic vocalizations and fear conditioning in male mice,273, 274 yet increases juvenile locomotor behavior and neophobia in both sexes.273 In summary, MIA appears to affect behavior in both males and females, though some research points to sex-specific vulnerabilities, particularly in males.
Table 2:
Summary of behavioral findings in studies of prenatal inflammation. Studies cited include rat and mouse models.
| Offspring Behavior | Findings from Studies with Both Sexes |
|---|---|
Vocalizations
|
- DEP+MS MIA increases vocalization number and decreases call frequency in male but not female neonates.274 - LPS MIA decreases vocalization number in male but not female neonates.272 - Poly I:C MIA reduces number of vocalizations emitted during juvenile play in both sexes.267 |
Social Behavior
|
- LPS and Poly I:C MIA reduce juvenile play in males and not females.247, 251, 267, 271, 272 - Poly I:C, Acute allergic MIA, and Chronic allergic MIA reduce sociability in both sexes.107, 245, 263, 264 |
Sex Behavior

|
- Acute Allergic MIA masculinizes female sex behavior and reduces male olfactory preference for females.265 |
Repetitive Behavior

|
- Chronic Allergic and Poly I:C MIA increase marble burying in both sexes.245, 263, 264, 267 |
Locomotor Activity
|
- In-utero LPS, DEP+MS, and Allergic MIA produce hyperactivity in both sexes.107, 263, 266, 270, 273 - Poly I:C MIA produces hypoactivity268 or hyperactivity267 in both sexes. |
Cognition
|
- DEP+MS MIA impairs fear conditioning only in males.273 - Acute Allergic MIA reduces cognitive flexibility in both sexes.107 |
DEP = diesel exhaust particle exposure; LPS = lipopolysaccharide; MIA = maternal immune activation; MS = maternal stress.
While causal manipulations are lacking, studies examining MIA effects in both sexes have found alterations to microglia in combination with such impaired behavior discussed above. Adult offspring exposed to Poly I:C on E15 display increases in microglia number and/or activation in the pons, thalamus, corpus callosum, and hippocampus.268 Notably, differential mast cell and microglia number/activation in the neonatal preoptic area is responsible for programming adult sex behavior,108, 156 and acute prenatal allergic inflammation produces female-specific increases in mast cell number and ameboid microglial tone in the neonatal POA, and this is followed by reversed sexual differentiation of POA dendritic spine density and masculinized sexual behavior in adulthood.265 A follow up in the same paradigm found impaired juvenile social play, late adolescent hyperactivity, and adult cognitive inflexibility in male and female offspring, and these behaviors were preceded by decreases in neonatal microglia colonization in brain regions relevant to these behaviors in both sexes.107 While only conducted in females, a separate study in a model of maternal allergic asthma found that differentially methylated regions in adolescent offspring were enriched for transcription factor binding sites related to regulation of microglial development and microglial inflammation (males not examined).275 Further analysis revealed that differentially expressed and methylated genes in this model significantly overlapped with a list of ASD risk genes,275 indicating that alterations to microglia function may be causally implicated in ASD.
Other work suggests that males and females may be differentially susceptible to certain immune-related consequences of prenatal inflammation. Generally, males may be predisposed to an elevated immune profile following MIA. For example, while prenatal Poly I:C on E9.5 increases C3 protein expression in the dentate gyrus of adult offspring of both sexes, it only produces microglia activation in males, despite female-specific increases in microglial CD68 and CD11b in the hippocampus.264 Prenatal DEP exposure increases inflammatory tone in the fetal male brain, decreasing brain IL-10 and elevating the number of ameboid microglia in the cortex and hippocampus.273 The opposite is true in the fetal female brain, where prenatal DEP elevates brain IL-10 and decreases the number ameboid microglia in the dentate gyrus.273, 274, 276 As juveniles, DEP males have elevated levels of cytokines, increased ameboid microglia, and also display increased neuron-microglia overlap.273, 276 Combined DEP + MS increases IL-1β/IL-10 ratio in the brain of adult male offspring, but decreases it in females.273, 274 Notably, as mentioned above, these studies found more male-specific behavioral alterations in anxiety-like behavior as adults, but both males and females exhibited hyperactivity and reduced social exploration as juveniles.274
Such an elevated immune profile may predispose males to have a hyperactive immune response following an acute challenge. A separate study employing an LPS model of in utero inflammation found reduced myelination of the corpus callosum and increased microglia number in adult offspring of both sexes.266, 270 However, some proximal and ultimate immune consequences were disparate between the sexes. IL-1β and TNFα were elevated in fetal male brains 48hrs following prenatal LPS challenge, a trend that was absent in females, and male offspring also displayed increased levels of TGFβ, C1q, and C3 in the brain as neonates, whereas female neonates had decreased levels of C3.266 Interestingly, these male offspring had potentiated central cytokine responses to acute administration of LPS in adulthood that were absent in females.266 Collectively, these findings suggests that males may be more susceptible to both inflammatory risk factors for and consequences of prenatal inflammation, which would make sense given genetic data on inflammatory markers discussed above.133 Additionally, given that LPS advances microglial maturity in males but not females,102, 104 exposure to immune challenges throughout life may explain why adult male brains have higher indices of microglial maturity, and why the brains of autistic individuals also display signs of elevated microglial maturity.104 It is therefore imperative that MIA studies should examine sex as a potential moderator of outcomes, given that 1) in humans, both males and females develop neurodevelopmental disorders, 2) MIA may induce sex-specific behavioral deficits, and 3) MIA may induce similar behavioral deficits in the sexes, but through different mechanisms.
4.3. Early life immune activation
Immune activation during the postnatal period also increases risk for neurodevelopmental disorders. Preterm born children who experience systemic inflammation in the first postnatal month have increased risk for ADHD or ASD-related symptoms.277, 278 In line with data showing that the development of atopic dermatitis and asthma in early life increases risk for subsequent diagnosis of both ASD and ADHD,175, 179 a rodent model of chronic allergic asthma beginning in the neonatal period found repetitive behavior and impaired sociality in adult (but not juvenile) animals.279 These animals also had impaired microglial engulfment of postsynaptic markers and reduced expression of engulfment-related genes during the peak pruning period in the hippocampus, and this was followed by an excess of excitatory synapses in the hippocampus.279 A separate model of early life infection with E. coli increases cytokine and microglial expression in the hippocampus of neonatal males (females not examined),280 causes hypomyelination of subcortical white matter and motor cortex in juvenile males,281 and increases levels of microglial markers and hyperreactive astrocytes in the adult hippocampus.280, 282 Behavioral sequalae include locomotor hyperactivity and impaired coordination as juveniles and adults,281 as well as impaired learning and memory following acute LPS challenge.280, 282 Blockade of IL-1β synthesis or microglial activation prior to LPS challenge prevented the combined effects of neonatal infection + LPS on learning and memory,280, 283 collectively suggesting that microglia-mediated mechanisms may underlie impairments induced by neonatal inflammation, at least in males.
Notably, diets that are high in fat induce systemic and chronic low-grade inflammation in both brain and body,284 and gestational obesity is a risk factor for ADHD.285 Thus, perinatal exposure to high fat diet (HFD) through manipulation of maternal chow during pregnancy and lactation is yet another relevant model of early inflammation. As such, perinatal HFD produces a hypodopaminergic condition in the mesocorticolimbic pathway in adult males (females not examined).286 This paradigm also decreases motivation to earn reward and increases impulsivity in adults male and female offspring.287 Male and female HFD offspring also display elevated concentrations of cysteine in the prefrontal cortex, consistent with oxidative stress.288 Notably, many of these deficits can be rescued in both sexes through dietary supplementation with methyl donors, which are nutrients that supply methyl groups important for cellular function and protein synthesis.289, 290 However, some effects of perinatal HFD appear to be male-biased. Examination of gene expression in the prefrontal cortex of male offspring found that impulsivity related to overexpression of DNMT1 (females not examined),287 and this may represent a compensatory mechanism for decreased DNA methylation in these males (females not examined).286 A follow up study found that this was a male-specific effect, as perinatal HFD reduced DNA methylation in the male but not female prefrontal cortex, and this could be rescued with dietary supplementation of methyl donors.288
Other studies also suggest there may be sex differences following neonatal inflammation. Specifically, neonatal challenge with LPS increased anxiety- and depressive-like behaviors, risk-taking, and working memory deficits in peri-adolescent male mice, with the anxiety- and depressive-like behaviors persisting into adulthood. Conversely, at either timepoint, female mice only displayed deficits in sensorimotor gating.291 As adults, males also had increased nitrite levels and decreased parvalbumin in the hippocampus, while both sexes experienced increased levels of pro-inflammatory cytokines in the brain.291 A similar paradigm found impaired spatial memory in adult males but not females, though both sexes displayed social impairments as adolescents.292 Conversely, a recent study found that neonatal inflammation with LPS produces female-specific impairments in adult sociability and social discrimination, and this was combined with increased somatostatin cell number in the anterior cingulate cortex.293 As a whole, these studies suggest that males may be more susceptible to emotional and cognitive related impairments following neonatal inflammation, whereas females may or may not be more susceptible to social impairment. More work needs to be done to ascertain the consistency of sex differences in these domains, as well as whether they may vary by age of inflammation, age at assessment, or other factors.
5. Concluding Remarks & Ways Forward
Autism and ADHD are the two most common neurodevelopmental disorders diagnosed in children. Both conditions are diagnosed more commonly in males, and sex differences in symptomology, onset, comorbidities, and immunological abnormalities have been documented. Despite this, most clinical and preclinical studies on immune-related mechanisms and endpoints of neurodevelopmental disorders have not examined sex as a biological variable (See Box 2 for our summary of the major knowledge gaps and high priority future goals). Overall, research that has investigated sex differences reveals that males and females may be differentially susceptible to certain neurodevelopmental disorder-associated risk factors, with males potentially being more vulnerable to immune-related risk factors for these conditions. Likewise, mechanisms underlying ASD and ADHD in males and females may be different and merit further investigation. Only by studying both males and females in both human studies as well as animal models and considering how sex interacts with gender norms and societal expectations to lead to diagnostic biases will we gain a complete picture of how neurodevelopmental disorders arise and grasp the full complexity of the etiology, symptomology and treatment strategies for these complex conditions.
Box 2: Major Knowledge Gaps.
Throughout this chapter, we have discussed what research has taught us regarding ASD, ADHD and animal models. However, whether many well-established research findings apply to females is still unknown. Given that neurodevelopmental disorders continue to affect both males and females, we believe the following questions should be priorities for scientific research moving forward.
To what extent does sex affect the developmental trajectory of ASD and ADHD, and how does this change our understanding of symptomology and prevalence? (Section 2)
Are males and females differentially susceptible to specific genetic risk factors or polygenic interactions for ASD and ADHD? (Sections 3.1 and 3.2)
Do the patterns of central and peripheral immune dysregulation in ASD and ADHD apply to females with these conditions or are they sex-specific? (Sections 3 and 4)
Does the maternal response to males and females during gestation modulate the developmental etiology of ASD or other NDDs?
Developing animal models that better reflect sex-specific genetic risk and protective factors for NDDs to dissect mechanisms at play in NDD etiology, and studying them across the lifespan and in combination with environmental risk exposures (Section 4.2).
Funding:
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program. Any opinions, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Identity-first language will be used when referring to autistic individuals and person-first language will be used when referring to individuals with ADHD to represent and respect the wishes of those communities, respectively.11 Additionally, while many of the studies cited in this chapter refer to “boys,” “girls,” “men,” and “women,” the authors will consistently refer to “males” and “females” to reflect that these studies examined cis-gender individuals.
Conflict of Interest Statement: The authors declare no competing financial interests
6. References
- 1.Diagnostic and Statistical Manual of Mental Disorders (5th ed.). 2013, Washington, DC: The American Psychiatric Association. [Google Scholar]
- 2.Zablotsky B, et al. , Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics, 2019. 144(4): p. e20190811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaroff CM and Uhm SY, Prevalence of autism spectrum disorders and influence of country of measurement and ethnicity. Soc Psychiatry Psychiatr Epidemiol, 2012. 47(3): p. 395–8. [DOI] [PubMed] [Google Scholar]
- 4.Hertz-Picciotto I and Delwiche L, The Rise in Autism and the Role of Age at Diagnosis. Epidemiology, 2009. 20(1): p. 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polanczyk GV, et al. , ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol, 2014. 43(2): p. 434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciberras E, et al. , Prenatal Risk Factors and the Etiology of ADHD—Review of Existing Evidence. Current Psychiatry Reports, 2017. 19(1). [DOI] [PubMed] [Google Scholar]
- 7.Rice CE, et al. , Evaluating Changes in the Prevalence of the Autism Spectrum Disorders (ASDs). Public Health Reviews, 2012. 34(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vovou F, Hull L, and Petrides KV, Mental health literacy of ADHD, autism, schizophrenia, and bipolar disorder: a cross-cultural investigation. Journal of Mental Health, 2021. 30(4): p. 470–480. [DOI] [PubMed] [Google Scholar]
- 9.Zablotsky B, Black LI, and Blumberg SJ, Estimated Prevalence of Children with Diagnosed Developmental Disabilities in the United States, 2014–2016. 2017. [PubMed]
- 10.Danielson ML, et al. , Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J Clin Child Adolesc Psychol, 2018. 47(2): p. 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.1,000 people surveyed, survey says…, in THE OARACLE. 2020, The Organization for Autism Research: Online. [Google Scholar]
- 12.DSM-5 Autism Spectrum Disorder [Fact Sheet]. 2013.
- 13.Miller JN and Ozonoff S, The external validity of Asperger disorder: Lack of evidence from the domain of neuropsychology. Journal of Abnormal Psychology, 2000. 109(2): p. 227–238. [PubMed] [Google Scholar]
- 14.Mayes SD, Calhoun SL, and Crites DL, Journal of Abnormal Child Psychology, 2001. 29(3): p. 263–271. [DOI] [PubMed] [Google Scholar]
- 15.Werling DM and Geschwind DH, Sex differences in autism spectrum disorders. Curr Opin Neurol, 2013. 26(2): p. 146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Wijngaarden-Cremers PJ, et al. , Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. J Autism Dev Disord, 2014. 44(3): p. 627–35. [DOI] [PubMed] [Google Scholar]
- 17.Andersson GW, Gillberg C, and Miniscalco C, Pre-school children with suspected autism spectrum disorders: do girls and boys have the same profiles? Res Dev Disabil, 2013. 34(1): p. 413–22. [DOI] [PubMed] [Google Scholar]
- 18.Mayes SD, Castagna PJ, and Waschbusch DA, Sex Differences in Externalizing and Internalizing Symptoms in ADHD, Autism, and General Population Samples. Journal of Psychopathology and Behavioral Assessment, 2020. 42(3): p. 519–526. [Google Scholar]
- 19.Wiggins LD, et al. , Evaluation of sex differences in preschool children with and without autism spectrum disorder enrolled in the study to explore early development. Res Dev Disabil, 2021. 112: p. 103897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasca BC, et al. , Sex Differences in Externalizing and Internalizing Symptoms of Children with ASD. J Autism Dev Disord, 2020. 50(9): p. 3245–3252. [DOI] [PubMed] [Google Scholar]
- 21.Giarelli E, et al. , Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J, 2010. 3(2): p. 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandy W, et al. , Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord, 2012. 42(7): p. 1304–13. [DOI] [PubMed] [Google Scholar]
- 23.May T, Cornish K, and Rinehart N, Does gender matter? A one year follow-up of autistic, attention and anxiety symptoms in high-functioning children with autism spectrum disorder. J Autism Dev Disord, 2014. 44(5): p. 1077–86. [DOI] [PubMed] [Google Scholar]
- 24.May T, Cornish K, and Rinehart NJ, Gender Profiles of Behavioral Attention in Children With Autism Spectrum Disorder. J Atten Disord, 2016. 20(7): p. 627–35. [DOI] [PubMed] [Google Scholar]
- 25.Szatmari P, et al. , Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet, 2012. 159B(1): p. 5–12. [DOI] [PubMed] [Google Scholar]
- 26.Kaat AJ, et al. , Sex differences in scores on standardized measures of autism symptoms: a multisite integrative data analysis. J Child Psychol Psychiatry, 2021. 62(1): p. 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbett BA, et al. , Camouflaging in Autism: Examining Sex-Based and Compensatory Models in Social Cognition and Communication. Autism Res, 2021. 14(1): p. 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter AS, et al. , Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 2007. 37: p. 86–97. [DOI] [PubMed] [Google Scholar]
- 29.Sipes M, et al. , Gender differences in symptoms of Autism Spectrum Disorders in toddlers. Research in Autism Spectrum Disorders, 2011. 5(4): p. 1465–1470. [Google Scholar]
- 30.Solomon MB and Herman JP, Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav, 2009. 97(2): p. 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrop C, et al. , Visual attention to faces in children with autism spectrum disorder: are there sex differences? Molecular Autism, 2019. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLennan JD, Lord C, and Schopler E, Sex differences in higher functioning people with autism. Journal of Autism and Developmental Disorders, 1993. 23: p. 217–227. [DOI] [PubMed] [Google Scholar]
- 33.Mahendiran T, et al. , Sex Differences in Social Adaptive Function in Autism Spectrum Disorder and Attention-Deficit Hyperactivity Disorder. Front Psychiatry, 2019. 10: p. 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai MC, et al. , Quantifying and exploring camouflaging in men and women with autism. Autism, 2017. 21(6): p. 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rynkiewicz A, et al. , An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism, 2016. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiller RM, Young RL, and Weber N, Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism, 2016. 20(1): p. 75–84. [DOI] [PubMed] [Google Scholar]
- 37.Willcutt EG, The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics, 2012. 9(3): p. 490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biederman J, et al. , Influence of Gender on Attention Deficit Hyperactivity Disorder in Children Referred to a Psychiatric Clinic. American Journal of Psychiatry, 2002. 159: p. 36–42. [DOI] [PubMed] [Google Scholar]
- 39.Rucklidge JJ, Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am, 2010. 33(2): p. 357–73. [DOI] [PubMed] [Google Scholar]
- 40.Gershon J, A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders, 2002. 5(3): p. 143–154. [DOI] [PubMed] [Google Scholar]
- 41.Arnett AB, et al. , Sex differences in ADHD symptom severity. J Child Psychol Psychiatry, 2015. 56(6): p. 632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottosen C, et al. , Sex Differences in Comorbidity Patterns of Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry, 2019. 58(4): p. 412–422 e3. [DOI] [PubMed] [Google Scholar]
- 43.Carlson CL, Tamm L, and Gaub M, Gender Differences in Children With ADHD, ODD, and Co-Occurring ADHD/ODD Identified in a School Population. Journal of the American Academy of Child & Adolescent Psychiatry, 1997. 36(12): p. 1706–1714. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen K and Levander S, Untreated ADHD in Adults. Journal of Attention Disorders, 2009. 12(4): p. 353–360. [DOI] [PubMed] [Google Scholar]
- 45.Balint S, et al. , Attention deficit hyperactivity disorder (ADHD): gender- and age-related differences in neurocognition. Psychol Med, 2009. 39(8): p. 1337–45. [DOI] [PubMed] [Google Scholar]
- 46.Rucklidge JJ and Tannock R, Psychiatric, Psychosocial, and Cognitive Functioning of Female Adolescents With ADHD. Journal of the American Academy of Child & Adolescent Psychiatry, 2001. 40(5): p. 530–540. [DOI] [PubMed] [Google Scholar]
- 47.Chronis-Tuscano A, et al. , Very Early Predictors of Adolescent Depression and Suicide Attempts in Children With Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry, 2010. 67(10): p. 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masi A, et al. , Cytokine levels and associations with symptom severity in male and female children with autism spectrum disorder. Mol Autism, 2017. 8: p. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz E, et al. , Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol Psychiatry, 2011. 16(12): p. 1213–20. [DOI] [PubMed] [Google Scholar]
- 50.Martin J, et al. , Sex-specific manifestation of genetic risk for attention deficit hyperactivity disorder in the general population. J Child Psychol Psychiatry, 2018. 59(8): p. 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray AL, et al. , Sex differences in ADHD trajectories across childhood and adolescence. Dev Sci, 2019. 22(1): p. e12721. [DOI] [PubMed] [Google Scholar]
- 52.Gaub M and Carlson CL, Gender Differences in ADHD: A Meta-Analysis and Critical Review. Journal of the American Academy of Child & Adolescent Psychiatry, 1997. 36(8): p. 1036–1045. [DOI] [PubMed] [Google Scholar]
- 53.Williamson D and Johnston C, Gender differences in adults with attention-deficit/hyperactivity disorder: A narrative review. Clin Psychol Rev, 2015. 40: p. 15–27. [DOI] [PubMed] [Google Scholar]
- 54.Breedlove SM, Sexual Differentiation of the Human Nervous System. Annual Review of Psychology, 1994. 45: p. 389–418. [DOI] [PubMed] [Google Scholar]
- 55.Nugent BM, et al. , Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience, 2015. 18(5): p. 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smail PJ, et al. , The Fetal Hormonal Environment and its Effect on the Morphogenesis of the Genital System. Pediatric Andrology, 1981. 7: p. 9–19. [Google Scholar]
- 57.McCarthy MM, Estradiol and the Developing Brain. Physiological Reviews, 2008. 88: p. 91–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lephart E, A review of brain aromatase cytochrome P450. Brain Research Reviews, 1996. 22(1): p. 1–26. [PubMed] [Google Scholar]
- 59.Wallen K, Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in Neuroendocrinology, 2005. 26(1): p. 7–26. [DOI] [PubMed] [Google Scholar]
- 60.George FW and Ojeda SR, Changes in Aromatase Activity in the Rat Brain during Embryonic, Neonatal, and Infantile Development*. Endocrinology, 1982. 111(2): p. 522–529. [DOI] [PubMed] [Google Scholar]
- 61.Lauber ME, Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. 1994. 135(4): p. 1661–1668. [DOI] [PubMed] [Google Scholar]
- 62.Pheonix CH, et al. , Organizational action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology, 1959. 65: p. 369–382. [DOI] [PubMed] [Google Scholar]
- 63.Cesta CE, et al. , Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychological Medicine, 2020. 50(4): p. 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherskov A, et al. , Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Translational Psychiatry, 2018. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knickmeyer R, et al. , Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm Behav, 2006. 50(1): p. 148–53. [DOI] [PubMed] [Google Scholar]
- 66.Auyeung B, et al. , Fetal Testosterone Predicts Sexually Differentiated Childhood Behavior in Girls and in Boys. Psychological Science, 2009. 20(2): p. 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auyeung B, et al. , Foetal testosterone and autistic traits in 18 to 24-month-old children. Molecular Autism, 2010. 1(1): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baron-Cohen S, et al. , Elevated fetal steroidogenic activity in autism. Mol Psychiatry, 2015. 20(3): p. 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baron-Cohen S, et al. , Foetal oestrogens and autism. Mol Psychiatry, 2020. 25(11): p. 2970–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day DB, et al. , Prenatal sex hormones and behavioral outcomes in children. Psychoneuroendocrinology, 2020. 113: p. 104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lombardo MV, et al. , Sex-specific impact of prenatal androgens on social brain default mode subsystems. Mol Psychiatry, 2020. 25(9): p. 2175–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross JL, et al. , Behavioral and Social Phenotypes in Boys With 47,XYY Syndrome or 47,XXY Klinefelter Syndrome. Pediatrics, 2012. 129(4): p. 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kung KT, et al. , No relationship between prenatal androgen exposure and autistic traits: convergent evidence from studies of children with congenital adrenal hyperplasia and of amniotic testosterone concentrations in typically developing children. J Child Psychol Psychiatry, 2016. 57(12): p. 1455–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jamnadass ESL, et al. , The perinatal androgen to estrogen ratio and autistic-like traits in the general population: a longitudinal pregnancy cohort study. Journal of Neurodevelopmental Disorders, 2015. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitehouse AJ, et al. , Perinatal testosterone exposure and autistic-like traits in the general population: a longitudinal pregnancy-cohort study. Journal of Neurodevelopmental Disorders, 2012. 4(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarachana T, et al. , Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS One, 2011. 6(2): p. e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu VW, et al. , Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Molecular Autism, 2015. 6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginhoux F, et al. , Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science, 2010. 330(6005): p. 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verney C, et al. , Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. Journal of Anatomy, 2010. 217(4): p. 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bennett ML, et al. , New tools for studying microglia in the mouse and human CNS. Proceedings of the National Academy of Sciences, 2016. 113(12): p. E1738–E1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frost JL and Schafer DP, Microglia: Architects of the Developing Nervous System. Trends Cell Biol, 2016. 26(8): p. 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ratnayake U, et al. , Cytokines and the neurodevelopmental basis of mental illness. Front Neurosci, 2013. 7: p. 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vezzani A and Viviani B, Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology, 2015. 96: p. 70–82. [DOI] [PubMed] [Google Scholar]
- 84.Shigemoto-Mogami Y, et al. , Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci, 2014. 34(6): p. 2231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson LH, Warden S, and Lenz KM, Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun, 2017. 64: p. 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deverman BE and Patterson PH, Cytokines and CNS development. Neuron, 2009. 64(1): p. 61–78. [DOI] [PubMed] [Google Scholar]
- 87.Ueno M, et al. , Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci, 2013. 16(5): p. 543–51. [DOI] [PubMed] [Google Scholar]
- 88.Nelson LH, Peketi P, and Lenz KM, Microglia Regulate Cell Genesis in a Sex-dependent Manner in the Neonatal Hippocampus. Neuroscience, 2021. 453: p. 237–255. [DOI] [PubMed] [Google Scholar]
- 89.Marín-Teva J, et al. , Microglia promote the death of developing purkinje cells. Neuron, 2004. 41: p. 535–547. [DOI] [PubMed] [Google Scholar]
- 90.Wakselman S, et al. , Developmental Neuronal Death in Hippocampus Requires the Microglial CD11b Integrin and DAP12 Immunoreceptor. Journal of Neuroscience, 2008. 28(32): p. 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ravichandran KS, Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways. Immunity, 2011. 35(4): p. 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sierra A, et al. , Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell, 2010. 7(4): p. 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cunningham CL, Martinez-Cerdeno V, and Noctor SC, Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci, 2013. 33(10): p. 4216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molliver ME, Kostovic I´, and Van Der Loos H, The development of synapses in cerebral cortex of the human fetus. Brain Research, 1973. 50(2): p. 403–407. [DOI] [PubMed] [Google Scholar]
- 95.Huttenlocher PR and Dabholkar AS, Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology, 1998. 387(2): p. 167–178. [DOI] [PubMed] [Google Scholar]
- 96.Paolicelli RC, et al. , Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science, 2011. 333(6048): p. 1456–1458. [DOI] [PubMed] [Google Scholar]
- 97.Schafer DP, et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyamoto A, et al. , Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun, 2016. 7: p. 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weinhard L, et al. , Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun, 2018. 9(1): p. 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parkhurst C, et al. , Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell, 2013. 155(7): p. 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Squarzoni P, et al. , Microglia modulate wiring of the embryonic forebrain. Cell Rep, 2014. 8(5): p. 1271–9. [DOI] [PubMed] [Google Scholar]
- 102.Matcovitch-Natan O, et al. , Microglia development follows a stepwise program to regulate brain homeostasis. Science, 2016. 353(6301): p. aad8670. [DOI] [PubMed] [Google Scholar]
- 103.Thion MS, et al. , Biphasic Impact of Prenatal Inflammation and Macrophage Depletion on the Wiring of Neocortical Inhibitory Circuits. Cell Rep, 2019. 28(5): p. 1119–1126 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hanamsagar R, et al. , Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia, 2017. 65(9): p. 1504–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guneykaya D, et al. , Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep, 2018. 24(10): p. 2773–2783 e6. [DOI] [PubMed] [Google Scholar]
- 106.Han J, et al. , Uncovering sex differences of rodent microglia. Journal of Neuroinflammation, 2021. 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Breach MR, et al. , Maternal allergic inflammation in rats impacts the offspring perinatal neuroimmune milieu and the development of social play, locomotor behavior, and cognitive flexibility. Brain Behav Immun, 2021. 95: p. 269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lenz KM, et al. , Microglia are essential to masculinization of brain and behavior. J Neurosci, 2013. 33(7): p. 2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.VanRyzin JW, et al. , Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron, 2019. 102(2): p. 435–449 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwarz JM, Sholar PW, and Bilbo SD, Sex differences in microglial colonization of the developing rat brain. J Neurochem, 2012. 120(6): p. 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weinhard L, et al. , Sexual dimorphism of microglia and synapses during mouse postnatal development. Dev Neurobiol, 2018. 78(6): p. 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amateau SK and McCarthy MM, Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci, 2004. 7(6): p. 643–50. [DOI] [PubMed] [Google Scholar]
- 113.Pellis SM, Pellis VC, and Bell HC, The Function of Play in the Development of the social Brain. The American Journal of Play, 2010: p. 278–296. [Google Scholar]
- 114.Vanderschuren LJ and Trezza V, What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr Top Behav Neurosci, 2014. 16: p. 189–212. [DOI] [PubMed] [Google Scholar]
- 115.Kopec AM, et al. , Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun, 2018. 9(1): p. 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosin JM, Vora SR, and Kurrasch DM, Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav Immun, 2018. 73: p. 682–697. [DOI] [PubMed] [Google Scholar]
- 117.VanRyzin JW, et al. , Temporary Depletion of Microglia during the Early Postnatal Period Induces Lasting Sex-Dependent and Sex-Independent Effects on Behavior in Rats. eNeuro, 2016. 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nelson LH and Lenz KM, Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res, 2017. 316: p. 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhan Y, et al. , Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci, 2014. 17(3): p. 400–6. [DOI] [PubMed] [Google Scholar]
- 120.Zürcher NR, et al. , [11C]PBR28 MR–PET imaging reveals lower regional brain expression of translocator protein (TSPO) in young adult males with autism spectrum disorder. Molecular Psychiatry, 2021. 26(5): p. 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki K, et al. , Microglial Activation in Young Adults With Autism Spectrum Disorder. JAMA Psychiatry, 2013. 70(1): p. 49. [DOI] [PubMed] [Google Scholar]
- 122.Vargas DL, et al. , Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol, 2005. 57(1): p. 67–81. [DOI] [PubMed] [Google Scholar]
- 123.Morgan JT, et al. , Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry, 2010. 68(4): p. 368–76. [DOI] [PubMed] [Google Scholar]
- 124.Tetreault NA, et al. , Microglia in the cerebral cortex in autism. J Autism Dev Disord, 2012. 42(12): p. 2569–84. [DOI] [PubMed] [Google Scholar]
- 125.Voineagu I, et al. , Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature, 2011. 474(7351): p. 380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta S, et al. , Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun, 2014. 5: p. 5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parikshak NN, et al. , Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature, 2016. 540(7633): p. 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edmonson C, Ziats MN, and Rennert OM, Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Molecular Autism, 2014. 5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gandal MJ, et al. , Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science, 2018. 359(6376): p. 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Velmeshev D, et al. , Single-cell genomics identifies cell type–specific molecular changes in autism. Science, 2019. 364(6441): p. 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu Z-X, et al. , Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nature Communications, 2020. 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung H, et al. , Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nature Neuroscience, 2018. 21(9): p. 1218–1228. [DOI] [PubMed] [Google Scholar]
- 133.Werling DM, Parikshak NN, and Geschwind DH, Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun, 2016. 7: p. 10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, et al. , Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl Psychiatry, 2020. 10(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jacquemont S, et al. , A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet, 2014. 94(3): p. 415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khakh BS and Deneen B, The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci, 2019. 42: p. 187–207. [DOI] [PubMed] [Google Scholar]
- 137.Matejuk A and Ransohoff RM, Crosstalk between astrocytes and microglia: an overview. Frontiers in Immunology, 2020. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Amateau SK and McCarthy MM, Sexual Differentiation of Astrocyte Morphology in the Developing Rat Preoptic Area. Journal of Neuroendocrinology, 2002. 14(11): p. 904–910. [DOI] [PubMed] [Google Scholar]
- 139.Santos-Galindo M, et al. , Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biology of Sex Differences, 2011. 2(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller RH and Raff MC, Fibrous and Protoplasmic Astrocytes are Biochemically and Develompentally Distinct. Journal of Neuroscience, 1984. 4(2): p. 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vainchtein ID, et al. , Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science, 2018. 359(6381): p. 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia P, et al. , Histamine triggers microglial responses indirectly via astrocytes and purinergic signaling. Glia, 2021. [DOI] [PubMed] [Google Scholar]
- 143.Galli SJ and Tsai M, IgE and mast cells in allergic disease. Nat Med, 2012. 18(5): p. 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khalil M, et al. , Brain mast cell relationship to neurovasculature during development. Brain Res, 2007. 1171: p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lambract-Hall M, Dimitriadou V, and Theoharides TC, Migration of mast cells in the developing rat brain. Developmental Brain Research, 1990. 56: p. 151–159. [DOI] [PubMed] [Google Scholar]
- 146.Silver R and Curley JP, Mast cells on the mind: new insights and opportunities. Trends Neurosci, 2013. 36(9): p. 513–21. [DOI] [PubMed] [Google Scholar]
- 147.Wernersson S and Pejler G, Mast cell secretory granules: armed for battle. Nat Rev Immunol, 2014. 14(7): p. 478–94. [DOI] [PubMed] [Google Scholar]
- 148.Marszalek PE, et al. , Kinetics of Release of Serotonin from Isolated Secretory Granules. 1.Ion Exchange Determines the DiffusivityofSerotonin. Biophysical Journal, 1997. 73: p. 1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galli SJ, Nakae S, and Tsai M, Mast cells in the development of adaptive immune responses. Nat Immunol, 2005. 6(2): p. 135–42. [DOI] [PubMed] [Google Scholar]
- 150.Dong H, Zhang X, and Qian Y, Mast cells and neuroinflammation. Med Sci Monit Basic Res, 2014. 20: p. 200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ballardini N, et al. , Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. British Journal of Dermatology, 2013. 168(3): p. 588–594. [DOI] [PubMed] [Google Scholar]
- 152.Osman M, et al. , Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Primary Care Respiratory Journal, 2007. 16(1): p. 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Acker WW, et al. , Prevalence of food allergies and intolerances documented in electronic health records. Journal of Allergy and Clinical Immunology, 2017. 140(6): p. 1587–1591.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mackey E, et al. , Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biology of Sex Differences, 2016. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mackey E, et al. , Perinatal androgens organize sex differences in mast cells and attenuate anaphylaxis severity into adulthood. Proc Natl Acad Sci U S A, 2020. 117(38): p. 23751–23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lenz KM, et al. , Mast Cells in the Developing Brain Determine Adult Sexual Behavior. J Neurosci, 2018. 38(37): p. 8044–8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Joshi A, et al. , Sex differences in the effects of early life stress exposure on mast cells in the developing rat brain. Horm Behav, 2019. 113: p. 76–84. [DOI] [PubMed] [Google Scholar]
- 158.Nautiyal KM, et al. , Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A, 2008. 105(46): p. 18053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nautiyal KM, et al. , Serotonin of mast cell origin contributes to hippocampal function. Eur J Neurosci, 2012. 36(3): p. 2347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chee SS and Menard JL, The histaminergic H1, H2, and H3 receptors of the lateral septum differentially mediate the anxiolytic-like effects of histamine on rats’ defensive behaviors in the elevated plus maze and novelty-induced suppression of feeding paradigm. Physiol Behav, 2013. 116–117: p. 66–74. [DOI] [PubMed] [Google Scholar]
- 161.Chee SS, Menard JL, and Dringenberg HC, Behavioral anxiolysis without reduction of hippocampal theta frequency after histamine application in the lateral septum of rats. Hippocampus, 2014. 24(6): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 162.Larson AA, et al. , Spontaneous locomotor activity correlates with the degranulation of mast cells in the meninges rather than in the thalamus: disruptive effect of cocaine. Brain Res, 2011. 1395: p. 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tanioka D, et al. , Intracranial mast cells contribute to the control of social behavior in male mice. Behav Brain Res, 2021. 403: p. 113143. [DOI] [PubMed] [Google Scholar]
- 164.Theoharides T, C., Autism Spectrum Disorders and Mastocytosis. International Journal of Immunopathology and Pharmacology, 2009: p. 859–865. [DOI] [PubMed] [Google Scholar]
- 165.Chang HY, et al. , Allergic diseases in preschoolers are associated with psychological and behavioural problems. Allergy Asthma Immunol Res, 2013. 5(5): p. 315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chua RXY, et al. , Understanding the Link Between Allergy and Neurodevelopmental Disorders: A Current Review of Factors and Mechanisms. Front Neurol, 2020. 11: p. 603571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen M-H, et al. , Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: A nationwide population-based study. Research in Autism Spectrum Disorders, 2013. 7(2): p. 205–212. [Google Scholar]
- 168.Kotey S, Ertel K, and Whitcomb B, Co-occurrence of autism and asthma in a nationally-representative sample of children in the United States. J Autism Dev Disord, 2014. 44(12): p. 3083–8. [DOI] [PubMed] [Google Scholar]
- 169.Lyall K, et al. , Asthma and Allergies in Children With Autism Spectrum Disorders: Results From the CHARGE Study. Autism Res, 2015. 8(5): p. 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zerbo O, et al. , Immune mediated conditions in autism spectrum disorders. Brain Behav Immun, 2015. 46: p. 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roth N, et al. , Coincidence of Attention Deficit Disorder and Atopic Disorders in Children: Empirical Findings and Hypothetical Background. Journal of Abnormal Child Psychology, 1991. 19(1): p. 1–13. [DOI] [PubMed] [Google Scholar]
- 172.Schmitt J, Buske-Kirschbaum A, and Roessner V, Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy, 2010. 65(12): p. 1506–1524. [DOI] [PubMed] [Google Scholar]
- 173.Fasmer OB, et al. , Comorbidity of Asthma With ADHD. Journal of Attention Disorders, 2011. 15(7): p. 564–571. [DOI] [PubMed] [Google Scholar]
- 174.Mogensen N, et al. , Association between childhood asthma and ADHD symptoms in adolescence - a prospective population-based twin study. Allergy, 2011. 66(9): p. 1224–1230. [DOI] [PubMed] [Google Scholar]
- 175.Chen MH, et al. , Is atopy in early childhood a risk factor for ADHD and ASD? a longitudinal study. J Psychosom Res, 2014. 77(4): p. 316–21. [DOI] [PubMed] [Google Scholar]
- 176.Chen MH, et al. , Comorbidity of Allergic and Autoimmune Diseases Among Patients With ADHD. J Atten Disord, 2017. 21(3): p. 219–227. [DOI] [PubMed] [Google Scholar]
- 177.Wang LJ, et al. , Attention deficit-hyperactivity disorder is associated with allergic symptoms and low levels of hemoglobin and serotonin. Sci Rep, 2018. 8(1): p. 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jiang X, et al. , Early food allergy and respiratory allergy symptoms and attention-deficit/hyperactivity disorder in Chinese children: A cross-sectional study. Pediatr Allergy Immunol, 2018. 29(4): p. 402–409. [DOI] [PubMed] [Google Scholar]
- 179.Liao TC, et al. , Comorbidity of Atopic Disorders with Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder. J Pediatr, 2016. 171: p. 248–55. [DOI] [PubMed] [Google Scholar]
- 180.Bechmann I, Galea I, and Perry VH, What is the blood–brain barrier (not)? Trends in Immunology, 2007. 28(1): p. 5–11. [DOI] [PubMed] [Google Scholar]
- 181.D’Mello C and Swain MG, Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. 2016, Springer International Publishing. p. 73–94. [DOI] [PubMed] [Google Scholar]
- 182.Torres AR, Westover JB, and Rosenspire AJ, HLA Immune Function Genes in Autism. Autism Res Treat, 2012. 2012: p. 959073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saxena V, et al. , Structural, genetic, and functional signatures of disordered neuro-immunological development in autism spectrum disorder. PLoS One, 2012. 7(12): p. e48835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abdallah MW, et al. , Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol, 2012. 252(1–2): p. 75–82. [DOI] [PubMed] [Google Scholar]
- 185.Ashwood P, et al. , Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun, 2011. 25(1): p. 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Enstrom A, et al. , Detection of IL-17 and IL-23 in Plasma Samples of Children with Autism. Am J Biochem Biotechnol, 2008. 4(2): p. 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.<Xie et al., 2017.pdf>.
- 188.Xie J, et al. , Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget, 2016. 8(47): p. 82390–82398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Masi A, et al. , Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry, 2015. 20(4): p. 440–6. [DOI] [PubMed] [Google Scholar]
- 190.Saghazadeh A, et al. , A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res, 2019. 115: p. 90–102. [DOI] [PubMed] [Google Scholar]
- 191.Ashwood P, et al. , Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol, 2008. 204(1–2): p. 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Okada K, et al. , Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry, 2007. 31(1): p. 187–90. [DOI] [PubMed] [Google Scholar]
- 193.Luckheeram RV, et al. , CD4+T Cells: Differentiation and Functions. Clinical and Developmental Immunology, 2012. 2012: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Careaga M, et al. , Immune Endophenotypes in Children With Autism Spectrum Disorder. Biol Psychiatry, 2017. 81(5): p. 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ahmad SF, et al. , Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol Neurobiol, 2017. 54(6): p. 4390–4400. [DOI] [PubMed] [Google Scholar]
- 196.Moaaz M, et al. , Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-beta) in children with autism spectrum disorder. J Neuroimmunol, 2019. 337: p. 577071. [DOI] [PubMed] [Google Scholar]
- 197.Gupta S, et al. , Th1- and Th2-like cytokines in CD4 and CD8 T cells in autism. Journal of Neuroimmunology, 1998. 85: p. 106–109. [DOI] [PubMed] [Google Scholar]
- 198.Basheer S, et al. , Immune aberrations in children with Autism Spectrum Disorder: a case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology, 2018. 94: p. 162–167. [DOI] [PubMed] [Google Scholar]
- 199.Rose DR, et al. , T cell populations in children with autism spectrum disorder and co-morbid gastrointestinal symptoms. Brain, Behavior, & Immunity - Health, 2020. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mortimer N, et al. , Transcriptome profiling in adult attention-deficit hyperactivity disorder. Eur Neuropsychopharmacol, 2020. 41: p. 160–166. [DOI] [PubMed] [Google Scholar]
- 201.van Dongen J, et al. , Epigenome-wide Association Study of Attention-Deficit/Hyperactivity Disorder Symptoms in Adults. Biol Psychiatry, 2019. 86(8): p. 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yilmaz M, et al. , Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci, 2021. 24(2): p. 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Drtilkova I, et al. , Clinical and molecular-genetic markers of ADHD in children. Neuroendocrinology Letters, 2008. 29(3): p. 320–327. [PubMed] [Google Scholar]
- 204.Smith TF, et al. , Angiogenic, neurotrophic, and inflammatory system SNPs moderate the association between birth weight and ADHD symptom severity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 2014. 165(8): p. 691–704. [DOI] [PubMed] [Google Scholar]
- 205.Lasky-Su J, et al. , Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 2008. 147B(8): p. 1345–1354. [DOI] [PubMed] [Google Scholar]
- 206.Oades RD, et al. , Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism - effects of medication. Behavioral and Brain Functions, 2010. 6(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Donfrancesco R, et al. , Serum cytokines in paediatric neuropsychiatric syndromes: focus on Attention Deficit Hyperactivity Disorder. Minerva Pediatrica, 2016. [DOI] [PubMed] [Google Scholar]
- 208.Oades RD, et al. , Attention-deficit hyperactivity disorder (ADHD) and glial integrity: an exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behavioral and Brain Functions, 2010. 6(1): p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Joseph N, et al. , Oxidative Stress and ADHD: A Meta-Analysis. J Atten Disord, 2015. 19(11): p. 915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sanchez-Mora C, et al. , Epigenetic signature for attention-deficit/hyperactivity disorder: identification of miR-26b-5p, miR-185–5p, and miR-191–5p as potential biomarkers in peripheral blood mononuclear cells. Neuropsychopharmacology, 2019. 44(5): p. 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mazon-Cabrera R, Vandormael P, and Somers V, Antigenic Targets of Patient and Maternal Autoantibodies in Autism Spectrum Disorder. Front Immunol, 2019. 10: p. 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cabanlit M, et al. , Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci, 2007. 1107: p. 92–103. [DOI] [PubMed] [Google Scholar]
- 213.Goines P, et al. , Autoantibodies to cerebellum in children with autism associate with behavior. Brain, Behavior, and Immunity, 2011. 25(3): p. 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zou T, et al. , Autoantibody and autism spectrum disorder: A systematic review. Research in Autism Spectrum Disorders, 2020. 75. [Google Scholar]
- 215.Rout UK, Mungan NK, and Dhossche DM, Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. Eur Child Adolesc Psychiatry, 2012. 21(3): p. 141–7. [DOI] [PubMed] [Google Scholar]
- 216.Hegvik TA, Husebye ES, and Haavik J, Autoantibodies targeting neurotransmitter biosynthetic enzymes in attention-deficit/hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry, 2014. 23(2): p. 115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Passarelli F, et al. , Anti-Purkinje cell antibody as a biological marker in attention deficit/hyperactivity disorder: a pilot study. J Neuroimmunol, 2013. 258(1–2): p. 67–70. [DOI] [PubMed] [Google Scholar]
- 218.Donfrancesco R, et al. , Anti-Yo Antibodies in Children With ADHD: First Results About Serum Cytokines. Journal of Attention Disorders, 2020. 24(11): p. 1497–1502. [DOI] [PubMed] [Google Scholar]
- 219.Cetin FH, et al. , Attention deficit hyperactivity disorder and anti-Purkinje autoantibodies: no link? Psychiatry and Clinical Psychopharmacology, 2019. 29(4): p. 435–440. [Google Scholar]
- 220.Giana G, et al. , Detection of auto-antibodies to DAT in the serum: interactions with DAT genotype and psycho-stimulant therapy for ADHD. J Neuroimmunol, 2015. 278: p. 212–22. [DOI] [PubMed] [Google Scholar]
- 221.Adriani W, et al. , Potential for diagnosis versus therapy monitoring of attention deficit hyperactivity disorder: a new epigenetic biomarker interacting with both genotype and auto-immunity. Eur Child Adolesc Psychiatry, 2018. 27(2): p. 241–252. [DOI] [PubMed] [Google Scholar]
- 222.Gonzalez-Gronow M, et al. , Catalytic autoantibodies against myelin basic protein (MBP) isolated from serum of autistic children impair in vitro models of synaptic plasticity in rat hippocampus. J Neuroimmunol, 2015. 287: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 223.Chen SW, et al. , Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behav Brain Res, 2016. 296: p. 61–69. [DOI] [PubMed] [Google Scholar]
- 224.Sacco R, Gabriele S, and Persico AM, Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Research: Neuroimaging, 2015. 234(2): p. 239–251. [DOI] [PubMed] [Google Scholar]
- 225.Nordahl CW, et al. , Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun, 2013. 30: p. 61–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jones KL, et al. , Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol Psychiatry, 2020. 25(11): p. 2994–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bruce MR, et al. , Sexually dimorphic neuroanatomical differences relate to ASD-relevant behavioral outcomes in a maternal autoantibody mouse model. Mol Psychiatry, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zerbo O, et al. , Maternal Infection During Pregnancy and Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 2015. 45(12): p. 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Han VX, et al. , Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry, 2021. 11(1): p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Instanes JT, et al. , Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol Psychiatry, 2017. 81(5): p. 452–459. [DOI] [PubMed] [Google Scholar]
- 231.Roberts AL, et al. , Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect, 2013. 121(8): p. 978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brown AS, et al. , Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Archives of General Psychiatry, 2004. 61(8): p. 774. [DOI] [PubMed] [Google Scholar]
- 233.Pearce BD, Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Molecular Psychiatry, 2001. 6(6): p. 634–646. [DOI] [PubMed] [Google Scholar]
- 234.Müller N, Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophrenia Bulletin, 2018. 44(5): p. 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lin A, et al. , The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophrenia Research, 1998. 32(1): p. 9–15. [DOI] [PubMed] [Google Scholar]
- 236.Saetre P, et al. , Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry, 2007. 7(1): p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Borrell J, et al. , Prenatal Immune Challenge Disrupts Sensorimotor Gating in Adult Rats Implications for the Etiopathogenesis of Schizophrenia. Neuropsychopharmacology, 2002. 26(2): p. 204–215. [DOI] [PubMed] [Google Scholar]
- 238.Meyer U, Feldon J, and Fatemi SH, In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev, 2009. 33(7): p. 1061–79. [DOI] [PubMed] [Google Scholar]
- 239.Canetta S, et al. , Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry, 2016. 21(7): p. 956–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McGrath J, et al. , Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiologic Reviews, 2008. 30(1): p. 67–76. [DOI] [PubMed] [Google Scholar]
- 241.Abel KM, Drake R, and Goldstein JM, Sex differences in schizophrenia. Int Rev Psychiatry, 2010. 22(5): p. 417–28. [DOI] [PubMed] [Google Scholar]
- 242.Kamitaki N, et al. , Complement genes contribute sex-biased vulnerability in diverse disorders. Nature, 2020. 582(7813): p. 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sekar A, et al. , Schizophrenia risk from complex variation of complement component 4. Nature, 2016. 530(7589): p. 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Meyer U, Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry, 2014. 75(4): p. 307–15. [DOI] [PubMed] [Google Scholar]
- 245.Schwartzer JJ, et al. , Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Transl Psychiatry, 2015. 5: p. e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sharkhuu T, et al. , Effects of prenatal diesel exhaust inhalation on pulmonary inflammation and development of specific immune responses. Toxicol Lett, 2010. 196(1): p. 12–20. [DOI] [PubMed] [Google Scholar]
- 247.Kirsten TB, et al. , Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J Neurosci Res, 2012. 90(10): p. 1903–12. [DOI] [PubMed] [Google Scholar]
- 248.Malkova NV, et al. , Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun, 2012. 26(4): p. 607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Choi GB, et al. , The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science, 2016. 351(6276): p. 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Smith SE, et al. , Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci, 2007. 27(40): p. 10695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kirsten TB, et al. , Prenatal Lipopolysaccharide Reduces Social Behavior in Male Offspring. Neuroimmunomodulation, 2010. 17: p. 240–251. [DOI] [PubMed] [Google Scholar]
- 252.Penteado SH, et al. , Prenatal lipopolysaccharide disrupts maternal behavior, reduces nest odor preference in pups, and induces anxiety: studies of F1 and F2 generations. Eur J Pharmacol, 2014. 738: p. 342–51. [DOI] [PubMed] [Google Scholar]
- 253.Mattei D, et al. , Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun, 2014. 38: p. 175–84. [DOI] [PubMed] [Google Scholar]
- 254.Kirsten TB and Bernardi MM, Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behav Brain Res, 2017. 331: p. 25–29. [DOI] [PubMed] [Google Scholar]
- 255.Kirsten TB, et al. , Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behavioural Brain Research, 2010. 211(1): p. 77–82. [DOI] [PubMed] [Google Scholar]
- 256.Ajendra J, et al. , IL-17A both initiates, via IFNγ suppression, and limits the pulmonary type-2 immune response to nematode infection. Mucosal Immunology, 2020. 13(6): p. 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hsiao EY, et al. , Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci U S A, 2012. 109(31): p. 12776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Smolders S, et al. , Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain Behav Immun, 2018. 73: p. 51–65. [DOI] [PubMed] [Google Scholar]
- 259.Missault S, et al. , The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun, 2014. 42: p. 138–46. [DOI] [PubMed] [Google Scholar]
- 260.O’Loughlin E, et al. , Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation, 2017. 14(1): p. 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Juckel G, et al. , Microglial activation in a neuroinflammational animal model of schizophrenia--a pilot study. Schizophr Res, 2011. 131(1–3): p. 96–100. [DOI] [PubMed] [Google Scholar]
- 262.Mattei D, et al. , Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry, 2017. 7(5): p. e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Schwartzer JJ, et al. , Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun, 2017. 63: p. 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hui CW, et al. , Prenatal Immune Challenge in Mice Leads to Partly Sex-Dependent Behavioral, Microglial, and Molecular Abnormalities Associated with Schizophrenia. Front Mol Neurosci, 2018. 11: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lenz KM, et al. , Prenatal Allergen Exposure Perturbs Sexual Differentiation and Programs Lifelong Changes in Adult Social and Sexual Behavior. Sci Rep, 2019. 9(1): p. 4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Makinson R, et al. , Intrauterine inflammation induces sex-specific effects on neuroinflammation, white matter, and behavior. Brain Behav Immun, 2017. 66: p. 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gzielo K, et al. , The Effect of Maternal Immune Activation on Social Play-Induced Ultrasonic Vocalization in Rats. Brain Sci, 2021. 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Van den Eynde K, et al. , Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res, 2014. 258: p. 179–86. [DOI] [PubMed] [Google Scholar]
- 269.Meyer U, et al. , The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci, 2006. 26(18): p. 4752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Makinson R, et al. , Exposure to in utero inflammation increases locomotor activity, alters cognitive performance and drives vulnerability to cognitive performance deficits after acute immune activation. Brain Behav Immun, 2019. 80: p. 56–65. [DOI] [PubMed] [Google Scholar]
- 271.Taylor PV, et al. , Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ, 2012. 3(1): p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Vitor-Vieira F, Vilela FC, and Giusti-Paiva A, Hyperactivation of the amygdala correlates with impaired social play behavior of prepubertal male rats in a maternal immune activation model. Behav Brain Res, 2021. 414: p. 113503. [DOI] [PubMed] [Google Scholar]
- 273.Bolton JL, et al. , Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect, 2013. 121(9): p. 1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bilbo SD, et al. , Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol, 2018. 299(Pt A): p. 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Vogel Ciernia A, et al. , Microglia from offspring of dams with allergic asthma exhibit epigenomic alterations in genes dysregulated in autism. Glia, 2018. 66(3): p. 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Bolton JL, et al. , Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front Synaptic Neurosci, 2017. 9: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Allred EN, et al. , Systemic Inflammation during the First Postnatal Month and the Risk of Attention Deficit Hyperactivity Disorder Characteristics among 10 year-old Children Born Extremely Preterm. Journal of Neuroimmune Pharmacology, 2017. 12(3): p. 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Korzeniewski SJ, et al. , Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Translational Psychiatry, 2018. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Saitoh BY, et al. , Early postnatal allergic airway inflammation induces dystrophic microglia leading to excitatory postsynaptic surplus and autism-like behavior. Brain Behav Immun, 2021. 95: p. 362–380. [DOI] [PubMed] [Google Scholar]
- 280.Bilbo SD, et al. , Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci, 2005. 25(35): p. 8000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lieblein-Boff JC, et al. , Neonatal E. coli infection causes neuro-behavioral deficits associated with hypomyelination and neuronal sequestration of iron. J Neurosci, 2013. 33(41): p. 16334–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bilbo SD, et al. , Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci, 2005. 119(1): p. 293–301. [DOI] [PubMed] [Google Scholar]
- 283.Williamson LL, et al. , Microglia and memory: modulation by early-life infection. J Neurosci, 2011. 31(43): p. 15511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Guillemot-Legris O, et al. , High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation, 2016. 13(1): p. 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rodriguez A, et al. , Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes (Lond), 2008. 32(3): p. 550–7. [DOI] [PubMed] [Google Scholar]
- 286.Vucetic Z, et al. , Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology, 2010. 151(10): p. 4756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Grissom NM, et al. , Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology, 2015. 40(6): p. 1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.McKee SE, et al. , Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J Neurochem, 2018. 145(5): p. 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.McKee SE, et al. , Methyl donor supplementation alters cognitive performance and motivation in female offspring from high-fat diet – fed dams. The FASEB Journal, 2017. 31(6): p. 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Carlin J, George R, and Reyes TM, Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS ONE, 2013. 8(5): p. e63549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Custodio CS, et al. , Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-lasting Sex- and Age-related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol Neurobiol, 2018. 55(5): p. 3775–3788. [DOI] [PubMed] [Google Scholar]
- 292.Macrae M, et al. , Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience, 2015. 298: p. 455–466. [DOI] [PubMed] [Google Scholar]
- 293.Smith CJ, et al. , Neonatal immune challenge induces female-specific changes in social behavior and somatostatin cell number. Brain Behav Immun, 2020. 90: p. 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]