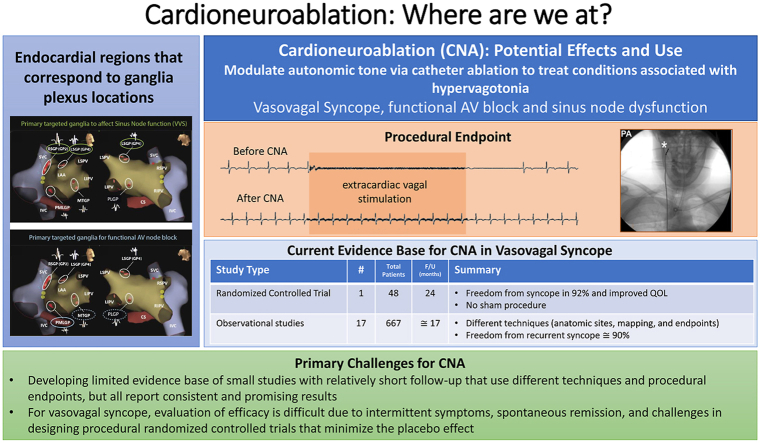
Abstract
Since its original description in 2005, catheter ablation techniques, commonly called cardioneuroablation, have emerged as a potential strategy for modulating autonomic function. Multiple investigators have provided observational data on the potential benefits of this technique in a variety of conditions associated with or exacerbated by increased vagal tone such as vasovagal syncope, functional atrioventricular block, and sinus node dysfunction. Patient selection, current techniques including the various mapping strategies, clinical experience, and limitations of cardioablation are reviewed. Finally, while cardioneuroablation has potential to be a treatment option for selected patients with symptoms mediated by hypervagotonia, the document outlines the important knowledge gaps that currently exist and the necessary next steps required before this technique can be widely implemented into clinical practice.
Keywords: Autonomic nervous system, Vasovagal syncope, Cardioneuroablation, Cardioneuralablation, Functional bradycardia
Graphical abstract
Key Findings.
-
▪
Endocardial ablation with a goal of vagal denervation (cardioneuroablation) is an emerging strategy for treating conditions associated with hypervagotonia such as vasovagal syncope.
-
▪
Multiple techniques have been proposed for identifying ablation targets and assessing the efficacy of cardioneuroablation.
-
▪
The current evidence base for the technique in patients with severe drug refractory vasovagal syncope is limited to small observational studies and one randomized controlled trial, but all have consistently reported a significant reduction in syncope after cardioneuroablation.
The impact of autonomic modulation of the heart in the setting of different pathophysiologic conditions such as ischemia, heart failure, and arrhythmias (including atrial fibrillation [AF]) has been the subject of study by multiple investigators.1, 2, 3, 4, 5, 6 The potential importance of the vagal nervous system in AF led to the concept of identifying AF nests, regions of tissue with a complex frequency spectrum that were associated with significant vagal effects.7 Although initially studied as a potential adjunct to AF ablation, investigators found that ablation at these sites led to vagal denervation of cardiac tissue and proposed that cardioneuroablation (CNA) could be a strategy for treating conditions associated with hypervagotonia.7, 8, 9 Since then, investigators have reported results from small- to moderate-sized nonrandomized patient cohorts and 1 randomized controlled trial on the use of CNA for treating conditions associated with symptomatic periods of increased vagal tone such as vasovagal syncope (VVS), functional atrioventricular (AV) block, and functional sinus node dysfunction (SND).7, 8, 9, 10, 11 This review developed collaboratively by a group of international investigators with experience in CNA provides information on the relevant anatomy, the current evidence base, and the limitations, techniques, and knowledge gaps for the procedure.
Anatomy of intrinsic cardiac autonomic nervous system relevant for CNA
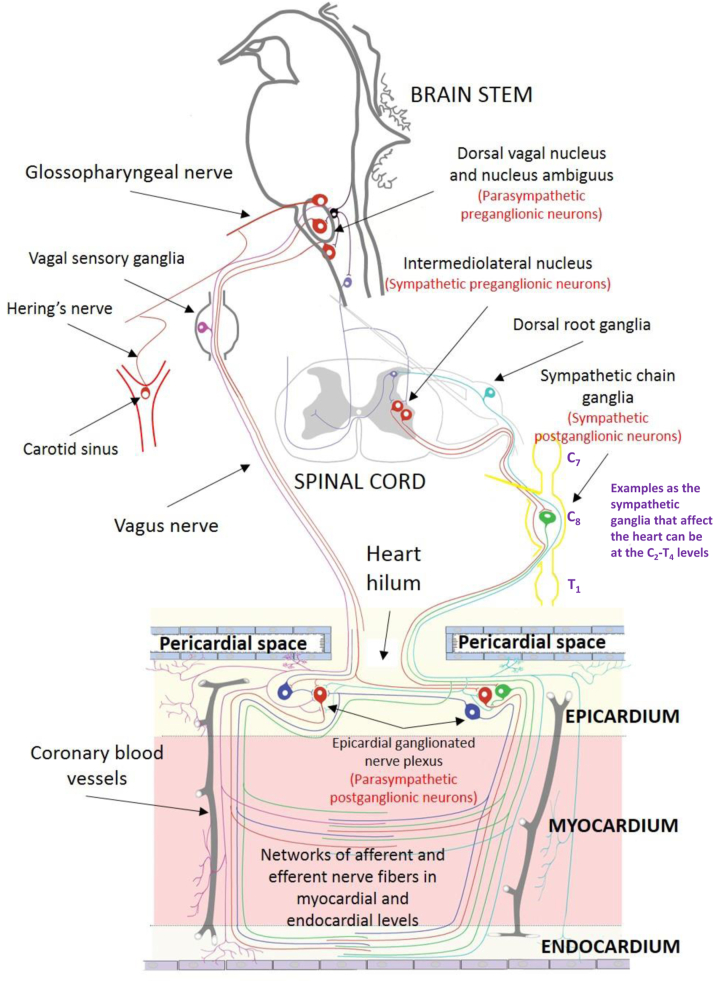
The cardiac autonomic nervous system (ANS) is complex and traditionally divided anatomically into an extrinsic component, composed of nerves that are not located in the heart and that provide connections between the central nervous system and the heart, and an intrinsic component, which consists of neurons located on the epicardial surface or embedded in the epicardial fat pad.12 Efferent fibers consist of at least 2 serially connected preganglionic and postganglionic plexi. The parasympathetic postganglionic fiber is short because its neural body is in the heart, mainly in the atrial wall and in the epicardial ganglionated plexi (GPs) (Figure 1). In contrast, the postganglionic sympathetic nerve is long because its cell body is in the paravertebral sympathetic chain (Figure 1). The cell body of the parasympathetic preganglionic nerve is nested in the medulla oblongata, nucleus ambiguous, and dorsal motor nucleus of the vagus nerve with long axons reaching the heart (Figure 1). According to the studies on the mammalian heart using histologic examination of heart sections, groups of autonomic ganglia in different sites of atria are called GPs.13
Figure 1.
A schematic representation of the cardiac autonomic nervous system in humans.
Recent studies by staining of intrinsic cardiac ANS on the whole heart demonstrated that the human heart contains more than 1500 ganglia and is under the control of epicardial autonomic ganglia and neurons that extend from these ganglia toward the sinoatrial and AV nodes and specific atrial and ventricular regions.14 Despite epicardial localization of autonomic ganglia, there is a highly dense network of sensory and efferent neurons at the myocardial and endocardial levels (Figure 1).12,15 Parasympathetic neurons run parallel to the longitudinal axis of the muscle fibers and are more numerous in the subendocardial area of the atrium than in the subepicardial area of the atrium.16
Several studies in animal models have confirmed that damage to the epicardial fat pads with different techniques can abolish the Bezold-Jarisch reflex.17,18 Histologic studies have identified morphologically necrotic and damaged postganglionic neurons and staining indicators of neuronal death (increased tyrosine hydroxylase–negative and TUNEL [terminal deoxynucleotidyl transferase dUTP nick end labeling]-positive cells) after ablation of epicardial fat pads.17,19,20
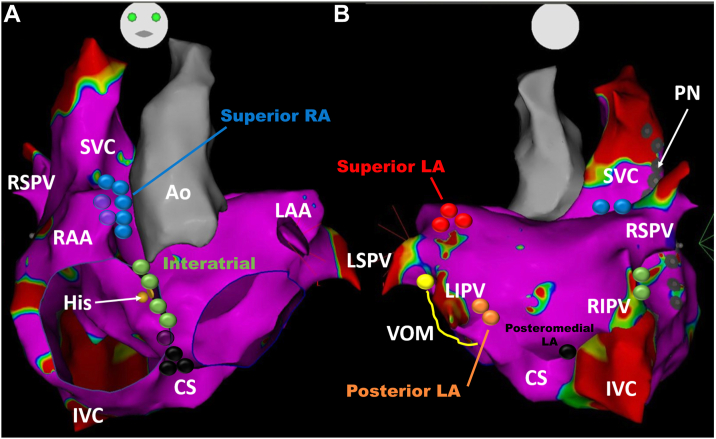
Based on Armour’s GP nomenclature,13 we propose the following classification for atrial locations that generally contain clusters with the highest numbers of autonomic ganglia: (1) the superior right atrial GP (the inferior portion of the superior vena cava-aortic ganglia), (2) the superior left atrial GP, (3) the posterior right atrial GP, (4) the posteromedial left atrial GP, (5) the interatrial septal GP, and (6) the posterolateral left atrial GP (Figure 2).8,21,22
Figure 2.
Schematic view of ganglionated plexi (GPs) distribution in relation to a 3-dimensional electroanatomic map showing anterior (A) and posterior (B) views of the left atrium (LA). The posterior right atrial (RA) ganglia are not shown. Spheres with different colors demonstrate sites of grouping epicardial ganglia defined as GPs: (1) the superior RA GPs labeled with blue color located on the posterosuperior surface of the RA adjacent to the junction of the superior vena cava (SVC) and the RA, (2) the superior LA GPs labeled with red on the posterosuperior surface of the LA between the pulmonary veins, (3) the posterior RA GPs located adjacent to the interatrial groove (not shown), (4) the posteromedial LA GPs labeled with black color on the posteromedial surface of the LA, (5) the interatrial septal GPs labeled with green color consisting of fusion and extensions of the posterior RA GPs and the posteromedial LA GPs, and (6) the posterolateral LA GPs labeled with orange color identified on the posterolateral surface of the LA. The course of the phrenic nerve (PN) is shown by the gray sphere and the vein of Marshall (VOM) in yellow. Ao = aorta; CS = coronary sinus; His = bundle of His; IVC = inferior vena cava; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RAA = right atrial appendage; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
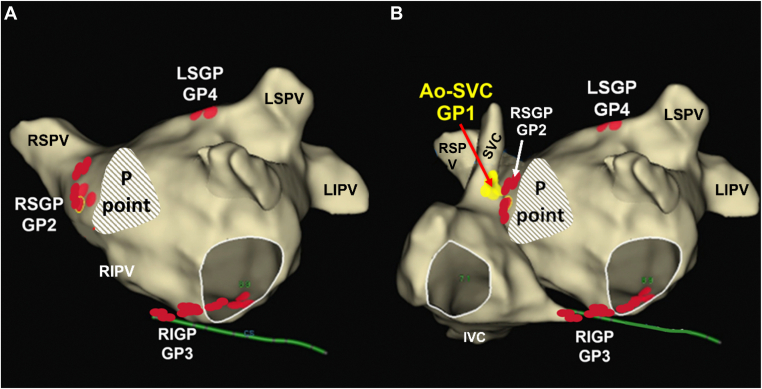
Although the parasympathetic postganglionic neurons comprise hundreds of autonomic ganglia and up to 7 GPs, 4–5 of them usually are targeted during CNA (and, as discussed subsequently, have varied among investigators). The vein of Marshall is also considered part of the intrinsic cardiac ANS, and parasympathetic fibers from the vein of Marshall innervate surrounding left atrial structures and the coronary sinus.23 The postulated distribution of GPs and innervation routes of these atrial GPs using clinical electrophysiologic mapping techniques in a patient is shown in Figure 3. It should be emphasized that although there are general locations for atrial GPs, the specific GP location can only be presumed and that the size, and number of neurons within GP vary significantly from individual to individual. Because the specific anatomic GPs defined previously cannot be precisely confirmed during an ablation, the putative endocardial locations for targeting GPs during catheter ablation are often referred to by investigators with a separate variable nomenclature. For example, RSGP or GP2 are used to refer to the left-sided, superior, and posterior portions of the interatrial atrial GP, RIGP or GP3 corresponds to the posteromedial left atrial GP, LSGP or GP4 denotes the superior left atrial GP, and the P point is identified as the more anterior portions of the interatrial septal GP.
Figure 3.
Location of the putative endocardial ablation sites for the 4 main ganglionated plexi (GPs) and P point in a 3-dimensional electroanatomical model. Electroanatomical model of the left atrium (A) and electroanatomical model of the right and left atria (B): the GP between the superior vena cava and aorta (Ao-SVC) (GP1), the right superior GP (RSGP) (GP2), the right inferior GP (RIGP) (GP3), and the left superior GP (LSGP). In some patients, there may be an extended GP4 or even an left inferior GP. In this nomenclature, right and left describe the relative position in the left atrium and numbered GPs are used because the sites on the electroanatomic map represent the putative sites of the GPs. IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Physiological experiments have demonstrated selective anatomic innervation for sinoatrial and AV nodes that could impact ablation strategies in individual patients (Figure 4). While removal of the epicardial ganglia around the coronary sinus ostium eliminated vagally mediated negative dromotropic effects without suppressing the vagal modulation of sinoatrial node function, removal of the epicardial ganglia and nerves around the right pulmonary veins blunted vagally mediated negative chronotropic effect without affecting vagal inhibition of AV conduction.24 The largest number and density of epicardial ganglia that supply the sinoatrial node are usually located at the junction of the superior vena cava with the right atrium, GP2, and GP4. The maximum density of neural tissue appears to be in the thick upper interatrial septum with some ramifications. This anatomical distribution may be why biatrial ablation is needed for complete denervation of the sinoatrial node in most cases.7,25,26 The endocardial nerves from autonomic ganglia to the AV node are extremely small, and in one anatomic study of porcine hearts, the parasympathetic nerve route of the AV node could not be specifically identified.27
Figure 4.
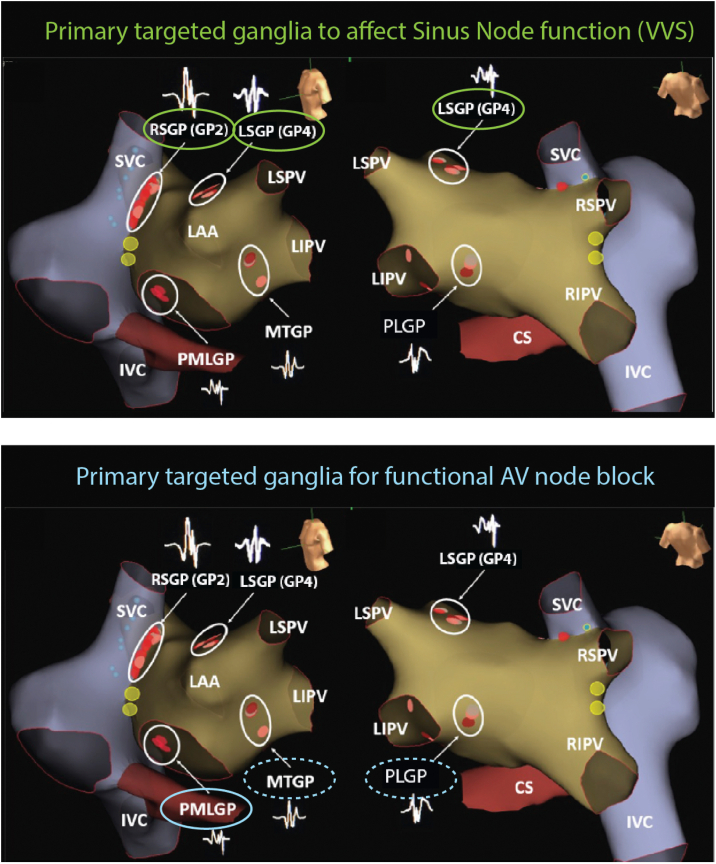
Summary of the primary ganglionated plexi (GPs) targeted based on the intended clinical effect (though additional ablation in other GP are often required). (Top) GPs predominantly innervating the sinus node: right superior GP (RSGP) (GP2) and left superior GP (LSGP) (GP4). Targeted for cardioinhibitory vagovagal syncope (VVS) and sinus node dysfunction. (Bottom) GPs predominantly innervating the atrioventricular (AV) node: the posteromedial left GP (PMLGP), the Marshall tract GP (MTGP) situated on ridge between the left atrial appendage (LAA) and left pulmonary veins, and the posterolateral GP (PLGP). These GPs are targeted for functional AV block, with the PMLGP being the predominant target (solid oval). CS = coronary sinus; IVC = inferior vena cava; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Current evidence base for clinical application
Since the original description of CNA in 2005 by Pachon and colleagues,7 this treatment strategy has been adopted gradually by interested clinicians around the globe. Therefore, the clinical evidence has been accumulated from observational data. Currently, there is only 1 randomized controlled trial and multiple observational studies but no large registries that have evaluated the use of CNA for cardioinhibitory VVS or other vagally mediated bradyarrhythmias. However, several observational studies have confirmed the reproducibility of the technique, but information on the long-term impact of the technique remains limited (Table 1).
Table 1.
Major studies for CNA in patients with vasovagal syncope
| Study | Study design | Number of patients | Age (y) | Follow-up duration (mo) | Technique | Results |
|---|---|---|---|---|---|---|
| LA (±RA) | ||||||
| Piotrowski et al, 202328 | RCT | 48 | 38 ± 10 | 24 | LA and RA EGM guided | Syncope in 8% in the CNA group, 54% in control group; improved QOL with CNA |
| Pachon et al, 201121 | Observational | 43 | 33 ± 15 | 45 ± 22 | LA, RA: Spectral and anatomic | 80% syncope free |
| Pachon et al, 202029 | Observational | 25 | 36 ± 19 | 24 | LA, RA: Spectral and anatomic | No recurrent syncope |
| Hu et al, 201911 | Observational | 115 | 43 ± 17 | 21 ± 13 | LA: Anatomic and HFS | 92% without recurrent syncope or presyncope |
| Aksu et al, 202010 | Observational | 51 | 36 ± 12 | 15 (8–29) | LA and RA: EGM and HFS | No recurrent syncope |
| Huang et al, 202030 | Observational | 49 | 42 ± 16 | 16 | RA and LA anatomic | 92% without recurrent syncope or presyncope |
| Xu et al, 202231 | Observational | 108 | 51 ± 15 | 8 | LA: anatomic and HFS | 84% syncope-free |
| Tung et al, 202232 | Observational (abstract) | 71 | 47 | 8.5 | LA/RA varied | 82% syncope-free |
| RA only | ||||||
| Debruyne et al, 202133 | Observational | 20 | 41 ± 19 | 6 | RA anatomic (with imaging) | 95% reduction in syncope burden |
| Calo et al, 202122 | Observational | 18 | 37 ± 11 | 34 ± 6 | RA anatomic and EGM | Recurrent syncope in 17% and 28% with only prodromal episodes |
| Candemir et al, 202234 | Observational | 23 | 41 ± 13 | 10 ± 2.9 | RA anatomic | 96% syncope-free |
Values are mean ± SD or median (interquartile range), unless otherwise indicated.
CNA = cardioneuroablation; EGM = electrogram; HFS = high-frequency stimulation; QOL = quality of life; RA = right atrium; RCT = randomized controlled trial.
CNA for VVS and mixed populations
Subclassification of different types of VVS is based on the response to head-up tilt test (HUT): (1) type 1 (mixed response), (2) type 2A (cardioinhibitory without asystole), (3) type 2B (cardioinhibitory with asystole >3 seconds), and (4) type 3 (pure vasodepressor response).35 The first report on CNA by Pachon and colleagues7 evaluated a mixed patient population of 21 patients (4 women, 48 ± 16 years of age) with VVS (n = 6), functional AV block (n = 7), and SND (n = 13) who underwent CNA using spectral mapping, and over 9 ± 4 months of follow-up, all had resolution of symptoms, without any procedural complications. The same group then reported a larger series of 43 VVS patients (18 women, 33 ± 15 years of age) with significant cardioinhibition on HUT. The pre-enrollment syncope burden was 5 ± 2 per patient. During 45 ± 22 months of follow-up, 3 patients had recurrent syncope, 2 had a vasodepressor, and 1 was undefined. Postablation HUT was positive in 4 cases with a mixed response, and specifically, the observed rhythms were sinus bradycardia (heart rate ≥40 beats/min) without any pauses.21,36 Considering that CNA does not directly affect vascular autonomic nerves, CNA will likely not be appropriate for VVS patients with type 3 HUT response. Moreover, cardiac deceleration capacity, which is derived from a novel analysis for heart rate variability, may serve as a potential tool to monitor cardiac vagal activity and to identify VVS patients.26 Recently, Tu and colleagues37 found that baseline nighttime deceleration capacity≥10 ms could identify patients with VVS in whom CNA might be beneficial.
Yao and colleagues38 reported similarly high success rates in 10 patients (7 women, 50 ± 6 years of age) with symptomatic VVS over a mean follow-up of 30 ± 16 months. In their study, high-frequency stimulation (HFS) was used to identify GP sites. The same group extended their study cohort by additional 47 patients (28 women, 42 ± 14 years of age) in whom CNA was performed anatomically at empirical sites of GPs.26 No statistical differences were found between HFS and anatomically guided ablation, either in freedom from syncope (100% vs 89.4%, P = .348) or recurrent prodromes (5%0 vs 77%, P = .167). A subsequent study evaluated the efficacy and safety of left atrial CNA in 115 patients (69 women, 43 ± 18 years of age) with VVS and positive HUT (75% of patients with mixed response). Over a follow-up of 21 ± 13 months, 92% of patients were free of syncope. Interestingly, patients with type 3 HUT response (14%) also showed benefit from can.11
Aksu and colleagues39 reported 22 patients presenting with symptomatic functional bradyarrhythmias (VVS, AVN, or SND) who underwent CNA targeting 3 main GPs using right and left atrial access.39 The ablation sites were identified by electrogram mapping, verified by HFS, and ablated until atrial electrical potentials were eliminated. The patients with VVS and SND were free from the new syncopal episodes at a mean 12.3 ± 3.4 months and 9.5 ± 3.1 months follow-up, respectively. Six of the 7 patients with functional AV block had improvement in AV conduction as assessed by follow-up ambulatory electrocardiography monitoring.
The efficacy of CNA through a right-sided approach (preferentially ablating the right GP) was studied by Debruyne and colleagues33 in 20 patients with syncope. Patients were assigned to a group with positive HUT (n = 12) or to a group with documented pause ≥3 seconds (n = 8). After limited anatomically guided ablation supported by the computed tomography scan merged with electroanatomical map, syncope burden was reduced by 95% at 6-month follow-up (P < .001). A purely empirical right atrial anatomical approach was studied by Calo and colleagues22 in a cohort of 18 young patients (mean age 36.9 ± 11.2 years). At a mean follow-up of 34.1 ± 6.1 months, 16.6% subjects experienced syncope and 27.7% experienced only prodromal episodes. A similar strategy was employed by Mesquita and colleagues,40 who enrolled 13 patients with functional bradyarrhythmias (median age of 51 years) and performed catheter ablation of the right GP using the support of 3-dimensional electroanatomical mapping. No patients had a recurrence of symptoms or significant bradyarrhythmia during a median follow-up of 8.4 months.
The first observational study comparing CNA vs medical therapy for cardioinhibitory VVS enrolled 101 patients, with 51 undergoing CNA and 50 receiving conservative therapy.10 All patients had a Vasovagal Syncope International Study type 2B response or type 1 response with >3 seconds asystole. Recurrence rates were compared between 19 propensity-matched pairs during a median follow-up of 22 months. Syncope recurrence was noted in 8 of 19 conservative therapy patients compared with 2 of 19 in the CNA group. CNA was associated with a hazard ratio of 0.23 (95% confidence interval 0.03–0.99, P = .049) for syncope recurrence. The 4-year syncope-free survival was 86% in the CNA group compared with 50% in the conservative therapy group.
A meta-analysis of the observational data found a 92% freedom of syncope after CNA with no differences identified among different CNA techniques but more syncope associated with a right atrial–only approach when compared with left atrial or biatrial approaches.41 Regardless of the technical or anatomic strategy, the studies included in the meta-analysis were small (average 34 patients) and follow-up in the studies was relatively short (11 of 14 studies with follow-up ≤30 months and longest follow-up 45 months). Since publication of the meta-analysis, observational studies from additional investigators and the first randomized controlled trial for CNA have been published.28, 31, 32, 34, 42 The observational studies reported similar efficacy to prior reports and have also reported improvement in quality of life. In a randomized controlled study, 48 patients with treatment refractory VVS and several criteria including a cardioinhibitory response to tilt table testing were randomized to receive CNA or not.28 After 2-year follow-up, CNA was associated with a significant decrease in syncope (CNA group: 8% vs control group: 54%) and improved quality of life. The major limitation of the study is that it was unblinded without a sham procedure control arm, and a significant placebo effect cannot be ruled out.
CNA for AV block
Several investigators have reported on the use of CNA for treating functional AV block.7,8,43,44 In the largest case series to date that evaluated 241 patients with symptomatic AV block, Aksu and colleagues44 identified 31 (13%) patients with functional AV block that appeared to be vagally mediated. Functional AV block was identified by a series of tests, including improvement in AV conduction in response to atropine or exercise and absence of intra/infra-His conduction abnormalities at electrophysiology testing. All 31 patients had prior syncope and 17 (55%) had persistent AV block. Twenty-eight (90%) patients received biatrial CNA, whereas the remaining 3 received right-sided CNA. Acute resolution of AV block and abolition of atropine response was achieved in 30 (97%) patients. Over 19 ± 15 months of follow-up, 2 patients experienced recurrent AV block and underwent pacemaker implantation. The authors identified the posteromedial left GP as the critical area for parasympathetic innervation of the AV node.
CNA in SND
The role of CNA in patients with pure SND was explored in several studies.2,16,37,39,45,46 Zhao and colleagues45 evaluated the efficacy and safety of CNA in a selected group of 11 patients (average age 46 ± 11 years) experiencing symptomatic sinus bradycardia for ≥ 5 years. Patients with sinus pauses >2 seconds, lack of atropine response, and corrected sinus node recovery time >525 ms were excluded from the study cohort. Patients had significant improvement in sinus bradycardia–related symptom score at 12 months after the CNA.45 In a larger study by Qin and colleagues46 from the same medical center, the age-dependent effects of CNA were investigated in 62 patients with similar clinical characteristics and exclusion criteria. At 12 months, symptoms improved overall but this was significant only in patients <50 years of age. Similarly, overall quality of life assessed by the 36-item Short Form Survey improved in 7 of the 8 domains; however, in 3 of the 8 domains, only in those ≥50 years of age compared with improvement in all domains in younger patients.
Limitations of current evidence
It is important to understand the significant limitations of the current studies. Available studies are observational, and the majority lack an adequate control population. There are significant differences in patient selection criteria, overlap between indications, and technique. To date, only one randomized study has evaluated the efficacy and safety of CNA in patients with VVS. However, there are several ongoing randomized studies studying CNA designed to minimize the placebo effect in patients with VVS (NCT04755101) or are evaluating the effect of CNA in different patient populations (SND: NCT04149886). A recent survey of 118 international clinicians has confirmed widespread support for randomized controlled trials to identify patients who will potentially benefit from CNA.47 It is also critical to acknowledge that many patients with severe VVS are young and understanding the potential long-term consequences along with the potential placebo effect of any therapy, will be vital before widespread application of CNA.48, 47, 49, 50 Perhaps most important, there is a high spontaneous remission rates in patients with VVS, with 50% to 70% of patients experiencing no additional episodes.51 Finally, although the data are mixed, alternative strategies such as exercise, physical countermeasures, and pharmacologic therapy may improve symptoms associated with severe VVS without the potential long-term effects of ablation.52, 51, 52, 53, 54, 55
Table 2 summarizes the patient characteristics of patients who may benefit from CAN with the current evidence base.
Table 2.
Possible patient selection considerations for CNA in different conditions
| Condition | Patient considerations |
|---|---|
| Vasovagal syncope |
|
| Functional AV block |
|
| Functional sinus bradycardia |
|
AV = atrioventricular; CNA = cardioneuroablation.
Technique
Typically, patients are prepared for can using a similar approach to radiofrequency catheter ablation procedures for AF (Table 3). Table 4 provides a description of different techniques that have been used by different investigators to identify endocardial regions that correspond with potential GP locations.
Table 3.
Practical advice for performing CNA
| General |
|
|
|
|
|
|
|
| Special considerations for AV node denervation |
|
|
AF = atrial fibrillation; AV = atrioventricular; CNA = cardioneuroablation; GP = ganglionated plexus; PMLGP = posteromedial left ganglionated plexus.
See Table 4 for more detailed information.
Table 4.
Methods for GP identification, technique-specific endpoints, and technique-independent endpoints that can be used with any GP mapping strategies
| GP identification | Mapping technique | Technique specific endpoints | Technique independent endpoints |
|---|---|---|---|
| Anatomic | Construct a 3-dimensional model of the atria (with or without additional imaging modalities) and perform ablation at expected GP locations | Electrogram elimination or attenuation |
|
| High-frequency stimulation | Ablation at endocardial sites associated with a vagal response (transient AV block, asystole, or an increase in the R-R interval) with very rapid stimulation (20 Hz, 10–20 V, pulse duration 5 ms and <5-s total duration) | Elimination of the vagal response with ablation or post | |
| Electrogram morphology | Spectral analysis: Ablation at sites characterized by multiple harmonics with frequencies >80 Hz | Electrogram elimination or attenuation | |
| Fractionation: Identifying sites with ≥4 deflections, particularly with higher amplitudes (>0.7 mV) |
AV = atrioventricular; GP = ganglionated plexus.
Innervation mapping
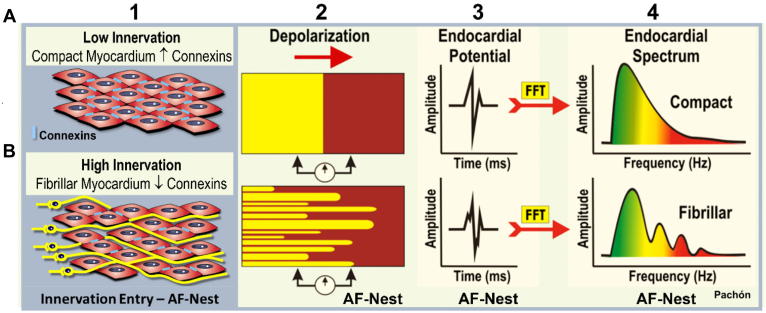
Several methods have been described for identifying endocardial sites associated with innervation (Table 4).7,56 In the original description of CNA in 2005, Pachon and colleagues7 identified 2 types of atrial tissue based on the frequency spectrum identified by fast Fourier transform of bipolar endocardial electrograms. Most atrial tissue (termed compact by the investigators) had a homogeneous spectrum centered around a single frequency (approximately 40 Hz), while atrial tissue located near the ganglia were characterized by multiple frequencies higher than 80 Hz and were termed fibrillar and AF nests (Figure 5), and served as a potential marker for the location of parasympathetic ganglia.29,57 Specialized software has been developed to automatically identify these using a fractionation threshold set by the operator and to tag these sites during the mapping process (Figure 6).57
Figure 5.
Basis of the innervation mapping. Electrical properties of the myocardium depending on the cell connection. A: Compact myocardium. B: Fibrillar myocardium. 1: myocardium histology draw; 2: conduction scheme; 3: time domain endocardial potential; 4: frequency domain endocardial potential (spectral analysis). The cells of the compact myocardium are very well connected with high connexin density, represented by small blue bars (1A). That provides an isotropic (homogeneous) conduction (2A) and a smooth potential (3A) and a smooth spectrum (4A). On the contrary, the entry of the nervous fibers into the myocardium and the presence of numerous micro-neurons (2B) change the cells connections, causing anisotropic conduction (2B) even without fibrosis (type 1 atrial fibrillation [AF] Nest). The endocardial potential may show fractionation (3B) and the spectrum typically segmented, with several frequency branches (4B), the conduction is heterogeneous as in a bunch of cells (type 1 AF nests).
Figure 6.
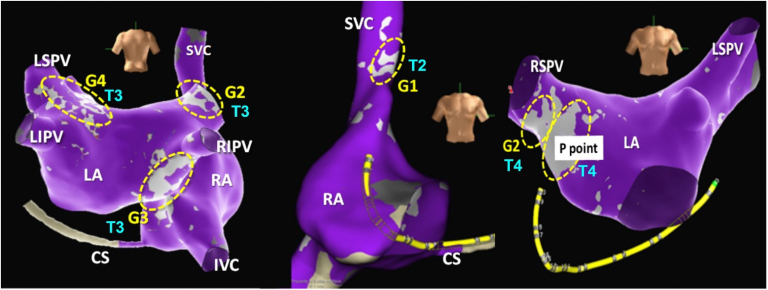
Fractionation mapping. Electroanatomical model showing fractionation map (light gray) and atrial walls (purple). Yellow dotted ellipses show ganglia GP1 to GP4. T2 to T4 indicate the fractionation map sensitivity and specificity threshold: the higher the number is, the more specific and less sensitive it is. This parameter must be adjusted by the operator to get the best view of the ganglionated plexus areas. Usually, the P point needs the highest threshold and GP1 the lowest. The software developed by Pachon and St Jude Medical (now Abbott Laboratories). IVC = inferior vena cava; LA = left atrium; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RA = right atrium; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Building on the original finding that AF nests are characterized by high frequencies, another approach, if specialized software is not available, is to set the high-pass and low-pass filters to 200 Hz and 500 Hz, respectively, and defining ganglia sites as those with fractionated multicomponent signal with ≥4 deflections.56 However, using this approach, low-amplitude fractionated electrograms (amplitudes <0.7 mV) may coincide with atrial scar, rather than with an area associated with high levels of innervation.56
HFS approach
The HFS was initially designed to identify GP location during circumferential pulmonary vein isolation for AF. In this technique, HFS with frequency of 20 Hz, voltage of 10 to 20 V, and pulse duration of 5 ms is delivered to each GP site. During HFS, the existence of a positive vagal response, defined as transient ventricular asystole, AV block, or R-R interval increased by 50%, demonstrates vagal innervation sites.11,58, 59, 60 The main limitation of this method is the inadvertent induction of AF.
Anatomic approach
The anatomical approach is based on the assumption of the anatomical location of the main GPs.7,26,60 There are up to 7 major GPs located in protuberances or grooves of the heart wall, such as interatrial tissues, connective folding between the atrium and pulmonary veins, tissues adjacent to coronary arteries, and epicardial AV tissues. Because atrial wall thickness ranges from 3 to 5 mm, the epicardial GPs can be ablated from the endocardium. In many but not all cases, anatomic ablation of the presumptive regions of 3 or 4 main GPs is enough to obtain a good result (Figure 3).7,26,46,60
Assessing denervation
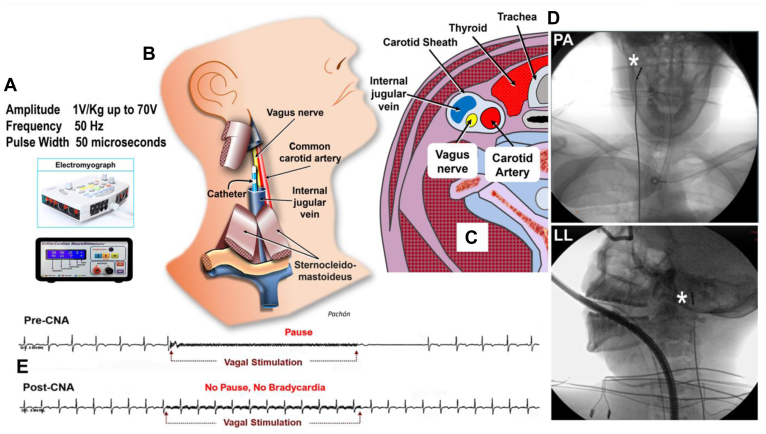
Regardless of ablation technique, denervation assessment is fundamental for effective CNA and as an endpoint for the procedure. Modification of electrophysiological parameters and atropine challenge at ablation completion are indirect and may not be totally reliable. While elimination of the vagal response with HFS at endocardial sites that preablation were associated with a vagal response has been used as a sign of successful CNA, extracardiac vagal stimulation (ECVS) is a technique for CNA that reproduces the cardioinhibition associated with a head-up-tilt-test and may be repeated at different times during the CNA procedure until the cardioinhibition response is abolished.11,54,58, 59, 60 ECVS is performed by advancing a catheter in the right internal jugular vein close to the jugular foramen (Figure 7). Vagus nerve capture is obtained without direct contact with the nerve by rapid stimulation (50 Hz) with pulse width of 50 ms and an amplitude of 1 V/kg (up to a maximum of 70 V).29,57 ECVS can be done repetitively during the procedure to assess progress of denervation. ECVS is usually performed in the right internal jugular vein, but stimulation in the left internal jugular vein can also be additionally performed, and the optimal location for ECVS may be facilitated by the use of ultrasound, and can assess both sinus node behavior and AV conduction.61,62 In some cases, ECVS can be used to assess the specific effect of CNA. For example, in a patient undergoing CNA for functional AV block, ECVS may be used to confirm absence of AV block without impacting sinus node function.62 Response to ECVS may be a strategy to facilitate reproducibility of CNA and consistency among different centers. While ECVS has been shown to be an important endpoint by some investigators, information on the best endpoint for CNA procedures still remains incomplete.
Figure 7.
A: Optimized vagal stimulation parameters. B: Drawing showing the lead inside the internal jugular vein and its closeness to the vagus nerve. The closest site is usually located near the jugular foramen at the base of the cranium. C: Cervical cross-section scheme showing the carotid sheath and the relationship between the vagus nerve and the internal jugular vein. D: Anteroposterior (top) and lateral (bottom) radiographs showing the position of the lead just below the right jugular foramen to proceed with the stimulation of the right vagus nerve. E: Electrocardiography of extracardiac vagal stimulation before cardioneuroablation (CNA) showing long sinus arrest (top), and again, extracardiac vagal stimulation after CNA showing no more vagal response (bottom) (see Video). Asterisk indicates catheter tip. LL = left lateral; PA = lateral.
Regardless of the strategy used, CNA is a procedure that may have a significant associated placebo effect similar to the initial studies evaluating the impact of permanent pacing in VVS.45,46 Thus, denervation should be confirmed during and at the end of the procedure and the results will guide whether continued ablation is required.
Preventing reinnervation
Reinnervation is a process that may limit the benefit of this therapy. In a canine model, Oh and colleagues63 delivered extensive radiofrequency energy with an epicardial approach to the fat pads that acutely eliminated the vagal effects on the sinus node, atrial tissue, and AV node, only to note that these denervation effects disappeared completely at retesting at 4 weeks. However, partial recovery of a previously severely symptomatic autonomic response may be acceptable if important cardioinhibition is prevented. Due to the widespread distribution of nerve endings, there is a significant overlap among GPs. Therefore, the denervation of one GP may be counteracted and compensated by the neighbors and by the numerous surrounding micro-GPs. Specific procedural endpoints may be important, as one group has reported that abolishing vagal effects by ECVS assessment provided a long-term clinical benefit even in the setting of reinnervation.29
Anesthesia considerations
Almost all agents used for general anesthesia will impact the ANS but generally not to the point that it will impact identifying ablation targets or assessing procedural endpoints. However, it is recommended that the level of anesthesia is controlled with bispectral values between 40 and 50. Values <40 represent an undesirable deep hypnotic state that may potentially significantly interfere with autonomic nervous tone. Conscious sedation (intravenous administration of midazolam and sufentanil) is also an acceptable option, for it would not directly affect the vagal response during CNA.
Knowledge Gaps
At this time there are different techniques employed for CNA including location (right atrial, left atrial, or biatrial), ablation strategies (anatomic vs GP identification), and procedural endpoints. As CNA continues to develop, heterogeneity will almost necessarily exist as investigators explore distinct aspects of the procedure and which patients benefit the most. However, as the evidence base becomes more robust, development of relatively standardized approaches and agreement on acute and clinically relevant long-term endpoints will be critical, particularly in the design and interpretation of results of randomized controlled trials.
The design of randomized controlled trials is particularly relevant in VVS given the intermittent nature of symptoms, placebo effect for therapies, and the complex pathophysiology with interindividual variability. These issues are compounded by the younger age of many patients with VVS and the relatively short follow-up (≤2 years) in published studies to date. For all of these reasons, the likely required inclusion criteria for any randomized controlled trial evaluating CNA for VVS will include patients with frequent debilitating symptoms due to cardioinhibition, a positive response to atropine, and prior failure of conservative approaches and medical therapy.
Interest in CNA originally developed as a potential therapy for the treatment of AF. Continued research from multiple groups over the past several decades has emphasized the potential impact of the ANS in AF, and it may be that CNA will develop as an adjunctive therapy for selected patients with AF.64, 65, 66
Conclusion
Since its original description in 2005, CNA has emerged as a potential therapy for diseases and symptoms associated with hypervagotonia. Multiple investigators have described beneficial effects associated with CNA in patients with severely symptomatic VVS and developing evidence suggests a benefit in those patients with functional bradycardia. However, almost all of the evidence is observational with short follow-up and must be balanced by past experience with permanent pacing and the high rate of spontaneous remission in patients with VVS. Given the limitations in the evidence, at this time, adoption of this technique requires careful programmatic development including specific protocols for identifying appropriate patients, technique, and comprehensive follow-up. Ideally, all patients who undergo CNA and are not included in a clinical trial should be included in registries that outline the technical aspects of the procedure including endpoints, identify procedural complications and immediate physiologic impact, and include long-term monitoring for complications and outcomes. Within this structure for acquiring real world data, this information along with results from future randomized controlled trials will delineate the appropriate use and best methods for CNA, and the broader medical community will then better understand how to assimilate this technique more generally into clinical care.
Acknowledgments
The authors thank T.J. Lobo, C.P.M. Zelia, C.T.C. Pachon, C. Higuti, T.G. Santillana, J.C.A. Zerpa, F. Ortencio, R. Amarante, R.F. Silva, and T. Osorio for their work in producing the video accompanying this article.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Jose Carlos Pachon, Enrique Indalecio Pachon, Tolga Aksu, and Fred Kusimoto have no conflicts of interest to disclose. Rakesh Gopinathannair has served as a consultant for or received honoraria from Abbott Medical, Boston Scientific, Biosense Webster, and Sanofi; and served on the advisory board for PaceMate (no compensation). Josef Kautzner has served as a speaker for, served on the advisory board for, and received consultant fees from Abbott, Bayer, Biosense Webster, Biotronik, Boehringer Ingelheim, CubeVision, Medtronic, Mylan, Pfizer, and ProMed CS. Yan Yao has served as a speaker and received consultant fees from Abbott, Biosense Webster, Boston Scientific, and Medtronic.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Appendix. Supplementary Data
References
- 1.Goldberger J.J., Arora R., Buckley U., Shivkumar K. Autonomic nervous system dysfunction: JACC Focus Seminar. J Am Coll Cardiol. 2019;73:1189–1206. doi: 10.1016/j.jacc.2018.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerknecht E.H. The history of the discovery of the vegatative (autonomic) nervous system. Med Hist. 1974;18:1–8. doi: 10.1017/s0025727300019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 4.Loomis T.A., Krop S. Auricular fibrillation induced and maintained in animals by acetylcholine or vagal stimulation. Circ Res. 1955;3:390–396. doi: 10.1161/01.res.3.4.390. [DOI] [PubMed] [Google Scholar]
- 5.Scherf D., Romano F.J., Terranova R. Experimental studies on auricular flutter and auricular fibrillation. Am Heart J. 1948;36:241–251. doi: 10.1016/0002-8703(48)90403-7. [DOI] [PubMed] [Google Scholar]
- 6.Cannata D., Narbonne N.B. Clinical observations on the role of the vegetative nervous system in the pathogenesis of atrial fibrillation. Cardiologia (Basel) 1958;32:329–345. doi: 10.1159/000165836. [DOI] [PubMed] [Google Scholar]
- 7.Pachon J.C., Pachon E.I., Pachon J.C., et al. "Cardioneuroablation"--new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Pachon M.J.C., Pachon M.E.I., Lobo T.J., et al. Syncopal high-degree AV block treated with catheter RF ablation without pacemaker implantation. Pacing Clin Electrophysiol. 2006;29:318–322. doi: 10.1111/j.1540-8159.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 9.Pachon -M.J.C. Cardioneuroablation for neurocardiogenic syncope. Heart Rhythm. 2019;16:1552–1553. doi: 10.1016/j.hrthm.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Aksu T., Guler T.E., Bozyel S., Yalin K., Gopinathannair R. Usefulness of post-procedural heart rate response to predict syncope recurrence or positive head up tilt table testing after cardioneuroablation. Europace. 2020;22:1320–1327. doi: 10.1093/europace/euaa230. [DOI] [PubMed] [Google Scholar]
- 11.Hu F., Zheng L., Liang E., et al. Right anterior ganglionated plexus: the primary target of cardioneuroablation? Heart Rhythm. 2019;16:1545–1551. doi: 10.1016/j.hrthm.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Aksu T., Gopinathannair R., Gupta D., Pauza D.H. Intrinsic cardiac autonomic nervous system: What do clinical electrophysiologists need to know about the "heart brain"? J Cardiovasc Electrophysiol. 2021;32:1737–1747. doi: 10.1111/jce.15058. [DOI] [PubMed] [Google Scholar]
- 13.Armour J.A., Murphy D.A., Yuan B.X., Macdonald S., Hopkins D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Pauza D.H., Skripka V., Pauziene N., Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Aksu T., Gupta D., Pauza D.H. Anatomy and physiology of intrinsic cardiac autonomic nervous system: Da Vinci anatomy card #2. J Am Coll Cardiol Case Rep. 2021;3:625–629. doi: 10.1016/j.jaccas.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano H., Okada R., Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y., Zhao W., Yang Z.-J., et al. Catheter ablation of cardiac fat pads attenuates Bezold-Jarisch reflex in dogs. J Cardiovasc Electrophysiol. 2011;22:573–578. doi: 10.1111/j.1540-8167.2010.01922.x. [DOI] [PubMed] [Google Scholar]
- 18.Chiou C.W., Zipes D.P. Selective vagal denervation of the atria eliminates heart rate variability and baroreflex sensitivity while preserving ventricular innervation. Circulation. 1998 Jul 28;98:360–368. doi: 10.1161/01.cir.98.4.360. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y., Jiang Z., Tsai W.C., et al. Ganglionated plexi and ligament of Marshall ablation reduces atrial vulnerability and causes stellate ganglion remodeling in ambulatory dogs. Heart Rhythm. 2016;13:2083–2090. doi: 10.1016/j.hrthm.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padmanabhan D., Naksuk N., Killu A.K., et al. Electroporation of epicardial autonomic ganglia: safety and efficacy in medium-term canine models. J Cardiovasc Electrophysiol. 2019;30:607–615. doi: 10.1111/jce.13860. [DOI] [PubMed] [Google Scholar]
- 21.Pachon M.J.C., Pachon M.E.I., Cunha Pachon M.Z., Lobo T.J., Pachon M.J.C., Santillana P.T.G. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace. 2011;13:1231–1242. doi: 10.1093/europace/eur163. [DOI] [PubMed] [Google Scholar]
- 22.Calo L., Rebecchi M., Sette A., et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: a long-term follow-up prospective study. J Interv Card Electrophysiol. 2021;61:499–510. doi: 10.1007/s10840-020-00840-9. [DOI] [PubMed] [Google Scholar]
- 23.Ulphani J.S., Arora R., Cain J.H., et al. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293:H1629–H1635. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 24.Randall W.C., Ardell J.L., O'Toole M.F., Wurster R.D. Differential autonomic control of SAN and AVN regions of the canine heart: structure and function. Prog Clin Biol Res. 1988;275:15-31. [PubMed] [Google Scholar]
- 25.Pauziene N., Pauza D.H., Stropus R. Morphology of human intracardiac nerves: an electron microscope study. J Anat. 2000;197:437–459. doi: 10.1046/j.1469-7580.2000.19730437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng L., Sun W., Liu S., et al. The diagnostic value of cardiac deceleration capacity in vasovagal syncope. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008659. [DOI] [PubMed] [Google Scholar]
- 27.Batulevicius D., Skripka V., Pauziene N., Pauza D.H. Topography of the porcine epicardiac nerve plexus as revealed by histochemistry for acetylcholinesterase. Auton Neurosci. 2008;138:64–75. doi: 10.1016/j.autneu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Piotrowski R., Baran J., Sikorska A., Krynski T., Kulakowski P. Cardioneuroablation for reflex syncope: efficacy and effects on autonomic cardiac regulation-a prospective randomized trial. J Am Coll Cardiol EP. 2023;9:85–95. doi: 10.1016/j.jacep.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Pachon -M.E.I., Pachon-Mateos J.C., Higuti C., et al. Relation of fractionated atrial potentials with the vagal innervation evaluated by extracardiac vagal stimulation during cardioneuroablation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.007900. [DOI] [PubMed] [Google Scholar]
- 30.Huang X., Chen Y., Huang Y., et al. Comparative effects of intensive ganglionated plexus ablation in treating paroxysmal atrial fibrillation and vasovagal syncope. Clin Cardiol. 2020;43:1326–1333. doi: 10.1002/clc.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L., Zhao Y., Duan Y., et al. Clinical efficacy of catheter ablation in the treatment of vasovagal syncope. J Clin Med. 2022;11:5371. doi: 10.3390/jcm11185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung R, Locke AH, Shah AD, et al. Feasibility and safety of catheter-based cardioneural ablation: results from the multicenter CAN registry. Presented at Heart Rhythm 2022; April 29–May 1, 2022; San Francisco, CA.
- 33.Debruyne P., Rossenbacker T., Janssens L., et al. Durable physiological changes and decreased syncope burden 12 months after unifocal right-sided ablation under computed tomographic guidance in patients with neurally mediated syncope or functional sinus node dysfunction. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.009747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Candemir B., Baskovski E., Beton O., et al. Procedural characteristics, safety, and follow-up of modified right-sided approach for cardioneuroablation. Anatol J Cardiol. 2022;26:629–636. doi: 10.5152/AnatolJCardiol.2022.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brignole M., Menozzi C., Del Rosso A., et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace. 2000;2:66–76. doi: 10.1053/eupc.1999.0064. [DOI] [PubMed] [Google Scholar]
- 36.Pachon -M.J.C., Pachon -M.E.I., Pachon C.T.C., et al. Long-term evaluation of the vagal denervation by cardioneuroablation using Holter and heart rate variability. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008703. [DOI] [PubMed] [Google Scholar]
- 37.Tu B., Wu L., Hu F., et al. Cardiac deceleration capacity as an indicator for cardioneuroablation in patients with refractory vasovagal syncope. Heart Rhythm. 2022;19:562–569. doi: 10.1016/j.hrthm.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Yao Y., Shi R., Wong T., et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 39.Aksu T., Golcuk E., Yalin K., Guler T.E., Erden I. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. doi: 10.1111/pace.12756. [DOI] [PubMed] [Google Scholar]
- 40.Mesquita D., Parreira L., Carmo P., et al. Anatomic guided ablation of the atrial right ganglionated plexi is enough for cardiac autonomic modulation in patients with significant bradyarrhythmias. Indian Pacing Electrophysiol J. 2021;21:327–334. doi: 10.1016/j.ipej.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandenberk B., Lei L.Y., Ballantyne B., et al. Cardioneuroablation for vasovagal syncope: a systematic review and meta-analysis. Heart Rhythm. 2022 Jun doi: 10.1016/j.hrthm.2022.06.017. 16 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Baysal E., Mutluer F.O., Dagsali A.E., Kumrulu U.C., Huang H.D., Aksu T. Improved health-related quality of life after cardioneuroablation in patients with vasovagal syncope. J Interv Card Electrophysiol. 2022 Nov 11 doi: 10.1007/s10840-022-01420-9. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Fukunaga M., Wichterle D., Peichl P., Aldhoon B., Čihák R., Kautzner J. Differential effect of ganglionic plexi ablation in a patient with neurally mediated syncope and intermittent atrioventricular block. Europace. 2017;19:119–126. doi: 10.1093/europace/euw100. [DOI] [PubMed] [Google Scholar]
- 44.Aksu T., Gopinathannair R., Bozyel S., Yalin K., Gupta D. Cardioneuroablation for treatment of atrioventricular block. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.121.010018. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L., Jiang W., Zhou L., et al. Atrial autonomic denervation for the treatment of long-standing symptomatic sinus bradycardia in non-elderly patients. J Interv Card Electrophysiol. 2015;43:151–159. doi: 10.1007/s10840-015-9981-8. [DOI] [PubMed] [Google Scholar]
- 46.Qin M., Zhang Y., Liu X., Jiang W.F., Wu S.H., Po S. Atrial ganglionated plexus modification: a novel approach to treat symptomatic sinus bradycardia. J Am Coll Cardiol EP. 2017;3:950–959. doi: 10.1016/j.jacep.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Vandenberk B., Morillo C.A., Sheldon R.S., Chew D.S., Aksu T., Raj S.R. Clinician needs and perceptions about cardioneuroablation for recurrent vasovagal syncope: an international clinician survey. Heart Rhythm. 2021;18:2160–2166. doi: 10.1016/j.hrthm.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Connolly S.J., Sheldon R., Roberts R.S., Gent M. The North American Vasovagal Pacemaker Study (VPS). A randomized trial of permanent cardiac pacing for the prevention of vasovagal syncope. J Am Coll Cardiol. 1999;33:16–20. doi: 10.1016/s0735-1097(98)00549-x. [DOI] [PubMed] [Google Scholar]
- 49.Connolly S.J., Sheldon R., Thorpe K.E., et al. VPS II Investigators. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: Second Vasovagal Pacemaker Study (VPS II): a randomized trial. JAMA. 2003;289:2224–2229. doi: 10.1001/jama.289.17.2224. [DOI] [PubMed] [Google Scholar]
- 50.Brignole M., Menozzi C., Moya A., et al. International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation. 2012 May 29;125:2566–2571. doi: 10.1161/CIRCULATIONAHA.111.082313. [DOI] [PubMed] [Google Scholar]
- 51.Pournazari P., Sahota I., Sheldon R. High remission rates in vasovagal syncope: systematic review and meta-analysis of observational and randomized studies. J Am Coll Cardiol EP. 2017;3:384–392. doi: 10.1016/j.jacep.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Abdelazeem B., Abbas K.S., Manasrah N., Amin M.A., Mohammed S.M., Mostafa M.R. Yoga as a treatment for vasovagal syncope: a systematic review and meta-analysis. Complement Ther Clin Pract. 2022;48 doi: 10.1016/j.ctcp.2022.101579. [DOI] [PubMed] [Google Scholar]
- 53.Vyas A., Swaminathan P.D., Zimmerman M.B., Olshansky B. Are treatments for vasovagal syncope effective? A meta-analysis. Int J Cardiol. 2013 Sep 1;167:1906–1911. doi: 10.1016/j.ijcard.2012.04.144. [DOI] [PubMed] [Google Scholar]
- 54.Lei L.Y., Raj S.R., Sheldon R.S. Midodrine for the prevention of vasovagal syncope: a systematic review and meta-analysis. Europace. 2022;24:1171–1178. doi: 10.1093/europace/euab323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mai W., Kusumoto F. Advanced atrioventricular block due to hypervagotonia: treatment with hyoscyamine. HeartRhythm Case Rep. 2022;8:343–346. doi: 10.1016/j.hrcr.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aksu T., Guler T.E., Mutluer F.O., Bozyel S., Golcuk S.E., Yalin K. Electroanatomic-mapping-guided cardioneuroablation versus combined approach for vasovagal syncope: a cross-sectional observational study. J Interv Card Electrophysiol. 2019;54:177–188. doi: 10.1007/s10840-018-0421-4. [DOI] [PubMed] [Google Scholar]
- 57.Pachon M.J.C., Pachon M.E.I., Santillana P.T.G., et al. Simplified method for vagal effect evaluation in cardiac ablation and electrophysiological procedures. J Am Coll Cardiol EP. 2015;1:451–460. doi: 10.1016/j.jacep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Hou Y., Zhou Q., Po S.S. Neuromodulation for cardiac arrhythmia. Heart Rhythm. 2016;13:584–592. doi: 10.1016/j.hrthm.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Scanavacca M., Pisani C.F., Hachul D., et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 60.Hu F., Yao Y. Cardioneuroablation in the management of vasovagal syncope, sinus node dysfunction, and functional atrioventricular block - techniques. J Atr Fibrillation. 2020;13:119–123. doi: 10.4022/jafib.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piotrowski R., Zuk A., Baran J., Sikorska A., Krynski T., Kulakowski P. Ultrasound-guided extracardiac vagal stimulation-new approach for visualization of the vagus nerve during cardioneuroablation. Heart Rhythm. 2022;19:1247–1252. doi: 10.1016/j.hrthm.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Pachon -M.J.C., Ortencio F.A., Pachon -M.E.I., et al. Treatment of symptomatic functional atrioventricular block by cardioneuroablation as an alternative to pacemaker implantation. J Am Coll Cardiol Case Rep. 2022;4:990–995. doi: 10.1016/j.jaccas.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh S., Zhang Y., Bibevski S., Marrouche N.F., Natale A., Mazgalev T.N. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006;3:701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Stavrakis S., Po S. Ganglionated plexi ablation: physiology and clinical applications. Arrhythm Electrophysiol Rev. 2017;6:186–190. doi: 10.15420/aer2017.26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanna P., Buch E., Stavrakis S., et al. Neuroscientific therapies for atrial fibrillation. Cardiovasc Res. 2021;117:1732–1745. doi: 10.1093/cvr/cvab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park H.W., Shen M.J., Lin S.F., Fishbein M.C., Chen L.S., Chen P.S. Neural mechanisms of atrial fibrillation. Curr Opin Cardiol. 2012;27:24–28. doi: 10.1097/HCO.0b013e32834dc4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.