Abstract
Background
Hybrid MRI linear accelerators (MR-Linac) might enable individualized online adaptation of radiotherapy using quantitative MRI sequences as diffusion-weighted imaging (DWI). The purpose of this study was to investigate the dynamics of lesion apparent diffusion coefficient (ADC) in patients with prostate cancer undergoing MR-guided radiation therapy (MRgRT) on a 1.5T MR-Linac. The ADC values at a diagnostic 3T MRI scanner were used as the reference standard.
Patients and and methods
In this prospective single-center study, patients with biopsy-confirmed prostate cancer who underwent both an MRI exam at a 3T scanner (MRI3T) and an exam at a 1.5T MR-Linac (MRL) at baseline and during radiotherapy were included. Lesion ADC values were measured by a radiologist and a radiation oncologist on the slice with the largest lesion. ADC values were compared before vs. during radiotherapy (during the second week) on both systems via paired t-tests. Furthermore, Pearson correlation coefficient and inter-reader agreement were computed.
Results
A total of nine male patients aged 67 ± 6 years [range 60 – 67 years] were included. In seven patients, the cancerous lesion was in the peripheral zone, and in two patients the lesion was in the transition zone. Inter-reader reliability regarding lesion ADC measurement was excellent with an intraclass correlation coefficient of (ICC) > 0.90 both at baseline and during radiotherapy. Thus, the results of the first reader will be reported. In both systems, there was a statistically significant elevation of lesion ADC during radiotherapy (mean MRL-ADC at baseline was 0.97 ± 0.18 × 10−3 mm2/s vs. mean MRL-ADC during radiotherapy 1.38 ± 0.3 × 10−3 mm2/s, yielding a mean lesion ADC elevation of 0.41 ± 0.20 × 10−3 mm2/s, p < 0.001). Mean MRI3T-ADC at baseline was 0.78 ± 0.165 × 10−3 mm2/s vs. mean MRI3T-ADC during radiotherapy 0.99 ± 0.175 × 10−3 mm2/s, yielding a mean lesion ADC elevation of 0.21 ± 0.96 × 10−3 mm2/s p < 0.001). The absolute ADC values from MRL were consistently significantly higher than those from MRI3T at baseline and during radiotherapy (p < = 0.001). However, there was a strong positive correlation between MRL-ADC and MRI3T-ADC at baseline (r = 0.798, p = 0.01) and during radiotherapy (r = 0.863, p = 0.003).
Conclusions
Lesion ADC as measured on MRL increased significantly during radiotherapy and ADC measurements of lesions on both systems showed similar dynamics. This indicates that lesion ADC as measured on the MRL may be used as a biomarker for evaluation of treatment response. In contrast, absolute ADC values as calculated by the algorithm of the manufacturer of the MRL showed systematic deviations from values obtained on a diagnostic 3T MRI system. These preliminary findings are promising but need large-scale validation. Once validated, lesion ADC on MRL might be used for real-time assessment of tumor response in patients with prostate cancer undergoing MR-guided radiation therapy.
Keywords: prostate carcinoma, MRI, adaptive radiotherapy, image guidance, MR-Linac, ADC
Introduction
Radiotherapy (RT) is a curative treatment option for patients with localized prostate cancer.1 MR-guided radiotherapy (MRgRT) enables improved soft tissue contrast and enhances accuracy of treatment planning.2 In this context, the hybrid magnetic resonance 1.5T scanner with a linear accelerator MR-Linac (MRL) is currently being used in centers around the world to perform high-precision MRgRT with daily plan adaptations based on anatomical MR sequences.3,4 Furthermore, functional MRI sequences such as diffusion weighted imaging (DWI) are being additionally taken into account for radiotherapy planning, as they provide valuable “real-time” functional information.5 The apparent diffusion coefficient (ADC) of a tumor lesion has been shown to function as a biomarker for prostate cancer on diagnostic scanners.6 MRL presents a novel opportunity to integrate ADC-values of a tumor lesion into daily plan adaptations and individualize radiotherapy.7 A prerequisite is the clinical translatability of ADC-measurements on MRL to a “gold standard” 3T diagnostic scanner (MRI3T).
In a previous study, it was demonstrated that ADC measurements of a region of interest in intraprostatic tumor lesions on MRL correlated with corresponding measurements on a diagnostic 3T MRI scanner (MRI3T).8 In that analysis, the MRIs on both scanners were performed prior to treatment initiation. However, as an initial step to evaluate, whether ADC measurements on an MRL might function as a biomarker enabling response assessment under RT, the longitudinal stability of ADC data gained on an MRL should be examined.
The purpose of this study is to longitudinally investigate the dynamics of lesion ADC in patients with prostate cancer undergoing MR-guided radiation therapy on an MR-Linac using the ADC values at a 3T MRI scanner as a reference standard.
Patients and methods
Participant sample, study design and MRI technique
All patients included in this prospective study were recruited in the M-base Pro 1.09 or M-base HyPro 2.0 at our institution (ClinicalTrials.gov Identifiers: NCT02724670; NCT03880851). The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the medical faculty of Tuebingen University (No. 022/2016BO1, 14.03.2016 and No. 920/2018BO1, 10.07.2019). Informed consent was obtained from all subjects involved in the study. All patients consented to prospectively undergo multiple MRIs on an MRL and additionally on a MRI3T at several points prior to and during RT. The aforementioned studies each examine a novel MR-adaptive concept for radiotherapy of primary localized prostate cancer. Between February 2019 and October 2021, 9 patients with biopsy-confirmed prostate cancer and available MRL and MRI3T data sets prior to RT and under RT were included. All patients were treated daily on a 1.5T MRL (Elekta UnityTM, Philips, Stockholm, Sweden).10 All patients were treated according to national guidelines with either 39 × 2 Gy per fraction over eight weeks (M-base 1.0 study, n = 3 patients) or 20 × 3 Gy per fraction over four weeks (M-base Hypro 2.0 study, n = 6 patients) and additional neoadjuvant androgen deprivation therapy (ADT) of six months for intermediate risk patients and 24–36 months for high risk patients.11 The time point of the MRL and MRI3T was during week 2 of RT in both treatment protocols. MRI technique, specifications and acquisition parameters of the examinations on both systems have been previously described.8 Most study participants in this study were used in the prior publication8, but only examinations prior to RT were analyzed. No lesion ADC dynamics during RT were reported in the previous study.8
Lesion ADC evaluation
The ADC maps for MRL and MRI3T for each patient, prior to and during radiotherapy, were independently presented to two readers (reader 1, a board certified radiation oncologist with 8 years of experience reader 2, a radiology resident with 4 years of experience). Both readers placed an elliptic region-of-interest (ROI) within the lesions for each patient in MRL and MRI3T image sets. A dedicated workstation (GE Healthcare Centricity™ PACS RA1000, Milwaukee WI, USA) was utilized for image analysis using a dedicated software (syngo.via, Siemens Healthcare, Erlangen, Germany).
Statistical analysis
Continuous variables were reported as mean and standard deviation. Paired t-tests were used for pair-wise pre- vs. during-treatment comparisons, as well as MRL vs. MRI-3T. Intraclass correlation coefficient (ICC, two-way, absolute agreement) was used to compute inter-reader agreement. An ICC of less than 0.4 signalizes poor agreement, of 0.40 to 0.59 indicates fair agreement, of 0.60 to 0.74 good agreement, and an ICC of 0.75 to 1.00 signalizes excellent agreement.12 Pearson Correlation coefficient was used to compare lesion ADC between MRL and MRI3T. Level of significance was set at 0.05. Statistical analyses were performed using SPSS (v26.0, IBM-Corp, Armonk, NY, USA).
Results
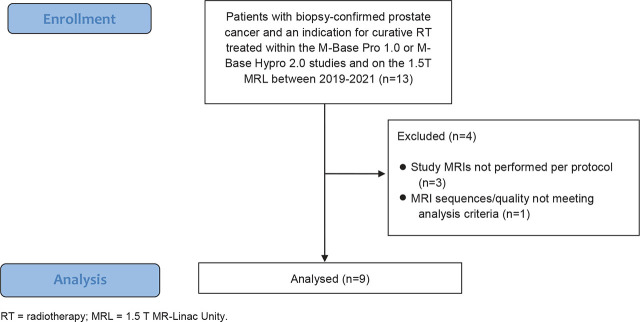
A total of nine patients were included. Figure 1 delineates the the inclusion/exclusion process.
FIGURE 1.
Flow diagram illustrating the inclusion/exclusion process.
Table 1 summarizes patients’ characteristics. For each patient, the two imaging examinations prior to and during RT were successfully performed and evaluated.
The mean elapsed time between the baseline MRL exam and the MRL exam during radiotherapy was 26 days ± 14 days. The mean time difference between MRI3T and MRL examinations at baseline was 1.7 days ± 1.7 days. Similarly, the mean time difference between MRI3T and MRL examinations during radiotherapy was was 1.7 days ± 1.3 days.
Inter-reader reliability regarding lesion ADC measurement was excellent with ICC > 0.90 both at baseline and during radiotherapy (ICC for MRL at baseline was 0.927 and during radiotherapy was 0.976; ICC for MRI3T at baseline was 0.978 and during radiotherapy was 0.998).
For reader 1, in both systems, there was a statistically significant elevation of lesion ADC during radiotherapy (Figure 2). Mean MRL-ADC at baseline was 0.97 ± 0.18 mm2/s vs. mean MRL-ADC during radiotherapy 1.38 ± 0.3 mm2/s, yielding a mean lesion ADC elevation of 0.41 ± 0.20 mm2/s, p <0.001. Mean MRI3T-ADC at baseline was 0.78 ± 0.165 mm2/s vs. mean MRI3T-ADC during radiotherapy 0.99 ± 0.175 mm2/s, yielding a mean lesion ADC elevation of 0.21 ± 0.96 mm2/s p <0.001.
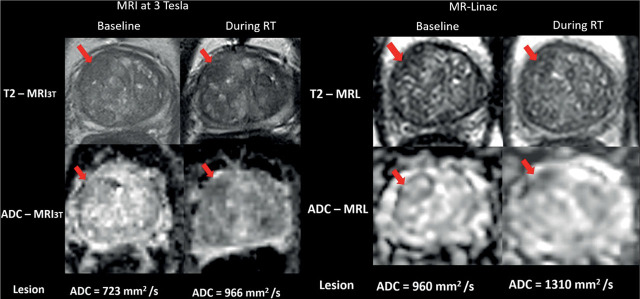
FIGURE 2.
T2-weighted MR images (scan time: 2 minutes) and apparent diffusion coefficient (ADC) maps of a 66-year-old male patient with prostate cancer in the antero-apical region of the transition zone (red arrows) at baseline (left column) and during radiotherapy (right column) as recorded on MR-Linac (MRL) and on standard MRI at 3T (MRI3T). The figure shows similar dynamics of lesion ADC elevation during radiotherapy.
The ADC values at MRL were consistently significantly higher than MRI3T at baseline and during radiotherapy (p < 0.01). However, there was a strong positive correlation between MRL-ADC and MRI3T-ADC at baseline (r = 0.798, p = 0.01) and during radiotherapy (r = 0.863, p = 0.003) (Figure 3).
FIGURE 3.
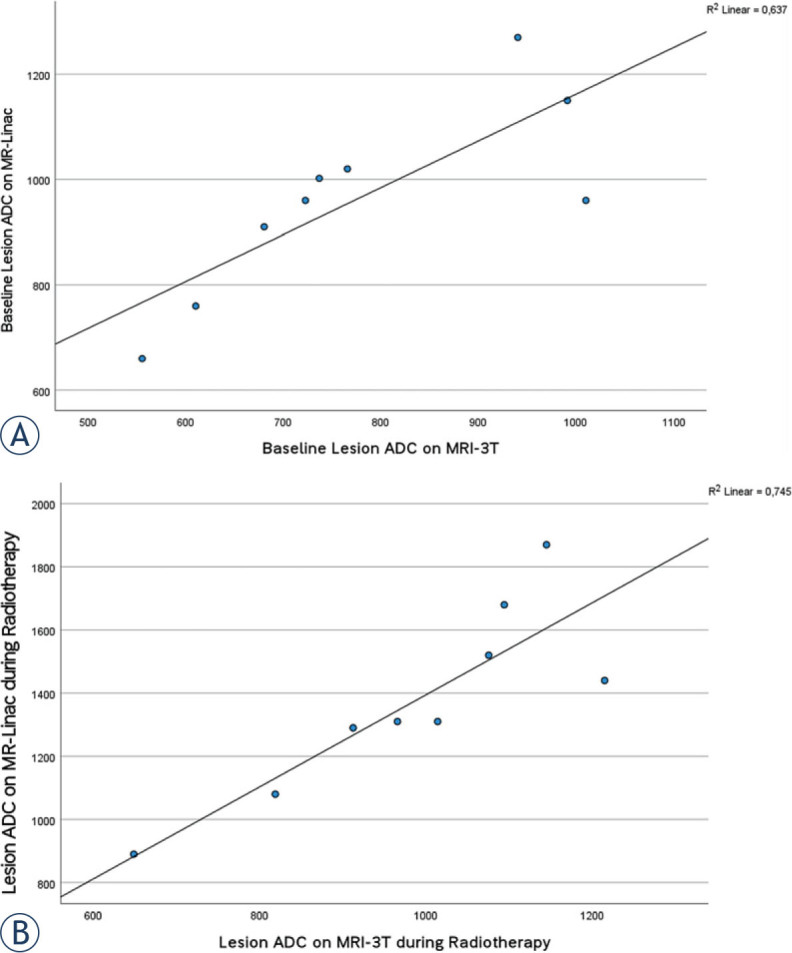
Scatter plots with a fitted line for ADC values as recorded on MR-Linac and MRI3T for reader 1. (A) at baseline, (B) during radiotherapy (week 2). These plots illustrate the strong positive correlation between MRL-ADC and MRI3T-ADC at both time points.
Similarly, for reader 2, in both systems, there was a statistically significant elevation of lesion ADC during radiotherapy (mean MRL-ADC at baseline was 1.0 ± 0.23 × 10−3 mm2/s vs. mean MRLADC during radiotherapy 1.36 ± 0.30 × 10−3 mm2/s, yielding a mean lesion ADC elevation of 0.36 ± 0.17 × 10−3 mm2/s, p <0.001). Mean MRI3T-ADC at baseline was 0.78 ± 0.17 × 10−3 mm2/s vs. mean MRI3TADC during radiotherapy 1.0 ± 0.183 × 10−3 mm2/s, yielding a mean lesion ADC elevation of 0.22 ± 0.129 × 10−3 mm2/s p <0.001).
The ADC values at MRL were consistently significantly higher than MRI3T at baseline and during radiotherapy (p < 0.001). However, there was a strong positive correlation between MRL-ADC and MRI3T-ADC at baseline (r = 0.872, p = 0.002) and during radiotherapy (r = 0.788, p = 0.012).
Discussion
This prospective study compared lesion ADC values in patients with prostate carcinoma undergoing MR-guided radiotherapy on an MRL at 1.5 T to a diagnostic scanner at 3T. Absolute values of lesion ADC measurements differed while dynamics in the context of radiation therapy were comparable between the scanners. In both systems, there was a statistically significant elevation of lesion ADC during radiotherapy with a strong positive correlation of lesion ADC between the scanners.
ADC changes during radiotherapy
ADC changes of the intraprostatic tumor are to be expected both during radiotherapy and during ADT and the correlation between ADC and prostate cancer aggressiveness has been shown before on diagnostic scanners.17,18 The mean ADC-values calculated in this study on both scanners are similar to values found in the literature: Tamada et al. reported mean ADC values of (untreated) tumor regions of 1.02 ± 0.25 × 10−3 mm2/s for the peripheral zone and of 0.94 ± 0.21 × 10−3 mm2/s for the transitional zone of the prostate.13 Van Schie et al. reported median ADC values in the tumor scanned on a diagnostic scanner of 1.08 ± 0.39 × 10−3 mm2/s (mean ± SD) prior to treatment and assessed changes of ADC prior to a hypofractionated RT and then weekly during RT in 73 patients in a similar manner as performed in our study. The group found a (non-significant) median increase of the ADC-value in the tumor of 7% for patients with concurrent ADT and a median increase of 20% for patients without ADT.14
ADC as biomarker for response assessment and role of MRL
Moreover, ADC values and -changes have been shown to function as biomarkers for RT response in prostate cancer patients.6,15 Radiomics approaches seem promising in assessing response to RT, as performed by Abdollahi et al. prior to vs. after RT.16 In all of these studies and in prostate cancer diagnostics, a 3 T MRI scanner has been established as the gold standard for mpMRI.17 MRI3T leads to optimal diagnostic images, often aided by suppression of peristalsis via intravenous application of butyl scopolamine or other agents. In contrast, on a 1.5 T MRL, the utilized sequences are optimized for fast and geometrically accurate image acquisition in an online workflow without routine administration of peristalisis suppressing medications or contrast agents. These possible limitations, in addition to technical differences of the hybrid system to diagnostic scanners18, pose the question whether an MRL can deliver comparable functional information during RT of prostate cancer.
In principle, the hybrid system offers fertile ground for further plan adaption in prostate carcinoma patients based on mpMRT findings such as ADC values since it offers daily MR-guided plan adaptations. Treatment individualization and plan adaptation under RT are a focus of research in other tumor entities as well.7,19,20 Longitudinal diffusion MRI on a 0.35 T hybrid system was already performed in small series for several other tumor entities with promising results (Yang et al.: three head and neck cancer patients and three sarcoma patients21; Shaverdian et al.: three rectal cancer patients22). On the 1.5 T MRL, Lawrence et al. report a high ADC repeatability and comparability to a diagnostic 1.5 T scanner for 59 patients with central nervous system tumors.23 Habrich et al. used a test-retest approach on 11 patients with head and cancers and showed to a high repeatability of ADC measurements on the 1.5 T MRL.24 In the context of prostate carcinoma, Habrich et al. also examined intravoxel incoherent motion (IVIM) and dynamic contrast enhanced (DCE) MRI changes over the course of a moderately hypofractionated RT in 20 patients, also indicating that longitudinal measurements of functional imaging parameters is feasible and could be used for response assessment in the future.25
However, concerning intraprostatic tumor lesions, the verification of longitudinal stability of ADC measurements on an MRL as performed in this study in a comparison to a latest generation 3 T diagnostic scanner has to the best of our knowledge not been performed yet.
In a previous study, we tested the clinical applicability based on qualitative and quantitative parameters of prostate MR images on an MRL against a MRI3T at one point of time prior to starting RT. We were able to show a promising and comparable result of T2 weighted image quality, and lesion conspicuity and we reported comparable lesion ADC measurements between MRL and MRI3T.8
With the current work, we demonstrate longitudinal comparability and reliability between the two system during RT. This represents a necessary basis for future analyses of lesion changes over time on the MRL and confirms its potential for individualized treatment adaptations such as dose painting26 and response assessment during treatment.
This study has limitations. Firstly, the small sample size of patients who underwent multiple prostate imaging at both devices and the fact that only one time-point during radiotherapy was used for analysis. Multiple time-points during the course of radiotherapy should be analyzed to further validate the stability and comparability of ADC measurements. However, logistic challenges hindered further validation with an MRI3T at more than one time point. Secondly, treatment regimens differed in this population (either 20 × 3 Gy oder 39 × 2 Gy, additional neoadjuvant ADT in 3 patients). Thirdly, the DWI acquisition parameters did not fully conform to the published recommendations of the MR-linac consortium, which were published after we had already included the patients in our study and predefinded the technical aspects of the utilized sequences.27
Nonetheless, this study depticts the reality and the challenges of clinical routine and its preliminary findings could be considered novel. Indeed, further prospective studies examining mpMRI data under RT and correlating those with clinical endpoints are desirable to advance individualized radiation treatment.
In conclusion, lesion ADC as measured on MRL increased significantly during radiotherapy and lesion ADC measurements on both systems showed similar dynamics. These preliminary findings are promising but need large-scale validation. Once validated, lesion ADC on MRL might be used as a biomarker for real-time assessment of tumor response in patients with prostate cancer undergoing MR-guided radiation therapy.
Acknowledgements
This research project was partially financed through a research grant of the DFG (German Research Council, Grant MU 4603/1-1 | OT 534/3-1, Package No. 997/1). Arndt-Christian Mueller is a recipient of the IIT program (Mbase Pro 1.0 study) of the Medical Faculty of Tuebingen University (AKF 345-1-0). Haidara Almansour is a scholarship recipient of the junior clinician scientist program of the medical faculty of Tuebingen University (no. 461-0-0). The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Disclosure: The authors' institution operates a Linac Unity (Elekta, Sweden). The Department of Radiation Oncology Tübingen receives financial and technical support by Elekta, Philips, Siemens, Dr. Sennewald Medizintechnik, Kaiku Health, TheraPanacea, PTW and ITV in the context of research cooperation's. The aforementioned entities had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no further conflicts of interest.
References
- 1.Mottet N, van den Bergh RCN, Briers E, van den Broeck T, Cumberbatch MG, de Santis M. et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Pathmanathan AU, McNair HA, Schmidt MA, Brand DH, Delacroix L, Eccles CL. et al. Comparison of prostate delineation on multimodality imaging for MR-guided radiotherapy. Br J Radiol. 2019;92:20180948. doi: 10.1259/bjr.20180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlop A, Mitchell A, Tree A, Barnes H, Bower L, Chick J. et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegener D, Thome A, Paulsen F, Gani C, Boldt J, Butzer S. et al. First experience and prospective evaluation on feasibility and acute toxicity of online adaptive radiotherapy of the prostate bed as salvage treatment in patients with biochemically recurrent prostate cancer on a 1.5 T MR-linac. J Clin Med. 2022;11:4651. doi: 10.3390/jcm11164651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grégoire V, Guckenberger M, Haustermans K, Lagendijk JJW, Ménard C, Pötter R. et al. Image-guidance in radiation therapy for better cure of cancer. Mol Oncol. 2020;14:1470–91. doi: 10.1002/1878-0261.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decker G, Mürtz P, Gieseke J, Träber F, Block W, Sprinkart AM. et al. Intensity-modulated radiotherapy of the prostate: dynamic ADC monitoring by DWI at 3.0T. Radiother Oncol. 2014;113:115–20. doi: 10.1016/j.radonc.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Thorwarth D, Ege M, Nachbar M, Mönnich D, Gani C, Zips D. et al. Quantitative magnetic resonance imaging on hybrid magnetic resonance linear accelerators: Perspective on technical and clinical validation. Phys Imaging Radiat Oncol. 2020;16:69–73. doi: 10.1016/j.phro.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almansour H, Afat S, Fritz V, Schick F, Nachbar M, Thorwarth D. et al. Prospective image quality and lesion assessment in the setting of MR-guided radiation therapy of prostate cancer on an MR-linac at 1.5 T: a comparison to a standard 3 T MRI. Cancers. 2021;13:1533. doi: 10.3390/cancers13071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegener D, Zips D, Thorwarth D, Weiß J, Othman AE, Grosse U. et al. Precision of T2 TSE MRI-CT-image fusions based on gold fiducials and repetitive T2 TSE MRI-MRI-fusions for adaptive IGRT of prostate cancer by using phantom and patient data. Acta Oncologica. 2019;58:88–94. doi: 10.1080/0284186X.2018.1518594. [DOI] [PubMed] [Google Scholar]
- 10.Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM. et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–9. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.[Guideline program oncology. Interdisciplinary guideline of quality S3 for early detection, diagnosis and therapy of the various stages of prostate carcinoma.] [German] Deutsche Krebsgesellschaft, D.K., AWMF; 2019. p. 345. Long version 5.1: [Google Scholar]
- 12.Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol. 2012;8:23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamada T, Sone T, Jo Y, Toshimitsu S, Yamashita T, Yamamoto A. et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: Comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008;28:720–6. doi: 10.1002/jmri.21503. [DOI] [PubMed] [Google Scholar]
- 14.van Schie MA, van Houdt PJ, Ghobadi G, Pos FJ, Walraven I, de Boer HCJ. et al. Quantitative MRI changes during weekly ultra-hypofractionated prostate cancer radiotherapy with integrated boost. Front Oncol. 2019;9:1264. doi: 10.3389/fonc.2019.01264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Jiao Y, Zhang Y, Ren X, Li P, Yu Q. et al. Imaging-based individualized response prediction of carbon ion radiotherapy for prostate cancer patients. Cancer Manag Res. 2019;11:9121–31. doi: 10.2147/CMAR.S214020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdollahi H, Mofid B, Shiri I, Razzaghdoust A, Saadipoor A, Mahdavi A. et al. Machine learning-based radiomic models to predict intensity-modulated radiation therapy response, Gleason score and stage in prostate cancer. Radiol Med. 2019;124:555–67. doi: 10.1007/s11547-018-0966-4. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ. et al. PI-RADS prostate imaging – reporting and data system: 2015, version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorwarth D, Low DA. Technical challenges of real-time adaptive MR-guided radiotherapy. Front Oncol. 2021;11:634507. doi: 10.3389/fonc.2021.634507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeke S, Mönnich D, van Timmeren JE, Balermpas P. MR-guided radiotherapy for head and neck cancer: current developments, perspectives, and challenges. Front Oncol. 2021;11:616156. doi: 10.3389/fonc.2021.616156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boldrini L, Intven M, Bassetti M, Valentini V, Gani C. MR-guided radiotherapy for rectal cancer: current perspective on organ preservation. Front Oncol. 2021;11:619852. doi: 10.3389/fonc.2021.619852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Cao M, Sheng K, Gao Y, Chen A, Kamrava M. et al. Longitudinal diffusion MRI for treatment response assessment: preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys. 2016;43:1369–73. doi: 10.1118/1.4942381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaverdian N, Yang Y, Hu P, Hart S, Sheng K, Lamb J. et al. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol. 2017;90:20160739. doi: 10.1259/bjr.20160739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence LSP, Chan RW, Chen H, Keller B, Stewart J, Ruschin M. et al. Accuracy and precision of apparent diffusion coefficient measurements on a 1.5 T MR-Linac in central nervous system tumour patients. Radiother Oncol. 2021;164:155–62. doi: 10.1016/j.radonc.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Habrich J, Boeke S, Nachbar M, Nikolaou K, Schick F, Gani C. et al. Repeatability of diffusion-weighted magnetic resonance imaging in head and neck cancer at a 1.5 T MR-Linac. Radiother Oncol. 2022;174:141–8. doi: 10.1016/j.radonc.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Habrich J, Boeke S, Nachbar M, Nikolaou K, Schick F, Gani C. et al. Longitudinal correlations between intravoxel incoherent motion (IVIM) and dynamic contrast-enhanced (DCE) MRI during radiotherapy in prostate cancer patients. Front Oncol. 2022;12:897130. doi: 10.3389/fonc.2022.897130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Heide UA, Houweling AC, Groenendaal G, Beets-Tan RG, Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging. 2012;30:1216–23. doi: 10.1016/j.mri.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooreman ES, van Houdt PJ, Keesman R, Pos FJ, van Pelt VWJ, Nowee ME. et al. ADC measurements on the Unity MR-linac - A recommendation on behalf of the Elekta Unity MR-linac consortium. Radiother Oncol. 2020;153:106–13. doi: 10.1016/j.radonc.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]