Abstract
Background
Postoperative cognitive dysfunction (POCD) is a clinically frequent postoperative complication in the elderly, which is mainly manifested by the occurrence of cognitive dysfunction after anesthetized surgery in patients. To explore the involvement of C/EBPα in microglial polarization in sevoflurane anesthesia induced cognitive impairment in aged rats.
Methods
Sprague-Dawley (SD) rats were anesthetized by inhalation of 3% sevoflurane for 6 h to establish the POCD model. The histopathological structure of hippocampus was observed by hematoxylin and eosin (HE) staining. Associative learning and memory function and spatial learning and memory function were assessed by conditioned fear test and water maze test. The concentrations of inflammatory factors in the hippocampus were measured by ELISA. The levels of microglial activation marker (Iba1) and microglial M1 (CD86) and M2 (CD206) polarization markers were determined by immunofluorescence staining and RT-qPCR, respectively. The transcriptional regulation of HDAC1 by C/EBPα was confirmed by dual luciferase reporter assay and ChIP assay.
Results
Sevoflurane-induced pathomorphological damage in the hippocampal tissue of aged rats, accompanied by elevated expression of C/EBPα. Silencing of C/EBPα alleviated hippocampal histopathological injury, inhibited M1 microglial activation and the expression of M1 marker CD86, enhanced the expression of M2 marker CD206. C/EBPα transcriptionally activated HDAC1. Knockdown of C/EBPα downregulated the expression of HDAC1 and STAT3 phosphorylated proteins, which inhibited the pro-inflammatory factors (IL-6 and TNF-α) and accelerated anti-inflammatory factors (IL-10 and TGF-β) secretion. In addition, silencing of C/EBPα caused rats to have a delayed freezing time in contextual conditioned fear, a shorter escape latency, and an increased number of platform crossings.
Conclusion
Inhibition of C/EBPα promotes the M2 polarization of microglia and reduces the production of pro-inflammatory cytokines to alleviate the cognitive dysfunction of sevoflurane-induced elderly rats by HDAC1/STAT3 pathway.
Keywords: Sevoflurane, C/EBPα, Microglial polarization, Cognitive impairment, HDAC1/STAT3 pathway
Introduction
Postoperative cognitive dysfunction (POCD) is a clinical complication that often occurs in elderly patients after surgery. POCD mainly occurs 5–7 days after surgery, and patients older than 65 years have a short-term POCD incidence of approximately 20%–40% and a long-term POCD incidence of approximately 10% after undergoing noncardiac surgery (Evered & Silbert, 2018; Berger et al., 2018). Cognitive decline in older patients after anaesthetic surgery is characterized by mild thought impairment, inability to concentrate, memory impairment, and reduced ability to learn executive and language comprehension. Although the pathophysiological mechanism of POCD is still unclear. Studies increasingly believe that aging has become an important and independent risk factor for POCD (Lin et al., 2020). Age related brain decline can produce a variety of behavioral defects (Mufson et al., 2016; Thomas, 2016). Sevoflurane is a widely used inhalation anesthetic in clinical practice. Studies have shown that sevoflurane can activate neuroinflammation, aggravate nervous system damage, and lead to long-term neurocognitive dysfunction. (Shao et al., 2020; Chen et al., 2020). Therefore, it is of great practical importance to elucidate the mechanism of sevoflurane neurotoxicity and may help to prevent cognitive dysfunction.
Microglia, an important component in the central nervous system (CNS), belong to the type of glial cells, which function similarly to macrophages and are considered the most primary one of the immune defenses in the CNS. Accumulating evidence suggests that microglial activation in the CNS is heterogeneous and can be divided into two opposite types: M1 phenotype and M2 phenotype (Tang & Le, 2016; Guo, Wang & Yin, 2022). Activated microglia can play dual roles depending on the activated phenotype, therefore, microglia can produce either cytotoxic M1 or neuroprotective M2. When microglia achieve an M1 phenotype, large amounts of inflammatory cytokine products including IL-1β, TNF-α, IL-6 are produced. However, the anti-inflammatory cytokines IL-4, IL-10, IL-13, and TGF-β, among others, are released when the M2 phenotype is present. Morphological changes in activated microglia and an increase in inflammatory factors are observed in the aging brain. Morphological alterations have also been suggested as a potential mechanism for age-related neurodegeneration (Crotti & Ransohoff, 2016; Costa et al., 2021). It has been confirmed that neuroinflammation leads to the occurrence of POCD by promoting the release of inflammatory factors in the periphery and the disruption of the blood–brain barrier, reaching the center to cause the occurrence of central inflammation as well as the apoptosis of neurons. Previous studies have shown that sevoflurane promotes microglial M1 activation suppresses microglial M2 activation Sevoflurane suppresses microglial M2 polarization (Pei, Wang & Li, 2017). However, the underlying mechanism of sevoflurane-regulated microglial polarization in the aging brain remains undefined.
The transcription factor CCAAT/enhancer binding protein (C/EBP) is a family of leucine zipper transcription factors, and as the first discovered C/EBP family member, C/EBPα is mapped to 19q 13.1 on human chromosome. As a transcription factor with important biological functions, C/EBPα is distributed in several tissues and participate in the regulation of biological processes such as cell differentiation, proliferation by DNA methylation, and energy metabolism. C/EBPα was highly expressed in the renal tissue of diabetic nephropathy rats; Sinomenine reduced IL-18 and IL-1β production by decreasing C/EBPα to improve renal glomerular endothelial dysfunction (Zhang & Wang, 2022). In experimental autoimmune encephalomyelitis, C/EBPα was a downstream gene of miR-124 and increased in activated microglia, contributing to CNS inflammation (Ponomarev et al., 2011). Notably, the expression of C/EBPα was substantially increased in M1-polarized microglia, and inhibition of C/EBPα contributed to the M2 polarization of microglia thus ameliorating intracerebral hemorrhage induced-inflammatory injury (Yu et al., 2017). However, whether C/EBPα play a role by regulating microglial activation in sevoflurane induced postoperative cognitive impairment has not been reported.
In this study, a POCD model of aged rats was established by anesthesia with inhaled sevoflurane to explore the effects of sevoflurane-induced anesthesia on C/EBPα protein expression levels, microglia activation levels and inflammatory factors levels in the hippocampus of aged rats, and to find potential intervention target molecules for POCD.
Materials & Methods
Animals and model
A total of 80 male SD rats (550–650 g, age 19–22 months) were provided by the Animal Experiment Center of Xi’an Jiaotong University (License Number: SCXK (Shaanxi) 2020-001). They were housed in ventilated cages (485*350*200 mm; temperature: 21–23 °C, humidity: 50% ± 5%) containing three to seven rats per cage with sufficient supply of food and water, at normal circadian rhythm, and adaptively housed for one week before the experiment. The rats were fasted for 12 h before the experiment and had free access to water. Using random number table method, rats were randomly divided into four groups (n = 20): control group, 3% sevoflurane group (Sevo), 3% sevoflurane + sh-NC group (Sevo + sh-NC), and 3% sevoflurane + sh-C/EBPα group (Sevo + sh-C/EBPα). The sequences of sh-C/EBPα and sh-NC was synthesized by Genepharma, Co., Ltd (Shanghai, China). After anesthetizing rats with 1% pentobarbital sodium solution (50 mg/Kg), 5 µL of lentivirus encoded sh-C/EBPα (5′-GTG CGC AAG AGC CGA GAT AAA-3′) at a titer of 2.0×109 TU/ml was injected into the left lateral ventricle of the rats and its non-targeting sequence sh-NC (5′- TTC TCC GAA CGT GTC ACG T -3′). Control and Sevo groups were injected with PBS solution 5 µL. One day after the injection, the control group inhaled 30% oxygen/air for 6 h, and the Sevo, Sevo + sh-NC, and Sevo + sh-C/EBPα groups inhaled a mixture of 3% sevoflurane and 30% oxygen/air for 6 h (Wang et al., 2021). Rats were placed in an anesthetizing chamber with two interfaces: one was connected to a sevoflurane vaporizer and the other was connected to a multi-gas monitor. The concentration of sevoflurane and oxygen was monitored using a gas analyzer (Dash 4000; GE Healthcare, Milwaukee, WI) during sevoflurane anesthesia. After the end of sevoflurane anesthesia, it was confirmed by blood gas analysis (Kent Scientific Corp., Torrington, CT, USA) that the arterial blood gases or pH values were not changed during the inhalation of sevoflurane anesthesia in rats. After cognitive testing, rats were sacrificed by intraperitoneal injection of pentobarbital sodium solution (85 mg/kg). This experiment was approved by the Ethics Committee of Shaanxi Provincial People’s Hospital.
Staining methods
Hematoxylin-eosin (HE) staining
Paraffin sections of hippocampal tissues (5 µm) were obtained, deparaffinized by xylene and hydrated by adding graded concentrations of ethanol. Then, the sections were stained with hematoxylin solution for 5 min, and subsequently incubated with eosin solution for 1 min. After graded ethanol and dimethylbenzene treatment neutral resin was used to block the sections. The pathological structure of rat hippocampal tissue was observed under a light microscope (magnification, ×200; Nikon Corporation, Tokyo, Japan).
Immunofluorescence staining
After xylene deparaffinization, the hippocampus tissue sections were washed by 0.1M PBS and microwave repaired for 15 min, blocked with 10% normal goat serum for 20 min, followed by the addition of 300 µL of primary antibodies (rabbit anti-rat Iba-1; 1:200; ab153696, Abcam, Cambridge, UK) for overnight incubation. After washing by PBS, sections were incubated with a fluorescent secondary antibody (Alexa Fluor® 488-labeled Goat anti rabbit; 1:300; ab150077, Abcam, Cambridge, UK) for 2 h in the dark, washed three times with PBS, and mounted using anti-fluorescence quencher containing DAPI. Ultimately, a confocal laser-scanning microscope was utilized to observe and photograph the sections (magnification, ×200; Olympus FV1000, Olympus, Tokyo, Japan).
Cognitive testing
Morris water maze task
Ten rats from each group were randomly selected for the water maze experiment 24 h after sevoflurane inhalation (Vorhees & Williams, 2006). The location navigation experiment was conducted from day 1 to 5, rats were placed into a pool 150 cm deep 60 cm from either quadrant facing the wall of the pool, and the platform was placed in the middle of the pool, and the time that rats found the platform was scored as the escape latency. If the rats still did not find the platform within 90 s, the rats were guided to the platform and remained on the platform for 15 s. The escape latency was recorded as 90 s, which was averaged from four measurements per day. On day 6, a spatial exploration experiment was performed, the platform was withdrawn, and rats were placed into the water from either quadrant facing the wall of the pool, and the number and swimming speed at which they crossed the platform in 90 s were recorded.
Fear-conditioning test
The conditioned fear test can be used to examine associative learning and memory functions in rats (Zhang et al., 2022). Conditioned fear experiments were performed on days 12 and 13 after sevoflurane inhalation. Rats were individually placed into the conditioned fear box and allowed rats to freely explore the box for 100 s. Rats were then given three groups of stimulation. Each group was given a sound stimulus 5,000 Hz, 85 dB, 30 s, immediately followed by a shock of 0.8 Ma, 2 s. Each set of stimuli was separated by 1 min. After the stimulation ended, the rats were allowed to continue to stay in the box for 30 s. The rats were removed. After 24 h of the testing, the rats were placed back in the same box and removed 6 min later, and the freezing time of the rats during the 6 min session was recorded. After 2 h, another box was taken with the odors in the box and the lights all differed from the first. Rats were placed into a second box and given three sets of sound stimuli: 5,000 Hz, 85 dB, 30 s, with 1 min interval between each set of stimuli. The rats’ freezing time was recorded.
ELISA
After 50 mg of frozen hippocampal tissue was added to 1.5 ml of saline and ground to a slurry using a homogenizer, the supernatant was collected by centrifugation at 12,000 rpm/min for 15 min at 4 °C, and IL-6 (SP12279), IL-10 (SP12294), TGF-β (SP12253), and TNF-α (SP12250) in hippocampal tissue was detected according to the instructions of ELISA kits (Wuhan Saipei Biotechnology Co., Ltd., Wuhan, China).
Western blotting
After 50 mg of frozen hippocampal tissue was lysed in protein lysis buffer (containing 1% protease inhibitors) on ice to extract total protein, protein concentration was measured using bicinchoninic acid (Beyotime, Shanghai, China). Denatured protein samples were obtained at 50 µg after row separation by electrophoresis, go to a polyvinylidene fluoride membrane. After blocking treatment, 1:1 000 dilution of primary antibodies were added, respectively, and placed for overnight incubation at 4 °C. The following day, after washing the membrane, a 1:5 000 dilution of secondary antibody was added and incubated for 2 h at room temperature. After exposure was developed by chemiluminescence, the gray levels of the bands of interest were determined using image J software, and the ratio obtained from the gray levels of the internal reference GAPDH was defined as the relative amount of the protein of interest.
RT-qPCR
After 50 mg of frozen hippocampal tissue was harvested and RNA was extracted by the Trizol method (15596026, Invitrogen, Carlsbad, CA, USA), it was reverse transcribed to synthesize cDNA (Yang, Liu & Chu, 2020). The mixed reaction system was preheated at 95 °C for 6 min followed by 40 cycle stages: 60 °C for 30 s after 95 °C for 10 s. The mRNA expressions of CD86 and CD206 in hippocampal tissues were detected by 2−△△CT assay with U6 as internal references.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using the EZ chip kit (Millipore, Burlington, MA, USA) according to the manufacturer’s protocol. DNA protein crosslinks were generated by adding 1% formaldehyde to cells and incubating for 10 min. Cross linked chromatin DNA was then sonicated to yield chromatin fragments of 200-300 base pairs that were incubated with anti-C/EBPα (Abcam, Cambridge, UK) or IgG at 4 °C. The promoter region of HDAC1 was examined by RT-qPCR.
Luciferase reporter assay
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was employed to co-transfect HEK-293T cells with the luciferase report vectors, containing promoter serial truncations of HDAC1, and vector or C/EBPα overexpression vector (pEX3-C/EBPα). After HEK-293T cells transfection of 24 h, the luciferase activity was detected based on the manufacturer’s instructions with the Luciferase Assay Reporter System (Promega, Madison, WI, USA).
Statistical analysis
SPSS 23 statistics was used for data analysis. After the Shapiro Wilk test, normally distributed measurement data were expressed as mean ± standard deviation, and comparisons among multiple groups were performed by one-way ANOVA, while comparisons between two groups were performed by t-test. P < 0.05 was considered statistically significant.
Results
Sevoflurane-induced anesthesia promoted C/EBPα expression in aged rats
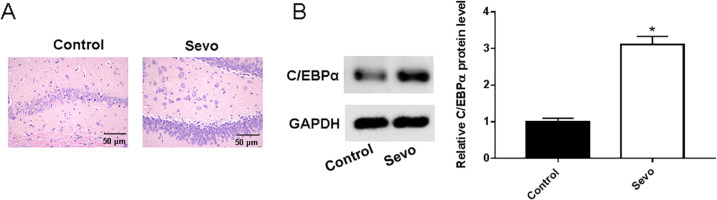
A sevoflurane-induced anesthesia model was constructed in aged rats. HE staining showed that the morphology of neuronal cells in the hippocampus of control rats was normal, and the cells were arranged in an orderly and regular manner. Compared with the control group, the neuronal cells in the hippocampus tissue of the Sevo group were unevenly distributed and irregularly arranged, and there were a large number of pyknosis phenomena (Fig. 1A). Expression of C/EBPα protein after sevoflurane anesthesia in aged rats was examined. Our results showed that sevoflurane anesthesia-induced a significant increase in C/EBP α protein expression in the hippocampus of aged rats. (Fig. 1B).
Figure 1. Sevoflurane exacerbated the aging-induced upregulation of C/EBPα expression in the rat hippocampus.
(A) Hippocampal histopathological findings of rats in each group; (B) Expression of C/EBPα protein level in the hippocampus of rats in each group. An asterisk (*) indicates that P < 0.05.
Knockdown of C/EBPα inhibited microglial activation and phenotypic changes in aged rats after sevoflurane anesthesia
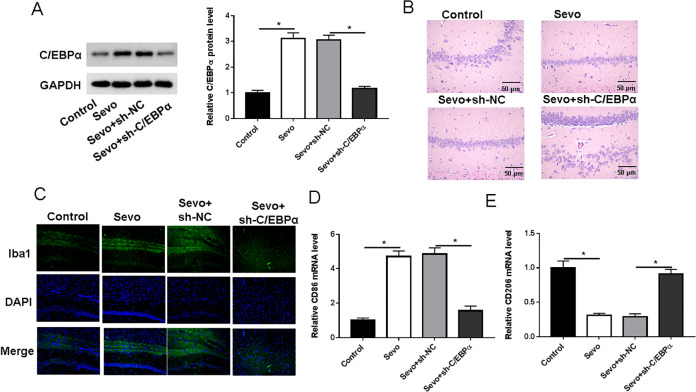
Microglial activation in the aging brain causes neuronal damage in neurodegenerative diseases, promoting age-related cognitive impairment. To investigate the role of C/EBPα in microglia, short-hairpin RNA against C/EBPα (sh-C/EBPα) or corresponding negative control (sh-NC) were injected into the lateral ventricles of rats before sevoflurane treatment. The protein levels of C/EBP α in the hippocampus were examined in aged rats after sevoflurane anesthesia by Western blotting. The results showed that C/EBPα interference could memorably reduce the expression level of C/EBPα in hippocampal tissues of sevoflurane-induced rats (Fig. 2A). Hippocampal neurons in both the Sevo and Sevo + sh-NC groups were reduced in number and disordered in arrangement, cells shrunk and appeared conical or triangular in shape with disrupted nuclear positioning or lysis and deepened staining. In the Sevo + sh-C/EBP α group, the number of hippocampal neurons was increased and more regularly arranged, and most of the cells had a normal morphology with regular nuclei and individual cells shrunk (Fig. 2B). Further, we uncovered that anaesthetic surgery induced a significant increase in the number of Iba1 positive cells, that is, increased microglial activation, in the hippocampus of aged rats. After injection of sh-C/EBPα, the number of Iba1 positive cells in Sevo + sh-C/EBPα group was dramatically decreased (Fig. 2C). The function of activated microglia depends on its phenotype. We used RT-qPCR to identify two distinct phenotypic markers of microglia in the hippocampus of aged rats. Sevoflurane anesthesia significantly increased CD86 (microglial M1 type marker) and decreased CD206 (microglial M2 type marker) expression. Injection of sh-C/EBPα resulted in a significant downregulation of CD86 and upregulation of CD206 in the hippocampus of aged rats (Fig. 2D and 2E).
Figure 2. Loss of C/EBPα reduced sevoflurane-induced microglial activation and polarization in aged rats.
(A) Western blotting measurement results of C/EBPα protein level in hippocampus. (B) Hippocampal histopathological findings of rats in each group; (C) Immunofluorescence measurement results of Iba1 in hippocampus. (D and E) RT-qPCR measurement results of CD86 and CD206 mRNA levels in hippocampus. An asterisk (*) indicates that P < 0.05.
C/EBPα transcriptionally activated HDAC1
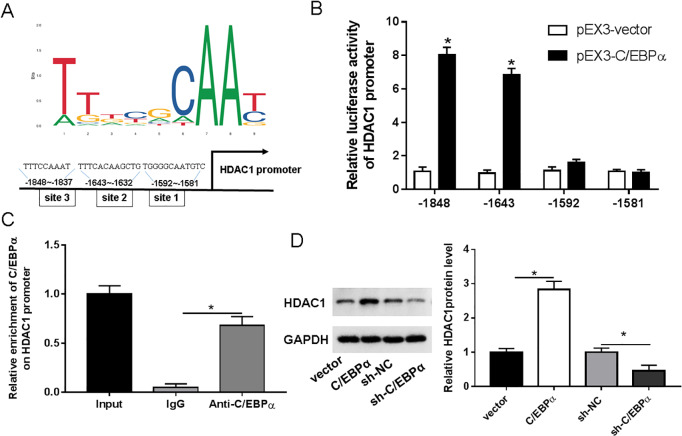
There is already evidence that inhibition of HDAC1 can ameliorate neuroinflammation and cognitive dysfunction in neurodegenerative models. We found the binding motif of C/EBPα by Jaspar database, which predicted its three binding sites (P1, P2 and P3) to the promoter of HDAC1 (Fig. 3A). Luciferin reporter assays showed no significant difference in the luciferase activity of the vector containing the P1 and P3 binding sites compared with the vector group, whereas the luciferase activity of the vector containing the P2 binding site was significantly higher (Fig. 3B). ChIP experiments further indicated that C/EBPα bound to the promoter region of HDAC1 (Fig. 3C). Analysis of the western blotting demonstrated that an elevation of C/EBP α also led to an efficient rise in HDAC1 expression, while interference of C/EBPα deceased HDAC1 expression (Fig. 3D).
Figure 3. C/EBPα was involved in regulating HDAC1 expression.
(A) Schematic representation of C/EBPα binding motif and C/EBPα binding sites on the promoter region of HDAC1; (B) Luciferase reporter assay measurement results of relative luciferase activities of HDAC1 promoter; (C) ChIP measurement results of the enrichment of C/EBPα on HDAC1 promoter; (D) Western blotting measurement results of HDAC1protein level. An asterisk (*) indicates that P < 0.05.
Knockdown of C/EBPα regulated the expression of inflammatory cytokines via HDAC1/STAT3 pathway in aged rats after sevoflurane anesthesia
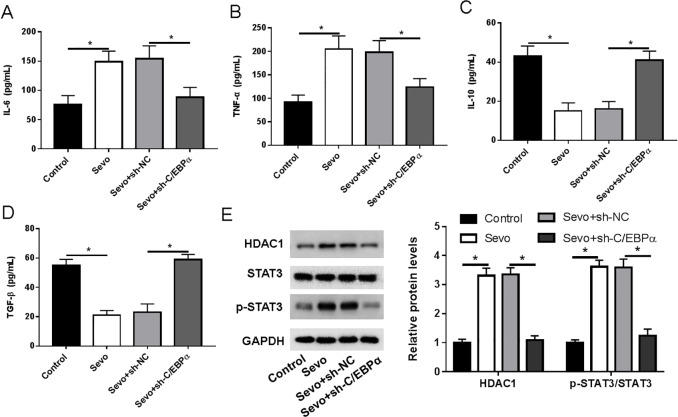
The release of inflammatory factors by activated microglia can cause synaptic and neuronal damage in the hippocampal tissue and thus participate in POCD. To further confirm the regulation of inflammatory responses by C/EBPα in aged rats after anesthesia, we examined the production of inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-10 and TGF- β) in the hippocampus region, respectively. The expression levels of IL-6 and TNF-α in the hippocampal tissues of aged rats were increased after sevoflurane anesthesia, whereas the expression levels of IL-10 and TGF- β were decreased after sevoflurane anesthesia. Silencing of C/EBPα inhibited the up-regulation of IL-6 and TNF-α expression and the down-regulation of IL-10 and TGF- β expression induced by sevoflurane anesthesia (Figs. 4A–4D). Furthermore, activation of the HDAC1/STAT3 pathway contributes to the activation of microglia and promotes neuroinflammatory responses. The western blotting results revealed that sevoflurane anesthesia-induced a significant increase in the HDAC1 protein and STAT3 phosphoprotein levels in the hippocampus of aged rats. After injection of sh-C/EBPα, the level of HDAC1 protein and STAT3 phosphoprotein in Sevo + sh-C/EBPα group was dramatically decreased (Fig. 4E).
Figure 4. Loss of C/EBPα reduced sevoflurane-induced inflammatory cytokines via HDAC1/STAT3 pathway in aged rats.
(A–D) ELISA measurement results of IL-6, THF-α, IL-10 and THF- β levels in hippocampus. (E) Western blotting measurement results of HDAC1 and STAT3 phosphorylation protein levels in hippocampus. An asterisk (*) indicates that P < 0.05.
Knockdown of C/EBPα prevented the impairment of cognitive function induced by sevoflurane anesthesia in aged rats
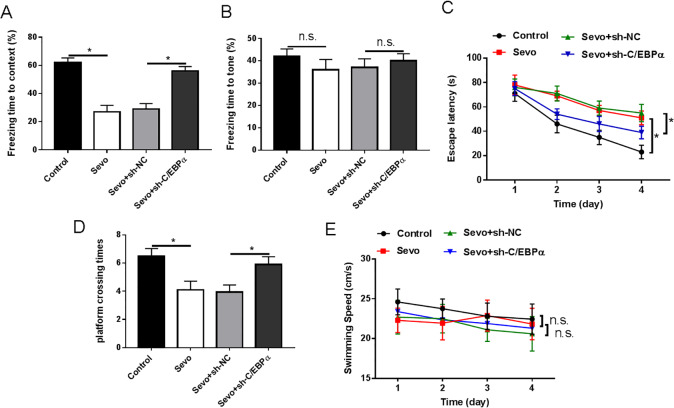
To investigate associative learning and memory abilities in aged rats, we performed a conditioned fear test in rats after sevoflurane anesthesia. The results of the study showed that rats in the sevoflurane anesthesia group had reduced freezing time in contextual fear compared with the control group, whereas no significant difference was found in freezing time in voice fear. Compared with the Sevo + sh-NC group, aged rats in the Sevo + sh-C/EBPα group showed prolonged freezing time in contextual fear, with no obvious difference in freezing time in acoustic fear (Figs. 5A and 5B). Next, we used MWM (Morris water maze) to detect the spatial learning and memory ability of sevoflurane-induced aged rats. The results of the MWM study showed that in the testing, as well as in the control group, the aged rats in the Sevo group had significantly longer escape latencies and fewer times to traverse the platform on the 4th day. Compared with rats in the Sevo + sh-NC group, rats in the Sevo + sh-C/EBPα group had significantly shorter escape latencies and increased number of platform crossings on day 4. In addition, the swimming speed differences during the experimental period among the four groups of rats were not statistically significant (Figs. 5C–5E).
Figure 5. Loss of C/EBPα alleviated sevoflurane-induced cognitive impairment in aged rats.
(A) FCT assay measurement results of freezing time in contextual fear of rats; (B) FCT assay measurement results of freezing time in voice fear of rats; (C) MWM assay measurement results of escape latencies of rats; (D) MWM assay measurement results of the number of platform crossings of rats; (E) MWM assay measurement results of swimming speed of rats. An asterisk (*) indicates that P < 0.05.
Discussion
Advantages of sevoflurane such as low blood gas partition coefficient and less respiratory irritation to patients are widely used in clinic. Sevoflurane has induced neuroinflammation by stimulating inflammatory factors such as IL-1β and TNF-α, and has also induced neur oxidative damage by activating calcium dependent proteases to produce a large amount of reactive oxygen species, which has promoted neuronal apoptosis, leading to cognitive dysfunction. Sevoflurane is widely used in clinical anesthesia and can further aggravate nervous system injury on the basis of surgical factors. Found in cases undergoing cholecystectomy that inhalational anesthesia with sevoflurane results in postoperative cognitive dysfunction in patients was more likely than intravenous anesthesia with propofol (Geng, Wu & Zhang, 2017). Compared to propofol anesthesia regimens, patients who underwent cardiac surgery with external circulation under sevoflurane anesthesia had a significantly higher incidence of postoperative cognitive dysfunction and risk of adverse effects (Tang et al., 2019). Therefore, an in-depth understanding of the mechanism of sevoflurane-induced cognitive dysfunction is of great importance for clinical better prevention and treatment of the occurrence of POCD.
Most of the current studies on the effects of sevoflurane on POCD focus on middle-aged and elderly patients. Previous studies have shown that aged rats showed impairment in cognitive function when they inhaled sevoflurane at a concentration of higher than or equal to 3% for 6 h (Shao et al., 2020; Yin et al., 2022; Xu & Qian, 2020). Therefore, 3% sevoflurane inhalation for 6 h was used for treatment in this experiment. After inhalation of sevoflurane, aged rats showed cognitive impairment, uneven and irregular distribution of neuronal cells in the hippocampus, increased expression of C/EBPα, activation of microglia, and elevated secretion of inflammatory factors.
Studies have shown that C/EBPs play an essential role during brain development. C/EBPα was lowly expressed in the hippocampal tissue of diabetic rats and might be one of the important molecular targets that contribute to neurodegeneration in diabetes (Kazkayasi et al., 2013). Pan et al. (2013) revealed that IL-13 significantly enhanced C/EBPα, which further promoted activated microglia death, thereby preventing cerebral inflammation and neuronal injury. In Alzheimer’s disease, NF-κB was one of the substrates of C/EBP β, and the increased C/EBPβ promoted NF-κB p65 nuclear translocation, contributing to NF-κB mediated inflammatory reaction and neuronal degeneration, thus aggravating cognitive impairment of APP/PS1 rats (Yi-Bin et al., 2022). Our data showed that after interfering with the expression of C/EBPα in rat hippocampal tissues in vivo, the rats showed significantly shorter escape latency, significantly increased number of crossing the platform, significantly lower concentration of inflammatory factors in hippocampal tissues, and improved cell pathological morphology in the water maze test, suggesting that C/EBPα may play some role in sevoflurane-induced cognitive dysfunction in rats.
Histone deacetylase 1 (HDAC1) belongs to the histone deacetylase family and has the ability to regulate chromatin structure and transcription, and most importantly, it can participate in the occurrence of inflammatory responses. For example, enhancing the activity of HDAC1 contributed to the conversion of microglia M1 to M2 phenotype by decreasing KLF4 deacetylation, thus promoting neuroinflammation (Ji et al., 2019). Wang et al. (2017) reported that H19 enhanced TNF-α, IL-1 β and reduced IL-10 by driving HDAC1-dependent M1 microglial polarization in ischemic stroke. There is also evidence that HDAC1 affected cognitive impairment by inducing neuroinflammation. Inhibition of HDAC1 improved microglia overgrowth and pro-inflammatory factor hypersecretion by upregulation of Tet2 to treat memory impairment and cognitive symptoms in aged Alzheimer’s disease-related rats (Li et al., 2020). In anesthesia/surgery induced POCD, HDAC1 was enriched in the hippocampus, accompanied by a severe inflammatory response and learning and memory impairment (Yang et al., 2020). In traumatic spinal cord injury, parthenolide facilitated shift from M1 to M2 polarization of microglia by inactivation of STAT3 via downregulation of HDAC1 (Gaojian et al., 2020). Yang et al. (2020) uncovered that loss of HDAC1 exerted protective effects in POCD via partially inactivation of JAK2/STAT3 signal pathway. In current study, after inhalation of 3% sevoflurane for 6 h in aged rats, the expression levels of HDAC1 and phosphorylated proteins of STAT3 in hippocampal tissues were increased, the polarity of microglia shifted from M2 to proinflammatory M1 phenotype, and the secretion of proinflammatory factors was increased, while the secretion of anti-inflammatory factors was decreased. However, knockdown of C/EBPα reduced the expression of inflammatory phenotype of microglia, improved neuroinflammatory response and reversed cognitive dysfunction by selectively enhancing microglial function after M2 polarization.
These findings lay a new theoretical basis for generating insights into the roles of C/EBPα and are meaningful for further clinical usage in POCD treatment. In addition, these results also suggest that sevoflurane can induce cognitive impairment in elderly patients, and alternatives can be used instead of sevoflurane when used clinically However, this experimental study did not conduct the exploration of the effects of surgical trauma combined with sevoflurane on superimposed cognitive function in rats, and in vitro cell experiments were not performed to jointly verify the conclusions, which has some limitations.
Conclusions
Sevoflurane activates microglial M1 polarization and inflammatory responses, leading to hippocampal tissue damage and the occurrence of POCD by promoting C/EBPα expression. Inhibition of C/EBPα can downregulate the HDAC1/STAT3 pathway, promote microglia to M2 type transition, inhibit inflammatory responses, attenuate hippocampal histopathological and morphological damage, and improve cognitive function in aged rats. In addition, alleviating the prevention and treatment of central neuroinflammatory responses may provide new ideas for the prevention or treatment of POCD in the elderly.
Supplemental Information
Funding Statement
This work was supported by the Shaanxi Provincial People’s Hospital Science and Technology Development Incubation Fund (2022YJY-34) and the Shaanxi Natural Science Basic Research Program (2023-JC-QN-0951). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Zhao Xu conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Xi Yao conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Yikang Zhao analyzed the data, prepared figures and/or tables, and approved the final draft.
Bo Yao performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Shaanxi Provincial People’s Hospital approved the study.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.
References
- Berger et al. (2018).Berger M, Schenning KJ, Brown CHt, Deiner SG, Whittington RA, Eckenhoff RG, Angst MS, Avramescu S, Bekker A, Brzezinski M, Crosby G, Culley DJ, Eckenhoff M, Eriksson LI, Evered L, Ibinson J, Kline RP, Kofke A, Ma D, Mathew JP, Maze M, Orser BA, Price CC, Scott DA, Silbert B, Su D, Terrando N, Wang DS, Wei H, Xie Z, Zuo Z. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesthesia and Analgesia. 2018;127(6):1406–1413. doi: 10.1213/ANE.0000000000003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen Y, Zhang P, Lin X, Zhang H, Miao J, Zhou Y, Chen G. Mitophagy impairment is involved in sevoflurane-induced cognitive dysfunction in aged rats. Aging. 2020;12(17):17235–17256. doi: 10.18632/aging.103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa et al. (2021).Costa J, Martins S, Ferreira PA, Cardoso AMS, Guedes JR, Peça J, Cardoso AL. The old guard: age-related changes in microglia and their consequences. Mechanisms of Ageing and Development. 2021;197:111512. doi: 10.1016/j.mad.2021.111512. [DOI] [PubMed] [Google Scholar]
- Crotti & Ransohoff (2016).Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44(3):505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Evered & Silbert (2018).Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesthesia and Analgesia. 2018;127(2):496–505. doi: 10.1213/ANE.0000000000003514. [DOI] [PubMed] [Google Scholar]
- Gaojian et al. (2020).Gaojian T, Dingfei Q, Linwei L, Xiaowei W, Zheng Z, Wei L, Tong Z, Benxiang N, Yanning Q, Wei Z, Jian C. Parthenolide promotes the repair of spinal cord injury by modulating M1/M2 polarization via the NF-κB and STAT 1/3 signaling pathway. Cell Death Discovery. 2020;6(1):97–112. doi: 10.1038/s41420-020-00333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, Wu & Zhang (2017).Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. Journal of Clinical Anesthesia. 2017;38:165–171. doi: 10.1016/j.jclinane.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Guo, Wang & Yin (2022).Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Frontiers in Aging Neuroscience. 2022;14:815347. doi: 10.3389/fnagi.2022.815347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji et al. (2019).Ji J, Wang J, Yang J, Wang XP, Huang JJ, Xue TF, Sun XL. The intra-nuclear SphK2-S1P axis facilitates M1-to-M2 shift of microglia via suppressing HDAC1-mediated KLF4 deacetylation. Frontiers in Immunology. 2019;10:1241. doi: 10.3389/fimmu.2019.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazkayasi et al. (2013).Kazkayasi I, Burul-Bozkurt N, Önder S, Kelicen-Ugur P, Pekiner C. Effects of experimental diabetes on C/EBP proteins in rat hippocampus. Sciatic Nerve and Ganglia, Cellular and Molecular Neurobiology. 2013;33(4):559–567. doi: 10.1007/s10571-013-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020).Li L, Qiu Y, Miao M, Liu Z, Li W, Zhu Y, Wang Q. Reduction of Tet2 exacerbates early stage Alzheimer’s pathology and cognitive impairments in 2 ×Tg-AD mice. Human Molecular Genetics. 2020;29(11):1833–1852. doi: 10.1093/hmg/ddz282. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2020).Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Experimental Gerontology. 2020;130:110791. doi: 10.1016/j.exger.2019.110791. [DOI] [PubMed] [Google Scholar]
- Mufson et al. (2016).Mufson EJ, Malek-Ahmadi M, Snyder N, Ausdemore J, Chen K, Perez SE. Braak stage and trajectory of cognitive decline in noncognitively impaired elders. Neurobiology of Aging. 2016;43:101–110. doi: 10.1016/j.neurobiolaging.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. (2013).Pan HC, Yang CN, Hung YW, Lee WJ, Tien HR, Shen CC, Sheehan J, Chou CT, Sheu ML. Reciprocal modulation of C/EBP-α and C/EBP-β by IL-13 in activated microglia prevents neuronal death. European Journal of Immunology. 2013;43(11):2854–2865. doi: 10.1002/eji.201343301. [DOI] [PubMed] [Google Scholar]
- Pei, Wang & Li (2017).Pei Z, Wang S, Li Q. Sevoflurane suppresses microglial M2 polarization. Neuroscience Letters. 2017;655:160–165. doi: 10.1016/j.neulet.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Ponomarev et al. (2011).Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nature Medicine. 2011;17(1):64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao et al. (2020).Shao A, Fei J, Feng S, Weng J. Chikusetsu saponin IVa alleviated sevoflurane-induced neuroinflammation and cognitive impairment by blocking NLRP3/caspase-1 pathway. Pharmacological Reports. 2020;72(4):833–845. doi: 10.1007/s43440-020-00078-2. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2019).Tang S, Huang W, Zhang K, Chen W, Xie T. Comparison of effects of propofol versus sevoflurane for patients undergoing cardiopulmonary bypass cardiac surgery. Pakistan Journal of Medical Sciences. 2019;35(4):1072–1075. doi: 10.12669/pjms.35.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang & Le (2016).Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Molecular Neurobiology. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Thomas (2016).Thomas M. Age-related differences of neural connectivity during mental rotation. International Journal of Psychophysiology. 2016;101:33–42. doi: 10.1016/j.ijpsycho.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Vorhees & Williams (2006).Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2021).Wang F, Li C, Shao J, Ma J. Sevoflurane induces inflammation of microglia in hippocampus of neonatal rats by inhibiting Wnt/β-Catenin/CaMKIV pathway. Journal of Pharmacological Sciences. 2021;146(2):105–115. doi: 10.1016/j.jphs.2021.02.004. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang J, Zhao H, Fan Z, Li G, Ma Q, Tao Z, Wang R, Feng J, Luo Y. Long noncoding RNA H19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-dependent M1 microglial polarization. Stroke. 2017;48(8):2211–2221. doi: 10.1161/STROKEAHA.117.017387. [DOI] [PubMed] [Google Scholar]
- Xu & Qian (2020).Xu Z, Qian B. Sevoflurane anesthesia-mediated oxidative stress and cognitive impairment in hippocampal neurons of old rats can be ameliorated by expression of brain derived neurotrophic factor. Neuroscience Letters. 2020;721:134785. doi: 10.1016/j.neulet.2020.134785. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2020).Yang CX, Bao F, Zhong J, Zhang L, Deng LB, Sha Q, Jiang H. The inhibitory effects of class I histone deacetylases on hippocampal neuroinflammatory regulation in aging mice with postoperative cognitive dysfunction. European Review for Medical and Pharmacological Sciences. 2020;24(19):10194–10202. doi: 10.26355/eurrev_202010_23240. [DOI] [PubMed] [Google Scholar]
- Yang, Liu & Chu (2020).Yang ZY, Liu J, Chu HC. Effect of NMDAR-NMNAT1/2 pathway on neuronal cell damage and cognitive impairment of sevoflurane-induced aged rats. Neurological Research. 2020;42(2):108–117. doi: 10.1080/01616412.2019.1710393. [DOI] [PubMed] [Google Scholar]
- Yi-Bin et al. (2022).Yi-Bin W, Xiang L, Bing Y, Qi Z, Fei-Tong J, Minghong W, Xiangxiang Z, Le K, Yan L, Ping S, Yufei G, Ye X, Chun-Yan W. Inhibition of the CEBP β-NF κB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death & Disease. 2022;13(4):318. doi: 10.1038/s41419-022-04765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2022).Yin C, Zhang Q, Zhao J, Li Y, Yu J, Li W, Wang Q. Necrostatin-1 against sevoflurane-induced cognitive dysfunction involves activation of BDNF/TrkB pathway and inhibition of necroptosis in aged rats. Neurochemical Research. 2022;47(4):1060–1072. doi: 10.1007/s11064-021-03505-9. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu A, Zhang T, Duan H, Pan Y, Zhang X, Yang G, Wang J, Deng Y, Yang Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-α pathway in intracerebral hemorrhage. Immunology Letters. 2017;182:1–11. doi: 10.1016/j.imlet.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2022).Zhang D, Yang Y, Yang Y, Liu J, Zhu T, Huang H, Zhou C. Severe inflammation in new-borns induces long-term cognitive impairment by activation of IL-1β/KCC2 signaling during early development. BMC Medicine. 2022;20(1):235. doi: 10.1186/s12916-022-02434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Wang (2022).Zhang L, Wang J. Sinomenine alleviates glomerular endothelial permeability by activating the C/EBP-α/claudin-5 signaling pathway. Human Cell. 2022;35(5):1453–1463. doi: 10.1007/s13577-022-00750-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental File.