Abstract
Objective:
Periods of low-amplitude electroencephalographic (EEG) signal (quiescence) are present during both anesthetic-induced burst suppression (BS) and postictal generalized electroencephalographic suppression (PGES). PGES following generalized seizures induced by electroconvulsive therapy (ECT) has been previously linked to antidepressant response. The commonality of quiescence during both BS and PGES motivated trials to recapitulate the antidepressant effects of ECT using high doses of anesthetics. However, there have been no direct electrographic comparisons of these quiescent periods to address whether these are distinct entities.
Methods:
We compared periods of EEG quiescence recorded from two human studies: BS induced in 29 healthy adult volunteers by isoflurane general anesthesia and PGES in 11 patients undergoing right unilateral ECT for treatment-resistant depression. An automated algorithm allowed detection of EEG quiescence based on a 10-microvolt amplitude threshold. Spatial, spectral, and temporal analyses compared quiescent epochs during BS and PGES.
Results:
The median (interquartile range) voltage for quiescent periods during PGES was greater than during BS (1.81 (0.22) microvolts vs 1.22 (0.33) microvolts, p < 0.001). Relative power was greater for quiescence during PGES than BS for the 1–4 Hz delta band (p < 0.001), at the expense of power in the theta (4–8 Hz, p < 0.001), beta (13–30 Hz, p = 0.04) and gamma (30–70 Hz, p = 0.006) frequency bands. Topographic analyses revealed that amplitude across the scalp was consistently higher for quiescent periods during PGES than BS, whose voltage was within the noise floor.
Conclusions:
Quiescent epochs during PGES and BS have distinct patterns of EEG signals across voltage, frequency, and spatial domains.
Significance:
Quiescent epochs during PGES and BS, important neurophysiological markers for clinical outcomes, are shown to have distinct voltage and frequency characteristics.
Keywords: Electroencephalography, Electroconvulsive therapy, Major depressive disorder, Seizures, Postictal generalized, electroencephalographic suppression, Anesthesia, Burst suppression
1. Introduction
Quiescence—periods of low-amplitude electroencephalographic (EEG) signal—has been viewed as a neurophysiological marker of clinical outcomes.
Postictal generalized EEG suppression (PGES) is a period of low-amplitude EEG activity that often follows generalized seizures. The clinical criteria for PGES require an EEG amplitude of no more than 10 microvolts (mcV), discounting artifact and noise (Lhatoo et al., 2010). PGES duration has been viewed as a biomarker for the risk of sudden unexplained death in epilepsy (SUDEP) (Carlson, 2011; Lhatoo et al., 2010). Moreover, PGES following ECT-induced seizures is a putative marker for the efficacy of ECT (Mayur, 2006; Nobler et al., 2000), as longer duration of PGES correlates with a greater antidepressant response (Azuma et al., 2007; Azuma et al., 2011; Robin et al., 1985). Conversely, less ideal clinical responses to ECT have been associated with shorter duration of PGES (Nobler et al., 1993). Given the potential clinical significance of PGES, neurophysiologic mechanisms underlying PGES are an active area of investigation (Bauer et al., 2017). The persistence of local intracranial EEG activity during PGES recorded after epileptic seizures suggests that cortical activity may not be truly isoelectric during PGES (Altenmuller et al., 2016). Furthermore, automated algorithms for detecting intermittent periods of PGES following ECT-induced generalized seizures pave the way for further clinical outcome investigations (Hickman et al., 2020; Hickman et al., 2021).
Burst suppression (BS) is an EEG pattern defined by periods of quiescence punctuated by high-amplitude epochs (bursts) (Derbyshire et al., 1936). The periods of quiescence appear isoelectric and reflect interruption of thalamocortical activity (Steriade et al., 1994). EEG quiescence is commonly seen intraoperatively during general anesthesia (Hagihira, 2015). BS has been viewed as a harbinger of poor cognitive outcomes in the perioperative and critical care settings (Andresen et al., 2014; Fritz et al., 2016; Plaschke et al., 2010; Soehle et al., 2015; Watson et al., 2008). While BS has been shown to predict adverse clinical outcomes, it also has been utilized as a target for pharmacologic termination of status epilepticus (Krishnamurthy and Drislane, 1999) and neuroprotection during select surgical procedures (Keenan et al., 2016; Young et al., 1989). Thus, quiescence during BS may have important clinical implications and underlying neurophysiologic mechanisms.
Given that the quiescence during BS resembles that of PGES, anesthetic doses compatible with surgery have been trialed as an intervention for treatment-resistant major depression. Data supporting antidepressant efficacy of anesthetic BS (Brown et al., 2018; Carl et al., 1988; Engelhardt et al., 1993; Greenberg et al., 1987; Langer et al., 1995; Langer et al., 1985; Mickey et al., 2018; Weeks et al., 2013) have been countered by reports showing a lack of sufficient antidepressant response (García-Toro et al., 2001; Greenberg et al., 1987). One possibility accounting for these discrepancies is that quiescent periods during anesthetic BS and PGES are distinct entities with varied relationships to different clinical outcomes, including antidepressant response. Comparisons of these EEG patterns are needed, irrespective of neuropsychiatric outcomes.
It remains unknown whether BS and PGES differ across amplitude, frequency, or spatial topography. Our objective here is to directly compare quiescent periods during isoflurane-induced BS with the PGES following ECT-induced generalized seizures.
2. Methods
2.1. Participants and study design
2.1.1. Study 1: BS in healthy adult volunteers under isoflurane general anesthesia
Data were acquired through a multisite investigation, Reconstructing Consciousness and Cognition (ReCCognition, NCT01911195). Locations included the University of Michigan; Washington University School of Medicine in St. Louis; and the University of Pennsylvania Perelman School of Medicine. Data collection from thirty participants occurred from July 2013 to February 2015. Details of inclusion criteria, exclusion criteria, and study design are previously described (Maier et al., 2017). Healthy volunteers aged 20–40 years with American Society of Anesthesiologists (ASA) Physical Status Classification I or II were recruited. The median age of Study 1 participants was 26 years (IQR 24–29.5) with 62% males (18 out of 29). Unconsciousness was induced using a propofol infusion (5-minute sequential periods of 100, 200, and 300 mcg/kg/min). A laryngeal mask airway was placed after induction of general anesthesia. Participants then inhaled isoflurane for three hours at 1.3 multiples of the minimum alveolar concentration (MAC), adjusted for age (Nickalls and Mapleson, 2003). One participant, a 23-year-old female, was excluded due to greater than one hour of missing EEG data. Cognitive task performance data are reported elsewhere (Mashour et al., 2021).
2.1.2. Study 2: PGES following generalized seizures induced by ECT
These data were obtained as part of an investigation on the recovery of EEG markers and cognition following ECT-induced seizures (RCC2, ClinicalTrials.gov NCT02761330) (Palanca et al., 2018). Fifteen patients with treatment-resistant unipolar or bipolar depression were recruited, with data acquisition between May 2016 and August 2018. These adult participants were fluent in English and able to provide written informed consent. Potential participants were excluded if they had 1) cognitive impairment sufficient to impair testing prior to ECT, 2) a diagnosis of schizophrenia or schizoaffective disorder, 3) blindness or deafness, or 4) an inadequate seizure during etomidate general anesthesia, defined as bilateral spike-and-wave complexes present for less than 10 seconds. For Study 2, the median age of participants was 37 years (IQR 30–47) with 36% males (4 out of 11). All patients, referred for right unilateral (RUL) ECT, underwent an initial dose-charge titration session under etomidate general anesthesia (0.2 mg/kg bolus) as per protocol for standard of care ECT. A Thymatron System IV was used to deliver RUL ECT, using a current of 0.9 amperes and a pulse width of 0.3 milliseconds. During dose-charge titration, charge was increased until production of seizure (5% total charge: 24.9 millicoulombs, 10 Hz stimulation, 4.6 second duration; 10% total charge: 50.8 millicoulombs, 20 Hz stimulation, 4.65 second duration, 15% total charge: 75.6 millicoulombs, 20 Hz stimulation, 6.98 second duration). During subsequent treatments, patients received six times the charge effective for inducing a generalized seizure during the dose-charge titration session. Patients then participated in a blinded cross-over design, with a goal of two sessions per condition. The three conditions included: ECT following 0.2 mg/kg bolus of etomidate, ECT following 2–2.5 mg/kg bolus of ketamine, or sham ECT following 2–2.5 mg/kg bolus of ketamine in which no seizures were induced. EEG data in this study were culled from 50 sessions in which seizures were recorded from 11 patients.
Both Study 1 and Study 2 received ethics committee approval independently. Written informed consent was obtained from each participant prior to study procedures.
2.2. EEG acquisition and pre-processing
EEG acquisition was performed using 32-, 64-, or 128-electrode Geodesics Sensor Nets (Magstim, Eden Prairie, MN, USA). Sensors were filled with Elefix electrode paste (Nihon Kohden America, Inc., Irvine, CA, USA). Recordings (500 Hz sampling, Cz reference) were processed using EEGLAB (Delorme and Makeig, 2004) in MATLAB (MathWorks, Natick, MA). For noise analysis, recordings were performed with melons instrumented for high-density EEG, as phantoms. Pre-processing included 1–70 Hz bandpass filtering using a Butterworth IIR filter, 60-Hz notch filtering, and temporal downsampling to 250 Hz. Visual inspection allowed the removal of bad channels, which were subsequently estimated using spherical spline interpolation. Independent component analysis (ICA), using the EEGLAB plugin, allowed detection and removal of electrocardiographic artifacts.
2.3. Automated detection of quiescent periods during PGES and BS
We applied a recently developed and validated voltage-based algorithm for detecting PGES in the initial 30 seconds (Hickman et al., 2020) and five minutes (Hickman et al., 2021) following ECT-induced generalized seizures. Detection of quiescent epochs of EEG was performed in one-second non-overlapping moving windows using custom-written MATLAB scripts. EEG data were first re-referenced to generate 19 virtual channels in the international 10–20 EEG system. This allowed subsequent common processing of data acquired across sensor nets with varied electrode numbers. Second, EEG data from 19 virtual channels were utilized to generate EEG signals in the bipolar longitudinal montage commonly used by epileptologists for clinical interpretation. The maximum rectified EEG amplitude was evaluated across all virtual channels in one-second windows. Quiescent periods in the EEG (BS or PGES) were detected when the maximum rectified amplitude was less than 10 microvolts in at least 11 of the 18 EEG bipolar longitudinal montage virtual channels; this number of channels was an optimal threshold for matching the ratings of boardcertified epileptologists (Hickman et al., 2020). We excluded channels without detected quiescence from all analyses except spatial analyses, to compare signals at the sensor level, and to minimize the risk of Type I error (artificial difference between quiescent periods during BS and PGES).
2.4. Temporal analysis
To assess the temporal relationship of EEG activity during quiescent periods during BS and PGES, we first quantified the median values of rectified EEG amplitude in one-second intervals for each detected period of quiescence (Hickman et al., 2021). We then calculated correlation coefficients between median EEG amplitude and time for quiescent periods during BS and PGES. To test whether such temporal relationships are significantly different than zero, null hypothesis testing using Mann-Whitney U-test was performed on correlation coefficients.
2.5. Spectral analysis
The Chronux Toolbox (Mitra and Bokil, 2008) was utilized to compute multi-taper spectral estimation, using 3 tapers and a time-bandwidth product of 5. Power spectral density (PSD) was calculated as the averaged spectrogram across one-second non-overlapping quiescent epochs and channels. Computed PSD was utilized to calculate EEG total power and quantities in conventional frequency bands delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–70 Hz). Relative power was calculated as the ratio of specific band power to the total power across all frequencies. Given that EEG voltage varies with age (Kreuzer et al., 2020), our goal was to normalize EEG power at an individual level, partially accounting for the observed age differences among participants of the two studies.
2.6. Spatial visualization and analysis
To visualize the EEG data over the scalp, we used the 19 virtual channels derived from the international 10–20 EEG system, with signal at each sensor referenced to the average. Spatial maps of median power during quiescent periods were rendered on a generic head model, using Brainstorm (Tadel et al., 2011). Subsequent analyses comparing power during quiescent periods of BS and PGES were carried out at the sensor level.
2.7. Statistical analysis
Mann-Whitney U-tests were utilized to test for differences in medians between groups. Pearson correlation coefficients were calculated to evaluate changes in the rectified EEG voltage as a function of time. Bonferroni’s method was utilized to correct for multiple comparisons, with adjusted p-values reported whenever applicable. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. EEG voltage is consistently lower during quiescent periods of BS compared to PGES
We first assessed quiescent periods during BS and PGES, leveraging an automated algorithm (Hickman et al., 2020). Representative examples of single-channel EEG time-series during BS and PGES are illustrated in Fig. 1A and Fig. 1B, respectively. EEG time-series for all channels, re-referenced to the average EEG signal across all channels, during BS and PGES, are provided in the Supplementary Information. Using identical methodology, quiescent periods during BS appear consistently low in amplitude whereas those of PGES are shorter and difficult to discern visually. To compare the amplitude of EEG quiescence during BS and PGES, we evaluated the median rectified EEG signals across one-second non-overlapping windows. We noted significantly lower voltage for quiescence during BS than during PGES (Fig. 1C, Mann-Whitney U-tests, z-statistic = 6.03, p < 0.001). Noise analysis, using recordings from melons as phantoms, demonstrated that the signals within BS quiescent periods are within the noise floor (Mann-Whitney U-test, p = 0.11) while signals within PGES quiescent periods are not (Mann-Whitney U-test, p = 0.001). Although signals during quiescence of BS are likely noise, we have included metrics of power for quiescent periods during BS to have consistent and systematic comparisons between quiescent periods during BS and PGES.
Fig. 1.
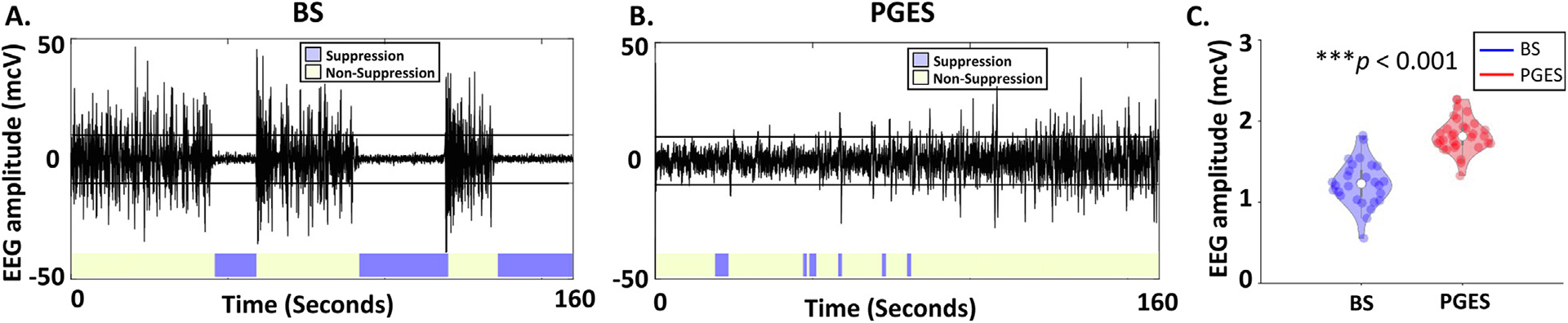
Electroencephalogram (EEG) signals during Burst Suppression (BS) and Postictal Generalized EEG Suppression (PGES). (A) Single-channel EEG time-series during BS induced by isoflurane. (B) Postictal EEG records following seizure termination. Purple area demonstrates the EEG activity within [-10,10] microvolts. The horizontal lines in panels (A) and (B) represent +/−10 mcV. (C) Comparison of quiescent periods of BS versus PGES in the voltage domain. Each dot in the violin plot represents the median of rectified EEG amplitude across quiescent channels and across one-second windows detected as PGES or isoelectric epoch of BS for a given session. The median rectified amplitude is lower for epochs EEG quiescence during BS (N = 29) compared to PGES (N = 31), using Mann-Whitney U-test (p < 0.001).
3.2. Amplitude during EEG quiescence does not vary over time for either BS or PGES
We tested the variability of the EEG amplitude across time during the progression of quiescent EEG intervals of BS and PGES. Example time-series of EEG data during BS induced by isoflurane anesthesia and PGES following seizure termination after ECT did not demonstrate a change in amplitude during either periods of quiescence (blue) in both Fig. 2A and Fig. 2B. Correlation coefficients (r) were computed from the median voltage during the quiescent periods and time variable, using quiescent periods during both BS (Supplementary Fig. 1A, r = 0.13, linear regression line in red) and PGES (Supplementary Fig. 1B, r = 0.023, linear regression line in red). Relative proportion of correlation values for quiescent periods during BS and PGES are demonstrated in Fig. 2C (median = −0.11, green line) and Fig. 2D (median = 0.04, green line). Similarly, relative proportion of linear regression slope for quiescent periods during BS and PGES is demonstrated in Supplementary Fig. 2A (median = −2.7 × 10−5, green line) and Supplementary Fig. 2B (median = 7.6 × 10−4, green line). Across all sessions/subjects, EEG amplitudes during quiescent periods were not correlated with time during either BS (Mann-Whitney U-test, p = 0.25) or PGES (Mann-Whitney U-test, p = 0.70). Similarly, regression slope coefficients during quiescent periods of BS (Mann-Whitney U-test, p = 0.24) and PGES (Mann-Whitney U-test, p = 0.58) were not different than zero, consistent with no significant linear change in the signal amplitude over time.
Fig. 2.
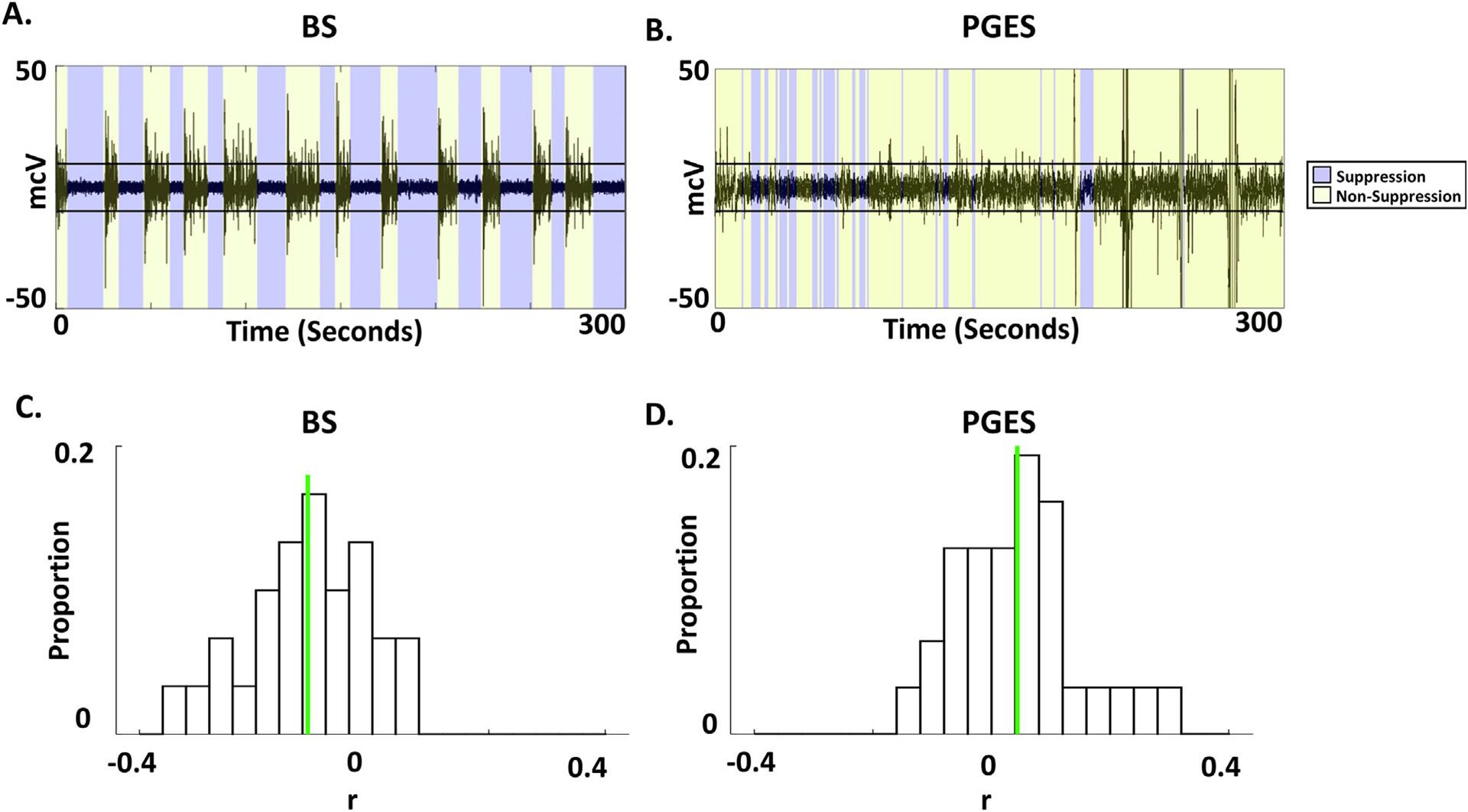
Temporal characterization of quiescent periods during Burst Suppression (BS) and Postictal Generalized EEG Suppression (PGES). (A) Single-channel EEG data illustrating quiescent periods punctuated by periods of higher amplitude bursts during isoflurane general anesthesia. These periods align with EEG quiescence (blue) or otherwise (yellow), as detected using an automated algorithm. (B) Five-minute epoch following seizure termination with detected PGES. Interspersed periods of quiescence (blue) were detected with the same automated algorithm and amplitude threshold (10 microvolts). (C) Correlations were assessed between the rectified EEG amplitude and time. Relative proportion of correlation values between the median of rectified EEG amplitude and time across low-amplitude channels during BS quiescent periods. Green line shows the median, −0.11, which is not statistically significantly different from zero (Mann-Whitney U-test, p = 0.25). (D) Relative proportion of correlation values, as in panel (C), are provided for PGES intervals with the median (0.04, green line) not statistically significantly different from zero (Mann-Whitney U-test, p = 0.70).
3.3. Quiescent epochs during BS and PGES have distinct spectral content
Representative time–frequency spectrograms of the EEG data during BS and PGES are demonstrated in Fig. 3A and Fig. 3B, respectively. Despite being recorded from an older population, total power during quiescent periods during PGES was greater than during BS (Fig. 3C, Mann-Whitney U-test, p < 0.001). Power spectral density (PSD) and relative PSD averages demonstrate the frequency profiles of the quiescent periods during BS and PGES (Supplementary Fig. 3A and 3D). We compared quiescent periods during BS and PGES at different frequency bands including delta (1–4 Hz), theta (4–8 Hz), alpha (8–13), beta (13–30), and gamma (30–70 Hz). Mann-Whitney U-test with Bonferroni’s correction showed that power was greater during PGES quiescence for the delta band (p < 0.001), theta band (p < 0.001), alpha band (p < 0.001), beta band (p < 0.001), and gamma band (p = 0.002) (Supplementary Fig. 3B). In contrast, relative power was greater for PGES quiescence in the delta band (p < 0.001) at the expense of power in the theta band (p < 0.001), beta band (p = 0.04) and gamma bands (p = 0.006, illustrated in Fig. 3E). Relative power in the alpha band was not significantly different between quiescent periods during BS and PGES (p = 0.2). These findings are robust in subgroup analysis carried out to evaluate sex as a contributing biological variable (Supplementary Fig. 4). We also compared phantom recordings from melons, and quiescent periods during PGES at different frequency bands. Comparisons of the PGES and phantom recordings confirmed that power was greater during PGES quiescence for the delta band (p = 0.009, median difference = 0.78 mcV2), theta band (p = 0.007, median difference = 0.52 mcV2), alpha band (p = 0.007, median difference = 0.3 mcV2), beta band (p = 0.007, median difference = 0.29 mcV2), and gamma band (p = 0.007, median difference = 0.08 mcV2). These results from Mann-Whitney U-test, survived Bonferroni’s correction for all five frequency bands (p < 0.01).
Fig. 3.
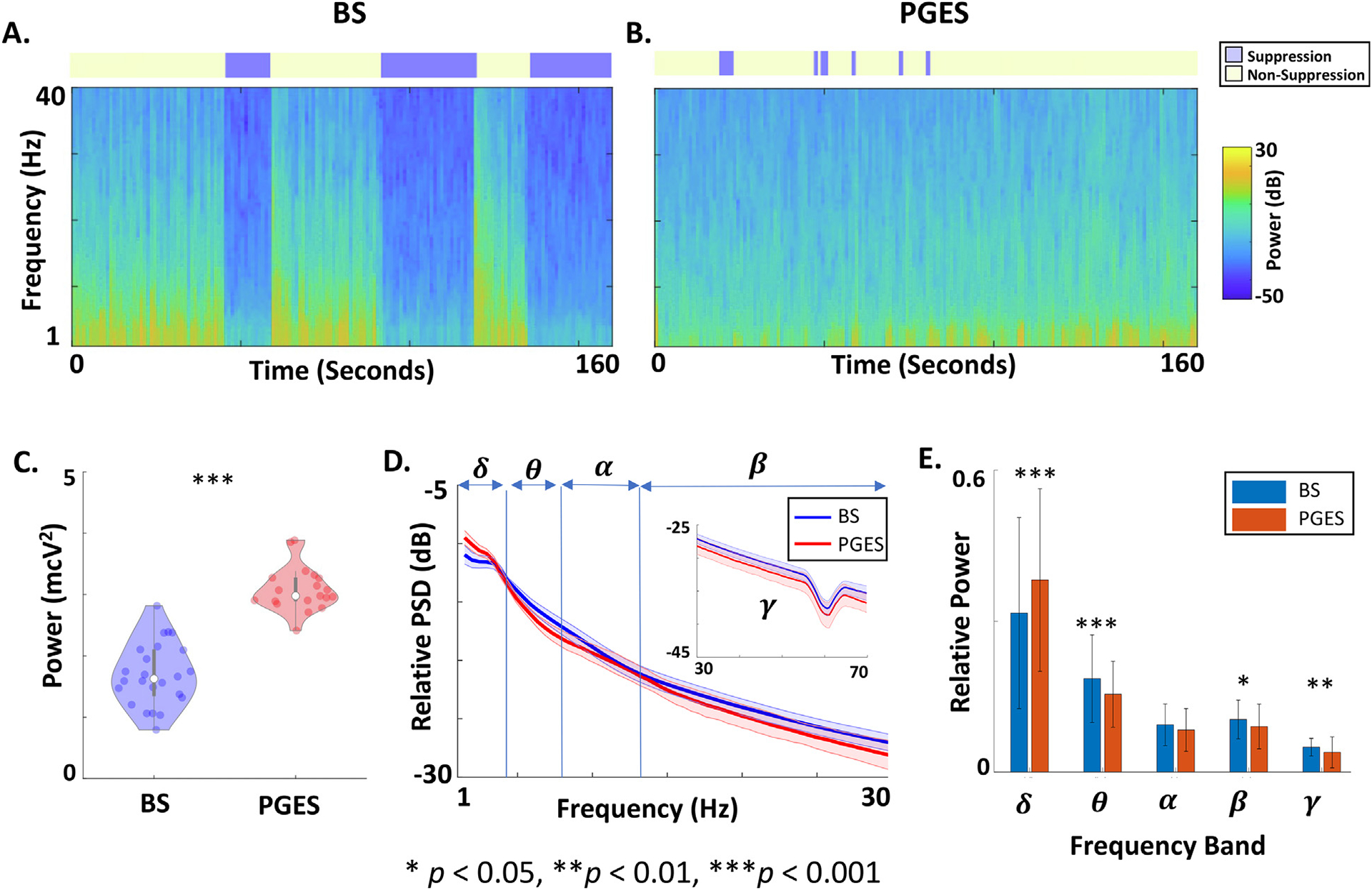
Frequency comparison of quiescent periods during Burst Suppression (BS) and Postictal Generalized EEG Suppression (PGES). (A) Time-frequency spectrogram of the EEG data in Fig. 1A displaying the distribution of power across frequencies over time. The detected quiescent periods of EEG utilizing the automated algorithm is represented as the blue rectangles above the spectrogram. Quiescent epochs (blue) show a reduction in broadband power compared to bursts (yellow). (B) The spectrogram of the EEG data in Fig. 1B showing frequency content of quiescent epochs of PGES (blue). (C) Violin plot comparing total power of quiescent periods during BS and PGES. Each dot represents total power averaged across quiescent channels for a given treatment session. (D) Relative power spectral density (PSD) comparing quiescent periods during BS and PGES. The thick lines represent the average values across treatment sessions and quiescent channels. The shaded areas demonstrate the standard deviations. (E) Bar plot comparing relative power of quiescent periods during BS and PGES at different frequency bands including delta (δ: 1–4 Hz), theta (θ: 4–8 Hz), alpha (α: 8–13), beta (β: 13–30), gamma (γ: 30–70 Hz). The error bars show the standard deviation. ***: p < 0.001, **: p < 0.01, *: p < 0.05.
3.4. Quiescent periods during BS have diffusely lower scalp EEG power compared to those of PGES
To assess the spatial distribution of EEG power during the quiescent periods of BS and PGES intervals, we used the international 10–20 montage re-referenced to the average EEG signal across all the channels. Fig. 4A–B shows EEG power with front and top view of the scalp during quiescent periods. EEG power at the sensor level was significantly lower for quiescent periods during BS compared to PGES (Mann-Whitney U-test with Bonferroni correction, p < 0.05 for all 19 channels).
Fig. 4.
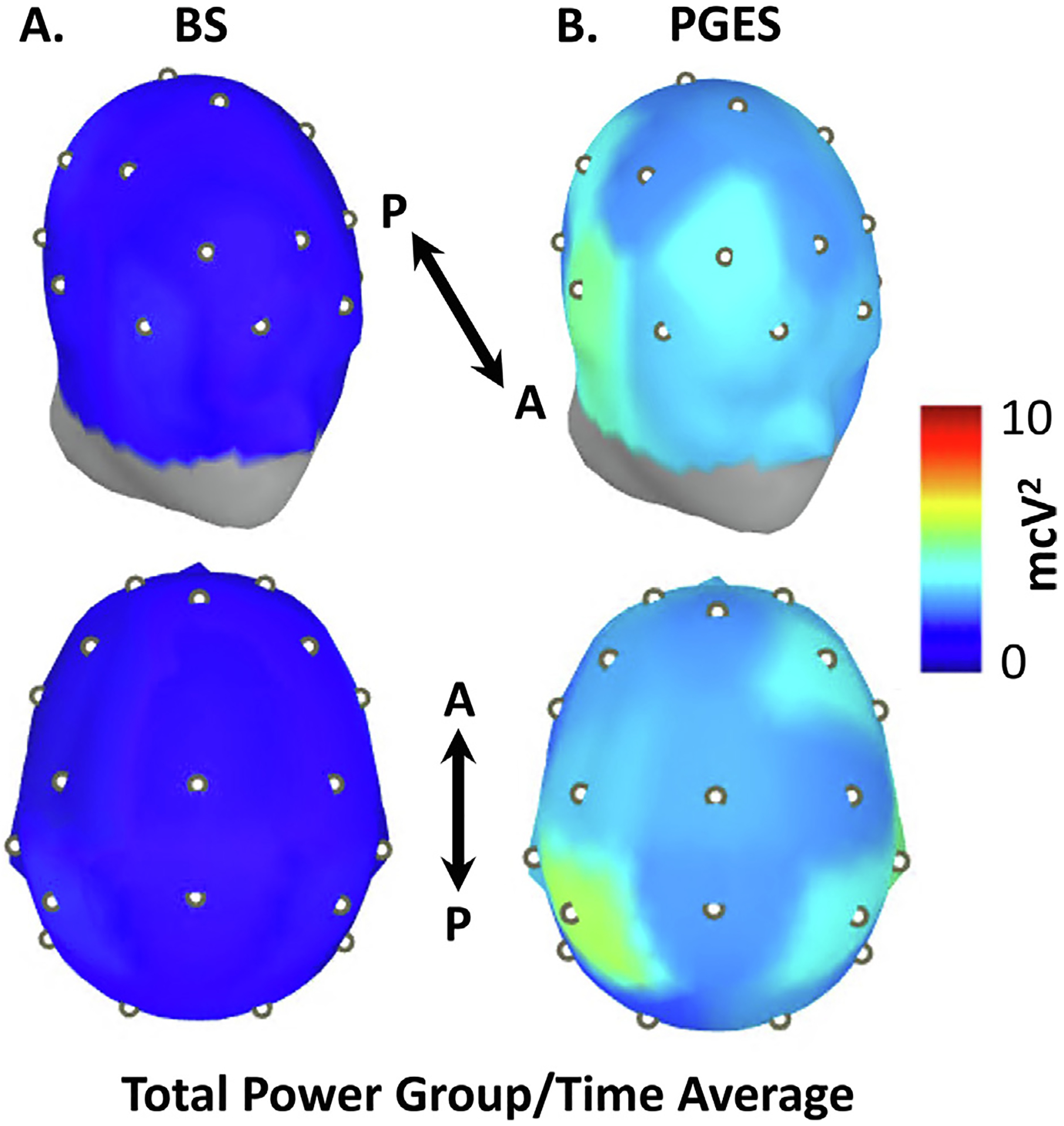
Group-level spatial comparison of quiescent periods of Burst Suppression (BS) and Postictal Generalized EEG Suppression (PGES). Electrode locations corresponding to the international 10–20 montage are utilized to visualize EEG total power across 19 scalp sensor locations (white dots). For each sensor, EEG data was re-referenced to the average signal, with the median power calculated across all 1-second quiescent epochs. (A) Oblique frontal and superior views of EEG power across the scalp for isoelectric periods of BS. (B) Oblique frontal and superior view of EEG power across the scalp for PGES intervals. At the sensor level, total EEG power is diffusely greater during quiescent epochs of PGES than those of BS (Mann-Whitney U-test with Bonferroni correction, p < 0.05 for all 19 sensors). Anterior (A) and posterior (P) aspects are indicated.
4. Discussion
4.1. Summary of findings
We quantitatively compared quiescence EEG periods recorded during isoflurane-induced BS and ECT-induced PGES. The signal amplitudes of both do not vary significantly over time. Despite this similarity, we noted consistently higher amplitude for the quiescent periods during PGES compared to those of BS, with more power distributed in delta frequency band at the expense of power in the theta, beta, and gamma bands. Across the spatial domain, higher total EEG power was observed at the sensor level for the quiescent periods during PGES compared to those of BS. Thus, while apparent similarities between PGES and BS have inspired investigations into the psychotherapeutic uses of anesthetics (Brown et al., 2018; Engelhardt et al., 1993; García-Toro et al., 2001; Greenberg et al., 1987; Langer et al., 1995; Langer et al., 1985; Mickey et al., 2018; Weeks et al., 2013), our data challenge the original assumption that quiescence following ECT-induced seizures can be considered “electrocortical silence” identical to the quiescent periods during BS (Langer et al., 1985).
4.2. Clinical importance of PGES
PGES remains a clinically significant pattern for both therapeutic and pathologic seizures. Longer periods of PGES have been associated with improvement in depression scores (Azuma et al., 2007; Suppes et al., 1996). Studies have demonstrated the utility of ictal EEG power and postictal suppression duration in optimizing ECT parameters such as stimulus intensity (Krystal et al., 1995) and ECT montage (unilateral or bilateral ECT) (Krystal et al., 2000). Longer duration of EEG suppression after ECT has also been associated with longer reorientation time during recovery after ECT (Sartorius et al., 2021). Recently, an automatic voltage-based algorithm was developed to detect PGES following existing PGES rating criteria used by expert epileptologists (Hickman et al., 2020). Utilization of this automatic algorithm revealed that PGES occurred intermittently for several minutes following seizure termination (Hickman et al., 2021). Anesthesia type (ketamine vs etomidate) appears to impact expression of PGES, with greater PGES duration after ECT with ketamine compared to etomidate (Erdil et al., 2015; Erdogan Kayhan et al., 2012; Hickman et al., 2021; Hoyer et al., 2014; Zavorotnyy et al., 2017). PGES during isoflurane anesthesia would be challenging to study as inhaled anesthetics are typically not used to induce unconsciousness during ECT. Finally, comparisons between PGES evoked by ECT-induced seizures and epileptic tonic-clonic seizures are a future avenue of investigation given that the former may offer a model system for understanding the latter (Pottkamper et al., 2021).
PGES may also serve as an important marker for the risk of sudden unexplained death in epilepsy (SUDEP) but the relationship remains unclear. While a longer duration of PGES has been linked to a greater risk of SUDEP (Lhatoo et al., 2010; Moseley et al., 2013), recent studies have not supported this association (Kang et al., 2017; Lamberts et al., 2013; Odom and Bateman, 2018; Surges et al., 2011). It is possible that advances in quantitative characterization of PGES (Hickman et al., 2020; Hickman et al., 2021; Theeranaew et al., 2018) will clarify the relationship between this EEG marker and clinical outcomes.
4.3. Clinical importance of BS
BS has been hypothesized as a marker of adverse clinical outcomes in certain clinical settings, but this association remains unclear. The time spent in this state has been associated with an increased risk of delirium in surgical patients (Fritz et al., 2016; Soehle et al., 2015) and in critically ill patients undergoing pharmacologic sedation (Andresen et al., 2014). In fact, Watson et al. have shown that BS may be an independent predictor of increased risk of mortality in critically ill patients (Watson et al., 2008). Intraoperative BS (Bruhn et al., 2000) has also been associated with poor postoperative outcomes (Plaschke et al., 2010), including delirium after cardiac surgery (Pedemonte et al., 2020). Recent investigations have further qualified these relationships (Fritz et al., 2020; Fritz et al., 2018). Interestingly, duration of BS induced by isoflurane was not correlated with trajectories of cognitive recovery in healthy volunteers following emergence from general anesthesia (Shortal et al., 2019). Overall, BS remains an important EEG marker for a wide range of clinical conditions requiring further investigation on associations to clinical outcomes and cognitive function..
4.4. Mechanistic implications
Despite their apparent similarities as intermittent periods of quiescence, the consistent differences in voltage and power for BS and PGES suggest distinct underlying neural mechanisms. The augmentation in the proportion of delta band power with concomitant reductions in power within the theta, beta and gamma bands have implications for potential underlying circuit oscillators. Considering that BS and PGES are induced through different pathways (i.e., high doses of anesthesia vs the combination of anesthesia, electrical stimulation, and recent induction of high amplitude seizure activity), the distinction between these two patterns of quiescence in different domains could open the door for rigorous experimental and computational investigations on underlying mechanisms. Computational modeling of BS (Ching et al., 2012) supports the theory that this pattern occurs secondary to neuronal metabolic processes (Liu and Ching, 2017). While recent computational modeling work has also investigated how epileptic seizure dynamics relate to PGES (Bauer et al., 2017), no investigations have compared PGES and BS via biophysical modeling. Applying parallel computational modeling methods during the state transitions for these patterns of quiescence may further elucidate the underlying similarities and differences between PGES and BS.
4.5. Strengths and limitations
We leveraged high-density EEG datasets to compare quiescent periods during BS and PGES, using an automated algorithm for detecting EEG quiescence (Hickman et al., 2020). We excluded high-amplitude epochs from non-quiescent channels to maximize rigor and not to potentially inflate differences between BS and PGES. This multidisciplinary study bridges a gap between clinical specialties of anesthesiology (BS) and psychiatry/neurology (PGES), with EEG patterns quantitatively compared across the domains of time, frequency, and space.
An important limitation of the current study is that the BS and PGES data do not originate from the same participants. Patients with PGES had treatment-resistant MDD, whereas those with anesthetic-induced BS were healthy volunteers. Participants in the BS dataset were also younger than those in the ECT dataset. Age could thus potentially explain some of the difference in amplitude during quiescent periods. Additionally, different anesthetics were present during the quiescent periods of BS (isoflurane) and PGES (etomidate or ketamine). However, rodent studies of BS induced by isoflurane and etomidate suggest that our findings are unlikely to be due to anesthetic agent alone; unlike our findings, this past research showed a lower residual peak-to-peak voltage for the quiescence during etomidate BS compared to isoflurane BS (Akrawi et al., 1996). Another limitation of ours study is that PGES data were characterized from patients undergoing right-unilateral ECT. PGES characteristics could differ for bilateral or bifrontal ECT, a topic of current investigation (ClinicalTrials.gov NCT04451135).
5. Conclusions
The patterns of EEG quiescence characteristic during BS and PGES are not identical across time, frequency, and space. EEG amplitude and power during PGES is consistently greater than during BS, at an individual channel level and across the entire scalp. The distribution of spectral content across conventional frequency bands varies for these two states of EEG quiescence.
Supplementary Material
HIGHLIGHTS.
We compared quiescent epochs in human EEG acquired during anesthetic BS and PGES following ECT-induced generalized seizures.
Quiescent periods during PGES showed greater amplitude than during BS with stronger relative delta power across the scalp.
Quantitative analyses reveal distinct profiles for PGES and BS quiescent periods across voltage, frequency, and spatial domains.
Acknowledgments
We appreciate the feedback and comments from Maxwell R. Muench. We also thank members of the Reconstructing Consciousness and Cognition Phase 1 (RCC) and Phase 2 (RCC2).
Funding
We appreciate funding by the James S. McDonnell Foundation (GAM, MBK, MSA), the McDonnell Center for Systems Neuroscience (MK, NBF, BJAP), National Institute of Mental Health K01 MH128663 (MK), National Institutes of Health Clinical and Translational Science Award (CTSA) program, TL1 TR002344 (LBH).
Footnotes
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2022.07.493.
References
- Akrawi WP, Drummond JC, Kalkman CJ, Patel PM. A comparison of the electrophysiologic characteristics of EEG burst-suppression as produced by isoflurane, thiopental, etomidate, and propofol. J Neurosurg Anesthesiol 1996;8 (1):40–6. [DOI] [PubMed] [Google Scholar]
- Altenmuller DM, Schulze-Bonhage A, Elger CE, Surges R. Local brain activity persists during apparently generalized postictal EEG suppression. Epilepsy Behav 2016;62:218–24. [DOI] [PubMed] [Google Scholar]
- Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med 2014;42 (10):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Fujita A, Sato K, Arahata K, Otsuki K, Hori M, et al. Postictal suppression correlates with therapeutic efficacy for depression in bilateral sine and pulse wave electroconvulsive therapy. Psychiatry Clin Neurosci 2007;61(2):168–73. [DOI] [PubMed] [Google Scholar]
- Azuma H, Yamada A, Shinagawa Y, Nakano Y, Watanabe N, Akechi T, et al. Ictal physiological characteristics of remitters during bilateral electroconvulsive therapy. Psychiatry Res 2011;185(3):462–4. [DOI] [PubMed] [Google Scholar]
- Bauer PR, Thijs RD, Lamberts RJ, Velis DN, Visser GH, Tolner EA, et al. Dynamics of convulsive seizure termination and postictal generalized EEG suppression. Brain 2017;140(3):655–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Zanos P, Wang L, Elmer GI, Gould TD, Shepard PD. Isoflurane but Not Halothane Prevents and Reverses Helpless Behavior: A Role for EEG Burst Suppression? Int J Neuropsychopharmacol 2018;21(8):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn J, Bouillon TW, Shafer SL. Bispectral index (BIS) and burst suppression: revealing a part of the BIS algorithm. J Clin Monit Comput 2000;16(8):593–6. [DOI] [PubMed] [Google Scholar]
- Carl C, Engelhardt W, Teichmann G, Fuchs G. Open comparative study with treatment-refractory depressed patients: electroconvulsive therapy–anesthetic therapy with isoflurane (preliminary report). Pharmacopsychiatry 1988;21 (6):432–3. [DOI] [PubMed] [Google Scholar]
- Carlson C Generalized Postictal EEG Background Suppression: A Marker of SUDEP Risk. Epilepsy Curr 2011;11(3):86–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A 2012;109(8):3095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- Derbyshire AJ, Rempel B, Forbes A, Lambert EF. The effects of anesthetics on action potentials in the cerebral cortex of the cat. Am J Physiol 1936;116(3):577–96. [Google Scholar]
- Engelhardt W, Carl G, Hartung E. Intra-individual open comparison of burst-suppression-isoflurane-anaesthesia versus electroconvulsive therapy in the treatment of severe depression. Eur J Anaesthesiol 1993;10(2):113–8. [PubMed] [Google Scholar]
- Erdil F, Ozgul U, Colak C, Cumurcu B, Durmus M. Effect of the Addition of Ketamine to Sevoflurane Anesthesia on Seizure Duration in Electroconvulsive Therapy. J ECT 2015;31(3):182–5. [DOI] [PubMed] [Google Scholar]
- Erdogan Kayhan G, Yucel A, Colak YZ, Ozgul U, Yologlu S, Karlidag R, et al. Ketofol (mixture of ketamine and propofol) administration in electroconvulsive therapy. Anaesth Intensive Care 2012;40(2):305–10. [DOI] [PubMed] [Google Scholar]
- Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, et al. Intraoperative Electroencephalogram Suppression Predicts Postoperative Delirium. Anesth Analg 2016;122(1):234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BA, King CR, Ben Abdallah A, Lin N, Mickle AM, Budelier TP, et al. Preoperative Cognitive Abnormality, Intraoperative Electroencephalogram Suppression, and Postoperative Delirium: A Mediation Analysis. Anesthesiology 2020;132 (6):1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BA, Maybrier HR, Avidan MS. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth 2018;121(1):241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Toro M, Segura C, Gonzalez A, Perello J, Valdivia J, Salazar R, et al. Inefficacy of burst-suppression anesthesia in medication-resistant major depression: a controlled trial. J ECT 2001;17(4):284–8. [DOI] [PubMed] [Google Scholar]
- Greenberg LB, Gage J, Vitkun S, Fink M. Isoflurane Anesthesia Therapy: A Replacement for ECT in Depressive Disorders? Convuls Ther 1987;3(4):269–77. [PubMed] [Google Scholar]
- Hagihira S Changes in the electroencephalogram during anaesthesia and their physiological basis. Br J Anaesth 2015;115(Suppl 1):i27–31. [DOI] [PubMed] [Google Scholar]
- Hickman LB, Hogan RE, Labonte AK, Kafashan M, Chan CW, Huels ER, et al. Voltage-based automated detection of postictal generalized electroencephalographic suppression: Algorithm development and validation. Clin Neurophysiol 2020;131(12):2817–25. [DOI] [PubMed] [Google Scholar]
- Hickman LB, Kafashan M, Labonte AK, Chan CW, Huels ER, Guay CS, et al. Postictal generalized electroencephalographic suppression following electroconvulsive therapy: Temporal characteristics and impact of anesthetic regimen. Clin Neurophysiol 2021;132(4):977–83. [DOI] [PubMed] [Google Scholar]
- Hoyer C, Kranaster L, Janke C, Sartorius A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci 2014;264(3):255–61. [DOI] [PubMed] [Google Scholar]
- Kang JY, Rabiei AH, Myint L, Nei M. Equivocal significance of post-ictal generalized EEG suppression as a marker of SUDEP risk. Seizure 2017;48:28–32. [DOI] [PubMed] [Google Scholar]
- Keenan JE, Wang H, Ganapathi AM, Englum BR, Kale E, Mathew JP, et al. Electroencephalography During Hemiarch Replacement With Moderate Hypothermic Circulatory Arrest. Ann Thorac Surg 2016;101(2):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer M, Stern MA, Hight D, Berger S, Schneider G, Sleigh JW, et al. Spectral and Entropic Features Are Altered by Age in the Electroencephalogram in Patients under Sevoflurane Anesthesia. Anesthesiology 2020;132(5):1003–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia 1999;40(6):759–62. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Holsinger T, Weiner RD, Coffey CE. Prediction of the utility of a switch from unilateral to bilateral ECT in the elderly using treatment 2 ictal EEG indices. J ECT 2000;16(4):327–37. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Weiner RD, Coffey CE. The ictal EEG as a marker of adequate stimulus intensity with unilateral ECT. J Neuropsychiatry Clin Neurosci 1995;7 (3):295–303. [DOI] [PubMed] [Google Scholar]
- Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, Thijs RD. Postictal generalized EEG suppression: an inconsistent finding in people with multiple seizures. Neurology 2013;81(14):1252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Karazman R, Neumark J, Saletu B, Schonbeck G, Grunberger J, et al. Isoflurane narcotherapy in depressive patients refractory to conventional antidepressant drug treatment. A double-blind comparison with electroconvulsive treatment. Neuropsychobiology 1995;31(4):182–94. [DOI] [PubMed] [Google Scholar]
- Langer G, Neumark J, Koinig G, Graf M, Schonbeck G. Rapid psychotherapeutic effects of anesthesia with isoflurane (ES narcotherapy) in treatment-refractory depressed patients. Neuropsychobiology 1985;14(3):118–20. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68(6):787–96. [DOI] [PubMed] [Google Scholar]
- Liu S, Ching S. Homeostatic dynamics, hysteresis and synchronization in a low-dimensional model of burst suppression. J Math Biol 2017;74(4):1011–35. [DOI] [PubMed] [Google Scholar]
- Maier KL, McKinstry-Wu AR, Palanca BJA, Tarnal V, Blain-Moraes S, Basner M, et al. Protocol for the Reconstructing Consciousness and Cognition (ReCCognition) Study. Front Hum Neurosci 2017;11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA, Palanca BJ, Basner M, Li D, Wang W, Blain-Moraes S, et al. Recovery of consciousness and cognition after general anesthesia in humans. Elife. 2021;10: e59525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayur P Ictal electroencephalographic characteristics during electroconvulsive therapy: a review of determination and clinical relevance. J ECT 2006;22 (3):213–7. [DOI] [PubMed] [Google Scholar]
- Mickey BJ, White AT, Arp AM, Leonardi K, Torres MM, Larson AL, et al. Propofol for Treatment-Resistant Depression: A Pilot Study. Int J Neuropsychopharmacol 2018;21(12):1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Bokil H. Observed Brain Dynamics. 1st ed. New York: Oxford University Press; 2008. [Google Scholar]
- Moseley BD, So E, Wirrell EC, Nelson C, Lee RW, Mandrekar J, et al. Characteristics of postictal generalized EEG suppression in children. Epilepsy Res 2013;106(1–2):123–7. [DOI] [PubMed] [Google Scholar]
- Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth 2003;91(2):170–4. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Luber B, Moeller JR, Katzman GP, Prudic J, Devanand DP, et al. Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. I. Global analyses. J ECT 2000;16(3):211–28. [DOI] [PubMed] [Google Scholar]
- Nobler MS, Sackeim HA, Solomou M, Luber B, Devanand DP, Prudic J. EEG manifestations during ECT: effects of electrode placement and stimulus intensity. Biol Psychiatry 1993;34(5):321–30. [DOI] [PubMed] [Google Scholar]
- Odom N, Bateman LM. Sudden unexpected death in epilepsy, periictal physiology, and the SUDEP-7 Inventory. Epilepsia 2018;59(10):e157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanca BJA, Maybrier HR, Mickle AM, Farber NB, Hogan RE, Trammel ER, et al. Cognitive and Neurophysiological Recovery Following Electroconvulsive Therapy: A Study Protocol. Front Psychiatry 2018;9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte JC, Plummer GS, Chamadia S, Locascio JJ, Hahm E, Ethridge B, et al. Electroencephalogram Burst-suppression during Cardiopulmonary Bypass in Elderly Patients Mediates Postoperative Delirium. Anesthesiology 2020;133 (2):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 2010;36(12):2081–9. [DOI] [PubMed] [Google Scholar]
- Pottkamper JCM, Verdijk J, Hofmeijer J, van Waarde JA, van Putten M. Seizures induced in electroconvulsive therapy as a human epilepsy model: A comparative case study. Epilepsia Open 2021;6(4):672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A, Binnie CD, Copas JB. Electrophysiological and hormonal responses to three types of electroconvulsive therapy. Br J Psychiatry 1985;147(6):707–12. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Karl S, Zapp A, Putschogl F, Bumb JM, Reinwald J, et al. Duration of Electroconvulsive Therapy Postictal Burst Suppression Is Associated With Time to Reorientation. J ECT 2021;37(4):247–9. [DOI] [PubMed] [Google Scholar]
- Shortal BP, Hickman LB, Mak-McCully RA, Wang W, Brennan C, Ung H, et al. Duration of EEG suppression does not predict recovery time or degree of cognitive impairment after general anaesthesia in human volunteers. Br J Anaesth 2019;123(2):206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol 2015;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol 1994;90(1):1–16. [DOI] [PubMed] [Google Scholar]
- Suppes TWA, Carmody T, Gordon E, Gutierrez-Esteinou R, Hudson JL, Pope HG. Is postictal electrical silence a predictor of response to electroconvulsive therapy? J Affect Disord 1996;41:51–8. [DOI] [PubMed] [Google Scholar]
- Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav 2011;21(3):271–4. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci 2011;2011 879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeranaew W, McDonald J, Zonjy B, Kaffashi F, Moseley BD, Friedman D, et al. Automated Detection of Postictal Generalized EEG Suppression. IEEE Trans Biomed Eng 2018;65(2):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med 2008;36(12):3171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks HR 3rd, Tadler SC, Smith KW, Iacob E, Saccoman M, White AT, et al. Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. PLoS One 2013;8(7): e69809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WL, Solomon RA, Pedley TA, Ross L, Schwartz AE, Ornstein E, et al. Direct cortical EEG monitoring during temporary vascular occlusion for cerebral aneurysm surgery. Anesthesiology 1989;71(5):794–9. [DOI] [PubMed] [Google Scholar]
- Zavorotnyy M, Kluge I, Ahrens K, Wohltmann T, Kohnlein B, Dietsche P, et al. S - ketamine compared to etomidate during electroconvulsive therapy in major depression. Eur Arch Psychiatry Clin Neurosci 2017;267(8):803–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


