Abstract
Among the cytokines regulating immune cells, interleukin 9 (IL-9) has gained considerable attention for its ability to act on multiple cell types as a regulator of beneficial and pathologic immune responses. Yet, it is still not clearly defined how IL-9 impacts immune responses. IL-9 demonstrates a remarkable degree of tissue-specific functionality and has cellular sources that vary by tissue site and the context of the inflammatory milieu. Here, we provide perspective to summarize the biological activities of IL-9 and highlight cell type-specific roles in the immune pathogenesis of diseases. This perspective will be important in defining the diseases where targeting IL-9 as a therapeutic strategy would be beneficial, and where it has the potential to complicate clinical outcomes.
Introduction to IL-9 responsiveness
The function of interleukin 9 (IL-9) has been a puzzle almost since its discovery. While it was first associated with type 2 immune responses, subsequently identified roles for IL-9 in autoimmunity and tumor immunity have prevented it from being neatly categorized as a component of type 1, type 2, or type 3 (type 17) immunity. Moreover, there are no immune responses yet identified where IL-9 is the predominant cytokine being produced, first suggesting that there are no ‘type 9’ immune responses, and second supporting a paradigm where the outcomes of IL-9 stimulation are impacted by responses to other factors in the environment. While there are many beneficial and pathologic immune responses where IL-9 is critical, the outcome of the response to IL-9 varies with how the responding cell integrates the cytokine milieu.
IL-9, initially named P40, was first identified as a T cell and mast cell growth factor, functions that have now been demonstrated in vivo in multiple models (1). The source of IL-9 was initially associated with T cells, but mast cells, neutrophils, basophils, eosinophils, and innate lymphoid cells can also produce varying amounts of IL-9 (1). Cytokines greatly contribute to regulating IL-9 expression. In vivo approaches using neutralizing antibodies against IL-4, IL-33, and TGFβ effectively diminish IL-9 synthesis, implying a regulatory role in IL-9 production (2–4). In vitro cell culture systems of mouse and human cells confirmed that IL-4, IL-33, and TGFβ directly induce IL-9 production in CD4+ T cells and mast cells (3–7). In addition, IL-9 expression in CD4+ T cells can be further enhanced by IL-1, IL-2, IL-25, or Activin A; while IFNγ or IL-23 can diminish IL-9 expression levels (5, 8–10). Several TNFSF members also induce IL-9 production (OX40, TL1A, GITRL) (11–13). At the molecular level, Il9 gene expression is promoted by transcription factors that include PU.1 (14), IRF4 (15), BATF (16), Smad (7), Gcn5 (17), IRF8 (18), NF-κB (12, 13), ETV5(19), ERG (20), STAT5 (21), STAT6 (17), and other factors (22), acting through several cis-regulatory elements (23–25), Taken together, IL-9 and IL-9-producing cells can be influenced by the cytokine milieu to enhance or reduce IL-9 production by targeting the Il9 gene through a multitude of transcription factors. This is likely important as it is still not clear if the source of IL-9 is a factor in the outcome of the response. It is possible and perhaps likely that the IL-9-producing cell itself secretes other cytokines or expresses other surface molecules that impact the outcome in the responding cell.
IL-9 utilizes a heterodimeric receptor consisting of IL-9Rα and common γ chain to allow for the activation of intracellular signaling cascades. IL-9R is expressed on hematopoietic cells including T cells, B cells, myeloid cells, mast cells, and epithelial cells (26). IL-9Rα expression is variable, with some cells, mast cells for example, expressing abundant surface receptor regardless of activation state, while other cells express receptor in a differentiation-specific manner. IL-9Rα is also expressed on non-hematopoietic cells including airway and intestinal epithelial cells, smooth muscle cells, and keratinocytes (27–29). The lack of γc on non-hematopoietic cells suggests that there could be another receptor complex which would provide a structural basis to further diversify the response to IL-9.
An understanding of the secreting cell, the responding cell, and the context for receiving the IL-9 signal begins to provide the foundation for determining IL-9 function. In this review we will dissect the cell-type specific functions of IL-9 in allergic inflammation, tumor immunity, autoimmunity, and infection. We will further highlight where there are direct effects of IL-9 on a particular cell type with the goal of providing a picture of the cellular landscape from the perspective of IL-9.
Lymphocytes
As noted above, IL-9 was originally identified as a T cell growth factor based on in vitro experiments (30). There are numerous models where IL-9 promotes inflammation or tumor immunity where the effect on T cell expansion can be observed following neutralization of IL-9 (5, 6, 31, 32). In a mouse model of allergic asthma, the absence of IL-9/IL-9R signaling significantly reduced CD4+ T cell numbers, demonstrating that IL-9 can affect T lymphocytes (31, 33). However, despite mouse and human T cells having abundant expression of Il9r/IL9R (6, 29), whether these observations are due to direct effects of IL-9 on T cells is still unclear.
IL-9 also impacts CD4+ T cell responses by altering their cytokine profile and their ability to respond to the environment (Figure 1). CD4+ Th17 cells are key IL-9 responders in a mouse model of experimental autoimmune encephalomyelitis (EAE); however, the exact function of IL-9 in this disease remains controversial. In some studies, mice deficient in IL-9 signaling (Il9−/− and Il9r−/−) were resistant to the induction of EAE, which correlated with reduced Th17 cells, central nervous system (CNS) cellular infiltration, and less production of the inflammatory cytokine IL-17, IFNγ, and TNFα (6, 34, 35). In agreement with this, neutralizing IL-9 antibody effectively suppressed the incidence and severity of EAE (34). Specifically, blockade of IL-9 led to reduced C-C chemokine receptor expression of CCR2, CCR5, and CCR6 which could contribute to the reduced T cell migration in the CNS and suppressed myelin-specific Th1 and Th17 differentiation (5, 6). The ability of IL-9 to promote Th17 development in vitro (5) suggests that the effects in vivo on T cells are indirect and an effect of diminished inflammation. In contrast, the decrease in Treg frequency observed with anti-IL-9 mAb treatment support the role of IL-9 on Tregs described in Elyaman et al (5). Thus, in addition to its role of suppressing Th1 and Th17 induction, blockade of IL-9 can reduce the suppressive capacity of Tregs thereby allowing for T cell proliferation and effector function to support EAE induction (5). Under certain conditions, IL-9 may play contrasting roles in disease development that may be due to cell type-specific effects. Moreover, studies utilizing different methodologies such as monoclonal or polyclonal antibodies, dosage of rIL-9 or anti-IL-9 antibodies, or varying timing and routes of administration could lead to differences in experimental outcomes. These studies highlight the importance of understanding the regulation of IL-9 signaling in development of inflammatory diseases. Accordingly, studies are still needed to investigate the direct effects that IL-9 exerts on CD4+ T cell subsets.
Figure 1:
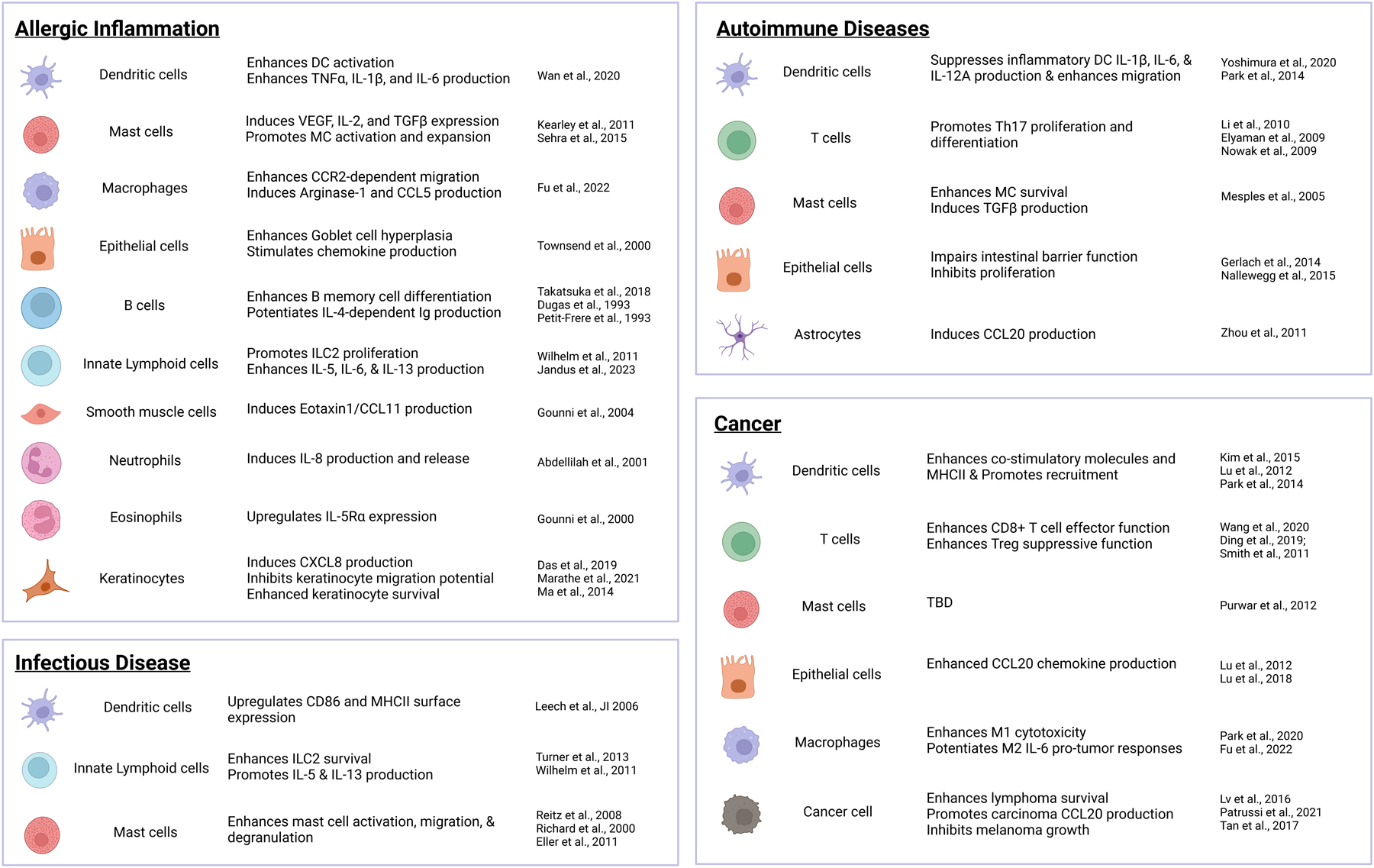
Cell-type specific functions of IL-9 in inflammatory diseases. Responses of the indicated cell types to IL-9 are divided by type of immune response. Citations for each cell-type response is indicated at right.
The effects of IL-9 on CD8+ T cells have been best characterized on cytotoxic CD8+ T cells (CTL) in anti-tumor responses against breast cancer, colorectal cancer, and melanoma. IL-9 can act as an immunostimulatory cytokine to modulate CTL numbers and cytolytic functions. Notably, IL-9R expression on CD8+ T cells correlates with lower expression of the exhaustion markers PD-1 and TIM-3 when examined ex vivo in breast cancer patients and healthy controls, supporting the role for IL-9 in enhancing CTL antitumor responses (36). Moreover, exogenous IL-9 can increase expression of IL-9R in CD8+ T cells and changes the balance of cytokine production, lowering IFNγ and increasing IL-2 and IL-17 (36). This effect may have an autocrine function to promote proliferation of CD8+ T cells. Indeed, CD8+ T cells isolated from patient colorectal cancer tissues can be expanded in the presence of IL-9 (37). The overall decrease in inhibitory receptors may suggest that these CD8+ T cells are less susceptible to immune suppressive mechanisms in tumor microenvironment, thus allowing for greater effector function by enhancing cytokine expression. In vivo IL-9 blockade promoted melanoma progression in a murine melanoma model; moreover, in vitro IL-9 blockade in human PBMCs led to diminished cytolytic molecules, granzyme B and perforin, in CD8+ T cells (38). This is consistent with a study using a glucocorticoid-induced tumor necrosis factor receptor-related protein agonist that can trigger IL-9 expression transiently at the early stages of tumor progression in a murine model of CT26 colon cancer. In this model, they showed that tumor-bearing mice treated with IL-9 neutralizing antibody had reduced granzyme B, TNFα, IFNγ, and CD107a expression in tumor-specific CD8+ T cells (13). These data indicate that IL-9 can contribute to initiating and maintaining antitumor immunity by modulating CD8+ T cell cytotoxicity (39) and this likely contributes to the ability of IL-9-secreting CD8+ T cells to promote highly efficient anti-tumor responses (Figure 1).
IL-9R is expressed in resting and activated germinal center B cells (40). Overexpression of IL-9 can promote B cell responses by enhancing baseline and antigen-specific antibody production of IgG and IgE (40, 41) (Figure 1). IL-9 induces STAT3 and STAT5 activation in B cells to potentiate IgG and IgE production (42) (Figure 1). Consistent with this, in an allergic inflammation model, deficiency in IL-9R reduced CD40-dependent proliferation and plasma cell differentiation of B memory cells thus severely diminishing recall antibody production (43) (Figure 1).
IL-9R is dispensable for type 2 innate lymphoid cell (ILC2) maintenance in naïve mice but required for survival of activated ILC2 in a mouse model of Nippostrongylus brasiliensis lung infection (44). In N. brasiliensis infection, ILC2 accumulation, amphiregulin, IL-5 and IL-13 production is dependent upon IL-9R signaling (44, 45) (Figure 1). IL-9 enhances BCL3-dependent anti-apoptotic protein expression to enhance ILC2 survival (44, 46). Patients with mastocytosis have fewer IL-9R+ ILC2 which correlated with lower plasma IL-9, higher IgE levels, and decreased circulating Tregs (47) (Figure 1). CD25+ ILC stimulated with IL-9 exhibited enhanced IL-5, IL-6, and IL-13 production compared to unstimulated controls, and in parallel neutralizing IL-9 antibodies led to diminished cytokine production but did not impact ILC number (45) (Figure 1). Furthermore, in the lung tissues of house dust mite (HDM)-challenged mice, anti-IL-9 neutralizing antibodies led to diminished ILC2 numbers that are likely similar to the reduced ILC2 proliferation also observed in Il9−/− mice, and changes in gene expression of cytokines and other effectors (31, 33). Together, reports suggest IL-9 directly impacts ILC expansion and effector function.
IL-9R is expressed in a number of lymphoma cell lines: diffuse large-B cell lymphoma, mantle cell lymphoma, and human acute T cell leukemia Jurkat cell lines (48). In vitro studies showed that IL-9 directly induced proliferation and inhibited apoptosis in lymphoma cell lines (48, 49). In support of this, in vivo studies demonstrate that blockade of IL-9 decreases lymphoma cell proliferation (50, 51) (Figure 1).
Mast cells
Initial studies characterizing IL-9 function identified mast cells as one of the main targets (52). IL-9R+ mucosal (mouse) or tryptase-expressing (human) mast cells account for most IL-9 responsive mast cells within murine or human tissue at homeostasis. Mast cell phenotypes are likely influenced by signals from the tissue microenvironment, with IL-9 being highly expressed in mucosal tissues.
In murine models of allergic airway inflammation, blockade of IL-9 led to diminished lung mast cell numbers and impaired mast cell activation and decreased expression of the profibrotic mediators, TGFβ, VEGF, and FGF2 (53, 54). Similarly, blockade of IL-9 in a mouse model of renal disease, nephrotoxic serum nephritis, effectively reduced mast cell numbers in the kidney-draining lymph nodes leading to increased disease (55). These findings demonstrate that the effects of IL-9 on mast cells are tissue-specific and is strictly regulated in various diseases.
Subsequent work in mice deficient for either IL-9 or IL-9Rα showed defective expansion mast cells in the bone marrow, peritoneal cavity, lung, and intestines in response to infection or following the induction of allergic airway inflammation (23, 56–58). As previously noted, IL-9 has been shown to exert both helpful and harmful effects, depending on the context. In situations where mast cells are helpful, such as in host defense against infection, deficiency in IL-9 can be detrimental to clearance of infection. This is observed in a mouse model of a parasitic infection with Strongyloides ratti (S. ratti) where IL-9R-deficient mice exhibited decreased mast cell activation and degranulation which correlated with greater parasite burden (59) (Figure 1). When IL-9 is overexpressed using IL-9-transgenic mice or treatment with rIL-9, mice have enhanced mucosal mast cell responses that promote worm expulsion (60, 61). However, other studies have found that IL-9 can exacerbate mast cell-dependent allergic inflammation. In models of allergic diseases, mice deficient in IL-9 signaling had fewer mast cell numbers and mast cell protease (MCPT1) expression (62–64). Using a food allergy or allergic airway inflammation model, mice deficient in IL-9 signaling were protected against antigen-induced systemic anaphylaxis and airway hyperresponsiveness, demonstrating that IL-9 can contribute to outcomes of allergic disease by promoting mast cell expansion and enhancing mast cell responses (62–64) (Figure 1). Since IL-9 is highly expressed in mucosal sites and IL-9 responsive mast cells are poised in mucosal tissues, it is likely that the microbiome can contribute to the development of inflammatory diseases by acting through mast cells (65–67). It is still not clear how the IL-9/mast cell axis impacts local microbiome.
In vitro evidence suggests that IL-9 has direct effects on mast cells. These studies revealed that IL-9 can induce VEGF secretion in human mast cells (68), and IL-2 and TGFβ1 expression in murine mast cells (56, 69) (Figure 1). Since these secreted factors could have pro-inflammatory downstream effects, it is possible that IL-9 can also indirectly contribute to exacerbating inflammation. Although the role of mast cells in tumor immunity has been extensively studied, exactly how IL-9 modulates mast cell anti-tumor responses remains to be clearly defined.
Antigen Presenting Cells (Monocytes, Macrophages, and Dendritic cells)
Myeloid cells other than mast cells have not been classically thought of as important IL-9 responding cells. Yet, IL-9R is easily detected in monocytes, macrophages, bone marrow-derived dendritic cells (BMDC), myeloid dendritic cells, and plasmacytoid dendritic cells (70, 71). Notably, in the CNS, cells committed to resolve inflammation such as nonclassical monocytes and plasmacytoid dendritic cells express lower levels of IL-9R compared to other monocytes or myeloid dendritic cells. The ability of IL-9 to promote inflammation by altering myeloid cell function is likely linked to environmental impacts on IL-9R expression.
There are numerous studies where IL-9 can have direct effects on isolated myeloid cells in vitro (70–72). For instance, human macrophages stimulated with IL-9 express reduced inflammatory markers CD45, CD68, CD14, and CD11b, suggesting that IL-9 can regulate macrophage migration and phagocytic activity (70) (Figure 1). Similarly, LPS-stimulated monocytes and alveolar macrophages stimulated with IL-9 obtained an anti-inflammatory phenotype with enhanced TGFβ production and diminished TNF-α and IL-10 release (71, 72) (Figure 1). This anti-inflammatory function of IL-9 on monocytes and macrophages supports its relevance as a therapeutic target to dampen severe inflammation, specially observed in autoimmune diseases such as multiple sclerosis.
Many of these in vitro responses can be recapitulated in vivo when IL-9 activity is blocked. In a mouse model of EAE, blockade of IL-9 using anti-IL-9 neutralizing antibodies attenuated EAE inflammation by decreasing IL-6 producing macrophage in the CNS and regional lymph node (6). Similarly, neutralizing anti-IL-9 antibodies inhibited the production of reactive oxygen intermediated in activated human blood monocytes and alveolar macrophages, consequently inhibiting oxidative burst responses which may be required for regulating lung tissue injury (71–73). In cancer, blockade of IL-9 downregulated co-stimulatory and MHCII molecules and decreased DC cross-presentation capacity thereby diminishing tumor-specific CTL responses (13, 74) (Figure 1).
In the absence of an IL-9R conditional allele, some of the best experiments to define cell type specific effects have used adoptive transfer to interrogate function. As we’ve described earlier, IL-9 likely regulates neuroinflammation by dampening inflammatory myeloid cells. Indeed, adoptive transfer of Il9r−/− dendritic cells to wild type (WT) mice led to exacerbated autoimmune neuroinflammation disease development that correlated with increased GM-CSF-producing T cells in the CNS (75) (Figure 1). In contrast, in a chronic model of airway inflammation, IL-9R deficiency in Il9r−/− mice protects against allergic inflammation, suggesting that IL-9 promotes pro-inflammatory functions in lung macrophages. Adoptive transfer of interstitial macrophages into Il9r−/− mice led to greater inflammation observed with higher lung cell number and eosinophil infiltration into the allergic lung (76). Moreover, the transfer of wild type macrophages to Il9r−/− mice was able to rescue the development of airway inflammation that was lost in the absence of IL-9 signaling, further supporting the pro-inflammatory role of IL-9 on macrophages (76). IL-9 also impacted the ability of monocytes to be recruited to the lung (76). Among IL-9 regulated genes, arginase 1 (ARG1) in macrophages was critical for IL-9-mediated inflammation (76) (Figure 1). Parallel observations were made in a lung cancer model where IL-9 signaling in macrophages promoted the development of tumors in the lung (77). This was in contrast to a B16 lung cancer model where the tumor was engineered to overexpress IL-9, resulting in increased CD80 and CD86 expression in lung, spleen, and peritoneal-derived macrophages, driving M1 macrophage polarization which enhances anti-tumor cytotoxicity (78) (Figure 1). These disparate effects may be due to gating of differing populations of macrophages or ectopic effects of IL-9 in the tissue microenvironment. Nonetheless, these results highlight the importance of the amount of IL-9 present in the milieu and as noted earlier, the source of IL-9 may impact the outcome of an IL-9 response.
Granulocytes (Neutrophils and Eosinophils)
At steady-state, neutrophils do not respond to IL-9 as they lack surface expression of IL-9Rα (79). Notably, IL-9Rα was detected intracellularly but was not expressed at the surface-level in neutrophils from healthy patients (80). However, during an inflammatory response, the expression of IL-9Rα was variable, but detected in human BAL-derived and PBMC-derived neutrophils, demonstrating that expression of IL-9Rα can be induced and can be regulated by the tissue microenvironment (80). Although the precise mechanism regulating IL-9Rα expression on neutrophils remains to be explored, IL-9 neutralizing antibodies can reduce blood neutrophil cell numbers in a mouse model of allergic airway inflammation in Balb/C mice (81). Functionally, IL-9 can induce the production and release of IL-8 in neutrophils, which may function to promote recruitment of other inflammatory cells (80) (Figure 1).
IL-9 is a known enhancer of IL-5-driven airway eosinophilia by promoting eosinophil precursor maturation in the bone marrow (82). IL-9Rα on human eosinophils was detected at the transcriptional and protein level (83, 84). Functionally, IL-9 can enhance IL-5Rα expression thereby enhancing eosinophil development and can inhibit eosinophil apoptosis in a dose-dependent manner (83, 84) (Figure 1). During an acute model of allergic airway inflammation, administering one dose of neutralizing monoclonal anti-IL-9 antibody reduced bone marrow mature eosinophils, but was unable to attenuate the development of eosinophilia in the lung tissue, suggesting that the effects of IL-9 can be tissue-specific and may likely be regulated by IL-9R expression (81). In support of this, Il9−/− mice exhibited reduced HDM-induced eosinophil infiltration into the airways compared to WT control mice (33). It is still unclear if the effects observed in vivo on eosinophils are direct or indirect.
Non-hematopoietic cells (Epithelial cells, Smooth muscle cells, Keratinocytes, Tumor cells, Glial cells, and Hepatocytes)
Although it has not been well characterized, IL-9 can also act on airway epithelial cells and smooth muscle cells (85–87). Similar to other cell types, IL-9R is not typically expressed on epithelial cells, yet expression of IL-9R can be increased during an inflammatory response (88). Intratracheal administration of IL-9 increased mucus production in the airways shown by Periodic acid–Schiff (PAS)-stained epithelial cells as well as expression of mucin-related genes, MUC2 and MUC5AC (87) (Figure 1). In an allergic model, IL-9 can stimulate airway epithelial and smooth muscle cell chemokine production: CCL2, CCL3, CCL7, CCL11/Eotaxin, and CCL12, which can allow for cellular infiltration of other immune cells into inflamed tissues (85, 89, 90) (Figure 1). Moreover, IL-9 can also regulate intestinal barrier function by inducing claudin-2, a transmembrane protein important for regulating barrier permeability, in intestinal epithelial cells treated with rIL-9 in vitro (88) (Figure 1). Subsequent research further exploring the role of IL-9 in vivo expanded upon IL-9-dependent and IL-9-independent effects on epithelial cells. In particular, IL-9 can regulate mucus production that is independent of IL-5 and IL-13. Moreover, IL-9-deficient mice (Il9−/−) are unable to increase goblet cell production despite normal IL-13 expression, indicating that IL-9 can promote goblet cell metaplasia independent of IL-13 (91). This is consistent with research utilizing neutralizing antibodies in which antibody blockade of IL-9, but not IL-5 or IL-13, inhibited mucus production in an allergic airway inflammation model in dogs (86) (Figure 1). Thus, IL-9 can regulate airway epithelial cells and smooth muscle cells; however, how IL-9 signals downstream of IL-9R requires further exploration. In addition, the role of IL-9 in epithelial cells and smooth muscle cells at different tissues such as in the trachea, remains to be explored.
IL9R expression is constitutively low in human keratinocytes, but it can be induced during type 2 inflammatory conditions (92, 93). This is best depicted in individuals with atopic dermatitis expressing increased IL-9R expression on epidermal keratinocytes compared to healthy controls (93). These findings demonstrate that keratinocytes are IL-9 responsive and suggest that IL-9 can contribute to allergic skin inflammation. Several studies have utilized in vitro culture assays and IL-9 neutralizing antibodies to show that IL-9 can regulate keratinocyte expansion, cytokine production, and migration (92–96) (Figure 1). Stimulation of human primary keratinocytes (HPK) with IL-9 enhanced survival by reducing reactive oxygen species-mediated apoptosis, whereas in another report IL-9 induced Ki67 expression to promote HPK proliferation (92, 97) (Figure 1). Furthermore, IL-9 can promote HPK cytokine secretion as observed in vitro where IL-9-stimulated HPK exhibited increased IL-8 (CXCL8) production, and vascular endothelial growth factor secretion (93–95) (Figure 1). One recent study has also demonstrated that treatment with rIL-9 can inhibit IFNγ and IL-17A-induced HPK migratory potential by regulating the actomyosin cytoskeleton (96) (Figure 1).
IL-9 acts on neurons to enhance murine newborn neocortex neural survival by inhibiting expression of Bax, a pro-apoptotic molecule (98). IL-9 also induced TGFβ1 expression in cultured neurons that exacerbates the brain lesion development observed in infants with cerebral palsy (69). IL-9R is constitutively expressed on astrocytes (99), and treatment with IL-9 induces CCL20 production which can enhance Th17 migration and contribute to EAE induction (99) (Figure 1). Nonetheless, although other neural cells express IL-9R, the contribution of IL-9 signaling to neural cell-mediated pathology is still unclear (99).
Of the diseases mentioned above, there is still a need to define the exact mechanism of the role of IL-9 in diseases in which IL-9 may intensify or attenuate inflammatory responses. In the case of systemic lupus erythematosus, patients exhibited significantly higher mRNA and protein levels of IL-9 compared to healthy controls, suggesting that IL-9 is associated with promoting autoimmune inflammation (100). Yet, in a mouse model of ethanol-induced alcoholic liver injury (ALI), treatment with IL-9 can relieve the injury and reduce expression of inflammatory markers, IL-6 and TNFα, indicating that IL-9 may have an anti-inflammatory role in ALI (101). However, IL-9 may also be regulated at distinct phases of disease pathogenesis. This is evident in plasma samples from a patient population with acute or chronic stage of HIV infection. Notably, levels of IL-9 were significantly reduced with the progression of HIV-1 infection (102).
IL-9 as a therapeutic target
Blockade of IL-9 has been shown to be very effective in pre-clinical models. In murine models of intermittent allergic asthma, anti-IL-9 treatment provided strong protection against airway reactivity in a recall response (31). Anti-IL-9 is also effective in models of chronic allergen challenge (103, 104). There is also support for IL-9 being central to the pathology associated with the allergic asthmatic phenotype in patients (105). Anti-IL-9 biologics for the treatment of allergic asthma have been shown to be effective at improving asthma symptom scores in individuals with mild-to-moderate asthma; however, there were mixed results in its efficacy in the greater asthmatic population (106, 107). When MEDI-528, a humanized mAb against IL-9, was administered to patients with mild-to-moderate asthma, they observed a trend towards improved asthma symptom scores and reduced asthma exacerbation rates, while in their phase II study with patients with moderate-to-severe asthma did not experience improved clinical activities or quality of life (106, 107). However, these studies were limited by short periods of treatment, and that allergic vs. non-allergic asthma patients were not considered separately. It is likely that asthmatic patients should be assessed for IL-9 levels in the bloodstream prior to anti-IL-9 treatment to specifically target individuals who have IL-9-driven asthmatic responses. Indeed, this may identify specific endotypes of asthma where IL-9-targeted therapies might be most effective.
Modifying IL-9 production and responses has taken a number of approaches. Retinoic acid was used to antagonize Th9-derived IL-9 and effectively reduce allergic airway inflammation (24). An IL-9R inhibitor (rhIL-9-ETA fusion toxin) effectively targets malignant cells, including Hodgkin’s lymphoma, non-Hodgkin lymphoma and acute myeloid leukemia (108). IL-9Rα neutralizing antibodies were used to treat leukemia cells from several patients, demonstrated effective inhibition of leukemia cell proliferation (109). Bioniz therapeutics developed an antagonist to IL-2, IL-9, and IL-15 (BNZ132-1 now referred to as BNZ-1), while not affecting IL-4, IL-7, or IL-21 signaling (110, 111). However, inhibition of IL-9 was achieved at concentrations 10 times higher than those that resulted in inhibition of IL-2 and IL-15 (110). Whether immunotherapies involving targeting IL-9 signaling through neutralizing IL-9 or IL-9R inhibitors can be translated into the clinic requires further testing.
In contrast to blocking IL-9, adoptive cell transfer (ACT) of IL-9-producing cells has shown promise for treatment of some tumors (9, 112–115). IL-9-producing CD4+ Th9 and CD8+ Tc9 cells have shown great efficacy in pre-clinical models for cancer immunotherapies. Remarkably, tumor-specific Th9 cells present a less exhaustive and long-lived effector phenotype in comparison to antitumor effector Th1 and Th17 cells (113, 114). These studies indicate that ACT of IL-9-producing T cells can retain a strong antigen-specific antitumor effect, and this effect is clearly observed in a preclinical tumor model in which Th9 transfer was effective against melanoma (116). In melanoma patients, immune checkpoint blockade treatments targeting CTLA-4 or PD-1 using ipilimumab or nivolumab, respectively, were more effective in individuals that had elevated levels of IL-9 or IL-9-producing T cells (38, 117). Together, ACT of IL-9-producing cells presents a promising therapeutic approach for cancer treatment. Moreover, cytokines in the tumor microenvironment may also contribute to IL-9/Th9/Tc9 efficacy. Notably, IL-33 can enhance IL-9 production in murine Th9/Tc9 to enhance its antitumor activity by maintaining a central memory phenotype and upregulating cytolytic molecules, perforin and granzyme B (118). Immunotherapy with CD4+ and CD8+ chimeric antigen receptor (CAR) T cells polarized under Th9-culture conditions have enhanced antitumor activity against hematological malignancies (113, 119). This strategy is IL-9-dependent, as blockade of IL-9 after CAR Tc9 transfer attenuated anti-tumor activity (113). Nevertheless, the use of ACT/CAR T cell therapies in anticancer therapy is not without limitations, including how pre-therapy IL-9 levels and IL-9 signaling on other immune cells may impact responses to ACT/CAR T therapy. Thus, further investigation on the safety and efficacy of this approach is needed prior to clinical application.
IL-9 remains an attractive target in multiple pathologic conditions. Greater efforts in precision therapy will likely be necessary to identify the patients that will benefit most from agents that block IL-9 signaling or from adoptive cell therapies that provide IL-9 in the microenvironment to benefit immunity.
Conclusions
The IL-9/IL-9R pathway has the potential to exert both pro-inflammatory and anti-inflammatory cell type-specific effects that contribute to its dual roles in disease development (Figure 1). Despite significant therapeutic advances over the last decade, the paucity of direct evidence from animal and clinical studies for the role of IL-9 in specific biological responses remains a challenge. The strongest evidence for the direct actions of IL-9 on cells highlights mast cells, innate lymphoid cells, and macrophages as mediators of disease outcomes. This is assuredly not an exhaustive list and as more detailed studies are performed, the effects of IL-9 on specific cell types and their contributions to inflammation and immunity. Better understanding of IL-9 signaling pathways and what distinguishes cell-type specific effects will provide a more complete view on how IL-9 exerts its pro-inflammatory or anti-inflammatory function in each physiological setting.
Acknowledgments
This work was supported by Public Health Service grants from the National Institutes of Health (R01 AI057459 and AI129241 to M.H.K.). A.P. was supported by the National Institutes of Health grant T32 AI060519.
Abbreviations
- ACT
adoptive cell transfer
- BMDC
bone marrow-derived dendritic cells
- CAR
chimeric antigen receptor
- CCR
C-C chemokine receptor
- CTL
cytotoxic T lymphocyte
- EAE
experimental autoimmune encephalomyelitis
- IFNγ
Interferon gamma
- IL
Interleukin
- ILC
innate lymphoid cell
- Th
T helper
- TNFα
Tumor necrosis factor alpha
- HDM
house dust mite
- HIV
human immunodeficiency virus
- HPK
Human primary keratinocyte
- SLE
systemic lupus encephalitis
- WT
wild type
References
- 1.Goswami R, and Kaplan MH. 2011. A brief history of IL-9. J. Immunol 186: 3283–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessner A, Blum H, and Röllinghoff M. 1993. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology 189: 419–435. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Uyttenhove C, Van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, and Stockinger B. 2008. Transforming growth factor-β’reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol 9: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 4.Blom L, Poulsen BC, Jensen BM, Hansen A, and Poulsen LK. 2011. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PloS one 6: e21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, Van Snick J, Renauld J-C, and Khoury SJ. 2009. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. U.S.A 106: 12885–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, and Noelle RJ. 2009. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med 206: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomar S, Ganesan V, Sharma A, Zeng C, Waggoner L, Smith A, Kim CH, Licona-Limón P, Reinhardt RL, Flavell RA, Wang Y-H, and Hogan SP. 2021. IL-4–BATF signaling directly modulates IL-9 producing mucosal mast cell (MMC9) function in experimental food allergy. J Allergy Clin Immunol 147: 280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, and Rüde E. 1994. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J. Immunol 153: 3989–3996. [PubMed] [Google Scholar]
- 9.Végran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rébé C, Dérangère V, Ryffel B, Kato M, Prévost-Blondel A, Ghiringhelli F, and Apetoh L. 2014. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol 15: 758–766. [DOI] [PubMed] [Google Scholar]
- 10.Jones CP, Gregory LG, Causton B, Campbell GA, and Lloyd CM. 2012. Activin A and TGF-beta promote T(H)9 cell-mediated pulmonary allergic pathology. J Allergy Clin Immunol 129: 1000–1010.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, Yang X-P, Cruz AC, Penumetcha P, Hayes ET, Pelletier M, Gabay O, Walsh M, Ferdinand JR, Keane-Myers A, Choi Y, O’Shea JJ, Al-Shamkhani A, Kaplan MH, Gery I, Siegel RM, and Meylan F. 2015. The TNF-Family Ligand TL1A and Its Receptor DR3 Promote T Cell–Mediated Allergic Immunopathology by Enhancing Differentiation and Pathogenicity of IL-9–Producing T Cells. J. Immunol 194: 3567–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky EJ, Fu Y-X, Choi Y, Walsh MC, and Li XC. 2012. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat. Immunol 13: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I-K, Kim B-S, Koh C-H, Seok J-W, Park J-S, Shin K-S, Bae E-A, Lee G-E, Jeon H, Cho J, Jung Y, Han D, Kwon BS, Lee H-Y, Chung Y, and Kang C-Y. 2015. Glucocorticoid-induced tumor necrosis factor receptor–related protein co-stimulation facilitates tumor regression by inducing IL-9–producing helper T cells. Nat. Med 21: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 14.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, and Kaplan MH. 2010. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 11: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, and Bopp T. 2010. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33: 192–202. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Wang J, Panangipalli G, Ulrich BJ, Koh B, Xu C, Kharwadkar R, Chu X, Wang Y, Gao H, Wu W, Sun J, Tepper RS, Zhou B, Janga SC, Yang K, and Kaplan MH. 2020. STAT5 promotes accessibility and is required for BATF-mediated plasticity at the Il9 locus. Nat. Commun 11: 4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami R, and Kaplan MH. 2012. Gcn5 is required for PU. 1-dependent IL-9 induction in Th9 cells. J. Immunol 189: 3026–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamiya T, Ichiyama K, Kotani H, Fukaya T, Sekiya T, Shichita T, Honma K, Yui K, Matsuyama T, Nakao T, Fukuyama S, Inoue H, Nomura M, and Yoshimura A. 2013. Smad2/3 and IRF4 play a cooperative role in IL-9–producing T cell induction. J. Immunol 191: 2360–2371. [DOI] [PubMed] [Google Scholar]
- 19.Koh B, Hufford MM, Pham D, Olson MR, Wu T, Jabeen R, Sun X, and Kaplan MH. 2016. The ETS family transcription factors Etv5 and PU. 1 function in parallel to promote Th9 cell development. J. Immunol 197: 2465–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharwadkar R, Ulrich BJ, Chu M, Koh B, Hufford MM, Fu Y, Birdsey GM, Porse BT, Randi AM, and Kaplan MH. 2023. ERG Functionally Overlaps with Other Ets Proteins in Promoting TH9 Cell Expression of Il9 during Allergic Lung Inflammation. J. Immunol 210: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J-X, Du N, Li P, Kazemian M, Gebregiorgis T, Spolski R, and Leonard WJ. 2017. Critical functions for STAT5 tetramers in the maturation and survival of natural killer cells. Nat. Commun 8: 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan MH 2017. The transcription factor network in Th9 cells. In Seminars in immunopathology. Springer. 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh B, Abdul Qayum A, Srivastava R, Fu Y, Ulrich BJ, Janga SC, and Kaplan MH. 2018. A conserved enhancer regulates Il9 expression in multiple lineages. Nat. Commun 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz DM, Farley TK, Richoz N, Yao C, Shih H-Y, Petermann F, Zhang Y, Sun H-W, Hayes E, Mikami Y, Jiang K, Davis FP, Kanno Y, Milner JD, Siegel RM, Laurence A, Meylan F, and O’Shea JJ. 2019. Retinoic acid receptor alpha represses a Th9 transcriptional and epigenomic program to reduce allergic pathology. Immunity 50: 106–120. e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Fan Y, Li J, Zhang X, Lou X, Dou Y, Shi X, Lan P, Xiao Y, Minze L, and Li XC. 2018. Guidance of super-enhancers in regulation of IL-9 induction and airway inflammation. J. Exp. Med 215: 559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J-O, Lee H, and Kim YS. 2020. Crosstalk between the Producers and Immune Targets of IL-9. Immune Netw. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciccia F, Guggino G, Ferrante A, Cipriani P, Giacomelli R, and Triolo G. 2016. Interleukin-9 and T helper type 9 cells in rheumatic diseases. Clin. Exp. Immunol 185: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Al-Khalili Szigyarto C, Odeberg J, Djureinovic D, Ottosson J, Hober S, Alm T, Edqvist P-H, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, J. M., Hamsten M, von K, Forsberg M, Persson L, Johansson F, Zwahlen M, von G, Nielsen J, and Pontén F. 2015. Tissue-based map of the human proteome. Science 347: 1260419. [DOI] [PubMed] [Google Scholar]
- 29.Human Protein Atlas.
- 30.Uyttenhove C, Simpson RJ, and Van Snick J. 1988. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc. Natl. Acad. Sci. U.S.A 85: 6934–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulrich BJ, Kharwadkar R, Chu M, Pajulas A, Muralidharan C, Koh B, Fu Y, Gao H, Hayes TA, Zhou H-M, Goplen NP, Nelson AS, Liu Y, Linnemann AK, Turner MJ, Licona-Limon P, Flavell RA, Sun J, and Kaplan MH. 2022. Allergic airway recall responses require IL-9 from resident memory CD4+ T cells. Science Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SE, Hoelzinger DB, Dominguez AL, Van Snick J, and Lustgarten J. 2011. Signals through 4–1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol. Immunother 60: 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Lan F, Yang Y, Xu Y, Chen Y, Qin X, Lv Z, Wang W, Ying S, and Zhang L. 2022. The absence of IL-9 reduces allergic airway inflammation by reducing ILC2, Th2 and mast cells in murine model of asthma. BMC Pulm. 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Nourbakhsh B, Ciric B, Zhang G-X, and Rostami A. 2010. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J. Immunol 185: 4095–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Nourbakhsh B, Cullimore M, Zhang GX, and Rostami A. 2011. IL‐9 is important for T‐cell activation and differentiation in autoimmune inflammation of the central nervous system. Eur. J. Immunol 41: 2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding P, Zhu R, Cai B, Zhang J, Bu Q, and Sun D-W. 2019. IL-9-producing CD8+ T cells represent a distinctive subset with different transcriptional characteristics from conventional CD8+ T cells, and partially infiltrate breast tumors. Int. J. Biochem. Cell Biol 115: 105576. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Lu Y, Chen L, Gao T, Yang Q, Zhu C, and Chen Y. 2020. Th9 cells are subjected to PD-1/PD-L1-mediated inhibition and are capable of promoting CD8 T cell expansion through IL-9R in colorectal cancer. Int. Immunopharmacol 78: 106019. [DOI] [PubMed] [Google Scholar]
- 38.Nonomura Y, Otsuka A, Nakashima C, Seidel JA, Kitoh A, Dainichi T, Nakajima S, Sawada Y, Matsushita S, Aoki M, Takenouchi T, Fujimura T, Hatta N, Koreeda S, Fukushima S, Honda T, and Kabashima K. 2016. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology 5: e1248327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan J, Wu Y, Huang L, Tian Y, Ji X, Abdelaziz MH, Cai W, Dineshkumar K, Lei Y, Yao S, Sun C, Su Z, Wang S, and Xu H. 2021. ILC2-derived IL-9 inhibits colorectal cancer progression by activating CD8+ T cells. Cancer Lett. 502: 34–43. [DOI] [PubMed] [Google Scholar]
- 40.Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, and Mazer BD. 2007. Expression of IL-9 receptor α chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 120: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 41.Vink A, Warnier G, Brombacher F, and Renauld J-C. 1999. Interleukin 9–induced in vivo expansion of the B-1 lymphocyte population. J. Exp. Med 189: 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dugas B, Renauld JC, Pène J, Bonnefoy JY, Peti‐Frère C, Braquet P, Bousquet J, Van Snick J, and Mencia‐Huerta JM. 1993. Interleukin‐9 potentiates the interleukin‐4‐induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur. J. Immunol 23: 1687–1692. [DOI] [PubMed] [Google Scholar]
- 43.Takatsuka S, Yamada H, Haniuda K, Saruwatari H, Ichihashi M, Renauld J-C, and Kitamura D. 2018. IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat. Immunol 19: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 44.Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, and Stockinger B. 2013. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med 210: 2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, and Stockinger B. 2011. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 12: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard M. l., Louahed J, Demoulin J-B, and Renauld J-C. 1999. Interleukin-9 regulates NF-κB activity through BCL3 gene induction. Blood 93: 4318–4327. [PubMed] [Google Scholar]
- 47.Jandus P 2023. IL-9R loss in innate lymphoid cell type 2 (ILC2) reflects Treg impairment in mastocytosis patients. J. Investig. Allergol. Clin. Immunol 33. [DOI] [PubMed] [Google Scholar]
- 48.Lv X, Feng L, Ge X, Lu K, and Wang X. 2016. Interleukin-9 promotes cell survival and drug resistance in diffuse large B-cell lymphoma. J. Exp. Clin. Cancer Res 35: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Z-J, Zhou Q, Yin W, Yuan M-L, Yang W-B, Xiong X-Z, Zhang J-C, and Shi H-Z. 2012. Differentiation and immune regulation of IL-9− producing CD4+ T cells in malignant pleural effusion. Am. J. Respir. Crit. Care Med 186: 1168–1179. [DOI] [PubMed] [Google Scholar]
- 50.Qiu L, Lai R, Lin Q, Lau E, Thomazy DM, Calame D, Ford RJ, Kwak LW, Kirken RA, and Amin HM. 2006. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood 108: 2407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavorgna A, Matsuoka M, and Harhaj EW. 2014. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-κB activation and T-cell transformation. PLoS pathog. 10: e1004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renauld JC, Kermouni A, Vink A, Louahed J, and Van Snick J. 1995. Interleukin-9 and its receptor: involvement in mast cell differentiation and T cell oncogenesis. J Leukoc Biol 57: 353–360. [DOI] [PubMed] [Google Scholar]
- 53.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, and Humbles AA. 2011. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am. J. Respir. Crit. Care Med 183: 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, and Kaplan MH. 2015. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol 136: 433–440.e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, and Wolf AM. 2011. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J. Immunol 186: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, Borghi M, Puccetti M, De Zuani M, Pucillo CE, Paolicelli G, Zelante T, Renauld J-C, Bereshchenko O, Sportoletti P, Lucidi V, Chiara MC, Colombo C, Fiscarelli E, Lass-Flörl C, Majo F, Ricciotti G, Ellemunter H, Ratclif L, Nicola VT, Napolioni V, and Romani L 2017. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat. Commun 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, Austen KF, and Gurish MF. 2009. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J. Immunol 183: 5251–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen C-Y, Lee J-B, Liu B, Ohta S, Wang P-Y, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP, and Wang Y-H. 2015. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity 43: 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reitz M, Hartmann W, Rüdiger N, Orinska Z, Brunn M-L, and Breloer M. 2018. Interleukin-9 promotes early mast cell-mediated expulsion of Strongyloides ratti but is dispensable for generation of protective memory. Sci. Rep 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faulkner H, Renauld J-C, Van Snick J, and Grencis R. 1998. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect. Immun 66: 3832–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Licona-Limón P, Henao-Mejia J, Temann AU, Gagliani N, Licona-Limón I, Ishigame H, Hao L, De’Broski RH, and Flavell RA. 2013. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39: 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, and Hogan SP. 2008. IL-9–and mast cell–mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med 205: 897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld J-C, and Hogan SP. 2010. Differential roles for the IL-9/IL-9 receptor α-chain pathway in systemic and oral antigen–induced anaphylaxis. J Allergy Clin Immunol 125: 469–476. e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pajulas A, Fu Y, Cheung CCL, Chu M, Cannon A, Alakhras N, Zhang J, Ulrich BJ, Nelson AS, Zhou B, and Kaplan MH. Interleukin-9 promotes mast cell progenitor proliferation and CCR2-dependent mast cell migration in allergic airway inflammation. [DOI] [PMC free article] [PubMed]
- 65.Afrin LB, and Khoruts A. 2015. Mast cell activation disease and microbiotic interactions. Clin. Ther 37: 941–953. [DOI] [PubMed] [Google Scholar]
- 66.Kasakura K, Takahashi K, Itoh T, Hosono A, Momose Y, Itoh K, Nishiyama C, and Kaminogawa S. 2014. Commensal bacteria directly suppress in vitro degranulation of mast cells in a MyD88-independent manner. Biosci. Biotechnol. Biochem 78: 1669–1676. [DOI] [PubMed] [Google Scholar]
- 67.Forsythe P, Wang B, Khambati I, and Kunze WA. 2012. Systemic effects of ingested Lactobacillus rhamnosus: inhibition of mast cell membrane potassium (IKCa) current and degranulation. PloS one 7: e41234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Vasiadi M, Therianou A, and Theoharides TC. 2012. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PloS one 7: e33271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mesplès B, Fontaine RH, Lelièvre V, Launay J-M, and Gressens P. 2005. Neuronal TGF-β1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice. Neurobiol. Dis 18: 193–205. [DOI] [PubMed] [Google Scholar]
- 70.Donninelli G, Saraf-Sinik I, Mazziotti V, Capone A, Grasso MG, Battistini L, Reynolds R, Magliozzi R, and Volpe E. 2020. Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain. J. Neuroinflammation 17: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pilette C, Ouadrhiri Y, Van Snick J, Renauld J-C, Staquet P, Vaerman J-P, and Sibille Y. 2002. IL-9 inhibits oxidative burst and TNF-α release in lipopolysaccharide-stimulated human monocytes through TGF-β. J. Immunol 168: 4103–4111. [DOI] [PubMed] [Google Scholar]
- 72.Pilette C, Ouadrhiri Y, Van Snick J, Renauld J-C, Staquet P, Vaerman J-P, and Sibille Y. 2002. Oxidative burst in lipopolysaccharide-activated human alveolar macrophages is inhibited by interleukin-9. Eur. Respir. J 20: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 73.Johnson K, and Ward P. 1981. Role of oxygen metabolites in immune complex injury of lung. J. Immunol 126: 2365–2369. [PubMed] [Google Scholar]
- 74.Wan J, Wu Y, Ji X, Huang L, Cai W, Su Z, Wang S, and Xu H. 2020. IL-9 and IL-9-producing cells in tumor immunity. Cell Commun. Signal 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshimura S, Thome R, Konno S, Mari ER, Rasouli J, Hwang D, Boehm A, Li Y, Zhang G-X, Ciric B, and Rostami A. 2020. IL-9 controls central nervous system autoimmunity by suppressing GM-CSF production. J. Immunol 204: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu Y, Wang J, Zhou B, Pajulas A, Gao H, Ramdas B, Koh B, Ulrich BJ, Yang S, Kapur R, Renauld JC, Paczesny S, Liu Y, Tighe RM, Licona-Limón P, Flavell RA, Takatsuka S, Kitamura D, Tepper RS, and Kaplan MH. 2022. An IL-9–pulmonary macrophage axis defines the allergic lung inflammatory environment. Sci. Immunol 7: eabi9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu Y, Pajulas A, Wang J, Zhou B, Cannon A, Cheung CCL, Zhang J, Zhou H, Fisher AJ, Omstead DT, Khan S, Han L, Renauld JC, Paczesny S, Gao H, Liu Y, Yang L, Tighe RM, Licona-Limón P, Flavell RA, Takatsuka S, Kitamura D, Bilgicer B, Sears CR, and Kaplan MH. 2022. Mouse pulmonary interstitial macrophages mediate the pro-tumorigenic effects of IL-9. Nat. Commun 13: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park SM, Lee J-O, Lee H, and Kim YS. 2020. Interleukin-9 inhibits lung metastasis of melanoma through stimulating anti-tumor M1 macrophages. Mol. Cells 43: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Girard D, Boiani N, and Beaulieu AD. 1998. Human neutrophils express the interleukin-15 receptor α chain (IL-15Rα) but not the IL-9Rα component. J. Clin. Immunol 88: 232–240. [DOI] [PubMed] [Google Scholar]
- 80.Abdelilah SG, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides NC, Levitt RC, and Hamid Q. 2001. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J. Immunol 166: 2768–2774. [DOI] [PubMed] [Google Scholar]
- 81.Sitkauskiene B, Rådinger M, Bossios A, Johansson A-K, Sakalauskas R, and Lötvall J. 2005. Airway allergen exposure stimulates bone marrow eosinophilia partly via IL-9. Respir. Res 6: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louahed J, Zhou Y, Maloy WL, Rani PU, Weiss C, Tomer Y, Vink A, Renauld J-C, Van Snick J, and Nicolaides NC. 2001. Interleukin 9 promotes influx and local maturation of eosinophils. Blood 97: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 83.Gounni AS, Gregory B, Nutku E, Aris F, Latifa K, Minshall E, North J, Tavernier J, Levit R, and Nicolaides N. 2000. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood 96: 2163–2171. [PubMed] [Google Scholar]
- 84.Gounni AS, Nutku E, Koussih L, Aris F, Louahed J, Levitt RC, Nicolaides NC, and Hamid Q. 2000. IL-9 expression by human eosinophils: regulation by IL-1β and TNF-α. J Allergy Clin Immunol 106: 460–466. [DOI] [PubMed] [Google Scholar]
- 85.Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, and Shan L. 2004. IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J. Immunol 173: 2771–2779. [DOI] [PubMed] [Google Scholar]
- 86.Longphre M, Li D, Gallup M, Drori E, Ordonez C, Redman T, Wenzel S, Bice D, Fahy J, and Basbaum C. 1999. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Investig 104: 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Louahed J, Toda M, Jen J, Hamid Q, Renauld J-C, Levitt RC, and Nicolaides NC. 2000. Interleukin-9 upregulates mucus expression in the airways. Am. J. Respir. Cell Mol 22: 649–656. [DOI] [PubMed] [Google Scholar]
- 88.Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr H-A, Wirtz S, Vieth M, Waisman A, Rosenbauer F, McKenzie ANJ, Weigmann B, and Neurath M. 2014. TH9 cells that express the transcription factor PU. 1 drive T cell–mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol 15: 676–686. [DOI] [PubMed] [Google Scholar]
- 89.Dong Q, Louahed J, Vink A, Sullivan CD, Messler CJ, Zhou Y, Haczku A, Huaux F, Arras M, Holroyd KJ, Renauld JC, Levit R, and Nicolaides NC. 1999. IL‐9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur. J. Immunol 29: 2130–2139. [DOI] [PubMed] [Google Scholar]
- 90.Baraldo S, Faffe DS, Moore PE, Whitehead T, McKenna M, Silverman ES, Panettieri RA Jr, and Shore SA. 2003. Interleukin-9 influences chemokine release in airway smooth muscle: role of ERK. Am. J. Physiol. Lung Cell Mol 284: L1093–L1102. [DOI] [PubMed] [Google Scholar]
- 91.Temann U-A, Geba GP, Rankin JA, and Flavell RA. 1998. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med 188: 1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niehues H, Smits JP, Rodijk-Olthuis D, Schalkwijk J, and van den Bogaard E. 2017. Keratinocyte proliferation and differentiation on IL-9 stimulation: An explorative in vitro study. [DOI] [PubMed]
- 93.Hong C-H, Chang K-L, Wang H-J, Yu H-S, and Lee C-H. 2015. IL-9 induces IL-8 production via STIM1 activation and ERK phosphorylation in epidermal keratinocytes: A plausible mechanism of IL-9R in atopic dermatitis. J. Dermatol. Sci 78: 206–214. [DOI] [PubMed] [Google Scholar]
- 94.Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, and Yu J. 2014. Possible pathogenic role of T helper type 9 cells and interleukin (IL)‐9 in atopic dermatitis. Clin. Exp. Immunol 175: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, Fishelevich R, Temann A, Licona-Limón P, Girardi M, Flavell RA, and Gaspari AA. 2014. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J. Invest. Dermatol 134: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Das S, Srinivasan S, Srivastava A, Kumar S, Das G, Das S, Dwivedi A, Karulkar A, Makkad K, Bilala R, Gupta A, Sawant A, Nayak C, Tayalia P, and Purwar R. 2019. Differential influence of IL-9 and IL-17 on actin cytoskeleton regulates the migration potential of human keratinocytes. J. Immunol 202: 1949–1961. [DOI] [PubMed] [Google Scholar]
- 97.Marathe S, Dhamija B, Kumar S, Jain N, Ghosh S, Dharikar JP, Srinivasan S, Das S, Sawant A, Desai S, Khan F, Syiemlieh A, Munde M, Nayak C, Gandhi M, Kumar A, Srivastava S, Venkatesh KV, Barthel SR, and Purwar R. 2021. Multiomics analysis and systems biology integration identifies the roles of IL-9 in keratinocyte metabolic reprogramming. J. Invest. Dermatol 141: 1932–1942. [DOI] [PubMed] [Google Scholar]
- 98.Fontaine R, Cases O, Lelievre V, Mesples B, Renauld J-C, Loron G, Degos V, Dournaud P, Baud O, and Gressens P. 2008. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell Death Differ 15: 1542–1552. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, Iwakura Y, Mizuno T, and Suzumura A. 2011. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. J. Immunol 186: 4415–4421. [DOI] [PubMed] [Google Scholar]
- 100.Ouyang H, Shi Y, Liu Z, Feng S, Li L, Su N, Lu Y, and Kong S. 2013. Increased interleukin-9 and CD4+ IL-9+ T cells in patients with systemic lupus erythematosus. Mol. Med. Rep 7: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 101.Meng H, Niu R, You H, Wang L, Feng R, Huang C, and Li J. 2022. Interleukin-9 attenuates inflammatory response and hepatocyte apoptosis in alcoholic liver injury. Life Sci. 288: 120180. [DOI] [PubMed] [Google Scholar]
- 102.Gorenec L, Lepej SZ, Grgic I, Planinic A, Bes JI, Vince A, and Begovac J. 2016. The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb. Pathog 97: 125–130. [DOI] [PubMed] [Google Scholar]
- 103.Kim MS, Cho K-A, Cho YJ, and Woo S-Y. 2013. Effects of interleukin-9 blockade on chronic airway inflammation in murine asthma models. Allergy, Asthma & Immunol Res 5: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Antoniu SA 2010. MEDI-528, an anti-IL-9 humanized antibody for the treatment of asthma. Curr. Opin. Mol. Ther 12: 233–239. [PubMed] [Google Scholar]
- 105.Seumois G, Ramírez-Suástegui C, Schmiedel BJ, Liang S, Peters B, Sette A, and Vijayanand P. 2020. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci. Immunol 5: eaba6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, Robbie GJ, White WI, White B, and Molfino NA. 2011. Safety profile and clinical activity of multiple subcutaneous doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulm. 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, and Molfino NA. 2013. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir. Res 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klimka A, Barth S, Drillich S, Wels W, van Snick J, Renauld J, Tesch H, Bohlen H, Diehl V, and Engert A. 1996. A deletion mutant of Pseudomonas exotoxin-A fused to recombinant human interleukin-9 (rhIL-9-ETA’) shows specific cytotoxicity against IL-9-receptor-expressing cell lines. Cytokine and Mol. Ther 2: 139–146. [PubMed] [Google Scholar]
- 109.Chen J, Petrus M, Bryant BR, Phuc Nguyen V, Stamer M, Goldman CK, Bamford R, Morris JC, Janik JE, and Waldmann TA. 2008. Induction of the IL-9 gene by HTLV-I Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood 111: 5163–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frohna PA, Ratnayake A, Doerr N, Basheer A, Al‐Mawsawi LQ, Kim WJ, Zapata JC, Wu X, Waldmann TA, Azimi N, and Tagaya Y. 2020. Results From a First‐in‐Human Study of BNZ‐1, a Selective Multicytokine Inhibitor Targeting Members of the Common Gamma (γc) Family of Cytokines. J. Clin. Pharmacol 60: 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nata T, Basheer A, Cocchi F, van Besien R, Massoud R, Jacobson S, Azimi N, and Tagaya Y. 2015. Targeting the binding interface on a shared receptor subunit of a cytokine family enables the inhibition of multiple member cytokines with selectable target spectrum. J. Biol. Chem 290: 22338–22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, Yang J, Qian J, and Yi Q. 2012. Th9 cells promote antitumor immune responses in vivo. J. Clin. Investig 122: 4160–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, Qian J, and Yi Q. 2014. Tumor-specific IL-9–producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl. Acad. Sci. U.S.A 111: 2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y, Wang Q, Xue G, Bi E, Ma X, Wang A, Qian J, Dong C, and Yi Q. 2018. Th9 cells represent a unique subset of CD4+ T cells endowed with the ability to eradicate advanced tumors. Cancer Cell 33: 1048–1060. e1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xue G, Zheng N, Fang J, Jin G, Li X, Dotti G, Yi Q, and Lu Y. 2021. Adoptive cell therapy with tumor-specific Th9 cells induces viral mimicry to eliminate antigen-loss-variant tumor cells. Cancer Cell 39: 1610–1622. e1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, and Kupper TS. 2012. Robust tumor immunity to melanoma mediated by interleukin-9–producing T cells. Nat. Med 18: 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forget M-A, Haymaker C, Hess KR, Meng YJ, Creasy C, Karpinets T, Fulbright OJ, Roszik J, Woodman SE, Kim YU, Sakellariou-Thompson D, Bhatta A, Wahl A, Flores E, Thorsen S, Tavera R, Ramachandran R, Gonzalez A, Toth C, Wardell S, Mansaray R, Patel V, Carpio D, Vaughn C, Farinas C, Velasquez P, Hwu W-J, Patel S, Davies M, Diab A, Glitza I, Tawbi H, Wong M, Cain S, Ross M, Lee J, Gershenwald J, Lucci A, Royal R, Cormier J, Wargo J, Radvanyi L, Torres-Cabala C, Beroukhim R, Hwu P, Amaria R, and Bernatchez C. 2018. Prospective Analysis of Adoptive TIL Therapy in Patients with Metastatic Melanoma: Response, Impact of Anti-CTLA4, and Biomarkers to Predict Clinical OutcomeImpact of CTLA4 Checkpoint Blockade on TIL ACT. Clin. Cancer Res 24: 4416–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramadan A, Griesenauer B, Adom D, Kapur R, Hanenberg H, Liu C, Kaplan MH, and Paczesny S. 2017. Specifically differentiated T cell subset promotes tumor immunity over fatal immunity. J. Exp. Med 214: 3577–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L, Bi E, Ma X, Xiong W, Qian J, Ye L, Su P, Wang Q, Xiao L, Yang M, Lu Y, and Yi Q. 2020. Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nat. Commun 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


