Abstract
Aims/Introduction
Regular screening for diabetic retinopathy is essential. This study aimed to show the process and current situation of diabetic retinopathy screening prescribed by physicians (internists) and ophthalmologists for Japanese patients with diabetes.
Materials and Methods
This retrospective cohort study used data from the Japanese National Database of Insurance Claims between April 2016 and March 2018. Ophthalmology visits and fundus examinations are defined using specific medical procedure codes. The proportion of ophthalmology visits for patients with diabetic medication and for fundus examination among those who visited ophthalmologists was calculated in the fiscal year 2017. A modified Poisson regression analysis was carried out to identify factors associated with retinopathy screening. Similarly, quality indicators by prefectures were also calculated.
Results
Among 4,408,585 patients receiving diabetic medications (57.8% men, 14.1% insulin use), 47.4% visited the ophthalmology department and 96.9% of those underwent fundus examination. Regression analysis showed that female sex, older age, insulin use, medical facilities with Japan Diabetes Society certification and large medical facilities were predictors of fundus examination. By prefecture, the ophthalmology consultation rate and the fundus examination ranged 38.5–51.0% and 92.1–98.7%, respectively.
Conclusions
Less than half of the patients who were prescribed antidiabetic medication by their physicians visited an ophthalmologist. However, most of the patients who visited an ophthalmologist had a fundus examination carried out. A similar tendency was noted for each prefecture. It is essential to reaffirm the necessity of recommending ophthalmologic examinations to physicians and healthcare professionals who care for patients with diabetes.
Keywords: Claims analysis, Diabetic retinopathy, Healthcare quality assessment
This study showed the process and current situation of diabetic retinopathy screening prescribed by physicians (internists) and ophthalmologists for Japanese patients with diabetes using a national database. Fewer than half of the patients who were prescribed antidiabetic medication by their physicians visited an ophthalmologist; however, most patients who visited an ophthalmologist had a fundus examination carried out. This finding implied that there is a chasm between physicians and ophthalmologists that should be improved.
INTRODUCTION
Diabetic retinopathy is one of the most prevalent diabetic comorbidities, which often causes visual loss in individuals with diabetes 1 . In recent decades, the incidence of visual impairment due to diabetic retinopathy has reduced as a result of better glucose management with new antidiabetic drugs, greater eye screening uptake and advances in diabetic retinopathy therapies, including antivascular endothelial growth factor injections 2 , 3 , 4 .
Diabetic retinopathy is typically asymptomatic before it develops into severe stages; hence, regular eye examinations are required to detect its onset and progression. Subsequent retinopathy screening is recommended to minimize the loss of vision due to the progression of diabetic retinopathy 5 , 6 , 7 , 8 . Nationwide systematic diabetic retinopathy screening programs exist in Iceland, the UK and Ireland, and systematic diabetic eye screening is also being promoted in other regions, including parts of mainland Europe, Asia and Africa 9 . In Japan, although some municipality insurers carry out systematic eye screenings for patients with diabetes or hypertension 10 , most diabetic eye screening relies on ad hoc referrals from physicians (internists) to ophthalmologists. We have previously reported that the number of patients in Japan undergoing retinopathy examination once a year who are prescribed antidiabetic drugs remains as low as 47% 11 . To increase the rate of fundus examination among patients with diabetes, it is essential to identify the reasons for the current low rates. It is especially important to break down the rate of fundus examination into the rate of ophthalmologist visits among patients with diabetes and that of patients undergoing fundus examination at the ophthalmologist office among those who visited ophthalmologists.
The Japanese National Database (NDB) 12 contains claimed data of almost all Japanese citizens, excluding data on medical expenses of patients who received welfare or those not covered by insurance; for example, preventive medicine or maternity expenses. In the present study, we used NDB data and investigated the annual proportion of patients with diabetes who visited an ophthalmologist, including those who visited an ophthalmologist for other reasons. We also investigated the proportion of patients who underwent fundus examination among those with diabetes who visited an ophthalmologist.
MATERIALS AND METHODS
The present cross‐sectional study used an anonymous, nationwide claims database in Japan called the NDB. With approximately 3,500 insurers 13 , Japan holds a universal health coverage that covers individuals based on their unique characteristics (e.g., age, region and job). NDB includes almost all claim data, except for fully publicly funded medical procedures and medical procedures not covered by medical insurance (e.g., data of individuals receiving preventive medicine, traffic accidents or maternity expenses). NDB comprises claims data submitted by each insurer to the government of Japan (the Ministry of Health, Labor and Welfare); these data are obtained from 98.4% of hospital claims, and 99.9% of pharmacy claims gathered from hospitals, clinics and pharmacies. It provides anonymous data for administrative and research purposes 12 .
This study was approved by the institutional review board of the National Center for Global Health and Medicine (NCGM‐G‐002492‐04). The institutional review board waived informed consent, because the data in the database were anonymized before being provided by the Ministry of Health, Labor and Welfare.
Patients
All definition variables have been explained in Table S1. Patients with diabetes were defined as those who received antidiabetic medication regularly. Antidiabetic medication was determined using the anatomical therapeutic chemical classification, and antidiabetic drugs were defined using the A10 code. Data on voglibose 0.2 mg tablets and epalrestat were excluded owing to their efficacy in diabetes prevention and diabetic neuropathy, respectively 14 .
The patient selection process is shown in Figure 1. First, we extracted data on adult patients with diabetes (≥20 years) who received antidiabetic medications regularly (at least one prescription every 3 months) in April 2017 and March 2018 (fiscal year [FY] 2017). We excluded the following patients: (1) those not prescribed antidiabetic medications in FY2016 (for the purpose of excluding those who were newly diagnosed with diabetes), (2) patients with admission history in FY2017, (3) patients who received comprehensive medical care, and (4) patients who had a disease name of blindness. We excluded patients with comprehensive medical care that could cause underreporting of the examinations carried out. Visual impairment was defined using International Classification of Diseases, 10th Revision 15 . The details on comprehensive medical care and visual impairment are shown in Table S1.
Figure 1.
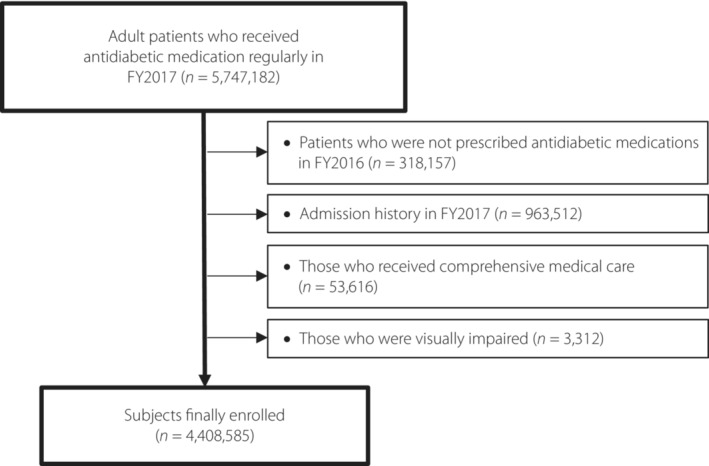
Flowchart of the selection of patients. FY, fiscal year.
The type of diabetes was defined using International Classification of Diseases, 10th Revision codes obtained from the medical claims data. Patients were diagnosed with type 1 diabetes mellitus if code E10 occurred at least once, and patients were diagnosed with type 2 diabetes mellitus and other types if the claims data had code E11‐14.
Outcome variables
According to Guidelines for the Treatment of Diabetic Retinopathy (1st edition) in Japan 16 , the outcome variable was the annual proportion of patients with ophthalmology visits and fundus examinations among those with diabetes. Proportions of the following were calculated: (1) patients who visited an ophthalmologist (A) among patients with diabetes (U), (2) patients undergoing fundus examination (B) among those who visited an ophthalmologist (A), and (3) patients undergoing fundus examination (B) among those with diabetes (U); the calculated proportions were A/U, B/A and B/U, respectively (Figure S1).
Predictors and covariates
Definitions of predictors and covariates are shown in Table S1. Patient characteristics and ophthalmology facilities were extracted from medical claims data, whereas prescription information and medical facilities for diabetic care were extracted from medical and pharmaceutical claims data.
Medical facilities for regular diabetes care for each patient were identified in the following order: (1) facilities where patients received the most antidiabetic prescriptions during the year, (2) Japan Diabetes Society (JDS)‐certified facilities (JDS‐certified facilities are the facilities having a diabetes training instructor and treatment or care for >200 patients with diabetes), (3) facilities with the greatest number of beds and medical facilities patients visited, and (4) facilities patients visited earlier in FY2017. Information on the number of beds, whether the medical facility was a JDS‐certified education facility and the location (prefecture) of the facility were also collected.
Ophthalmology facilities and departments for each of the patients were identified using ophthalmology‐related medical remuneration point codes during FY2017. Such facilities and departments were identified in the following order: (1) facilities where a patient received fundus examination, (2) where a patient visited most often, and (3) where a patient visited for the first time in FY2017.
Statistical analysis
First, we described the patient's characteristics, type of diabetes, antidiabetic medication, and the medical facilities visited for diabetes treatment and ophthalmology visits. Categorical variables are presented as numbers (percentages), whereas continuous variables are presented as means (standard deviation). For the primary analysis, we calculated the proportion of patients visiting ophthalmologists and the proportion of those who carried out a fundus examination among patients visiting ophthalmologists. The χ2‐test was used to compare these proportions by individual and facility characteristics. Next, a modified Poisson regression analysis 17 was carried out to identify factors associated with retinopathy screening based on the following: sex, age, insulin use and medical facilities prescribing antidiabetic medications.
The ophthalmology consultation rates of the patients and fundus examinations in each prefecture were investigated to validate the actual retinopathy screening condition. According to the Position Statement of the American Diabetes Association, for patients with no evidence of retinopathy for one or more annual eye examinations, examinations can be considered every 2 years 7 . Therefore, we analyzed the rate of ophthalmology consultation and retinopathy screening among patients with diabetes over 2 years (FY2016–17).
All statistical analyses were carried out using Stata 17.0 software (StataCorp, College Station, TX, USA), with P < 0.05 showing statistical significance.
RESULTS
Among 5,747,182 adult patients who regularly received antidiabetic medications in FY2017, 318,157 who were not prescribed antidiabetic medications in FY2016, 963,512 who had admission history, 53,616 who received comprehensive medical care and 3,312 who had visual impairment were excluded. The remaining 4,408,585 patients were eligible for the present analysis (Figure 1). Baseline characteristics are shown in Table 1. More than half of the patients with diabetes were men and aged >60 years. In addition, 85.9% of them did not use insulin, 97.3% had type 2 diabetes and most patients visited clinics for antidiabetic medication.
Table 1.
Patient characteristics and quality indicator of retinopathy screening
| Patients with diabetes | Visiting ophthalmologists | Not visiting Ophthalmologists | Quality indicator | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Fundus examination (+) | Fundus examination (−) | P‐value | P‐value | Proportion of ophthalmology visits among patients with diabetes | Proportion of fundus examination (+) among ophthalmology visits | Proportion of fundus examination (+) among patients with diabetes | |||
| U | A | B | A/B | U/A | A/U | B/A | B/U | |||
| Total | 4,408,585 | 2,091,372 | 2,025,841 | 65,531 | 2,317,213 | 47.4% | 96.9% | 46.0% | ||
| Sex | ||||||||||
| Male | 2,548,307 (57.8) | 1,106,171 (52.9) | 1,069,882 (52.8) | 36,289 (55.4) | <0.001 | 1,442,136 (62.2) | <0.001 | 43.4% | 96.7% | 42.0% |
| Female | 1,860,278 (42.2) | 985,201 (47.1) | 955,959 (47.2) | 29,242 (44.6) | 875,077 (37.8) | 53.0% | 97.0% | 51.4% | ||
| Age (years) | ||||||||||
| 20–29 | 12,268 (0.3) | 5,192 (0.3) | 4,937 (0.2) | 255 (0.4) | <0.001 | 7,076 (0.3) | <0.001 | 42.3% | 95.1% | 40.2% |
| 30–39 | 56,009 (1.3) | 21,477 (1.0) | 20,298 (1.0) | 1,179 (1.8) | 34,532 (1.5) | 38.4% | 94.5% | 36.2% | ||
| 40–49 | 257,881 (5.8) | 91,622 (4.4) | 87,085 (4.3) | 4,537 (6.9) | 166,259 (7.2) | 35.5% | 95.1% | 33.8% | ||
| 50–59 | 579,850 (13.2) | 211,773 (10.1) | 202,412 (10.0) | 9,361 (14.3) | 368,077 (15.9) | 36.5% | 95.6% | 34.9% | ||
| 60–69 | 1,194,242 (27.1) | 527,133 (25.2) | 509,753 (25.2) | 17,380 (26.5) | 667,109 (28.8) | 44.1% | 96.7% | 42.7% | ||
| 70–79 | 1,373,291 (31.2) | 741,928 (35.5) | 723,564 (35.7) | 18,364 (28.0) | 631,363 (27.3) | 54.0% | 97.5% | 52.7% | ||
| ≥80 | 935,044 (21.2) | 492,247 (23.5) | 477,792 (23.6) | 14,455 (22.1) | 442,797 (19.1) | 52.6% | 97.1% | 51.1% | ||
| Antidiabetic drug | ||||||||||
| Insulin (+) | 622,537 (14.1) | 387,637 (18.5) | 381,600 (18.8) | 6,037 (9.2) | <0.001 | 234,900 (10.1) | <0.001 | 62.5% | 98.4% | 61.3% |
| (−) | 3,786,048 (85.9) | 1,703,735 (81.5) | 1,644,241 (81.2) | 59,494 (90.8) | 2,082,313 (55.0) | 45.2% | 96.5% | 43.4% | ||
| Type of diabetes | ||||||||||
| Type 1 | 84,537 (1.9) | 54,590 (2.6) | 53,685 (2.7) | 905 (1.4) | <0.001 | 29,947 (1.3) | <0.001 | 64.6% | 98.3% | 63.5% |
| Type 2 | 4,288,849 (97.3) | 2,031,523 (97.1) | 1,967,219 (97.1) | 64,304 (98.1) | 2,257,326 (97.4) | 47.4% | 96.8% | 45.9% | ||
| Unknown | 35,199 (0.8) | 5,259 (0.3) | 4,937 (0.2) | 322 (0.5) | 29,940 (1.3) | 14.9% | 93.9% | 14.0% | ||
| Diabetic medical facilities | ||||||||||
| JDS‐certified education facility | 542,971 (12.3) | 325,969 (15.6) | 318,444 (15.7) | 7,525 (11.5) | <0.001 | 217,002 (9.4) | <0.001 | 60.0% | 97.7% | 58.7% |
| Non‐JDS‐certified education facility | 3,865,614 (87.7) | 1,765,403 (84.4) | 1,707,397 (84.3) | 58,006 (88.5) | 2,100,211 (90.6) | 45.7% | 96.7% | 44.2% | ||
| Facilities with ophthalmology department | 1,637,771 (37.2) | 900,916 (43.1) | 876,314 (43.3) | 24,602 (37.5) | <0.001 | 736,855 (31.8) | <0.001 | 55.0% | 97.3% | 53.5% |
| Facilities without ophthalmology department | 2,770,814 (62.9) | 1,190,456 (56.9) | 1,149,527 (56.7) | 40,929 (62.5) | 1,580,358 (68.2) | 43.0% | 96.6% | 41.5% | ||
| No. beds (diabetes medical facilities) | ||||||||||
| 0–19 | 2,833,848 (64.3) | 1,267,072 (60.6) | 1,225,320 (60.5) | 41,752 (63.7) | <0.001 | 1,566,776 (67.6) | <0.001 | 44.7% | 96.7% | 43.2% |
| 20–99 | 293,206 (6.7) | 135,564 (6.5) | 130,612 (6.5) | 4,952 (7.6) | 157,642 (6.8) | 46.2% | 96.4% | 44.6% | ||
| 100–100 | 413,660 (9.4) | 203,002 (9.7) | 196,420 (9.7) | 6,582 (10.0) | 210,658 (9.1) | 49.1% | 96.8% | 47.5% | ||
| ≥200 | 847,520 (19.2) | 477,340 (22.8) | 465,340 (23.0) | 12,000 (18.3) | 370,180 (16.0) | 56.3% | 97.5% | 54.9% | ||
| Unknown | 20,351 (0.5) | 8,394 (0.4) | 8,149 (0.4) | 245 (0.4) | 11,957 (0.5) | 41.3% | 97.1% | 40.0% | ||
| Prefecture | ||||||||||
| Hokkaido | 204,365 | 92,445 | 85,144 | 7,301 | 111,920 | 45.2% | 92.1% | 41.7% | ||
| Aomori | 59,209 | 28,075 | 27,025 | 1,050 | 31,134 | 47.4% | 96.3% | 45.6% | ||
| Iwate | 54,562 | 26,919 | 26,509 | 410 | 27,643 | 49.3% | 98.5% | 48.6% | ||
| Miyagi | 89,280 | 42,691 | 41,736 | 955 | 46,589 | 47.8% | 97.8% | 46.7% | ||
| Akita | 46,451 | 21,849 | 21,455 | 394 | 24,602 | 47.0% | 98.2% | 46.2% | ||
| Yamagata | 44,516 | 19,823 | 19,216 | 607 | 24,693 | 44.5% | 96.9% | 43.2% | ||
| Fukushima | 83,339 | 36,654 | 35,114 | 1,540 | 46,685 | 44.0% | 95.8% | 42.1% | ||
| Ibaraki | 110,739 | 54,501 | 52,206 | 2,295 | 56,238 | 49.2% | 95.8% | 47.1% | ||
| Tochigi | 79,647 | 36,096 | 34,688 | 1,408 | 43,551 | 45.3% | 96.1% | 43.6% | ||
| Gunma | 75,184 | 33,762 | 32,535 | 1,227 | 41,422 | 44.9% | 96.4% | 43.3% | ||
| Saitama | 231,864 | 108,660 | 105,674 | 2,986 | 123,204 | 46.9% | 97.3% | 45.6% | ||
| Chiba | 206,824 | 102,099 | 98,993 | 3,106 | 104,725 | 49.4% | 97.0% | 47.9% | ||
| Tokyo | 394,272 | 198,531 | 191,857 | 6,674 | 195,741 | 50.4% | 96.6% | 48.7% | ||
| Kanagawa | 259,416 | 123,657 | 120,281 | 3,376 | 135,759 | 47.7% | 97.3% | 46.4% | ||
| Niigata | 85,169 | 41,569 | 40,456 | 1,113 | 43,600 | 48.8% | 97.3% | 47.5% | ||
| Toyama | 41,418 | 18,377 | 17,370 | 1,007 | 23,041 | 44.4% | 94.5% | 41.9% | ||
| Ishikawa | 43,585 | 18,415 | 17,021 | 1,394 | 25,170 | 42.3% | 92.4% | 39.1% | ||
| Fukui | 30,058 | 11,566 | 10,953 | 613 | 18,492 | 38.5% | 94.7% | 36.4% | ||
| Yamanashi | 29,716 | 13,705 | 13,154 | 551 | 16,011 | 46.1% | 96.0% | 44.3% | ||
| Nagano | 77,337 | 34,915 | 32,985 | 1930 | 42,422 | 45.1% | 94.5% | 42.7% | ||
| Gifu | 78,411 | 37,241 | 36,300 | 941 | 41,170 | 47.5% | 97.5% | 46.3% | ||
| Shizuoka | 140,453 | 66,949 | 65,288 | 1,661 | 73,504 | 47.7% | 97.5% | 46.5% | ||
| Aichi | 261,141 | 131,975 | 130,014 | 1961 | 129,166 | 50.5% | 98.5% | 49.8% | ||
| Mie | 69,291 | 34,380 | 33,819 | 561 | 34,911 | 49.6% | 98.4% | 48.8% | ||
| Shiga | 43,700 | 19,974 | 19,561 | 413 | 23,726 | 45.7% | 97.9% | 44.8% | ||
| Kyoto | 78,410 | 37,586 | 36,671 | 915 | 40,824 | 47.9% | 97.6% | 46.8% | ||
| Osaka | 283,471 | 134,841 | 132,539 | 2,302 | 148,630 | 47.6% | 98.3% | 46.8% | ||
| Hyogo | 185,281 | 89,594 | 88,091 | 1,503 | 95,687 | 48.4% | 98.3% | 47.5% | ||
| Nara | 48,481 | 24,093 | 23,774 | 319 | 24,388 | 49.7% | 98.7% | 49.0% | ||
| Wakayama | 38,158 | 17,311 | 16,915 | 396 | 20,847 | 45.4% | 97.7% | 44.3% | ||
| Tottori | 20,974 | 9,001 | 8,754 | 247 | 11,973 | 42.9% | 97.3% | 41.7% | ||
| Shimane | 26,510 | 12,674 | 12,343 | 331 | 13,836 | 47.8% | 97.4% | 46.6% | ||
| Okayama | 71,535 | 32,769 | 32,089 | 680 | 38,766 | 45.8% | 97.9% | 44.9% | ||
| Hiroshima | 104,222 | 49,206 | 48,186 | 1,020 | 55,016 | 47.2% | 97.9% | 46.2% | ||
| Yamaguchi | 54,071 | 25,890 | 25,284 | 606 | 28,181 | 47.9% | 97.7% | 46.8% | ||
| Tokushima | 31,005 | 13,612 | 13,285 | 327 | 17,393 | 43.9% | 97.6% | 42.8% | ||
| Kagawa | 40,676 | 18,990 | 18,177 | 813 | 21,686 | 46.7% | 95.7% | 44.7% | ||
| Ehime | 51,726 | 24,634 | 23,829 | 805 | 27,092 | 47.6% | 96.7% | 46.1% | ||
| Kochi | 28,472 | 12,174 | 11,810 | 364 | 16,298 | 42.8% | 97.0% | 41.5% | ||
| Fukuoka | 166,802 | 79,876 | 77,269 | 2,607 | 86,926 | 47.9% | 96.7% | 46.3% | ||
| Saga | 30,820 | 13,751 | 13,104 | 647 | 17,069 | 44.6% | 95.3% | 42.5% | ||
| Nagasaki | 48,522 | 21,938 | 20,851 | 1,087 | 26,584 | 45.2% | 95.0% | 43.0% | ||
| Kumamoto | 68,679 | 30,509 | 28,695 | 1814 | 38,170 | 44.4% | 94.1% | 41.8% | ||
| Oita | 44,443 | 20,163 | 19,386 | 777 | 24,280 | 45.4% | 96.1% | 43.6% | ||
| Miyazaki | 43,103 | 18,339 | 17,238 | 1,101 | 24,764 | 42.5% | 94.0% | 40.0% | ||
| Kagoshima | 62,610 | 28,852 | 27,880 | 972 | 33,758 | 46.1% | 96.6% | 44.5% | ||
| Okinawa | 40,667 | 20,741 | 20,317 | 424 | 19,926 | 51.0% | 98.0% | 50.0% | ||
Data shown as n (%). JDS, Japan Diabetes Society.
Rate of visiting ophthalmologists and fundus examination
The proportion of patients who visited ophthalmologists (A/U) in FY2017 was 47.4%. Of those who visited ophthalmologists, 96.9% of the patients (B/A) underwent fundus examination. The proportion of patients undergoing ophthalmology visit (A/U) was lower among men (43.4% vs 53.0%, P < 0.001), non‐insulin users (45.2% vs 62.5%, P < 0.001) and patients with type 2 diabetes (type 1 diabetes 64.6%; type 2 diabetes 47.4%; unknown 14.9%, P < 0.001). Patients who visited ophthalmologists often were those who cared for their diabetes at JDS‐certified education facilities (60.0% vs 45.7%, P < 0.001), facilities with an ophthalmology department (55.0% vs 43.0%, P < 0.001) and facilities with a large number of beds (0–19 beds 44.7%; 20–99 beds 46.2%; 100–199 beds 49.1%; 200 beds 56.3%; unknown 41.3%, P < 0.001). In contrast, the proportion of patients undergoing fundus examination among those who visited ophthalmologists (B/A) was high regardless of patient characteristics or medical facilities for diabetes care (Table 1). At 2 years, 56.2% of patients had had visited and ophthalmologist, and 97.1% underwent fundus examination among those who visited an ophthalmologist (Table S2).
The proportion of patients who visited ophthalmologists by prefectures (A/U) was 38.5–51.0% (Table 1). Out of the patients who visited ophthalmologists, 92.1–98.7% underwent fundus examination (B/U) in each prefecture.
The proportions of ophthalmology visits (A/U) stratified by sex are shown in Figure S2. The proportion of ophthalmology visits was consistently higher among women, irrespective of age (Figure S2a), insulin use and non‐insulin use (Figure S2b), any number of beds in medical facilities (Figure S2c), and JDS‐certificated education facilities of JDS or not (Figure S2d).
Predictors for conducting fundus examination
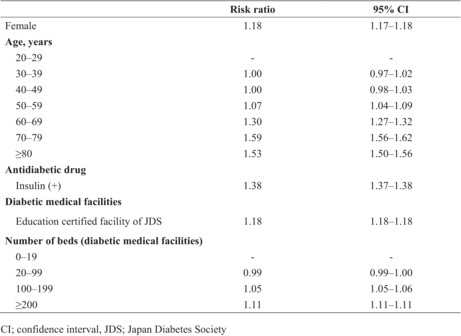
In the multivariable modified Poisson regression analysis, the following factors were found to be predictors of fundus examination: female sex (male vs female; adjusted risk ratio [aRR] 1.18, 95% confidence interval [CI] 1.17–1.18), older age (20s vs 30s: aRR 1.00, 95% CI 0.97–1.02; 20s vs 40s: aRR 1.00, 95% CI 0.98–1.03; 20s vs 50s: aRR 1.07, 95% CI 1.04–1.09; 20s vs 60s: aRR 1.30, 95% CI 1.27–1.32; 20s vs 70s: aRR 1.59, 95% CI 1.56–1.62; 20s vs >80s: aRR 1.53, 95% CI 1.50–1.56), insulin use (non‐insulin users vs insulin users: aRR 1.38, 95% CI 1.37–1.38), JDS facilities (JDS vs not JDS: aRR 1.18, 95% CI 1.18–1.18), large medical facilities (0–19 beds vs 20–99 beds: aRR 0.99, 95% CI 0.99–1.00; 0–19 beds vs 100–199 beds aRR 1.05, 95% CI 1.05–1.06; 0–19 beds vs >200 beds: aRR 1.11, 95% CI 1.11–1.11; Table 2).
Table 2.
Risk ratio for conducting fundus examination
| Risk ratio | 95% CI | |
|---|---|---|
| Female | 1.18 | 1.17–1.18 |
| Age (years) | ||
| 20–29 | – | – |
| 30–39 | 1.00 | 0.97–1.02 |
| 40–49 | 1.00 | 0.98–1.03 |
| 50–59 | 1.07 | 1.04–1.09 |
| 60–69 | 1.30 | 1.27–1.32 |
| 70–79 | 1.59 | 1.56–1.62 |
| ≥80 | 1.53 | 1.50–1.56 |
| Antidiabetic drug | ||
| Insulin (+) | 1.38 | 1.37–1.38 |
| Diabetic medical facilities | ||
| Education certified facility of JDS | 1.18 | 1.18–1.18 |
| No. beds (diabetic medical facilities) | ||
| 0–19 | ‐ | ‐ |
| 20–99 | 0.99 | 0.99–1.00 |
| 100–199 | 1.05 | 1.05–1.06 |
| ≥200 | 1.11 | 1.11–1.11 |
CI, confidence interval; JDS, Japan Diabetes Society.
Total n = 4,388,234.
DISCUSSION
The present study found that less than half of Japanese patients with diabetes who regularly received antidiabetic medications visited ophthalmologists in a year. Men and middle‐aged patients visited the ophthalmology department less frequently than women and younger patients. In contrast, the rate of fundus examinations among those who once visited ophthalmologists was very high. To the best of our knowledge, this is the first study assessing the proportion of patients undergoing diabetic retinopathy screening (ophthalmology visits and fundus examination) among patients visiting ophthalmologists.
The present results showed that middle‐aged men and non‐insulin users were significantly less likely to visit ophthalmologists. Furthermore, patients who underwent eye screening were more likely to be treated at more extensive medical facilities with a JDS certification or an ophthalmology department (Table 1). We found that the following were predictors of fundus examination from the modified Poisson regression analysis: female sex, older age, insulin use, medical facilities with a JDS certification and large medical facilities (Table 2).
Previous studies also reported female sex 18 , 19 , 20 , insulin use 18 and age >60 years 18 to be associated with a higher likelihood of receiving eye screening. Socioeconomic deprivation, which was not measured in the NDB, was also reported as a major reason for non‐attendance at eye screening 21 , 22 . Furthermore, a nationwide survey carried out in Korea reported that rural areas, low academic status, unawareness about diabetes care and a higher level of diabetic retinopathy were also associated with a lower likelihood of undergoing eye examination 20 . In Denmark, among those who were screened at least once, younger people, those who had been divorced and those with lower incomes had fewer return visits, and those with more severe diabetic retinopathy more often delayed attendance than recommended 23 . The present results highlight the importance of encouraging regular eye screening for patients who are younger, male, do not use insulin, and visit relatively small and non‐JDS‐certified medical facilities.
If glycemic control is preferable, eye screening at every 2 years is recommended for patients with diabetes without retinopathy 7 . We evaluated the rates of retinopathy screening at both 1‐ and 2‐year intervals. Because of the longer observation period, there was an increase in the number of patients who saw ophthalmologists and underwent fundus examinations; nevertheless, the number was still insufficient. Future research should additionally investigate the eye‐screening participation rate and cost over a longer time.
The present results show a chasm between physicians and ophthalmologists, in parallel with a few studies already highlighting this issue 24 , 25 , 26 , 27 . A study in Chiba Prefecture, located next to Tokyo, found that patients visiting diabetologists had a higher rate of continued ophthalmologic care than patients visiting non‐diabetologists (70.9% vs 56.5%) 25 . Additionally, an interview survey in the USA found that of those who did not receive a dilated eye examination, 82.2% visited a primary care physician during the year 26 . More than 80% of the patients with diabetes were not recommended regular eye examinations by a doctor in Hong Kong 24 . According to the Diabetic Retinopathy Barometer Report, which surveyed patients with diabetes and healthcare professionals working in diabetes care or ophthalmology in 41 countries, 27% of the patients had never discussed eye complications with a professional or had done so only after symptoms manifested 27 . According to these results, one of the fundamental issues of a low rate of eye screening in patients with diabetes can be the lack of encouragement from the physician rather than the omission of fundus examinations by ophthalmologists.
The rates of ophthalmologist visits varied by prefecture (Table 1), which might be due to limited geographical access to ophthalmologists and inadequate patient education. Careful observation and evidence‐based policy‐making by prefectures are critical to solving inequality. Introducing fundus photography by internists, telemedicine screening with non‐mydriatic fundus photography 28 and automated retinal image analysis 29 are other alternatives that could reduce barriers to ophthalmologic care. Expanding eye screening in municipal medical checkups might also help close the gap and could be monitored by a future study using medical checkup data in addition to claims data.
The strength of the present study is that the NDB covers almost all the insurance treatments in Japan, which has a universal health insurance system. Thus, we can track patient care across different medical facilities, which is generally difficult in hospital‐based studies. We believe that our results provide a basis for future systematic improvements in screening for diabetic retinopathy. Furthermore, it identifies a patient population unfamiliar to eye screening and generates solutions for more targeted initiatives.
However, the present study had some limitations. First, we extracted claims data on patients diagnosed with diabetes as they were receiving antidiabetic medications. As a result, we did not include patients who had not been prescribed antidiabetic medications. Although dilated and comprehensive eye examination are recommended for patients on diet and exercise therapy, they were not included in this study, and further research on such patients must be caried out. Second, we did not have clinical information, such as glycated hemoglobin level and clinical status of diabetic retinopathy or other complications. Glucose level negatively affects retinopathy; therefore, identifying the proportion of patients undergoing retinopathy screening by glycemic level should be assessed. The progression of other diabetes complications might also be a predictor of retinopathy. Although it is not sufficient, we assumed that insulin use controls the severity of diabetes in this analysis. Third, we did not consider patients who underwent non‐mydriatic fundus camera‐based examinations at the medical checkup as having undergone a fundus examination, because National Health Insurance did not routinely recommend these self‐medical checkups. However, as the municipality's eye screening program has expanded 10 , we must consider these uptake rates in future studies using alternative data, as they are not captured in claims data.
In conclusion, we showed that half of the patients taking antidiabetic medication did not visit an ophthalmologist within the first year, whereas almost all the patients who visited an ophthalmologist received a fundus examination. The screening rate remained constant during the 2‐year interval. In Japan, where systematic eye screening systems are non‐existent for patients with diabetes, it is necessary to resolve issues regarding the recommendation of ophthalmologist‐associated diabetes care from a physician or health provider.
Disclosure
The authors declare no conflict of interest.
Approval of the research protocol: This study conforms to the provision of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013), and was approved by the ethics committees of the National Center of Global Health and Medicine Center Hospital (NCGM‐G‐002492‐04).
Informed consent: As the data were anonymized, it was impossible to re‐identify patients in this study; opt‐out or opt‐in was therefore impossible and not required according to the ethical guidelines.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Venn diagram of visiting ophthalmologists and fundus examinations.
Figure S1 | Venn diagram of visiting ophthalmologists and fundus examinations.
Figure S2 | The proportion of visiting ophthalmologists by sex.
Table S1 | Definition of variables.
Table S2 | Data on 2 years of visiting ophthalmologists and fundus examination.
Table S3 | The REporting of studies Conducted using Observational Routinely‐collected Data statement – checklist of items, extended from the STrengthening the Reporting of OBservational studies in Epidemiology statement.
Acknowledgment
The authors thank all investigators involved in this study. We wrote this manuscript along with the Reporting of REporting of studies Conducted using Observational Routinely‐collected Data guideline in Table S3. This study was supported by the Health and Labor Sciences Research Grants (Comprehensive Research on Life‐Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus, H29‐Cardiovascular‐General‐004, 20FA1016). The funding agency had no role in the design or conduct of the study; collection, management, analysis and interpretation of data; preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication. This manuscript has not been published elsewhere and is not under consideration by another journal; however, a portion of the content was presented at the 24th Japanese Society of Ophthalmic Diabetology meeting in Tokyo, Japan.
Contributor Information
Takehiro Sugiyama, Email: tsugiyama@hosp.ncgm.go.jp.
Toshimasa Yamauchi, Email: tyamau@m.u-tokyo.ac.jp.
Takashi Kadowaki, Email: t-kadowaki@toranomon.kkr.or.jp.
References
- 1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990‐2020: A systematic review and meta‐analysis. Lancet Glob Health 2017; 5: e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BE. Are individuals with diabetes seeing better?: A long‐term epidemiological perspective. Diabetes 2010; 59: 1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morizane Y, Morimoto N, Fujiwara A, et al. Incidence and causes of visual impairment in Japan: The first nation‐wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol 2019; 63: 26–33. [DOI] [PubMed] [Google Scholar]
- 4. Scanlon PH. The contribution of the English NHS diabetic eye screening Programme to reductions in diabetes‐related blindness, comparisons within Europe, and future challenges. Acta Diabetol 2021; 58: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Younis N, Broadbent DM, Vora JP, et al. Incidence of sight‐threatening retinopathy in patients with type 2 diabetes in the Liverpool diabetic eye study: A cohort study. Lancet 2003; 361: 195–200. [DOI] [PubMed] [Google Scholar]
- 6. Misra A, Bachmann MO, Greenwood RH, et al. Trends in yield and effects of screening intervals during 17 years of a large UK community‐based diabetic retinopathy screening programme. Diabet Med 2009; 26: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 7. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017; 40: 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vujosevic S, Aldington SJ, Silva P, et al. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol 2020; 8: 337–347. [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama T HY, Yamada M. Trends in fundus examination as a result of the review of specific health checkups. MHLW grant system, 2020 (Japanese).
- 11. Sugiyama T, Imai K, Ihana‐Sugiyama N, et al. Variation in process quality measures of diabetes care by region and institution in Japan during 2015‐2016: An observational study of nationwide claims data. Diabetes Res Clin Pract 2019; 155: 107750. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health, Labour and Welfare . Website regarding national database of health insurance claims and specific health checkups of Japan. 2020. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/reseputo/index.html Accessed March 12, 2023 (Japanese).
- 13. Ministry of Health, Labour and Welfare. Basic Data on Medical Insurance . Medical Expenditures in Fiscal Year 2017. 2019. Available from: https://www.mhlw.go.jp/content/kiso_h29.pdf Accessed March 12, 2023 (Japanese).
- 14. WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index 2022, 2022. Available from: https://www.whocc.no/atc_ddd_index/ Accessed March 12, 2023.
- 15. International Statistical Classification of Diseases and Related Health Problems 10th Revision, 2016. Available from: https://icd.who.int/browse10/2016/en Accessed March 12, 2023.
- 16. Committee for Clinical Guidelines, the Japanese Society of Ophthalmic Diabetology . Diabetic retinopathy clinical practice guidelines (1st edition). Nippon Ganka Gakkai Zasshi 2020; 12: 953–981 (Japanese). [Google Scholar]
- 17. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 18. Kawamura T, Sato I, Tamura H, et al. Influence of comorbidities on the implementation of the fundus examination in patients with newly diagnosed type 2 diabetes. Jpn J Ophthalmol 2018; 62: 68–76. [DOI] [PubMed] [Google Scholar]
- 19. Kreft D, McGuinness MB, Doblhammer G, et al. Diabetic retinopathy screening in incident diabetes mellitus type 2 in Germany between 2004 and 2013 ‐ a prospective cohort study based on health claims data. PLoS One 2018; 13: e0195426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One 2013; 8: e62991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chou CF, Sherrod CE, Zhang X, et al. Barriers to eye care among people aged 40 years and older with diagnosed diabetes, 2006‐2010. Diabetes Care 2014; 37: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kashim RM, Newton P, Ojo O. Diabetic retinopathy screening: A systematic review on Patients' non‐attendance. Int J Environ Res Public Health 2018; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thykjaer AS, Andersen N, Bek T, et al. Attendance in a national screening program for diabetic retinopathy: A population‐based study of 205,970 patients. Acta Diabetol 2022; 59: 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lian J, McGhee SM, Gangwani RA, et al. Awareness of diabetic retinopathy and its association with attendance for systematic screening at the public primary care setting: A cross‐sectional study in Hong Kong. BMJ Open 2018; 8: e019989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenichi Sakurai MM, Takatsuna Y, Kuribayashi N, et al. Survey on the actual conditions of medical Care for Diabetic Retinopathy by internal medicine and ophthalmic medical institutions in Chiba prefecture‐ a Report from Chiba Council for Promotion of countermeasures against diabetes. J Jpn Diab Soc 2020; 63: 163–171 (Japanese). [Google Scholar]
- 26. Gibson DM. Estimates of the percentage of US adults with diabetes who could Be screened for diabetic retinopathy in primary care settings. JAMA Ophthalmol 2019; 137: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The Diabetic Retinopathy Barometer Report Global Findings. Available from: https://drbarometer.com/evidence/explore‐the‐data/global‐findings/ Accessed 1 September 2022.
- 28. Jani PD, Forbes L, Choudhury A, et al. Evaluation of diabetic retinal screening and factors for ophthalmology referral in a telemedicine network. JAMA Ophthalmol 2017; 135: 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norgaard MF, Grauslund J. Automated screening for diabetic retinopathy ‐ a systematic review. Ophthalmic Res 2018; 60: 9–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Venn diagram of visiting ophthalmologists and fundus examinations.
Figure S1 | Venn diagram of visiting ophthalmologists and fundus examinations.
Figure S2 | The proportion of visiting ophthalmologists by sex.
Table S1 | Definition of variables.
Table S2 | Data on 2 years of visiting ophthalmologists and fundus examination.
Table S3 | The REporting of studies Conducted using Observational Routinely‐collected Data statement – checklist of items, extended from the STrengthening the Reporting of OBservational studies in Epidemiology statement.


