Abstract
INTRODUCTION
Exercise training is crucial in the management of nonalcoholic fatty liver disease (NAFLD); however, whether it can achieve clinically meaningful improvement in liver fat is unclear. We investigated the association between exercise training and the achievement of validated thresholds of MRI-measured treatment response.
METHODS:
Randomized controlled trials in adults with NAFLD were identified through March 2022. Exercise training was compared with no exercise training. The primary outcome was ≥30% relative reduction in MRI-measured liver fat (threshold required for histologic improvement in nonalcoholic steatohepatitis activity, nonalcoholic steatohepatitis resolution, and liver fibrosis stage). Different exercise doses were compared.
RESULTS:
Fourteen studies (551 subjects) met inclusion criteria (mean age 53.3 yrs; body mass index 31.1 kg/m2). Exercise training subjects were more likely to achieve ≥30% relative reduction in MRI-measured liver fat (odds ratio 3.51, 95% confidence interval 1.49–8.23, P = 0.004) than those in the control condition. An exercise dose of ≥750 metabolic equivalents of task min/wk (e.g., 150 min/wk of brisk walking) resulted in significant treatment response (MRI response odds ratio 3.73, 95% confidence interval 1.34–10.41, P = 0.010), but lesser doses of exercise did not. Treatment response was independent of clinically significant body weight loss (>5%).
DISCUSSION:
Independent of weight loss, exercise training is 3 and a half times more likely to achieve clinically meaningful treatment response in MRI-measured liver fat compared with standard clinical care. An exercise dose of at least 750 metabolic equivalents of task-min/wk seems required to achieve treatment response. These results further support the weight-neutral benefit of exercise in all patients with NAFLD.
Keywords: steatohepatitis, clinical trial, steatosis, physical activity, lifestyle modification
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of liver disease worldwide, affecting in upward of 30% of the global population (1). Defined by at least 5% liver fat in the absence of secondary causes of steatosis, NAFLD remains a disease without an effective drug treatment or cure. NAFLD encompasses 2 distinct histologic diseases: nonalcoholic fatty liver, the non-progressive form, and nonalcoholic steatohepatitis (NASH), the progressive type. Over time, patients with NASH can develop liver fibrosis and progress to cirrhosis or develop hepatocellular carcinoma, both of which can require lifesaving liver transplantation (2,3).
In the absence of an effective drug therapy or cure, lifestyle modification with dietary change and increased physical activity remains crucial to improve patient-oriented outcomes in this common condition and to prevent the consequences of end-stage liver disease or primary liver cancer (4). Physical activity, and in particular exercise training (a type of physical activity which is planned, structured, repetitive, and intended to improve or maintain physical fitness), has many benefits for patients with NAFLD. Regular physical activity or intentional exercise training can improve liver fat, physical fitness, body composition, vascular biology, and health-related quality of life in patients with NAFLD and NASH (5,6). Although these benefits have been established by multiple randomized clinical trials (RCTs) and the impact of exercise training on liver fat reduction has been aggregated by several recent systematic reviews (7–10), it remains unclear whether exercise training can achieve ≥30% relative reduction in liver fat, the minimal clinically important difference (MCID), which is the validated threshold required for histologic response, NASH resolution, or liver fibrosis improvement (11,12). This indirect evidence is incredibly important to understand because almost all exercise-based clinical trials conducted to date in patients with NAFLD have not included liver histology as an end point. In addition, the dose of exercise required to significantly reduce liver fat, including the achievement of this MCID threshold, also remains unknown, again owing in part to exercise-based clinical trial design where direct comparisons of different exercise prescriptions are also lacking.
In light of this, there is a clear unmet need to better understand how effective exercise training truly is, given that it remains the cornerstone of medical management for patients with NAFLD. Moreover, a better understanding of the minimum dose required to achieve the amount of liver fat reduction that surrogates for histologic response may allow for better adherence to and motivation for completing exercise training programs. For these reasons, the aim of our systematic review and meta-analysis was to assess the evidence for MRI-measured liver reduction in response to exercise training, including whether a ≥30% relative reduction can be achieved across different doses of exercise. The primary outcome was to examine the association between exercise training and a relative decline in liver fat ≥30%. The secondary outcome of this study was to examine exercise training’s impact on absolute and relative change in liver fat and to compare different exercise doses.
MATERIALS AND METHODS
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, we performed a systematic review of the existing medical literature (13). This systematic review was registered with The International Prospective Register of Systematic Reviews, which is an open access online database of systematic review protocols on health-related topics (CRD42021242542). This systematic review did not require institutional review board approval.
Identification of studies and searches
A detailed search was conducted by a medical librarian (A.H.) using indexing languages, including Medical Subject Headings and free-text terms for NAFLD, NASH, MRI, and exercise. The search strategy is found in the Supplementary Digital Content (see Supplementary Table 1, http://links.lww.com/AJG/C796). We searched the following through March 2022: PubMed MEDLINE, Cochrane, CINAHL, Scopus, Web of Science, and SPORT Discus. Multiple clinical trial registries were searched including ClinicalTrials.gov. After this, search results were combined into an Endnote database (version X8.2, Clarivate Analytics, PA) for reference management and then imported into the Rayyan web and mobile app for systematic reviews (Qatar Computing Research Institute, Doha, Qatar) (14). In addition, the reference lists for all eligible studies were screened, as were identified systematic reviews and meta-analyses, to identify any other eligible studies.
Study selection
Studies were chosen if they met the following inclusion criteria: (i) study design: randomized controlled trials in human subjects; (ii) population: adults (age >18 years) with NAFLD; (iii) exposure: exercise training program; and (iv) outcome measures: provision of data on the primary or secondary outcome measures. To fully extract the desired data, we excluded abstracts where study investigators were unable to provide additional information required. All study authors had access to the data, reviewed the final article, and approved the final article. The primary outcome was a relative reduction in MRI-measured liver fat ≥30%, the threshold required for histologic improvement (11,12). Although this threshold was derived from antisteatogenic drug studies in patients with NASH, this threshold has been applied to exercise-based interventions which are well-established to decrease MRI-measured liver fat (6–8,10,11). Secondary outcomes were absolute and relative change in MRI-measured liver fat and exercise dose.
Data extraction and risk of bias assessment
Study-level data were extracted from each study in this review and included author, country, study conditions (exercise training vs control), and study year. To perform an aggregate meta-analysis, additional data were extracted, including subject age, sex, body mass index (BMI), change in body weight, exercise adherence, and change in MRI-measured liver fat. As necessary, the authors were contacted for unpublished data (namely, relative reduction of ≥30% liver fat) to complete data extraction.
The Cochrane Risk of Bias Tool Version 2 was used to adjudicate study risk of bias (15). The Bias Tool Version 2 tool has 5 domains: (i) randomization process, (ii) derivations from intended intervention, (iii) missing outcome data, (iv) outcome measurement, and (v) selection of the reported result. For each domain, the reporting of each study is graded as yes, partly yes, partly no, no, or no information. Based on this assessment, each domain is assigned an overall risk of bias: (i) low, (ii) some concerns, and (iii) high.
Statistical analysis
The Cochrane Handbook for Systematic Reviews of Interventions was used to guide data analysis (16). The number of subjects who underwent MRI measurement of liver fat before and after exercise training was extracted for each study. Both the absolute and relative changes in MRI measured liver fat were extracted for each study with the corresponding SD. For the primary outcome, the number of subjects was extracted into 2 groups, those subjects who had a ≥30% reduction in MRI-measured liver fat vs those who did not.
For the secondary outcome of exercise dose, exercise dose was calculated according to the frequency, intensity, time, and type principles (FITT principles) from the American College of Sports Medicine and reported by the accepted standard of metabolic equivalents of task (MET) min/wk (17). After exercise dose calculation, the number of subjects was extracted into 2 groups, those subjects who achieved ≥750 MET-min/wk of exercise training vs those who did not. The 750 MET-min/wk dose of exercise training was chosen because this equates to 150 minutes per week of moderate-intensity physical activity, the amount recommended for patients with NAFLD by the clinical practice guidelines (4,18,19).
Review manager software (Rev-Man version 5.4; Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration; September 2020) was used to perform descriptive analysis of the studies identified, excluded, and included and quantitative meta-analysis. Where appropriate, the mean differences (absolute and relative liver fat) were calculated and pooled; odds ratios (ORs) between the 2 groups of subjects for each outcome were estimated by weighting the study-specific risk ratios by the inverse of their individual variance. DerSimonian and Laird random-effects models were used to calculate the corresponding 95% confidence intervals (CIs) (20,21). Between-study variability was assessed using the Cochran’s Q statistic (P < 0.05) (20,21). The I2 index was calculated to determine the proportion of heterogeneity accounted for by between-study variability (20,21). To assess publication bias, the post hoc funnel plot asymmetry and the Egger test were used (see Supplementary Figure S2, http://links.lww.com/AJG/C796) (22).
RESULTS
Study selection
After removing duplicates, the search identified a total of 521 abstracts and titles. After the review of all titles, abstracts, and full-study texts, a total of 14 studies met inclusion criteria (Figure 1) (6,23–35). The Supplementary Digital Content (see Supplementary Table 2, http://links.lww.com/AJG/C796) denotes citations and reasons for study exclusion after the full-text publication review.
Figure 1.
PRISMA diagram. The initial search retrieved 521 reports, of which 14 were included in this systematic review with meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study and patient characteristics
Five hundred fifty-one subjects were included from the 14 studies meeting the criteria for this review. Study and patient characteristics are summarized in Table 1. Study intervention duration ranged from 4 to 52 weeks and included the following types of exercise intervention: aerobic (n = 10), high-intensity interval training (n = 3), resistance training (n = 1), and aerobic training plus resistance training (n = 2). The subject mean average age was 53.3 years (range 41–62 years); 47% of participants were female. The subject mean BMI was 31.1 kg/m2 (range 27.1–40.0 kg/m2). No study achieved clinically significant weight loss required for histologic response (7%–10%), and the mean weight loss was – 2.8% (range +1.1% to −4.5%) for exercise intervention.
Table 1.
Characteristics of included studies
| First author (yr) and country | Length | Subjects | Demographics | Supervision | Exercise frequency | Exercise intensity | Exercise session time | Exercise type | Weight loss | Adherence | MRI method |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hallsworth et al. (2011), UK | 8 wks | Sedentary NAFLD (n = 19) Exercise (n = 11) Control (n = 8) |
Exercise 52 yrs Control 62 yrs BMI: Exercise 32.2 kg/m 2 Control 32.2 kg/m2 Male: NR |
Yes | 3 d/wk | Vigorous (70% RM) | 45–60 min | RT | 0.0 kg (0.0%) | All participants completed the 8-wk program showing “good adherence” | MR-S |
| Sullivan et al. (2012), USA | 16 wks | NAFLD with obesity (n = 18) Exercise (n = 12) Control (n = 6) |
Age: Exercise 48 yrs Control 49 yrs BMI: Exercise 37.1 kg/m2 Control 40.0 kg/m2 Male: Exercise 33% Control 17% |
Yes | 5 d/wk | Moderate(45%–55%VO2peak) | 30–60 min | Aerobic | NR | NR | MR-S |
| Pugh et al. (2013), UK | 16 wks | NAFLD (n = 11) Exercise (n = 6) Control (n = 5) |
Age: Exercise 45 yrs Control 51 yrs BMI: Exercise31.0 kg/m 2 Control 30.0 kg/m2 Male: 54% |
Yes | 3–5 d/wk | Moderate(30%–60%MHR) | 30–45 min | Aerobic | −2.2 kg (−2.4%) | NR | MR-S |
| Pugh et al. (2014), UK | 16 wks | NAFLD with obesity (n = 21) Exercise (n = 13) Control (n = 8) |
Overall 48 yrs BMI 31.0 kg/m2 Male: 52% |
Yes | 3 d/wk | Moderate (30%–60% HRR) | 30–45 min | Aerobic | −2.1 kg (−2.4%) | NR | MR-S |
| Hallsworth et al. (2015), UK | 12 wks | NAFLD (n = 23) Exercise (n = 12) Control (n = 11) |
Age: Exercise 54 yrs Control 52 yrs BMI: Exercise 31.0 kg/m2 Control 31.0 kg/m2 Male: NR |
No | 3 d/wk | Vigorous (75%–80% RM) | 30–40 min | HIIT | −1.4 kg (−1.5%) | NR | MR-S |
| Cuthbertson et al. (2016), UK | 16 wks | Sedentary NAFLD (n = 50) Exercise (n = 30) Control (n = 20) |
Age: Exercise 52 yrs Control 50 yrs BMI: Exercise 30.7 kg/m2 Control 29.7 kg/m2 Male: Exercise 77% Control 80% |
Yes | 3–5 d/wk | Moderate (30%–60% HRR) | 30–45 min | Aerobic | −2.5 kg (−2.6%) | NR | MR-S |
| Zhang et al. (2016), China | 52 wks | NAFLD with obesity (n = 220) Exercise (n = 146, moderate-vigorous n = 73, moderate n = 73) Control (n = 74) |
Age: Exercise 54 yrs Control 54 yrs BMI: Exercise 28.1 kg/m2 Control 28.0 kg/m2 Male: Exercise 30% Control 38% |
Yes | 5 d/wk | Aerobic: moderate-vigorous (65%–80%MHR) Aerobic: moderate (45%–55% MHR) | 30 min | Aerobic | Aerobic (moderate): 2.6 kg −3.7% Aerobic (moderate-vigorous): 3.2 kg −4.5% | 94% adherence (participating in ≥80%) Of exercise sessions) | MR-S |
| Shojaee-Moradie et al. (2016), UK | 16 wks | Male patients with NAFLD (n = 27) Exercise (n = 15) Control (n = 12) |
Exercise 52 yrs Control 53 yrs BMI: Exercise31.6 kg/m2 Control 31.7 kg/m2 Male: 100% |
Yes | 4-5 d/wk | Moderate (40%–60% HRR) | 20–60 min | Aerobic | −4.0 kg (−3.8%) | NR | MR-S |
| Cheng et al. (2017), China | 12 wks | NAFLD with impaired fasting glucose (n = 40) Exercise (n = 22) Control (n = 18) |
Age: Exercise 60 yrs Control 59 yrs BMI: Exercise 27.3 kg/m2 Control 27.1 kg/m2 Male: Exercise 21% Control 24% |
Yes | 2–3 d/wk | Moderate-vigorous (60%–75% VO2peak) | 30–60 min | Aerobic | −1.0 kg (−1.4%) | NR | MR-S |
| Houghton et al. (2017), UK | 12 wks | Biopsy-proven NASH (n = 24) Exercise (n = 12) Control (n = 12) |
Exercise 52 yrs Control 51 yrs BMI: Exercise: 33.0 kg/m2 Control: 33.0 kg/m2 Male: NR |
Yes | 3 d/wk | Aerobic: vigorous (90% MHR) RT: vigorous (60%–70% RM) | 45–60 min | Aerobic + RT | +1.0 kg (+1.1%) | NR | MR-S |
| Winn et al. (2018), USA | 4 wks | Sedentary NAFLD with overweight/obesity (n = 21) Exercise (n = 16, 8 HIIT, 8 AT) Control (n = 5) |
Age: HIIT 41 yrs AT 46 yrs Control 51 yrs BMI: HIIT 33.8kg/m2AT 40.3 kg/m2 Control 30.3 kg/m2 Male: NR |
Yes | 4 d/wk | Aerobic: moderate (55% VO2peak) HITT: vigorous (80% VO2peak) | 45–60 min | Aerobic HIIT | Aerobic −1.0 kg HIIT −0.3 kg | NR | MR-S |
| Brouwers et al. (2018), Netherlands | 12 wks | Sedentary NAFLD with obesity (n = 22) Exercise (n = 11) Control (n = 11) |
Age: Exercise 55 yrs Control 58 yrs BMI: Exercise 30.9 kg/m2 Control 29.5 kg/m2 Male: 100% |
Yes | 3 d/wk (2d at,1d RT) | Aerobic: vigorous RT) (70% Wmax) RT: 60% MVC | 30 min | Aerobic + RT | Exercise −0.9 kg Control + 0.25 kg | 95% Exercise session attendance | MR-S |
| Abdelbasset et al. (2020), Egypt | 8 wks | NAFLD with diabetes and obesity (n = 47) Exercise (n = 31,16 HIIT, 15 AT) Control (n = 16) |
Age: HIIT 54 yrs AT 55 yrs Control 55 yrs BMI: HIIT 36.3kg/m2AT 36.7 kg/m2 Control 35.9 kg/m2 Male: HIIT 63% AT 53% Control 56% |
No | 3 d/wk | Aerobic:moderate (60%-70% VO2max) HIIT: vigorous (80%–85% VO2max) | 40–50 min | Aerobic HIIT | NR | NR | MR-S |
| Stine et al. (2021), USA | 20 weeks | Sedentary biopsyproven NASH (n = 28) Exercise (n = 18) Control (n = 10) |
Exercise 53 yrs Control 45 yrs BMI: Exercise 34.3 kg/m2 Control 35.1 kg/m2 Male: Exercise 33% Control 50% |
Yes | 5 d/wk | Moderate (45%–55% VO2peak) | 45 min | Aerobic | −2.5 kg (2.6%) | 82% Overall completion 89% Exercise adherence (completion of ≥80% of exercise sessions) | MRI-PDFF |
BMI, body mass index; HIIT, high-intensity interval training; HRR, heart rate reserve; MHR, maximum heart rate; MVC, maximal voluntary contraction; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PDFF, proton density fat fraction; RM, repetition maximum; S, spectroscopy; Wmax, maximal work-load.
Risk of bias assessment
The majority of included studies were adjudicated to have a low risk of bias (Table 2). Four studies were judged overall to have some concern bias. Three studies (24,32,33) had some concerns with the randomization process in that the exercise and control groups were not well matched for baseline age (24), cardiorespiratory fitness level (24,32), BMI (33), or MRI-measured liver fat (33). The study by Cuthbertson et al. (29) had some concerns for bias due to missing data because 9 subjects in the control condition declined the end of study measurement. With the exception of the studies by Cheng et al. (31) and Abdelbasset et al. (34), which blinded study investigators to the group assignment during testing and analysis, all other studies had some concerns with the measurement of clinical outcomes because neither study investigators nor participants were blinded to the exercise intervention. The Supplementary Digital Content (see Supplementary Figure S1, http://links.lww.com/AJG/C796) summarizes the risk of bias assessment.
Primary outcome: ≥ 30% relative reduction in MRI-measured liver fat
Seven studies contributed data to the analysis of the primary outcome with 152 subjects. The pooled rate of ≥30% relative reduction in MRI-measured liver fat was 34% for exercise training and 13% for the control condition. Meta-analysis found that exercise training subjects had higher odds of achieving ≥30% relative reduction in MRI-measured liver fat (pooled OR 3.51, 95% CI 1.49–8.23, P = 0.004) when compared with standard-of-care controls (Figure 2). No heterogeneity was observed (I2 = 0%).
Figure 2.
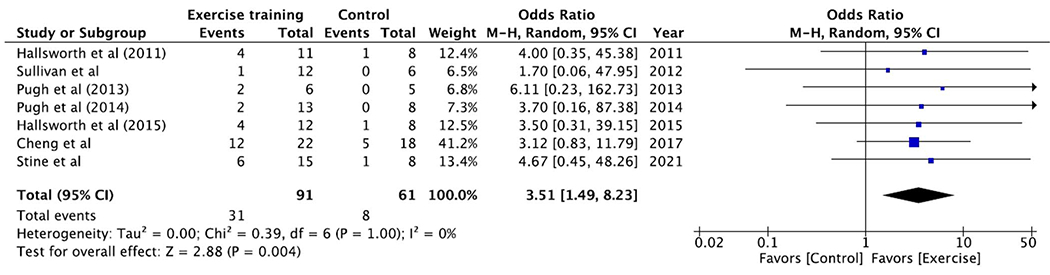
Exercise training achieves threshold of MRI-measured liver fat reduction that predicts histologic treatment response. Exercise training subjects had higher odds of achieving ≥30% relative reduction in MRI-measured liver fat (pooled OR 3.51, 95% CI 1.49–8.23, P = 0.004) when compared with standard-of-care controls. CI, confidence interval; OR, odds ratio.
Secondary outcomes: absolute and relative change in MRI-measured liver fat
Fourteen studies with 551 subjects (338 exercise training and 213 control condition) were included in the secondary outcome analysis of absolute change in MRI-measured liver fat. The mean change in absolute liver fat was −6.7% for exercise training vs −0.8% for the control condition. Meta-analysis demonstrated that the pooled mean difference in absolute change in MRI-measured liver fat for exercise training vs the control condition was −5.8%, 95% CI −8.1 to −3.6 (P < 0.001) (Figure 3). Significant heterogeneity was observed (I2 = 90%).
Figure 3.
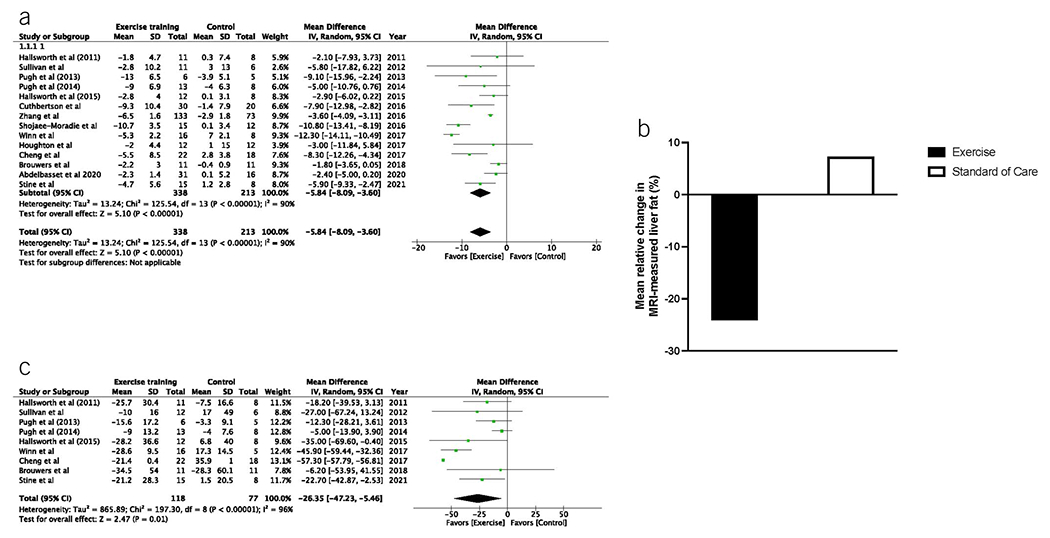
Exercise training leads to greater absolute and relative reduction in MRI-measured liver fat than standard clinical care. (a) The pooled mean difference in absolute change in MRI-measured liver fat for exercise training vs the control condition was −5.8%, 95% CI −8.1% to −3.6% (P < 0.001). (b) Mean relative change in liver fat was −24.1% for exercise training vs +7.3% for the control condition. (c) The pooled mean difference in relative change in MRI-measured liver fat for exercise training vs the control condition was −26.4%, 95% CI −47.2% to −5.5% (P = 0.010). CI, confidence interval.
For the secondary outcome of relative change in MRI-measured liver fat, 9 studies with 195 subjects (118 exercise training and 77 control condition) were able to be analyzed. The mean relative change in liver fat was −24.1% for exercise training vs + 7.3% for the control condition. Meta-analysis demonstrated that the pooled mean difference in relative change in MRI-measured liver fat for exercise training vs the control condition was −26.4%, 95% CI −47.2% to −5.5% (P = 0.010) (Figure 3). Although significant heterogeneity was observed (I2 = 96%), it should be noted that in each study that was included, the effect estimate favored exercise intervention.
Secondary outcomes: exercise dose of ≥750 MET-min/wk
Exercise dose was able to be calculated for all 14 studies totaling 551 subjects. Subjects who were prescribed ≥750 MET-min/wk of exercise training had a −8.0% (95% CI −8.8 to −3.7, P < 0.001) absolute and −28.9% (95% CI −56.2% to ℒ1.7%, P = 0.040) relative mean difference in MRI-measured liver fat vs −4.1% (95% CI −6.1 to −1.9, P ≤ 0.001) and −22.8% (95%CI – 33.2 to −12.4, P < 0.001) for subjects prescribed <750 MET-min/wk (Figure 4). An exercise dose of ≥750 MET-min/wk led to a ≥30% relative reduction in MRI-measured liver fat in 39.3% of subjects vs 25.7% for a dose <750 MET-min/wk. Meta-analysis showed that exercise training subjects prescribed an exercise dose ≥750 MET-min/wk had higher odds of achieving ≥30% relative reduction in MRI-measured liver fat (pooled OR 3.73, 95% CI 1.34–10.31, P = 0.010) when compared with standard-of-care controls (Figure 5) or when compared with those prescribed <750 MET-min/wk (OR 3.17, 95% CI 0.69–14.57, P = 0.140). No heterogeneity was observed (I2 = 0%).
Figure 4.
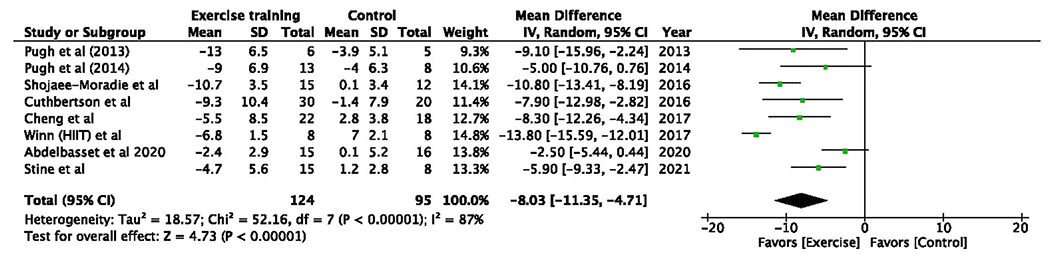
Exercise dose of ≥750 MET-min/wk leads to greater reduction in absolute and relative MRI-measured liver fat. (a) Subjects who were prescribed ≥750 MET-min/wk of exercise training had a −8.0% (95% CI −8.8 to −3.7, P < 0.001) absolute and −28.9% (95% CI −56.2% to −1.7%, P = 0.040) relative mean difference in MRI-measured liver fat vs −4.1% (95% CI −6.1 to −1.9, P < 0.001) and −22.8% (95% CI −33.2 to −12.4, P = 0.005) for subjects prescribed <750 MET-min/wk. (b) Exercise dose ≥750 MET-min/wk led to a ≥30% relative reduction in MRI-measured liver fat in 39.3% of subjects vs 25.7% for a dose <750 MET-min/wk. CI, confidence interval; MET, metabolic equivalent of task.
Figure 5.
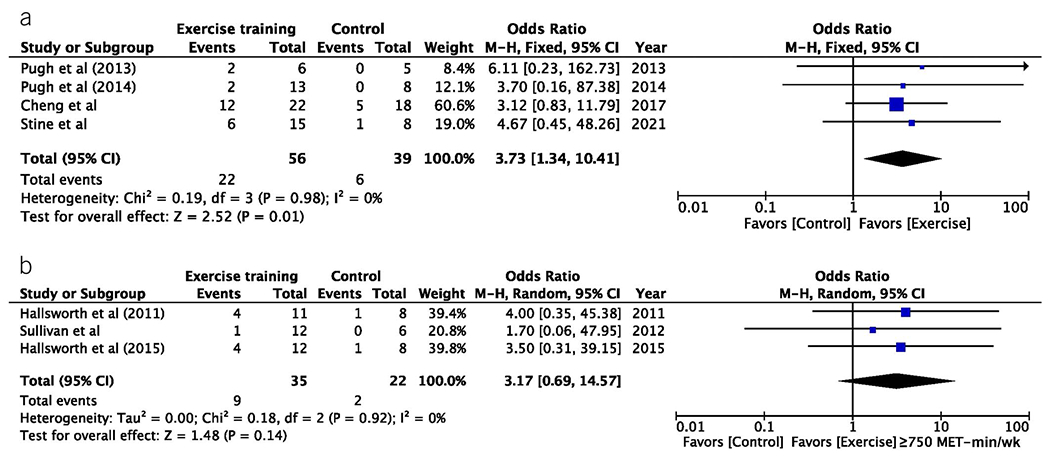
Exercise dose of ≥750 MET-min/wk leads to greater achievement of MRI-measured liver fat reduction threshold for histologic treatment response. (a) Meta-analysis showed exercise training subjects prescribed an exercise dose ≥750 MET-min/wk had higher odds of achieving ≥30% relative reduction in MRI-measured liver fat (pooled OR 3.73, 95% CI 1.34–10.31, P = 0.010). (b) Those prescribed <750 MET-min/wk did not achieve statistically significant rates of ≥30% relative reduction in MRI-measured liver fat (OR 3.17, 95% CI 0.69–14.57, P = 0.140). CI, confidence interval; MET, metabolic equivalent of task; OR, odds ratio.
Sensitivity analysis
Because the study by Zhang et al. (23) was significantly longer and larger in size than any of the other studies, we elected to perform a sensitivity analysis and to exclude this study. Sensitivity analysis demonstrated that the conclusions of the primary and secondary analyses as presented were unchanged. Figures for the sensitivity analysis are found in the Supplementary Digital Content (see Supplementary Materials, http://links.lww.com/AJG/C796).
DISCUSSION
This systematic review and meta-analysis found a consistent body of evidence from a large number of highly rigorous international RCTs demonstrating that exercise training can achieve clinically significant improvement in imaging biomarkers routinely used to monitor treatment response in patients with NAFLD. Patients with NAFLD who participate in an exercise training program are substantially more likely to reduce the amount of fat in their liver. Regular exercise training leads to the achievement of clinically meaningful reductions in MRI-measured liver fat by a factor of 3.5 when the validated MCID of ≥30% relative reduction is used to surrogate for histologic response, including improvement in NASH activity and liver fibrosis stage regression. Importantly, the ability of exercise training to achieve clinically meaningful improvement in MRI-measured liver fat is independent of significant weight loss. The high-quality evidence and low overall bias of this study provide substantial methodologic rigor to support further confidence in prescribing exercise as medicine to patients with NAFLD and NASH to achieve the intended clinical response. Moreover, this study also found an exercise dose of ≥750 MET-min/wk, which equates to 150 min/wk of moderate-intensity physical activity, such as brisk walking or light cycling, remains necessary to reach the threshold of response required to improve histologic outcomes. Importantly, when this exercise dose was prescribed, the rate at which ≥30% relative reduction in MRI-measured liver fat was achieved is similar to that previously reported for early-phase NASH drug trials which studied largely antisteatogenic medications (11,36).
In the context of current recommendations and evidence
At this time, there is no consensus from leading professional societies regarding the optimal physical activity program or exercise prescription for patients with NAFLD with varying recommendations across each of the American College of Sports Medicine (ACSM) FITT principles of exercise prescription. Despite these differences, the current clinical guidelines from the ACSM, American Gastroenterology Association, and European Association for the Study of the Liver all agree on one thing: at least 150 min/wk of moderate-intensity aerobic activity, such as brisk walking or light cycling, is recommended to all patients with NAFLD and NASH (4,18,19). However, these recommendations are based largely on expert opinion because head-to-head clinical trials directly comparing different exercise doses remain largely undertaken and indirect evidence with previous systematic reviews and meta-analyses have not been conducted to compare different exercise doses.
In this study, we are the first on a large-scale across multiple highly rigorous RCTs to confirm the current expert opinion-based guideline recommendations that an exercise dose of 750 MET-min/wk, which equates to 150 min/wk of moderate-intensity physical activity, remains necessary for clinical improvement because this is the dose of exercise required to reach the threshold of MRI-liver fat reduction that surrogates for improved histologic outcomes. We would suggest that as clinicians counsel patients with NAFLD, especially the subset of patients with NASH and liver fibrosis, this minimum amount of physical activity be included in each and every counseling session and reinforced with exercise professionals who routinely develop exercise prescriptions to administer the intended dose of exercise. Having an established dose to target is important to allow exercise professionals to vary each component of FITT exercise prescription for a personalized medicine approach, which may address an important barrier to NAFLD and NASH improvement by improving adherence to lifestyle modification.
As lifestyle modification is currently viewed as a vehicle for clinically significant weight loss, our findings of a weight loss independent benefit of exercise training suggest that we should reframe our thinking about the role of lifestyle modification, and in particular our intent with increasing physical activity through exercise training. This study adds to our understanding by demonstrating that liver fat can be reduced without clinically significant weight loss and can occur in parallel to other weight loss independent benefits of exercise training including gain in cardiorespiratory fitness, change in body composition with loss of adipose tissue and gain in lean muscle mass, and improvement in vascular biology with reversal of endothelial dysfunction, an established biomarker of cardiovascular risk (5).
Strengths and limitations
This is the only systematic review and meta-analysis to (i) calculate a pooled measure of effect for exercise training’s ability to achieve the validated MCID for noninvasive imaging biomarkers required for histologic response; (ii) analyze exercise training programs by dose of exercise delivered, improving on previous attempts to investigate this which focus instead on each component of exercise dose (e.g., FITT) separately; and (iii) extend the existing medical literature through contacting and receiving additional quantitative data not reported in the original peer-reviewed articles from 6 studies, including the standardized provision of relative change in liver fat and achievement of the ≥30% relative reduction threshold.
Further investigation into this topic is unlikely to change our conclusions, given the methodologically robust assessment performed in this study and full-confidence in these findings can be put forth into clinical practice and future lifestyle modification-based clinical trial design. The inclusion of unpublished data enables us to ensure that our meta-analysis is comprehensive and contains the information required to make this strong conclusion, a fact that is further supported by the findings of little or no publication bias.
We do recognize that there are limitations to our work. Missing data are problematic for all systematic reviews with meta-analysis and not all studies reported data for the 30% MRI-measured liver fat cutoff. Our study is also unable to confirm whether the intended exercise dose was truly delivered because exercise adherence was not routinely reported or systematically captured in most of the included RCTs. We were also unable to extract data for direct evidence of exercise’s independent benefit on liver histology because no included study used liver histology as an end point (except the NASHFit trial (6) which included a small subgroup of 3 subjects who underwent a pilot liver biopsy feasibility study after 20 weeks of exercise training (37)). Other limitations include that significant heterogeneity was encountered for several end points, which may be attributed to differences in study intervention where exercise training programs were not consistent across each of the ACSM’s FITT Principles of Exercise Prescription nor study population (e.g., most studies included all stages of NAFLD and only 1 study limited exclusively to NASH) or differences in MRI methods (one study used MRI-proton density fat fraction while all others MR-spectroscopy). Dietary interventions were also highly variable or ignored by individual study designs, and we recognize that in all likelihood, the combination of dietary change and exercise training together would be expected to be more effective than either alone. There was also a lack of allocation concealment across all included studies, which can introduce bias in the results achieved. Despite these limitations, our pooled measure of effect for all outcomes was highly statistically significant and again, of high confidence.
CONCLUSION
This meta-analysis demonstrates that exercise training is 3 and a half times more likely to achieve a ≥30% relative reduction in MRI-measured liver fat, the validated threshold that surrogates for histologic response with improvement in NASH activity and liver fibrosis regression. Moreover, an exercise dose of ≥750 MET-min/wk is most effective to achieve this important threshold of treatment response. These results provide further support of the benefit of prescribing exercise as medicine to all patients with NAFLD, including those with NASH who are most in need disease modifying therapies. Further research is needed to validate this indirect evidence and to determine whether exercise training can lead directly to histologic improvement and also to directly compare the impact of different exercise doses head-to-head.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
Exercise training is crucial in nonalcoholic fatty liver disease management; however, whether it can achieve clinically meaningful improvement in liver fat is unclear, especially without modest weight loss.
WHAT IS NEW HERE
Independent of weight loss, regular exercise is 3.5 times more likely to achieve clinically meaningful liver fat reduction, especially if >750 metabolic equivalents of task-min/wk (e.g., 150 min/wk of walking) are completed.
Exercise should no longer be viewed as a vehicle for weight loss.
Weight-neutral liver fat improvement should be targeted when prescribing exercise with at least 750 metabolic equivalents of task-min/wk of exercise recommended.
Financial support:
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award No. K23DK131290 (J.G.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.L. receives funding support from NCATS (5UL1TR001442), NIDDK (U01DK061734, U01DK130190, R01DK106419, R01DK121378, R01DK124318, and P30DK120515), NHLBI (P01HL147835), and NIAAA (U01AA029019) (Loomba).
Potential competing interests:
J.S.: Research Funding from Astra Zeneca, Galectin, Grifols Inc, Noom Inc, and Novo Nordisk. R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 Bio, Terns Pharmaceuticals, and Viking Therapeutics. In addition to his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C796
REFERENCES
- 1.Younossi ZM. Non-alcoholic fatty liver disease–a global public health perspective. J Hepatol 2019;70(3):531–44. [DOI] [PubMed] [Google Scholar]
- 2.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2019;70(2):487–95. [DOI] [PubMed] [Google Scholar]
- 3.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: Risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48(7):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review. Gastroenterology 2021;160(3):912–8. [DOI] [PubMed] [Google Scholar]
- 5.Thorp A, Stine JG. Exercise as medicine: The impact of exercise training on nonalcoholic fatty liver disease. Curr Hepatol Rep 2020;19(4):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stine JG, Schreibman IR, Faust AJ, et al. NASHFit: A randomized controlled trial of an exercise training program to reduce clotting risk in patients with NASH. Hepatology 2022;76(1):172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker CJ, Martinez-Huenchullan SF, D’Souza M, et al. Effect of exercise on hepatic steatosis: Are benefits seen without dietary intervention? A systematic review and meta-analysis. J Diabetes 2021;13(1):63–77. [DOI] [PubMed] [Google Scholar]
- 8.Fernández T, Viñuela M, Vidal C, et al. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One 2022;17(2):e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabag A, Barr L, Armour M, et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: A systematic review and meta-analysis. J Clin Endocrinol Metab 2022; 107(3):862–81. [DOI] [PubMed] [Google Scholar]
- 10.Babu AF, Csader S, Lok J, et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: A systematic review and meta-analysis. Nutrients 2021;13(9): 3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stine JG, Munaganuru N, Barnard A, et al. Change in MRI-PDFF and histologic response in patients with nonalcoholic steatohepatitis: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; 19(11):2274–83e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamaki N, Munaganuru N, Jung J, et al. Clinical utility of 30% relative decline in MRI-PDFF in predicting fibrosis regression in non-alcoholic fatty liver disease. Gut 2022;71(5):983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 2019;366:14898. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPTTJ, Chandler J, Cumpston M, et al. (eds).Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (www.training.cochrane.org/handbook) (updated July 2019) Cochrane, 2019. [Google Scholar]
- 17.Bayles MPSA. ACSM’s Exercise Testing and Prescription.
- 18.European Association for the Study of the Liver EASL, European Association for the Study of Diabetes EASD, European Association for the Study of Obesity EASO. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 19.Being Active When You Have NAFLD. American College of Sports Medicine (ACSM)-Exercise is Medicine (EIM) (https://www.exerciseismedicine.org/assets/page_documents/EIM_Rx%20for%20Health_NAFLD.pdf).
- 20.Haidich AB. Meta-analysis in medical research. Hippokratia 2010; 14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 21.Murad MH, Montori VM, Ioannidis JPA, et al. How to read a systematic review and meta-analysis and apply the results to patient care: Users’ guides to the medical literature. JAMA 2014;312(2):171–9. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang HJ, He J, Pan LL, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med 2016;176(8):1074–82. [DOI] [PubMed] [Google Scholar]
- 24.Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60(9):1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan S, Kirk EP, Mittendorfer B, et al. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012;55(6):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh CJA, Cuthbertson DJ, Sprung VS, et al. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 2013;305(1):E50–8. [DOI] [PubMed] [Google Scholar]
- 27.Pugh CJA, Sprung VS, Kemp GJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol 2014;307(9):H1298–306. [DOI] [PubMed] [Google Scholar]
- 28.Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin Sci (Lond) 2015;129(12):1097–105. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2016;130(2):93–104. [DOI] [PubMed] [Google Scholar]
- 30.Shojaee-Moradie F, Cuthbertson DJ, Barrett M, et al. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J Clin Endocrinol Metab 2016;101(11):4219–28. [DOI] [PubMed] [Google Scholar]
- 31.Cheng S, Ge J, Zhao C, et al. Effect of aerobic exercise and diet on liver fat in pre-diabetic patients with non-alcoholic-fatty-liver-disease: A randomized controlled trial. Sci Rep 2017;7(1):15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2017;15(1): 96–102e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winn NC, Liu Y, Rector RS, et al. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity–a randomized trial. Metabolism 2018;78:128–40. [DOI] [PubMed] [Google Scholar]
- 34.Abdelbasset WK, Tantawy SA, Kamel DM, et al. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine (Baltimore) 2020;99(10):e19471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouwers B, Schrauwen-Hinderling VB, Jelenik T, et al. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am J Physiol Endocrinol Metab 2018;314(2): E165–73. [DOI] [PubMed] [Google Scholar]
- 36.Loomba R MRI-proton density fat fraction treatment response criteria in nonalcoholic steatohepatitis. Hepatology 2021;73(3):881–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stine JG, Xu D, Schmitz K, et al. Exercise attenuates ribosomal protein six phosphorylation in fatty liver disease. Dig Dis Sci 2020;65(11):3238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


