Abstract
Objective.
To determine whether a non-platinum chemotherapy doublet improves overall survival (OS) among patients with recurrent/metastatic cervical carcinoma.
Methods.
Gynecologic Oncology Group protocol 240 is a phase 3, randomized, open-label, clinical trial that studied the efficacy of paclitaxel 175 mg/m2 plus topotecan 0.75 mg/m2 days 1–3 (n = 223) vs cisplatin 50 mg/m2 plus paclitaxel 135 or 175 mg/m2 (n = 229), in 452 patients with recurrent/metastatic cervical cancer. Each chemotherapy doublet was also studied with and without bevacizumab (15 mg/kg). Cycles were repeated every 21 days until progression, unacceptable toxicity, or complete response. The primary endpoints were OS and the frequency and severity of adverse effects. We report the final analysis of OS.
Results.
At the protocol-specified final analysis, median OS was 16.3 (cisplatin-paclitaxel backbone) and 13.8 months (topotecan-paclitaxel backbone) (HR 1.12; 95% CI, 0.91–1.38; p = 0.28). Median OS for cisplatin-paclitaxel and topotecan-paclitaxel was 15 vs 12 months, respectively (HR 1.10; 95% CI,0.82–1.48; p = 0.52), and for cisplatin-paclitaxel-bevacizumab and topotecan-paclitaxel-bevacizumab was 17.5 vs 16.2 months, respectively (HR 1.16; 95% CI, 0.86–1.56; p = 0.34). Among the 75% of patients in the study population previously exposed to platinum, median OS was 14.6 (cisplatin-paclitaxel backbone) vs 12.9 months (topotecan-paclitaxel backbone), respectively (HR 1.09; 95% CI, 0.86–1.38;p = 0.48). Post-progression survival was 7.9 (cisplatin-paclitaxel backbone) vs 8.1 months (topotecan-paclitaxel backbone) (HR 0.95; 95% CI, 0.75–1.19). Grade 4 hematologic toxicity was similar between chemotherapy backbones.
Conclusions.
Topotecan plus paclitaxel does not confer a survival benefit to women with recurrent/metastatic cervical cancer, even among platinum-exposed patients. Topotecan-paclitaxel should not be routinely recommended in this population. NCT00803062.
Keywords: Cervical Cancer, Topotecan, Paclitaxel, Platinum, Recurrent
1. Introduction
Invasive cervical cancer continues to represent a major health problem among women in the United States and throughout the world. For 2023, the American Cancer Society estimates there will be 13,960 new cases and 4310 deaths [1] due to a disease that is preventable through screening via cervical cytology and/or high-risk human papillomavirus (HPV) DNA testing and vaccination using one of three available HPV vaccines developed using virus-like particle technology [2–4]. Globally, there are nearly 600,000 new cases annually, with over half of these patients dying each year [5]. With a median age of 54 years, many deaths occur among young women with small children at home and/or in the midst of professional careers [6].
While clinically early stage disease (i.e., FIGO stages IA2-IB2) can be treated by radical hysterectomy and bilateral pelvic lymphadenectomy followed by adjuvant chemoradiation if high-risk surgico-pathologic factors are identified, patients with locally advanced disease (FIGO stages IB3-IVA) require cisplatin-based radiosensitizing fractionated chemoradiation and high-dose-rate intracavitary brachytherapy to bring the total dose to point A (i.e., the parametria) to approximately 85 Gy [7]. Select women with very early stage cancers may be candidates for fertility-preserving radical trachelectomy with lymphadenectomy, and some with locally advanced tumors may opt for lateral ovarian transposition and/or oocyte harvesting prior to commencing radiotherapy. Patients with isolated, centrally-located recurrent disease following pelvic irradiation may be salvaged through total pelvic exenteration with urinary diversion [7].
In 2007, the phase 3, randomized clinical trial, Gynecologic Oncology Group (GOG) protocol 204, was closed for futility at interim analysis as none of the three investigational platinum-based chemotherapy doublets (cisplatin-topotecan, cisplatin-vinorelbine, cisplatin-gemcitabine) were expected to out-perform the control, cisplatin-paclitaxel [8]. Additionally, with widespread adoption of cisplatin-based chemoradiation protocols for locally advanced disease, acquired resistance to platinum at relapse became a concern. Accordingly, the GOG was tasked by the National Cancer Institute to identify a non-platinum chemotherapy doublet and/or novel targeted agents to study in women with recurrent, persistent, and metastatic cervical carcinoma. Although the SCOTCERV trial using gemcitabine-docetaxel had not matured [9], laboratory data by Bahadori, et al. suggested synergy between topotecan and microtubule-inhibiting agents [10] and a phase II trial of topotecan-paclitaxel by Tiersten, et al. had demonstrated activity in a preirradiated population of women with recurrent cervical cancer [11]. Vascular endothelial growth factor had also recently emerged as a viable target for anti-angiogenesis platforms, and based on clinical, pathologic, therapeutic, and molecular rationale, the fully, humanized, monoclonal antibody, bevacizumab, was vetted for what would be the GOG’s ninth phase 3 randomized trial in this patient population [12–17].
GOG-0240 activated in April 2009 and at the first interim analysis, topotecan-paclitaxel (with or without bevacizumab) did not significantly affect overall survival (OS) when compared with cisplatin-paclitaxel (with or without bevacizumab) (HR 1.20; 99% CI, 0.82–1.76) [12]. At the second interim analysis, the incorporation of bevacizumab was found to confer a survival advantage (17.0 vs 13.2 months; HR for death, 0.71; 98% CI, 0.54–0.95; p = 0.004) [12] and would directly lead to an approved indication in first-line treatment of recurrent/metastatic cervical cancer in over 60 countries on six continents. Here we present the protocol-specified analysis of OS using topotecan-paclitaxel.
2. Methods
2.1. Study design and participants
GOG-0240 is a phase III, open-label, randomized trial performed in the United States, Canada and in Spain through the GOG and Spanish Ovarian Cancer Group (GEICO). The study is listed at www.clinicaltrials.gov (NCI identifier #NCT00803062) and the master protocol is available at www.nrgoncology.org. Eligibility criteria included patients with metastatic, persistent, and recurrent cervical carcinoma. Patients with recurrent disease must not have been candidates for potentially curative pelvic exenteration. All cancers had central pathology review. GOG performance status scores of 0 or 1 (on a scale of 0 to 4 with 0 indicating that the patient is fully active and 1 indicating that the person is restricted in physically strenuous activities but ambulatory) were required, and patients had to have adequate renal, hepatic, and bone marrow function. All patients must have had measurable disease. Patients treated with chemotherapy for recurrence, and those with non-healing wounds, active bleeding conditions, and inadequately anticoagulated thromboembolism, were ineligible.
2.2. Procedures
Patients were randomly assigned to one of four treatment regimens which were repeated at 21-day intervals. Control treatment consisted of cisplatin (50 mg/m2) plus paclitaxel (135 or 175 mg/m2). The non-platinum doublet topotecan (0.75 mg/m2 days 1–3) plus paclitaxel (175 mg/m2) was included in two of the experimental arms. Each chemotherapy backbone was studied with and without bevacizumab (15 mg/kg day 1). Treatment was discontinued at the onset of disease progression, unacceptable adverse events (AEs), voluntary withdrawal by the patient, or upon complete response (CR).
Details related to disease assessment and tumor measurements using Response Evaluation Criteria in Solid Tumors (RECIST v.1) appear in the master protocol. Safety, as assessed by the National Cancer Institute’s Common Terminology Criteria for Adverse Events, was monitored during each cycle. Following treatment discontinuation, disease was assessed every three months for two years, followed by every six months for three years until progression. Three validated and sensitive instruments were used to measure health-related quality of life (HRQoL) and patient reported outcomes (PROs).
2.3. Statistical analysis
Assuming absence of interaction between experimental agents, the study utilized a 2 × 2 factorial design to investigate the ability of either regimen containing the non-platinum chemotherapy doublet (topotecan-paclitaxel) or anti-angiogenesis therapy (bevacizumab) to significantly impact patient outcomes. The study employed the intent-to-treat principle. Patients were prospectively stratified by GOG performance status (0 vs 1), prior radiosensitizing platinum, and disease status (recurrence/persistence versus stage IVB primary).
OS (based on a pooled analysis for each of the treatment factors under investigation) and the frequency and severity of AEs with each regimen were the primary endpoints. Secondary endpoints were to compare progression-free survival (PFS) and objective tumor responses among the different treatment arms. Differences in OS and PFS by factor level were assessed primarily by the log-rank test, stratified by clinical prognostic markers and the level of the other treatment factor [18]. Hazard ratios (HR) were estimated with a Cox proportional hazards model [19].
Exploratory analyses included a prospective validation of poor prognostic markers [15] identified in pooled analyses from prior studies, the impact of tumor histology, and several novel translational endpoints involving circulating tumor cells (CTC). For the Moore prognostic scoring system, patients were considered high risk if they had 4 or 5 equally weighted clinical factors (i.e., performance status >0, disease-free interval < 12 months, African American ethnicity, pelvic disease, and prior platinum exposure) [15]. Mid-risk patients had 2 or 3 factors, and low-risk patients had 0 or 1 factor [15]. CTC analyses were performed pre-cycle 1 and 36 days post-cycle 1. The median cut-off observed value of 7 CTCs/sample was used to separate those with high levels of CTCs from those with low levels of CTCs.
A sample size of approximately 450 was accrued to potentially observe 346 deaths in the final analysis to provide a study with 90% power when either factor was capable of reducing the hazard of death by at least 30%, while limiting the one-sided type I error for each to 2.5% (experiment-wise error rate >5% for the treatment regimens; none of the other exploratory analyses controlled for the experiment-wise error). An interim analysis was scheduled near 173 deaths to drop a factor or to stop the study for futility or to report regimen activity early according to the spending function in the event of dramatic improvement in survival [20,21]. A final analysis for survival required 346 deaths to have occurred. Since the study was designed with futility rules, the alternative hypotheses, critical regions, and p-values for the primary analyses of efficacy were listed as one-sided.
To monitor for unacceptable toxicity in the experimental arms, two 2-stage sequential toxicity analyses were embedded early in the conduct of the study (i.e., first 50 patients assigned to investigational treatment), with specific guidelines dictating when a meeting of the Data Safety Monitoring Board (DSMB) would need to be convened. AEs were reported until 30 days after the last study treatment and summarized for patients who received any therapy and submitted AE information. Changes in HRQoL and PROs were evaluated using a mixed model for analysis of repeated measures [22].
2.3.1. Reiteration of the interim analysis report
With the occurrence of 174 events, the pre-planned interim analysis was triggered and the DSMB concluded that topotecan was not a superior substitute for cisplatin when combined with paclitaxel by OS (HR 1.20; 99% CI, 0.82–1.76; p = 0.88). This information was communicated to physicians and patients participating in the study via ‘Dear Investigator’ and ‘Dear Patient’ letters prepared by the Study Chair. The analysis did not indicate that the topotecan regimens (with and without bevacizumab) performed worse than the cisplatin regimens by OS. Patients receiving therapy with any of the four arms of GOG 240 could remain on their current treatment assignment unless progression, unacceptable toxicity, or CR manifested. It was noted that the results concerning the topotecan regimens in GOG 240 were independent of bevacizumab in this study population. At the time of dissemination of the interim analysis results, GOG 240 had closed to accrual.
3. Results
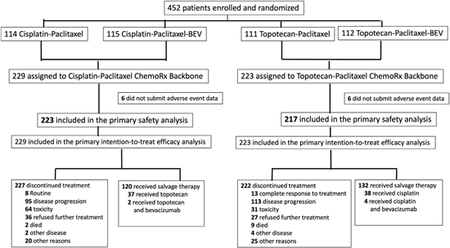
Between April 6, 2009, and January 3, 2012, a total of 452 eligible patients from 164 institutions in the United States and Spain were enrolled and randomized to one of two regimens containing the cisplatin-paclitaxel chemotherapy backbone (n = 229, 50.7%) and to one of two regimens containing the non-platinum chemotherapy doublet backbone of topotecan-paclitaxel (n = 223, 49.3%) (see CONSORT Diagram). On March 7, 2014, with 348 deaths, the protocol-specified 346 deaths had been exceeded to trigger the final analysis of OS. Patients were well-matched for GOG performance status, ethnicity, histology, disease status, and in-field pelvic recurrences (Table 1). Importantly, 337 (75%) of 452 patients had previously received platinum-based radiosensitizing chemotherapy, and this proportion was also evenly distributed between those receiving the two chemotherapy regimens. CONSORT Diagramdepicting the random allocation of patients to four treatment arms and subsequent attrition due to progression, toxicity, and complete response.
Table 1.
Baseline characteristics.
| Platinum-Based Chemotherapy Backbone N = 229 | Non-Platinum Chemotherapy Doublet Backbone N = 223 | |
|---|---|---|
| Age (years) | Median 46 (range, 20–85) | median 48 (range, 22–82) |
| Histology | ||
| Squamous | 71% | 65% |
| Adenocarcinoma | 19% | 19% |
| Adenosquamous | 9% | 10% |
| Other | 0% | 5% |
| Race | ||
| White | 78% | 77% |
| Black | 13% | 13% |
| Asian | 5% | 4% |
| Pacific Islander | 0.4% | 0.0% |
| Other | 3% | 6% |
| Disease status | ||
| Recurrent | 75% | 69% |
| Persistent | 9% | 14% |
| Metastatic | 16% | 17% |
| GOG performance status | ||
| 0 | 57% | 59% |
| 1 | 43% | 41% |
| Previous platinum-based radiosensitizing chemotherapy | 76% | 74% |
| Pelvic disease | 50% | 57% |
| Prior radiation therapy | 80% | 79% |
| Target lesion in radiation field | ||
| Yes | 39% | 40% |
| No | 60% | 59% |
| Unknown | 1% | 0% |
In the final analysis of OS, there was no significant survival benefit conferred by the non-platinum chemotherapy doublet backbone with median OS of 16.3 months (cisplatin-paclitaxel backbone with and without bevacizumab) and 13.8 months (topotecan-paclitaxel backbone with and without bevacizumab) (HR 1.12; 95% CI, 0.91–1.38; p = 0.28) (Fig. 1A). Updated PFS indicated a higher risk in the topotecan regimen (7.8 vs 6.0 months, respectively) (HR 1.24; 95% CI 1.02–1.50; p = 0.03) (Fig. 1B). The final OS of cisplatin-paclitaxel vs topotecan-paclitaxel was 15 vs 12 months, respectively (HR 1.10; 95% CI, 0.82–1.48;; p = 0.52) and the final OS of cisplatin-paclitaxel-bevacizumab vs topotecan-paclitaxel-bevacizumab was 17.5 vs 16.2 months, respectively (HR 1.16; 95% CI, 0.86–1.56; p = 0.34) (Figs. 1C–D).
Fig. 1.
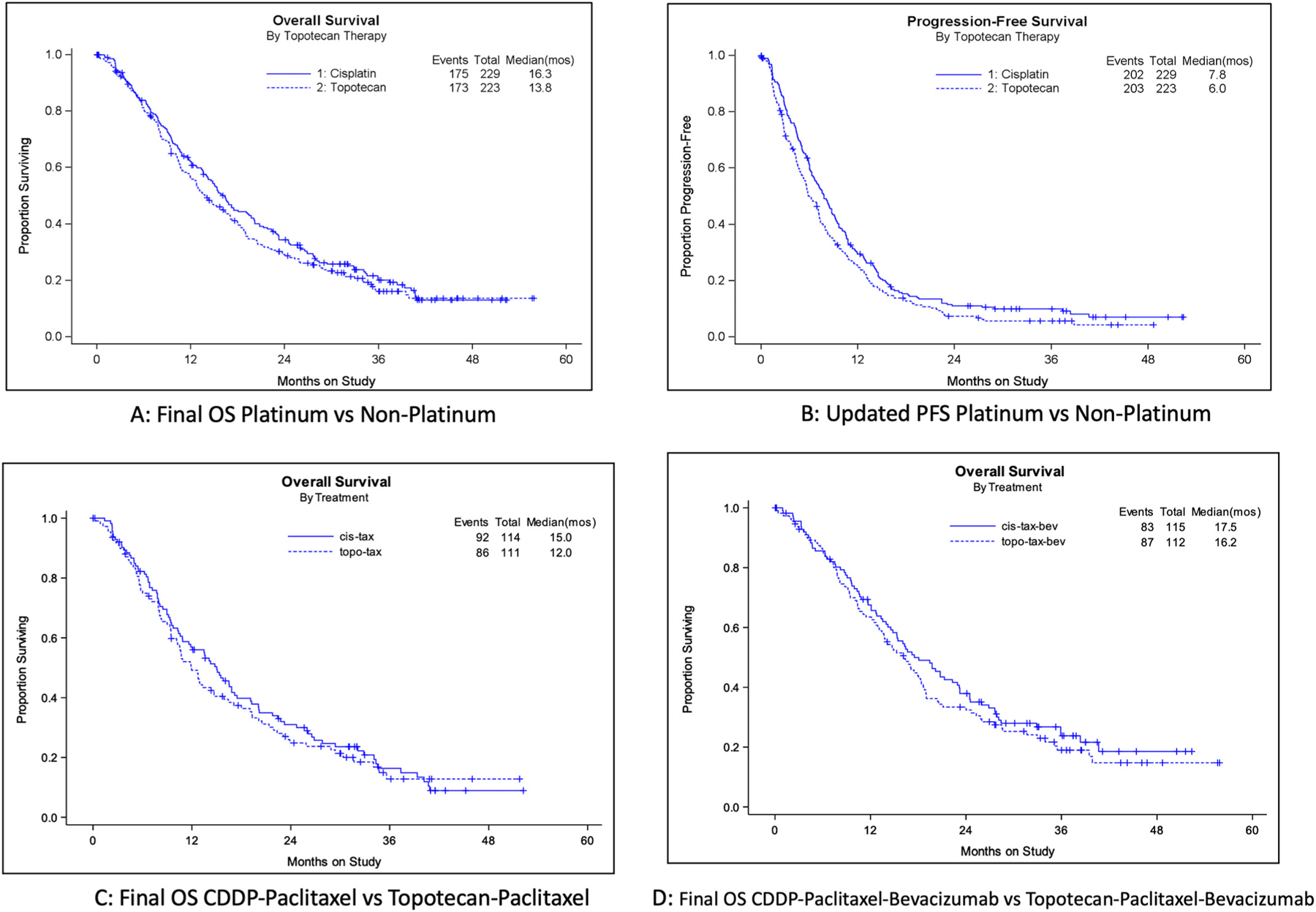

Kaplan-Meier curves comparing protocol-specified Final Overall Survival, updated Progression-Free Survival, and Post-Progression Survival between the cisplatin-containing chemotherapy backbone and the topotecan-containing chemotherapy backbone. Panel A: Final OS for the overall population comparing cisplatin-paclitaxel with and without bevaciaumab vs topotecan-paclitaxel with and without bevacizumab. Panel B: Updated PFS for the overall population comparing cisplatin-paclitaxel with and without bevaciaumab vs topotecan-paclitaxel with and without bevacizumab. Panel C: Final OS comparing cisplatin-paclitaxel without bevacizumab to topotecan-paclitaxel without bevacizumab. Panel D: Final OS comparing the cisplatin-paclitaxel-bevacizumab triplet to the topotecan-paclitaxel-bevacizumab triplet. Panel E: Final OS among the cohort of patients who previously were exposed to cisplatin for treatment of locally advanced disease using chemoradiation: cisplatin-paclitaxel with and without bevacizumab vs topotecan-paclitaxel with and without bevacizumab. Panel F: Final OS among the cohort of patients who were radiation naïve (i.e., presented with FIGO stage IVB disease): cisplatin-paclitaxel with and without bevacizumab vs topotecan-paclitaxel with and without bevacizumab. Panel G: Post-Progression Survival for the overall population comparing cisplatin-paclitaxel with and without bevaciaumab vs topotecan-paclitaxel with and without bevacizumab.
The final median OS for patients with prior platinum exposure treated with the cisplatin-paclitaxel backbone was 14.6 months and 12.9 months for the topotecan-paclitaxel backbone (HR 1.09; 95% CI, 0.86–1.38; p = 0.48) (Fig. 1E). Among those patients who had not received prior radiotherapy, the final OS was 20.2 vs 17.1 months for cisplatin-paclitaxel with and without bevacizumab vs topotecan-paclitaxel with and without bevacizumab, respectively (HR 1.25; 95% CI, 0.74–2.09; p = 0.40) (Fig. 1F). Post-progression survival was 7.9 vs 8.1 months, respectively (HR 0.95; 95% CI, 0.75–1.19; p = 0.63) (Fig. 1G).
A forest plot of prognostic factors appears in Fig. 2A. When analyzing the patient-reported outcomes using the Functional Assessment of Cancer Therapy-Cervix Trial Outcome Index (FACT-Cx TOI), there was no significant difference in HRQoL between treatment with either chemo-therapy backbone (Fig. 2B, difference 0.5; p = 0.66). Compared with the cisplatin-paclitaxel backbone, the topotecan-paclitaxel backbone was associated with less grade 3 or worse neurosensory toxicity (OR 0.25; 95% CI, 0.09–0.69; p = 0.004) but there were no differences in grade 3 or worse other neurotoxicity (OR 0.76; 95% CI, 0.26–2.24; p = 0.78) as measured with the FACT/GOG-Ntx subscale short form (difference, 0.43; p = 0.45). Similarly, no significant differences were observed complaining of grade 3 or worse pain in either backbone (OR 0.90; 95% CI, 0.5–1.63; p = 76?). Exploratory analyses of the Moore clinical scoring system were not informative when comparing the platinum-based and non-platinum chemotherapy backbones for high risk patients (median OS 8.0 vs 8.2 months, respectively; HR 0.81; 95% CI, 0.48–1.39), mid risk patients (16.5 vs 13.8 months; HR 1.16; 95% CI, 0.89–1.52), and low risk (26.0 vs 20.5 months; HR 1.10; 95% CI, 0.62–1.95) (Figs. 3A–C). There were also no significant differences in OS observed by histologic type and treatment assignation. The median OS among patients with adenocarcinoma alone was 23.2 (platinum-based backbone) vs 13.7 months (non-platinum backbone) (HR 1.58; 95% CI, 0.97–2.57) and the median OS for all adenocarcinoma (i.e., including adenosquamous histology) was 16.7 (platinum-based backbone) vs 14.7 months (non-platinum backbone) (HR 1.29; 95% CI, 0.88–1.90). The median OS among patients with squamous cell carcinoma alone was 16.1 (platinum-based backbone) vs 12.9 months (non-platinum backbone) (HR 1.12; 95% CI, 0.87–1.44), and the median OS for all squamous cell carcinomas (i.e., including adenosquamous histology) was 15.3 (platinum-based backbone) vs 13.8 months (non-platinum backbone) (HR 1.09; 95% CI, 0.85–1.38) (Figs. 3D–G).
Fig. 2.

Prognostic factors and Health-Related Quality of Life. Panel A: Forest plot of prognostic factors depicting distribution of the hazard of death for the experimental regimen (i.e., topotecan-paclitaxel chemotherapy backbone) and the control regimen (i.e., cisplatin-paclitaxel chemotherapy backbone).
Fig. 3.


Exploratory analyses comparing the Final Overall Survival and Progression-Free Survival of the cisplatin-containing chemotherapy backbone to the topotecan-containing chemo-therapy backbone. Panel A: Moore High-Risk clinical prognostic scoring system. Panel B: Moore Mid-Risk clinical prognostic scoring system. Panel C: Moore Low-Risk clinical prognostic scoring system. Panel D: Adenocarcinoma histology. Panel E: Adenocarcinoma plus Adenosquamous histology. Panel F: Squamous cell carcinoma histology. Panel G: Squamous cell carcinoma plus Adenosquamous histology. Panel H: Pre-Cycle 1 Low Circulating Tumor Cells Final OS. Panel I: Pre-Cycle 1 High Circulating Tumor Cells Final OS. Panel J: Pre-Cycle 1 Low Circulating Tumor Cells PFS. Panel K: Pre-Cycle 1 High Circulating Tumor Cells PFS. Panel L: Post-Cycle 1 Low Circulating Tumor Cells Final OS. Panel M: Post-Cycle 1 High Circulating Tumor Cells Final OS. Panel N: Post-Cycle 1 Low Circulating Tumor Cells PFS. Panel O: Post-Cycle 1 High Circulating Tumor Cells PFS.
For the evaluation of pre-cycle 1 CTC counts, the chemotherapy backbone used was not associated with a survival benefit when studying patients with low (17.2 vs 17.1 months) (HR 0.964;95% CI, 0.57–1.63) or high CTC counts (20.5 vs 17.1 months) (HR 1.32; 95% CI, 0.81–2.16). There was also no discernible impact on PFS based on precycle 1 CTC counts: low CTCs median PFS 6.0 vs 6.4 months (HR 0.82; 95% CI, 0.51–1.33); high CTCs median PFS 9.2 vs 7.3 months (HR 1.20; 95% CI, 0.76–1.88). For the CTC analyses 36 days post-cycle 1, the topotecan-containing chemotherapy backbone had a non-significant trend for worse survival in the cohort with low CTCs: median OS 27.9 vs 14.3 months (HR 1.98; 95% CI, 1.14–3.42). For high CTCs, the median OS was 17.2 vs 18.4 months (HR 0.89; 95% CI, 0.55–1.43). PFS also appeared worse in the topotecan-containing regimens in the low CTC post-therapy group: median PFS 10.5 vs 4.9 months (HR 1.88; 95% CI, 1.14–3.08). For high CTCs, the median PFS was 8.1 vs 8.2 months (HR 0.93; 95% CI, 0.60–1.44). These data appear in Figs. 3H–O.
3.1. Toxicology
The adverse events assigned to each of the chemotherapy backbones appear in Table 2. As stipulated in the protocol, there were no safety signals identified with dosing of the first 50 patients on the topotecan-paclitaxel backbone that required the DSMB to be convened. In the entire study population, there were no toxic deaths attributed to either chemotherapy backbone.
Table 2.
Adverse Events.
| Adverse event | Chemotherapy Backbone | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|---|
| Hematologic | |||||||
| Leukopenia | CDDP-Paclitaxel | 42 | 24 | 76 | 71 | 10 | 0 |
| Topotecan-Paclitaxel | 28 | 26 | 49 | 79 | 35 | 0 | |
| Neutropenia | CDDP-Paclitaxel | 54 | 19 | 42 | 49 | 59 | 0 |
| Topotecan-Paclitaxel | 47 | 6 | 24 | 61 | 79 | 0 | |
| Anemia | CDDP-Paclitaxel | 17 | 47 | 103 | 50 | 6 | 0 |
| Topotecan-Paclitaxel | 12 | 50 | 104 | 44 | 7 | 0 | |
| Thrombocytopenia | CDDP-Paclitaxel | 130 | 65 | 18 | 8 | 2 | 0 |
| Topotecan-Paclitaxel | 110 | 82 | 11 | 7 | 7 | 0 | |
| Non-hematologic | |||||||
| Nausea | CDDP-Paclitaxel | 76 | 85 | 44 | 18 | 0 | 0 |
| Topotecan-Paclitaxel | 100 | 77 | 34 | 6 | 0 | 0 | |
| Vomiting | CDDP-Paclitaxel | 142 | 37 | 32 | 11 | 1 | 0 |
| Topotecan-Paclitaxel | 165 | 33 | 13 | 6 | 0 | 0 | |
| Gastrointestinal | CDDP-Paclitaxel | 51 | 80 | 61 | 29 | 1 | 1 |
| Topotecan-Paclitaxel | 70 | 63 | 63 | 19 | 1 | 1 | |
| Metabolic | CDDP-Paclitaxel | 118 | 44 | 29 | 31 | 1 | 0 |
| Topotecan-Paclitaxel | 141 | 38 | 25 | 12 | 1 | 0 | |
| Neurosensory | CDDP-Paclitaxel | 84 | 75 | 44 | 20 | 0 | 0 |
| Topotecan-Paclitaxel | 85 | 91 | 36 | 5 | 0 | 0 | |
| Allergy | CDDP-Paclitaxel | 178 | 18 | 19 | 8 | 0 | 0 |
| Topotecan-Paclitaxel | 203 | 9 | 2 | 3 | 0 | 0 | |
Hematologic toxicity was similar between each chemotherapy backbone, with a trend for increased grade 3 and grade 4 leukopenia and neutropenia observed among patients treated with the topotecan-paclitaxel backbone. Although not statistically significant, there were more grade 3 nausea, vomiting, gastrointestinal, metabolic, neurosensory, and allergic events reported among patients treated with the cisplatin-paclitaxel chemotherapy backbone.
4. Discussion
The non-platinum chemotherapy doublet, topotecan-paclitaxel, did not improve survival over platinum among patients treated with first-line systemic therapy for recurrent and/or metastatic disease. As previously reported, the interaction term was not significant, indicating that there was no interaction between the two treatment regimens (non-platinum doublet and bevacizumab) under investigation. Interestingly, whereas high-risk Moore score patients treated with chemotherapy plus bevacizumab (using either chemotherapy backbones) derived the greatest survival benefit compared to those with mid-risk and low-risk scores, similar observations were not found when these exploratory analyses isolated the contribution of topotecan-paclitaxel. In addition, the contribution of topotecan-paclitaxel was not impacted by the presence (or absence) of circulating tumor cells.
Although QoL was not significantly impacted by topotecan-paclitaxel, there are notable differences in the adverse effect profiles attributed to each of the chemotherapy backbones. Incorporation of topotecan-paclitaxel with bevacizumab may represent a reasonable option for patients who wish to avoid the emetogenic, gastrointestinal, neurosensory, and metabolic toxicity which occur more frequently when cisplatin-paclitaxel is used. However, we should note that one year following the interim analysis report from GOG-0240, the Japanese Clinical Oncology Group reported on the non-inferiority of carboplatin when substituted for cisplatin as first line treatment of recurrent disease, particularly among patients who were platinum-naïve (JCOG 0505; NCT00295789) [23]. Carboplatin is relatively more tolerable than cisplatin, and the carboplatin-paclitaxel-bevacizumab triplet was found to have encouraging activity (61% ORR, median OS 25.0 months) and a fistula rate similar to GOG-0240 in the phase 2 CELICIA study (NCT024679070) [24]. The topotecan-paclitaxel-bevacizumab triplet may best be reserved for patients who manifest significant delayed hypersensitivity to the platinum drugs.
GOG-0240 represents a proof of concept of anti-angiogenesis therapy for cervical cancer and a proof of principle of supportive care. With the exhaustion of chemotherapy options studied in 9 phase 3 randomized trials of the GOG, these data may also be viewed as the capstone on systemic chemotherapy in this disease. When the trial launched in 2009, it was the only study evaluating a novel therapeutic agent in this disease. During the interim period, the Cervical Cancer Genome Atlas was published and novel amplifications were identified in the immune targets PD-L1 and PD-L2 [25]. Importantly, it was recognized that amplification of these immunologic sequences were triggered by viral integration of the human papillomavirus [26].
In 2018, the phase 2 study, KeyNote-158 (NCT02628067), led to accelerated approval of pembrolizumab as a second-line treatment option for PD-L1+ cervical cancer based on an objective response rate of 14% [27]. In 2021, the EMPOWER-Cervix/GOG-3016/ENGOT-Cx9 phase III randomized trial, reported a survival benefit using the anti-PD-1, cemiplimab, over physician’s choice chemotherapy in the second-line setting [28]. This was closely followed by the announcement that the phase 3 randomized study, KeyNote-826 (NCT03635567), met its dual primary endpoints of OS and PFS, demonstrating the clinical benefit conferred by the incorporation of pembrolizumab over chemotherapy alone in the first-line setting [29]. On October 13, 2021, the U.S. Food and Drug Administration approved pembrolizumab in combination with chemotherapy, with or without bevacizumab, for first-line treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1 as determined by the Combined Positive Score ≥ 1.
In summary, the non-platinum doublet of topotecan-paclitaxel did not demonstrate increased activity in patients previously exposed to platinum. While not statistically significant, the platinum-containing doublet was numerically superior to topotecan-paclitaxel for efficacy. Although the NCCN lists both chemotherapy backbones combined with bevacizumab as Category 1 based on interim analysis data, the final analysis of OS reported in this paper indicates that the non-platinum doublet should not be routinely recommended in this patient population.
HIGHLIGHTS.
Topotecan plus paclitaxel is not superior to cisplatin plus paclitaxel for recurrent/metastatic cervical cancer.
Topotecan-paclitaxel plus bevacizumab is not superior to cisplatin-paclitaxel plus bevacizumab for cervical cancer.
The non-platinum doublet, topotecan-paclitaxel, should not be routinely used for recurrent/metastatic cervical cancer.
Acknowledgements
This study was supported by the following National Cancer Institute and Genentech grants: NRG Oncology (1U10CA180822), NRG Operations (U10CA180868) and NCORP grant UG1CA189867.
The following NRG Oncology member institutions participated in the primary treatment studies: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Northwestern Memorial Hospital, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke’s Medical Center, Magee Women’s Hospital, SUNY Downstate Medical Center, University of Kentucky, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women’s Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Yale University, University of Wisconsin Hospital, Cancer Trials Support Unit, University of Texas - Galveston, Women and Infants Hospital, The Hospital of Central Connecticut, Georgia Core, Aurora Women’s Pavilion of West Allis Memorial Hospital, Grupo Espanol de Investigacion en Cancer de Ovario, University of California, San Francisco-Mt. Zion, St. Joseph’s Hospital and Medical Center (Arizona), and Community Clinical Oncology Program.
Declaration of Competing Interest
Dr. Tewari reports receiving honoraria for lectures, presentations, speakers’ bureaus for Merck, Astra Zeneca, Eisai, Seagen, Tesaro and Clovis. He also reports serving on Data Safety Monitoring Board/Advisory Board for Iovance.
Dr. Penson reports grants received from Array BioPharma Inc., AstraZeneca, Eisai Inc., Genentech, Inc., Regeneron, Sanofi-Aventis US LLC, Tesaro Inc. and Vascular Biogenics Ltd. Dr. Penson also reports receiving consulting fees from AstraZeneca, Eisai, GSK Inc., ImmunoGen Inc., Merck & Co, Mersana, Novacure, Roche Pharma, Sutro Biopharma, and Vascular Biogenics Ltd. and reports participating on Advisory Boards for AstraZeneca and Roche Pharma.
Dr. Oaknin reports grants to her institution from the following: Abbvie Deutschland Gmbh & Co Hg, Ability Pharmaceuticals, Advaxis, Agenus, Aprea Therapeutics AB, AstraZeneca AB, BeiGene USA, Inc., Belgian Gynecological Oncology Group (BGOG), Bristol-Myers Squibb International Corporation (BMSM Clovis Oncology, Corcept Therapeutics, Eisai, F. Hoffmann-La Roche, Grupo Española de Investigación en Cáncer de Ovario (GEICO)), Immunogen, Iovance Biotherapeutics, Lilly, Medimmune, Merck Healthcare, Merck Sharp & Dohme, Millennium Pharmaceuticals, Unipharm Research, Novartis Farmacéutica, Regeneron Pharmaceuticals, Seagen, Seattle Genetics, Sutro Biopharma, Tesaro, University Health Network, and Werastem, Regeneron Pharmaceuticals, Seagen, Seattle Genetics, Sutro Biopharma, Tesaro, University Health Network, and Werastem. Dr. Oaknin also reports receiving consulting fees from: Agenus, AstraZeneca Clovis Oncology, Inc., Corcept Therapeutics, Deciphera Pharmaceutical, Eisai Europe Limited, EMD Serono, Inc., F. Hoffmann-La Roche, GlaxoSmithKline, Immunogen, KL Logistics, Medison Pharma, Merck Sharp & Dohme de España, Mersana Therapeutics, Novocure GmbH, Pharma Mar, prIME Oncology, ROCHE FARMA, Sattucklabs, and Sutro Biopharma, Inc., and GEICO. Dr. Oaknin reports personally receiving honoraria for lectures, presentations and Speakers bureaus from ESMO, Edizioni Minerva Medica SpA, Doctaforum Servicios S.L and received support for travel from Roche, AstraZeneca, PharmaMar as well as Clovis. Dr. Oaknin participated on Data Safety Monitoring Board/Advisory Board for Agenus, AstraZeneca Clovis Oncology, Inc., Corcept Therapeutics, Deciphera Pharmaceutical, Eisai Europe Limited, EMD Serono, Inc., F. Hoffmann-La Roche, GlaxoSmithKline, GOG, Immunogen, KL Logistics, Medison Pharma, Merck Sharp & Dohme de España, Mersana Therapeutics, Novocure GmbH, Pharma Mar, prIME Oncology, ROCHE FARMA, Sattucklabs and Sutro Biopharma.
Dr. Leitao reports receiving consulting fees from Medtronic, honoraria for lectures from Intuitive Surgical, Inc. as an Ad-hoc Speaker and participating on an Advisory Board for JnJ/Ethicon.
Dr. Monk reports receiving consulting fees from the following: Acrivon, Adaptimune, Agenus, Akeso Bio, Amgen, Aravive, AstraZeneca, Bayer, Clovis, Easai, Elevar, EMD Merck, Genmab/Seagen, GOG Foundation, Gradalis, Heng Rui, UmmunoGen, Karyopharm, Iovance, Laekna, Macrogenics, Merck, Mersana, Myriad, Novartis, Novocure, OncoC4, Panavance, Pleris, Pfizer, Puma, Regeneron, Roche/Genentech, Sorrento, Tesaro/GSK, US Oncology Research, VBL, Verastem and Zentalis. He also reports receiving honoraria from: AstraZeneca, Merck, Myriad, Tesaro/GSK, Roche/Genentech, Clovis and Easai.
All other authors had no conflicts of interest to disclose with regard to this Study.
The authors wrote the manuscript and take responsibility for the accuracy and completeness of the reported data and for the fidelity of the document to the protocol. All patients provided written informed consent before enrollment. The study was sponsored by the National Cancer Institute, which provided the bevacizumab without charge.
References
- [1].Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023, CA Cancer J. Clin 73 (2023) 17–48. [DOI] [PubMed] [Google Scholar]
- [2].US Preventive Services Task Force, Curry SJN, Krist AH, Owens DK, et al. , Screening for cervical cancer: US preventive services task force recommendation statement, JAMA 320 (7) (2018) 675–686. [DOI] [PubMed] [Google Scholar]
- [3].Markowitz LE, Dunne EF, Saraiya M, et al. , Human papillomavirus vaccination: recommendations of the advisory committee on immunization practices (ACIP), MMWR Recomm. Rep 63 (RR-05) (2014) 1–30. [PubMed] [Google Scholar]
- [4].Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M, Validation of the 2018 FIGO cervical cancer staging system, Gynecol. Oncol 152 (1) (2019) 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].American Cancer Society, Cancer Facts and Figures, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html 2021.
- [6].Tewari KS, Monk BJ, Invasive Cervical Cancer, in: DiSaia PJ, Creasman WT (Eds.), Clinical Gynecologic Oncology, 9th ed. Elsevier, 2017. [Google Scholar]
- [7].Tewari KS, Monk BJ, Evidence-based treatment paradigms for management of invasive cervical carcinoma, J. Clin. Oncol 37 (27) (2019) 2472–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Monk BJ, Sill MW, McMeekin DS, et al. , Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervix al carcinoma: a gynecologic oncology group study, J. Clin. Oncol 27 (28) (2009) 4649–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Symonds RP, Davidson SE, Chan S, et al. , SCOTCERV: a phase II trial of docetaxel and gemcitabine as second line chemotherapy in cervical cancer, Gynecol. Oncol 23 (1) (2011) 105–109. [DOI] [PubMed] [Google Scholar]
- [10].Bahadori HR, Green MR, Catapano CV, Synergistic interaction between topotecan and microtubule-interfering agents, Cancer Chemother. Pharmacol 48 (3) (2001) 188–196. [DOI] [PubMed] [Google Scholar]
- [11].Tiersten AD, Selleck MJ, Hershman DL, et al. , Phase II study of topotecan and paclitaxel for recurrent, persistent, or metastatic cervical carcinoma, Gynecol. Oncol 92 (2) (2004) 635–638. [DOI] [PubMed] [Google Scholar]
- [12].Tewari KS, Sill MW, Long HJ 3rd, et al. , Improved survival with bevacizumab in advanced cervical cancer, N. Engl. J. Med 370 (8) (2014) 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Penson RT, Huang HQ, Wenzel LB, et al. , Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG oncology-gynecologic oncology group protocol 240), Lancet Oncol 16 (3) (2015) 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tewari KS, Sill MW, Penson RT, et al. , Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240), Lancet 390 (10103) (2017) 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tewari KS, Sill MW, Monk BJ, et al. , Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG oncology/GOG study, Clin. Cancer Res 21 (24) (2015) 5480–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Seamon LG, Java JJ, Monk BJ, et al. , Impact of tumour histology on survival in advanced cervical carcinoma: an NRG oncology/Gynaecologic oncology group study, Br. J. Cancer 118 (2) (2018) 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tewari KS, Sill MW, Monk BJ, et al. , Circulating tumor cells in advanced cervical cancer: NRG oncology – gynecologic oncology group study 240 (NCT00803062), Mol. Cancer Ther (2020). 10.1158/1535-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mantel N, Evaluation of survival data and two new rank order statistics arising in its consideration, Cancer Chemother. Rep 50 (3) (1996) 163–170. [PubMed] [Google Scholar]
- [19].Cox DR, Regression models and life-tables, J. R Stat. Soc. [B] 34 (1972) 187–220. [Google Scholar]
- [20].Wieand S, Schroeder G, O’Fallon JR, Stopping when the experimental regimen does not appear to help, Stat. Med 13 (13–14) (1994) 1453–1458. [DOI] [PubMed] [Google Scholar]
- [21].Gordan Lan KK, DeMets DL, Discrete sequential boundaries for clinical trials, Biometrika 70 (3) (1983) 659–663. [Google Scholar]
- [22].Diggle P, Liang KY, Zeger SL, Analysis of Longitudinal Data. Oxford, Clarendon Press, United Kingdom, 1993. [Google Scholar]
- [23].Kitagawa R, Katsumata N, Shibata T, et al. , Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505, J. Clin. Oncol 33 (19) (2015) 2129–2135. [DOI] [PubMed] [Google Scholar]
- [24].Redondo A, Colombo N, Dreosti LM, et al. , Primary results from CECILIA, a global single-arm phase 2 evaluate bevacizumab, carboplatin and paclitaxel for advanced cervical cancer, Gynecol. Oncol 159 (1) (2020) 142–149. [DOI] [PubMed] [Google Scholar]
- [25].The Cancer Genome Atlas Research Network, Integrated genomic and molecular characterization of cervical cancer, Nature 543 (7645) (2017) 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ojesina AI, Lichtenstein L, Freeman SS, et al. , Landscape of genomic alterations in cervical carcinomas, Nature 506 (7488) (2014) 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung HC, Ros W, Delord JP, et al. , Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KeyNote-158 study, J. Clin. Oncol 37 (17) (2019) 1470–1478. [DOI] [PubMed] [Google Scholar]
- [28].Tewari KS, Monk BJ, Vergote I, et al. , Survival with cemiplimab in recurrent cervical cancer, N. Engl. J. Med 386 (6) (2022) 544–555. [DOI] [PubMed] [Google Scholar]
- [29].Colombo N, Dubot C, Lorusso D, et al. , Pembrolizumab for persistent, recurrent, or metastatic cervical cancer, N. Engl. J. Med 385 (20) (2021) 1856–1867. [DOI] [PubMed] [Google Scholar]


