Abstract
Introduction
The purpose of this study was to evaluate the long-term outcomes of posterior capsular opacification (PCO) in highly myopic eyes and its influencing factors.
Methods
Patients undergoing phacoemulsification with intraocular lens implantation and followed up for 1–5 years were included in this prospective cohort study. The severity of PCO was evaluated using EPCO2000 software system, with the area of central 3.0 mm (PCO-3 mm) and within the capsulorhexis (PCO-C) both being analyzed. Percentage of eyes after Nd:YAG capsulotomy, as well as clinically significant PCO (defined as eyes with visual-impairing PCO or after capsulotomy), were also included as outcome variables.
Results
A total of 673 highly myopic eyes [axial length (AL) ≥ 26 mm] and 224 control eyes (AL < 26 mm) were analyzed. The mean follow-up time was 34.0 ± 9.0 months. PCO was more severe in highly myopic eyes compared with controls with regard to higher EPCO scores (P < 0.001 for both PCO-3 mm and PCO-C), higher capsulotomy rate (P = 0.001), higher clinically significant PCO rate (P < 0.001) and shorter PCO-free survival time (P < 0.001). Extreme myopia (AL ≥ 28 mm) would further aggravate PCO in terms of higher EPCO scores (PCO-3 mm: P = 0.017; PCO-C: P = 0.013) and higher clinically significant PCO rate (P = 0.024) compared with other myopic eyes. In highly myopic eyes, AL [odds ratio (OR) 1.124, P = 0.004] and follow-up duration (OR 1.082, P < 0.001) were independent risk factors for clinically significant PCO after cataract surgery.
Conclusion
Highly myopic eyes had more severe PCO in the long term. Longer AL and follow-up duration were associated with higher risk of PCO.
Clinical Trial Registration
The study was registered at ClinicalTrials.gov (NCT03062085).
Keywords: High myopia, Posterior capsular opacification, Nd:YAG capsulotomy
Key Summary Points
| Why carry out this study? |
| Posterior capsular opacification (PCO) is the most frequent long-term vision affected complication of cataract surgery. However, whether high myopia is a risk factor for PCO formation remains controversial. |
| In this study, we evaluated the long-term outcomes of PCO in highly myopic eyes and its influencing factors. |
| What was learned from the study? |
| PCO was more severe and frequent in highly myopic eyes compared with controls. |
| Longer axial length and follow-up duration were associated with higher risk of PCO in highly myopic eyes. |
| In highly myopic patients with higher risk of PCO, posterior capsular polishing should be properly performed to prevent PCO. |
Introduction
High myopia (HM), defined as eyes with axial length (AL) ≥ 26 mm, is currently gaining increasing attention worldwide [1]. It is characterized by early-onset nuclear cataract [2] and more complications after cataract surgery due to structural and intraocular microenvironment abnormalities [3, 4]. Posterior capsular opacification (PCO), with a reported incidence varying from 14.8–56.8% in 2–4 years of follow-ups [5–7], is the most frequent long-term complication of cataract surgery, which significantly affects visual outcome [1]. However, whether high myopia is a risk factor for PCO formation remains controversial.
Some studies suggested that high myopia was usually accompanied with a larger capsular bag [8, 9], and thus insufficient capsule-intraocular lens (IOL) adhesion occurred, lens epithelial cells (LECs) migrated, and PCO followed [1], whereas others claimed that myopia would not affect PCO development [7, 10]. In addition, studies have investigated risk factors for PCO and indicated that younger age, female sex, longer follow-up duration, diabetes, hydrophilic IOL, and surgical techniques would aggravate PCO development [1, 11–15], yet no studies have evaluated the risk factors for PCO in highly myopic population.
In this study, we sought to figure out whether high myopia would lead to more severe PCO and to further identify factors involved in their PCO formation. Our results could offer real-world clinical practice evidence regarding PCO in highly myopic population, in the hopes of helping with the long-term prognosis of phacoemulsification and IOL implantation, as well as in providing early diagnosis and treatment options for highly myopic patients.
Methods
Compliance with Ethics Guidelines
The Institutional Review Board of Eye and Ear, Nose and Throat (ENT) Hospital approved this prospective cohort study (no. 2020068). All procedures adhered to the Declaration of Helsinki and informed consent was obtained for using their clinical data and further follow-up from each patient at the first follow-up. The study was registered at ClinicalTrials.gov (NCT03062085).
Patient Selection
This prospective cohort study recruited highly myopic patients and control subjects who underwent phacoemulsification and IOL implantation and finished 1 month follow-up at our hospital from January 2016 to December 2020. These patients received the second follow-up at least 1 year postoperatively between May 2021 and December 2021. Inclusion criteria were patients with hydrophilic IOL implantation including Humanoptics MC X11 ASP (HumanOptics AG, Erlangen, Germany) or Rayner 920H (Rayner Intraocular lenses Ltd., Hove, East Sussex, UK), which are the major IOL types used in highly myopic eyes at our hospital, and with complete postoperative 1-month examination data (recorded as the first follow-up). Exclusion criteria are listed as follows: (1) age < 30 years old; (2) pupil diameter < 6 mm after mydriasis (3) history of trauma or other intraocular surgeries; (4) occurrence of severe intraoperative complications including posterior capsule rupture and corneal descemet membrane detachment, etc.; (5) occurrence of other visual-impairing circumstances including retinal detachment, severe retinoschisis, macular hole, vitreous hemorrhage, uveitis, etc.; and (6) intrinsic posterior capsular fibrosis observed during surgery. One eye was randomly selected from each patient if both eyes met criteria.
The included eyes were divided into control group (AL < 26 mm) and HM group (AL ≥ 26 mm), with the latter further divided into two subgroups according to AL (HM1: 26 mm ≤ AL < 28 mm; HM2: AL ≥ 28 mm, which was also known as extreme myopia [16, 17]).
Preoperative Examinations
Patients registered for cataract surgeries went through routine preoperative ophthalmic examinations including visual acuity assessment, intraocular pressure (IOP) measurements (Nidek, Tokyo, Japan), AL measurements (IOLMaster 500, Carl Zeiss AG, Oberkochen, Germany), and macular optical coherence tomography (OCT) examinations (Heidelberg Engineering, Heidelberg, Germany). Both uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) were recorded as Snellen values and converted to the logarithm of the minimum angle of resolution (logMAR) for analysis. If visual acuity was counting fingers or worse, the corresponding conversion was calculated as previously reported [18].
Surgical Technique
All procedures were performed by one experienced surgeon (Prof. Yi Lu). A 2.6 mm temporal clear corneal incision was made after topical anesthesia. Viscoelastic (DisCoVisc; Alcon Laboratories, Inc., Fort Worth, Texas, USA) was injected, followed by continuous curvilinear capsulorhexis, hydrodissection, phacoemulsification, cortical removal, posterior capsular polishing, and foldable hydrophilic IOL implantation. All the incisions were hydrated and no stitch was used. Routine postoperative medication was prescribed to all patients including Cravit eye drops (levofloxacin; Santen Pharmaceutical, Japan), prednisolone acetate (Allergen Pharmaceutical Ireland, Westport, Country Mayo, Ireland), and pranopulin (Pranoprofen; Senju Pharmaceutical Osaka, Japan) four times daily for 4 weeks.
Follow-Up
At both follow-ups, routine ophthalmic examinations including visual acuity assessment, refraction, slit-lamp examinations, IOP measurements (Nidek, Tokyo, Japan), and macular OCT evaluations were performed. The slit-lamp examination encompassed documentation of slit-beam, diffuse-, and retroilluminated-slit lamp photographs with maximum pupil mydriasis at a fixed illumination and magnification. The photographs were then used for PCO assessment. History of neodymium-doped yttrium aluminum garnet (Nd:YAG) capsulotomy conducted between the two follow-ups was also recorded. In total, 931 patients who met the criteria were recruited in this study, and 897 patients completed the second follow-up.
Posterior Capsular Opacification Evaluation
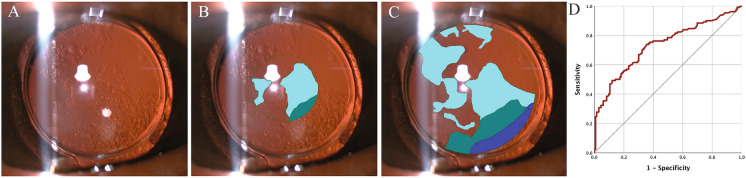
PCO was assessed with the Evaluation of Posterior Capsular Opacification 2000 program (EPCO2000, a software program developed by Manfred Tetz and Christophe Nimsgern, Universitats-Augenklinik Charite, Berlin, Germany), a quantitative morphological assessment of PCO [7, 15]. All the measurements were conducted by one experienced researcher who was masked to patient information. The central 3.0 mm zone (PCO-3 mm) and the zone within the capsulorhexis margin (PCO-C), regarded as central and peripheral PCO, respectively, were analyzed. The boundaries of the region of interest and each opaque area of the posterior capsule were drawn on the documented photographs with a computer mouse so that the fraction of the opaque area could be calculated automatically (Fig. 1A–C). The density of opacification was graded from 0 to 4: 0, none; 1, minimal; 2, mild; 3, moderate; and 4, severe. The individual EPCO score was calculated by multiplying the fraction of the opaque area involved by the density of the opacification [15].
Fig. 1.
Representative photographs of PCO grading using EPCO2000 and ROC curve to analyze the cut-off value of visual-impairing PCO-3 mm score. A Original image; B PCO evaluation of central 3.0 mm zone; C PCO evaluation of the entire IOL optic zone within the capsulorhexis margin. Light blue represents minimal opacification density, medium blue represents mild opacification density, and dark blue represents moderate opacification density. D ROC curve built by the PCO-3 mm score and the diagnosis of significant visual impairment in eyes with PCO-3 mm score > 0. Significant visual impairment was defined as a decrease in visual acuity of more than two lines on Snellen chart since their first follow-up. PCO posterior capsular opacification, ROC receiver operating characteristic
Additionally, in patients with PCO-3 mm score > 0, the PCO-3 mm score and the diagnosis of significant visual impairment, which was defined as a decrease in visual acuity of more than two lines on Snellen chart since their first follow-up [14], were used to build the receiver operating characteristic (ROC) curve (Fig. 1D). According to the ROC analysis, the cut-off value of visual-impairing PCO-3 mm score based on Youden index [19] was 0.1955, with sensitivity and specificity being 0.738 and 0.647, respectively. We then defined eyes with clinically significant PCO as having PCO-3 mm score ≥ 0.1955 or having Nd:YAG capsulotomy.
Statistical Analysis
Statistical analyses were performed with SPSS version 20.0 (IBM Inc., Chicago, IL, USA). Quantitative data are presented as means ± standard deviations (SD). The χ2 test was used to evaluate categorical variables. Student’s t-test was used to compare continuous parameters between two groups. Paired t-test was used to evaluate the change of ocular parameters between two visits. The Kaplan–Meier curve was generated to assess PCO-free survival analysis, with log-rank test used for difference inspection. Logistic regression analysis was also performed to analyze the influencing factors of PCO in highly myopic eyes. The final model was constructed with multivariate regression using backward stepwise selection, which was based on the significant influencing factors originating from univariate analysis. P-values of less than 0.05 were considered statistically significant.
Results
Patient Characteristics
In total, 673 highly myopic eyes from 673 cases and 224 control eyes from 224 cases were analyzed in this study. The mean follow-up duration of all patients was 34.0 ± 9.0 months, ranging from 13.0 to 57.1 months. Patient characteristics are presented in Table 1, and no significant differences were found between the HM and control groups or between the two HM subgroups with regard to age, gender, diabetes, IOL type, or follow-up duration (Student’s t-test or χ2 test, all P > 0.05). Pre- and postoperative BCVAs were worse in the HM group compared with the control (Student’s t-test, all P < 0.05). BCVA improved after cataract surgery at the first follow-up compared with the preoperative data (paired t-test 0.91 ± 0.59 versus 0.21 ± 0.33, P < 0.001), and regressed at the second follow-up compared with the first follow-up (paired t-test, 0.32 ± 0.48 versus 0.21 ± 0.33, P < 0.001).
Table 1.
Patient characteristics
| Control | HM | P-valuea | P-valueb | |||
|---|---|---|---|---|---|---|
| Total | Subgroup | |||||
| HM1 | HM2 | |||||
| Eyes (n) | 224 | 673 | 216 | 457 | NA | NA |
| Axial length (mm) | 23.78 ± 1.25 (21.40–25.94) | 29.36 ± 2.05 (26.01–35.63) | 27.06 ± 0.53 (26.01–27.99) | 30.45 ± 1.55 (28.02–35.63) | < 0.001c | < 0.001c |
| Age (years) | 64.8 ± 7.9 (31–83) | 64.1 ± 8.1 (30–83) | 64.7 ± 7.9 (30–81) | 63.8 ± 8.1 (39–83) | 0.423 | 0.170 |
| Gender (M/F) | 79/145 | 262/411 | 96/120 | 166/291 | 0.329 | 0.051 |
| Diabetes (with/without) | 23/201 | 57/616 | 21/195 | 36/421 | 0.413 | 0.423 |
| IOL (Humanoptics/Rayner) | 120/104 | 346/327 | 105/111 | 241/216 | 0.576 | 0.318 |
| BCVA0 (logMAR) | 0.72 ± 0.37 (0.22–1.52) | 0.92 ± 0.62 (0.22–4.00) | 0.81 ± 0.56 (0.22–3.00) | 0.97 ± 0.64 (0.22–4.00) | 0.006c | 0.019 |
| BCVA1 (logMAR) | 0.11 ± 0.19 (−0.08–1.00) | 0.23 ± 0.35 (−0.08–3.30) | 0.15 ± 0.21 (−0.08–1.30) | 0.27 ± 0.40 (−0.08–3.30) | < 0.001c | < 0.001c |
| BCVA2 (logMAR) | 0.13 ± 0.20 (−0.18–1.30) | 0.36 ± 0.49 (−0.18–3.30) | 0.25 ± 0.36 (−0.18–3.30) | 0.42 ± 0.54 (−0.18–3.30) | < 0.001c | < 0.001c |
| Follow-up duration (months) | 34.9 ± 8.9 (13.1–52.3) | 33.8 ± 9.1 (13.0–57.1) | 33.3 ± 8.9 (13.1–57.1) | 34.0 ± 9.2 (13.0–57.1) | 0.323 | 0.302 |
HM high myopia, HM1 high myopia with AL ≤ 28 mm, HM2 high myopia with AL > 28 mm, NA not applicable, M male, F female, IOL intraocular lens, BCVA0 best-corrected visual acuity before cataract surgery, BCVA1 best-corrected visual acuity at the first follow-up, BCVA2 best-corrected visual acuity at the second follow-up, LogMAR logarithm of the minimum angle of resolution
Data were presented as mean ± standard deviation (range) or proportion
aStudent’s t-test or χ2 test, difference between control group and HM group
bStudent’s t-test or χ2 test, difference between two HM subgroups
cSignificant (P < 0.05)
PCO Severity
PCO was not present in any eyes at the first follow-up. PCO severity at the second follow-up is presented in Table 2. Significantly higher EPCO score was observed in HM group both centrally and peripherally compared with controls (Student’s t-test, PCO-3 mm: 0.19 ± 0.38 versus 0.04 ± 0.11, P < 0.001; PCO-C: 0.24 ± 0.40 versus 0.08 ± 0.24, P < 0.001). Extreme myopia (AL ≥ 28 mm) could also aggravate PCO with regard to both central and peripheral PCO severity compared with other myopic eyes (Student’s t-test, both P < 0.05).
Table 2.
Comparisons of posterior capsular opacification and Nd:YAG capsulotomy rate in different axial length groups
| Control | High myopia | P-valuea | P-valueb | |||
|---|---|---|---|---|---|---|
| Total | Subgroup | |||||
| HM1 | HM2 | |||||
| PCO-3 mm | 0.04 ± 0.11 | 0.19 ± 0.38 | 0.14 ± 0.27 | 0.22 ± 0.43 | < 0.001c | 0.017c |
| PCO-C | 0.08 ± 0.24 | 0.24 ± 0.40 | 0.18 ± 0.31 | 0.27 ± 0.44 | < 0.001c | 0.013c |
| Nd:YAG capsulotomy rate | 11.2% (25/224) | 21.7% (146/673) | 18.5% (40/216) | 23.2% (106/457) | 0.001c | 0.193 |
| Clinically significant PCO rate | 18.3% (41/224) | 41.6% (280/673) | 35.2% (76/216) | 44.6% (204/457) | < 0.001c | 0.024c |
PCO-3 mm EPCO score within the central 3.0 mm zone, PCO-C EPCO score within the capsulorhexis margin, Nd:YAG neodymium-doped yttrium aluminum garnet. Control: AL < 26 mm; high myopia: AL ≥ 26 mm; HM1: 26 mm ≤ AL < 28 mm; HM2: AL ≥ 28 mm
Data were presented as mean ± standard deviation or proportion
aStudent’s t-test or χ2 test, difference between control group and HM group
bStudent’s t-test or χ2 test, difference between two HM subgroups
cSignificant (P < 0.05)
Nd:YAG Capsulotomy Rate and Clinically Significant PCO Rate
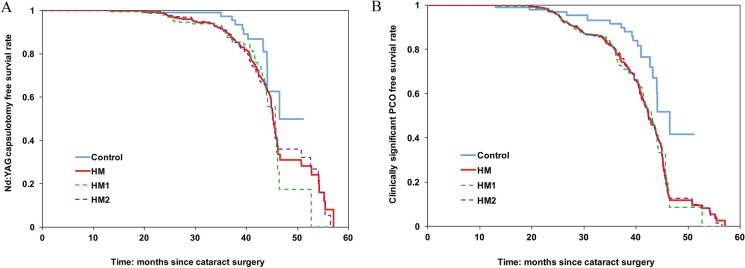
The overall Nd:YAG capsulotomy rate in HM group was significantly higher than in the control group (Table 2, χ2 test, 21.7% versus 11.2%, P = 0.001). According to Kaplan–Meier survival analysis, HM group showed significantly lower median Nd:YAG capsulotomy free survival time compared with the control group (Fig. 2A; 45.2 versus 47.4 months, P = 0.01).
Fig. 2.
Kaplan–Meier estimates of the Nd:YAG capsulotomy-free (A) and clinically significant PCO-free (B) survival probabilities according to axial length (AL). Control: AL < 26 mm; HM: AL ≥ 26 mm; HM1: 26 mm ≤ AL < 28 mm; HM2: ≥ 28 mm; Nd:YAG neodymium-doped yttrium aluminum garnet, PCO posterior capsular opacification
The overall rate of clinically significant PCO was also significantly higher in the HM group than in the control (Table 2, χ2 test, 41.6% versus 18.3%, P < 0.001). In addition, extreme myopia also showed a higher rate of clinically significant PCO than other highly myopic eyes (Table 2, χ2 test, P = 0.024).
According to Kaplan–Meier survival analysis, HM group showed significantly lower median clinically significant PCO-free survival time compared with control group (Fig. 2B; 42.3 versus 47.4 months, P < 0.001).
Influencing Factors of Clinically Significant PCO in Highly Myopic Eyes
Factors influencing PCO in highly myopic eyes were further investigated (Table 3). In highly myopic eyes, clinically significant PCO rate was significantly lower in patients aged > 70 years than the other two age groups (χ2 test with Bonferroni correction, age < 60 years versus age > 70 years, P = 0.002; age 60–70 years versus age > 70 years, P = 0.003). Clinically significant PCO rate kept increasing with time elapse in highly myopic eyes (χ2 test with Bonferroni correction, 1–2 years versus 2–3 years: P < 0.001; 2–3 years versus > 3 years: P = 0.001). Clinically significant PCO rate was higher in highly myopic eyes with IOL power < 10.0D than in eyes with IOL power ≥ 10.0D (χ2 test, P < 0.001). Gender, diabetes, IOL type, and IOL thickness did not show a significant correlation with clinically significant PCO rate in highly myopic eyes (χ2 test, all P > 0.05).
Table 3.
Analysis of influencing factors associated with posterior capsular opacification in highly myopic eyes
| Variables | Eyes (n) | Clinically significant PCO | P-value |
|---|---|---|---|
| Age (years) | |||
| < 60 | 192 | 46.4% (89/192) | |
| 60–70 | 339 | 44.0% (149/339) | |
| > 70 | 142 | 29.6% (42/142) | 0.004 |
| Gender | |||
| Male | 262 | 40.5% (106/262) | |
| Female | 411 | 42.3% (174/411) | 0.688 |
| Follow-up duration (years) | |||
| 1–2 | 128 | 15.6% (20/128) | |
| 2–3 | 244 | 36.1% (88/244) | |
| > 3 | 301 | 57.1% (172/301) | < 0.001 |
| Diabetes | |||
| With | 57 | 38.6% (22/57) | |
| Without | 616 | 41.9% (258/616) | 0.827 |
| IOL type | |||
| Humanoptics | 346 | 40.5% (140/346) | |
| Rayner | 327 | 42.8% (140/327) | 0.584 |
| IOL power (D) | |||
| < 10D | 376 | 48.7% (183/376) | |
| ≥ 10D | 297 | 32.7% (97/297) | < 0.001 |
| IOL thickness (mm) | |||
| < 0.74 mm | 342 | 43.6% (149/342) | |
| ≥ 0.74 mm | 331 | 39.6% (131/331) | 0.294 |
EPCO Evaluation of posterior capsular opacification, PCO-3 mm EPCO score within the central 3.0 mm zone, PCO-C EPCO score within the capsulorhexis margin, Nd:YAG neodymium-doped yttrium aluminum garnet, IOL intraocular lens
Data were presented as mean ± standard deviation or percentage (proportion)
Difference of clinically significant PCO rate among different groups was analyzed by χ2 test
According to the multivariate logistic regression analysis with backward stepwise selection, longer axial length and follow-up duration were independent risk factors for clinically significant PCO after cataract surgery in highly myopic eyes (both P < 0.01, Table 4).
Table 4.
Backward stepwise multivariate logistic regression analysis of independent risk factors for clinically significant posterior capsular opacification after cataract surgery in highly myopic eyes
| Parameter | OR | 95% CI of OR | P-value |
|---|---|---|---|
| Axial length | 1.124 | 1.038–1.218 | 0.004 |
| Follow-up duration | 1.082 | 1.061–1.103 | < 0.001 |
OR odds ratio, CI confidence interval
Discussion
As one of the most common complications after modern cataract surgery, PCO has been previously studied. However, whether high myopia would exacerbate PCO remained controversial, not to mention the relevant influencing factors. Our study took the lead in conducting a long-term prospective cohort study with a large sample size of highly myopic subjects. We made a quantitative evaluation of PCO, and separate analysis of Nd:YAG capsulotomy rate and clinically significant PCO rate. Our study showed that highly myopic eyes had more severe PCO in the long term, and longer AL and follow-up duration were associated with a higher risk of clinically significant PCO after cataract surgery in these eyes.
In this study, we defined clinically significant PCO by combining visual-impairing PCO and Nd:YAG capsulotomy with the help of ROC analysis, while previous studies generally put emphasis on either PCO score or Nd:YAG capsulotomy rate alone. Since PCO severity could only provide part of the truth after excluding cases with Nd:YAG capsulotomy, and Nd:YAG capsulotomy rate was not an accurate indicator of PCO since it might be affected by individual health behavior [15, 20], the clinically significant PCO provided a way out for the overall PCO assessment.
In our study, we found that more severe PCO occurred in HM group compared with control group, and it would be further aggravated in extreme myopia with regard to PCO severity and clinically significant PCO rate. Some previous studies supported the conclusion that longer AL leads to a higher incidence of PCO [1, 7, 14]; yet others claimed that AL was not associated with the degree of PCO [7, 10]. Such inconsistency was possibly brought by different measuring methods and a limited sample size. Our result was consistent with the opinions that highly myopic eyes were more likely to form incomplete capsule-IOL interaction due to larger capsular bags and thinner IOL implanted [1], compared with non-myopic eyes, resulting in poor barrier effect against LEC migration. Those residual cells in the capsular bag of highly myopic eyes gradually encroached from the periphery toward the visual axis and were responsible for most cases of PCO-related visual losses. In addition, the proinflammatory status in the aqueous humor of highly myopic eyes may also facilitate PCO formation [4, 21]. Thus, for highly myopic eyes, anti-inflammation eye drops may be used for a longer time during perioperative period and posterior capsular polishing should be properly performed to prevent PCO.
Among other previously proposed risk factors for PCO, only age and follow-up duration showed significance in highly myopic eyes in the present study. Our study showed that younger age was related to a higher clinically significant PCO rate in univariate analysis, which was consistent with the previous studies [12, 14]. This may be due to the more vigorous metabolism of young people and the faster migration of LECs in their eyes. However, patients with longer AL normally suffered from higher risk of early-onset, rapid-progressive nuclear cataract, and were generally diagnosed with cataract and received cataract surgery at an earlier age [2, 22]. Thus, the collinearity between AL and age might exclude age as an independent risk factor for clinically significant PCO in multivariate analysis. Other potential risk factors for PCO including gender, diabetes, and IOL type did not show significance in highly myopic eyes according to our study. In addition, a previous study showed that use of lower diopter IOLs was associated with increased incidence of clinically significant PCO [23], which was consistent with our univariate analysis’s result in highly myopic eyes. However, we did not find such a relationship in multivariate logistic regression analysis, suggesting that the risk of PCO may depend more on the AL than the IOL power, because lower diopter IOLs were usually used in higher myopic eyes. We did not find the correlation between IOL thickness and incidence of clinically significant PCO. This may be due to the difference of average thickness of two IOL types. Rayner 920H was thicker than Humanoptics MC X11 ASP with the same IOL power.
However, there were some limitations of this study. The sample size of the control group was much smaller than HM group. We only included two types of hydrophilic acrylic IOL in this study. Thus, we could not know the influence of IOL materials of PCO in highly myopic eyes. The current study was a single-center clinical study and needs to be verified in multiple centers in the future. In addition, patients with long-term multiple follow-ups at certain times would provide stronger evidence.
Conclusion
Our study indicated that highly myopic eyes had more severe PCO in the long-term follow-up compared with the controls. Longer AL and follow-up duration would increase the risk of PCO after cataract surgery in these eyes.
Acknowledgements
Funding
This study and its publication, including the journal’s Rapid Service Fee were funded by research grants from the National Natural Science Foundation of China (82122017, 81870642, 81970780, 81900838, 81470613 and 81670835), Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (No. 19441900700 and No.21S31904900), Clinical Research Plan of Shanghai Shenkang Hospital Development Center (No. SHDC2020CR4078, SHDC12019X08 and SHDC12020111), and the Fudan University “Outstanding 2025” Program and Double-E Plan of Eye & ENT Hospital of Fudan University (SYA202006).
Author Contributions
Xiangjia Zhu and Yi Lu designed the study. Wenwen He, Kaiwen Cheng, Liangliang Zhao, Shuyu Liu, Zhiqian Huang, Keke Zhang, Yu Du collected the data. Wenwen He, Kaiwen Cheng, Liangliang Zhao and Shuyu Liu analyzed and interpreted the data. Wenwen He and Shuyu Liu drafted the manuscript, and Xiangjia Zhu, Yi Lu and Xingtao Zhou substantively revised it. Xiangjia Zhu, Yi Lu and Xingtao Zhou reviewed the manuscript. All the authors have approved the final manuscript.
Disclosures
Wenwen He, Kaiwen Cheng, Liangliang Zhao, Shuyu Liu, Zhiqian Huang, Keke Zhang, Yu Du, Xingtao Zhou, Yi Lu, and Xiangjia Zhu declare that they have no competing interests.
Compliance with Ethics Guidelines
The design and conduction of the study were in accordance with the tenets of the Declaration of Helsinki, and were approved by the Institutional Review Board of Eye & Ear, Nose and Throat (ENT) Hospital of Fudan University (No. 2014055). All the participants were informed to use their clinical data and participate in further follow-up, and the data used for publication will not contain their personalized information.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Wenwen He, Kaiwen Cheng, Liangliang Zhao and Shuyu Liu contributed equally to this work.
Contributor Information
Yi Lu, Email: luyieent@163.com.
Xiangjia Zhu, Email: zhuxiangjia1982@126.com.
References
- 1.Zhao Y, Li J, Lu W, Chang P, Lu P, Yu F, et al. Capsular adhesion to intraocular lens in highly myopic eyes evaluated in vivo using ultralong-scan-depth optical coherence tomography. Am J Ophthalmol. 2013;155(3):484–491. doi: 10.1016/j.ajo.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Zhu XJ, Zhou P, Zhang KK, Yang J, Luo Y, Lu Y. Epigenetic regulation of αA-crystallin in high myopia-induced dark nuclear cataract. PLoS ONE. 2013;8(12):e81900. doi: 10.1371/journal.pone.0081900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyoshi T, Fujie S, Yoshida H, Iwamoto H, Tsukamoto H, Oshika T. Effects of capsular tension ring on surgical outcomes of premium intraocular lens in patients with suspected zonular weakness. PLoS ONE. 2020;15(2):e228999. doi: 10.1371/journal.pone.0228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu X, Zhang K, He W, Yang J, Sun X, Jiang C, et al. Proinflammatory status in the aqueous humor of high myopic cataract eyes. Exp Eye Res. 2016;142:13–18. doi: 10.1016/j.exer.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Elgohary MA, Dowler JG. Incidence and risk factors of Nd:YAG capsulotomy after phacoemulsification in non-diabetic and diabetic patients. Clin Exp Ophthalmol. 2006;34(6):526–534. doi: 10.1111/j.1442-9071.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 6.Güell JL, Rodriguez-Arenas AF, Gris O, Malecaze F, Velasco F. Phacoemulsification of the crystalline lens and implantation of an intraocular lens for the correction of moderate and high myopia: four-year follow-up. J Cataract Refract Surg. 2003;29(1):34–38. doi: 10.1016/S0886-3350(02)01944-2. [DOI] [PubMed] [Google Scholar]
- 7.Vasavada AR, Shah A, Raj SM, Raveen MR, Shah GD. Prospective evaluation of posterior capsule opacification in myopic eyes 4 years after implantation of a single-piece acrylic IOL. J Cataract Refract Surg. 2009;35(9):1532–1539. doi: 10.1016/j.jcrs.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Du Y, Li D, et al. Aberrant TGF-β1 signaling activation by MAF underlies pathological lens growth in high myopia. Nat Commun. 2021;12(1):2102. doi: 10.1038/s41467-021-22041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu X, He W, Zhang Y, Chen M, Du Y, Lu Y. Inferior decentration of multifocal intraocular lenses in myopic eyes. Am J Ophthalmol. 2018;188:1–8. doi: 10.1016/j.ajo.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K, Yoshida M, Hayashi H. Posterior capsule opacification in myopic eyes. J Cataract Refract Surg. 2006;32(4):634–638. doi: 10.1016/j.jcrs.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine (Baltimore) 2017;96(44):e8301. doi: 10.1097/MD.0000000000008301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindholm JM, Laine I, Tuuminen R. Intraocular lens power, myopia, and the risk of Nd:YAG capsulotomy after 15,375 cataract surgeries. J Clin Med. 2020;9(10):3071. doi: 10.3390/jcm9103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toda J, Kato S, Oshika T, Sugita G. Posterior capsule opacification after combined cataract surgery and vitrectomy. J Cataract Refract Surg. 2007;33(1):104–107. doi: 10.1016/j.jcrs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Tong N, Pan L, Jiang X, Li Y, Guo M, et al. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018;2018:9089285. doi: 10.1155/2018/9089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Praveen MR, Shah GD, Vasavada AR, Dave KH. The effect of single-piece hydrophobic acrylic intraocular lenses on the development of posterior capsule opacification. Am J Ophthalmol. 2015;160(3):470–478. doi: 10.1016/j.ajo.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Ohsugi H, Ikuno Y, Matsuba S, Ohsugi E, Nagasato D, Shoujou T, et al. Morphologic characteristics of macular hole and macular hole retinal detachment associated with extreme myopia. Retina. 2019;39(7):1312–1318. doi: 10.1097/IAE.0000000000002155. [DOI] [PubMed] [Google Scholar]
- 17.Rong X, He W, Zhu Q, Qian D, Lu Y, Zhu X. Intraocular lens power calculation in eyes with extreme myopia: comparison of Barrett Universal II, Haigis, and Olsen formulas. J Cataract Refract Surg. 2019;45(6):732–737. doi: 10.1016/j.jcrs.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Yang K, Xu L, Fan Q, Zhao D, Ren S. Repeatability and comparison of new Corvis ST parameters in normal and keratoconus eyes. Sci Rep. 2019;9(1):15379. doi: 10.1038/s41598-019-51502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belda JI, Daban JP, Elvira JC, O'Boyle D, Puig X, Perez-Vives C, et al. Nd:YAG capsulotomy incidence associated with five different single-piece monofocal intraocular lenses: a 3-year Spanish real-world evidence study of 8293 eyes. Eye (Lond) 2022;36(11):2205–2210. doi: 10.1038/s41433-021-01828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141(3):456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Li D, Du Y, He W, Lu Y. DNA hypermethylation-mediated downregulation of antioxidant genes contributes to the early onset of cataracts in highly myopic eyes. Redox Biol. 2018;19:179–189. doi: 10.1016/j.redox.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecht I, Dubinsky-Pertzov B, Karesvuo P, et al. Association between intraocular lens diopter and posterior capsular opacification. Clin Exp Ophthalmol. 2020;48:889–894. doi: 10.1111/ceo.13821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.