Abstract
Background
Supraventricular tachycardia (SVT) is a common arrhythmia. Infants with SVT are often admitted to initiate antiarrhythmics. Transesophageal pacing (TEP) studies can be used to guide therapy prior to discharge.
Objective
The objective of this study was to investigate the impact of TEP studies on length of stay (LOS), readmission, and cost in infants with SVT.
Methods
This was a 2-site retrospective review of infants with SVT. One site (Center TEPS) utilized TEP studies in all patients. The other (Center NOTEP) did not. Patients with structural heart disease, patients with gestational age <34 weeks, and patients diagnosed after 6 months were excluded. At Center TEPS, repeat TEP studies were performed after titration of medication until SVT was not inducible. Primary endpoints were LOS and readmission for breakthrough SVT within 31 days of discharge. Hospital reimbursement data were utilized for cost-effectiveness analysis.
Results
The cohort included 131 patients, 59 in Center TEPS and 72 in Center NOTEP. One patient was readmitted in Center TEPS vs 17 in Center NOTEP (1.6% vs 23.6%; P ≤ .001). Median LOS was longer for Center TEPS at 118.0 (interquartile range [IQR] 74.0–189.5) hours vs Center NOTEP at 66.9 (IQR 45.5–118.3) hours (P = .001). Twenty-one patients had multiple TEP studies. Median length of readmission for Center NOTEP was 65 (IQR 41–101) hours. Including readmission costs, utilization of TEP studies resulted in a probability-weighted cost of $45,531 per patient compared with $31,087 per patient without TEP studies.
Conclusion
Utilization of TEP studies was associated with decreased readmission rates but longer LOS and greater cost compared with SVT management without TEP studies.
Keywords: Cost, Hospital readmission, Supraventricular tachycardia, Pediatric electrophysiology, Transesophageal pacing
Key Findings.
-
▪
Readmission rates for infants with supraventricular tachycardia who are medically treated are high with an observe-and-discharge approach.
-
▪
By utilizing transesophageal pacing studies to guide medication therapy, readmissions for infants with supraventricular tachycardia can be significantly reduced.
-
▪
Utilization of transesophageal pacing studies is associated with greater cost, even when readmission costs are included.
Introduction
Supraventricular tachycardia (SVT) is the most common arrhythmia in infants.1 After initial diagnosis, most infants with SVT are admitted for inpatient care to initiate chronic antiarrhythmic therapy with the goal to prevent SVT recurrence and begin medication safely.2 A common practice after admission is to initiate antiarrhythmics, adjust medications based on early breakthrough SVT, and discharge patients after a period of observation.3 While this system works in many instances, some patients will have readmission secondary to breakthrough tachycardia. In an attempt to reduce early readmission for breakthrough arrhythmia, some centers utilize transesophageal pacing (TEP) studies prior to discharge while patients are on antiarrhythmics to guide medication therapy.4,5 If a patient has inducible tachycardia on TEP studies while receiving medication therapy, medications are titrated or changed and TEP studies are repeated until the patient is no longer inducible. The effectiveness of these 2 approaches and the impact on medical care and cost has not been compared. The objective of this study was to address this gap in the literature and evaluate the effect of TEP studies on hospital readmission rates and hospital length of stay and perform a cost-effectiveness analysis comparing these 2 approaches.
Methods
This was a retrospective chart review of infants with 1:1 re-entrant SVT diagnosed prior to 6 months of age. Patients were hospitalized between May of 2010 and October of 2021 at 1 of 2 pediatric institutions. The study was conducted with permission from local institutional review boards at both participating centers (#2020-0977 and #1697128-2). Due to the retrospective nature of this study, informed consent from patients was not sought, as only nonidentifiable clinical data were collected and analyzed. All research conducted during this study adhered to the Helsinki Declaration guidelines. Infants were admitted following diagnosis with medication initiation according to center practice. The first institution (Center NOTEP) used admission and discharge planning without the use of TEP studies. The second institution (Center TEPS) routinely utilized TEP studies screening in all infants after initiation of antiarrhythmic therapy but prior to hospital discharge. Patients were excluded if they had a primary diagnosis of atrial tachycardia, atrial fibrillation or atrial flutter, structural heart disease requiring surgical or catheter intervention, or gestational age <34 weeks.
Inpatient management
Patient care was delivered as part of routine institutional patient management at each center. Decisions regarding antiarrhythmic choice were determined clinically and based on patient presentation, tachycardia characteristics, and physician preference. No patients admitted at Center NOTEP underwent TEP studies, while all patients admitted at Center TEPS underwent TEP studies after 4 to 6 half-lives of the medication used, which was typically after 48 hours of antiarrhythmic treatment if no breakthrough tachycardia was detected. The protocol for TEP studies at Center TEPS consisted of placement of a 5F bipolar pacing catheter in the retro-cardiac position within the esophagus via the nares or oropharynx followed by a pacing protocol. The pacing protocol consisted of incremental atrial pacing and single and double atrial extrastimuli both off and on isoproterenol. If sustained SVT was inducible on TEP studies, defined as SVT that did not spontaneously terminate within 20 beats of tachycardia, this was considered a failure of the current medical treatment regimen. If there was a first failure, the dose of the initial agent, usually a beta-blocker, was adjusted. Once therapeutic efficacy of the new regimen was deemed stable, typically 48 hours after medication adjustment, the TEP was repeated. If the patient remained inducible then a second agent, typically flecainide, was added. This process was repeated until the patient was no longer inducible by TEP studies. Studies were performed with minimal sedation and most patients only received oral dextrose via a pacifier. At Center NOTEP, patients were discharged once arrhythmia free for 24 hours after medication was deemed to be at steady state, which was typically 48 hours after initiation.
At both sites, the electronic medical record was examined to gather baseline and initial hospitalization characteristics. These included patient age, race, ethnicity, sex, weight, length, gestational age, prenatal diagnosis, congenital heart disease not requiring intervention, maternal diabetes, age at admission, admission length and location, left ventricular ejection fraction on admission, type of and changes to medical therapy, number of TEP studies performed, utilization of extracorporeal membrane oxygenation, and if discharge was delayed due to factors not related to treatment of SVT. Follow-up data after initial discharge were also collected, including length of follow-up, readmission while on antiarrhythmics, duration and discontinuation of outpatient antiarrhythmic therapy, SVT recurrence after discontinuation of outpatient therapy, and catheter ablation therapy.
At each center, the diagnosis of SVT was confirmed by a pediatric electrophysiologist who reviewed an electrocardiogram tracing of each patient’s tachycardia at admission and compared it to an electrocardiogram in sinus rhythm. Patients were admitted to either an intensive care unit or the general cardiology floor depending on patient stability and center policy. Prematurity was defined as gestational age under 37 weeks. Left ventricular ejection fraction <55% on admission echocardiogram was defined as tachycardia-induced cardiomyopathy. If a patient was on stable medication therapy and suitable for discharge but was then not discharged for >24 hours due to comorbidities unrelated to SVT, this patient was deemed to have a delayed discharge and was not included in length of stay or cost-effectiveness analyses.
The first-line agent was most often propranolol and was initiated at 2 mg/kg/d divided every 8 hours. Other agents utilized included digoxin, dosed initially at 10 μg/kg/d divided over 2 doses; flecainide, dosed initially at 80 mg/m2/d divided every 8 hours; and amiodarone, dosed initially at 5 mg/kg/d daily after a load of 10 mg/kg/d. If an antiarrhythmic was discontinued in favor of another agent or if another agent was added to the current regimen, these were defined as changes in medication. Changes in route of administration, such as intravenous to oral, of 2 identical medications or medications with identical mechanisms of action, were not recorded as changes in medication. For each patient with a medication change, the reason for a change in medication was identified via review of progress and consultation notes in the electronic medical record and categorized as being secondary to breakthrough SVT while on current medication regimen, medication side effects, or results of TEP studies testing.
Postdischarge follow-up and readmission
Prior to discharge, all patients received teaching by the nursing staff on both medication administration and heart rate monitoring. All patients were contacted after discharge within 24 to 48 hours to ensure there were no barriers to medication access or administration. All patients had routine electrophysiology follow-up within 4–8 weeks after discharge and then routinely while on medication therapy. Early hospital readmission was the primary outcome in this study. Each patient was followed from the time of hospital discharge until the last outpatient visit at the participating institution. All hospital readmissions were tracked though the entire length of patient care. Early hospital readmission was defined as readmission within 31 days of discharge from initial SVT hospitalization. As part of routine care, all patients who had early recurrence of documented SVT were readmitted at both centers. The secondary outcome was hospital length of stay during initial admission. This was determined from the electronic health record and was calculated as the time between placement of admission and discharge orders. Length of stay was also calculated for all readmissions.
Statistical analysis
Median with interquartile range (IQR) or frequency and percentage were used to describe demographic, hospitalization, and follow-up variables. Differences between the Center NOTEP and Center TEPS cohorts were tested by Wilcoxon rank sum tests for continuous variables and chi-square tests for categorical variables. A Kaplan-Meier curve of freedom from readmission over the first year after initial hospital discharge was generated for the entire population, as well as each center. The log-rank test was used to evaluate differences in readmission between the centers. P values <.05 were considered statistically significant. All statistical analyses were performed using R statistical program (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
Cost analysis
To examine the financial implications of the strategy choice, a cost-effectiveness analysis was performed. The financial analysis was performed from the perspective of the healthcare sector, with costs being defined as payer cost according to consensus guidelines for the performance of cost-effectiveness analyses.6 Indirect costs, such as parent opportunity costs for readmission, which would be included in societal perspective, were not included. Costs were normalized to present value 2022 constant U.S. dollars using a 3% real discount rate. The unit of effectiveness was readmissions prevented and inpatient hospital days prevented. To this end, hospital reimbursement records for study subjects cared for at Center NOTEP were analyzed for all-inclusive procedural and inpatient care costs both for the initial admission and readmissions for SVT. Expenses included a subset of patients who required intensive care, as this represents the true spectrum of costs and thereby maintains external validity. However, patients whose hospitalization was prolonged for reasons other than SVT management were excluded from this cost analysis. Cost for the performance of TEP studies in an unrelated outpatient population was utilized as a surrogate for the cost of TEP studies in the study cohort. The cumulative costs and effectiveness of each strategy was probability weighted according to the clinical outcomes observed in the study subjects. To limit the extent to which state or institutional contract reimbursement differences impacted the results, the cost rates extracted from Center NOTEP were applied equally to the clinical courses experienced by patients at both centers.
Results
Baseline characteristics and initial hospitalization
A total of 131 patients were included in the study, 72 at Center NOTEP and 59 at Center TEPS. Table 1 demonstrates the cohort’s baseline characteristics. The overall population was predominantly male (64%), Caucasian (74%), and non-Hispanic (91%). Center TEP had a greater proportion of patients with Hispanic ethnicity than Center NOTEP (10 of 59 vs 2 of 71; P = .014). Median weight at presentation was 3.7 (IQR 3.3–4.2) kg, and the median weight at presentation was lower for Center NOTEP than for Center TEPS (3.5 kg vs 3.9 kg; P = .009). Gestational age was slightly less by 2 days at Center NOTEP than at Center TEPS (38.5 weeks vs 39 weeks; P = .033). There were no other differences in baseline characteristics between the 2 cohorts.
Table 1.
Baseline characteristics of infants admitted with supraventricular tachycardia
| Variable | All patients (N = 131) | By center |
P value | |
|---|---|---|---|---|
| NOTEP (n = 72) | TEPS (n = 59) | |||
| Male | 84 (64) | 41 (57) | 43 (73) | .087 |
| Admission weight, kg | 3.7 (3.3–4.2) | 3.5 (3.2–4.1) | 3.9 (3.5–4.3) | .009∗ |
| Admission length, cm | 51.5 (49.0–54.0) | 51.0 (48.2–53.5) | 51.7 (50.2–54.8) | .078 |
| Race | .13 | |||
| Caucasian | 96 (74) | 57 (79) | 39 (66) | |
| African American | 22 (17) | 11 (15) | 11 (19) | |
| Asian | 1 (0.8) | 0 (0) | 1 (1.7) | |
| Other/multiple | 11 (8.5) | 3 (4.2) | 8 (14) | |
| Unknown | 1 (0.8) | 1 (1.4) | 0 (0) | |
| Hispanic ethnicity | 12 (9.2) | 2 (2.8) | 10 (17) | .014∗ |
| Infant of diabetic mother | 22 (17) | 13 (18) | 9 (15) | .8 |
| Prematurity | 18 (14) | 14 (19) | 4 (6.8) | .066 |
| Gestational age, wk | 39.0 (37.4–39.5) | 38.5 (37.0–39.3) | 39.0 (38.0–4.0) | .033∗ |
| Noninterventional CHD | 11 (8.4) | — | — | |
| Prenatal diagnosis | 22 (17) | 14 (19) | 8 (14) | .5 |
| Age at diagnosis, d | 11.0 (3.0–29.0) | 12.5 (4.5–32.2) | 10.0 (3.0–25.0) | .4 |
Values are n (%) or median (interquartile range).
CHD = congenital heart disease; NOTEP = no transesophageal pacing; TEPS = transesophageal pacing studies.
P-values < .05.
Regarding admission location, 84 (64%) patients were admitted to an intensive care unit, and patients at Center NOTEP were more likely to be admitted to an intensive care unit (53 of 72 vs 31 of 59; P = .012). Table 2 demonstrates the cohort’s hospitalization characteristics. All patients were initiated on antiarrhythmic medications after admission, and there was no statistically significant difference between initial antiarrhythmic type. During initial admission, patients at Center TEPS were more likely to undergo an initial change in medication than were those at Center NOTEP (33 of 59 vs 24 of 72; P = .016). However, patients at Center NOTEP were more likely to have had multiple medication changes during initial admission (11 of 24 vs 4 of 33; P = .011). Upon discharge, patients at Center TEPS were more likely to be treated with a beta-blocker (54 of 59 vs 43 of 72; P < .001), be treated with flecainide (32 of 59 vs 15 of 72; P < .001), and be discharged on multiple medications (30 of 59 vs 5 of 72; P < .001) and were less likely to be treated with amiodarone (1 of 59 vs 10 of 72; P = .029). When length of admission was examined, patients at Center TEPS had a longer median length of admission compared with those at center NOTEP (118.0 [IQR 74.0–189.5] hours vs 66.9 [IQR 45.5–118.3] hours; P = .001). There were no other significant differences between the 2 centers regarding other hospitalization characteristics.
Table 2.
Hospitalization characteristics of infants admitted with supraventricular tachycardia
| Variable | All patients (N = 131) | By center |
P value | |
|---|---|---|---|---|
| NOTEP (n = 72) | TEPS (n = 59) | |||
| Length of admission, h | 91.4 (51.6–160.2) | 66.9 (45.5–118.3) | 118.0 (74.0–189.5) | .001∗ |
| Initial admission location | .021∗ | |||
| Cardiac intensive care unit | 57 (44) | 40 (56) | 17 (29) | |
| Neonatal intensive care Unit | 23 (18) | 12 (17) | 11 (19) | |
| Pediatric intensive care unit | 4 (3.1) | 1 (1.4) | 3 (5.1) | |
| Cardiac step down | 46 (35) | 19 (26) | 27 (46) | |
| Other location | 1 (0.8) | 0 (0) | 1 (1.7) | |
| Initial medication | ||||
| Beta-blocker | 110 (84) | 57 (79) | 53 (90) | .2 |
| Digoxin | 12 (9.2) | 10 (14) | 2 (3.4) | .077 |
| Flecainide | 4 (3.1) | 1 (1.4) | 3 (5.1) | .5 |
| Amiodarone | 4 (3.1) | 3 (4.2) | 1 (1.7) | .8 |
| Procainamide | 2 (1.5) | 2 (2.8) | 0 (0) | .6 |
| Medication change | 57 (44) | 24 (33) | 33 (56) | .016∗ |
| ECMO utilized | 3 (2.3) | 2 (2.8) | 1 (1.7) | >.9 |
| TICM on admission | 39 (30) | 21 (29) | 18 (31) | >.9 |
| Dysfunction prior to discharge | 6 (4.6) | 4 (5.6) | 2 (3.4) | .7 |
| Continuous antiarrhythmic at admission | 17 (13) | 8 (11) | 9 (15) | .7 |
Values are median (interquartile range) or n (%).
ECMO = extracorporeal membrane oxygenation; NOTEP = no transesophageal pacing; TEPS = transesophageal pacing studies; TICM = tachycardia-induced cardiomyopathy.
P-values < .05.
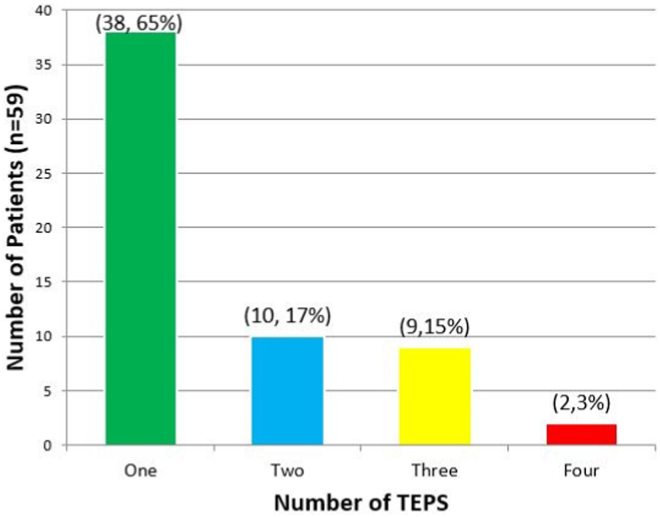
A total of 72 pacing studies were performed for the 59 patients at Center TEPS. Multiple TEP studies were performed in 21 patients, while 38 patients only had a single TEP study. A breakdown of number of TEP studies performed per patient is presented in Figure 1. There were no complications from any TEP studies.
Figure 1.
Bar graph demonstrating the number of transesophageal pacing (TEP) studies received by each patient at Center TEPS. The x-axis represents the number of TEP studies performed, while the y-axis is the number of patients who received that many TEP studies.
Readmission, recurrence, and postdischarge information
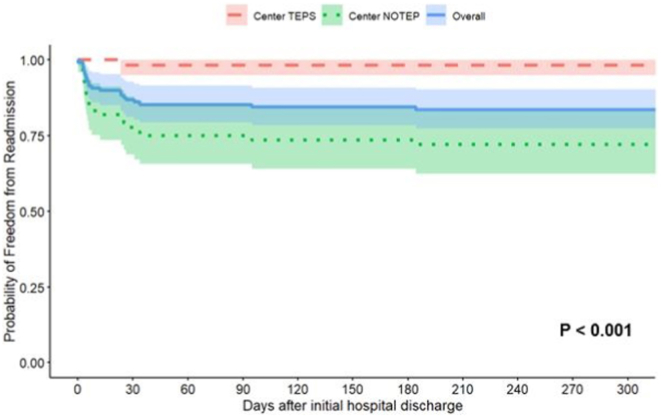
For the entire cohort, median follow-up after initial hospital discharge was 667 (IQR 346–1562) days. Table 3 demonstrates the cohort’s discharge and follow-up characteristics. One patient at Center TEPS did not have any follow-up after initial hospital discharge and was not included in the readmission analysis. Patients at Center TEPS were more likely to be discharged earlier from outpatient care, as median follow-up at Center TEPS was 368 (IQR 246–785) days compared with 942 (IQR 502–1728) days (P < .001) at Center NOTEP. A total of 18 (13.8%) patients were readmitted within 31 days of hospital discharge. Only 1 (1.7%) patient was readmitted at Center TEPS, while 17 (23.6%) patients were readmitted at Center NOTEP (1 of 58 vs 17 of 72; P < .001). Of the 17 patients readmitted at Center NOTEP within 31 days of discharge, 11 were readmitted within 1 week with minimal change in weight. Of the other 6 patients readmitted in this time period, 2 patients had weight-based medication doses less than discharge, 3 patients had weight-based medication doses greater than discharge after outpatient dose adjustment, and 1 patient had unchanged weight-based medication dose. Of these early patients with early readmission, all were on monotherapy and 12 were on beta-blocker, 2 were on flecainide, 2 were on digoxin, and 1 was on amiodarone. Caregivers reported no missed doses, but 3 reported vomiting around time of medication dose. A further 3 patients were readmitted between 32 and 200 days of discharge, all at Center NOTEP. The single Center TEPS readmission had recurrence at 23 days of age, and median recurrence at Center NOTEP was 6 (IQR 4–25) days. A Kaplan-Meier curve of freedom from readmission by center over the first year is presented in Figure 2. There was a statistically significant difference in freedom from readmission between Center TEPS and Center NOTEP (98.2% vs 73.6% at 150 days; log-rank P < .001).
Table 3.
Discharge and follow-up characteristics of infants admitted with supraventricular tachycardia
| Variable | All patients (N = 131) | By center |
P value | |
|---|---|---|---|---|
| NOTEP (n = 72) | TEPS (n = 59) | |||
| Discharge medication | ||||
| Beta-blocker | 97 (74) | 43 (60) | 54 (92) | <.001∗ |
| Digoxin | 9 (6.9) | 7 (9.7) | 2 (3.4) | .3 |
| Flecainide | 47 (36) | 15 (21) | 32 (54) | <.001∗ |
| Amiodarone | 11 (8.4) | 10 (14) | 1 (1.7) | .029∗ |
| Propafenone | 1 (0.8) | 1 (1.4) | 0 (0) | >.9 |
| No medication | 2 (1.5) | 2 (2.8) | 0 (0) | .6 |
| Discharge on multiple medications | 35 (27) | 5 (6.9) | 30 (51) | <.001∗ |
| Readmission within 31 d | 18 (13.8) | 17 (23.6) | 1 (1.7) | <.001∗ |
| Length of follow-up, d | 667 (346–1562) | 942 (502–1728) | 368 (246–785) | <.001∗ |
| Medications discontinued by study end | 112 (88) | 61 (87) | 51 (88) | >.9 |
| Age medications discontinued, mo | 12.0 (7.8–12.0) | 11.0 (8.0–13.0) | 12.0 (7.0–12.0) | .5 |
| Recurrence after medications discontinued | 10 (8.9) | 7 (11) | 3 (5.9) | .5 |
| Age of recurrence after medications discontinued, mo | 38.5 (10.0–49.0) | 36.0 (10.5–42.0) | 60.0 (34.5–78.0) | .3 |
| Patient underwent ablation | 7 (5.4) | 6 (8.3) | 1 (1.7) | .2 |
Values are n (%) or median (interquartile range).
NOTEP = no transesophageal pacing; TEPS = transesophageal pacing studies.
P-values < .05.
Figure 2.
Kaplan-Meier curve of freedom from readmission of supraventricular tachycardia patients. The red dashed line represents patients at the center with transesophageal pacing studies (Center TEPS), while the green dotted line represents patients at the center with no transesophageal pacing studies (Center NOTEP). The solid blue line is the overall cohort. Shaded areas are 95% confidence intervals.
The majority of patients at both centers stopped medication therapy by study endpoint, with 61 (85%) and 51 (88%) patients stopping at Center NOTEP and Center TEPS, respectively. The median age of medication cessation was similar between Center TEPS (12 [IQR 7–12] months) and Center NOTEP (11 [IQR 8–12] months) (P = .5). Ten patients had recurrence of tachycardia after medication cessation, 7 (11%) at Center NOTEP and 3 (5%) at Center TEPS (P = .5). Median age of recurrence was slightly older among patients at Center TEPS (60 [IQR 35–78] months) than at Center NOTEP (36 [IQR 11–42] months), although this did not reach statistical significance (P = .3). A total of 7 patients underwent ablation during the study period, 6 at Center NOTEP and 1 at Center TEPS (6 of 72 vs 1 of 58; P = .13). Only 2 patients underwent ablation during the first year of age, both at Center NOTEPS. One patient had 3 readmissions before ablation at 2 months of age after failure of multiple medications and 1 patient underwent ablation at 1 month of age during initial admission after inefficacy of multiple medications. All other patients received elective ablation at 3 years of age or older.
Cost analysis
Utilizing reimbursement data from Center NOTEP, an incremental cost-effectiveness ratio analysis was performed by comparing the cost of each center’s strategy with its efficacy at preventing readmissions. Using these data, a median daily rate of $8810.39 for initial admission was generated from 55 patients whose discharge was not delayed and who had accurate reimbursement accounts. We multiplied this daily rate with each centers median admission length in days (3 for Center NOTEP and 5 for Center TEPS). We then utilized the same strategy to obtain a median daily of rate of $6573.15 for readmission from 14 patients who had accurate reimbursement accounts pertaining to their readmission hospital course. We multiplied this value with the readmission probability of each center and a median readmission length of 3 days to obtain a probability weighted readmission cost. We then obtained reimbursement data for 7 patients not in the study population who received outpatient TEP studies at Center NOTEP for other indications to keep all reimbursement data tied to a single center. We then probability weighted the likelihood of multiple TEP studies based on the number of TEP studies performed for each patient. By summing the initial admission costs with probability-weighted TEP studies and readmission costs, we calculated a probability-weighted total strategy cost of $45,531.77 at Center TEPS and $31,087.16 at Center NOTEP. Thus, the routine utilization of TEP studies resulted in a $14,444.6 greater cost per patient, on average. By dividing the difference in cost of each strategy by the difference in efficacy of each strategy, the incremental cost-effectiveness ratio analysis demonstrated an average cost of $65,908.35 for routine utilization of TEP studies to prevent 1 readmission.
Discussion
SVT is the most common arrhythmia in infants, and early readmission is both a financial and emotional stress for both caregivers and healthcare systems. In this study, we examined the utility of TEP studies in preventing early hospital readmission in infants diagnosed with SVT and performed an analysis to determine the cost-effectiveness of differing discharge strategies. These data demonstrated several key factors in early readmission and the utility of TEP studies in discharge readiness. First, early readmission was common following SVT discharges in which observation was the only measure of discharge readiness. Second, the use of TEP studies in this cohort was associated with a significant reduction in early hospital readmission compared with patients with observation only prior to discharge. However, due to longer lengths of stay incurred by patients who underwent multiple TEP studies, as well as procedural costs, the utilization of TEP studies was associated with greater overall cost.
The need for early hospital readmission associated with observation only discharge strategies in infants with SVT is common, and the early recurrence rate in this study is similar to prior reports.7 Unfortunately, there are few inherent patient or arrhythmia characteristics that accurately predict readmission. Because of this, strategies to improve readmission risk assessment are of potential use. Early on, TEP studies were utilized in the management of SVT in various capacities including tachycardia termination, risk stratification, and basic electrophysiologic description.8, 9, 10 Subsequently, TEP studies were utilized for SVT medication assessment in the pediatric population, and Benson and colleagues11 used TEP studies to test efficacy of pharmacological therapy in children treated with quinidine. Shortly afterward, Benson and colleagues12 reported that many infants with SVT treated with digoxin were inducible on TEP studies and had a high rate of clinical recurrence. Other applications of TEP studies in pediatric SVT have been used, including assessing arrhythmia mechanism and age distribution in children,13 evaluating for SVT inducibility after discontinuing chronic antiarrhythmic therapy,14 evaluation of palpitations,15 and evaluating tachycardia substrate in newborns with a history of fetal tachycardia.5,16
In our study, TEP studies were utilized to test for continued inducibility of SVT in newly diagnosed infants undergoing inpatient treatment with antiarrhythmics in hopes of determining discharge readiness and risk for early readmission secondary to medication failure. Patients who remained inducible on their current antiarrhythmic therapy remained inpatient and received either an increase in medication dosage or an addition or substitution of another antiarrhythmic agent with the underlying assumption that these patients have a higher risk of recurrence in the outpatient setting. The use of TEP studies in SVT recurrence was investigated by Bonney and colleagues,17 who demonstrated that infants with inducible SVT on TEP studies at discharge were more likely to have outpatient recurrence.
Prior studies have found that TEP studies carry a high negative predictive value for assessing efficacy of clinical therapy. However, the positive predictive value was significantly lower.18 Our data correlated with these findings, as only a single patient was readmitted using a TEP strategy. However, 21 (35.6%) of the 59 patients required multiple TEP studies to achieve a negative endpoint, which resulted in prolonged hospital readmission and increased cost. Recently published work evaluating TEP studies in prenatally diagnosed infants with SVT found a 21% recurrence rate among a 19-patient cohort who received only 1 TEP while on esmolol. Some of these patients remained inducible on esmolol, suggesting that multiple TEP studies to achieve a negative endpoint may be necessary to prevent readmission.5 While there was a significant reduction in readmission, our cost analysis demonstrated a significantly increased probability-weighted total cost for utilizing TEP studies in all patients. To routinely employ TEP studies in this manner resulted in a $14,444.60 greater cost per patient, and $65,908.35 was needed to prevent 1 readmission. Given this value exceeds the probability-weighted cost of that resultant readmission, from a purely financial standpoint the TEP strategy is challenging to justify from the healthcare sector perspective.
However, this value considers neither the economic cost of caregiver distress from readmission19 nor the lost earnings and nonmedical expenses for caregivers.20 While difficult to measure, these societal cost factors may result in a lower relative cost of routinely utilizing TEP studies than our numbers suggest. Additionally, while the readmission rate for Center NOTEP is consistent with published values in the literature, some studies demonstrate higher admission rates,21 and utilization of these rates would improve the cost profile of the TEP strategy. Nonetheless, while the cost per patient utilizing TEP studies in all patients is significantly greater in this dataset, TEP was demonstrated to be an effective tool to prevent readmissions. Thus, a targeted approach to utilizing TEP studies may result in reduced readmissions while also improving the cost-effectiveness. The challenge is identifying which patients are more likely to be readmitted. Multiple prior studies have demonstrated that patients with ventricular pre-excitation are at a higher risk of readmission, and these patients would likely benefit from receiving TEP studies prior to discharge.21, 22, 23 Other patients who may be at risk include patients with a diagnosis of fetal tachycardia21,24,25 or who require multiple medications to manage,21,23,25 although evidence on these risk factors is mixed.22,23,26
In addition to those patients who are at greater risk of readmission, patients who are located farther from healthcare facilities or those with other comorbidities may benefit from the utilization of TEP studies to achieve a satisfactorily therapeutic medication regimen. Similarly, patients who have already been readmitted may benefit from TEP studies to prevent ongoing medication failure. Ultimately, providers at each center will have to weigh the costs and benefits of each approach and can engage in shared decision making with families to determine the ideal approach for each patient.
Limitations
This study has several limitations. Given that there were 2 sites involved in this study, some outcome variables may have been influence by practice variation between the 2 centers. Also, medication regimens varied, and some medications utilized in early study patients, such as digoxin, are less prevalent in the treatment of SVT today. At Center TEPS, TEP studies were performed after approximately 48 hours of treatment, which depending on medication used, may not have allowed for 5 half-lives prior to testing. Additionally, aside from asking patients at follow-up appointments and readmission, there was no formal mechanism for evaluating medication compliance, which may be a factor in patient recurrences. This study was retrospective, and therefore our information was limited to what could be extracted from the electronic health record.
Conclusion
This study evaluated the effect that a TEP predischarge strategy had on early hospital readmission and compared clinical effectiveness and cost-effectiveness of a TEP strategy to routine observation for the management of SVT in infants. These data demonstrated that patients who underwent titration of antiarrhythmic medications under the guidance of TEP studies had a significantly lower rate of readmission. However, the routine utilization of TEP studies was also associated with longer hospitalizations and greater costs, even when readmission costs were included. These data may aid physicians in determining if an observation only, routine TEP study utilization, or a hybrid strategy is most appropriate for their centers.
Acknowledgments
This work was conducted with support from the Heart Institute Research Core at Cincinnati Children’s Hospital.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts of interest or disclosures.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Due to the retrospective nature of this study, informed consent from patients was not sought as only nonidentifiable clinical data was collected and analyzed.
Ethics Statement
The study was conducted with permission from local institutional review boards at both participating centers (IRB# 2020-0977 and 1697128-2). All research conducted during this study adhered to the Helsinki Declaration guidelines.
References
- 1.Salerno J.C., Seslar S.P. Supraventricular tachycardia. Arch Pediatr Adolesc Med. 2009;163:268–274. doi: 10.1001/archpediatrics.2008.547. [DOI] [PubMed] [Google Scholar]
- 2.Chu P.Y., Hill K.D., Clark R.H., Smith P.B., Hornik C.P. Treatment of supraventricular tachycardia in infants: analysis of a large multicenter database. Early Hum Dev. 2015;91:345–350. doi: 10.1016/j.earlhumdev.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seslar S.P., Garrison M.M., Larison C., Salerno J.C. A multi-institutional analysis of inpatient treatment for supraventricular tachycardia in newborns and infants. Pediatr Cardiol. 2013;34:408–414. doi: 10.1007/s00246-012-0474-6. [DOI] [PubMed] [Google Scholar]
- 4.Drago F., Silvetti M.S., De Santis A., et al. Paroxysmal reciprocating supraventricular tachycardia in infants: electrophysiologically guided medical treatment and long-term evolution of the re-entry circuit. Europace. 2008;10:629–635. doi: 10.1093/europace/eun069. [DOI] [PubMed] [Google Scholar]
- 5.Michel M., Renaud C., Chiu-Man C., Gross G., Jaeggi E. Postnatal recurrence and transesophageal inducibility of prenatally treated fetal supraventricular tachycardia. Heart Rhythm. 2022;19:1343–1349. doi: 10.1016/j.hrthm.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Neumann P.J., Sanders G.D., Russell L.B., Siegel J.E., Ganiats T.G. 2nd ed. Oxford University Press; New York, NY: 2017. Cost effectiveness in health and medicine. [Google Scholar]
- 7.Tortoriello T.A., Snyder C.S., Smith E.O., Fenrich A.L., Jr., Friedman R.A., Kertesz N.J. Frequency of recurrence among infants with supraventricular tachycardia and comparison of recurrence rates among those with and without preexcitation and among those with and without response to digoxin and/or propranolol therapy. Am J Cardiol. 2003;92:1045–1049. doi: 10.1016/j.amjcard.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Montoyo J.V., Angel J., Valle V., Gausi C. Cardioversion of tachycardias by transesophageal atrial pacing. Am J Cardiol. 1973;32:85–90. doi: 10.1016/s0002-9149(73)80089-x. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher J.J., Smith W.M., Kerr C.R., et al. Esophageal pacing: a diagnostic and therapeutic tool. Circulation. 1982;65:336–341. doi: 10.1161/01.cir.65.2.336. [DOI] [PubMed] [Google Scholar]
- 10.Critelli G., Grassi G., Perticone F., Coltorti F., Monda V., Condorelli M. Transesophageal pacing for prognostic evaluation of preexcitation syndrome and assessment of protective therapy. Am J Cardiol. 1983;51:513–518. doi: 10.1016/s0002-9149(83)80090-3. [DOI] [PubMed] [Google Scholar]
- 11.Benson D.W., Jr., Dunnigan A., Sterba R., Benditt D.G. Atrial pacing from the esophagus in the diagnosis and management of tachycardia and palpitations. J Pediatr. 1983;102:40–46. doi: 10.1016/s0022-3476(83)80283-2. [DOI] [PubMed] [Google Scholar]
- 12.Benson D.W., Jr., Dunnigan A., Benditt D.G., Thompson T.R., Narayan A., Boros S. Prediction of digoxin treatment failure in infants with supraventricular tachycardia: role of transesophageal pacing. Pediatrics. 1985;75:288–293. [PubMed] [Google Scholar]
- 13.Ko J.K., Deal B.J., Strasburger J.F., Benson D.W., Jr. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 14.Blaufox A.D., Warsy I., D'Souza M., Kanter R. Transesophageal electrophysiological evaluation of children with a history of supraventricular tachycardia in infancy. Pediatr Cardiol. 2011;32:1110–1114. doi: 10.1007/s00246-011-9987-7. [DOI] [PubMed] [Google Scholar]
- 15.Brembilla-Perrot B., Groben L., Chometon F., et al. Rapid and low-cost method to prove the nature of no documented tachycardia in children and teenagers without pre-excitation syndrome. Europace. 2009;11:1083–1089. doi: 10.1093/europace/eup093. [DOI] [PubMed] [Google Scholar]
- 16.Naheed Z.J., Strasburger J.F., Deal B.J., Benson D.W., Jr., Gidding S.S. Fetal tachycardia: mechanisms and predictors of hydrops fetalis. J Am Coll Cardiol. 1996;27:1736–1740. doi: 10.1016/0735-1097(96)00054-x. [DOI] [PubMed] [Google Scholar]
- 17.Bonney W.J., Kannankeril P.J., Johns J.A., Fish F.A. Transesophageal pacing as a predictor for arrhythmia recurrence in infants with paroxysmal supraventricular tachycardia. Circulation. 2009;120:S600. [Google Scholar]
- 18.Rhodes L.A., Walsh E.P., Saul J.P. Programmed atrial stimulation via the esophagus for management of supraventricular arrhythmias in infants and children. Am J Cardiol. 1994;74:353–356. doi: 10.1016/0002-9149(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Rey R., Alonso-Tapia J., Colville G. Prediction of parental posttraumatic stress, anxiety and depression after a child's critical hospitalization. J Crit Care. 2018;45:149–155. doi: 10.1016/j.jcrc.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Chang L.V., Shah A.N., Hoefgen E.R., et al. H2O Study Group Lost earnings and nonmedical expenses of pediatric hospitalizations. Pediatrics. 2018;142 doi: 10.1542/peds.2018-0195. [DOI] [PubMed] [Google Scholar]
- 21.Moore J.A., Stephens S.B., Kertesz N.J., et al. Clinical predictors of recurrent supraventricular tachycardia in infancy. J Am Coll Cardiol. 2022;80:1159–1172. doi: 10.1016/j.jacc.2022.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Vari D, Kurek N, Zang H, Anderson JB, Spar DS, Czosek RJ. Outcomes in infants with supraventricular tachycardia: risk factors for readmission, recurrence and ablation. Pediatr Cardiol 2022 Oct 22 https://link.springer.com/article/10.1007/s00246-022-03035-3 [DOI] [PubMed]
- 23.Gilljam T., Jaeggi E., Gow R.M. Neonatal supraventricular tachycardia: outcomes over a 27-year period at a single institution. Acta Paediatr Aug. 2008;97:1035–1039. doi: 10.1111/j.1651-2227.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- 24.Bruder D., Weber R., Gass M., Balmer C., Cavigelli-Brunner A. Antiarrhythmic medication in neonates and infants with supraventricular tachycardia. Pediatr Cardiol. 2022;43:1311–1318. doi: 10.1007/s00246-022-02853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucking C., Michaelis A., Markel F., et al. Evaluation of clinical course and maintenance drug treatment of supraventricular tachycardia in children during the first years of life. A Cohort Study from Eastern Germany. Pediatr Cardiol. 2022;43:332–343. doi: 10.1007/s00246-021-02724-9. [DOI] [PubMed] [Google Scholar]
- 26.Guerrier K., Shamszad P., Czosek R.J., Spar D.S., Knilans T.K., Anderson J.B. Variation in antiarrhythmic management of infants hospitalized with supraventricular tachycardia: a multi-institutional analysis. Pediatr Cardiol. 2016;37:946–952. doi: 10.1007/s00246-016-1375-x. [DOI] [PubMed] [Google Scholar]