Management of infections in the liver transplant recipient starts with identifying the risk factors for potential opportunistic infections during the pretransplant evaluation setting. This involves identifying latent infections, assessing immunity, administering vaccinations, treatment, and control of chronic illness before transplant. This review helps summarize the evidence-based and expert recommendations for the screening, diagnosis, prevention, and treatment of cytomegalovirus (CMV), Epstein Barr virus (EBV), latent tuberculosis (TB), cryptococcus, and HIV in the liver transplant recipient.
CYTOMEGALOVIRUS
CMV is a common opportunistic infection that heavily contributes to the morbidity and mortality of liver transplant recipients.1 CMV infection is defined simply by the presence of CMV replication in tissue, blood, or other bodily fluids regardless of the presence of symptoms. CMV disease, however, is a CMV infection accompanied by clinical signs and symptoms, and further categorized into CMV syndrome manifesting as fever, malaise, leukopenia, or neutropenia and elevated aminotransferases or end-organ CMV disease (gastrointestinal disease, pneumonitis, hepatitis, myocarditis, and encephalitis).2
Risk factors and preventative strategies
Knowledge of CMV IgG serology in the transplant recipient and organ donor is paramount in categorizing the risk of post-transplant CMV disease (Table 1) and guiding prevention strategies.4 In addition, the degree of induction and maintenance of immunosuppressive medications also influences the risk of CMV disease after transplant.5
TABLE 1.
Risk category based on donor/recipient CMV serology
| High risk |
| Donor(+)/ recipient(−) Duration of CMV PPX: 3–6 mo |
| Moderate risk |
| Donor(+)/ recipient(+) Duration of CMV PPX: 3 mo Donor(−)/ recipient(+) Duration of CMV PPX: 3 mo |
| Low risk |
| Donor(−)/recipient(−) |
Abbreviation: CMV, cytomegalovirus; PPX, prophylaxis.
Without antiviral prophylaxis or a preemptive prevention strategy, CMV infection and disease typically occur during the first 3 months after transplant. Valganciclovir and IV ganciclovir are the preferred drugs for prophylaxis and are started in general within the first 10 days after transplant.6 While there are no randomized clinical trials to assess the optimal duration of CMV prophylaxis in the liver transplant recipient, the overall consensus is that the duration of prophylaxis is dependent on the CMV donor and recipient serology (Table 1).4 Preemptive treatment is an alternative CMV prevention strategy, which uses weekly CMV monitoring for 12 weeks post-transplant. Antiviral treatment is initiated for asymptomatic liver transplant recipients with positive CMV PCR to prevent disease.4 There is no widely applicable viral load threshold to guide preemptive therapy, and thresholds may be specific to the risk group.
Treatment
IV ganciclovir and valganciclovir are the first-line agents in the treatment of CMV disease (Figure 1).4 As with most post-transplant infections, cautious reduction in immunosuppression should be considered if feasible. Patients should receive weekly surveillance labs with CMV PCR to assess response to treatment along with a complete blood count and renal function to monitor for drug toxicity. Antiviral dosing should not be adjusted for leukopenia. Patients with CMV disease should receive the full therapeutic dose of antiviral therapy until the virus is at an undetectable level or below a predefined viral load. Although the efficacy is not proven, some centers provide secondary prophylaxis with valganciclovir for 1–3 months after the disease given the risk of recurrence.7
FIGURE 1.
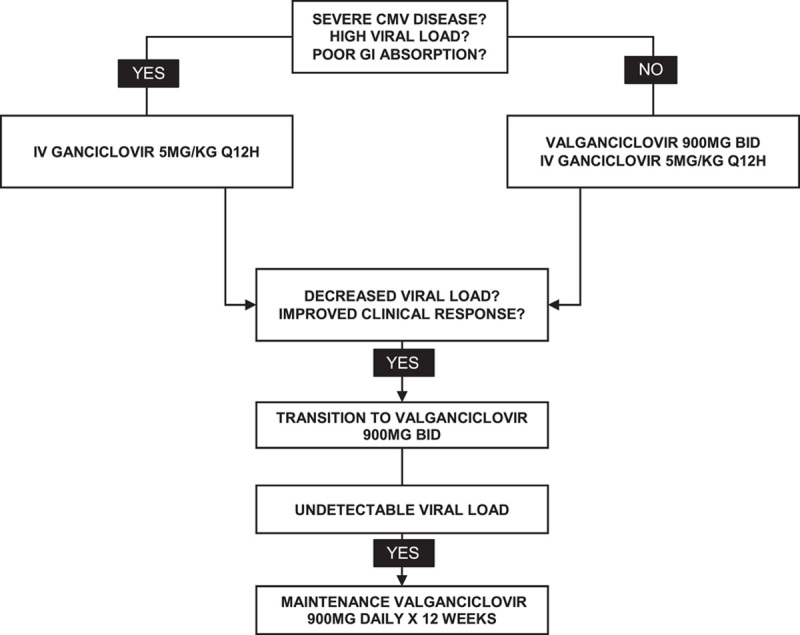
Recommended treatment algorithm for CMV disease. Ganciclovir and valganciclovir need to be renally dosed. Abbreviation: CMV, cytomegalovirus.
TUBERCULOSIS
TB is the second most common cause of death due to infection in the world.8 After inhalation of aerosol droplets of Mycobacterium tuberculosis, there are 4 possible outcomes: (1) immediate clearance, (2) primary disease, (3) latent tuberculosis infection (LTBI), or (4) reactivation of disease years after primary infection. The classic triad of fevers, night sweats, and weight loss may occur less often in transplant recipients. Although both primary and reactivation diseases occur most often in the lungs, ~30% of post-transplant cases are extrapulmonary and 16% are disseminated.9 Primary infection post-transplant can occur through donor-derived transmission or among individuals from endemic regions where there is a greater risk of exposure post-transplant. However, in most cases, active TB disease in the liver transplant recipient occurs through the reactivation of latent TB.
Diagnosis
LTBI is diagnosed by a positive tuberculin skin test or interferon gamma release assay, such as QuantiFERON-TB Gold or T-SPOT in the absence of active clinical disease. Caution should be taken in interpreting results among individuals already on immunosuppression as it can lead to false negatives.10 As a positive tuberculin skin test or interferon gamma release assay cannot distinguish latent TB from active TB, a positive test should be followed with pulmonary imaging.
Treatment
Successful treatment of active TB post-transplant, although possible, is challenging due to potential drug toxicities and major drug-drug interactions with immunosuppression. Accordingly, emphasis is made on the treatment of LTBI in the pretransplant setting. First-line regimens for LTBI include Isoniazid, Rifampin, and combination therapy with Isoniazid and Rifapentine (Table 2).11 As the duration of pretransplant LTBI treatment may cross over into the post-transplant setting, the timing of the administration of therapy requires balancing the risk and benefits on an individualized basis. In general, LTBI is a safe pretransplant but in the cases of an unstable patient, delaying treatment until post-transplant may be the safest option. First-line therapy for active TB post-transplant is nearly the same as that for the immunocompetent host although rifabutin is usually substituted for rifampin to minimize drug interactions with calcineurin inhibitors. The standard 4-drug regimen for active TB is isoniazid, rifampin, pyrazinamide, and ethambutol for the first two months followed by isoniazid and rifampin alone for an additional 4 months (Table 3).11
TABLE 2.
First-line regimens for LTBI
| Regimen | Dosing |
|---|---|
| Isoniazida | 5 mg/kg (maximum, 300 mg) orally for 9 mo |
| Rifampin | 10 mg/kg (maximum, 600 mg) orally for 4 mo |
| Isoniazida and Rifapentine | Isoniazid (orally once weekly for 3 mo; direct observation is preferred): 15 mg/kg, rounded up to the nearest 50 or 100 mg; 900 mg maximum Rifapentine (orally once weekly for 3 mo; direct observation is preferred): 10–14 kg: 300 mg 14.1–25.0 kg: 450 mg 25.1–32.0 kg: 600 mg 32.1–49.9 kg: 750 mg ≥50 kg: 900 mg maximum |
In conjunction with pyridoxine supplementation (25–50 mg daily) to prevent peripheral neuropathy.
Abbreviation: LTBI, latent tuberculosis infection.
TABLE 3.
First-line drugs for the treatment of active TB
| Drug | Common adverse events | Drug interactions |
|---|---|---|
| Isoniazid PO | Hepatotoxicity Neurotoxicity Cytopenias |
Corticosteroids Azole antifungals |
| Rifampin PO | Hepatotoxicity Cytopenias Red-orange body fluids Interstitial nephritis Rash |
Calcineurin inhibitors mTOR inhibitors Mycophenolate Corticosteroids Azole antifungals Atovaquone |
| Rifabutin PO | Same as Rifampin Uveitis |
Similar to Rifampin but less severe Clarithromycin |
| Rifapentine PO | Same as Rifampin Hypersensitivity reactions |
Similar to Rifampin |
| Pyrazinamide PO | Hepatotoxicity Cytopenias Hyperuricemia Interstitial nephritis |
Cyclosporine |
| Ethambutol | Hepatotoxicity Neurotoxicity Cytopenias |
— |
Abbreviation: mTOR, mammalian target of rapamycin; PO, per os; TB, tuberculosis.
EPSTEIN BARR VIRUS
EBV, a widely ubiquitous herpesvirus, plays a major role in the development of B-cell post-transplant lymphoproliferative disorders (PTLD) occurring within the first-year post-transplant. With a reported incidence of 3.8%, PTLD is one of the most devastating complications of liver transplant with 3 and 5-year overall survival rates of 61% and 38% among adult recipients.12
Risk factors and preventative strategies
A predisposing risk factor for the development of PTLD is the serological status of the recipient with the highest risk among EBV mismatched transplant recipients (donor seropositive/recipient seronegative).13 The use of chemoprophylaxis with acyclovir or ganciclovir or immunoprophylaxis with intravenous immunoglobulin among EBV mismatched recipients is controversial and not universally recommended in liver transplant recipients.14 In addition, there is not enough data to prescribe a universal protocol for immunosuppression reduction, switching to an mammalian target of rapamycin (mTOR) inhibitor or the addition of Rituximab in the liver transplant recipient.14
Treatment
Given its complexity, PTLD management and treatment should involve a multidisciplinary team that includes a transplant hepatologist, oncologist, and infectious disease physician. A stepwise strategy starting with the reduction of immunosuppression is the initial approach in PTLD management. In general, antimetabolites (Azathioprine and Mycophenolate) are discontinued, whereas calcineurin inhibitors are reduced by 30%–50%. There is insufficient evidence regarding switching to an mTOR inhibitor. Rituximab monotherapy is the next recommended treatment in individuals with progressive disease followed by cytotoxic chemotherapy.
CRYPTOCOCCOSIS
Cryptococcus, an environmental fungus found in soil, trees, and bird droppings, is the third most common invasive fungal infection in liver transplant recipients.15 Cryptococcus typically occurs within the first-year post-transplant due to reactivation of latent infection although primary infection post-transplant can occur.16 While cryptococcus has a predilection for the lungs and central nervous system, liver transplant recipients are at a sixfold higher risk of developing disseminated disease involving the skin, soft tissue, or bone.16 Clinical manifestations of pulmonary infection can range from asymptomatic infection or a simple pulmonary nodule to severe pneumonia with respiratory failure. Patients with central nervous system infection can present with prolonged headaches, fevers, malaise, altered mental status, or meningitis.
Risk factors and preventative strategies
While donor-derived infections can occur, prescreening donors and recipients has not been shown to be successful in prevention. As there is no specified high risk-group identified, routine antifungal prophylaxis against cryptococcosis is also not recommended.
Treatment
Diagnosis of cryptococcosis is made by isolation of Cryptococcus neoformans or Cryptococcus gatti in fungal culture from a clinical specimen, positive antigen testing, or direct detection of the fungus by India ink staining. In liver transplant recipients with suspected or proven cryptococcosis, a thorough examination of extrapulmonary sites of infection, including lumbar puncture with opening and closing pressures, blood, and urine culture along with other relevant tissue cultures is recommended to guide the choice and duration of antifungal agent (Table 4).16 Serial lumbar punctures are essential to reduce increased intracranial pressure in these patients.
TABLE 4.
Treatment of cryptococcus
| CNS disease disseminated disease severe pulmonary disease | Asymptomatic or mild-moderate pulmonary disease | |
|---|---|---|
| Induction | Liposomal Amphotericin B: 3–4 mg/kg/d Amphotericin B lipid complex: 5 mg/kg/d + Flucytosine 25 mg/kg PO q6h Duration: minimum 2 wk |
— |
| Consolidation | Fluconazole: 400–800 mg/d Duration: 8 wk |
— |
| Maintenance | Fluconazole: 200–400 mg/d Duration: 6–12 mo |
Fluconazole: 400 mg/d Duration: 6–12 mo |
Abbreviation: CNS, central nervous system.
HIV
Liver transplant is the standard of care among patients with end-stage liver disease with well-controlled HIV infection before transplant (Table 5).17 With the approval of the HIV Organ Policy Equity Act in 2013, HIV-infected organs can be transplanted into HIV-infected recipients under specific research criteria. Post-transplant outcomes of HIV-infected recipients are consistent with those of HIV-noninfected recipients.18 Although there is no data to determine the optimal time period of demonstrated control of infection, it is plausible that longer periods of control may potentially decrease the risk of rejection by decreased immune activation of the virus.19
TABLE 5.
Suggested criteria for liver transplantation in HIV-infected patients
| CD4 count >100 cells/μL (without history of OI) |
| CD4 count >200 cells/μL during 3 mo before OLT |
| Undetectable HIV viral load while on HAART |
| Detectable HIV viral load due to intolerance of HAART (HIV can be suppressed after OLT) |
| Compliance with stable HAART |
| Absence of OI |
Abbreviations: HAART, highly active antiretroviral therapy; OI, opportunistic infection.
Post-transplant management
Compared with the HIV-noninfected recipients, rejection rates are nearly 2–3 fold among HIV-infected liver transplant recipients.20 However, the optimal maintenance immunosuppression in this patient population is not well defined. Given the potential drug interactions with highly active antiretroviral therapy and immunosuppressants, careful consideration should be taken with the choice of antiretroviral therapy post-transplant; protease inhibitors are generally avoided. Integrase inhibitors (raltegravir, bictegravir, and dolutegravir) are the favored highly active antiretroviral therapy given the advantage of having no drug interactions and minimal toxicity.
CONCLUSION
Liver transplant recipients are susceptible to a variety of infections due to their immunocompromised state. Providers need to have a high index of suspicion to diagnose infections and initiate treatment early to prevent morbidity and mortality from these diseases. Identifying risk factors, serologic testing, and close consultation with transplant infectious disease physicians are essential in identifying and controlling latent infections before transplantation.
Acknowledgments
CONFLICTS OF INTEREST
The authors have no conflicts to report.
EARN MOC FOR THIS ARTICLE
Footnotes
Abbreviations: CMV, cytomegalovirus; CNS, central nervous system; EBV, Epstein Barr virus; HAART, highly active antiretroviral therapy; LTBI, latent tuberculosis infection; OI, opportunistic infection; PTLD, post-transplant lymphoproliferative disorder; TB, tuberculosis.
Contributor Information
Omobonike Oloruntoba-Sanders, Email: omobonike.oloruntoba@duke.edu.
Sajal D. Tanna, Email: sajal.tanna@inova.org.
REFERENCES
- 1. Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. 2018;78:1085–103. [DOI] [PubMed] [Google Scholar]
- 2. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- 3. Razonable R. Direct and indirect effects of cytomegalovirus: can we prevent them? Enferm Infecc Microbiol Clin. 2010;28:1–5. [DOI] [PubMed] [Google Scholar]
- 4. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13512. [DOI] [PubMed] [Google Scholar]
- 5. Gardiner BJ, Nierenberg NE, Chow JK, Ruthazer R, Kent DM, Snydman DR. Absolute lymphocyte count: a predictor of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis. 2018;67:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–20. [DOI] [PubMed] [Google Scholar]
- 7. Sullivan T, Brodginski A, Patel G, Huprikar S. The role of secondary cytomegalovirus prophylaxis for kidney and liver transplant recipients. Transplantation. 2015;99:855–9. [DOI] [PubMed] [Google Scholar]
- 8. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abad CLR, Razonable RR. Mycobacterium tuberculosis after solid organ transplantation: a review of more than 2000 cases. Clin Transplant. 2018;32:e13259. [DOI] [PubMed] [Google Scholar]
- 10. Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266–77. [DOI] [PubMed] [Google Scholar]
- 11. Subramanian AK, Theodoropoulos NM. Infectious Diseases Community of Practice of the American Society of Transplantation. Mycobacterium tuberculosis infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;33:e13513. [DOI] [PubMed] [Google Scholar]
- 12. Tajima T, Hata K, Haga H, Nishikori M, Umeda K, Kusakabe J, et al. Post-transplant lymphoproliferative disorders after liver transplantation: a retrospective cohort study including 1954 transplants. Liver Transpl. 2021;27:1165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–62. [DOI] [PubMed] [Google Scholar]
- 14. Allen UD, Preiksaitis JK. AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13652. [DOI] [PubMed] [Google Scholar]
- 15. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baddley JW, Forrest GN. on behalf of the AST Infectious Diseases Community of Practice. Cryptococcosis in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13543. [DOI] [PubMed] [Google Scholar]
- 17. Blumberg EA, Rogers CC. on behalf of the American Society of Transplantation Infectious Diseases Community of Practice. Solid organ transplantation in the HIV-infected patient: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13499. [DOI] [PubMed] [Google Scholar]
- 18. Locke JE, Durand C, Reed RD, MacLennan PA, Mehta S, Massie A, et al. Long-term outcomes after liver transplantation among human immunodeficiency virus-infected recipients. Transplantation. 2016;100:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husson J, Stafford K, Bromberg J, Haririan A, Sparkes T, Davis C, et al. Association between duration of human immunodeficiency virus (HIV)-1 viral suppression prior to renal transplantation and acute cellular rejection. Am J Transplant. 2017;17:551–6. [DOI] [PubMed] [Google Scholar]
- 20. Sawinski D, Goldberg DS, Blumberg E, Abt PL, Bloom RD, Forde KA. Beyond the NIH multicenter HIV transplant trial experience: outcomes of HIV+ liver transplant recipients compared to HCV+ or HIV+/HCV+ coinfected recipients in the United States. Clin Infect Dis. 2015;61:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


