Abstract
Glucagon has been defined as an ‘insulin counteracting hormone’, which raises blood glucose levels. Recent progress in basic research has shown that glucagon is closely involved in glucose and amino acid metabolism. Additionally, its secretion is intricately, but precisely, regulated by various mechanisms involving molecules in addition to glucose, thus showing its critical role in systemic nutrient metabolism. An innovative dual‐antibody‐linked immunosorbent assay for glucagon that improves measurement accuracy has been developed, and substantial clinical findings have been obtained using this new system. This discovery expanded the pathophysiological significance of glucagon and accelerated the development of its clinical applications in diabetes.
Keywords: α‐Cells, Amino acids Glucagon
Recent progress in basic research has shown that glucagon is closely involved in glucose and amino acid metabolism. Its secretion is intricately, but precisely, regulated by various mechanisms involving molecules in addition to glucose, thus showing its critical role in systemic nutrient metabolism. An innovative dual‐antibody‐linked immunosorbent assay for glucagon that improves measurement accuracy has been developed, and substantial clinical findings have been obtained using this new system.
INTRODUCTION
In metabolic diseases, including diabetes, impaired metabolism and utilization of various nutrients from food intake induce wide‐ranging complications, especially atherosclerosis and cerebral/cardiovascular diseases. Abnormalities in the secretion and actions of various hormones, specifically insulin, which controls systemic metabolic status, underlie the pathophysiology of these diseases. Therefore, these are intensively investigated, and the results have been applied to clinical therapeutics. Conversely, glucagon, a 29‐amino acid peptide hormone, produced and secreted by pancreatic islet α‐cells, has been recognized as an anti‐hypoglycemic and anti‐insulin hormone. Glucagon was vigorously investigated in the 1970s, owing to its significance in inducing hyperglycemia. Since then, insulin research has surpassed glucagon, possibly because of the lack of appropriate and effective interventional approaches to this hormone. However, in the 2010s, glucagon was re‐evaluated with the progress of incretin research and clinical applications, as glucagon was shown as an important target for incretin therapy. In particular, basic research targeting glucagon has preceded many novel and important scientific findings about glucagon. The development of new enzyme‐linked immunosorbent assay (ELISA) systems with improved accuracy also accelerates glucagon research in clinical settings, the pathological significance of glucagon in metabolic diseases and its potential for clinical applications. Here, we outlined the knowledge pertaining to glucagon based on recent findings from basic and clinical research, its clinical applications, and potential for therapeutic development in the future.
NEWLY DISCOVERED PHYSIOLOGICAL ROLE FOR GLUCAGON
It has been more than 100 years since the discovery of glucagon; however, recent achievements in intensive research have elucidated many previously unknown and unexpected findings about glucagon. Thus, glucagon has morphed from a conventional anti‐insulin and anti‐hypoglycemic agent to a comprehensive metabolic regulator with various physiological roles and clinical significance. Glucagon was first recognized as a contaminant of pancreatic extracts that temporally increased blood glucose levels after administration. Owing to the constant hyperglycemic effect of the pancreatic extract on administration, the presence of a physiological hyperglycemic substance in the pancreas was suggested, proven and named ‘glucagon’. Under this history of discovery, there have been many investigations to understand this hormone and elucidate its role in hyperglycemia in diabetes (for the history of glucagon discovery and advancement in research, refer to ‘Glucagon, from past to present: a century of intensive research and controversies’ by Scheen and Lefebvre) 1 .
Since its discovery, many studies have focused on the hyperglycemic effects of glucagon, identified its receptors, and elucidated the mechanisms of glucose output and gluconeogenesis, mainly in liver hepatocytes. Based on these findings, glucagon has been defined as an ‘anti‐hypoglycemic hormone’, through which secretion from α‐cells is increased during hypoglycemia promoting glucose release and production from the liver to increase the diminished blood glucose levels. However, the diverse physiological actions of glucagon suggest that its functional essence is not limited to mere blood glucose regulation (Figure 1). In particular, the recent discovery of glucagon's ability to promote amino acid metabolism in the liver has added to these new actions in energy regulation 2 , 3 . Interestingly, amino acids are shown to promote the proliferation of α‐cells, which produce and secrete glucagon 4 , 5 , showing functional interaction between amino acids and glucagon.
Figure 1.
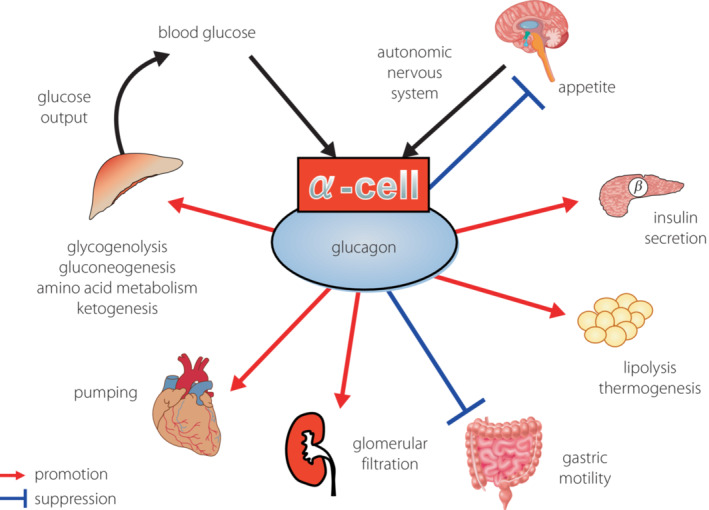
Physiological functions of glucagon.
Hence, the essential functions of glucagon are not limited to simply responding to hypoglycemia, but also include: (1) promoting nutrient metabolism and breakdown in energy‐storing organs, such as the liver and adipose tissue; (2) releasing energy and nutrient sources, mainly glucose, from these organs; and (3) delivering the sources to energy‐consuming organs, such as the central nervous system and skeletal muscles. Thus, glucagon is working as an ‘energy mobilizer’ that effectively circulates necessary energy and nutrients on demand 6 , 7 . In contrast, insulin, which ‘reduces blood glucose levels’, promotes glucose uptake and cellular activities, including protein synthesis in energy‐consuming organs, and simultaneously inhibits excessive energy release from energy‐storing organs. Blood glucose levels, which show the degree of circulating and moving energy, are ‘reduced’ according to the energy transport between organs. Taken together, insulin and glucagon do not counteract each other, but cooperate to facilitate the smooth flow of energy transfer in response to supply and demand (Figure 2).
Figure 2.
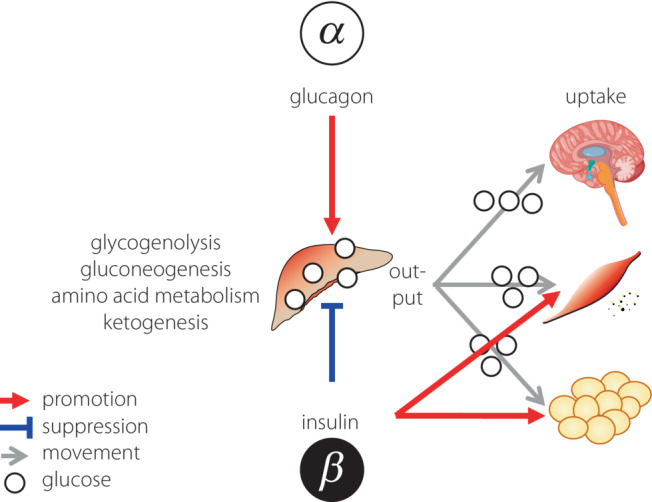
Role for glucagon as an ‘energy mobilizer’.
Glucagon secreted from α‐cells acts directly on neighboring β‐cells through glucagon‐like peptide‐1 (GLP‐1) and glucagon receptors in an intra‐islet paracrine manner, thereby promoting insulin secretion 8 . This fact lacks coherence if glucagon functionally counteracts insulin, but is rational if glucagon cooperates with insulin to orchestrate systemic energy transportation and metabolism, as described above. Indeed, fasting plasma glucagon levels were correlated with serum C‐peptide levels in hospitalized patients with type 2 diabetes 9 , suggesting that glucagon and insulin maintain a certain balance.
CLINICAL SIGNIFICANCE OF GLUCAGON AND IMPACT OF ITS DYSREGULATION
The role of incretin therapy is greatly expanding in the field of current clinical treatments for diabetes. GLP‐1 receptor agonists are now widely used because of their multipotent effects, including central nervous system‐mediated appetite suppression, delayed gastric emptying and weight loss. Additionally, they improve hyperglycemia through the glucose‐dependent enhancement of insulin secretion and suppression of excessive glucagon secretion. Several clinical trials have shown that this agent shows cardiovascular protective effects; thus, the American Diabetes Association Standards of Medical Care in Diabetes 2022 added a recommendation to positively introduce GLP‐1 receptor agonists or sodium–glucose transporter 2 inhibitors for patients with atherosclerosis and individuals with high risk of atherosclerosis. The clinical application of incretin therapies has enabled a two‐sided therapeutic approach for both glucagon and insulin from the previous one‐sided approach for insulin.
In the 1970s, since the glucagon radioimmunoassay (RIA) became available 10 , glucagon research was vigorously carried out in both basic and clinical fields. Consequently, it has been widely recognized that glucagon regulates the appropriate energy flow in balance with insulin (see Introduction), which is dysregulated in the state of diabetes. Therefore, it is involved in the pathogenesis of the disease 11 .
In the state of diabetes, glucagon secretion, which is supposed to be suppressed during hyperglycemia, shows a paradoxical increase, resulting in excessive hepatic glucose output, exacerbating postprandial hyperglycemia in combination with insulin deficiency 12 . This excessive action of glucagon is also involved in fasting hyperglycemia. In contrast, defective glucagon secretory responses to hypoglycemia often result in severe and prolonged hypoglycemia. Thus, dysregulated glucagon secretion in diabetes together with impaired insulin secretion and action, which is widely recognized as the central pathophysiology of diabetes, leads to further exacerbation of blood glucose variability. It has been conceptualized as a part of ‘bi‐hormonal disorder’, an abnormality in two critical glucose‐regulating hormones 13 . The insulin supply is almost completely dependent on exogenous administration in type 1 diabetes; hence, the imbalance between glucagon and insulin is more pronounced, worsening glycemic instability and fragility to fatal hypoglycemia. Thus, glucagon and its dysregulation are closely involved in major critical pathological states, such as hyperglycemia and hypoglycemia. In addition, the recent expansion in the use of incretin therapies emphasizes the pathological significance and clinical recognition of glucagon.
EXPLORING THE REGULATORY MECHANISM FOR GLUCAGON SECRETION AND ITS DYSREGULATION
Given the recent rise in the clinical significance of glucagon dysregulation in diabetes and metabolic disorders, there has been a resurgence in glucagon research, especially on secretion regulation, from physiological and pathological points of view. First, the physiological mechanisms of glucagon secretory control have been studied, including the impacts of nutrients, the nervous system and the endocrine system, and various regulatory mechanisms within the pancreatic islets by β‐cells and δ‐cells have been identified 14 . The discovery of intra‐islet regulatory mechanisms for glucagon secretion also shows the importance and necessity of the unique anatomical structure of the pancreatic islet, where a variety of endocrine cells that secrete different hormones gather in a characteristic manner of cellular distribution.
It has been widely accepted that glucagon secretion is enhanced and suppressed by hypoglycemia and hyperglycemia, respectively. However, the regulatory mechanism of glucagon secretion in α‐cells remained largely unknown until recently. In this context, basic research was carried out to clarify the underlying mechanism(s) of glucagon secretion using molecular biological techniques. In addition to regulation by nutrients, including glucose, glucagon secretion is also regulated by the nervous and endocrine systems, including GLP‐1, glucose‐dependent insulinotropic polypeptide (GIP) and somatostatin 15 . Furthermore, wide‐ranging molecular biological studies targeting α‐cells have shown that insulin 16 , zinc ions and gamma‐aminobutyric acid are involved in regulating glucagon both positively and negatively 17 . Interestingly, they are all secreted by β‐cells located close to glucagon‐secreting α‐cells, suggesting that β‐cells actively regulate glucagon secretion by α‐cells in an intra‐islet paracrine manner 18 .
Indeed, chemical β‐cell destruction by streptozotocin administration in healthy mice induces extreme hyperglycemia owing to severe insulin deficiency; however, plasma glucagon levels in this condition are unexpectedly elevated despite such hyperglycemia 18 . Interestingly, when phloridzin was administered to insulin‐deficient hyperglycemic mice to normalize blood glucose without using insulin, plasma glucagon levels normalized according to the decrease in blood glucose levels. This indicates the importance of the presence of neighboring β‐cells in regulating glucagon secretion 18 . Furthermore, α‐cell‐specific insulin receptor‐deficient mice, which do not show overt diabetes or obesity, displayed mildly, but significantly, elevated blood glucose levels and hyperglucagonemia in random‐fed states, hypersecretion of glucagon in response to amino acid stimulation and reduced glucagon secretion during long‐term fasting‐induced hypoglycemia, together with paradoxically increased glucagon secretion on refeeding 16 . These characteristic dynamics of abnormal glucagon secretion resemble the clinical state of type 2 diabetes. These results provide molecular evidence for an intra‐islet insulin‐mediated regulatory mechanism for α‐cell glucagon secretion in vivo.
As these physiological mechanisms for regulating glucagon secretion have been elucidated, the pathogenesis of abnormal glucagon secretion in diabetes was subsequently explored. Abnormal glucagon secretion owing to α‐cell glucose unresponsiveness, impairment of the regulatory nervous system and impairment of incretin action have been proposed. Among these, the possible impact of disorders in intra‐islet glucagon regulation was investigated. In our study, using a glucagon‐secreting cell line and isolated mouse islets, we confirmed high‐glucose‐induced hypersecretion of glucagon after prolonged incubation in a medium containing high glucose, mimicking hyperglycemia in diabetes. In parallel, we identified a decrease in intracellular insulin signaling, increased intracellular oxidative stress and upregulated stress‐responsive c‐Jun N‐terminal kinase signaling as an upstream mechanism 19 . Thus, like many insulin‐target organs, such as the liver and skeletal muscles, α‐cells are insulin‐sensitive, as they are exposed to large amounts of insulin from neighboring β‐cells. Insulin receptor expression in α‐cells has been substantial and comparable with that in hepatocytes 20 . Subsequently, under diabetic hyperglycemia, the α‐cell insulin action will be impaired, and the cells would enter a so‐called ‘insulin‐resistant’ state, thereby evoking the characteristic abnormality of glucagon secretion owing to an impaired response to insulin – the secretion of which changes dynamically in response to glycemic conditions.
Here, we propose a model for developing glucagon dysregulation through impaired insulin action on α‐cells induced by a chronic high‐glucose load in diabetes (Figure 3). In hyperglycemia, the increased insulin secreted by β‐cells acts on α‐cells in an intra‐islet paracrine manner and suppresses glucagon secretion by activating α‐cell insulin signaling. Conversely, in hypoglycemia, α‐cells promptly sense a decrease in insulin secretion from β‐cells and enhance glucagon secretion through the inactivation of insulin signaling in a ‘switch‐off’ manner. In contrast, in diabetes, β‐cell insulin secretion is often defective despite the stimulation of hyperglycemia, and insulin action on α‐cells is also reduced owing to impaired intracellular signaling, resulting in relatively excessive glucagon owing to deficient suppression. Conversely, in the state of hypoglycemia, β‐cell insulin secretion is not appropriately reduced because of treatment with insulin secretagogues or blunted secretion, and the α‐cells, which are also less sensitive to insulin, do not respond and promote glucagon secretion appropriately. This occurs because the cells cannot sense the reduction of surrounding insulin. In diabetes, both excessive and defective glucagon secretion is inexplicable, and this dysregulation of glucagon secretion further aggravates the disruption of glucose homeostasis in diabetes. The intra‐islet regulation of glucagon secretion and its dysregulation can be reasonably explained.
Figure 3.
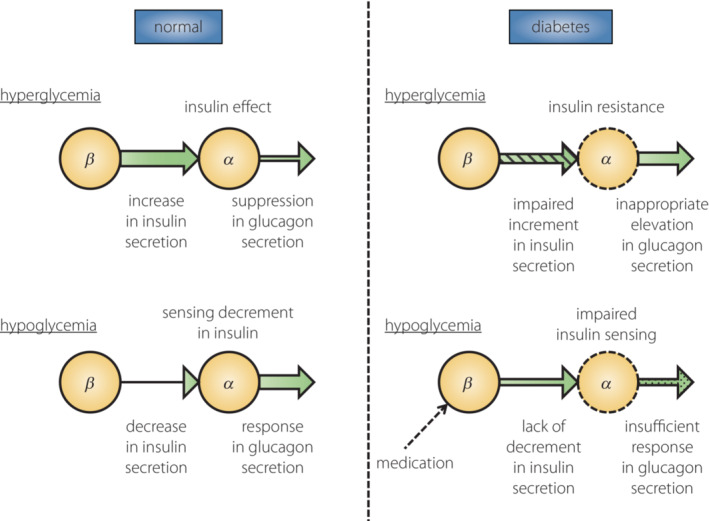
Model for the intra‐islet regulation of glucagon secretion from α‐cells by insulin. Normal state: In hyperglycemia, increased insulin secretion from β‐cells acts on α‐cells to suppress glucagon secretion. In hypoglycemia, decreased insulin secretion from β‐cells is sensed by α‐cells, which increases glucagon secretion. Diabetes state: In hyperglycemia, the lack of suppression of glucagon secretion because of impaired insulin secretion from β‐cells and insulin resistance in α‐cells leads to an abnormal elevation of glucagon. In hypoglycemia, the lack of decrease in insulin secretion from β‐cells, in addition to insulin insensitivity in α‐cells, results in an insufficient response in glucagon secretion.
CHANGING ROLES OF α‐CELLS: NEWLY DISCOVERED FUNCTIONS AND POSITIONING
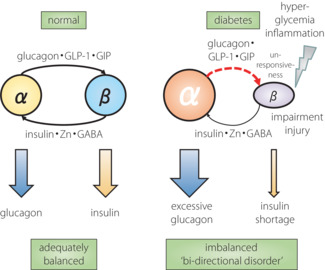
As presented above, many new findings regarding the physiological roles of glucagon, its clinical significance, and the mechanisms for secretion and dysregulation have been shown. The α‐cell has also attracted scientific attention as a research target because of its pathophysiological importance 21 . In addition to glucagon, α‐cells secrete GLP‐1 22 , GIP 23 and acetylcholine 24 , which then act on neighboring β‐cells, and promote insulin secretion and other β‐cell functions 21 . In particular, the importance of β‐cell GLP‐1 signaling activation by α‐cell‐derived products in insulin secretion stimulation and blood glucose regulation has been verified in animal models 25 . Regarding the regulatory mechanism for α‐cell proliferation, amino acids (especially glutamine) promote cellular proliferation through the amino acid transporter Slc38a5–mammalian target of rapamycin pathway 4 , 5 . In addition, α‐cells contain a subgroup showing active proliferation 26 , and possess the capacity for transdifferentiation into β‐cells, indicating their important role in the quantitative regulation of islet cells 27 . Therefore, α‐cells secrete glucagon, and are positioned as qualitative (insulin secretion) and quantitative (cellular number maintenance) coordinators of β‐cells inside the islets. Thus, α‐cells receive regulatory inputs from β‐cells, such as insulin, and simultaneously provide outputs to β‐cells, which in turn influence each other to regulate their functions; that is, the appropriate secretion of insulin and glucagon (Figure 2). In summary, β‐cells play an important role in regulating α‐cell function, and α‐cells play an important role in maintaining β‐cell function. Taken together, in the state of diabetes, insulin and glucagon are not independently dysregulated in their secretion. However, their interaction, especially the impaired action of β‐cells on α‐cells, induces disruption of the elegant functional balance between α‐ and β‐cells. This leads to characteristic abnormal secretion of both glucagon and insulin, together with abnormal quantitative regulation of α‐ and β‐cells, a ‘bi‐directional disorder’ in the islets (Figure 4).
Figure 4.
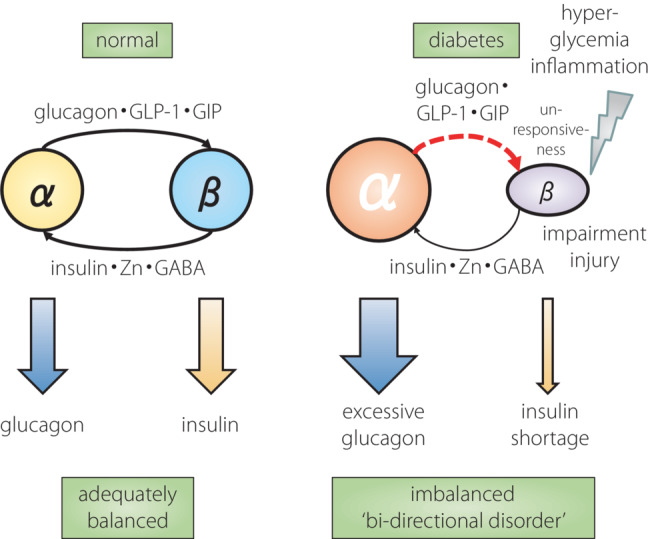
Model of balanced hormone secretion by adequate intra‐islet α–β interaction and its collapse by ‘bi‐directional disorder’. GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1.
With the recent development of single‐cell ribonucleic acid sequencing analysis, the heterogeneity of β‐cells has been well studied, while glucagon‐secreting α‐cells are proving to be heterogeneous as well. Using single‐cell ribonucleic acid sequencing analysis of healthy human islets, we found that the responsiveness to hypoglycemia, hyperglycemia and calcium stimulation varied among subpopulations of α‐cells, and that a set of genes was regulated by calcium stimulation in each α‐cell subpopulation 28 . In particular, α‐cell subpopulations that strongly express oxidative stress‐related genes have been identified. Evaluating the contribution of these differently responsive α‐cell clusters to the paradoxical secretion of glucagon in the context of diabetes would be beneficial.
Intriguingly, it has been proposed that (pro)glucagon transcript expression is not limited to α‐cells. Human islet single‐cell ribonucleic acid sequencing results showed that 3% of islet cells were copositive for insulin/glucagon 28 . Results from pseudotime analysis, a mathematical analysis of gene expression pattern transition, suggested that these copositive cells are not the result of differentiation or dedifferentiation from α‐ or β‐cells, but are an independent population of cells in the bona fide. In addition, studies using transgenic mice, in which immature and mature β‐cells can be visualized by fluorescent proteins, showed that there are two sites of β‐cell neogenesis; that is, the pancreatic ducts and blood vessels. They also showed that β‐cells on the duct side are positive for glucagon 29 , 30 . These new findings suggest the existence of cell types with various gradations of glucagon‐positive cells, and there might be a high degree of plasticity between α‐ and β‐cells. In recent years, glucagon‐secreting α‐cells have attracted attention as a cell source for β‐cell replacement therapy to treat diabetes. We successfully induced β‐cells from α‐cells in vivo by introducing specific transcription factors 31 . Detailed analysis of glucagon and α‐cells regarding cell differentiation and maturation will lead to the development of regenerative medicine for diabetes.
ADVANCES IN THE CLINICAL EVALUATION OF GLUCAGON AND FINDINGS
In contrast to the advances in basic research on glucagon and α‐cell biology, clinical research was one step behind, owing to the lack of reliable clinical assays for glucagon, which has a characteristic production manner (Figure 5) 32 . In the RIA method, which has been used since the 1970s, cross‐reactivity of the specific antibody recognizing the C‐terminus of glucagon with various proglucagon‐derived products is predicted. Additionally, the accuracy of the RIA method is controversial, as studies using molecular biological approaches have elucidated the characteristic production manner of glucagon and its related products. Under such situations, with a rising demand for improved measurement methods that override the currently existing problems for RIAs, a sandwich ELISA system using dual antibodies against the C‐terminus and N‐terminus of glucagon was developed. This ELISA system has been validated for its improved accuracy by the mass‐spectrometry method 33 , and has been widely used in clinical studies 34 . Many studies utilizing this new ELISA have re‐evaluated plasma glucagon levels in wide‐ranging states from physiological to pathological in patients with diabetes and controls. These results confirmed previously recognized behaviors of plasma glucagon according to the changes in glycemic condition and its dysregulation in patients with diabetes. Strikingly, the absolute plasma values of glucagon evaluated using the new ELISA were substantially lower than those using the RIA 35 . This shows that the previously measured and known values for plasma glucagon included certain ‘contamination’ with other substances, possibly other proglucagon‐derived products, such as oxyntomodulin and glicentin. The development of the new system has induced a surge in clinical evaluations of glucagon, and new data and findings are rapidly accumulating. First, the ELISA also reconfirmed the known glucagon hypersecretion under hyperglycemia during an oral glucose tolerance test in patients with type 2 diabetes, while the glucagon levels were decreased by glucose load, as expected in non‐diabetic controls 35 . However, as described above, the absolute values measured were much lower than those measured by the conventional RIA method. Therefore, the relative change in the values intensified. These results emphasize the pathological significance of the secretory changes in glucagon. Suppression of glucagon secretion by glucose load in normal individuals was confirmed in another study 36 . These are good examples of new assays that have stimulated and advanced clinical research in related areas.
Figure 5.
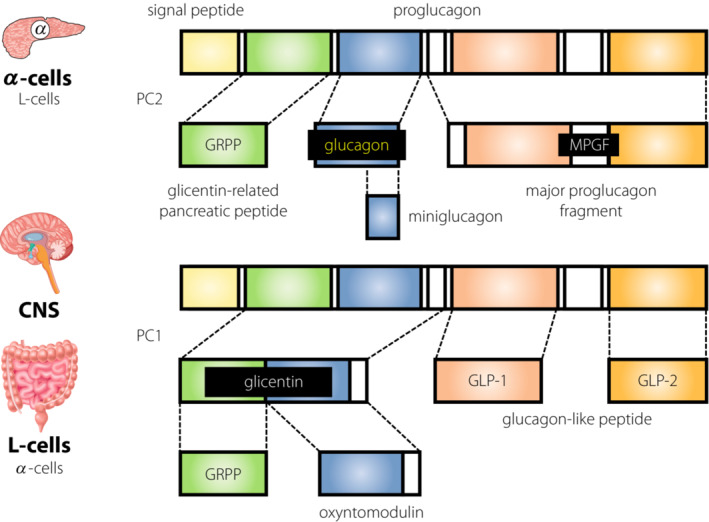
Production of glucagon and related peptides from proglucagon. GLP‐1, glucagon‐like peptide‐1; GLP‐2, glucagon‐like peptide‐2; GRPP, glicentin‐related pancreatic polypeptide; MPGF, major proglucagon fragment.
Amid the recent rapid expansion in the clinical application of the new glucagon assay, we investigated the clinical status of glucagon in type 1 diabetes 37 . First, plasma glucagon levels evaluated by ELISA at a random fed state in patients with type 1 diabetes were not correlated with blood glucose levels at the time of measurement and were widely dispersed without any constant trend. This suggests impaired secretory regulation of glucagon in response to glycemic status and its persistence 38 . Although this is a reconfirmation of previously proposed findings, the underlying mechanism of these abnormalities in glucagon secretion could be owing to the disruption of α‐ and β‐cell interactions in the islets. This disruption is mediated by shortage of insulin and other substances owing to β‐cell elimination, which is the central pathophysiology of type 1 diabetes mellitus. Thus, this research also provided clinical evidence of the importance of β‐cell function in regulating glucagon secretion, which has been proposed in various basic studies. In addition, plasma glucagon levels were significantly lower in the group of patients experiencing hypoglycemia unawareness, suggesting the contribution of impaired glucagon secretion. Interestingly, glucagon levels were not associated with HbA1c or other parameters of glucose metabolism, lipid metabolism, liver function, renal function or diabetic complications, but were strongly correlated only with serum blood urea nitrogen levels. This significant correlation between glucagon and blood urea nitrogen shows an association with amino acids, which are sources of nitrogen in the body. While amino acids stimulate glucagon secretion from α‐cells as an acute effect, glucagon promotes amino acid metabolism in the liver, resulting in an increased production of urea nitrogen 2 , 3 . Thus, the present study also clinically proves the functional involvement of glucagon in amino acid metabolism, which has been found in basic research. We also showed characteristic changes in plasma amino acid profiles, especially a large decrease in glutamate levels, in patients with type 1 diabetes 39 . Therefore, the development of the new glucagon assay system not only re‐evaluated the pathophysiological significance of glucagon, but also led to new medical and nutritional concepts in conjunction with basic research 34 .
FUTURE THERAPEUTIC APPROACHES TO DIABETES AND METABOLIC DISEASES USING GLUCAGON
The physiological significance of glucagon has been increasing, owing to its newly found functions in nutrient metabolism – both glucose and amino acids –, pathological involvement in metabolic diseases and clinical significance confirmed by many clinical studies using new assay methods. These advances in basic and clinical research on glucagon have prompted attempts to utilize glucagon for clinical diagnoses and develop various therapeutic approaches for metabolic diseases. Glucagon has been used to treat hypoglycemia because of its strong hyperglycemic effect. In contrast, in recent years, therapeutic effects by inhibiting glucagon action and secretion have been expected because of glucagon's involvement in the pathogenesis of diabetes, especially in the exacerbation of hyperglycemia. It has also exerted significant effects on incretin therapies, including the use of GLP‐1 receptor agonists. Tirzepatide, a recently developed co‐activator of GLP‐1 and GIP receptors, exerts certain effects that improve hyperglycemia, which could also be mediated by normalization of glucagon secretion in part 40 . Interestingly, by changing the concept of the role of glucagon as a ‘metabolic activator’ rather than a ‘hyperglycemia‐inducing factor’, glucagon receptor agonists have also been developed as a strategy for obesity treatment that effectively utilizes their energy expenditure‐promoting effects. Progress has also been made regarding glucagon as an anti‐hypoglycemic agent, with its conventional injectable form being improved to an easier nasally administered form. Its effectiveness in treating acute hypoglycemic complications, such as hypoglycemic coma, has been achieved 41 . In the future, it will be necessary to control the excessive secretion and action of glucagon in diabetes, and to appropriately utilize its physiological function to improve wide‐ranging metabolic disorders, including obesity and liver dysfunction.
Although reliable clinical evaluation of glucagon using new assay methods with improved accuracy has become widely available, there is currently no clear definition for glucagon reference values or unified sampling conditions. Currently, clinical samples are collected and evaluated in fasting, glucose tolerance, and protein and other dietary tolerance tests 42 , and glucagon levels are analyzed in combination with a variety of laboratory parameters, including blood glucose, insulin and C‐peptide 9 . Each has its advantages and disadvantages. However, extensive future work accumulating detailed knowledge on these parameters is still necessary to establish a simple and accurate standard method that reflects the pathophysiology and contributes to medical care for various metabolic diseases, similar to blood glucose and HbA1c in the past.
For a long time, glucagon has been widely utilized as an acute therapeutic agent for hypoglycemia, and has indeed saved numerous lives. However, glucagon administration is difficult, because it is provided in powder form in vials, and time‐consuming dissolution and loading in a syringe are required. In addition, patients with hypoglycemia cannot carry out these complicated protocols, and these steps cannot be carried out by other nearby persons with no specialized skills. To overcome these obstacles, a precast formulation of glucagon has been developed. However, there is still a strong hesitation to administer (through injectable formulations) glucagon to hyperglycemic individuals in the general public, even in emergencies. Under these circumstances, an intranasal glucagon formulation was introduced as a new form of administration that could be quickly applied in hypoglycemic emergencies. This formulation showed a short administration completion time and a higher success rate compared with conventional injectable formulations 43 . Therefore, it is very useful as a response to severe, sometimes life‐threatening, hypoglycemia.
On expanding the pathophysiological significance shown by intensive research, glucagon has attracted attention as a potential target for several metabolic diseases. Various glucagon receptor antagonists, from small molecule compounds to specific antibodies, have been developed to inhibit the strong effects of excessive glucagon in the liver, and have shown a certain improving effect with a low risk of hypoglycemia 44 . In addition, during their development, an interesting mechanism for the hypoglycemic effect of these agents was proposed, in which the compensatory increase in glucagon secretion owing to glucagon receptor antagonism acts on pancreatic β‐cell GLP‐1 receptors in an intra‐islet manner, thereby exerting an insulin‐secretion‐promoting effect 8 . In contrast, many reports show that the suppression of glucagon effects, especially the promotion of the energy‐mobilizing effect, worsens hepatic lipid accumulation and metabolic parameters, such as blood pressure, low‐density lipoprotein cholesterol and bodyweight 45 . Therefore, the development of these agents has been suspended owing to safety concerns. Thus, there are still issues to be resolved to optimize glucagon action.
Conversely, various glucagon receptor‐activating therapies are currently developed on the premise that glucagon enhances energy metabolism and mobilization, especially in treating obesity and metabolic dysfunction‐associated fatty liver disease. Glucagon receptor agonists, which promote the release of energy from the liver and adipose tissue by the pharmacological activation of glucagon signaling, can reduce lipid accumulation in these organs, but induce hyperglycemia because of excessive hepatic glucose output. In response, glucagon and GLP‐1 receptor dual‐agonists have been developed, and have shown significant effects on bodyweight reduction and improvement of glycemic status, despite the significantly higher incidence of gastrointestinal side‐effects 46 . Furthermore, glucagon, GLP‐1 and GIP receptor tri‐agonists have been developed 47 that exert certain effects on bodyweight reduction 48 . In addition, sodium–glucose transporter 2 inhibitors have been suggested to reduce hepatic fat accumulation and improve obesity by increasing glucagon levels. With further development, we expect glucagon‐targeted therapies to show effects, such as weight loss, and improvement in glucose metabolism and metabolic liver diseases 49 . This might lead to a bright future for the treatment of diabetes and obesity, for which insulin action and secretion are currently exclusive targets.
CONCLUSION
Knowledge pertaining to glucagon based on recent findings from basic and clinical research, its clinical applications, and potential for therapeutic development in the future was outlined in the present paper. In particular, the close relationship between glucagon and amino acid metabolism shows that glucagon might be involved not only in glucose, but also in protein metabolism. The pathophysiology of metabolic diseases, especially diabetes, has been discussed in the context of dysfunction in insulin secretion and action. However, glucagon, which is equally important to insulin in systemic energy metabolism, must also be considered as a central player in future medical treatment for diabetes and metabolic diseases.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: NA.
Informed consent: NA.
Registry and the registration no. of the study/trial: NA.
Animal studies: NA.
ACKNOWLEDGMENTS
This work was partially supported by JSPS KAKENHI (grant numbers 21K08576 to DK, 21K20902 and 22K16395 to SS) by the Ministry of Education, Culture, Sports, Science and Technology in Japan.
REFERENCES
- 1. Scheen AJ, Lefebvre PJ. Glucagon, from past to present: A century of intensive research and controversies. Lancet Diabetes Endocrinol 2023; 11: 129–138. [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ, Wewer Albrechtsen NJ, Pedersen J, et al. Glucagon and amino acids are linked in a mutual feedback cycle: The liver‐alpha‐cell axis. Diabetes 2017; 66: 235–240. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi Y, Seino Y. Regulation of amino acid metabolism and alpha‐cell proliferation by glucagon. J Diabetes Investig 2018; 9: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dean ED, Li M, Prasad N, et al. Interrupted glucagon signaling reveals hepatic alpha cell axis and role for L‐glutamine in alpha cell proliferation. Cell Metab 2017; 25: 1362–1373.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim J, Okamoto H, Huang Z, et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition‐induced pancreatic alpha cell hyperplasia in mice. Cell Metab 2017; 25: 1348–1361.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawamori D. Exploring the molecular mechanisms underlying alpha‐ and beta‐cell dysfunction in diabetes. Diabetol Int 2017; 8: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gromada J, Chabosseau P, Rutter GA. The alpha‐cell in diabetes mellitus. Nat Rev Endocrinol 2018; 14: 694–704. [DOI] [PubMed] [Google Scholar]
- 8. Capozzi ME, Wait JB, Koech J, et al. Glucagon lowers glycemia when beta‐cells are active. JCI Insight 2019; 4: e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosokawa Y, Kozawa J, Nishizawa H, et al. Positive correlation between fasting plasma glucagon and serum C‐peptide in Japanese patients with diabetes. Heliyon 2019; 5: e01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unger RH, Eisentraut AM, McCall CM, et al. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med 1959; 102: 621–623. [DOI] [PubMed] [Google Scholar]
- 11. Muller WA, Faloona GR, Aguilar‐Parada E, et al. Abnormal alpha‐cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970; 283: 109–115. [DOI] [PubMed] [Google Scholar]
- 12. Gerich JE, Lorenzi M, Karam JH, et al. Abnormal pancreatic glucagon secretion and postprandial hyperglycemia in diabetes mellitus. JAMA 1975; 234: 159–155. [PubMed] [Google Scholar]
- 13. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975; 1: 14–16. [DOI] [PubMed] [Google Scholar]
- 14. Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 2012; 153: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gromada J, Franklin I, Wollheim CB. Alpha‐cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84–116. [DOI] [PubMed] [Google Scholar]
- 16. Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 2009; 9: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawamori D, Welters HJ, Kulkarni RN. Molecular pathways underlying the pathogenesis of pancreatic alpha‐cell dysfunction. Adv Exp Med Biol 2010; 654: 421–445. [DOI] [PubMed] [Google Scholar]
- 18. Kawamori D, Akiyama M, Hu J, et al. Growth factor signalling in the regulation of alpha‐cell fate. Diabetes Obes Metab 2011; 13: 21–30. [DOI] [PubMed] [Google Scholar]
- 19. Katsura T, Kawamori D, Aida E, et al. Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS One 2017; 12: e0176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franklin I, Gromada J, Gjinovci A, et al. Beta‐cell secretory products activate alpha‐cell ATP‐dependent potassium channels to inhibit glucagon release. Diabetes 2005; 54: 1808–1815. [DOI] [PubMed] [Google Scholar]
- 21. Kawamori D. Alpha the versatile: Guardians of the islets. J Diabetes Investig 2019; 10: 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin‐6 enhances insulin secretion by increasing glucagon‐like peptide‐1 secretion from L cells and alpha cells. Nat Med 2011; 17: 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fujita Y, Wideman RD, Asadi A, et al. Glucose‐dependent insulinotropic polypeptide is expressed in pancreatic islet alpha‐cells and promotes insulin secretion. Gastroenterology 2010; 138: 1966–1975. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez‐Diaz R, Dando R, Jacques‐Silva MC, et al. Alpha cells secrete acetylcholine as a non‐neuronal paracrine signal priming beta cell function in humans. Nat Med 2011; 17: 888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chambers AP, Sorrell JE, Haller A, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 2017; 25: 927–934.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam CJ, Cox AR, Jacobson DR, et al. Highly proliferative alpha‐cell‐related islet endocrine cells in human pancreata. Diabetes 2018; 67: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stanojevic V, Habener JF. Evolving function and potential of pancreatic alpha cells. Best Pract Res Clin Endocrinol Metab 2015; 29: 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon JS, Sasaki S, Velghe J, et al. Calcium‐dependent transcriptional changes in human pancreatic islet cells reveal functional diversity in islet cell subtypes. Diabetologia 2022; 65: 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Sasaki S, Lee MYY, Wakabayashi Y, et al. Spatial and transcriptional heterogeneity of pancreatic beta cell neogenesis revealed by a time‐resolved reporter system. Diabetologia 2022; 65: 811–828. [DOI] [PubMed] [Google Scholar]
- 30. Sasaki S, Miyatsuka T. Heterogeneity of islet cells during embryogenesis and differentiation. Diabetes Metab J 2023; 47: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuoka TA, Kawashima S, Miyatsuka T, et al. Mafa enables Pdx1 to effectively convert pancreatic islet progenitors and committed islet alpha‐cells into beta‐cells in vivo. Diabetes 2017; 66: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue‐specific processing of proglucagon. Mol Endocrinol 1996; 10: 342–355. [DOI] [PubMed] [Google Scholar]
- 33. Miyachi A, Kobayashi M, Mieno E, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography‐high resolution mass spectrometry: Comparison with commercially available immunoassays. Anal Bioanal Chem 2017; 409: 5911–5918. [DOI] [PubMed] [Google Scholar]
- 34. Kawamori D. Beginning of a new era in glucagon research: Breakthrough by the new glucagon assay. J Diabetes Investig 2020; 11: 1123–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuo T, Miyagawa J, Kusunoki Y, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme‐linked immunosorbent assay. J Diabetes Investig 2016; 7: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horie I, Abiru N, Eto M, et al. Sex differences in insulin and glucagon responses for glucose homeostasis in young healthy Japanese adults. J Diabetes Investig 2018; 9: 1283–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawamori D, Katakami N, Takahara M, et al. Dysregulated plasma glucagon levels in Japanese young adult type 1 diabetes patients. J Diabetes Investig 2019; 10: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawamori D, Katakami N, Takahara M, et al. Consistency of plasma glucagon levels in patients with type 1 diabetes after a 1‐year period. J Diabetes Investig 2020; 11: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawamori D, Kageyama Y, Tanaka T, et al. Characteristic changes in plasma glutamate levels and free amino acid profiles in Japanese patients with type 1 diabetes mellitus. J Diabetes Investig 2023; 14: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yabe D, Kawamori D, Seino Y, et al. Change in pharmacodynamic variables following once‐weekly tirzepatide treatment versus dulaglutide in Japanese patients with type 2 diabetes (SURPASS J‐mono substudy). Diabetes Obes Metab 2023; 25: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thieu VT, Mitchell BD, Varnado OJ, et al. Treatment and prevention of severe hypoglycaemia in people with diabetes: Current and new formulations of glucagon. Diabetes Obes Metab 2020; 22: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kobayashi M, Satoh H, Matsuo T, et al. Plasma glucagon levels measured by sandwich ELISA are correlated with impaired glucose tolerance in type 2 diabetes. Endocr J 2020; 67: 903–922. [DOI] [PubMed] [Google Scholar]
- 43. Aranishi T, Nagai Y, Takita Y, et al. Usability of nasal glucagon device: Partially randomized caregiver and third‐party user experience trial with simulated administration at a Japanese site. Diabetes Ther 2020; 11: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lefebvre PJ, Paquot N, Scheen AJ. Inhibiting or antagonizing glucagon: Making progress in diabetes care. Diabetes Obes Metab 2015; 17: 720–725. [DOI] [PubMed] [Google Scholar]
- 45. Kazda CM, Frias J, Foga I, et al. Treatment with the glucagon receptor antagonist LY2409021 increases ambulatory blood pressure in patients with type 2 diabetes. Diabetes Obes Metab 2017; 19: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 46. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP‐1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double‐blind, ascending dose and phase 2a study. Lancet 2018; 391: 2607–2618. [DOI] [PubMed] [Google Scholar]
- 47. Urva S, Coskun T, Loh MT, et al. LY3437943, a novel triple GIP, GLP‐1, and glucagon receptor agonist in people with type 2 diabetes: A phase 1b, multicentre, double‐blind, placebo‐controlled, randomised, multiple‐ascending dose trial. Lancet 2022; 400: 1869–1881. [DOI] [PubMed] [Google Scholar]
- 48. Coskun T, Urva S, Roell WC, et al. LY3437943, a novel triple glucagon, GIP, and GLP‐1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab 2022; 34: 1234–1247.e9. [DOI] [PubMed] [Google Scholar]
- 49. Boland ML, Laker RC, Mather K, et al. Resolution of NASH and hepatic fibrosis by the GLP‐1R/GcgR dual‐agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab 2020; 2: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]


