Abstract
OBJECTIVE:
Polypharmacy increases the risk of drug-drug interactions and adverse drug events. As obesity and rates of obesity comorbid chronic conditions continue to rise, an improved understanding of whether children with obesity experience higher risk of polypharmacy is needed. This study aimed to compare chronic medication polypharmacy prevalence among children with and without a diagnosis of obesity.
METHODS:
We performed a cross-sectional examination of prescription data for children aged 2–18 years prescribed ≥1 chronic medication using the 2019 Marketscan Medicaid database. Children with documented obesity were identified using medical visit diagnosis codes. Chronic medications included any ≥30-day prescription with ≥2 dispensed refills. Polypharmacy was defined as the prescription of ≥2 chronic medications for ≥1 overlapping days. Chi square tests compared polypharmacy prevalence and the distribution of chronic medication classes between children with and without obesity. Logistic regression determined the adjusted odds ratio (aOR) of polypharmacy for children with obesity, adjusting for relevant demographic and clinical differences.
RESULTS:
Of 634,671 included children, 12.2% had documented obesity. More than one-half (52.7%) of children with obesity experienced polypharmacy compared with 47.6% of children without obesity (aOR 1.06 [95% confidence interval 1.04–1.06]). Chronic medication prescriptions, particularly for psychiatric and asthma medications, were more commonly prescribed among children with obesity than those without obesity.
CONCLUSIONS:
Children with documented obesity have higher polypharmacy prevalence than children without obesity. Clinicians must be aware of this risk and minimize inappropriate polypharmacy whenever possible. Future work should examine the consequences of polypharmacy, including drug-drug interactions and adverse drug events in children with obesity.
Keywords: Polypharmacy, obesity, drug interactions, chronic conditions
Introduction
Polypharmacy, defined as the prescription of ≥2 medications for 1 or more overlapping days1, is common among the general pediatric population in the United States (US), occurring in an estimated 30–35% of children in the outpatient setting.2,3 Although sometimes necessary for treatment of acute and chronic conditions, polypharmacy increases the risk of drug-drug interactions, adverse drug events, difficulty with medication adherence, and even hospitalizations.4,5
Obesity increases the risk of developing chronic health conditions that often require multiple medications for management.6,7 Such polypharmacy places obese children at risk of medication-related adverse drug events, including drug-drug interactions.1 Obesity itself may also increase the risk of experiencing adverse drug events as a result of altered pharmacokinetic and pharmacodynamic drug responses.8 Although obesity affects 1 in 5 American children9 and increases the risk of many chronic medical problems, the frequency of polypharmacy in children with obesity in the US is unknown. While a few studies have focused on psychotropic medication polypharmacy and obesity10,11,12, little is known about other medication classes. Additionally, the results of work defining polypharmacy prevalence varied due to the use of different study methodologies and definitions of polypharmacy.2
Obesity and polypharmacy may present additive risks for adverse drug events. This risk, coupled with evidence of more frequent polypharmacy associated with certain clinical factors or diagnoses (e.g., medical complexity), necessitate an examination of how children with obesity experience polypharmacy. We aimed to determine the prevalence of chronic medication polypharmacy among Medicaid-insured children with and without documented obesity. We also sought to determine whether clinical factors aside from weight status augmented the risk of experiencing polypharmacy in children.
Methods
Study Design
We conducted a cross-sectional study using the 2019 MarketScan Multi-state Medicaid Database (IBM Watson Health, Armonk, NY). This de-identified dataset contains detailed claims data from 11 geographically diverse states, including inpatient, outpatient, and pharmacy claims, and provides patient demographic and clinical characteristics. This study was deemed non-human subjects research by the Children’s Mercy Hospital Institutional Review Board.
Study Definitions
Chronic medications were identified from dispensed pharmacy claims for all unique medication prescriptions meeting the following accepted definition: any ≥30-day prescription fill with ≥2 subsequent dispensed refills.13 Chronic medications were categorized based on therapeutic class using the 2019 American Hospital Formulary Service classification compilation.
Children with documented obesity (hereafter referred to as children with obesity) were identified based on the presence of any International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) obesity diagnosis code associated with an encounter in the 2019 database (Supplemental Table 1). Having an ICD-10-CM code for obesity has been found to be highly specific and generally has a positive predictive value of >90%.14
Polypharmacy was defined as the prescription of ≥2 chronic medications for 1 or more overlapping days.1 This is the expert recommended definition provided by a scoping review published in 2018. The short overlap period of 1 or more days is recommended for children as adverse drug events may occur with even very short overlaps of drug pairs. We identified overlapping drug pairs using drug dispensed dates coupled with the length of the prescription and number of refills reported in the Marketscan database.
Study Population
We included children 2–18 years of age who were prescribed at least 1 chronic medication and were continuously enrolled in Medicaid fee-for-service and managed care plans for ≥11 months of 2019. Continuously enrollment was required to enable examination of annual prescription fill data.
Children with no chronic medication prescriptions and those without any Medicaid utilization during the study period were excluded. Children younger than 2 years of age were excluded because the Centers for Disease Control and Prevention definition of obesity does not include children younger than 2 years. Children with complex chronic conditions (CCCs) were also excluded using a prior developed algorithm15, as they are known to experience higher rates of polypharmacy.3
Study Outcomes and Measures
The primary outcome was the prevalence of polypharmacy in children with and without obesity. To determine if obesity was independently associated with risk of polypharmacy, we also examined the following demographic and clinical factors, determined a priori: patient age, race/ethnicity, sex, type and number of common obesity comorbid conditions, number of non-complex chronic diseases (excluding obesity), and number of mental health conditions. Obesity-associated comorbid conditions included: hypertension, polycystic ovarian syndrome (PCOS), pre-diabetes, diabetes mellitus, obstructive sleep apnea (OSA), and asthma.7 Dyslipidemia and non-alcoholic fatty liver disease were excluded from the list of obesity comorbid conditions for this study because they are considered CCCs. Non-complex chronic diseases, identified using the Agency for Healthcare Research and Quality (AHRQ) Chronic Condition Indicator, are conditions expected to last 12 months or longer and result in the need for ongoing intervention with medical products/services/equipment or place limitations on self-care, independent living, and social interactions.16 Obesity was excluded from the list of non-complex chronic diseases for this study. Mental health conditions were identified through ICD-10-CM codes for mental health conditions, and included diagnoses such as depression and anxiety, as well as autism spectrum disorder, neurocognitive conditions, and intellectual disability.17
Statistical Analysis
Demographic and clinical characteristics, as well as number and classes of chronic medications and polypharmacy prevalence, were summarized using frequencies and percentages overall and by weight category. These were compared across weight categories using Chi-square tests. Logistic regression was used to measure the association between obesity and polypharmacy, accounting for the demographic and clinical factors identified above. All analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC), and p<0.05 was considered statistically significant.
Results
Study population
We included the 634,671 children who were prescribed at least 1 chronic medication in the analysis (Figure 1). Most children were adolescents (46.8% age 12–18 years), male (56.3%), and non-Hispanic White (52.5%) (Table 1). Most children had 1 (43.9%) or 2–3 (40.3%) non-complex chronic diseases. A majority (61.4%) of the cohort had at least 1 mental health condition.
Figure 1.
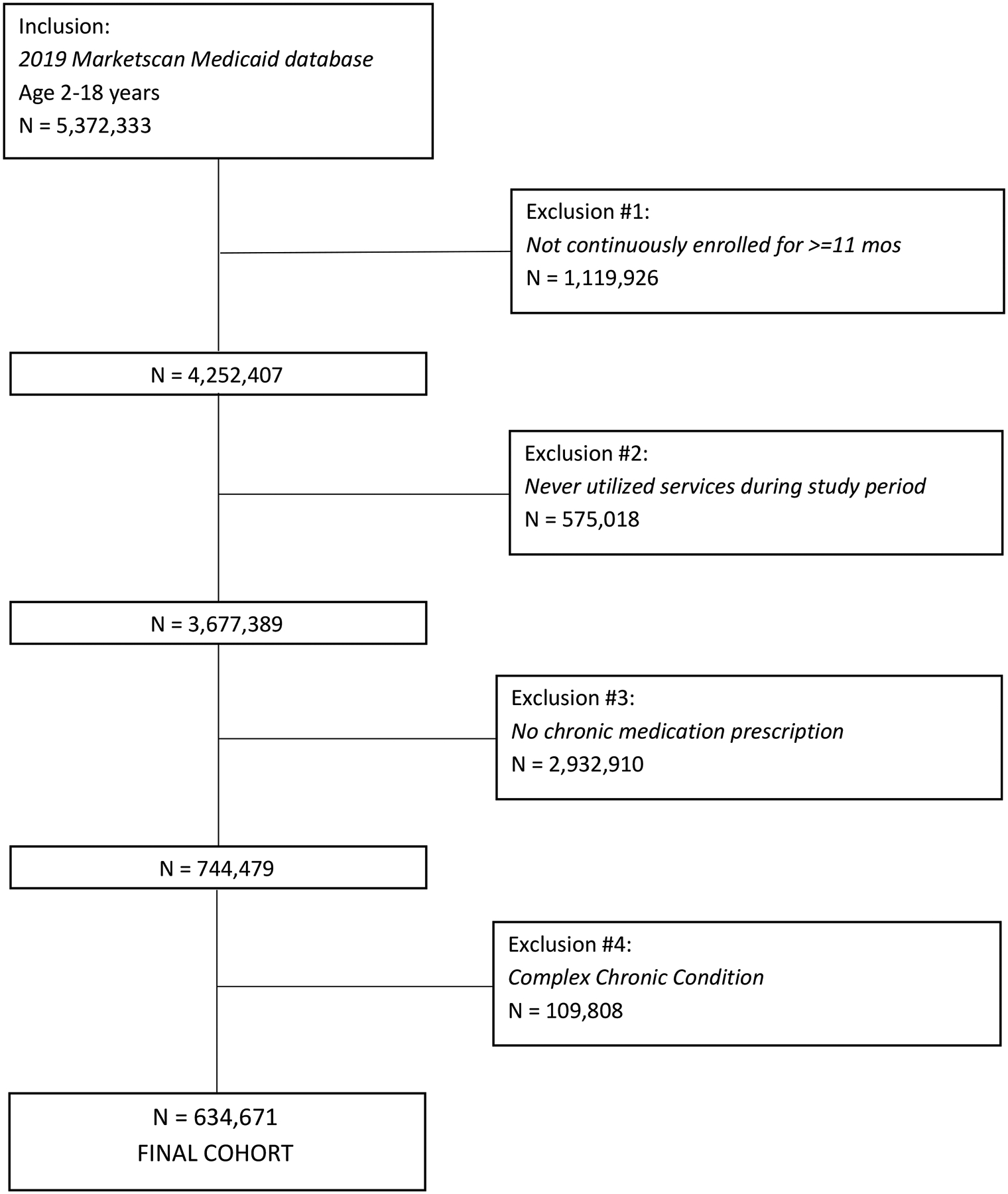
STROBE diagram
Table 1.
Demographic and Clinical Characteristics of study population†
| Overall | No Documented Obesity | Documented Obesity | ||
|---|---|---|---|---|
| N Children | 634671 | 557556 (87.8) | 77115 (12.2) | |
| Age | 2–5 years | 84655 (13.3) | 77827 (14) | 6828 (8.9) |
| 6–11 years | 252921 (39.9) | 224761 (40.3) | 28160 (36.5) | |
| 12–18 years | 297095 (46.8) | 254968 (45.7) | 42127 (54.6) | |
| Race/ethnicity | Non-Hispanic White | 333240 (52.5) | 295070 (52.9) | 38170 (49.5) |
| Non-Hispanic Black | 183861 (29) | 159139 (28.5) | 24722 (32.1) | |
| Hispanic | 44996 (7.1) | 38076 (6.8) | 6920 (9) | |
| Other | 72574 (11.4) | 65271 (11.7) | 7303 (9.5) | |
| Sex | Male | 357320 (56.3) | 317026 (56.9) | 40294 (52.3) |
| Number of non-complex chronic diseases (except obesity) | 0 | 67768 (10.7) | 62391 (11.2) | 5377 (7) |
| 1 | 278812 (43.9) | 251078 (45) | 27734 (36) | |
| 2–3 | 256078 (40.3) | 219320 (39.3) | 36758 (47.7) | |
| 4–5 | 29962 (4.7) | 23363 (4.2) | 6599 (8.6) | |
| 6+ | 2051 (0.3) | 1404 (0.3) | 647 (0.8) | |
| Number of mental health conditions | 0 | 244986 (38.6) | 214549 (38.5) | 30437 (39.5) |
| 1 | 150552 (23.7) | 133597 (24) | 16955 (22) | |
| 2–3 | 159684 (25.2) | 141140 (25.3) | 18544 (24) | |
| 4–5 | 56318 (8.9) | 49087 (8.8) | 7231 (9.4) | |
| 6+ | 23131 (3.6) | 19183 (3.4) | 3948 (5.1) | |
| Number of Obesity comorbid conditions | 0 | 386672 (60.9) | 349921 (62.8) | 36751 (47.7) |
| 1 | 222248 (35) | 189737 (34) | 32511 (42.2) | |
| 2 | 23883 (3.8) | 17177 (3.1) | 6706 (8.7) | |
| 3+ | 1868 (0.3) | 721 (0.1) | 1147 (1.5) | |
| Obesity comorbid conditions | Hypertension (Idiopathic) | 5400 (0.9) | 2918 (0.5) | 2482 (3.2) |
| PCOS | 1527 (0.2) | 643 (0.1) | 884 (1.1) | |
| Prediabetes | 3324 (0.5) | 1337 (0.2) | 1987 (2.6) | |
| Diabetes (Type II) | 2584 (0.4) | 1514 (0.3) | 1070 (1.4) | |
| OSA | 7954 (1.3) | 5208 (0.9) | 2746 (3.6) | |
| Asthma | 163797 (25.8) | 138131 (24.8) | 25666 (33.3) | |
| Depression | 91291 (14.4) | 76538 (13.7) | 14753 (19.1) | |
| Anxiety | 92923 (14.6) | 79511 (14.3) | 13412 (17.4) |
All comparisons statistically significant with p<0.001
PCOS – polycystic ovarian syndrome; OSA – obstructive sleep apnea
The prevalence of documented obesity was 12.2% (Table 1). Compared with children without obesity, those with obesity were more likely to be adolescents, non-Hispanic Black or Hispanic, and female. Approximately one-half of children with obesity (52.3%) had at least 1 obesity-associated comorbid condition. Asthma and mental health conditions were the most common chronic conditions among children with and without obesity, though the prevalence of these conditions was higher among children with obesity.
Chronic Medication Utilization
Children with obesity were more likely to have at least 1 chronic medication prescription during the study period compared to those without obesity (Table 2). Children with obesity more often received 5+ chronic prescriptions compared to those without obesity.
Table 2.
Number of Chronic Medications† by Weight Category
| Overall | No Documented Obesity | Documented Obesity | p value | ||
|---|---|---|---|---|---|
| Number chronic medications | 1 | 321827 (50.7) | 286201 (51.3) | 35626 (46.2) | <.001 |
| 2–4 | 265536 (41.8) | 232347 (41.7) | 33189 (43) | ||
| 5–9 | 45366 (7.1) | 37583 (6.7) | 7783 (10.1) | ||
| 10+ | 1942 (0.3) | 1425 (0.3) | 517 (0.7) |
Number of chronic medications does not factor in element of prescription overlap; therefore, this is not equivalent to polypharmacy
The top 10 most frequently prescribed classes of chronic medications were the same for both groups. Children with obesity received higher proportions of prescriptions for asthma/allergy medications, sympathomimetics, antidepressants, antipsychotics, ear/nose/throat anti-inflammatory medications, adrenals, and anti-convulsants (Figure 2). The most common classes of medications co-prescribed differed between children with and without obesity. Children with obesity were most likely to have co-prescriptions of allergy and asthma medications (e.g., antihistamines-leukotriene modifiers), whereas those without obesity had more co-prescriptions for stimulants and hypotensive agents (Table 3).
Figure 2.
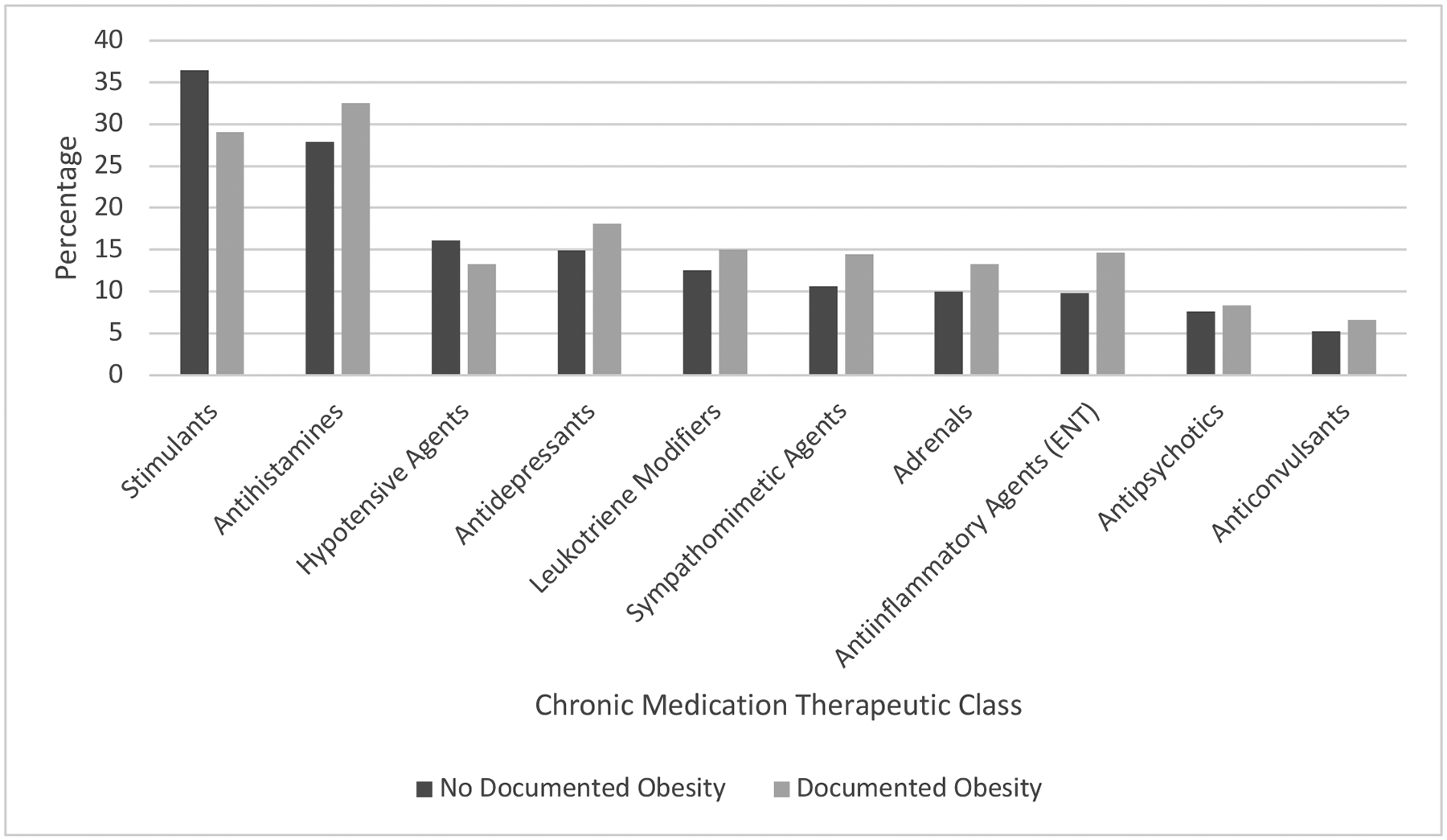
Proportions of most frequently prescribed chronic medication classes by weight category*
*All comparisons statistically significant with p<0.001
Table 3.
Most Common Co-Prescribed Chronic Medication Classes
| Medication Class 1† | Medication Class 2† | N | % |
|---|---|---|---|
| Hypotensive Agents | Stimulants | 67020 | 12.0 |
| Antihistamines | Leukotriene Modifiers | 38547 | 6.9 |
| Antidepressants | Stimulants | 38279 | 6.9 |
| Antihistamines | Anti-inflammatory Agents (ENT) | 36791 | 6.6 |
| Antihistamines | Stimulants | 33856 | 6.1 |
| Hypotensive Agents | Antidepressants | 29799 | 5.3 |
| Adrenals | Antihistamines | 28488 | 5.1 |
| Antipsychotics | Stimulants | 26431 | 4.7 |
| Adrenals | Leukotriene Modifiers | 25997 | 4.7 |
| Adrenals | Sympathomimetic Agents | 25212 | 4.5 |
| Medication Class 1† | Medication Class 2† | N | % |
|---|---|---|---|
| Antihistamines | Anti-inflammatory Agents (ENT) | 8737 | 11.3 |
| Antihistamines | Leukotriene Modifiers | 7837 | 10.2 |
| Hypotensive Agents | Stimulants | 7344 | 9.5 |
| Adrenals | Antihistamines | 6467 | 8.4 |
| Antidepressants | Stimulants | 6171 | 8.0 |
| Adrenals | Leukotriene Modifiers | 5665 | 7.3 |
| Antihistamines | Sympathomimetic Agents | 5580 | 7.2 |
| Hypotensive Agents | Antidepressants | 5495 | 7.1 |
| Adrenals | Sympathomimetic Agents | 5411 | 7.0 |
| Antidepressants | Antipsychotics | 5331 | 6.9 |
Common examples of drugs in each class: Stimulants (methylphenidate); Leukotriene modifiers (montelukast); Hypotensive agents (clonidine); Antihistamines (cetirizine); Antidepressants (sertraline); Sympathomimetic agents (albuterol, pseudoephedrine); Adrenals (budesonide, other steroids); Anti-inflammatory agents ENT (fluticasone); Antipsychotics (risperidone); Anticonvulsants (levetiracetam)
Factors Associated with Chronic Medication Polypharmacy
Polypharmacy occurred in 52.7% of children with obesity compared to 47.6% of those without obesity (p<0.001). After accounting for other demographic and clinical differences, obesity was independently associated with an increased risk of polypharmacy (adjusted odds ratio [aOR] 1.06, 95% confidence interval [CI] 1.04–1.08; Figure 3).
Figure 3.
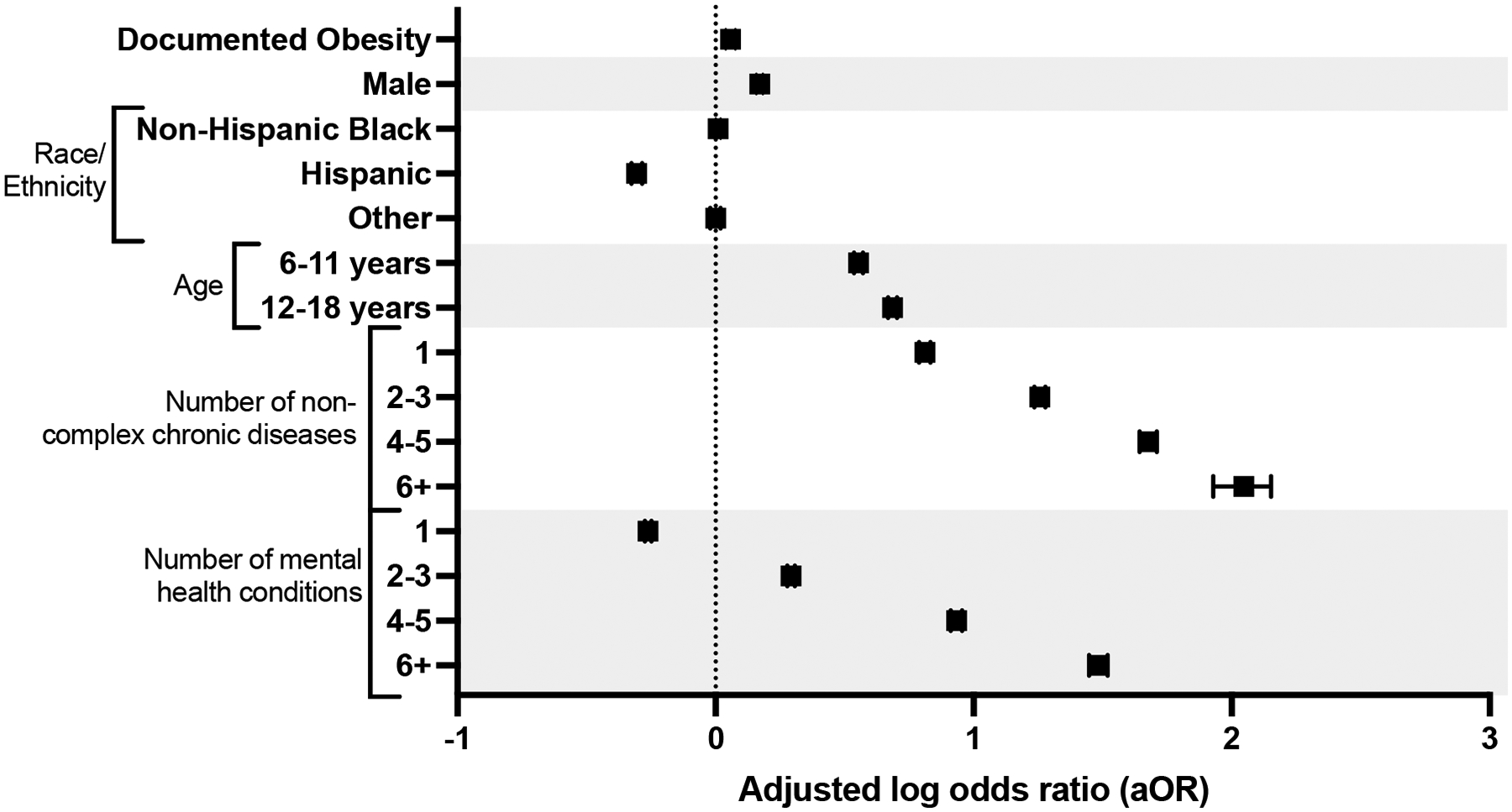
Adjusted odds of polypharmacy
*Adjusted for: age, sex, race/ethnicity, number of non-complex chronic conditions and number of mental health conditions. With 95% confidence intervals.
Other factors associated with an increased risk of polypharmacy included male sex, increasing age, and increasing medical and mental health complexity (Figure 3). As the number of non-complex chronic diseases increased, the risk of polypharmacy increased substantially. For example, children with 6+ non-complex chronic diseases had more than 7 times higher odds of experiencing polypharmacy compared to children with no non-complex chronic diseases (aOR 7.71, 95% CI 6.9–8.62). Children with increasing mental health complexity also experienced significantly higher odds of polypharmacy (6+ mental health conditions aOR 4.41, 95% CI 4.25–4.57).
Discussion
In this large, multicenter study of children 2–18 years, we found that children with obesity had increased odds of experiencing polypharmacy of chronic medications compared to children without obesity. Children with obesity were also more likely to receive chronic medication prescriptions in general and more often received medication prescriptions for asthma/allergies and mental health conditions.
Our study fills an important gap for children with obesity, with 52.7% of this group having polypharmacy, significantly more than children without obesity. Our findings align with prior work describing other clinical factors associated with risk of polypharmacy including: male sex, increasing age, and increasing medical complexity (in terms of number of chronic medical and mental health problems).3 In one prior study examining Medicaid-insured children from a single state, 35% of children experienced polypharmacy. However, the study cohort differed in important ways from ours which may partially explain difference in results, particularly the inclusion of children younger than 2 years of age and analysis of acute and chronic medication prescriptions.3 Prior work has shown that the type and depth of polypharmacy differs between early childhood and later in adolescence3,18, which our results confirm with adolescents experiencing 2x higher odds of polypharmacy compared to preschool aged children. Keast et. al. found that overweight or obese foster children in Oklahoma were more likely than foster children with a healthy weight to experience multi-class psychotropic medication polypharmacy.12 Our results add to these findings and provide a more comprehensive description of polypharmacy among a multi-state cohort of children with obesity.
Greater polypharmacy in children with obesity may be partially attributed to the presence of obesity-associated comorbid conditions, many of which may require pharmacotherapy. One-third of our cohort with obesity had asthma, and other prevalent obesity-associated comorbid conditions included mental health conditions like depression (19%) and anxiety (17%). The higher prevalence of these conditions is reflected in the classes of medications prescribed most frequently to this group. For example, antidepressants made up more than 18% of chronic medication prescriptions for children with obesity, compared to < 15% for those without obesity. Drugs used to treat obesity comorbid conditions (e.g., selective serotonin reuptake inhibitors, antihypertensives), are implicated in some of the most frequently encountered potential drug-drug interactions in outpatient populations, placing patients at risk for adverse drug events including serotonin syndrome and serious cardiac arrhythmias.19 Additionally, some drugs used to treat obesity-associated comorbid conditions, particularly antidepressants and antipsychotics, may predispose patients to developing obesity, highlighting the bidirectional nature of obesity and chronic medication prescriptions in some cases. Providers must be aware of contraindicated and high-risk drug combinations when prescribing treatment for common obesity comorbid conditions.
The increased odds of polypharmacy for children with obesity found in our study is alarming, as it may be putting this group at disproportionate risk of adverse drug events. While the observed differences in polypharmacy prevalence between children with and without obesity were relatively small, the rates of obesity, severe obesity9, and obesity-associated comorbid conditions continue to increase in the US.20,21 Therefore, clinicians must remain cognizant of risks posed by polypharmacy in children with obesity. In addition to higher risk of polypharmacy, children with obesity have physiologic differences that may result in alterations in how drugs are absorbed, distributed, metabolized, and eliminated. For example, altered organ blood flow, higher levels of systemic inflammation, and differences in liver metabolic enzyme activity, are likely responsible for known pharmacokinetic differences for a number of drugs used commonly in children with obesity.8,22,23 These pharmacokinetic differences, coupled with medical complexity of children with obesity experiencing polypharmacy, confer a potential for increased adverse drug events that requires further investigation.
Our results should be considered in the context of several limitations. We used ICD-10 codes to identify children with a diagnosis of obesity. Just over 12% of our study cohort had a diagnosis code for obesity, a smaller proportion than expected in a large group of Medicaid-insured children.9 While ICD-10 codes have a very high specificity (>99%) and positive predictive value, they have a relatively low sensitivity for capturing individuals with obesity.24,25 Thus, we likely underestimated the prevalence of obesity in our study, which would bias our results toward a null finding. Second, we did not include children with private or supplementary insurance and our findings generalize best to Medicaid-only insured children. In our analysis of chronic medications, we could not account for over-the-counter prescriptions, leading us to underestimate the proportion of polypharmacy in our cohort. It is unclear whether such misclassification would disproportionately affect children with obesity. We also did not include information regarding acute medication prescriptions, which would add to the risk of polypharmacy, and is an area in need of further study. Finally, our analysis was based on dispensed pharmacy claims, and we were unable to account for actual use of dispensed drugs. Despite these limitations, our findings advance knowledge of polypharmacy in children with obesity by providing a comprehensive analysis of polypharmacy prevalence, frequently prescribed drug classes, and factors associated with polypharmacy and obesity.
Conclusions
Children with obesity have a higher prevalence of polypharmacy compared to children without obesity. Children with obesity were more often prescribed medications for mental health conditions and asthma, both obesity-associated comorbid conditions. As obesity rates continue to climb, clinicians must be cognizant of the risks of polypharmacy for children with obesity. Future work should evaluate the relationship between polypharmacy, drug-drug interactions, and adverse drug events in children with obesity.
Supplementary Material
Supplemental Table 1. Obesity ICD-10 codes for inclusion
Acknowledgements:
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers K12 HL137943 (Dr. Antoon), the National Institute for Allergy and Infectious Diseases K24 AI148459 (Dr. Grijalva) and R01 AI125642 (Dr. Williams) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development K23 HD091295 (Dr. Feinstein) and K12 HD028827 (Dr. Tang Girdwood).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial Disclosures:
Dr. Grijalva has received consulting fees from Pfizer, Sanofi and Merck. Dr. Williams has received in-kind research support from Biomerieux.
Footnotes
Conflict of Interest Statement: The authors declared no conflict of interest.
REFERENCES
- 1.Bakaki PM, Horace A, Dawson N, et al. Defining pediatric polypharmacy: A scoping review. PloS One. 2018;13(11):e0208047. doi: 10.1371/journal.pone.0208047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker C, Feinstein JA, Ma X, et al. Variation of the prevalence of pediatric polypharmacy: A scoping review. Pharmacoepidemiol Drug Saf. 2019;28(3):275–287. doi: 10.1002/pds.4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feinstein JA, Feudtner C, Valuck RJ, Kempe A. The depth, duration, and degree of outpatient pediatric polypharmacy in Colorado fee-for-service Medicaid patients. Pharmacoepidemiol Drug Saf. 2015;24(10):1049–1057. doi: 10.1002/pds.3843 [DOI] [PubMed] [Google Scholar]
- 4.Golchin N, Johnson H, Bakaki PM, et al. Outcome measures in pediatric polypharmacy research: a scoping review. Drugs Ther Perspect Ration Drug Sel Use. 2019;35(9):447–458. doi: 10.1007/s40267-019-00650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthrie B, McCowan C, Davey P, Simpson CR, Dreischulte T, Barnett K. High risk prescribing in primary care patients particularly vulnerable to adverse drug events: cross sectional population database analysis in Scottish general practice. BMJ. 2011;342:d3514. doi: 10.1136/bmj.d3514 [DOI] [PubMed] [Google Scholar]
- 6.Leon G, de Klerk E, Ho J, et al. Prevalence of comorbid conditions pre-existing and diagnosed at a tertiary care pediatric weight management clinic. J Pediatr Endocrinol Metab JPEM. 2018;31(4):385–390. doi: 10.1515/jpem-2016-0245 [DOI] [PubMed] [Google Scholar]
- 7.Estrada E, Eneli I, Hampl S, et al. Children’s Hospital Association consensus statements for comorbidities of childhood obesity. Child Obes Print. 2014;10(4):304–317. doi: 10.1089/chi.2013.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harskamp-van Ginkel MW, Hill KD, Becker KC, et al. Drug Dosing and Pharmacokinetics in Children With Obesity: A Systematic Review. JAMA Pediatr. 2015;169(7):678–685. doi: 10.1001/jamapediatrics.2015.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. Published online February 26, 2018:e20173459. doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girand HL, Litkowiec S, Sohn M. Attention-Deficit/Hyperactivity Disorder and Psychotropic Polypharmacy Prescribing Trends. Pediatrics. 2020;146(1):e20192832. doi: 10.1542/peds.2019-2832 [DOI] [PubMed] [Google Scholar]
- 11.Horace AE, Golchin N, Knight EMP, et al. A Scoping Review of Medications Studied in Pediatric Polypharmacy Research. Paediatr Drugs. 2020;22(1):85–94. doi: 10.1007/s40272-019-00372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keast SL, Tidmore LM, Shropshire D, Nesser N, Lambert TL. Characterization of Chronic Multiclass Psychotropic Polypharmacy and Psychotherapy in Foster Care Youth in a State Medicaid Population. J Manag Care Spec Pharm. 2019;25(12):1340–1348. doi: 10.18553/jmcp.2019.25.12.1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein JA, Hall M, Antoon JW, et al. Chronic Medication Use in Children Insured by Medicaid: A Multistate Retrospective Cohort Study. Pediatrics. 2019;143(4):e20183397. doi: 10.1542/peds.2018-3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samadoulougou S, Idzerda L, Dault R, Lebel A, Cloutier AM, Vanasse A. Validated methods for identifying individuals with obesity in health care administrative databases: A systematic review. Obes Sci Pract. 2020;6(6):677–693. doi: 10.1002/osp4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chronic Condition Indicator (CCI) for ICD-10-CM (beta version). Accessed May 18, 2022. https://www.hcup-us.ahrq.gov/toolssoftware/chronic_icd10/chronic_icd10.jsp
- 17.Zima BT, Gay JC, Rodean J, et al. Classification System for International Classification of Diseases, Ninth Revision, Clinical Modification and Tenth Revision Pediatric Mental Health Disorders. JAMA Pediatr. 2020;174(6):620–622. doi: 10.1001/jamapediatrics.2020.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varimo E, Saastamoinen LK, Rättö H, Aronen ET. Polypharmacy in children and adolescents initiating antipsychotic drug in 2008–2016: a nationwide register study. Nord J Psychiatry. 2022;0(0):1–9. doi: 10.1080/08039488.2022.2042597 [DOI] [PubMed] [Google Scholar]
- 19.Holm J, Eiermann B, Kimland E, Mannheimer B. Prevalence of potential drug-drug interactions in Swedish pediatric outpatients. PLOS ONE. 2019;14(8):e0220685. doi: 10.1371/journal.pone.0220685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haegele JA, Foley JT, Healy S, Paller A. Prevalence of overweight among youth with chronic conditions in the United States: An update from the 2016 National Survey of Children’s Health. Pediatr Obes. 2020;15(4):e12595. doi: 10.1111/ijpo.12595 [DOI] [PubMed] [Google Scholar]
- 21.Avnieli Velfer Y, Phillip M, Shalitin S. Increased Prevalence of Severe Obesity and Related Comorbidities among Patients Referred to a Pediatric Obesity Clinic during the Last Decade. Horm Res Paediatr. 2019;92(3):169–178. doi: 10.1159/000504540 [DOI] [PubMed] [Google Scholar]
- 22.Kendrick JG, Carr RR, Ensom MHH. Pediatric Obesity: Pharmacokinetics and Implications for Drug Dosing. Clin Ther. 2015;37(9):1897–1923. doi: 10.1016/j.clinthera.2015.05.495 [DOI] [PubMed] [Google Scholar]
- 23.Kyler KE, Wagner J, Hosey-Cojocari C, Watt K, Shakhnovich V. Drug Dose Selection in Pediatric Obesity: Available Information for the Most Commonly Prescribed Drugs to Children. Paediatr Drugs. Published online August 20, 2019. doi: 10.1007/s40272-019-00352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo JG, Zeller MH, Wilson K, Inge T. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatr. 2009;154(3):327–331. doi: 10.1016/j.jpeds.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzow M, Homel P, Rhee K. Factors Associated With Documentation of Obesity in the Inpatient Setting. Hosp Pediatr. 2017;7(12):731–738. doi: 10.1542/hpeds.2017-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Obesity ICD-10 codes for inclusion


