Abstract
Antimicrobial resistance (AMR) has become a health, environmental, and economic threat around the globe. It is rising in Ethiopia. This analysis was designed to determine the current status of AMR on major bacterial pathogens, laboratory capacities, surveillance systems, and containment activities in the country. Data were collected from published literature and then supplemented by interviews with ten experts from key stakeholders. Data collections were guided by the AMR Situational Analysis Tool developed by Food Safety Officers at the Food Systems and Food Safety Division of the Food and Agriculture Organization of the United Nations. Published articles indicated the presence of gaps in knowledge, attitude, and practices by health professionals, students, and the community. AMR rates among E. coli, Salmonella, Staphylococci, and Campylobacter isolates ranged from 3.69‐88.41, 4.66–87.74, 17.03–85.08, and 8.41–86.63% to commonly prescribed antimicrobials, respectively. Microbiology laboratories are available. However, a considerable number of laboratories didn't have the basic equipment and consumables. AMR surveillance and reporting system have been established. The national strategic plan has been developed and updated three times. To contain AMR, a governance framework and regulations have been prepared. However, most of them were not fully implemented at all administrative levels. In conclusion, there was a high rate of AMR in the country; some activities have been conducted to prevent and contain AMR. However, more interventions and sustainable activities have to be performed to increase awareness, prevent and contain infectious diseases, rational use antimicrobials and generate more evidence in the country.
Keywords: Antimicrobial resistance, Containment, Ethiopia, Laboratory capacities, Situational analysis, Surveillance systems
Highlights
-
•
There is a high rate of AMR, especially against first-line drugs in the country.
-
•
There is a gap in the knowledge, attitude, and practices among prescribers and users of antimicrobials
-
•
Surveillance activities are more effectively conducted on samples from humans than from animals or the environment
-
•
Laboratories are available with various capacities, some of them lack the necessary items.
-
•
The national action plans, governance structure, and legal frameworks are available; however, they are not well implemented
1. Introduction
The discovery and use of antimicrobials in the 20th century are among the most important advancements in science. Several diseases affecting humans and animals have been controlled. The prevalence or incidence of several infectious diseases has been reduced and increased the success of medical procedures. Antimicrobials saved millions of lives, reduced disease burden, and improved patients' quality of life. Therefore, antimicrobials are extremely valuable resources [1,2]. Despite their importance, they are becoming ineffective in treating diseases due to the development of resistance by microorganisms. AMR occurs when commensal or disease-causing organisms survive upon exposure to a concentration of the drug that would normally kill or inhibit their growth. To some extent, a disease-causing organism can become resistant naturally by mutation, gene exchange, and recombination as a means of adaptation to the environment. However, overuse and misuse of antimicrobial agents have dramatically accelerated the emergence of AMR. Once the resistance capacities are developed, they can spread easily among humans, animals, and the environment [[3], [4], [5]].
AMR is becoming an important public health problem around the globe and several evidences showed that the problem is increasing. O'Neill [4,6] estimated that more than 700,000 people are dying every year. He added that if no action is taken, AMR induces the death of more than 10 million people, costs about 100 trillion United States Dollars (USD), and causes a 2–4% reduction in Gross Domestic Product (GDP) by 2050. Murray et al. [7] also reported that 1.27 million human deaths were associated with bacterial AMR worldwide in 2019.
It is found that patients with infections caused by a drug-resistant organism are at increased hospital stay and at risk of worse clinical outcomes which may extend up to death and cost more than patients infected with non-resistant strains of the same organism [8,9]. In addition to the negative impact of AMR on patients by increased morbidity and mortality, it increases the resource needs at the health institutes and the societal level [10]. Studies have indicated that the influence and the problem are high in low-income countries due to the high prevalence of infection, improper management, irrational uses and over-the-counter availability of antimicrobials, pollutants in the environment, agricultural residues, displacement or migration of people and animals, and absence of sufficient laboratories for isolation and antimicrobial susceptibility testing and regular surveillance [7,11,12].
Three international organizations (World Health Organization (WHO), the Food and Agriculture Organization (FAO), and World Organization for Animal Health (WOAH)) have joined forces to develop a Global Action Plan on AMR [13]. In the action plan, five strategic objectives namely improve awareness and understanding, strengthen the knowledge, and evidence, reduce the incidence of infection, optimize the use of antimicrobials, and sustainable investments to tackle AMR have been designed. Most member countries are using these strategic objectives to prepare their national action plan. To enhance awareness and promote the best practices among the general population, healthcare workers, and policymakers, “World Antimicrobial Awareness Week” has been declared. It is a global campaign that takes place each year in November. The establishment of the Global AMR Surveillance System (GLASS) is also another global initiative by the WHO to integrate surveillance data on antimicrobial use and resistance in humans. The system enables the harmonization of global reporting of national AMR and antimicrobial consumption data. It is working to enhance the data quality, completeness, and representativeness, and provide estimates of the impact of AMR [14]. Hence, since 2016 great attention has been given to AMR at the international level and it is valuable to analyze how countries are handling the issue to identify gaps and strengthen good practices.
Like in any developing country, the prevalence of drug resistance is high and rising in Ethiopia [[15], [16], [17]]. Resistant pathogens are detected in specimens from humans, animals, food, and the environment. Different attempts have been conducted by the government to mitigate AMR. Baseline studies have been conducted, the national strategic plan has been prepared and the country is among 109 countries that report and are enrolled in GLASS [18]. However, the momentum to tackle AMR is fluctuating now and then due to a lack of sustainable resources, and the shift of attention and resources to the COVID-19 pandemic. Additionally, COVID-19 itself increases antimicrobial production and consumption, the frequency of visiting health facilities, secondary infections, and the number of patients in the intensive care units [19]. Hence, assessing the extent of the problem, what has been done, and designing directions for the future are extremely valuable. Hence, this AMR situational analysis was conducted using a new and one health approach to get background information and an overview of the status of AMR on major bacterial pathogens, laboratory capacities, surveillance systems to provide the basis for setting priorities, and develop strategies to mitigate AMR in the country.
2. Methods
2.1. Sources of data
Two data sources had been used. First, data were extracted from published public literature, and then the information was supplemented by interviews of experts (focal persons) who were working in key institutions that have the potential to play roles in the prevention and containment of AMR.
2.2. Literature search
A desk review and literature search had been conducted to assess the status of AMR and laboratory facilities, surveillance, and mitigation activities in Ethiopia. Internet databases like Google Scholar, PubMed, and Science Direct were utilized by using a combination of sets of keywords like “Antimicrobial resistance” OR “antibiotic resistance” AND Ethiopia, “Antimicrobial resistance” OR “antibiotic resistance” OR “Awareness” OR “Practice” AND Ethiopia, “Antimicrobial resistance” OR “antibiotic resistance” AND “Escherichia coli” AND Ethiopia, “Antimicrobial resistance” OR “antibiotic resistance” “Salmonella” AND Ethiopia, “Antimicrobial resistance” OR “antibiotic resistance” “Staphylococcus” AND Ethiopia, “Antimicrobial resistance” OR “antibiotic resistance” “Campylobacter”, AND Ethiopia “Antimicrobial resistance” OR “antibiotic resistance” AND Ethiopia and “Antimicrobial resistance” OR “antibiotic resistance” OR laws, regulations, proclamations, rules, policies AND Ethiopia. The search queries were set based on medical subject headlines (MESH) and Boolean logic to retrieve as many published articles as possible.
To determine AMR status in the country, four major bacterial pathogens (Escherichia coli, Salmonella enterica, Staphylococcus aureus, and Campylobacter species) were selected based on their prevalence and zoonotic potential. Each bacterium was assessed individually.
2.2.1. Inclusion and exclusion criteria
To include in the study, the article should focus on AMR in Ethiopia. Published articles from 2012 to May 2022 were included to get updated information on the issue. Articles that did not focus on AMR, published before 2012, and conducted outside Ethiopia were excluded from the study.
2.2.2. Interviews
In-depth interviews were conducted with ten experts in stakeholders from government institutes, and international agencies. Key stakeholders have been identified based on their potential of having information about AMR and their potential roles in the containment of AMR. AMR experts and/or AMR focal persons in selected institutes were interviewed which lasted for about one and a half hours.
2.3. Data collection and analysis
Data collections were conducted from April 01 to May 31, 2022, and were guided by the AMR Situational Analysis Tool developed by experts in Food Safety Officers at the Food Systems and Food Safety Division of the Food and Agriculture Organization of the United Nations. The tool covered five major topics related to AMR including 1) a country overview of production, demographics, and disease burdens in people and their animals, 2) awareness, 3) practices, 4) evidence, and 5) governance. For data collection, each topic was placed on a separate Excel spread sheet. The data were extracted by two experts and checked by another expert. If there was a difference in the two data, the data were extracted again. After compiling the data in excel, the data were summarized using descriptive statistics and described using figures and tables. STATA version 14 software was used to calculate the pooled prevalences.
3. Results and discussions
3.1. An overview of production, demographics, and disease burdens
Ethiopia is an African country, located in the Eastern part of the continent, with a land area of about 1,127,127 km2. It is bordered on the North-northeast by Eritrea, on the East by Djibouti and Somalia, on the South by Kenya, and on the West and southwest by Sudan and South Sudan. Geographically, the country is located within 3o and 14o.8″ latitude; 33o and 48o longitude. The country has about 112 million people which grows at an annual rate of 2.57%. The life expectancy for Ethiopians is about 66.34 years. Most of the people (about 80%) are living in rural areas [20,21].
Currently, the country has 11 regional states (Tigray, Afar, Amhara, Oromia, Somali, Benishangul gumuz, South Nation Nationalities and People, Gambella, Harari, Sidama, and Southwest regions) and two city administrations (Addis Ababa and Dire Dawa). Each region is divided into zones and then zones into districts (woreda), and the last (smallest) administrative unit is Kebele which may encompass one or more villages.
The country ranks among the top five African countries in terms of livestock population with an estimated 70 million cattle, 43 million sheep, 52.5 million goats, and 8.1 million camels based on the latest Central Statistical Agency (Ethiopia) census [22]. These resources have multiple roles in the livelihood of many Ethiopians, particularly in the rural community. They provide food, inputs for crop production and soil fertility management, income, fuel, social functions and employment and foreign currency. Livestock contributes 15 to 17% of GDP and 35 to 49% of agricultural GDP and 37 to 87% of household incomes [23]. The demand for livestock products is increasing as a result of rapid population growth and rising per capita income [24]. To narrow the gap between demand and production, there is a huge interest to increase animal products. With the interest in boosting production, livestock owners are using different agricultural inputs including antimicrobials for therapeutic and non-therapeutic purposes often inappropriately. This activity may increase the chance of the development and spread of antimicrobial-resistant strains [25].
3.2. Major livestock diseases
Diverse agroclimatic zones, species of animals and minimum control or prevention measures are making the country a home for several livestock diseases. Hence, livestock diseases are among the four problems (nutrition, low productivity of local breeds, diseases, and poor husbandry practices) of livestock production in the country. The common bacterial diseases in the country are anthrax, blackleg, bovine/ovine pasteurellosis, mastitis, tuberculosis, brucellosis, contagious bovine pleuropneumonia, and contagious caprine pleuropneumonia [26]. Food and mouth disease, peste des petits ruminants, rabies, sheep and goatpox, camelpox, lumpy skin disease and orf are some of the common viral diseases [26]. Trypanosomiasis, liver fluke (fascioliasis), hydatidosis, lungworms, cysticercosis, toxoplasmosis, gastrointestinal nematodiasis, ticks and mange mites are common parasitic problems in the country [[26], [27], [28], [29]].
These diseases induce high mortality and reduce the productivity of animals, most of them are creating risks to public health and reducing domestic and international marketability [27]. The annual loss due to mortality ranges from 8 to 10% for cattle, 12–14% for sheep, 11–13% for goats and 56.9% for poultry. These figures are much higher for calves, lambs and kids [30]. The direct and indirect losses from livestock disease have significant economic, food security and livelihood impacts on livestock keepers and the national economy [31].
3.3. Human diseases
Several infectious and non-infectious diseases of humans are prevalent in the country. Infectious diseases like lower respiratory infections, diarrheal diseases, tuberculosis, HIV/AIDS and meningitis are among the top ten causes of death [32]. Almost 75% of the land is malarious and 68% of the population lives in areas at risk of malaria. Neglected tropical diseases like trachoma, schistosomiasis, anthrax, leishmaniasis and brucellosis [33,34] are prevalent. Diarrheal diseases due to rotavirus and enteric bacterial pathogens are also common in the country [35,36]. It has been also found that the country has the second-highest burden of zoonotic diseases in Africa [37]. Foodborne or waterborne diseases caused by Salmonella enterica, Shigella, Staphylococcus aureus, Campylobacter, Listeria and E. coli are also common in the country [38,39].
3.4. Awareness and practices related to antimicrobial use and resistance
Twenty published articles were found in the searched databases that assess the knowledge of healthcare professionals, livestock owners, patients, communities, and students. Of these articles, 90.00% of them reported the presence of a knowledge gap among these study participants. Seventeen articles assessed the attitude and 88.23% reported the presence of the gap. Seventeen articles assessed the practices and 94.12% of them reported malpractices (Table 1). All studies were cross-sectional and used questionnaires except one study which used retrospective data (recorded sheets in health facilities) to extract the data.
Table 1.
The number of published articles Assessing Knowledge, Attitude and Practice of different study populations regarding antimicrobial use and resistance.
| Targeted Study population | Knowledge |
Attitude |
Practice |
|||
|---|---|---|---|---|---|---|
| Number of studies assessing | Reporting Gap (n (%) | Number of studies assessing | Reporting Gap (n (%) | Number of studies assessing | Reporting Malpractices (n (%) | |
| Healthcare professionals | 6 | 4 (66.67) | 5 | 4 (80.00) | 3 | 2 (66.67) |
| Livestock owners | 3 | 3 (100.00) | 3 | 3 (100.00) | 3 | 3 (100.00) |
| Patients | 2 | 2 (100.00) | 1 | 1 (100.00) | 3 | 3 (100.00) |
| Communities | 4 | 4 (100.00) | 3 | 3 (100.00) | 6 | 6 (100.00) |
| Students | 5 | 5 (100.00) | 5 | 4 (80.00) | 2 | 2 (100.00) |
| Overall | 20 | 18 (90.00) | 17 | 15 (88.23) | 17 | 16 (94.12) |
N = number; % = percent.
3.5. Evidence of antimicrobial resistance
3.5.1. Escherichia coli
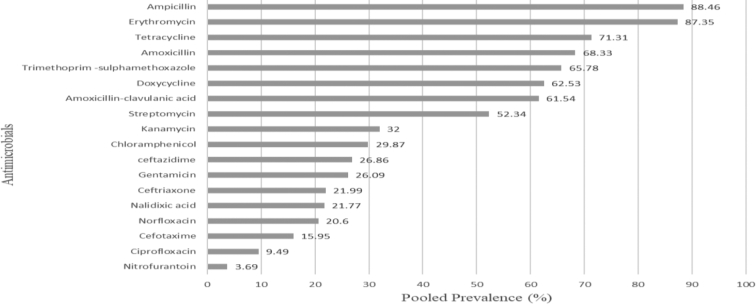
To determine the status of AMR of E. coli, 37 published articles were summarized. The majority (70.27%) of the studies were cross-sectional and on clinical specimens (64.86%). The reported prevalence ranged from 0.93 to 61.82% with a pooled prevalence of 10.98%. Antibiotic resistance was assessed on 18 commonly prescribed antimicrobial agents for the treatment of E. coli infection. E. coli isolates were highly resistant to ampicillin (88.46%), erythromycin (87.35%), and tetracycline (71.31%) (Fig. 1). A high prevalence of resistance in E. coli isolates was also reported by Alemu et al. [17] and Tuem et al. [40]. Other reports also indicated the highest percentage of resistance of E. coli isolates to antimicrobials like ampicillin and tetracycline [35].
Fig. 1.
Antimicrobial resistance patterns of E. coli isolates.
3.5.2. Salmonella enterica
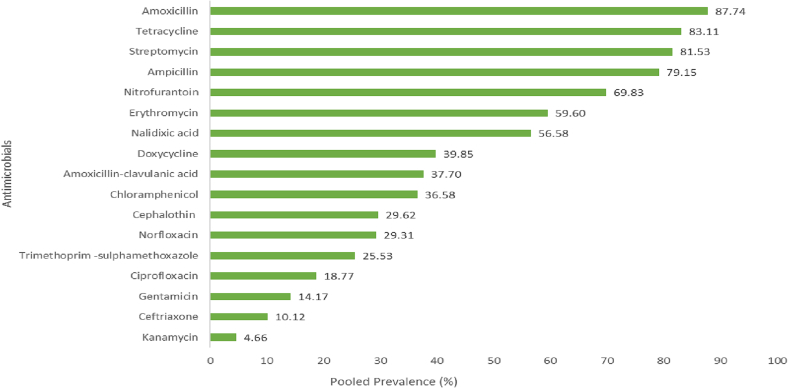
To determine the prevalence of AMR in S. enterica, 34 published articles were summarized. All studies were cross-sectional. The reported prevalence of the bacterium ranged from 1.26 to 24.3% with a pooled prevalence of 4.66%. Antibiotic resistance was assessed on 17 commonly used antimicrobials for the treatment of S. enterica infection. S. enterica isolates were highly resistant to amoxicillin (87.74%), tetracycline (83.11%) and streptomycin (81.53%) (Fig. 2). These percentages were in line with previous reports by Beyene et al. [35].
Fig. 2.
Antimicrobial resistance patterns of Salmonella enterica isolates.
3.5.3. Staphylococcus aureus
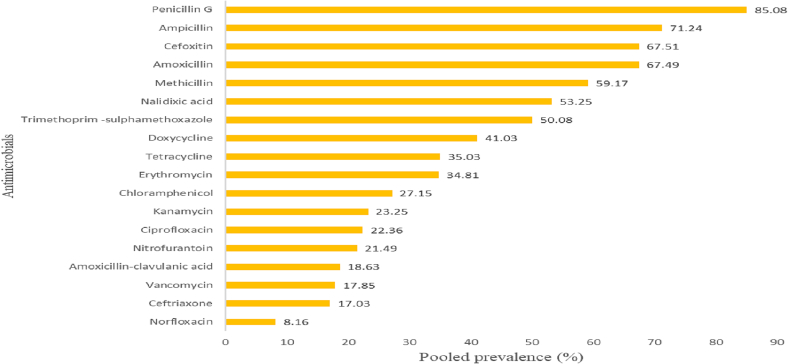
A total of 35 published articles were assessed to analyze the AMR patterns of S. aureus in the country. The reported prevalence ranged from 1.63 to 72.86% with 8.16% pooled prevalence. Published articles are indicating that S. aureus isolates were highly resistant to penicillin G (85.08%) and ampicillin (71.24%) (Fig. 3). In line with these results, Deyno et al [42] reported that S. aureus isolates were resistant to amoxicillin (77%), penicillin (76%), ampicillin (75%), tetracycline (62%), methicillin (47%), Trimethoprim-sulphamethoxazole (47%), doxycycline (43%), and erythromycin (41%) [41]. A high prevalence of resistant Staphylococcus species was also reported by Alemu et al. [17].
Fig. 3.
Antimicrobial resistance patterns of Staphylococcus aureus isolates.
3.5.4. Campylobacter species
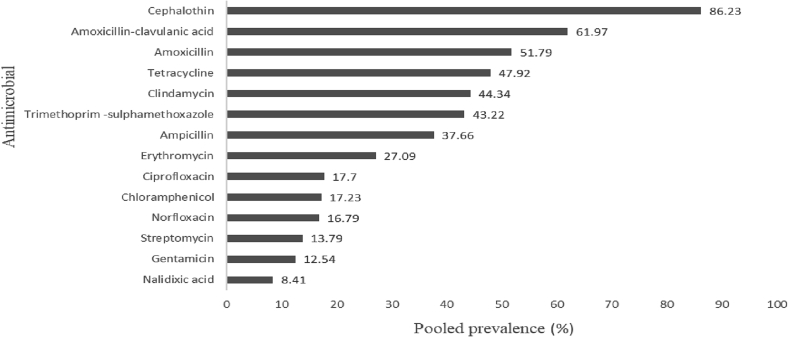
During our literature search, we got 12 articles that fulfill the inclusion criteria. The reported prevalence ranged from 6.05 to 54.62%, with a pooled prevalence of 13.67% in different sources of samples. The AMR status of the bacterium was assessed on 14 commonly used antimicrobial agents. A high prevalence of resistance was reported on drugs like cephalothin (86.23%), amoxicillin-clavulanic acid (61.97%) and amoxicillin (51.79%) (Fig. 4). These reports were in agreement with the resistant pattern of Campylobacter reported by Beyene et al. [35] among diarrheic patients. An increased proportion of antimicrobial-resistant isolates of Campylobacter species was also reported by Hlashwayo et al. [42] in sub-Saharan countries including Ethiopia.
Fig. 4.
Antimicrobial resistance patterns of Campylobacter species.
3.5.5. Multidrug resistance and special groups of resistant bacteria
The problem of drug resistance is exacerbated by some groups of organisms which are resistant to more than two groups of antimicrobial agents (multidrug resistance). Infections with multidrug-resistant bacteria are hard to treat since a few or even no treatment options remain. In some cases, healthcare providers must use antimicrobials that are more toxic and costly for the patient. Multidrug resistance facilitates the spread of AMR and complicates efforts to reduce resistance [43]. The prevalence of multidrug resistance on common pathogens is indicated in Table 2.
Table 2.
Prevalence of multidrug resistance on common pathogens.
Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-resistant Enterococci (VRE), carbapenemase-producing bacteria (CPB), AmpC β-lactamase-producing bacteria, extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-PE), multi-drug-resistant tuberculosis (MDR-TB) and multidrug-resistance Salmonella (MDRS) are special groups of bacteria which tend to be resistant to several groups of antimicrobial agents. They are grouped based on the type of bacteria, antimicrobial agent and mechanism of resistance. Most of these are listed as critical or high-priority pathogens by WHO [44]. Table 3 shows the prevalence of such groups of bacteria or resistance forms in Ethiopia.
Table 3.
Prevalence of special groups of bacteria or resistance forms in Ethiopia.
| S/n | Bacterial group/resistance form | Pooled prevalence (%) | Remark | Reference |
|---|---|---|---|---|
| 1. | Methicillin-resistant Staphylococcus aureus (MRSA) | 32.5 | [47] | |
| 30.9 | Nasal colonization | [48] | ||
| 40 | [49] | |||
| 2. | Vancomycin-resistant Staphylococcus aureus (VRSA) | 11 | [42] | |
| 3. | Vancomycin-resistant Enterococci (VRE) | 14.8 | [50] | |
| 4. | Carbapenemase-producing bacteria (CPB) | 2.4 | Food handlers (single study) | [51] |
| 5. | Extended-Spectrum β-lactamase Producing Enterobacteriaceae (ESBL-PE) | 18 | [52] | |
| 50 | ESBL-producing Gram-negative bacteria | [53] | ||
| 49 | Clinical samples | [54] |
3.6. Laboratory capacities
Different levels of laboratories with the capacity of isolating and conducting antimicrobial susceptibility tests are available in the country. They can be grouped as federal or regional, based on their location and mandate. Federal laboratories are found in or around the capital city (Addis Ababa) and relatively, they are better equipped than regional laboratories.
Four laboratories that were focused on primarily animal pathogens were assessed by a tool produced by FAO (Assessment Tool for Laboratories and AMR Surveillance Systems (ATLASS)) (https://www.fao.org/antimicrobial-resistance/resources/tools/fao-atlass/es/). The report revealed that the laboratories have different levels of capacities for isolating and conducting antimicrobial susceptibility tests. However, most of the laboratories didn't have the basic equipment needed for bacterial isolation (incubators, autoclaves, safety cabinets, fridges, and others), and were suffering from a lack of consumables (media, antimicrobial disks, sanitary equipment, personal protective equipment, and others) and lacking trained personnel. Except for some federal laboratories, all of them didn't have the facilities and abilities to run molecular procedures for the detection of AMR. To reduce the problems and improve the capacities of laboratories, the country has prepared strategic interventions like establishing a laboratory quality management system involving proficiency in testing and third-party accreditation, developing a laboratory information management system for federal and regional laboratories, collecting and stockpiling isolates of important pathogens for genetic sequencing and vaccine production [31].
3.7. Antimicrobial resistance surveillance and reporting system
AMR surveillance and reporting systems have been established in the country. The activities which are focused primarily on human pathogens are coordinated by Ethiopian Public Health Institute (EPHI) whereas the National Animal Health Diagnostic and Investigation Centre (NAHDIC) recently upgraded as Ethiopian Animal Health Institute |(EAHI) is the lead institute for pathogens from samples of animal origin. Sentinel sites (16 for humans) have been selected and priority pathogens for surveillance have been selected. The type of specimens to be collected (blood and urine) and standard operation procedures (SOPs) to follow had been determined. Capacity-building activities have been conducted on selected sites and a system to report and monitor the status has been established [45]. EPHI receives reports monthly from each site and provides feedback. Once a year, the institute reports the results to the National AMR Steering Committee (NASC) and GLASS.
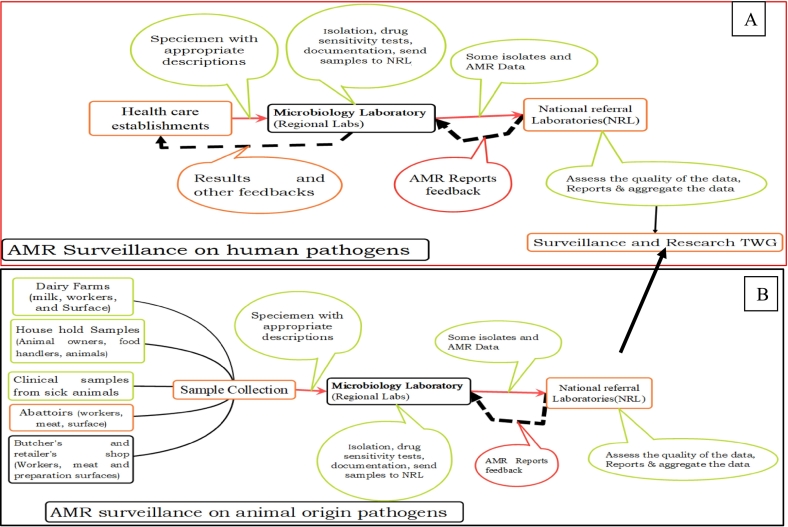
An assessment had been conducted on the laboratories that handle animal pathogens whether they have the potential to isolate bacteria and conduct AST, capacity building activities conducted; type of specimens (milk, abattoir, and cloacal swabs) and target pathogens (Escherichia coli, Salmonella enterica and Staphylococcus aureus) have been identified and standard operating procedures (SOPs) prepared. A document on integrated national AMR and residue surveillance plans in animal health, plant, food safety, and environment sectors of Ethiopia was prepared and implemented since 2019. FAO-Ethiopia [46] assessed the national AMR surveillance system in the food and agriculture sectors and reported the commencement of the system and the presence of enabling situations. We didn't get published or unpublished data which were generated primarily from surveillance AMR from samples of animal origin. Fig. 5 depicts AMR surveillance structures on human and animal potential pathogens.
Fig. 5.
Antimicrobial resistance (AMR) surveillance structures on human (A) and animal (B) pathogens (The structure for animal pathogens (B) is not fully functional, NRL = National Referral Laboratories); (TWG = Technical Working Group).
Harant [47] assessed the transparency and accountability of national action plans (NAP) on AMR in 15 African countries and found that Ethiopia has publicly accessible the NAP, surveillance data, and responsible bodies. However, progress reports and responsible person(s) per sector were partly available and there was no sufficient fund allocated for the activities related to AMR. Ethiopia is also among the countries in the world that are reporting AMR to the Global AMR and Use surveillance system (GLASS) [18]. Documents or published articles are available on activities conducted primarily on human pathogens [45].
Even though the surveillance and reporting system has been commenced, the activities have been challenged by the absence of sustainable investment, the absence of a budget to cover more sites in the country, and some laboratories lack the capacity to isolate pathogens and conduct an antimicrobial susceptability test, the absence of a continuous and sustainable supply of consumable items [45].
3.8. Antimicrobial resistance prevention and containment activities
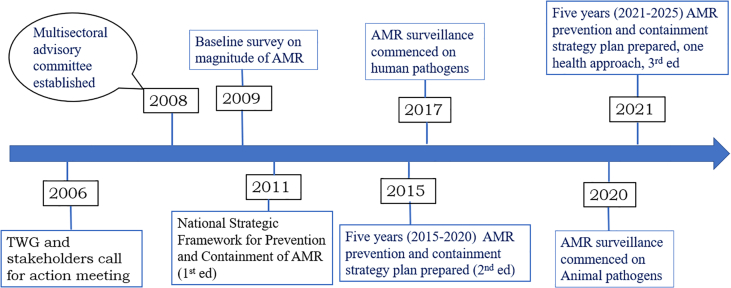
To reduce the impact, emergence and spread of AMR, several activities have been conducted in the country. In 2009, baseline studies on the magnitude of AMR were conducted. The survey found that most bacteria commonly involved in causing infections in humans and animals were showing a considerable degree of resistance to commonly used first-line antibacterial agents [48]. Following that survey, several awareness-creation activities were conducted by dissemination and press releases using local mass media and languages. A multi-institutional and multi-disciplinary AMR containment advisory committee has been established in 2008. The first national strategic framework for the prevention and containment of AMR was prepared in 2011. In 2015, a five years (2015–2020) AMR prevention and containment strategy plan was updated in line with the Global Action Plan (GAP) for the prevention and containment of AMR [13]. The national AMR containment strategy plan has been revised in 2021 by following a One Health approach which will be used as a guide till 2025. This strategic plan has been approved and co-signed by the Ministry of Health, Ministry of Agriculture, and Commission for Environment, Forest, and Climate Change. The plan has 5 strategic objectives, 22 initiatives, 66 interventions, and 180 operational plans [49]. The chronological sequences of the key events for the prevention and containment of AMR in Ethiopia are indicated in Fig. 6.
Fig. 6.
The chronological sequence of the key activities for prevention and containment of antimicrobial resistance (AMR) in Ethiopia, (TWG = Technical Working Group)
3.8.1. Governance and legislation
The country-level governance framework for the prevention and containment of AMR in the country is governed by the national inter-ministerial committee which has a secretariat and steering committee. The steering committee leads and receives information from technical working groups (TWG). The TWGs are divided into six primary tasks and responsible ministries and institutes were assigned to each task.
The presence of appropriate and strong rules, regulations and policies ensures the manufacture, trade, and use of antimicrobials, and protects and promotes animal health, animal production, the environment and public health at large. To perform core regulatory functions efficiently, the regulatory bodies require having adequate regulatory tools, appropriate organizational structure, adequately trained human resources, sustainable finance, adequate awareness of the community and effective cooperation between different regulatory bodies and with other law enforcing bodies. Tools for regulatory activities (policies, rules, and legislation) should be available and implemented both at the federal and regional levels [50].
Policies, rules, and regulations are available regarding antimicrobial use and resistance in the country. One of the prominent documents is proclamation number 728/2011. The title of this proclamation is ‘The Veterinary Drug and Feed Administration and Control Proclamation. It is a proclamation that was prepared to control and administer veterinary drugs and feed. It deals with antimicrobial quality standards, registration, packaging and labeling, prescription, distribution, and disposal of veterinary pharmaceuticals. The proclamation enabled the establishment of a regulatory authority that is responsible to control and assure the quality, safety and efficacy of veterinary drugs [51]. Proclamation number 661/2009 is another proclamation entitled Food, Medicine and Health Care Administration and Control Proclamation which was produced for the administration and control of food, drugs and health care providers of humans [52]. Proclamation number 267/2002 is a proclamation about animal disease prevention and control which was produced to control and prevention of animal diseases [53].
Standard Veterinary Treatment Guidelines for Ethiopia is a job aid prepared for the appropriate treatment of animal-related problems and diseases in the country. The first edition was prepared in 2006 and has been revised in 2020 and is currently available both in hard and soft copies [54]. A veterinary drug list for Ethiopia has also been prepared [55]. This document shows the name and composition of veterinary drugs that can be manufactured, imported, distributed, and used in the country.
There are also guidelines prepared to be used by professionals in human medicine; the most prominent ones are guidelines for the drug administration and control authority of Ethiopia, standard treatment guidelines for general hospitals, a practical guide to antimicrobial stewardship programs in Ethiopian hospitals, pharmaceutical products traceability master data guideline, a risk-based guideline for post-marketing quality surveillance of medicines in Ethiopia, and guideline for registration of low-risk medicines [56,57].
Hence, several regulatory and other documents are available in the country for human and animal health sectors. However, the prominent limitations are on their full implementation. Delayed registration time, negligence in the certification and inspection of drug distribution channels, unavailability of traditional medicine registration platforms, unaccredited quality control tests, and poor monitoring of drug residues in food of animal origin are the major limitations in the implementation of regulatory functions [50].
Some legislations were prepared at the federal level, but due to the autonomy of regions, they didn't implement all, hence they either adopt the federal one or prepare their own. This problem was highlighted by Zeru [50] who recommended the harmonization of the regulatory activities and improvement of the level of communication and cooperation between the federal and regional regulatory bodies. He also emphasized the importance of awareness creation programs in the legislation and regulatory activities to pull all stakeholders to work together for better implementations.
3.8.2. Advocacy and awareness raising
Using the governance framework, awareness-creation activities were conducted. Yearly, World Antimicrobial Awareness Week (WAAW) has been celebrated in the presence of higher officials from the Ministry of Health, Agriculture, and the Environment. Several capacity-building activities in terms of human and material supplies have been conducted in the country. Trainings have been given to professionals and workshops were also organized. Infection prevention and control activities, antimicrobial stewardship programs, waste management and legal frameworks were among the key issues during the activities. However, these activities cover mainly central areas and rarely reach remote and rural areas. Additionally, their impact has not been assessed whether they are bringing a tangible change or not.
Occasional awareness-enhancing activities have also been delivered for veterinary professionals and paraprofessionals. For example, from 27 to 28 November 2019, training on antimicrobial use and resistance had been given to animal health professionals by the Ethiopian Veterinary Drug and Feed Administration (VDFACA) currently upgraded to Ethiopian Agricultural Authority, the United Kingdom's Veterinary Medicines Directorate, and the Food and Agriculture Organization of the United Nations (FAO). Such types of trainings have also been delivered at different sites aiming to cover wider areas in the country (https://www.fao.org/ethiopia/news/detail-events/ar/c/1253964/). Here and there trainings and workshops have also been given or organized by other organizations like universities or regional offices. However, such trainings have limited capacity to include all health professionals, and livestock producers. Hence, wide awareness gaps and malpractices have been reported in the country among students, professionals and livestock owners [[58], [59], [60], [61]].
3.8.3. Infection prevention and control activities
Infection prevention and control activities are conducted by private and public (government) veterinary clinics for diseases of animals. More prevention activities like mass vaccination are primarily conducted by public services. The ministry of agriculture has branches that usually extends from the federal to the lowest administrative units (kebeles), and professionals with variable level of education and training have been assigned to each establishment [62].
Vaccination is the most dominant disease control and prevention activity in the country. National Veterinary Institute (NVI) is a government-owned institute that develops and produces almost all vaccines for animal in the country. The institute is producing more than 8 bacterial and 15 viral vaccines against common animal diseases [63].
Both private and governmental institutes are also playing a role in the control and prevention of human diseases. By implementing health policy and preventive strategies, there have been improvements in the country's health parameters like life expectancy, neonatal, infant, under-five and maternal mortalities. Despite these signs of progress, the country is still facing a high burden of diseases and has been unable to achieve universal health coverage [64].
3.8.4. Antimicrobial stewardship programs
The antimicrobial stewardship program includes a set of coherent actions aimed at promoting responsible antimicrobial use in humans and animals. It is a means to enhance the knowledge, attitude, and practice of professionals and the community regarding AMR and use. It helps to use antimicrobials rationally and take measures that can reduce the emergence and spread of AMR [65]. In the country, 60 human health establishments started the program, however, most of them are not active and didn't fulfill international standards. To solve the problem, three hospitals were benchmarked and used as a model to share their experiences with other health service providers by the Ministry of Health. We didn't come across such an initiative in animal health establishments.
3.8.5. Waste management
Appropriate waste management is helpful for disease control and prevention and protects the environment from antimicrobial pollution which is critical to combat the rising levels of AMR. There are rules and guidelines regarding waste management (waste from the farm, industries, and healthcare facilities). Examples: Proclamation number 513/2007 has been prepared regarding the management of solid waste. Proclamation number 1090/2018 is also a proclamation regarding hazardous waste management. Some health service providers post and attempt to practice the five key waste management practices (minimization, segregation, collection, temporary storage, transportation, and disposal by incineration). However, the general sanitary status of the country is very low [66] and the proportion of the population getting safe portable water is relatively low [67]. It is estimated that only 42% of the population is using basic drinking water and only 11% of the population are getting sanitary services [68].
4. Limitations of the study
The study didn't have serious limitations. However, the pooling of articles having a different method for the detection of AMR resistance may not be always precise.
5. Conclusions
Antimicrobials are very precious resources for the prevention and control of infectious diseases. Maintaining their efficacy must be among the top priority of activities in human, agriculture and environmental sectors. The development of resistance by disease-causing organisms is the main threat that affects the effectiveness of antimicrobials. It is accelerated by the misuse and overuse of antimicrobials. High rates of AMR have been reported on pathogens like E. coli, S. enterica. S. aureus and Campylobacter species.
Laboratories are available in the country for isolation of the common disease-causing agents and conducting antimicrobial susceptibility tests. However, most of them are less equipped and lack well-qualified personnel and almost all have a problem of getting consumable items for laboratory activities.
AMR surveillance activities have been commenced; sites, types of specimens and target bacterial pathogens have been identified. Capacity-building activities in terms of boosting the skill of laboratory workers and the availability of items have been conducted. More efficiently conducted for human samples. The surveillance activities are at an early stage for samples from animals. It hasn't been conducted for environmental samples for surveillance purposes.
Different activities have been conducted to prevent and contain AMR in the country by preparing the NAP. Attempts were made to generate evidence, create awareness, reduce the burden of diseases, optimize the use of antimicrobials, and establish governance frameworks. However, a formal assessment hasn't been conducted to see their impacts on minimizing the emergence and spread of AMR.
Therefore, to reduce the emergence and spread of AMR, more evidence must be generated by strengthening the AMR surveillance system in all sectors (human, animal, and environment), and more awareness creation, prevention, and containment of infectious diseases, rational use of antimicrobials must be conducted.
Author contributions
AMB, JPF, TA, and JL play a role in the conceptualization and formulation of the data accusation methods. GGD and GM collect data from published literatures. AMB conducted the interview, analyzed the data, and prepared the draft manuscript. JPF, TA, GGD, and GM review and edit the draft manuscript.
Funding
This work was partly funded by MARS.
Declaration of Competing Interest
The authors would like to declare the absence of any conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100527.
Contributor Information
Achenef Melaku Beyene, Email: achenefmela@yahoo.com, tbeyene11@mail.com.
Tenaw Andualem, Email: tenaw.tadege@fao.org.
Jeffrey LeJeune, Email: jeffrey.lejeune@fao.org.
Jorge Pinto Ferreira, Email: jorge.pintoferreira@fao.org.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Dixon J., Duncan C.J.A. Importance of antimicrobial stewardship to the English National Health Service, A Review. Infect. Drug Resist. 2014;7:145–152. doi: 10.2147/IDR.S39185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kardos N., Demain A.L. Penicillin: the medicine with the greatest impact on therapeutic outcomes. Appl. Microbiol. Biotechnol. 2011;92:677–687. doi: 10.1007/s00253-011-3587-6. [DOI] [PubMed] [Google Scholar]
- 3.OIE . 2016. World Organization for Animal Health. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. 12, rue de Prony, 75017 Paris, France. [Google Scholar]
- 4.O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations The Review on Antimicrobial Resistance. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf Available at:
- 5.Prestinaci F., Pezzotti P., A. Pantosti antimicrobial resistance: a global multifaceted phenomenon Pathog. Glob. Health. 2016;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations 2014. https://wellcomecollection.org/works/rdpck35v/items Available at:
- 7.Christopher J.L., Murray K.S., Ikuta F., Sharara L., Swetschinski, Aguilar G.R., Gray A., Han C., Bisignano C., et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard D.H., Scott R.D., Packard R., Jones D. Vol. 36. 2003. The Global Impact of Drug Resistance CID; pp. S4–S10. s. [DOI] [PubMed] [Google Scholar]
- 9.Serra-burriel M., Keys M., Campillo-Artero C., Agodi A., Barchitta M., Gikas A., et al. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227139. s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman N.D., Temkin E., Carmeli Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016;22:416–422. doi: 10.1016/j.cmi.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Okeke I.N., Klugman K.P., Bhutta Z.A., Duse A.G., Jenkins P., O’Brien T.F., Pablos-Mendez A., Laxminarayan R. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect. Dis. 2005;5:568–580. doi: 10.1016/S1473-3099(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 12.Pokharel S., Raut S., Adhikari B. Tackling antimicrobial resistance in low-income and middleincome countries. BMJ Glob. Health. 2019;4:4–6. doi: 10.1136/bmjgh-2019–002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Global action plan on antimicrobial resistance. 2015. https://apps.who.int/iris/rest/bitstreams/864486/retrieve 978 92 4 150976 3. Avaialble at.
- 14.Velazquez-meza M.E., Galarde-lópez M., Carrillo-Quiróz B., Alpuche-Aranda C.M., et al. Vet. World. 2022;15:743–749. doi: 10.14202/vetworld.2022.743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moges F., Endris M., Mulu A., Tessema B., Belyhun Y., Shiferaw Y., Huruy K., Unakal C., A. Kassu the growing challenges of antibacterial drug resistance in Ethiopia. J. Glob. Antimicrob. Resist. 2014;2(3):148–154. doi: 10.1016/j.jgar.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Muhie O.A. Antibiotic use and resistance pattern in Ethiopia: systematic review and meta-analysis. Int.J. Microbiol. 2019 doi: 10.1155/2019/2489063. 2489063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemu B., Assefa A., Bedasa M., Amenu K., Wieleand B. Antimicrobial resistance in Ethiopia: A systematic review and meta-analysis of prevalence in foods, food handlers, animals, and the environment. One Heal. 2021;13 doi: 10.1016/j.onehlt.2021.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Global antimicrobial resistance and use surveillance system (GLASS) report 2021 Geneva, 978-92-4-002733-6. 2021. https://apps.who.int/iris/rest/bitstreams/1350455/retrieve Available at:
- 19.Seethalakshmi P.S., et al. Science of the total environment delineating the impact of COVID-19 on antimicrobial resistance : An Indian perspective. Sci. Total Environ. 2022;818 doi: 10.1016/j.scitotenv.2021.151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CSA, Central Statistical Agency Ethiopia Population Size by Sex, Region, Zone, and Wereda. 2021. https://www.statsethiopia.gov.et/population-projection/ Available at:
- 21.EEA, Ethiopian Economics Association Economic Development, Population Dynamics, and Welfare. 2021. https://www.africaportal.org/publications/state-ethiopian-economy-202021-economic-development-population-dynamics-and-welfare/ Available at:
- 22.CSA Central Statistical Agency Agricultural sample survey 2020/21. volume ii, report on livestock and livestock characteristics. Stat. Bull. 2021;589 https://www.statsethiopia.gov.et/population-projection/ Addis Ababa, Ethiopia. Available at: [Google Scholar]
- 23.Endalew B., Ayalew Z. Assessment of the role of livestock in Ethiopia: A review. Am. J. Sci. Res. 2016;11(5):405–410. [Google Scholar]
- 24.Shapiro B., Gebru G., Desta S., Negassa A., Negussie K., Aboset G., Mechal H. International Livestock Research Institute (ILRI); Addis Ababa, Ethiopia: 2015. Ethiopia Livestock Master Plan Roadmaps for Growth and Transformation: A Contribution to the Growth and Transformation Plan II (2015–2020) 92–9146–425–2. [Google Scholar]
- 25.Alemu B., Lemma M., Magnusson U., Wieland B., Mekonnen M., Mulema A. 2019. Community Conversations on Antimicrobial Use and Resistance in Livestock International Livestock Research Institute (ILRI) Addis Ababa, Ethiopia. [Google Scholar]
- 26.Seyoum B., E. Teshome major transboundary disease of ruminants and their economic effect in Ethiopia. Glob. J. Med. Res. 2017;17(2):27–36. 2249-4618. [Google Scholar]
- 27.Birhanu T. Prevalence of the major infectious animal diseases affecting livestock trade industry in Ethiopia. J. Biol. Agric. Healthc. 2014;4:2014. 2225-093X. [Google Scholar]
- 28.Bekele A., Alemu D., Teklewold T., Moore H.L., Hodge C., Berg S. Strategies for animal disease control in Ethiopia: A review of policies, regulations, and actors. J. Toxicol. Environ. Heal. Sci. Rev. 2018;10:256–265. doi: 10.5897/JVMAH2018.0711. [DOI] [Google Scholar]
- 29.Pieracci E.G., Hall A.J., Gharpure R., Haile A., Walelign E., Deressa A., Bahiru G., Kibebe M., Walke H., Belay E. Prioritizing zoonotic diseases in Ethiopia using a one-health approach. One Heal. 2016;2:131–135. doi: 10.1016/j.onehlt.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fentie T., Guta S., Mekonen G., Temesgen W., Melaku A., Asefa G., Tesfaye S. Assessment of major causes of calf mortality in urban and periurban dairy production system of Ethiopia vet. Med. Int. 2020:1–7. doi: 10.1155/2020/3075429. 3075429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MoA (Ministry of Agriculture, Ethiopia) and ILRI (International Livestock Research Institute) Animal Health Strategy and Vision for Ethiopia, Addis Ababa, Ethiopia. 2013. 92–9146–408–2. [Google Scholar]
- 32.CDC, Center for Disease Control and Prevention CDC in Ethiopia, Factsheet. 2021. www.cdc.gov/globalhealth/countries/ethiopia
- 33.Mehari S., Zerfu B., Desta K. Prevalence and risk factors of human brucellosis and malaria among patients with fever in malaria-endemic areas, attending health institutes in Awra and Gulina district, Afar, Ethiopia. BMC Infect. Dis. 2021;21:942. doi: 10.1186/s12879-021-06654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahiru G., Bekele A., Seraw B., Boulanger L., Ali A. Human and animal anthrax in Ethiopia : A retrospective record review 2009-2013. Ethiop. Vet. J. 2016;20(2):75–85. [Google Scholar]
- 35.Beyene A., Gezachew M., Mengesha D., Yousef A., Gelaw B. Prevalence and drug resistance patterns of Gram-negative enteric bacterial pathogens from diarrheic patients in Ethiopia: A systematic review and meta-analysis. PLoS One. 2022;17 doi: 10.1371/journal.pone.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damtie D., Melku M., Tessema B., Vlasova A.N. Prevalence and genetic diversity of Rotaviruses. Viruses Rev. 2020;12:62. doi: 10.3390/v12010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grace D., Mutua F., Ochungo P., Kruska R., Jones K. Mapping of Poverty and Likely Zoonoses Hotspots. 2012. https://cgspace.cgiar.org/bitstream/10568/21161/4/ZooMap_July2012_final.pdf Available at:
- 38.Ayana Z., Yohannis M., Abera Z. Food-Borne bacterial diseases in Ethiopia. Acad. J. Nutr. 2015;4:62–76. [Google Scholar]
- 39.Hussen S., Mulatu G., Kassa Z.Y. Prevalence of Shigella species and its drug resistance pattern in Ethiopia: a systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2019;18:22. doi: 10.1186/s12941-019-0321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuem K.B., Gebre A.K., Atey T.M., Bitew H., Yimer E.M., D. F. Berhe drug resistance patterns of Escherichia coli in Ethiopia: a meta-analysis. Biomed. Res. Int. 2018 doi: 10.1155/2018/4536905. 4536905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deyno S., Fekadu S., Astatkie A. Resistance of Staphylococcus aureus to antimicrobial agents in Ethiopia: a meta-analysis. Antimicrob. Resist. Infect. Control. 2017;6:85. doi: 10.1186/s13756-017-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hlashwayo D.F., Sigau’que B., Noormahomed E.V., Afonso S.M.S., Mandomando I.M., Bila C.G. A systematic review and meta-analysis revealed that Campylobacter spp and antibiotic resistance are widespread in humans in sub-Saharan Africa. PLoS One. 2021;16 doi: 10.1371/journal.pone.024. e0245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wartu J.R., Butt A.Q., Suleiman U., Adeke M., Tayaza F.B., Musa B.J., J. Baba multidrug resistance by microorganisms: a review. Sci. World J. 2019;14(4):49–56. [Google Scholar]
- 44.WHO (World Health Organization) Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2019. https://www.quotidianosanita.it/allegati/allegato4135670.pdf Available at:
- 45.Ibrahim R.A., Teshal A.M., Dinku S.F., Abera N.A., Negeri A.A., Desta F.G., Seyum E.T., Gemeda A.W., Keficho W.M. Antimicrobial resistance surveillance in Ethiopia: Implementation experiences and lessons learned Prioritising antimicrobial resistance in Ethiopia. Afr. J. Lab. Med. 2018;7:a770. doi: 10.4102/ajlm.v7i2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FAOE (Food and Agricultural Organization, Ethiopia) 30 July-3 August 2018. Assessment of the National Antimicrobial Resistance Surveillance System in Food and Agriculture Sectors: Towards Global Surveillance of Antimicrobial Resistance, Mission Report. [Google Scholar]
- 47.Harant A. Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob. Resist. Infect. Control. 2022;11:1–15. doi: 10.1186/s13756-021-01040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DACA (Drug Administration and Control Authority, Ethiopia) Antimicrobial Use, Resistance and Containment Baseline Survey Syntheses of Findings. 2009. https://www.medbox.org/pdf/5e148832db60a2044c2d3892 Available at:
- 49.MOH, Ministry of Health . In: Ethiopia Antimicrobial Resistance Prevention and Containment Strategic Plan: The One Health Approach 2021–2025. 3rd ed., editor. 2021. [Google Scholar]
- 50.Zeru H. College of Health Science, Addis Ababa University; Ethiopia: 2020. Assessment of Veterinary Drugs Regulatory Framework in Ethiopia MSc Thesis, School of Pharmacy. [Google Scholar]
- 51.Ethiopian Parliament Proclamation No. 728/2011, Veterinary Drug and Feed Administration and Control Proclamation. 2012. https://www.lawethiopia.com/index.php/proclamations/2215-proclamation-no-728
- 52.Ethiopian Parliament Food, Medicine and Health Care Administration and Control Proclamation no. 661/2009 Fed. Negarit Gaz., 16th year, no. No.9. 2010. pp. 5157–5191. [Google Scholar]
- 53.Ethiopian Parliament, Proclamation No. 267/2002 Animal Diseases Prevention and Control Proclamation Negarit Gazeta, 6(14) 2002. p. 1694. [Google Scholar]
- 54.VDFACA (Veterinary Drug and Feed Administration and Control Authority, Ethiopia) Standard Veterinary Treatment Guidelines for Ethiopia. 2nd ed. 2020. Addis Ababa, Ethiopia. [Google Scholar]
- 55.VDFACA (Veterinary Drug and Feed Administration and Control Authority, Ethiopia) Ethiopian Veterinary Drugs List. 2nd ed. 2019. Addis Ababa, Ethiopia. [Google Scholar]
- 56.FNHACA (Food, Nutrition and Health Administration and Control Authority), Ethiopia A Practical Guide to Antimicrobial Stewardship Program in Ethiopian Hospitals. 2018. Addis Ababa Ethiopia. [Google Scholar]
- 57.FMHACA (Food, Medicine and Health Care Administration and Control Authority Administration and Control Authority, Ethiopia) Standard Treatment Guidelines. 3rd ed. 2014. Addis Ababa, Ethiopia. [Google Scholar]
- 58.Gemeda B.A., Amenu K., Magnusson U., Dohoo I. Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front. Vet. Sci. 2020;7:1–15. doi: 10.3389/fvets.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fetensa G., Wakuma B., Tolossa T., Fekadu G., Bekuma T.T., Fayisa L., Etafa W., Bekela T., Besho M., Hiko N., Bekele M.B., Worku D., Yadesa G., Tsegaye R. Knowledge and attitude towards antimicrobial resistance of graduating health science students of Wollega University. re. 2020;13:3937–3944. doi: 10.2147/IDR.S264481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seid M.A., Hussen M.S. Knowledge and attitude towards antimicrobial resistance among final year undergraduate paramedical students at University of Gondar, Ethiopia. BMC Infect. Dis. 2018;18:312. doi: 10.1186/s12879-018-3199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teshome D., Id G., Bekele D., Mulate B., Id G.G. Knowledge, attitude and practice of animal producers towards antimicrobial use and antimicrobial resistance in Oromia zone, northeastern Ethiopia. PLoS One. 2021;16 doi: 10.1371/journal.pone.02. e0251596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooper P. 2016. Review of Animal Health Service Delivery in the Mixed Crop-Livestock System in Ethiopia Mixed Crop-Livestock System in Ethiopia International Livestock Research Institute, Nairobi, Kenya. 92–9146–461–9. [Google Scholar]
- 63.NVI (National Veterinary Institute) Brochure. 2020. [Google Scholar]
- 64.Ministry of Health . 2019, November. Ethiopia Essential Health Services Package of Ethiopia Addis Ababa, Ethiopia; pp. 1–156. [Google Scholar]
- 65.WHO, World Health Organization Health Worker Education and Training on Antimicrobial Resistance Geneva, Organization. 2019. https://apps.who.int/iris/bitstream/handle/10665/329380/9789241516358-eng.pdf?sequence=1&isAllowed=y License: CC BY-NC-SA 3.0 IGO. 2019, Available at:
- 66.Beyene A., Hailu T., Faris K., Kloos H. Current state and trends of access to sanitation in Ethiopia and the need to revise indicators to monitor progress in the Post-2015 era. BMC Public Health. 2015;15:451. doi: 10.1186/s12889-015-1804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andualem Z., Dagne H., Azene Z.N., Taddese A.A., Dagnew B., Fisseha R., Muluneh A.G., Yeshaw Y. Households access to improved drinking water sources and toilet facilities in Ethiopia: a multilevel analysis based on the 2016 Ethiopian Demographic and Health Survey. BMJ. 2021;10 doi: 10.1136/bmjopen-2020-042071. e042071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shore R. Water in Crisis-Spotlight Ethiopia, The Water Project. 2022. https://thewaterproject.org/water-crisis/water-in-crisis-ethiopia Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.