Abstract
Background
Fatigue is a common problem in immune-mediated inflammatory disease (IMID) patients, significantly impacting their quality of life.
Objectives
In this study, we describe the pattern and characteristics of fatigue as a patient-reported adverse drug reaction (ADR) of biologics, and compared patient and treatment characteristics with patients reporting other ADRs or no ADRs.
Methods
In this cohort event monitoring study, the description and characteristics of fatigue reported as a possible ADR in the Dutch Biologic Monitor were assessed and analysed for commonly recurring themes or patterns. Baseline and treatment characteristics of patients with fatigue and patients reporting other ADRs or no ADRs were compared.
Results
Of 1382 participating patients, 108 patients (8%) reported fatigue as an ADR of a biologic. Almost half of these patients (50 patients, 46%) described episodes of fatigue during or shortly after biologic injection, which often recurred following subsequent injections. Patients with fatigue were significantly younger than patients with other ADRs or patients without ADRs (median age for patients with fatigue, 52 years; median age for patients with other ADRs, 56 years; and median age for patients without ADRs, 58 years); significantly more often smoked (25% vs. 16% and 15%); used infliximab (22% vs. 9% and 13%), rituximab (9% vs. 3% and 1%) or vedolizumab (6% vs. 2% and 1%); and significantly more often had Crohn’s disease (28% vs. 13% and 13%) and other comorbidities (31% vs. 20% and 15%). Patients with fatigue significantly less frequently used etanercept (12% vs. 29% and 34%) or had rheumatoid arthritis (30% vs. 45% and 43%).
Conclusions
IMID patients may experience fatigue as a postdosing effect of biologics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-023-00592-8.
Key Points
| Fatigue is a common complaint among patients with immune-mediated inflammatory diseases (IMIDs), but it is not well known as an adverse drug reaction (ADR) of biologics. |
| In this study, fatigue was the most frequently reported ADR of biologics by patients with IMIDs, and many of these patients described a pattern of recurring fatigue after biologic injection. |
| Evaluating the clinical pattern of fatigue may aid in understanding the potential contribution of a biologic in patients experiencing fatigue. |
Introduction
Patients with immune-mediated inflammatory diseases (IMIDs) frequently experience fatigue, significantly impacting their quality of life [1, 2]. The reported prevalence of fatigue in IMIDs varies from 19 to 72%, depending on IMID and disease status, compared with 9–25% in healthy adults [3]. It can be persistent and continuously present with sudden episodes of an overwhelming loss of energy and feeling exhausted [2, 3]. Fatigue reduces the ability of physical and mental effort. Although fatigue is an important aspect of IMIDs, not all factors contributing to fatigue have been elucidated and treatment remains difficult.
It is well known that various factors may contribute to experiencing fatigue in patients with IMIDs, such as the disease itself and behavioural and psychological factors [3–7]. Multimorbidity, pain, depression and disability have been associated with fatigue in rheumatic diseases [3, 4, 8–10]. Anti-tumour necrosis factor (TNF) treatment as well as other biologic treatment have demonstrated improvements in fatigue in patients with rheumatoid arthritis and other IMIDs [11–17]. However, reducing disease activity alone is not always sufficient to improve fatigue. Rheumatoid arthritis patients who achieved remission using anti-TNF therapy may continue to report fatigue [18]. Conversely, an increased risk of fatigue has been described with anti-TNF therapy in inflammatory bowel disease, especially during long-term treatment [19, 20].
In a previous study, we reported that 100 of 1369 patients (7%) with IMIDs who participated in the prospective Dutch Biologic Monitor reported fatigue as an adverse drug reaction (ADR) of their biologic treatment [21–23]. Fatigue has previously been labelled as an ADR in the European Summary of Product Characteristics (SmPC) for infliximab only, and not for other TNFα inhibitors [24–28]. For interleukin inhibitors and other biologics used in IMIDs, fatigue has been labelled as an ADR in the European SmPCs of abatacept, brodalumab, canakinumab, rituximab, secukinumab, ustekinumab and vedolizumab [29–35]. Fatigue is mentioned as an adverse reaction in the FDA drug labels of infliximab, certolizumab pegol, brodalumab, ustekinumab, rituximab and vedolizumab [36–41]. Little is known about the pattern and characteristics of fatigue as an ADR of biologics. Because fatigue is a commonly reported complaint with IMIDs, it may remain unnoticed as an ADR or may, perhaps mistakenly, be attributed to the disease rather than biologic therapy. As patients with more severe disease are treated with more intensive therapies, including biologics, it may be challenging to distinguish the contribution of the underlying disease from the potential contribution of the biologic or other therapies. In this study, we aimed to further understand fatigue as an ADR of biologics by assessing the pattern of the reported fatigue and the characteristics of the patients reporting fatigue in the Dutch Biologic Monitor. Therefore we aimed to (1) describe the pattern and characteristics of patient-reported fatigue; and (2) identify differences in baseline and treatment characteristics between patients reporting fatigue as a potential ADR and patients reporting other ADRs or no ADRs.
Methods
Study Design
An observational cohort event monitoring study of fatigue reported as an ADR of biologics in the Dutch Biologic Monitor.
Dutch Biologic Monitor
The Dutch Biologic Monitor is a prospective cohort event system for monitoring patient-reported ADRs attributed to biologics [21, 22]. Nine Dutch hospitals participated in the Dutch Biologic Monitor. Between 1 January 2017 and 31 December 2020, consecutive patients using one of the monitored biologics, mainly for IMIDs, were invited to participate by the healthcare professionals (HCP) of the respective hospitals. Patients were eligible to participate from age 18 years or older, with access to the internet, and proficient in the Dutch language. Participating patients were asked to complete a comprehensive web-based baseline questionnaire covering demographic information (sex, date of birth, weight, height, smoking habits: daily, weekly, monthly or less, never), biologic, start date of the biologic, indication for the biologic, combination therapy, comorbidities at baseline and ADRs they attributed to the biologic (Supplementary Table 1 in the Online Resource). Multiple options could be selected for indication for biologic therapy, combination therapy and comorbidities. The originator or, when available, biosimilars of the biologics were included. Subsequent questionnaires after baseline focused exclusively on biologic use, combination therapy and ADRs, and included identical questions on these topics. The baseline and subsequent questionnaire translated into English are presented in the Online Resource. Questionnaires were sent out bimonthly and patients received reminders if they had not completed the questionnaire within 7 days and 14 days. Patients could withdraw from the monitor at any time and no more questionnaires were sent in case the previous questionnaire had expired (after 21 days).
Ethical approval of the Dutch Biologic Monitor was waived for the Dutch Medical Research Involving Human Subjects Act (WMO) by the Medical Research Ethical Committee of Brabant, The Netherlands (NW2016-66). All participants received information about the Dutch Biologic Monitor prior to participation and signed a digital informed consent form.
Adverse Drug Reaction (ADR) Assessment
Patients were asked if they experienced any ADRs that they attributed to the biologic in each questionnaire. For each reported ADR, patients were asked for additional information. This included a description of the ADR using an open text field to reduce reporting bias, current status of the ADR (recovered, improving, aggravating, no change), start and stop date of the ADR if applicable, additional information about the ADR in an open text field, contact with an HCP, treatment or other actions taken by the HCP, self-initiated action by the patient following the ADR, the experienced ADR burden using a 5-point Likert type scale ranging from 1 (no burden) to 5 (very high burden) and an explanation of the ADR burden using an open text field. In the open text field for additional information about the ADR, patients were asked to further explain the ADR, which included the following suggested questions: How often do you experience this ADR? At which specific moments do you experience this ADR? Is there a specific pattern [21]? ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) terminology (version 23.0) by trained pharmacovigilance assessors following standard practice [42].
Data Collection
Fatigue as an ADR was defined as all reported ADRs with the MedDRA preferred term (PT) ‘Fatigue’. We selected all questionnaires from patients reporting fatigue as a possible ADR of their biologic. Additionally, we selected questionnaires from patients reporting other ADRs on MedDRA PT level, and questionnaires from patients reporting no ADRs.
Data Analysis
To identify characteristics of the reported fatigue as an ADR of biologics, we assessed patient’s descriptions of the course of fatigue in any questionnaire, the status of fatigue in the last completed questionnaire, HCP contact following fatigue in any questionnaire, treatment or other actions taken by the HCP in any questionnaire, self-initiated action reported in any questionnaire, hospitalization following fatigue in any questionnaire, and the ADR burden of fatigue in all questionnaires. Since the course of ADRs was described by patients in open text fields, this was subjected to thematic analysis by Jvl and NJ for patterns or commonly recurring themes in the course of fatigue in different patients. Discrepancies were discussed for consensus. A causal association between the biologic and fatigue was assessed by applying the Naranjo Probability Scale in a case-by-case manner [43].
Baseline and treatment characteristics were compared between patients reporting fatigue as an ADR and patients who did not report fatigue as an ADR, to investigate potential differences between these patients. Patients who did not report fatigue were divided into two groups: patients reporting other ADRs and patients reporting no ADR at all. The following baseline characteristics were included: age, sex, body mass index (BMI), smoking status (ever or never) and comorbidities. The following treatment characteristics were included: biologic, indication for biologic and combination therapy. Differences between patients reporting fatigue and patients with other ADRs or no ADRs were analysed using the Mann–Whitney U test for continuous variables that were not normally distributed, or ordinal variables such as burden. Continuous normally distributed variables were analysed using independent t tests, and categorical variables were analysed using Fisher’s exact test. Normality was assessed with histograms and the Kolmogorov–Smirnov test. Statistical analyses were performed in IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA).
Results
Of 1382 consecutive participating patients in the Dutch Biologic Monitor, 730 patients (53%) reported 2035 unique ADRs they experienced with biologics. The most frequently reported ADR on MedDRA PT level was fatigue. In total, 108 patients (15%) of 730 patients with ADRs reported fatigue (Table 1). All 108 patients reporting fatigue collectively completed a total of 813 questionnaires, with a median of five completed questionnaires per patient (range 1–24 questionnaires).
Table 1.
Demographics of patients reporting fatigue as an adverse drug reaction of biologics
| No. of patients | 108 (100) |
| Age, years (median [IQR]) | 52 [39–63] |
| Female sex | 66 (61) |
| Smoking | 27 (25) |
| BMI, kg/m2 (median [IQR]) | 25.4 [22.7–27.5] |
| Biologica | |
| Adalimumab | 30 (28) |
| Infliximab | 24 (22) |
| Etanercept | 13 (12) |
| Rituximab | 10 (9) |
| Tocilizumab | 8 (7) |
| Vedolizumab | 6 (6) |
| Ustekinumab | 6 (6) |
| Dupilumab | 4 (4) |
| Abatacept | 3 (3) |
| Certolizumab pegol | 2 (2) |
| Anakinra | 2 (2) |
| Secukinumab | 1 (1) |
| Golimumab | 1 (1) |
| Indication for biologic use | |
| Rheumatoid arthritis | 32 (30) |
| Psoriatic arthritis | 15 (14) |
| Axial spondyloarthritis | 11 (10) |
| Crohn’s disease | 30 (28) |
| Ulcerative colitis | 5 (5) |
| Psoriasis | 6 (6) |
| Other indication | 17 (16) |
| Combination therapy | |
| Methotrexate | 24 (22) |
| Corticosteroidsb | 21 (19) |
| Thiopurinesc | 12 (11) |
| Aminosalicylatesd | 9 (8) |
| Hydroxychloroquine | 5 (5) |
| Leflunomide | 2 (2) |
| No combination therapy | 45 (42) |
| Comorbidities | |
| Cardiovascular disorder | 23 (21) |
| Hypercholesterolaemia | 15 (14) |
| Respiratory disorder | 14 (13) |
| Psychiatric disorder | 11 (10) |
| Nervous system disorder | 3 (3) |
| Cancer | 2 (2) |
| Other comorbidity | 33 (31) |
| No comorbidity | 30 (28) |
Data are expressed as n (%) unless otherwise specified
BMI body mass index, IQR interquartile range, ADR adverse drug reaction
aOne patient reported fatigue as an ADR of infliximab and adalimumab, and one patient reported fatigue as an ADR of abatacept and rituximab
bCorticosteroids include predniso(lo)ne, hydrocortisone, methylprednisolone
cThiopurines include azathioprine, mercaptopurine and thioguanine
dAminosalicylates include sulfasalazine and mesalamine
Patterns of Fatigue
Postdosing fatigue was a common theme in the patients’ descriptions of the course of fatigue. Almost half (50 patients, 46%) of the 108 patients reporting fatigue as an ADR described a pattern of fatigue specifically occurring during or shortly after administration of the biologic. Of these, 41 patients (82%) described that fatigue recurred following more than one injection. Almost all patients describing this postdosing fatigue, recovered or partially improved from fatigue within 1 week after biologic administration (48 of 50 patients). Seven of these 50 patients described that the severity of fatigue sometimes also increased in the week before biologic administration (Fig. 1). Five patients specifically explained that they always experienced fatigue during their chronic disease but the fatigue was more severe shortly after the biologic administration.
Fig. 1.
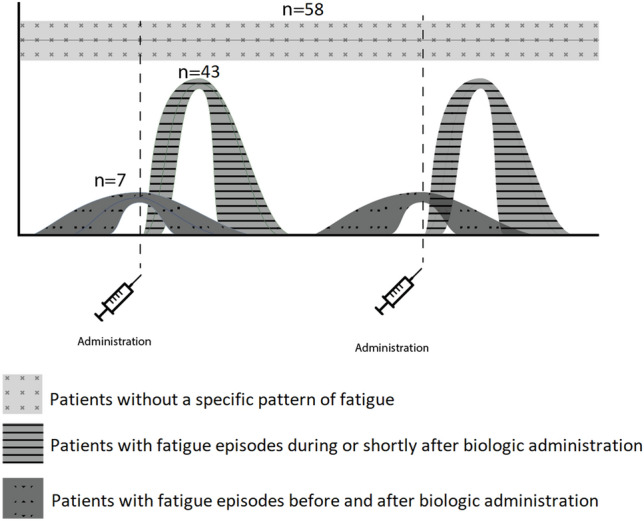
Described patterns in the course of fatigue as an adverse drug reaction of biologics
No specific pattern was described by the 58 patients (54%) without the postdosing pattern. A common description of the course of fatigue in these patients was continuously or daily present fatigue, with variation in severity.
Consequences of Fatigue
A total of 78 of 108 patients (72%) reported HCP contact following fatigue, with dose adjustments in 13 patients (12%) and discontinuation in seven patients (6%) (Table 2). Four of the 13 patients describing dose adjustments experienced postdosing fatigue. In four cases, this dose adjustment was a decrease in administration frequency, and in five cases it was an increase in administration frequency. In nine patients, fatigue (temporarily) improved or resolved following the dose adjustment. The dose adjustment was not always initiated due to fatigue but could have also been due to reasons other than an ADR.
Table 2.
Characteristics of patient-reported fatigue as an ADR of biologics (N = 108)
| No. of patients reporting HCP contact following fatigue | 78 (72) |
| Specialist doctor | 65 (60) |
| General practitioner | 27 (25) |
| Nurse | 35 (32) |
| Other | 4 (4) |
| No. of patients who reported an HCP action | 78 (72) |
| Discontinuation | 7 (6) |
| Dose adjustment | 13 (12) |
| Treatment | 10 (9) |
| Referral | 5 (5) |
| Mentioned, no other action | 39 (36) |
| Othera | 17 (16) |
| No. of patients with a status of ‘fatigue’ in the last completed questionnaire | |
| Recovered | 28 (26) |
| Improving | 17 (16) |
| No change | 54 (50) |
| Aggravating | 9 (8) |
| No. of patients reporting fatigue and hospitalization | 3 (3) |
| No. of patients reporting self-initiated action following fatigue | 63 (58) |
| Naranjo score | |
| Doubtful | 5 (5) |
| Possible | 75 (70) |
| Probable | 28 (26) |
| Certain | 0 (0) |
| Mean ADR burden score ± SD | 2.8 ± 0.9 |
Data are expressed as n (%) unless other specified
ADR adverse drug reaction, HCP healthcare professional, SD standard deviation
aOther HCP actions: further examination, 6; adjusting concomitant therapy, 6; other therapy, 3
Seven patients reported (temporary) discontinuation of the biologic following fatigue, including two patients with postdosing fatigue. Five patients improved or recovered from fatigue after discontinuation, including two patients with postdosing fatigue. Three patients specifically mentioned that the biologic was discontinued because of one or more ADRs.
Ten patients (13%) reported that the fatigue was treated following HCP contact, including three patients with postdosing fatigue. Treatment was specified as iron infusion by two patients, while the other patients did not further specify treatment. Four patients described improvements of fatigue after treatment, including one patient treated with iron supplementation and one patient with postdosing fatigue.
Three patients reported hospitalization following fatigue. In an explanation in an open text field, hospitalization was associated with other underlying problems in two patients. One patient described hospitalization for a liver procedure and one patient described hospitalization for a cardiac procedure. The third patient did not further explain the hospitalization.
The outcome of the Naranjo assessment was probable in 28 cases (26%) and possible in 75 cases (70%).
Burden of Fatigue
The mean ADR burden of fatigue was 2.8 (standard deviation [SD] 0.9) on a 5-point Likert-type scale from 1 (no burden) to 5 (very high burden). The mean ADR burden experienced by patients with postdosing fatigue (2.6 ± 0.9) was lower than the mean ADR burden of fatigue in patients without this pattern (3.0 ± 1.0; p < 0.001). The mean ADR burden of fatigue (2.8 ± 0.9) was higher than the mean burden of other ADRs (2.4 ± 1.0; p < 0.001) (Table 3). Patients elucidated the experienced burden of fatigue with various explanations. Fatigue reduced quality of life, led to limitations in daily activities and affected work productivity and concentration. It also led to difficulties in combining and planning work with a social and personal life and to struggles in enjoying life. Moreover, patients explained that fatigue led to a depressed mood.
Table 3.
Characteristics of patients reporting fatigue as an ADR compared with patients with other ADRs and patients without ADRs
| Patients with fatigue | Patients with other ADRs | p-value | Patients without ADRs | p-value | |
|---|---|---|---|---|---|
| No. of patients | 108 (100) | 622 (100) | 652 (100) | ||
| Age, years (median [IQR]) | 52 [39–63] | 56 [45–64] | 0.02 | 58 [48–67] | < 0.001 |
| Female sex | 66 (61) | 407 (65) | 0.39 | 331 (51) | 0.05 |
| Smoking | 27 (25) | 99 (16) | 0.03 | 98 (15) | 0.02 |
| BMI, kg/m2 (median [IQR]) | 25.4 [22.7–27.5] | 25.1 [22.5–28.4] | 0.83 | 25.8 [23.2–29.0] | 0.11 |
| Biologic | |||||
| Adalimumab | 30 (28) | 225 (36) | 0.10 | 238 (37) | 0.08 |
| Infliximab | 24 (22) | 54 (9) | < 0.001 | 82 (13) | 0.01 |
| Etanercept | 13 (12) | 180 (29) | < 0.001 | 220 (34) | < 0.001 |
| Rituximab | 10 (9) | 19 (3) | 0.01 | 5 (1) | < 0.001 |
| Tocilizumab | 8 (7) | 30 (5) | 0.25 | 12 (2) | 0.004 |
| Ustekinumab | 6 (6) | 25 (4) | 0.44 | 33 (5) | 0.81 |
| Vedolizumab | 6 (6) | 12 (2) | 0.04 | 7 (1) | 0.01 |
| Other | 13 (12) | 116 (19) | 0.10 | 78 (12) | 1.00 |
| Indication | |||||
| Rheumatoid arthritis | 32 (30) | 277 (45) | 0.004 | 279 (43) | 0.01 |
| Psoriatic arthritis | 15 (14) | 95 (15) | 0.77 | 132 (20) | 0.15 |
| Axial spondyloarthritis | 11 (10) | 83 (13) | 0.44 | 78 (12) | 0.75 |
| Crohn’s disease | 30 (28) | 78 (13) | < 0.001 | 86 (13) | < 0.001 |
| Ulcerative colitis | 5 (5) | 30 (5) | 1.00 | 25 (4) | 0.60 |
| Psoriasis | 6 (6) | 27 (4) | 0.61 | 50 (8) | 0.55 |
| Other indication | 17 (16) | 64 (10) | 0.06 | 37 (6) | 0.001 |
| Combination therapya | |||||
| Methotrexate | 24 (22) | 173 (28) | 0.24 | 221 (34) | 0.02 |
| Corticosteroidsb | 21 (19) | 111 (18) | 0.69 | 93 (14) | 0.19 |
| Thiopurinesc | 12 (11) | 45 (7) | 0.17 | 58 (9) | 0.47 |
| Aminosalicylatesd | 9 (8) | 51 (8) | 1.00 | 39 (6) | 0.39 |
| Hydroxychloroquine | 5 (5) | 33 (5) | 1.00 | 36 (6) | 0.82 |
| Leflunomide | 2 (2) | 42 (7) | 0.05 | 23 (4) | 0.56 |
| No combination therapy | 45 (42) | 264 (42) | 0.92 | 240 (37) | 0.34 |
| Comorbidity | |||||
| Cardiovascular disorder | 23 (21) | 155 (25) | 0.47 | 162 (25) | 0.47 |
| Hypercholesterolaemia | 15 (14) | 93 (15) | 0.88 | 117 (18) | 0.34 |
| Respiratory disorder | 14 (13) | 77 (12) | 0.88 | 75 (12) | 0.63 |
| Psychiatric disorder | 11 (10) | 49 (8) | 0.45 | 31 (5) | 0.04 |
| Nervous system disorder | 3 (3) | 19 (3) | 1.00 | 19 (3) | 1.00 |
| Cancer | 2 (2) | 15 (2) | 1.00 | 14 (2) | 1.00 |
| Other comorbidity | 33 (31) | 126 (20) | 0.02 | 99 (15) | < 0.001 |
| No comorbidity | 30 (28) | 213 (34) | 0.22 | 230 (35) | 0.15 |
| Mean burden score ± SD | 2.8 ± 0.9 | 2.4 ± 1.0 | < 0.001 | ||
Data are expressed as n (%) unless otherwise specified
ADR adverse drug reaction, BMI body mass index, IQR interquartile range, SD standard deviation
aCombination therapy at the time of reporting the ADR for the first time. For the patients without ADRs, the reported combination therapy at any time during participation was included
bCorticosteroids include predniso(lo)ne, methylprednisolone, hydrocortisone
cThiopurines include azathioprine, thioguanine, mercaptopurine
dAminosalicylates include mesalamine, sulfasalazine
Patients Reporting Fatigue as an ADR Compared with Patients Reporting Other ADRs or no ADRs
The characteristics of patients reporting fatigue as an ADR compared with patients reporting other ADRs or no ADRs are summarized in Table 3. Patients reporting fatigue were younger and more frequently smoked. Patients reporting fatigue as an ADR more frequently used infliximab, rituximab or vedolizumab, more frequently had other comorbidities and more frequently used a biologic for Crohn’s disease. Patients with fatigue less frequently used etanercept or less frequently used a biologic for rheumatoid arthritis than patients with other ADRs or patients without an ADR.
We also found differences between patients with fatigue and patients without ADRs. Patients with fatigue more frequently used tocilizumab, more frequently had a psychiatric comorbidity and less frequently used concomitant methotrexate. These differences were not found between patients with fatigue and patients with other ADRs.
Discussion
In this study, we investigated fatigue reported by patients as an ADR of biologics in the Dutch Biologic Monitor, a unique system for collecting patient-reported data on ADRs attributed to biologics. Fatigue was the most frequently reported ADR and patients included clear descriptions on the course and characteristics. This addresses the magnitude of patients experiencing fatigue as an ADR, while fatigue is not a labelled ADR in the European product information of all biologics monitored in the Dutch Biologic Monitor [25–28, 44].
Half of the patients described a similar pattern of recurring postdosing fatigue that resolved within 1 week after biologic administration. Although the pharmacological mechanism is not clear, this pattern substantiates a role of biologics in the manifestation of fatigue in these patients and supports fatigue as a potential ADR, comparable with the well-known gastrointestinal ADRs after methotrexate administration [45]. Treatment adjustments may decrease fatigue since some patients described improvements after discontinuation or dose adjustments. Some patients described increased fatigue in the week before biologic administration. In these patients, fatigue may be related to an increase in disease activity in the week before administration. This suggests that fatigue may sometimes emerge from a suboptimal biologic dose interval rather than an ADR. Improved fatigue after adjustments in concomitant drugs suggests that fatigue may sometimes be related to concomitantly used drugs rather than the biologic itself. Fatigue may also be a symptom of underlying medical problems that seemed apparent in some patients describing iron treatment. The different descriptions of the course of fatigue experienced by patients address the importance for HCPs to discuss and evaluate the course and characteristics with their patients and assess all potential factors contributing to fatigue to be able to optimize treatment and improve quality of life.
Patients reporting fatigue were younger and more frequently used a biologic for Crohn’s disease. This is in line with a previous study addressing adverse symptoms with anti-TNF therapy in patients with inflammatory bowel disease, and may suggest that patients with Crohn’s disease more often experience fatigue during biologic use than patients with other IMIDs [19]. Patients with fatigue also more frequently used infliximab, rituximab or vedolizumab. These biologics are mostly administered intravenously, and for which infusion related reactions are well known [46, 47]. In these patients, fatigue could be a symptom of an infusion-related reaction, similar to immediate adverse reactions following intravenous immunoglobulin administration [48]. In intravenously administered biologics, premedication, such as antihistamines or corticosteroids, may also play a role in the manifestation of fatigue [49]. It can also be postulated that immunogenicity might influence fatigue; however, we did not collect these data in the Dutch Biologic Monitor and, as far as we know, this association has not been described in previous studies [50, 51]. Patients with fatigue more frequently smoked than patients with other or no ADRs, and more frequently had psychiatric comorbidities than patients without ADRs. Smoking as well as psychiatric and depressive disorder have been associated with fatigue in IMIDs in previous studies, which is in line with our findings [52–56]. Even though this does not support fatigue as an ADR of biologics, it does not exclude a role of biologics in the manifestation of fatigue and supports the notion that many factors may contribute to fatigue in IMID patients [3, 7].
Interestingly, the mean ADR burden of fatigue was higher than the mean burden of other ADRs combined. The mean ADR burden of postdosing fatigue was lower than the mean ADR burden of fatigue without this specific pattern. Fatigue without this pattern implies the manifestation of chronic fatigue, which patients experienced as more burdensome. We cannot confirm these differences are clinically relevant as a standardized tool for measuring ADR burden is not yet available [57, 58]. However, the differences were considered clinically relevant by the expert panel involved in the Dutch Biologic Monitor and this is supported by the descriptions patients provided explaining the significant impact that fatigue has on their lives. Given the challenges in improving patients’ quality of life, HCPs should take the potential contribution of biologics into account.
The strengths of our study include the prospective nature of monitoring ADR information in a multicentre setting in patients using various biologics for different IMIDs, which makes data on different IMIDs comparable. Assessing unfiltered patient-reported information on ADRs is a novelty and improves our understanding of the course of ADRs and the patient perspective on experiencing ADRs. A relationship was considered possible or probable in almost all cases following the widely used Naranjo assessment, although the reliability of this tool has been questioned [59, 60]. Even though we cannot confirm a causal relationship between fatigue and biologics, the specific descriptions of a recurring postdosing pattern provide valuable information for HCPs. Because of the heterogeneity of the patients participating in the Dutch Biologic Monitor, we did not investigate risk factors for reporting fatigue as an ADR of biologics. The complexity of all factors involved in the manifestation of fatigue should be investigated in more detail for each biologic or group of biologics to better understand the contribution of different biologics in postdosing fatigue. The same applies to patient groups that may be more prone to suffer from fatigue as an ADR of biologics.
Although IMID patients frequently experience fatigue aside from biologics, a significant number of patients related fatigue to biologic use in this multicentre study that included a large number of patients with various IMIDs using a variety of biologics. This implies that a diverse group of patients associate fatigue with biologics across The Netherlands.
Conclusion
This is the first study to describe postdosing fatigue reported by patients as an ADR of various biologics for the treatment of IMIDs. Fatigue as an ADR of biologics may remain unrecognized or may automatically be attributed to the underlying disease. The specific recurring pattern after each administration suggests a contribution of biologics in the manifestation of fatigue. Since fatigue has a significant burden on patients, this previously unknown knowledge may be helpful for HCPs in understanding the experienced fatigue by their patients, and may assist in evaluating all possible factors contributing to fatigue. Distinguishing the relative contribution of underlying disease and treatment of the disease may be challenging. Evaluating the course of the symptoms may abate this challenge and may contribute to optimizing and personalizing biologic therapy to ultimately improve quality of life.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
Jette van Lint, Naomi Jessurun, Michael Nurmohamed, Bart van den Bemt and Eugene van Puijenbroek declare no conflicts of interest. Sander Tas reports grants and/or personal fees from Abbvie, Arthrogen, AstraZeneca, BMS, Celgene, Galapagos, GSK, MSD, Pfizer, Roche, and Sanofi-Genzyme, all outside the submitted work. Frank Hoentjen has served on advisory boards, or as speaker or consultant for Abbvie, Celgene, Janssen-Cilag, MSD, Takeda, Celltrion, Teva, Sandoz, and Dr Falk; and has received unrestricted grants from Dr Falk, Janssen-Cilag, and Abbvie. Martijn van Doorn has received consulting fees or honorarium from Novartis, Abbvie, Pfizer, Leopharma, Sanofi, Lilly, Janssen, Celgene, and BMS; has received a grant and payment for lectures, including service on speakers bureaus, from Novartis, Sanofi, and Janssen, outside the submitted work. Harald Vonkeman reports grants and/or personal fees from AbbVie, Amgen, AstraZeneca, BMS, Celgene, Celltrion, Galapagos, Gilead, GSK, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi-Genzyme, and UCB, all outside the submitted work.
Funding
The Dutch Biologic Monitor work was supported by the Netherlands Organisation for Health Research and Development (ZonMw) [grant number 848050005]. No funding was received for this study.
Author contributions
JvL, NJ, and BvdB contributed to the conception and design of this study. JvL and NJ contributed to the analysis and interpretation of the data and drafting of the paper. BvdB, ST, MvD, MN, EvP, FH, and HV critically revised the paper for intellectual content. All authors approved the final version and agree to be accountable for all aspects of this work.
Ethics approval
Ethical approval of the Dutch Biologic Monitor was waived for the Dutch Medical Research Involving Human Subjects Act (WMO) by the Medical Research Ethical Committee of Brabant, The Netherlands (NW2016-66).
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
Patient consent to participate
All participants received information about the Dutch Biologic Monitor prior to participation and signed a digital informed consent form.
Patient consent to publish
Not applicable.
Code availability
Not applicable.
References
- 1.Korte SM, Straub RH. Fatigue in inflammatory rheumatic disorders: pathophysiological mechanisms. Rheumatology (Oxford) 2019;58(Suppl 5):v35–v50. doi: 10.1093/rheumatology/kez413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavarría C, Casanova MJ, Chaparro M, et al. Prevalence and factors associated with fatigue in patients with inflammatory bowel disease: a multicentre study. J Crohns Colitis. 2019;13(8):996–1002. doi: 10.1093/ecco-jcc/jjz024. [DOI] [PubMed] [Google Scholar]
- 3.Graff LA, Walker JR, Russell AS, et al. Fatigue and quality of sleep in patients with immune-mediated inflammatory disease. J Rheumatol Suppl. 2011;88:36–42. doi: 10.3899/jrheum.110902. [DOI] [PubMed] [Google Scholar]
- 4.Druce KL, Basu N. Predictors of fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 5):v29–v34. doi: 10.1093/rheumatology/kez346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz P. Causes and consequences of fatigue in rheumatoid arthritis. Curr Opin Rheumatol. 2017;29(3):269–276. doi: 10.1097/BOR.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 6.Grimstad T, Norheim KB. Fatigue in inflammatory bowel disease. Tidsskr Nor Laegeforen. 2016;136(20):1721–1724. doi: 10.4045/tidsskr.16.0134. [DOI] [PubMed] [Google Scholar]
- 7.Swain MG. Fatigue in chronic disease. Clin Sci (Lond) 2000;99(1):1–8. doi: 10.1042/cs0990001. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM, 3rd, Myasoedova E, Gunderson TM, et al. Multimorbidity and fatigue in rheumatoid arthritis: a cross-sectional study of a population-based cohort. Rheumatol Ther. 2020;7(4):979–991. doi: 10.1007/s40744-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minnock P, Veale DJ, Bresnihan B, et al. Factors that influence fatigue status in patients with severe rheumatoid arthritis (RA) and good disease outcome following 6 months of TNF inhibitor therapy: a comparative analysis. Clin Rheumatol. 2015;34(11):1857–1865. doi: 10.1007/s10067-015-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haugeberg G, Hoff M, Kavanaugh A, et al. Psoriatic arthritis: exploring the occurrence of sleep disturbances, fatigue, and depression and their correlates. Arthritis Res Ther. 2020;22(1):198. doi: 10.1186/s13075-020-02294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida C, Choy EH, Hewlett S, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev. 2016;2016(6):CD008334. doi: 10.1002/14651858.CD008334.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip RML, Yim CW. Role of interleukin 6 inhibitors in the management of rheumatoid arthritis. J Clin Rheumatol. 2021;27(8):e516–e524. doi: 10.1097/RHU.0000000000001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoie IM, Dalen I, Omdal R. Effect of biological treatment on fatigue in psoriasis: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20(4):493–502. doi: 10.1007/s40257-019-00434-w. [DOI] [PubMed] [Google Scholar]
- 14.Shim J, Dean LE, Karabayas M, et al. Quantifying and predicting the effect of anti-TNF therapy on axSpA-related fatigue: results from the BSRBR-AS registry and meta-analysis. Rheumatology (Oxford) 2020;59(11):3408–3414. doi: 10.1093/rheumatology/keaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvien TK, Conaghan PG, Gossec L, et al. secukinumab provides sustained reduction in fatigue in patients with ankylosing spondylitis: long-term results of two phase III randomized controlled trials. Arthritis Care Res (Hoboken) 2022;74(5):759–767. doi: 10.1002/acr.24517. [DOI] [PubMed] [Google Scholar]
- 16.Reilly E, McGrogan A, Sengupta R. Evaluating patient-reported fatigue and serum biomarkers in axial spondyloarthritis. Rheumatology (Oxford) 2020;59(10):3111–3113. doi: 10.1093/rheumatology/keaa115. [DOI] [PubMed] [Google Scholar]
- 17.Laganà B, Vinciguerra M, D'Amelio R. Modulation of T-cell co-stimulation in rheumatoid arthritis: clinical experience with abatacept. Clin Drug Investig. 2009;29(3):185–202. doi: 10.2165/00044011-200929030-00005. [DOI] [PubMed] [Google Scholar]
- 18.Druce KL, Bhattacharya Y, Jones GT, et al. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2016;55(10):1786–1790. doi: 10.1093/rheumatology/kew241. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Lin X, Zhao Q, et al. Adverse symptoms with anti-TNF-alpha therapy in inflammatory bowel disease: systematic review and duration-response meta-analysis. Eur J Clin Pharmacol. 2015;71(8):911–919. doi: 10.1007/s00228-015-1877-0. [DOI] [PubMed] [Google Scholar]
- 20.Vogelaar L, van’t Spijker A, van Tilburg AJ, et al. Determinants of fatigue in Crohn's disease patients. Eur J Gastroenterol Hepatol. 2013;25(2):246–251. doi: 10.1097/MEG.0b013e32835aba83. [DOI] [PubMed] [Google Scholar]
- 21.van Lint JA, Jessurun NT, Hebing RCF, et al. Patient-reported burden of adverse drug reactions attributed to biologics used for immune-mediated inflammatory diseases. Drug Saf. 2020;43(9):917–925. doi: 10.1007/s40264-020-00946-z. [DOI] [PubMed] [Google Scholar]
- 22.Kosse LJ, Jessurun N, Hebing RCF, et al. Patients with inflammatory rheumatic diseases: quality of self-reported medical information in a prospective cohort event monitoring system. Rheumatology (Oxford) 2020;59(6):1253–1261. doi: 10.1093/rheumatology/kez412. [DOI] [PubMed] [Google Scholar]
- 23.Van Lint J, Bakker T, Ubbink J, et al. OP0208: patients report fatigue as an adverse drug reaction of biologics. Ann Rheum Dis. 2020;79(Suppl 1):129–130. doi: 10.1136/annrheumdis-2020-eular.1310. [DOI] [Google Scholar]
- 24.Remicade® Product Information: European Medicines Agency [updated 12 Nov 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf.
- 25.Enbrel® Product Information: European Medicines Agency [updated 25 Mar 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf.
- 26.Humira® Product Information: European Medicines Agency [updated 29 Jan 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf.
- 27.Cimzia® Product Information: European Medicines Agency [updated 7 Apr 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf.
- 28.Simponi® Product Information: European Medicines Agency [updated 11 Nov 2020; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/simponi-epar-product-information_en.pdf.
- 29.Orencia® Product Information: European Medicines Agency [updated 6 Oct 2020; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/orencia-epar-product-information_en.pdf.
- 30.Kyntheum® Product Information: European Medicines Agency [updated 27 Jul 2020; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/kyntheum-epar-product-information_en.pdf.
- 31.Ilaris® Product Information: European Medicines Agency [updated 25 Feb 2020; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/ilaris-epar-product-information_en.pdf.
- 32.Mabthera® Product Information: European Medicines Agency [updated 25 Mar 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf.
- 33.Cosentyx® Product Information: European Medicines Agency [updated 25 Mar 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf.
- 34.Stelara® Product Information: European Medicines Agency [updated 12 Mar 2021; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf.
- 35.Entyvio® Product Information: European Medicines Agency [updated 30 Nov 2020; cited 21 Apr 2021]. https://www.ema.europa.eu/en/documents/product-information/entyvio-epar-product-information_en.pdf.
- 36.Remicade® Product Label: US FDA [updated 10 May 2021; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103772s5401lbl.pdf.
- 37.Cimzia® Product Label: US FDA [updated 22 Dec 2022; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125160s305lbl.pdf.
- 38.Siliq® Product Label: US FDA [updated 15 Feb 2017; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf.
- 39.Stelara® Product Label: US FDA [updated 29 Jul 2022; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761044s010lbl.pdf.
- 40.Truxima® Product Label: US FDA [updated 04 Feb 2022; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761088s018lbl.pdf.
- 41.Entyvio® Product Label: US FDA [updated 17 Jun 2022; cited 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125476Orig1s046lbl.pdf.
- 42.MedDRA Maintenance and Support Services Organization. Medical Dictionary for Regulatory Activities: Northrop Grumman Corporation. http://www.meddra.org/.
- 43.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 44.RoActemra® Product Information: European Medicines Agency [updated 14 Sep 2020; cited 11 May 2021]. https://www.ema.europa.eu/en/documents/product-information/roactemra-epar-product-information_en.pdf.
- 45.Ćalasan MB, van den Bosch OF, Creemers MC, et al. Prevalence of methotrexate intolerance in rheumatoid arthritis and psoriatic arthritis. Arthritis Res Ther. 2013;15(6):R217. doi: 10.1186/ar4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul F, Cartron G. Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol. 2019;15(4):383–389. doi: 10.1080/1744666X.2019.1562905. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015;9(9):806–815. doi: 10.1093/ecco-jcc/jjv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katz U, Achiron A, Sherer Y, et al. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6(4):257–259. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Church MK, Church DS. Pharmacology of antihistamines. Indian J Dermatol. 2013;58(3):219–224. doi: 10.4103/0019-5154.110832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bots SJ, Parker CE, Brandse JF, et al. Anti-drug antibody formation against biologic agents in inflammatory bowel disease: a systematic review and meta-analysis. BioDrugs. 2021;35(6):715–733. doi: 10.1007/s40259-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31(4):299–316. doi: 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skoie IM, Dalen I, Ternowitz T, et al. Fatigue in psoriasis: a controlled study. Br J Dermatol. 2017;177(2):505–512. doi: 10.1111/bjd.15375. [DOI] [PubMed] [Google Scholar]
- 53.Ibn Yacoub Y, Amine B, Laatiris A, et al. Fatigue and severity of rheumatoid arthritis in Moroccan patients. Rheumatol Int. 2012;32(7):1901–1907. doi: 10.1007/s00296-011-1876-0. [DOI] [PubMed] [Google Scholar]
- 54.Conley S, Proctor DD, Jeon S, et al. Symptom clusters in adults with inflammatory bowel disease. Res Nurs Health. 2017;40(5):424–434. doi: 10.1002/nur.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wessely S, Chalder T, Hirsch S, et al. Psychological symptoms, somatic symptoms, and psychiatric disorder in chronic fatigue and chronic fatigue syndrome: a prospective study in the primary care setting. Am J Psychiatry. 1996;153(8):1050–1059. doi: 10.1176/ajp.153.8.1050. [DOI] [PubMed] [Google Scholar]
- 56.McCallum SM, Batterham PJ, Calear AL, et al. Associations of fatigue and sleep disturbance with nine common mental disorders. J Psychosom Res. 2019;123:109727. doi: 10.1016/j.jpsychores.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Rolfes L, van Hunsel F, Taxis K, et al. The impact of experiencing adverse drug reactions on the patient's quality of life: a retrospective cross-sectional study in the Netherlands. Drug Saf. 2016;39(8):769–776. doi: 10.1007/s40264-016-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolfes L, Haaksman M, van Hunsel F, et al. Insight into the severity of adverse drug reactions as experienced by patients. Drug Saf. 2020;43(3):291–293. doi: 10.1007/s40264-019-00890-7. [DOI] [PubMed] [Google Scholar]
- 59.Agbabiaka TB, Savović J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31(1):21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher RM, Kirkham JJ, Mason JR, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS One. 2011;6(12):e28096. doi: 10.1371/journal.pone.0028096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.


