Abstract
The pandemic of Gompertz virus disease remains a pressing issue in agricultural production. Moreover, the dynamics of various infectious diseases is usually investigated by the method of mathematical modelling. A new mathematical model for dynamics on Gompertz virus disease impulsive system is proposed and analyzed in this paper. We prove the dynamic characteristics about the permanence and globally exponential stability of Gompertz virus disease model. Moreover, we also give the sufficient condition that one positive solution which satisfies if exists. Eventually, numerical simulations are utilized to validate the validity of the theoretically analyzed conclusion in this paper.
Subject terms: Computational biology and bioinformatics, Ecology
Introduction
It is well-known that a nonlinear system model usually has a rich dynamic behavior which has been payed attention to by many researchers1–6. Moreover, in recent years, many researchers have paid attention to biological control7–16.
Due to many cases that we need to describe the biological dynamics with sudden changes as well as other phenomena, impulsive differential equations play important part17–19. In consideration of hybrid nature, different kinds of evolutionary processes in real-world life are usually described by impulsive systems, and these evolutionary processes usually state a big sudden change at some moments20–23. Some researchers stated that differential equations with impulse do well in describing biological control, and thought that differential equations with impulsive effect are more practical than ordinary differential equations in stating practical problems24,25. Liang et al.26 proposed the impulsive control of Leslie predator-prey model, and investigated the dynamic properties of the proposed mathematical biology model. The herbivore-plankton model with cannibalism was proposed by Fang et al.27, and the corresponding heteroclinic bifurcations and three order-1 periodic orbits were analyzed. A new impulsive state feedback model was proposed, and the management strategy with impulsive control was developed28. Wang and Chen29 proposed a new microbial pesticide model and analyzed the condition of the existence of corresponding periodic orbit. Li and Chen30 investigated the dynamics of the impulsive turbidostat model, and also studied the characteristic of the proposed mathematical biology model. Li et al.31 proposed a new water hyacinth fish impulsive control model, and investigated the local stability and global dynamics of the proposed ecological system. Li et al.32 proposed a new Filippov predator-prey model, and analyzed the corresponding dynamics using the Filippov theory knowledge. Qin et al.33 proposed a new non-smooth Filippov ecosystem with group defense and also studied the stability of equilibria and the bifurcation phenomenon numerically. Discontinuous plant disease models with a non-smooth separation line were investigated and discussed by Li et al.34. Khan et al., proposed some new hepatitis B epidemic models and novel corona virus disease models and investigated their dynamic characteristics35–40.
In fact, exploiting viruses and the release of pest population simultaneously, some research studies have been attached to devote the control of pests9,41. First of all, introducing pathogens into the pest population is expected to produce an epidemic and then become endemic. Moreover, the infected pests are released to the periodic impulsive pest population. However, the crops will not be affected by the infected pest. The vulnerable pests are infected by direct contact with infected pests or being exposed to an infected environment. Then the pest population and its death will be affected to some extent42,43. In recent years, the pandemic of Gompertz virus disease remains a pressing issue in epidemiological ecosystem. Mathematical modelling is an important method in investigating the dynamic characteristics of epidemiological ecosystem. Additionally, due to the hybrid nature, in order to well describe different kinds of real-world evolutionary processes, impulsive systems play important role because it states sudden change at some moments. This article will try to develop a new epidemiological ecosystem with impulsive effect and investigate its dynamics for bio-control. Additionally, it is assumed that the pest population will grow according to the logic curve without the disease9,44,45. Then we investigate the global attractiveness and permanence of the proposed model.
The remainder of this paper is provided as follows. In section “Mathematical model”, the Gompertz virus disease model with impulsive effect is established. In section “Global attractiveness”, utilizing the comparison theorem and Floquet theory of impulsive differential equation, the global attractiveness of the proposed Gompertz virus disease model is strictly proved. In section “Uniform persistence”, we obtain the criteria for the uniform persistence of the proposed Gompertz virus disease model. In section “Numerical simulations”, many numerical results are given to prove the validity of the obtained results. Finally, some conclusions are summarized.
Mathematical model
In this section, we will present the newly developed Gompertz virus disease model with impulsive effect.
The total population of insect pest is denoted by N(t), and it is divided into five subgroups such as I(t), E(t), S(t), R(t) and A(t). The susceptible pest is denoted by S(t); E(t) denotes the exposed pest; the infected (symptomatic) pest is denoted by I(t); A(t) represents the asymptotically infected pest; the recovered or the removed pest is denoted by R(t). The class M(t) denotes the reservoir or the seafood place or market.
In this paper, the impulsive introduction of disinfectants and the pulse vaccination strategy in the environment are investigated for the control of the Gompertz virus epidemic, and we also assume that the proposed model satisfies:
and respectively denotes the natural death rate and the birth rate of the pest.
The susceptible pest S(t) acquires Gompertz virus infection by ingesting environmental virus from the contaminated reservoir or seafood place or market, through the term given by where is the disease transmission coefficient.
The proportion of asymptomatic infection is denoted by . The transmission rate of the infected is denoted by denotes the transmission rate after completing the incubation period. K represents the pathogen concentration.
and respectively denote the pest in I(t) and A(t) joining R(t) with the removal or recovery rate, and Gompertz virus induced mortality rate of infected individuals is denoted by .
denotes the susceptible pest infected after the interaction with M(t), in which the disease transmission coefficient between S(t) and E(t) is represented by
The parameters and respectively denote the infected symptomatic and the asymptomatically infected contributing the Gompertz virus into the seafood market M(t). The removing rate of the Gompertz virus from the seafood market M(t) is given by the rate d. The natural growth rate of the Gompertz virus is denoted by b. We assume through this paper.
The parameter denotes the part of susceptible pest inoculated by the vaccine at times in which n is a positive integer, and the impulsive period between two consecutive pulse vaccinations is denoted by T. nT represents the pulse vaccination at the time of multiple periods. R(t) represents the temporary immunity which contains the recovered individual and the vaccinated individual.
- The death of the Gompertz virus dues to the environmental sanitation. Therefore, considering the pulse inoculation of disinfectant in the environment at the time of nT, the death rate of the virus is denoted by The model is expressed as:
1
For (1), all the coefficients are greater than 0. Based on
| 2 |
then the population size will change with t. If , we have
Similarly, we have
Then we have that when t takes the sufficiently large value,
By replacing with R(t), then
| 3 |
We provide the initial condition for (3) as:
| * |
Next, we will study the dynamic properties of (3). For biological considerations, (3) is investigated in the following closed set and this set is given as
In fact, it is not difficult to check that has a positively invariant property about (3). For further discussion, some important assumptions and definitions are given as follows.
We assume that the fixed solution and the arbitrary solution of (3) are respectively given by
and
Definition 1
, we have that when t takes the sufficiently large value, Then (3) has the uniform persistence.
Definition 2
For arbitrary , If , then has the global attractivity.
Definition 3
and when there is Moreover, if , and then Then has the characteristic of stability.
Definition 4
When the fixed solution has the characteristic of stability and global attractivity, then is called globally asymptotical stable. If there is the disease-free periodic solution for (3) which also has the characteristic of globally asymptotical stability, the Gompertz virus will finally disappear.
Definition 5
(Equicontinuty) For if we can find a positive parameter which satisfies holds true when and x(t) is said to be equicontinuous when
For further analysis conveniently, the following lemma is given as follows.
Lemma 1
46 We consider
| 4 |
where and
Thus we can obtain that for the periodic solution is given as
where
exists and is globally asymptotically stable, unique and positive.
Global attractiveness
In this section, we will discuss the global attractiveness of the Gompertz virus disease model with impulsive effect (3).
It means that the asymptotically infectious individuals permanently die out. Based on this prerequisite, we can transform (3) as:
| 5 |
Then we can obtain
Next, we will prove that for the susceptible S(t), there is one periodic solution. Firstly, we consider
| 6 |
It follows from Lemma 1 that a unique globally asymptotical periodic solution of (6) exists, and it is given as
| 7 |
Therefore, there exists one infection-free periodic solution which is given by
Theorem 1
For system (3), has global attractivity for , in which
Proof
Since , can be chosen such that
| 8 |
According to then there exists satisfying the following inequality
| 9 |
By (3), then
When and we investigate
| 10 |
For system (10), we can obtain the globally asymptotical stable periodic solution as utilizing Lemma 1, and also there is such that when ,
| 11 |
Then
Thus
| 12 |
Therefore, for , there is , satisfying when .
Then, according to (3), we can obtain that if
i.e.
Hence,
| 13 |
Similarly, for , there is , satisfying
when .
Exploiting the fourth one of (3), the following inequality can be obtained as
for all
Obviously
| 14 |
Therefore, for arbitrary , there is satisfying when
Next, according to the sixth one of (3), we get
Obviously,
| 15 |
Therefore, for , there is satisfying at the case
Eventually, we can obtain
when
When , the following comparative model will be investigated.
| 16 |
Obviously,
Exploiting the corresponding lemma of25, we can obtain that if , there is such that
| 17 |
Because and and they are extremely small, we can obtain by (9) and (17) that
| 18 |
From (9) and (12)–(15), we can get the following conclusion:
For , there is satisfying
when
When , it follows from (3) that
when and
Considering
| 19 |
based on Lemma 1, its unique asymptotical stable periodic solution can be computed as
when in which
According to25, there exists satisfying
| 20 |
for
Because and take the very small value, then we have
| 21 |
when
By (12)–(15), (18) and (21), the infection-free periodic solution is the globally attractive. Based on Theorem 1, we can obtain some assertions as follows.
Proposition 1
- The infection-free periodic solution which is given by is globally attractive, when
- If and
is globally attractive, where
Proposition 2
If
is globally attractive.
Uniform persistence
In this section, we will discuss the sufficient conditions for the Gompertz virus disease model with impulsive effect. In order to obtain uniform persistence, the following lemma needs to be proved first.
Lemma 2
If then for arbitrary doesn’t hold true for all where
Proof
Herein we use the method of contradiction. We assume that there is which satisfies when
| 22 |
When
| 23 |
and
| 24 |
By (23) and (24), then and when then
and
for arbitrary
Thus, when
It is not difficult to check that there is such that
Hence, when and we can obtain
For the case of and
| 25 |
It follows from Lemma 1 that
is globally attractive, in which
Thus, there exists a constant satisfying that when
| 26 |
According to (26) and (3), we can obtain
| 27 |
Integrating (27) from nT to considering that is arbitrary small and , then we can get
Using we get
Thus
This is the contradiction with that E(t) is bounded. Thus, there is such that and the above prediction is proved.
Theorem 2
When for system (3), there will be satisfying that there exists some positive solution which satisfies
Proof
Define
According to Lemma 2, is discussed based on the following cases.
Firstly, when t takes sufficiently large value.
Secondly, E(t) vibrates about when t takes sufficiently large value.
For case 1, we have Immediately, we have the conclusion of Theorem 2.
For case 2, let and be sufficiently large satisfying
If , since
and
we have ().
When then
if .
Similarly, we can prove
when and
when .
In the following, the conclusion that holds true when will be proved.
Herein we use the method of contradiction, and it means there is a positive constant satisfying if
and
Exploiting (3), and , then
This is a contradiction. So when
Theorem 3
If we have the conclusion that model (3) is permanent.
Proof
We assume the arbitrary solution of (3) is denoted by
If t takes a sufficiently large value, we can obtain
Using the same method, we have
where
( is sufficiently small).
When t takes a sufficiently large value, we derive
Therefore
where
When t is sufficiently large, we can obtain
So
where
According to Theorem 2, when t takes sufficiently large value, then
Therefore
where
( is sufficiently small).
Set
It follows from Theorem 3 and the analysis above that belongs to the globally attractive region of For the system (3), the positive solution satisfying the condition (*) will finally enter and remain in i.e., the system will be permanent.
According to Theorem 2 and Theorem 3, the following assertions can be easily obtained.
Proposition 3
If , system (3) will be permanent, i.e., the Gompertz virus will be an endemic disease, in which
When , system (3) will be permanent, and the Gompertz virus will be an endemic disease, in which
It follows from Theorem 1 and Theorem 3 that the Gompertz virus will not appear when , and when the Gompertz virus will be uniformly persistent.
Numerical simulations
In this section, the Runge-Kutta method is exploited to perform numerical simulations of Gompertz virus disease model with impulsive effect. Numerical simulations are presented to validate the analyzed conclusion from the view of impulsive differential equation theory.
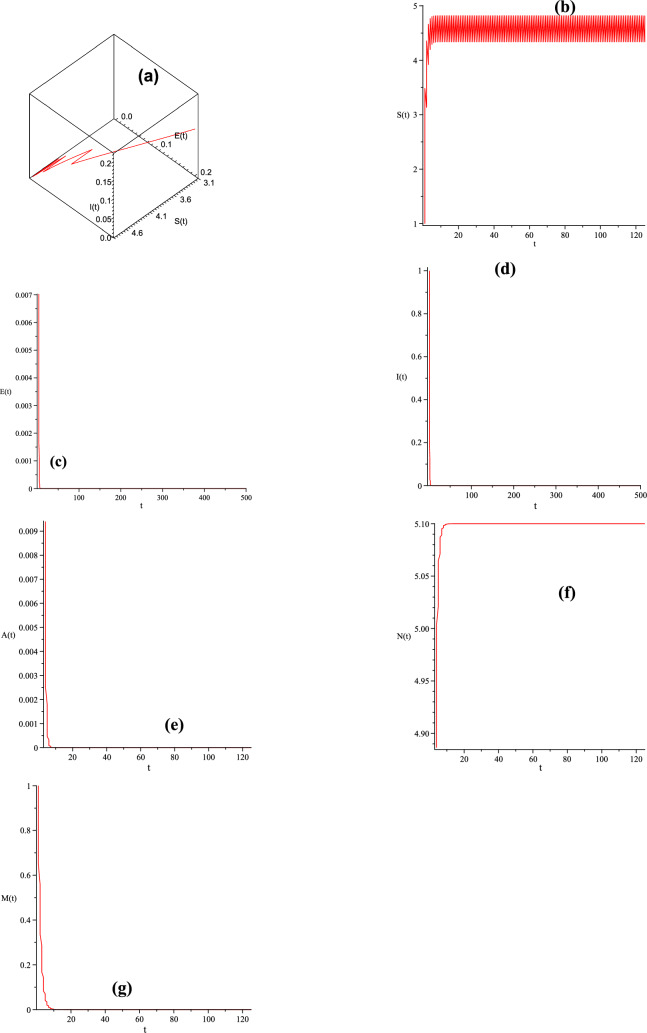
Firstly, when we choose we compute which satisfies the condition of Theorem 1. By Theorem 1, the infection-free periodic solution of (3) is globally asymptotically stable. Figure 1 also demonstrates this conclusion: The periodic solution has the characteristic of global attractivity. It can be shown from Fig. 1 that S(t) goes to oscillatory; E(t), I(t), A(t) and M(t) go to extinction; N(t) goes to some stable value. It implies that the susceptible pest population oscillate with a positive amplitude, i.e., they will not go extinct. Additionally, the infective pests almost will go extinct and have little effect on the crop, which can be shown in Fig. 1.
Figure 1.
Dynamical behavior of system (3) with and all the parameters satisfy (a) (I(t), E(t), S(t)); (b) trajectory of the susceptible pest S(t); (c) trajectory of the exposed pest E(t); (d) trajectory of the infected pest I(t); (e) trajectory of the asymptotically infected pest A(t); (f) trajectory of the total population of pest N(t); (g) trajectory of the reservoir or the seafood place or market M(t).
Moreover, when we choose we compute which satisfies the condition of Theorem 3. By Theorem 3, we can obtain that the proposed model is permanent, which can be shown in Fig. 2. Figure 2 also demonstrates this conclusion: The periodic solution has the characteristic of permanence. It can be shown in Fig. 2 that M(t), A(t), I(t), E(t) and S(t) go to oscillatory; N(t) goes to some stable value. It means that all the species will not go extinct and will almost maintain a stable value, which can be shown in Fig. 2.
Figure 2.
Dynamical behavior of system (3) with and all the parameters satisfy (a) (I(t), E(t), S(t)); (b) trajectory of the susceptible pest S(t); (c) trajectory of the exposed pest E(t); (d) trajectory of the infected pest I(t); (e) trajectory of the asymptotically infected pest A(t); (f) trajectory of the total population of pest N(t); (g) trajectory of the reservoir or the seafood place or market M(t).
From the view of ecology, infective pests almost have no effect on crops, but the pests that have a significant impact on the crops will become extinct. This will also demonstrate the effectiveness of scientific control. Actually, the purpose of scientific control is not to eradicate pests, but to control the number of pests to a certain level.
Conclusions
A new mathematical biological model on the Gompertz virus impulsive system is proposed in this paper. This proposed model considers the factors of the recovered or the removed population, asymptotically infected, infected (symptomatic), exposed and susceptible population. Utilizing the Floquet theory, we strictly prove the infection-free periodic solution of the proposed model is globally attractive if It means that the infective pests almost will go extinct and have little effect on the crop. Moreover, we also obtain that the system (3) is permanent if Hence, we exploit the impulsive control method based on the effect of the Gompertz virus on pest such that and then drive the virus to extinction. In addition, we can also control that the susceptible, exposed, infected (symptomatic), asymptotically infected population and the Gompertz virus in the market or seafood place or the reservoir oscillate with a positive amplitude. Actually, the scientific impulsive control is not to eradicate pests, but to control the number of pests to a certain level. Therefore, the proposed impulsive control strategy in this paper is very effective in impulsive control for the Gompertz virus model. Considering that time delays unavoidably exist in the transmission of the impulsive information and sampling in lots of practical cases, the proposed system will be extended to the delayed impulsive control for the Gompertz virus disease model in the future.
Acknowledgements
This work was supported by the Open Fund of Hubei Key Laboratory of Hydroelectric Machinery Design and Maintenance (2019KJX12).
Author contributions
L.W. contributed to the conception of the study. A.S. contributed significantly to data analysis and manuscript preparation; Y.X. helped to perform the analysis with constructive discussions.
Data availibility
Data will be made available on request from the corresponding author (Youxiang Xie).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guo SJ, Chen YM, Wu JH. Two-parameter bifurcations in a network of two neurons with multiple delays. J. Differ. Equ. 2008;244:444–486. doi: 10.1016/j.jde.2007.09.008. [DOI] [Google Scholar]
- 2.Ji W, Zhang H, Qiu J. Fuzzy affine model-based output feedback controller design for nonlinear impulsive systems. Commun. Nonlinear Sci. Numer. Simul. 2019;79:104894. doi: 10.1016/j.cnsns.2019.104894. [DOI] [Google Scholar]
- 3.Driessche PV, Zou X. Global attractivity in delayed Hopfield neural network models. SIAM J. Appl. Math. 1998;58:1878–1890. doi: 10.1137/S0036139997321219. [DOI] [Google Scholar]
- 4.Wang LJ, Han X. Stability and Hopf bifurcation analysis in bidirectional ring network model. Commun. Nonlinear Sci. Numer. Simul. 2011;16:3684–3695. doi: 10.1016/j.cnsns.2010.12.022. [DOI] [Google Scholar]
- 5.Wang BX, Jian JG. Stability and Hopf bifurcation analysis on a four-neuron BAM neural network with distributed delays. Commun. Nonlinear Sci. Numer. Simul. 2010;15:189–204. doi: 10.1016/j.cnsns.2009.03.033. [DOI] [Google Scholar]
- 6.Jiang XW, Li JH, Li B, Yin W, Sun L, Chen XY. Bifurcation, chaos, and circuit realisation of a new four-dimensional memristor system. De Gruyter. 2021 doi: 10.1515/ijnsns-2021-0393. [DOI] [Google Scholar]
- 7.Gao L, Wang D, Zong G. Exponential stability for generalized stochastic impulsive functional differential equations with delayed impulses and Markovian switching. Nonlinear Anal. Hybrid Syst. 2018;30:199–212. doi: 10.1016/j.nahs.2018.05.009. [DOI] [Google Scholar]
- 8.Yu HG, Zhong SM, Agarwal RP, Sen SK. Effect of seasonality on the dynamical behavior of an ecological system with impulsive control strategy. J. Franklin Inst. 2011;348:652–670. doi: 10.1016/j.jfranklin.2011.01.009. [DOI] [Google Scholar]
- 9.Wang LJ, Xie YX, Fu JQ. The dynamics of natural mortality for pest control model with impulsive effect. J. Franklin Inst. 2013;350:1443–1461. doi: 10.1016/j.jfranklin.2013.03.008. [DOI] [Google Scholar]
- 10.Zou L, Xiong ZL, Shu ZP. The dynamics of an eco-epidemic model with distributed time delay and impulsive control strategy. J. Franklin Inst. 2011;348:2332–2349. doi: 10.1016/j.jfranklin.2011.06.023. [DOI] [Google Scholar]
- 11.Xie YX, Yuan ZH, Wang LJ. Dynamic analysis of pest control model with population dispersal in two patches and impulsive effect. J. Comput. Sci. 2014;5:685–695. doi: 10.1016/j.jocs.2014.06.011. [DOI] [Google Scholar]
- 12.Xie YX, Wang LJ, Deng QC, Wu ZJ. The dynamics of an impulsive predator-prey model with communicable disease in the prey species only. Appl. Math. Comput. 2017;292:320–335. [Google Scholar]
- 13.Liang JH, Tang SY, Cheke RA. Beverton-Holt discrete pest management models with pulsed chemical control and evolution of pesticide resistance. Commun. Nonlinear Sci. Numer. Simul. 2016;36:327–341. doi: 10.1016/j.cnsns.2015.12.014. [DOI] [Google Scholar]
- 14.Shah K, Abdeljawad T, Jarad F, Al-Mdallal Q. On nonlinear conformable fractional order dynamical system via differential transform method. Comput. Model. Eng. Sci. 2023;136:1457–1472. [Google Scholar]
- 15.Li B, Eskandari Z, Avazzadeh Z. Strong resonance bifurcations for a discrete-time preyCpredator model. J. Appl. Math. Comput. 2023;69:2421–2438. doi: 10.1007/s12190-023-01842-2. [DOI] [Google Scholar]
- 16.Li B, Eskandari Z, Avazzadeh Z. Dynamical behaviors of an SIR epidemic model with discrete time. Fract. Fract. 2022;659:1–17. [Google Scholar]
- 17.Shah K, Abdalla B, Abdeljawad T, Gul R. Analysis of multipoint impulsive problem of fractional-order differential equations. Bound. Value Probl. 2023 doi: 10.1186/s13661-022-01688-w. [DOI] [Google Scholar]
- 18.Sitthiwirattham T, Gul R, Shah K, Mahariq I, Soontharanon J, Ansari KJ. Study of implicit-impulsive differential equations involving Caputo-Fabrizio fractional derivative. AIMS Math. 2021;7(3):4017–4037. doi: 10.3934/math.2022222. [DOI] [Google Scholar]
- 19.Shah K, Ahmad I, Nieto JJ, Rahman GU, Abdeljawad T. Qualitative investigation of nonlinear fractional coupled pantograph impulsive differential equations. Qual. Theory Dyn. Syst. 2022;21:131. doi: 10.1007/s12346-022-00665-z. [DOI] [Google Scholar]
- 20.Wang YQ, Lu JQ. Some recent results of analysis and control for impulsive systems. Commun. Nonlinear Sci. Numer. Simul. 2020;80:104862. doi: 10.1016/j.cnsns.2019.104862. [DOI] [Google Scholar]
- 21.Guan Z, Chen G, Jian M. On delayed impulsive Hopfield neural networks. Neural Netw. 1999;12(2):273–280. doi: 10.1016/S0893-6080(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Shen J, Rakkiyappan R. Persistent impulsive effects on stability of functional differential equations with finite or infinite delay. Appl. Math. Comput. 2018;329:14–22. [Google Scholar]
- 23.Lakshmikantham V, Simeonov P. Theory of Impulsive Differential Equations. World Scientific; 1989. [Google Scholar]
- 24.Bainov, D.D. & Simeonov, P.S. Impulsive differential equations: Periodic solutions and application. In Pitman Monographs and Surveys in Pure and Applied Mathematics, vol. 66, Longman Science and Technical, Harlow, UK (1993).
- 25.Lakshmikantham V, Bainov D, Simeonov P. Theory of Impulsive Differential Equations. World Scientific Publisher; 1989. [Google Scholar]
- 26.Liang Z, Zeng X, Pang G, et al. Periodic solution of a Leslie predator-prey system with ratio-dependent and state impulsive feedback control. Nonlinear Dyn. 2017;89(4):2941–2955. doi: 10.1007/s11071-017-3637-4. [DOI] [Google Scholar]
- 27.Fang D, Pei Y, Lv Y, et al. Periodicity induced by state feedback controls and driven by disparate dynamics of a herbivore-plankton model with cannibalism. Nonlinear Dyn. 2017;90(4):2657–2672. doi: 10.1007/s11071-017-3829-y. [DOI] [Google Scholar]
- 28.Zhang T, Meng X, Liu R, et al. Periodic solution of a pest management Gompertz model with impulsive state feedback control. Nonlinear Dyn. 2014;78(2):921–938. doi: 10.1007/s11071-014-1486-y. [DOI] [Google Scholar]
- 29.Wang T, Chen L. Nonlinear analysis of a microbial pesticide model with impulsive state feedback control. Nonlinear Dyn. 2011;65(1–2):1–10. doi: 10.1007/s11071-010-9828-x. [DOI] [Google Scholar]
- 30.Li Z, Chen L. Periodic solution of a turbidostat model with impulsive state feedback control. Nonlinear Dyn. 2009;58(3):525–538. doi: 10.1007/s11071-009-9498-8. [DOI] [Google Scholar]
- 31.Li WJ, Ji JC, Huang LH. Global dynamics analysis of a water hyacinth fish ecological system under impulsive control. J. Franklin Inst. 2022;359:10628–10652. doi: 10.1016/j.jfranklin.2022.09.030. [DOI] [Google Scholar]
- 32.Li WX, Chen YM, Huang LH, Wang JF. Global dynamics of a filippov predator-prey model with two thresholds for integrated pest management. Chaos Solit. Fract. 2022;157:111881. doi: 10.1016/j.chaos.2022.111881. [DOI] [Google Scholar]
- 33.Qin WJ, Tan XW, Tosato M, Liu XZ. Threshold control strategy for a non-smooth filippov ecosystem with group defense. Appl. Math. Comput. 2019;362:1–18. [Google Scholar]
- 34.Li WX, Huang LH, Wang JF. Dynamic analysis of discontinuous plant disease models with a non-smooth separation line. Nonlinear Dyn. 2020;99(2):1675–1697. doi: 10.1007/s11071-019-05384-w. [DOI] [Google Scholar]
- 35.Khan T, Khan A, Zaman G. The extinction and persistence of the stochastic hepatitis B epidemic model. Chaos Solit. Fract. 2018;108:123–128. doi: 10.1016/j.chaos.2018.01.036. [DOI] [Google Scholar]
- 36.Khan T, Qian ZS, Ullah R, Alwan BA, Zaman G, Al-Mdallal QM, Khatib YE, Kheder K. The transmission dynamics of hepatitis B virus via the fractional-order epidemiological model. Complexity. 2021;2021:8752161. doi: 10.1155/2021/8752161. [DOI] [Google Scholar]
- 37.Khan T, Zaman G, Chohan MI. The transmission dynamic and optimal control of acute and chronic hepatitis B. J. Biol. Dyn. 2017;11:172–189. doi: 10.1080/17513758.2016.1256441. [DOI] [PubMed] [Google Scholar]
- 38.Khan T, Zaman G, El-Khatib Y. Modeling the dynamics of novel coronavirus (COVID-19) via stochastic epidemic model. Results Phys. 2021;24:104004. doi: 10.1016/j.rinp.2021.104004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan T, Ullah R, Zaman G, Alzabut J. A mathematical model for the dynamics of SARS-CoV-2 virus using the Caputo-Fabrizio operator. Math. Biosci. Eng. 2021;18(5):6095–6116. doi: 10.3934/mbe.2021305. [DOI] [PubMed] [Google Scholar]
- 40.Ullah R, Mdallal QA, Khan T, Ullah R, Alwan BA, Faiz F, Zhu QX. The dynamics of novel corona virus disease via stochastic epidemiological model with vaccination. Sci. Rep. 2023;13:3805. doi: 10.1038/s41598-023-30647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LM, Chen LS, Nieto JJ. The dynamics of an epidemic model for pest control with impulsive effect, Journal of. Nonlinear Anal. Real World Appl. 2010;11:1374–1386. doi: 10.1016/j.nonrwa.2009.02.027. [DOI] [Google Scholar]
- 42.Liu JN, Qi Q, Liu B, Gao SJ. Pest control switching models with instantaneous and non-instantaneous impulsive effects. Math. Comput. Simul. 2023;205:926–938. doi: 10.1016/j.matcom.2022.10.027. [DOI] [Google Scholar]
- 43.Djuikem C, Grognard F, Touzeau S. Impulsive modelling of rust dynamics and predator releases for biocontrol. Math. Biosci. 2023;356:108968. doi: 10.1016/j.mbs.2023.108968. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Y, Bosch FVD. The dynamics of an eco-epidemic model with biological control. Ecol. Model. 2003;168:203–214. doi: 10.1016/S0304-3800(03)00197-2. [DOI] [Google Scholar]
- 45.Rhodes CJ, Anderson RM. Forest-fire as a model for the dynamics of disease epidemics. J. Franklin Inst. 1998;335:199–211. doi: 10.1016/S0016-0032(96)00096-8. [DOI] [Google Scholar]
- 46.Sisodiya OS, Misraa OP, Dharb J. Dynamics of cholera epidemics with impulsive vaccination and disinfection. Math. Biosci. 2018;298:46–57. doi: 10.1016/j.mbs.2018.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request from the corresponding author (Youxiang Xie).