Abstract
Introduction:
Hepatitis B surface antigen (HBsAg) loss is associated with improved long-term outcomes of patients with chronic hepatitis B but is infrequently achieved with current monotherapies. We assessed whether combination strategies that included treatment withdrawal enhanced HBsAg loss.
Methods:
A randomized (1:1) trial of tenofovir disoproxil fumarate (TDF) for 192 weeks with or without peginterferon (PegIFN) alfa-2a for the first 24 weeks, followed by withdrawal of TDF at week 192 with 48 weeks of off-treatment follow-up to week 240. The primary endpoint was HBsAg loss at week 240.
Results:
Of 201 participants (52% HBeAg-positive,12%/6% genotype A/A2, 7% cirrhosis) randomized to TDF+PegIFN (n=102) or TDF alone (n=99) 6 participants had lost HBsAg at the end of the treatment phase (week 192), 5 (5·3%) in the combination group and 1 (1·0%) in the TDF alone group (p=0·09). By week 240, 9 participants had cleared HBsAg, 5·3% in combination and 4·1% in monotherapy arms (p=0·73). HBsAg decline and loss occurred earlier with TDF+PegIFN than TDF, with a ≥1-logIU/mL qHBsAg decline by week 24 in 28% compared to 6% in TDF (p=0.04). HBsAg loss occurred in 7 of 12 (58%) with HBV subgenotype A2 (all HBeAg-positive) compared to only 2 of 189 (1%) with other HBV genotypes, and in 8 of 93 (8·6%) HBeAg-positive versus 1 of 87 (1·1%) HBeAg-negative.
Discussion:
Peginterferon combined TDF followed by protocolized TDF withdrawal led to earlier but not higher percentages of HBsAg clearance. Pre-treatment HBeAg positivity and subgenotype A2 were strongly associated with HBsAg clearance.
Source of funding:
NIH-NIDDK ClinicalTrials.gov ID NCT01369212
Keywords: HBeAg, genotype, quantitative HBsAg, flare, discontinuation
INTRODUCTION
Chronic infection with the hepatitis B virus (HBV) is a global health problem that afflicts 296 million people and results in an estimated 900,000 deaths each year primarily from decompensated cirrhosis or hepatocellular carcinoma.1 While the past two decades have witnessed significant advances in the treatment of chronic hepatitis B (CHB), there is an appreciable need for therapies that yield higher rates of functional cure. Defined as sustained loss of hepatitis B surface antigen (HBsAg) and undetectable HBV DNA off treatment, functional cure is associated with the best long-term survival and lowest incidence of hepatocellular carcinoma.2
Currently approved therapies include nucleoside analogues (NAs) and peginterferon (PegIFN) alfa-2a.3 NAs rapidly reduce serum HBV DNA, normalize serum aminotransferase levels, improve liver histology, and reduce liver-related adverse events.4 However, NAs have minimal if any effect on covalently closed circular HBV DNA, that resides in the nucleus of infected hepatocytes as episomal DNA, and very low rates of HBsAg clearance. PegIFN directly inhibits HBV transcription and is associated with activation of interferon-inducible genes that influence innate and adaptive immune responses to HBV, facilitating clearance of cccDNA5 and achieving higher rates of HBsAg loss in the short-term compared to NAs, though overall rates are low.6 Combining the two drug classes is a potential strategy to improve rates of HBsAg loss. A recent meta-analysis reported a pooled rate of HBsAg loss of 9% (95% CI: 7-12%)7 among patients treated with PegIFN and NA combination therapy, but with moderate heterogeneity contributing to uncertainty. Another strategy used to enhance HBsAg clearance is withdrawal of NAs after years of sustained HBV DNA suppression.8 Interpreting withdrawal study results is hampered by variable NA exposure before withdrawal 9–14, heterogeneity in HBeAg status at NA therapy initiation and differences in retreatment criteria. Furthermore, few studies have used a prospective study design.10,12,15 No prior study has examined whether these two strategies -- PegIFN added to NA followed by systematic withdrawal of NA after an interval of sustained viral suppression -- can enhance the probability of HBsAg loss.
The Hepatitis B Research Network (HBRN) Immune-Active Trial was designed to evaluate whether these two strategies (addition of PegIFN to NA and NA withdrawal) enhance HBsAg loss.
METHODS
Study design
This was a randomized (1:1) parallel-group trial comparing the safety and efficacy of PegIFN alfa-2a (180 μg subcutaneously once weekly) for the initial 24 weeks combined with TDF (300 mg orally once daily) for 192 weeks compared to TDF alone for 192 weeks (ClinicalTrials.gov ID NCT01369212). After completing 192 weeks of treatment, participants meeting criteria for treatment discontinuation stopped TDF treatment and were followed for another 48 weeks (total duration of treatment and follow up was up to 240 weeks). Participants who did not meet criteria or who refused discontinuation remained on TDF treatment for another 48 weeks (to week 240).
The study was conducted by the HBRN, a multicenter network funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, to prospectively study the natural history of chronic hepatitis B (CHB) and to conduct clinical trials in both children and adults. This trial was conducted at 21 clinical sites (12 consortium sites and nine subsites) in the United States and one in Canada, with the University of Pittsburgh serving as Data Coordinating Center.
Participants
Participants who met the following criteria were eligible: (1) at least 18 years of age; (2) chronic HBV infection based on detection of HBsAg or HBV DNA >1,000 IU/mL for a minimum of 24 weeks or histological evidence of CHB; (3) HBeAg-positive or negative; (4) HBV DNA levels ≥1000 IU/mL on 2 occasions at least 4 weeks apart within 32 weeks of randomization; and (5) at least two elevated serum alanine aminotransferase (ALT) levels (>45 U/L for males and >30 U/L for females) at least 4 weeks and no more than 32 weeks apart with the second being within 8 weeks before randomization. All participants underwent liver biopsy within 144 weeks of randomization and were required to have a histology activity index (HAI) score ≥3 or fibrosis score ≥1 by the modified Ishak system.16 Absence of hepatocellular carcinoma was required as shown by alpha-fetoprotein levels ≤20ng/mL within 8 weeks and negative abdominal imaging within 28 weeks of randomization.
Key exclusion criteria were: ALT >450 U/L for males and >300 U/L for females; treatment with PegIFN or NA within 48 weeks of randomization; more than 48 weeks of therapy with NA for hepatitis B at any time in the past; a past or current history of hepatic decompensation or hepatocellular carcinoma; platelet count <90,000 /mm3, absolute neutrophil count <1500 /mm3 (<1000/mm3 for African-Americans), direct bilirubin >0·5 mg/dL, albumin <3·5 g/L, INR >1·5, estimated creatinine clearance <60 mL/minute; human immunodeficiency virus, hepatitis D or hepatitis C virus co-infection; or pre-existing medical conditions that could be exacerbated by interferon therapy. A full list of inclusion and exclusion criteria is provided in Supplemental materials.
Randomization and masking
Randomization was performed centrally using a web-based, computer-generated randomization sequence following Efron’s biased coin randomization approach to ensure balance across strata defined by HBeAg status (positive/negative), genotype (A vs. non-A) and cirrhosis (present vs. absent). The randomization algorithm was implemented on the web server maintained by the data coordinating center. For each participant enrolled in the trial, site coordinators entered the strata information on the secured randomization website and the algorithm would generate the treatment assignment for that participant. Participants and investigators were not masked to treatment allocation.
Sample size and power analysis
Initially, the sample size 188 per arm of the study was determined to achieve 80% power for detecting a difference of 10% (5% in TDF and 15% in combination treatment) in the primary endpoint percentage of participants achieving HBsAg loss at week 240 with a two-sample two-sided Wald test under maximum 5% type I error, with an estimated 25% dropout. However, this sample size was later revised as the literature showed even lower rate for HBsAg loss with TDF therapy. Moreover, a 25% dropout was deemed an overestimate and was reduced to 10%. The revised targeted sample size was 100 participants per arm, which provided 81% power to detect a difference of 11% (4% in TDF vs. 15% in Combination) using a log-rank test under 5% level of significance.
Intervention and Procedures
After initiating therapy, participants were seen in follow up at 4-week intervals for the first 12 weeks and at 12-week intervals thereafter. Participants who discontinued TDF at or before week 192, were seen every 4 weeks for 24 weeks after stopping treatment and then every 12 and 24 weeks until week 240. More frequent visits and testing were performed for participants who met criteria for ALT flares. Participants not meeting eligibility criteria or who refused discontinuation, continued treatment with TDF and were followed every 12 weeks from weeks 192 to 240. Additional study visits occurred at the discretion of the investigator for participants having side effects related to treatment, adherence issues, or ALT flares.
Initially, criteria for TDF discontinuation at 192 weeks were absence of cirrhosis at study entry and HBV DNA <1000 IU/mL from weeks 168 to192 of TDF treatment, irrespective of HBeAg status. However, after two participants who were HBeAg-positive at the time of TDF withdrawal had severe flares accompanied by jaundice, the protocol was amended to require HBeAg negativity at and after week 144 to qualify for withdrawal. The protocol was further amended to require the presence of anti-HBe at or after week 144 to meet criteria for treatment discontinuation. During off-treatment follow-up, participants meeting criteria for re-treatment were offered reinstitution of therapy with TDF 300 mg daily. Retreatment criteria included any clinical decompensation, total bilirubin ≥3·0 mg/dL or direct bilirubin ≥1·0 mg/dL or persistent (duration varied by level of ALT elevation) elevation of HBV DNA and ALT values. Details included in Supplemental materials.
Study efficacy endpoints were based on quantitative HBeAg and HBsAg (Elecsys; Roche Molecular Systems, Branchburg, NJ) assays performed at the HBRN central virology laboratory at the University of Washington with lower limits of detection (LLODs) of 0·3 IU/mL for HBeAg and 0·05 IU/mL for HBsAg. For the primary endpoint based on HBsAg (described below), central lab results were used unless not available in which case a local lab result was used. For one participant at week 192, the central HBsAg test was positive but the local test was negative along with several serial negative results before and after week 192 from the central lab. This participant was considered to be HBsAg negative at week 192. HBV DNA was tested centrally using a real-time PCR assay (COBAS Ampliprep/COBAS TaqMan Test, version 2·0; Roche Molecular Systems) with a lower limit of quantification of 20 IU/mL and an LLOD of 10 IU/mL. HBV genotyping was performed at the Molecular Epidemiology and Bioinformatics Laboratory in the Division of Viral Hepatitis at the Centers for Disease Control and Prevention using mass spectrometry17, or was available locally.
Outcomes
The primary efficacy endpoint was the absence of detectable HBsAg at study week 240. Secondary endpoints were HBsAg loss at week 192, HBeAg loss at weeks 192 and 240, number of adverse events (AEs) and serious AEs and ALT flares. An on-treatment ALT flare was defined as an ALT elevation ≥300 U/L in men and ≥200 U/L in women and greater than 3 times baseline (day 0) value. Finally, in assessing the result of TDF withdrawal, a combined endpoint of HBV DNA <1000 IU/mL and normal ALT while not receiving TDF therapy at week 240, was examined to reflect an inactive CHB phenotype that would not require retreatment.
Statistical Analysis
The detailed statistical analysis plan is provided in the study protocol. Briefly, participant features across arms were characterized by medians and quartiles for continuous variables and by frequencies and percentages for categorical variables.
The primary endpoint was the percentage of participants who lost HBsAg by week 240 estimated using the product limit (Kaplan-Meier) method and compared using a two-sided two-sample Wald test. The secondary endpoint of the cumulative percentage of participants with HBsAg loss over time was compared using log-rank test.
Further secondary binary endpoints included HBeAg loss (among HBeAg-positive participants), HBV DNA <1000 IU/mL, HBV DNA <20 IU/mL, normalized ALT, and combined endpoint of HBV DNA <1000 IU/mL and normalized ALT at weeks 192 and 240. Percentages of participants with each event in the two treatment groups were compared using chi-square test or Fisher’s exact test as appropriate. The analyses of secondary endpoints were treated as exploratory so adjustments were not made for multiple comparison.
Some post-hoc analyses are reported. Changes in quantitative HBsAg between baseline and week 192, and between baseline and week 240 were compared between treatment groups using Wilcoxon’s rank-sum test. Unadjusted and adjusted associations between baseline characteristics and the binary outcome of ≥1 log HBsAg decline from baseline to week 240 were investigated using log-binomial regression. The results were reported as relative risks and 95% confidence intervals. For variable selections for adjusted analyses, we used stepwise approach. Change in qHBsAg over time from baseline, by treatment group and by baseline HBeAg status (negative/positive) were graphically described using medians and the 25th and 75th percentiles.
Primary analyses were performed using all randomized participants with endpoint data (differs by endpoint) The original sample size calculations accounted for participants lost to follow up for various reasons, and the rate of missing was similar in the two treatment groups. Sensitivity analyses of results based on multiple imputations and inverse-probability-weighting are presented in supplemental information. All authors had access to the study data and reviewed and approved the final manuscript.
Role of the funding source
The protocol was approved by the HBRN Steering Committee and the Institutional Review Boards (Research Ethics Board in the case of the Canadian site) of the participating sites, and all participants provided written informed consent. The study was overseen by an independent data safety monitoring board (DSMB) appointed by the NIDDK to monitor the clinical studies of the HBRN. TDF (Viread®) was kindly provided by Gilead Sciences, Foster City, CA, PegIFN-alfa2a (Pegasys®) by Roche Genentech, San Francisco, CA, and assays for HBV DNA by Roche Diagnostics, Indianapolis, IN, but these entities had no role in study design, data collection, data analysis or interpretation or the writing of this report.
RESULTS
Participants
A total of 281 adults were assessed for eligibility and 201 were enrolled; 102 were randomized to TDF alone and 99 to TDF plus PegIFN between December 1, 2012, and November 30, 2015 (Figure 1). The majority of participants were male (65%), Asian (83%), with a median age of 41 years. At enrollment, 52% of participants were HBeAg-positive, 12% had genotype A and 7% had cirrhosis. The median (IQR) ALT was 71 U/L (49,119), HBV DNA 6·5 log10 IU/mL (5·2, 8·1) and qHBsAg 3·7 (3·0, 4·3) log10 IU/mL. Baseline characteristics of the two groups were well balanced (Table 1).
Figure 1: Flow of Participants During the Study.

A total of 102 were randomized to TDF alone and 99 to TDF plus PegIFN. In the combination group, one participant withdrew prior to any study drug and 6 dropped out prior to week 192; in the TDF group, 6 dropped out before week 192. Of the 201 participants, 188 (94%) reached week 192. Of those reaching week 192, 111 were initially withdrawn from TDF treatment: 51 in the TDF group and 60 in the combination group. A total of 9 participants resumed treatment due to protocol changes for safety and 12 were restarted on therapy due to relapse. A total of 187 reached week 240, and missing rates did not differ significantly between treatment arms.
Note: In CONSORT, we have included people with any information by week 240. Not all participants followed had all labs and hence sample size varied by the variable analyzed.
Table 1:
Baseline Characteristics of Treatment Groups
| Characteristic at Randomization | TDF+PegIFN (N=99) | TDF (N=102) |
|---|---|---|
|
| ||
| Male, n (%) | 59 (60) | 71 (70) |
|
| ||
| Age (years), median (IQR) | 41 (34, 50) | 41 (34, 49) |
|
| ||
| BMI kg/m2, median (IQR) | 24 (22, 27) | 25 (23, 28) |
|
| ||
| Race, n (%) | ||
| White | 7 (7) | 9 (9) |
| Black | 7 (7) | 9 (9) |
| Asian | 85 (86) | 80 (80)) |
| Other/mixed | 0 | 2 (2) |
|
| ||
| HBeAg positive, n (%) | 49 (49) | 54 (53) |
|
| ||
| qHBeAg (log10IU/mL), median (IQR)* | 2·6 (1·6, 3·2) | 3·0 (1·9, 3·3) |
|
| ||
| HBV genotype, n (%) | ||
| A1 | 4 (4) | 8 (8) |
| A2 | 5 (5) | 7 (7) |
| B | 50 (51) | 37 (26) |
| C | 36 (36) | 32 (31) |
| D | 1 (1) | 12 (12) |
| E | 3 (3) | 5 (5) |
| F | 0 (0) | 1 (1) |
|
| ||
| HBV DNA (log10 IU/mL), median (IQR) | 6·3 (5·2, 7·9) | 6·7 (5·2, 8·2) |
|
| ||
| qHBsAg (log10IU/mL), median (IQR) | 3·6 (3·0, 4·9) | 3·9 (3·0, 4·5) |
|
| ||
| ALT U/L, median (IQR) | ||
| Male | 75 (57,117) | 82 (59, 135) |
| Female | 66·5 (47·5, 117·5) | 71 (49, 129) |
|
| ||
| Platelet count (x103/mm3), median (IQR) | 196 (161, 227) | 192 (170, 233) |
|
| ||
| Total bilirubin (mg/dL), median (IQR) | 0·7 (0·5, 0·8) | 0·6 (0·5, 0·8) |
|
| ||
| White cell count, (x103/mm3)m=, median (IQR) | 5·2 (4·5, 6·1) | 5·6 (4·8, 6·5) |
|
| ||
| Hemoglobin (g/dL), median (IQR) | 14·5 (13·7, 15·6) | 14·8 (13·7, 15·6) |
|
| ||
| Creatinine clearance (mL/min/1.73m2), median (IQR) | 96 (86, 111) | 106 (93, 117) |
|
| ||
| Albumin (g/dL), median (IQR) | 4·3 (4·0, 4·6) | 4·2 (4·1, 4·5) |
|
| ||
| Histologic Activity Index (HAI) ≥3, n (%) | 96 (97) | 97 (95) |
|
| ||
| Ishak score 5-6, n (%) | 7 (7) | 7 (7) |
Only among HBeAg positive.
After randomization, two participants were identified as having NA therapy for at least 24 weeks within 48 weeks of randomization. These two erroneously enrolled participants, both in the TDF group, remained in the study and were included in the analysis. In the combination group, one participant withdrew prior to any study drug and 6 more dropped out prior to week 192; in the TDF group, 6 dropped out before week 192 (Figure 1). One participant met the definition for virologic breakthrough on treatment and was switched to TDF-emtricitabine at week 69. There was no genotypic confirmation of resistance and the participant’s HBV DNA decreased but did not become undetectable on TDF-emtricitabine by week 240. Of the 201 participants, 188 (94%) reached week 192, 187 (90%) reached week 240, and the percentages who did not meet the milestone weeks did not differ significantly between treatment arms.
Of 188 participants reaching week 192, 111 qualified and were initially withdrawn from TDF treatment: 51 of 96 (53%) in the TDF group and 60 of 92 (65%) in the combination group (Figure 1). However, 9 (5 in the TDF group and 4 in the combination group) were placed back on treatment because of protocol changes for safety (i.e., the requirement of HBeAg loss and anti-HBe acquisition at least 48 weeks prior to withdrawal), leaving 102 withdrawn from TDF (46 in TDF alone group and 56 in the combination group). Of the 102 participants who met the modified protocol criteria for withdrawal of TDF, 12 (5 of 56 in the combination group and 7 of 46 in the TDF group) were restarted on therapy before week 240 due to relapse (Figure 2).
Figure 2: Status of Patients from Week 192 (Eligible for Withdrawal) to Week 240 (End of Follow-up).
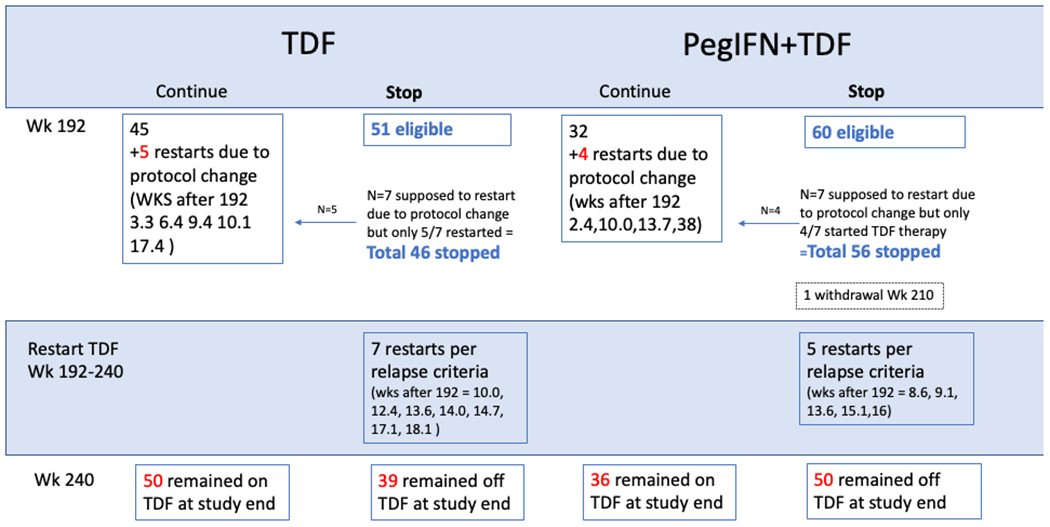
Of 188 participants reaching week 192, 111 qualified and were initially withdrawn from TDF treatment: 53% in the TDF group and 65% in the combination group. However, 9 (5 in the TDF group and 4 in the combination group) were placed back on treatment because of protocol changes for safety. Of the 102 participants who met the modified protocol criteria for withdrawal of TDF, 12 (5 of 56 in the combination group and 7 of 46 in the TDF group) restarted TDF before week 240 due to relapse.
Efficacy
HBsAg Loss
HBsAg Loss by Week 240:
By week 240, 9 HBsAg losses were observed, including 5 (5·3%) in the combination group versus 4 (4·1%) in the TDF alone group (p=0·72) (Figure 3A). HBsAg loss tended to occur earlier in the combination group (all prior to week 144) compared to TDF alone arm (3 of 4 after week 192). Characteristics of the 9 participants who lost HBsAg by week 240 are shown in Table 2, highlighting that 8 of the 9 were HBeAg-positive at study entry and 7 of 9 were also HBV subgenotype A2.
Figure 3: Cumulative Incidence of Key Serologic Endpoints.
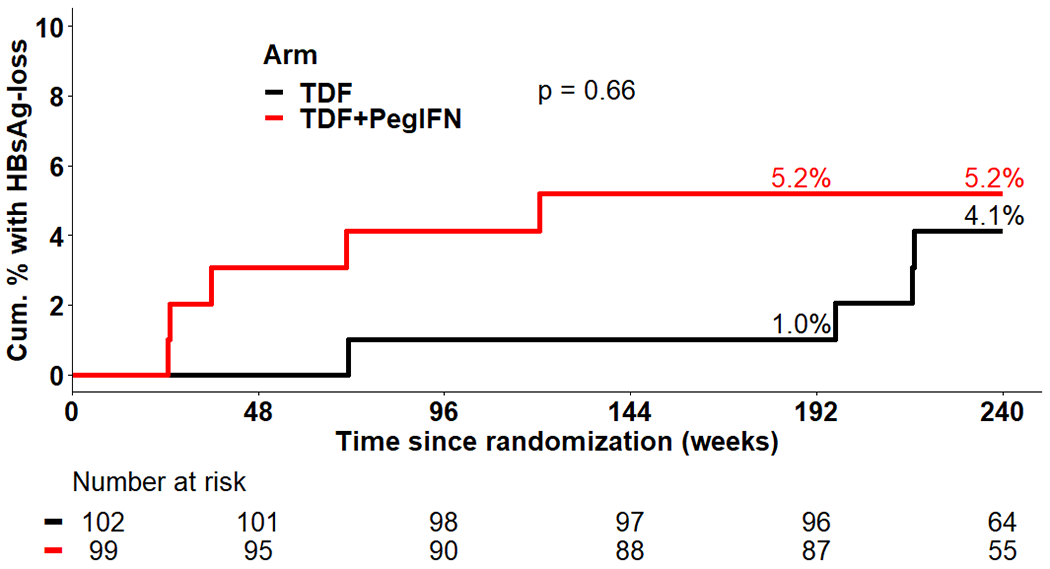

A: The cumulative percentage achieving HBsAg loss at week 240 was 5·3% in the TDF+PegIFN group and 4·1% in the TDF alone group (p=0·66).
B: The cumulative percentage achieving HBeAg loss at week 240 was 66·2% in the TDF+PegIFN group and 46·2% in the TDF alone group (p=0·009).
Table 2:
Characteristics of Patients Achieving HBsAg Loss
| ID | Treatment | Age in years | Sex | Race | HBeAg at Start of Treatment | Geno type | HBV DNA log10 IU/mL | qHBsAg log10 IU/mL | qHBeAg log10 IU/mL | ALT U/L | ISHAK | Week of HBsAg Loss | Status at time of HBsAg Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | TDF alone | 66·7 | F | White | Positive | A2 | 8·6 | 5·1 | 3·6 | 129 | 2 | 71 | On treatment |
| B | TDF alone | 45·9 | M | Black | Positive | A2 | 7·3 | 4·1 | 2·2 | 94 | 3 | 217 | Off treatment |
| C | TDF alone | 41·2 | M | White | Positive | A2 | 8·6 | 5·0 | 3·4 | 92 | 6 | 197 | On treatment |
| D | TDF alone | 37·5 | M | White | Positive | A2 | 8·8 | 4·9 | 3·2 | 66 | 2 | 217 | Off treatment |
| E | TDF+PegIFN | 37·2 | F | Asian | Negative | C | 4·2 | 2·3 | N/A | 91 | 0 | 71 | On treatment |
| F | TDF+PegIFN | 53·2 | M | White | Positive | A2 | 8·4 | 5·2 | 3·4 | 88 | 4 | 25 | On treatment |
| G | TDF+PegIFN | 60·0 | M | White | Positive | A2 | 8·5 | 5·1 | 3·2 | 66 | 2 | 36 | On treatment |
| H | TDF+PegIFN | 53·3 | F | Black | Positive | A2 | 8·4 | 4·8 | 3·5 | 54 | 5 | 25 | On treatment |
| I | TDF+PegIFN | 34·4 | F | Asian | Positive | B | 8·1 | 4·5 | 3·3 | 56 | 1 | 121 | On treatment |
ALT, alanine aminotransferase; HAI, hepatitis activity index; PegIFN, peginterferon; TDF, tenofovir disoproxil fumarate
HBsAg Loss by Withdrawal Status:
Of the 111 participants who withdrew from treatment, HBsAg loss had occurred before week 192 in 5 (3 with genotype A2) and then occurred in 2 more patients (both with genotype A2 having received TDF alone) between weeks 192 and 240. Among the 77 participants who were never withdrawn from treatment, HBsAg loss occurred before week 192 in one and between weeks 192 and 240 in one; both had genotype A2 and were not withdrawn because of cirrhosis on pre-treatment liver biopsy.
Factors Associated with HBsAg Loss:
Factors significantly associated with achieving HBsAg negativity in univariable analysis included non-Asian race, HBeAg positivity at baseline HBeAg, subgenotype A2, and higher levels of HBV DNA and HBsAg, but not age nor serum ALT (Table 3). AST levels, liver histologic features, and treatment arm were not significantly associated with HBsAg loss (data not shown). The limited number with HBsAg loss precluded multivariable analysis, however, HBeAg positivity and genotype A2 appear to the major factors associated with HBsAg loss.
Table 3:
HBsAg Loss at Week 240 by Participant Characteristics
| Subgroup (at randomization) | N (%) in the subgroup with HBsAg data available at week 240 | N (%) in the subgroup with HBsAg loss** | P value |
|---|---|---|---|
|
| |||
| Age Stratum (years) | 0·35 | ||
| 10-<30 | 27 (15·0) | 0 | |
| 30-<40 | 56 (31·1) | 3 (5·6) | |
| 40-<50 | 54 (30·0) | 2 (3·8) | |
| 50-<60 | 29 (16·1) | 2 (6·9) | |
| >60 | 14 (7·8) | 2 (16·7) | |
|
| |||
| Sex | 0·72 | ||
| Male | 117 (65·0) | 5 (4·3) | |
| Female | 63 (35·0) | 4 (6·3) | |
|
| |||
| Race | <0·001 | ||
| Asian | 146 (81·1) | 2 (1·4) | |
| Non-Asian | 34 (18·9) | 7 (20·6) | |
|
| |||
| HBeAg status | 0·032 | ||
| Negative | 87 (48·3) | 1 (1·1) | |
| Positive | 93 (51·7) | 8 (8·6) | |
|
| |||
| Genotype | <0·0001 | ||
| A1 | 10 (5·6) | 0 (0·.0) | |
| A2 | 12 (6·7) | 7 (58·3) | |
| B | 78 (43·3) | 1 (1·3) | |
| C | 59 (32·8) | 1 (1·7) | |
| D/E/F | 21 (11·7) | 0 (0·0) | |
|
| |||
| ALT (X ULN) * | 0·25 | ||
| <1ULN | 2 (1·1) | 0 (0.0) | |
| 1-<2 ULN | 43 (23·9) | 0 (0·0) | |
| 2-<5 ULN | 94 (52·2) | 8 (8·5) | |
| ≥5 ULN | 41 (22·8) | 1 (2·4) | |
|
| |||
| HBV DNA log10 IU/mL | 0·005 | ||
| ≤5 | 38 (21·1) | 1 (2·6) | |
| 5-8 | 90 (50·0) | 1 (1·1)> | |
| >8 | 52 (28·9) | 7 (13·5) | |
|
| |||
| qHBsAg log10 IU/mL | 0·008 | ||
| ≤3 | 42 (23·3) | 1 (2·4) | |
| 3-4 | 63 (35·0) | 0 (0·0) | |
| ≥4 | 75 (41·7) | 8 (10·7) | |
ALT ULN adjusted for sex (females 20-ULN and males 30-ULN)
HBsAg negativity at week 240 occurred in 8 of 93 (8·6%) participants who were initially HBeAg-positive, but only one of 87 (1·1%) who were initially HBeAg-negative (p=0·04). Furthermore, HBsAg loss occurred in 7 of 12 (58·3%) participants with genotype A2. HBsAg loss was rare in patients with other genotypes (2 of 168: 1·2%), occurring in 1 of 87 with genotype B and 1 of 68 with genotype C, both of whom received combination therapy
HBeAg Loss
Among HBeAg-positive participants (n=103), 64% achieved HBeAg negativity by week 192 in the combination therapy group compared to 33% in the TDF alone group (p=0·001), a difference that persisted but was smaller at week 240: (66%) vs (46%) (p=0·04) (Figure 3B). The median time to HBeAg loss being 144 weeks in the combination therapy group compared to >240 weeks in the TDF alone group (log-rank p=0·01).
Supplementary sensitivity analyses for missing data using multiple imputation and inverse-probability-weighting yielded similar conclusions for the primary and secondary outcomes (Supplementary Material on handling missing data).
Change in qHBsAg
Decline in qHBsAg occurred earlier with combination therapy than with TDF alone, with a ≥1-log IU/mL qHBsAg decline by week 24 in 28% on combination therapy compared to 6% in TDF arm (p=0·04) but the differences in mean qHBsAg decline by treatment group were lost after PegIFN was discontinued, such that percentages with decline were not significantly different at week 192 or 240 (Table 4).
Table 4:
Secondary Outcomes by Treatment Group at Weeks 192 and 240*
| Week 192 | Week 240 | |||||
|---|---|---|---|---|---|---|
| Outcomes | TDF+PegIFN (N=99) | TDF (N=102) | P value | TDF+PegIFN (N=99) | TDF (N=102) | P value |
| HBV DNA <1000 IU/mL | N=91 | N=96 | N=89 | N=92 | ||
| n (%) | 90 (98·9%) | 95 (99·0%) | >0·99 | 65 (73·0%) | 66 (71·.7%) | 0·87 |
| HBV DNA <20 IU/mL | N=91 | N=96 | N=89 | N=92 | ||
| n (%) | 87 (95·6%) | 82 (85·4%) | 0·022 | 48 (53·9%) | 46 (50·0%) | 0·65 |
| Normal ALT levels (M ≤30, F ≤20 U/L) | N=91 | N=96 | N=89 | N=92 | ||
| n (%) | 51 (56·0%) | 37 (38·5%) | 0·018 | 41 (46·1%) | 38 (41·3%) | 0·55 |
| HBV DNA<1000 IU/mL and normal ALT levels | N=90 | N=96 | N=89 | N=92 | ||
| n (%) | 49 (54·4%) | 37 (38·5%) | 0·038 | 32 (36·0%) | 35 (38·0%) | 0·88 |
| HBV DNA <1000 IU/mL and HBeAg negative ** | N=46 | N=51 | N=45 | N=49 | ||
| n (%) | 28 (60.9%) | 14 (27.5%) | 0.001 | 25 (55.6%) | 18 (36.7%) | 0.10 |
| HBV DNA <1000 IU/mL, normal ALT and HBeAg negative ** | N=45 | N=51 | N=45 | N=49 | ||
| n (%) | 13 (28.9%) | 8 (15.7%) | 0.14 | 15 (33.3%) | 9 (18.4%) | 0.11 |
| ≥1 log decline in qHBsAg levels | N=91 | N=96 | N=86 | N=91 | ||
| n (%) | 23 (25·3%) | 26 (27·1%) | 0·87 | 28 (32·6%) | 27 (29.7%) | 0·75 |
| Change in qHBsAg from baseline (log10 IU/mL) | N=91 | N=96 | N=86 | N=91 | ||
| Mean (STD) | -0·84 (1·36) | -0·78 (1·12) | 0·74 | -1·07 (1·50) | -0·95 (1·39) | 0·57 |
| Change in qHBeAg from baseline (log10 IU/mL) ** | N=46 | N=51 | N=43 | N=48 | ||
| Mean (STD) | -2·51 (1·40) | -2·18 (1·26) | 0·22 | -2·49 (1·39) | -2·47 (1·20) | 0·95 |
Sample sizes for outcome measures vary according to the availability of the respective measures at the specified time point.
Only among HBeAg positive participants at enrollment.
The mean decline and the corresponding 95% confidence intervals for qHBsAg levels are shown separately for the HBeAg-positive and HBeAg-negative and by treatment group in Figure 4. The decline in qHBsAg levels was more rapid and greater among HBeAg-positive than HBeAg-negative participants. By 192 weeks the mean (SD) change in HBsAg levels in the HBeAg-positive participants was 1·19 (1·66) (combination arm) and 1·12 (1·38) (TDF only arm) vs only 0·47 (0·86) (combination arm) and 0·38 (0·48) (TDF only arm) in the HBeAg-negative participants. Thus, HBeAg status but not treatment arm was associated with overall qHBsAg decline in univariable or multivariable models (sTable 1).
Figure 4: Change in qHBsAg Over Time in HBeAg-positive and HBeAg-negative Participants, by Treatment Group.

Data presented are means and 95% confidence intervals. The decline in qHBsAg occurred earlier with combination therapy than TDF alone but was not statistically different at week 240. The mean (SD) change in qHBsAg was less in those who were initially HBeAg-negative (−0·43 [SD = 0·69] log IU/mL) than in those who were HBeAg-positive (−1·16 [SD = 1·51]), with no difference by treatment group (p=0·59).
HBV DNA Undetectability
At week 192, when all participants were still on TDF treatment, 5 of 92 (4·9%) participants in the combination group and 1 of 96 (1·0%) in the TDF alone group were HBsAg negative (p=0·11) and 95% had HBV DNA <20 IU/mL in the combination group versus 85% in the TDF alone group (p=0·02) (Table 4). ALT normalization was higher with combination therapy than with TDF alone at week 192 also (56% vs 39%, p=0·02). However, at week 240 after the withdrawal phase, these outcomes were less frequent and similar in the two treatment arms, with percentages with HBV DNA <20 IU/mL of 54% and 50% (p=0·65) and with normal ALT in 46% and 41% (p=0·55) in the combination and TDF alone groups, respectively. The percentage achieving a combined response of normal ALT and HBV DNA <1000 IU/mL at week 240 was also similar between the two treatment arms (36% versus 38%, p=0·88). The week 240 outcomes included both those who were withdrawn and those continued or restarted on therapy and thus reflect the combined efficacy of the treatment strategies under study.
Inactive Chronic Hepatitis B
Among the 102 withdrawn from treatment, 5 were missing ALT or HBV DNA at week 240, and among those with available data, the percentage that met criteria for inactive CHB at week 240 were 17 of 53 (32%) in the combination versus 11 of 44 (25%) in the TDF group (p=0·50).
Safety
Of 280 adverse events (AEs) recorded amongst the 201 participants, 153 (55%) were classified as mild and 249 (89%) as unrelated to treatment (sTable 2). A total of 66% of participants had AEs in the combination group versus 52% in the TDF alone group. In the combination group, half the AEs were during the first 24 weeks while PegIFN was being given. After week 192, the percentages of participants with AEs were 12% and 28% in the combination versus TDF alone groups, respectively; with the percentage of participants reporting serious AEs comparable, 3% vs. 2% (sTable 3). The most common laboratory abnormalities noted (during treatment and after NA withdrawal) were ALT, AST and creatinine elevations in both treatment groups, and leukopenia and thrombocytopenia during PegIFN treatment in combination group.
The percentages of participants with serious AEs judged to be probably or definitely related to TDF were 4 (1%), and 4 (1%); and to PegIFN were 27 (10%) and 26 (9%), respectively. Definitely related serious or life-threatening AEs related to TDF were hepatic decompensation (n=2, both in withdrawal phase). Severe (definite and probable) AEs related to PegIFN included depression (n=2); hematologic, hepatobiliary, neurologic and ophthalmic (each n=1). The two participants with hepatic decompensation were HBeAg-positive at time of withdrawal of TDF and developed severe ALT flares with bilirubin ± prothrombin time elevation. Both recovered after reinstitution of TDF therapy.
In total, 6% (6/102) in the TDF alone group and 29% (29/99) in the combination therapy group had at least one dose reduction, interruption or discontinuation during the treatment phase. Of the treatment discontinuations, 5 were for TDF (1 for virologic breakthrough, remainder due to participant preference/site nonparticipation), 14 were for PegIFN (most commonly participant preference; 8 for AEs). Two participants discontinued both drugs (1 for AE of hearing loss).
ALT Flares:
An ALT flare was defined as an ALT level ≥300 U/L for males or ≥ 200 U/L for females. A total of 58 flares occurred during the course of the trial in a total of 52 participants. Two of the flares were observed at the screening visit while 11 more flares occurred at the randomization visit (baseline). In the comparison of flares between treatment groups or between HBeAg positives and negatives, we have excluded the (2 + 11) 13 flares that were observed prior to randomization, leaving us with 45 flares from a total of 40 participants.
During the first 192 weeks of the trial (after baseline and before the withdrawal phase), 16 flares occurred in 14 participants (7.0%). One participant had 3 flares at weeks 85, 108, and 145. If we consider only the time to first flare, median (min, max) time to first flare among these 14 participants with a post-baseline flare before week 192 was 8.4 (4.0, 85.0) weeks. Three of these participants belonged to TDF arm whereas the remaining 11 were in the combination arm (2.9% vs. 11.1%, p = 0.03) and 5 out of 98 (5.1%) were HBeAg-negative at randomization and 9 out of 103 (8.7%) HBeAg-positive (p = 0.41).
A total of 27 participants had 29 flares (2 participants with repeat flares) between week 192 and 240. The earliest flare was observed at week 200 and the latest during week 234, with median time to first flare being 203.6 weeks. All 27 participants belonged to the 111 participants who were eligible to withdraw based on initial withdrawal criteria (no flares were reported in the 77 participants who never withdrew treatment). Thus, among these 111 participants, 27 (24.3%) had at least one flare. By treatment arm, the percentages of participants experiencing flares in the withdrawal phase was 16/51 (31.4%) in the TDF arm and 11/60 (18.3%) in the combination arm (p = 0.13). The percentages of participants with flares during withdrawal phase did not differ significantly by HBeAg status at randomization [HBeAg-positive: 11/36 (30.6%) vs. HBeAg-negative: 16/75 (21.3%), p=0.34].
In the subset of participants who met the amended eligibility criteria and withdrew from treatment and did not have HBsAg loss prior to withdrawal (n = 92), 23 (25%) participants had flares following withdrawal. Most (20/23) of these participants had their first flare at or prior to week 208 visit. No statistically significant differences were observed by the original treatment assignments (13/43 or 30.2% in TDF, 10/49 or 20.4% in Combination arm, p = 0.34) or by HBeAg status at randomization (8/21 or 38.1% in HBeAg+ vs. 15/71 or 21.1% in HBeAg-, p = 0.15).
DISCUSSION
In this prospective randomized controlled trial of TDF combined with PegIFN for the initial 24 weeks versus TDF alone followed by protocolized withdrawal of TDF after 4 years, the frequency of functional cure (HBsAg loss) at week 240 was low and not significantly different between the treatment groups: 4·1% of the TDF versus 5·3% of the combination group. However, there were differences in the timing of HBsAg loss, with those treated with TDF+PegIFN achieving HBsAg loss earlier, whereas the TDF alone group exhibited more HBsAg loss after TDF withdrawal. These results support the concept that immune modulation, either with use of PegIFN or with NA withdrawal, can enhance HBsAg loss but combining this two “immune modulatory” strategies did not lead to enhanced rates of HBsAg loss. However, this study highlights a finite therapy approach with treatment discontinuation included in the treatment plan, a feature relevant to study design with new therapeutic agents.
In this study HBV subgenotype A2 was shown to play a major role in HBsAg loss. Thus, while subgenotype A2 was present in only 6% of trial participants, it accounted for 78% of those who achieved HBsAg loss. While previous studies have reported higher rates of HBsAg and HBeAg loss in patients with genotype A18–21, those studies did not evaluate responses by HBV subgenotype. Epidemiologic data regarding subgenotypes of A, indicate that A2 is most common in Northern Europeans with chronic hepatitis B, whereas A1 is found largely in African, Middle Eastern and Asian patients.22,23 In this study, HBsAg loss occurred in 4 of 6 HBeAg positive patients with genotype A2 treated with TDF alone (at weeks 71 to 217), and in all 3 such patients receiving combination therapy (at weeks 25 to 121). This association of HBsAg loss with subgenotype A2 was present only in those who were HBeAg positive. The two participants with subgenotype A2 and HBeAg who did not lose HBsAg did clear HBeAg. These results indicate that genotyping assays need to include means of discriminating between the subgenotypes A1 and A2. The association also should stimulate studies as to why genotype A2 is so sensitive to antiviral suppression and seems to be affected by concurrent use of interferon.
Inclusion of HBeAg-positive and HBeAg-negative immune active CHB participants afforded an important opportunity to compare outcomes by HBeAg status. A striking finding was that HBsAg loss was very infrequent among HBeAg-negative participants – occurring in only 1 of 102 participants. The scarcity of HBsAg loss after NA withdrawal among HBeAg-negative persons in this study is compatible with results from studies from Asia24 and Canada12 where the populations were predominantly of Asian race, in whom HBsAg loss after 1-3 years of NA withdrawal was ≤5%.25 This stands in contrast to prior studies from Europe of HBeAg-negative participants, who were predominantly White and with longer duration of TDF treatment before withdrawal, reporting HBsAg loss in ~20% of participants after 3 years or longer follow-up.10,25–27 Whether additional HBsAg loss would be observed after a longer follow up after stopping NA is unknown.10,24,27,28 The inclusion of ~50% participants initially HBeAg-positive and a higher rate of HBsAg loss in these participants is the likely reason for the somewhat counterintuitive findings that high serum HBsAg and HBVDNA levels at start of treatment were associated with higher rates of HBsAg loss. Nonetheless, the rates of HBsAg loss among participants who were initially HBeAg-positive remained low in both treatment groups.
Regarding safety, participants experienced typical side effects related to PegIFN leading to dose reductions or discontinuation in 29% of those treated with combination therapy compared to 6% of the participants treated with TDF only. For those treated with PegIFN, ALT flares were clinically mild and more frequent during the first 24 to 48 weeks of treatment. In contrast, among participants treated with TDF alone, most flares occurred after treatment withdrawal. Severe flares with jaundice occurred after TDF withdrawal, but these participants were HBeAg-positive at time of withdrawal. HBeAg positivity and lack of anti-HBe positivity may be factors influencing risk of flares in withdrawal studies.29 This led to modifications in our protocol to required study participants to be HBeAg-negative and anti-HBe positive for at least 48 weeks to be eligible for withdrawal. Among participants who met all the amended eligibility criteria – HBeAg-negative, anti-HBe positive, HBV DNA <1000 IU/mL for at least 6 months, and absence of cirrhosis – flares following NA withdrawal were seen in 25% and none were associated with decompensation. Rare but severe consequences of withdrawal of NA therapy have been reported in other studies.15
In addition to HBsAg loss, achievement of inactive chronic hepatitis B is a highly desirable endpoint of finite treatment. Prior studies suggested ~50% of HBeAg-negative patients withdrawn from NA therapy10,12,15 can achieve this endpoint. In our study, this proportion was lower, with slightly less than one-third meeting criteria for inactive disease. Whether this proportion is high enough to justify a trial of NA withdrawal is likely to vary among providers and patients – highlighting the need for NA withdrawal to be an individualized decision.
This study had limitations. First, the duration of PegIFN treatment may have been too short to result in effective immune control and subsequent HBsAg loss.6,30 However, longer duration of PegIFN will lead to more AEs and reduces provider and patient acceptability. Furthermore, as shown in cumulative incidence data (Figures 3A and 3B), differences in HBsAg and HBeAg responses began at week 24 when PegIFN was stopped and continued thereafter. Second, the duration of follow-up of 48 weeks after TDF withdrawal may have been too short to show a clear effect on HBsAg loss.15,10,27 Nonetheless, important strengths of the study included enrollment of both HBeAg-positive and -negative participants, and the diversity of HBV genotypes which demonstrated the unique and strong HBV treatment effect in HBeAg-positive participants with subgenotype A2. In addition, the prospective design and standardized criteria for TDF withdrawal and re-initiation allowed detail analysis of predictive factors of HBsAg loss. Lastly, this study included a large cohort representative of racially diverse North Americans with chronic hepatitis B, which helps with generalizability of the results.
This study highlights the importance of carefully considering key patient characteristics in the discussion regarding NA withdrawal. As in other studies, patients with cirrhosis should not be considered, particularly given the frequency of ALT flares and potential risk of decompensation. Contrary to other studies which focused on patients who were HBeAg-negative at the start of NA treatment, our study found that these patients who were predominantly Asians had negligible rates of HBsAg loss and thus seem poorly served by an NA withdrawal strategy. Finally, genotype is relevant here, with genotype A2, showing a high likelihood of success with finite therapy.
In conclusion, while the combined strategy of adding PegIFN to TDF and then withdrawing TDF after 4 years yielded low rates of HBsAg loss, an important influence of baseline HBeAg status and HBV genotypes was illuminated. Further, this study reflects the new direction of HBV therapeutics that focuses on finite courses of therapy and the endpoint of HBsAg loss and highlights how patient heterogeneity needs to be considered. Ultimately, future HBV therapies may need to be more individualized, and in this context, a better understanding of the virologic basis for the enhanced response, as defined by HBsAg loss, in patients infected with genotype A2 and poor response to current and possibly future antiviral therapies in other genotypes may help to advance therapy of this challenging disease.
Supplementary Material
STUDY HIGHLIGHTS:
What is Known:
NA therapy very infrequently leads to HBsAg loss and strategies to increase HBsAg loss (i.e. functional cure) are highly desirable.
Combination therapy of NA plus peginterferon and NA withdrawal have been reported to increase rates of HBsAg loss but studies conducted in North American populations are lacking.
What is New Here:
A strategy that combines NA with peg-interferon AND protocolized withdrawal of NAs after 4 years yielded low rates of HBsAg loss.
the timing of HBsAg loss differs by treatment strategy, with those treated with TDF+PegIFN achieving HBsAg loss earlier, whereas the TDF alone group exhibited more HBsAg loss after TDF withdrawal.
HBV subgenotype A2 is highly associated with HBsAg loss, highlighting the importance of subgenotyping data in interpreting outcomes with any HBV therapy.
Grant support:
The HBRN was funded as a Cooperative Agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the following investigators: Lewis R. Roberts, MB, ChB, PhD (U01-DK082843), Anna Suk-Fong Lok, MD (U01-DK082843), Steven H. Belle, PhD, MScHyg (U01-DK082864), Kyong-Mi Chang, MD (U01-DK082866), Michael W. Fried, MD (U01-DK082867), Adrian M. Di Bisceglie, MD (U01-DK082871), William M. Lee, MD (U01-DK082872), Harry L. A. Janssen, MD, PhD (U01-DK082874), Daryl T-Y Lau, MD, MPH (U01-DK082919), Richard K. Sterling, MD, MSc (U01-DK082923), Steven-Huy B. Han, MD (U01-DK082927), Robert C. Carithers, MD (U01-DK082943), Mandana Khalili, MD (U01-DK082944), Kathleen B. Schwarz, MD (U01-DK082916), an interagency agreement with NIDDK: Lilia M. Ganova-Raeva, PhD (A-DK-3002-001) and support from the intramural program, NIDDK, NIH: Marc G. Ghany, MD. Additional funding to support this study was provided to Kyong-Mi Chang, MD, the Immunology Center, (NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306, NIH Public Health Service Research Grant M01-RR00040), Richard K. Sterling, MD, MSc (UL1TR000058, NCATS (National Center for Advancing Translational Sciences, NIH), Norah A. Terrault, MD, MPH (CTSA Grant Number UL1TR000004), Michael W. Fried, MD (CTSA Grant Number UL1TR001111), Anna Suk-Fong Lok (CTSA Grant Number UL1RR024986, U54TR001959), and Kathleen B. Schwarz, MD (CTSA Grant Number UL1TR000423). Additional support was provided by Gilead Sciences, Inc. and Roche Molecular Systems through Cooperative Research and Development Agreements (CRADAs) with the NIDDK, and Roche/Genentech through a Clinical Trials Agreement (CTA) with the NIDDK.
The HBRN: Minnesota Alliance for Research in Chronic Hepatitis B Consortium: Mohamed A. Hassan, MD (University of Minnesota, Minneapolis, MN). University of Toronto Consortium: Joshua Juan, MD (Toronto General Hospital, Toronto, Ontario), Jordan Feld, MD, MPH (Toronto General Hospital, Toronto, Ontario), Colina Yim, NP, MN (Toronto General Hospital, Toronto, Ontario). HBV CRN North Texas Consortium: William M. Lee, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Carol S. Murakami, MD (Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center at Dallas, Dallas, TX), Son Do, MD (University of Texas Southwestern, Dallas, TX). Los Angeles Hepatitis B Consortium: Steven-Huy B. Han, MD (David Geffen School of Medicine, UCLA, Los Angeles, CA), Tram T. Tran, MD (Cedars Sinai Medical Center, Los Angeles, CA). Michigan Hawaii Consortium: Robert J. Fontana, MD (University of Michigan, Ann Arbor, MI), Naoky Tsai, MD (The Queen’s Medical Center, University of Hawaii, Honolulu, HI), Barak Younoszai, DO (The Queen’s Medical Center, University of Hawaii, Honolulu, HI). Chapel Hill, NC Consortium: Andrew Muir, M.D. (Duke University Medical Center, Durham, NC), Donna Evon, Ph.D. (University of North Carolina at Chapel Hill, Chapel Hill, NC), Jama M. Darling, MD (University of North Carolina at Chapel Hill, NC). PNW/Alaska Clinical Center Consortium: Robert C. Carithers, MD (University of Washington Medical Center, Seattle WA), Margaret Shuhart, M.D. (Harborview Medical Center, Seattle WA), Kris V. Kowdley, MD (Virginia Mason Medical Center, Seattle WA), Chia C. Wang, MD (Virginia Mason Medical Center, Seattle WA). Virginia Commonwealth University Medical Center: Velimir A. Luketic, MD (Virginia Commonwealth University Health System, Richmond, VA). Liver Diseases Branch, NIDDK: T. Jake Liang, MD (National Institutes of Health, Bethesda, MD). Liver Disease Research Branch, NIDDK: Edward Doo, MD (National Institutes of Health, Bethesda, MD), Jay H. Hoofnagle, MD (National Institutes of Health, Bethesda, MD). Immunology Center: Kyong-Mi Chang, MD, (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA), Jang-June Park, PhD (University of Pennsylvania Perelman School of Medicine, Philadelphia, PA). Data Coordinating Center: Wendy C. King, PhD (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA). Central Pathology: David Kleiner, MD, PhD. (Center for Cancer Research, National Cancer Institute, NIH, Bethesda, MD).
Conflicts of Interest:
Terrault: Institutional grant support from GSK, Roche-Genentech, Gilead Sciences, Helio Health; serves on DSMB for Moderna
Lok AS: Institutional grant support from Bristol-Myers Squibb and Gilead Sciences, and TARGET, and previously served as advisor/consultant to Gilead and Roche-Genentech. Currently serves as advisor/consultant to Arbutus, ClearB, Enanta, Enochian, GNI, GlaxoSmithKline, Janssen, Novo Nordisk (DSMB), TARGET (unpaid), and Virion.
Wahed AS: Consultant for Merck, Inc., through the Department of Biostatistics, University of Pittsburgh.
Ghany MG: Nothing to disclose
Wong DK: Nothing to disclose
Fried M: Serves as Chief Medical Officer for Target RWE and receives personal compensation and is a stockholder in the company. He reports grants paid to the University of North Carolina from Gilead, Abbvie, National Institutes of Health and Merck, outside the submitted work.
Cooper SL: Nothing to disclose
DiBisceglie A: Nothing to disclose
Lisker-Melman M: Speaker Bureau: Gilead, AbbVie
Perrillo R: Nothing to disclose
Sterling RK: Grant support from Abbott, AbbVie, Roche, and Gilead and serves on a DSMB for Pfizer and AskBio.
Khalili M: Institutional grant support from Gilead Sciences Inc and Intercept Pharmaceutical, consultant Gilead Sciences Inc.
Chung RT: Institutional grant support from Gilead, BMS, Janssen, Roche, GSK
Lau D: Research support: Gilead, Abbott Diagnostic, Janssen Pharmaceutica. Consultant: Abbott Diagnostics
Patel K: Institutional grant: Gilead Sciences, Intercept, Madrigal; Advisory Board: Gilead Sciences, Novo-Nordisk, Intercept
Roberts L: Grant support from ARIAD Pharmaceuticals, Bayer, BTG International, Exact Sciences, Gilead Sciences, Glycotest, Inc., RedHill, Inc., Target PharmaSolutions, and Wako Diagnostics; Consultant for Bayer, Exact Sciences, Gilead Sciences, GRAIL, Inc., QED Therapeutics and TAVEC.
Belle S: Nothing to Disclose
Janssen HLA: Received grants from: AbbVie, Arbutus, Bristol Myers Squibb, Gilead Sciences, Janssen, Medimmune, Merck, Roche./ Consultant for: Arbutus, Arena, Enyo, Gilead Sciences, GlaxoSmithKline, Janssen, Medimmune, Merck, Roche, Vir Biotechnology Inc., Viroclinics.
Abbreviations:
- PegIFN
peginterferon
- AE
adverse events
- ALT
alanine aminotransferase
- Anti-HBe
antibody to hepatitis B e antigen
- Anti-HBs
antibody to hepatitis B surface antigen
- CHB
chronic hepatitis B
- HAI
histology activity index
- HBRN
Hepatitis B Research Network
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
- IQR
interquartile range
- NA
nucleos(t)ide analogue
- TDF
tenofovir disoproxil fumarate
References:
- 1.World Health Organization. Hepatitis B. wwwwhoint.
- 2.Liu F, Wang XW, Chen L, Hu P, Ren H, Hu HD. Systematic review with meta-analysis: development of hepatocellular carcinoma in chronic hepatitis B patients with hepatitis B surface antigen seroclearance. Aliment Pharmacol Ther 2016;43:1253–61. [DOI] [PubMed] [Google Scholar]
- 3.Terrault NA, Lok AS, McMahon BJ, et al. Update on Prevention, Diagnosis, and Treatment and of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018;67:1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 2011;8:275–84. [DOI] [PubMed] [Google Scholar]
- 5.Bruder Costa J, Dufeu-Duchesne T, Leroy V, et al. Pegylated Interferon alpha-2a Triggers NK-Cell Functionality and Specific T-Cell Responses in Patients with Chronic HBV Infection without HBsAg Seroconversion. PloS one 2016;11:e0158297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcellin P, Ahn SH, Ma X, et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon alpha-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 2016;150:134–44 e10. [DOI] [PubMed] [Google Scholar]
- 7.Qiu K, Liu B, Li SY, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther 2018;47:1340–8. [DOI] [PubMed] [Google Scholar]
- 8.Kao JH, Jeng WJ, Ning Q, et al. APASL guidance on stopping nucleos(t)ide analogues in chronic hepatitis B patients. Hepatol Int 2021;15:833–51. [DOI] [PubMed] [Google Scholar]
- 9.Sonneveld MJ, Park JY, Kaewdech A, et al. Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE). Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 10.Berg T, Simon KG, Mauss S, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol 2017;67:918–24. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Chi H, Yu T, et al. Off-Treatment Hepatitis B Virus (HBV) DNA Levels and the Prediction of Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B: A Prospective Stop Study. J Infect Dis 2017;215:581–9. [DOI] [PubMed] [Google Scholar]
- 12.Liem KS, Fung S, Wong DK, et al. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: results from a randomised controlled trial (Toronto STOP study). Gut 2019;68:2206–13. [DOI] [PubMed] [Google Scholar]
- 13.Seto WK, Hui AJ, Wong VW, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut 2015;64:667–72. [DOI] [PubMed] [Google Scholar]
- 14.Papatheodoridis GV, Rigopoulou EI, Papatheodoridi M, et al. DARING-B: discontinuation of effective entecavir or tenofovir disoproxil fumarate long-term therapy before HBsAg loss in non-cirrhotic HBeAg-negative chronic hepatitis B. Antivir Ther 2018;23:677–85. [DOI] [PubMed] [Google Scholar]
- 15.Hirode G, Choi HS, Chen CH, et al. Off-therapy response after nucleos(t)ide analogue withdrawal in patients with chronic hepatitis B: An international, multi-center, multi-ethnic cohort (RETRACT-B study). Gastroenterology 2021. [DOI] [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 17.Ganova-Raeva L, Ramachandran S, Honisch C, Forbi JC, Zhai X, Khudyakov Y. Robust hepatitis B virus genotyping by mass spectrometry. J Clin Microbiol 2010;48:4161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123–9. [DOI] [PubMed] [Google Scholar]
- 19.Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002–9. [DOI] [PubMed] [Google Scholar]
- 20.Flink HJ, van Zonneveld M, Hansen BE, et al. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol 2006;101:297–303. [DOI] [PubMed] [Google Scholar]
- 21.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442–55. [DOI] [PubMed] [Google Scholar]
- 22.Zehender G, Svicher V, Gabanelli E, et al. Reliable timescale inference of HBV genotype A origin and phylodynamics. Infect Genet Evol 2015;32:361–9. [DOI] [PubMed] [Google Scholar]
- 23.Wolf JM, Pereira V, Simon D, Lunge VR. Temporal and geographic spreading of hepatitis B virus genotype A (HBV-A) in Brazil and the Americas. J Viral Hepat 2021;28:1130–40. [DOI] [PubMed] [Google Scholar]
- 24.Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2018;68:425–34. [DOI] [PubMed] [Google Scholar]
- 25.Hall SAL, Vogrin S, Wawryk O, et al. Discontinuation of nucleot(s)ide analogue therapy in HBeAg-negative chronic hepatitis B: a meta-analysis. Gut 2021. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Lopez M, Lens S, Pallett LJ, et al. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol 2021;74:1064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012;143:629–36 e1. [DOI] [PubMed] [Google Scholar]
- 28.Pan HY, Pan HY, Chen L, et al. Ten-year follow-up of hepatitis B relapse after cessation of lamivudine or telbivudine treatment in chronic hepatitis B patients. Clin Microbiol Infect 2015;21:1123 e1–9. [DOI] [PubMed] [Google Scholar]
- 29.Gara N, Tana MM, Kattapuram M, et al. Prospective Study of Withdrawal of Antiviral Therapy in Patients with Chronic Hepatitis B after Prolonged Virological Response. Hepatol Commun 2021;5:1888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampertico P, Vigano M, Di Costanzo GG, et al. Randomised study comparing 48 and 96 weeks peginterferon alpha-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 2013;62:290–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


